Abstract
Background
Other studies have reported high rates of depression and anxiety among human T-lymphotropic virus type 1 (HTLV-1) infected subjects, and have even suggested that HTLV-I causes psychiatric disease.
Study design and methods
We interviewed HTLV-I, HTLV-II and demographically similar HTLV seronegative blood donors with the Mini-International Neuropsychiatric Interview (MINI). Prevalences of major depression and generalized anxiety disorder in each group were calculated and compared to published U.S. population data. Adjusted odds ratios (aOR) and 95% confidence intervals (95% CI) controlling for educational achievement, alcohol intake and self-reported health status were calculated with multivariate logistic regression.
Results
Major depression was diagnosed in 5 (5.4%) of 93 HTLV-I positive subjects (aOR = 2.19, 95% CI 0.63–7.55) and 17 (6.6%) of 256 HTLV-II positive subjects (aOR = 1.61, 95% CI 0.66–3.927), compared to 12 (2.1%) of 585 HTLV seronegative blood donors. The prevalence of major depression among infected subjects was comparable to the 6.7% prevalence in the U.S. general population. Generalized anxiety disorder was diagnosed in 5 (5.4%) HTLV-I positive subjects (OR= 2.32, 95% CI 0.74–7.26) and 12 (4.7%) HTLV-II positive subjects (OR = 1.65 95% CI 0.68–4.01), compared to 15 (2.6%) seronegatives and 3.1% in the U.S. general population.
Conclusion
Major depression and generalized anxiety disorder were not significantly more prevalent among HTLV-I and HTLV-II infected former blood donors after controlling for health status and other confounding variables. HTLV seronegative blood donors had lower prevalence of these conditions than the U.S. population, probably due to a “healthy blood donor effect”.
Keywords: HTLV-I Infections, HTLV-II Infections, Depression, Anxiety Disorders, Mental Disorders
Introduction
Depression has been associated with several chronic viral infections including human immunodeficiency virus (HIV)1,2 hepatitis B virus (HBV) 3,4 and hepatitis C virus (HCV) 5,6,7. These observations have been attributed to a variety of factors, including the psychological stress of living with a morbid and transmissible disease, side effects of treatment, neurological changes caused by progression of disease as well as the role of pre-existing depression or other psychiatric disorders in influencing the frequency of high risk behaviors that can result in infection with these viruses.
Although less familiar to patients and physicians than the above-mentioned viruses, human T-lymphotropic viruses types I and II (HTLV-I and –II) were the first recognized human retroviral infections8. HTLV-I causes adult T-cell leukemia (ATL), HTLV associated myelopathy (HAM) and HTLV associated uveitis, while HTLV-II causes HAM and is also associated with pulmonary and urinary tract symptoms as well as increased mortality9,10. Modes of transmission for HTLV I/II are similar to other blood borne pathogens and include sexual contact, blood transfusion, breast feeding, and use of contaminated needles for intravenous injection.
A couple of recent studies have found increased prevalence of psychiatric symptoms among HTLV seropositives. Carvalho used the Mini International Neuropsychiatric Interview, Brazilian Version (MINI) to assess psychiatric disorders among 50 HTLV-1 seropositive patients in Salvador and other cities in the state of Bahia, Brazil. Twenty-one subjects (42%) had psychiatric co-morbidity, including 15 (30%) with depression and 7 (14%) with general anxiety11. Subjects with symptoms of HTLV diseases had higher rates of psychiatric co-morbidity than those who were non-symptomatic (depression 35% vs. 25%; general anxiety 15% vs. 13%). Similarly, Stumpfstudied the relationship between HTLV-1 and depression in Belo Horizonte, Brazil in 2004–2005 using the Brazilian MINI administered within cohort of 74 HTLV-I seropositive and 24 seronegative blood donor candidates 12. They found a significantly higher prevalence of depression among the HTLV compared to seronegative subjects (39% vs. 8%; p = .0005). Both Brazilian studies yielded results much higher than 1 year prevalence estimates in the Brazilian population, namely 4.5% for major depression and 1.3% for generalized anxiety disorder 13,14. Carvalho et al. suggested a causal role for HTLV-I infection in the pathogenesis of depression in their subjects.
These provocative findings led us to assess the prevalence of depression and anxiety in our own long term cohort of HTLV I and-II infected United States (U.S.) blood donors and demographically matched HTLV seronegative controls. We attempted to replicate and extend the findings of the Brazilian investigators.
Methods
Study design and subjects
We studied subjects from a cohort of 155 HTLV-I seropositive, 387 HTLV-II seropositive, and 799 seronegative blood donors enrolled in 1990–1992 as part of the HTLV Outcomes Study (HOST). At enrollment, participants were age 18 or older, HIV seronegative and were blood donors at one of 5 geographically dispersed U.S. blood centers. HTLV-I and HTLV-II infection status was determined by enzyme immunoassay, followed by type-specific Western blot or polymerase chain reaction. Seronegative controls were demographically matched to positive subjects by age, sex, race/ethnicity, type of blood donation (whole blood, autologous, or apheresis) and blood center. Subjects were followed with in-person visits or mail/telephone questionnaires approximately every two years since enrollment. Each visit included a health questionnaire including questions on overall health status, a screening neurological exam, and phlebotomy for CBC testing and repository storage. Questions pertaining to overall health status were similar to those contained in the Third International Health and Nutrition Examination Survey.15,16 Current symptomatology pertinent to diseases known or suspected to be related to HTLV-I or II was assessed.
During the eighth study visit in July 2007 through February 2009 we added an assessment for depression and anxiety using an adaptation of the Mini-International Neuropsychiatric Interview (MINI), a diagnostic interview designed for use by non-physician technicians who are not psychiatrists or doctoral level psychologists. The MINI interviews were added to the standard health questionnaire collected for the study and performed by trained nurse counselors in person or by telephone. Interviewers were not blinded to the serostatus of the study subjects. We also added questions regarding the use of anti-depressant and anti-anxiety medications.
Psychiatric assessment
The MINI is a short and easily administered instrument which was designed as a screening tool for use in research and clinical settings to diagnose psychiatric disorders according to the frameworks set out in Diagnostic and Statistical Manual (DSM),4th edition American Psychiatric Association, 2000 and International Classification of Diseases (ICD)-10 psychiatricdisorders (World Health Organization 1992). It consists ofstandardized, structured, closed-end questions which are read verbatim to the interviewees. Psychiatric diagnoses are made according to the number of affirmativereplies to specific questions. Sheehan et al, 1998 performed several parallel studies to test the validity of MINI diagnoses17. Concordance between the clinician rated MINI and the structured clinical interview for DSM axis 1 disorders (SCIP-P) was very good or good for major depressive disorder and generalized anxiety disorder and in a lower range, but acceptable for dysthymia. Concordance between the MINI and the composite international diagnostic interview (CIDI) was very good for major depressive disorder, and slightly lower but acceptable for generalized anxiety disorder. Agreement between the MINI diagnoses generated by general practitioners and expert psychiatrist diagnoses was found in 85% of the patients, and was the highest for major depressive disorder, generalized anxiety disorder and social phobia.
To reduce the time of this assessment and focus on the scope of the current study (depression and anxiety) weonly included questions pertaining to major depressive disorder, dysthymia, and generalized anxiety disorder. We compared our results to previously reported point prevalence estimates of major depression and generalized anxiety disorders in the adult U.S. population which were derived from interviews of a probability sample of U.S. adults conducted between February 2001 and April 2003 using the CIDI18.
Statistical Analysis
Initially, six binary dependent variables were included in the analysis: current major depressive episode, recurrent major depressive episode, current dysthymia, generalized anxiety disorder, current anti-depressive medication intake, and current anti-anxiety medication intake. Afterward current major depressive episode and recurrent major depressive episode variables were combined into one variable called major depression, due to a small number of affected subjects. Each dependent variable was analyzed for its association with either HTLV-I or HTLV-II compared to seronegative status. Other independent variables included demographic characteristics, education, alcohol consumption per week and self-reported overall health status were added to the analysis as potential predictors. Current injection drug use variable was excluded from analysis due to the small number of subjects admitting this behavior. Predictors were categorized as follows: age, approximate tertiles, 34–50, 51–64, and =>65 years; alcohol intake, approximate tertiles, 0, 0–1, and >1 drinks per week; education as high school, some college or technical school, and college degree.
Multivariable logistic regression (PROC LOGISTIC, SAS Institute, Cary NC), was used to calculate odds ratios and 95% confidence intervals for the association of HTLV-I and HTLV-II with each outcome variable, adjusted for covariates. For each HTLV type and psychiatric disorder a separate multivariable model was run, and the chi-square goodness-of-fit test was used to assess the fit of each model. Due to the fact that gender and race did not show significant effects on outcome variables, they were dropped from model. Finally, we ran multivariate models while adding overall health, work loss days, disabled/unable to work, pneumonia, and bronchitis derived from the main health questionnaire. Overall health was the only health related variable to show a significant effect on outcome variables, so it was kept in the final model.
Additionally, we compared results from the MINI in Visit 8 to General Well Being Scale (GWB) data gathered from the cohort during Visit 2 in 1992–1994. The GWB is a standardized, self-administered, psychological measure consisting of questions concerning the “presence, severity, or frequency of some clinical symptoms that are generally considered important in making assessments of subjective well-being”.19 GWB items are scaled, yielding numerical scores on 6 subscales as well as an overall General Well Being score. Considerable evidence confirms the correlational validity of the GWB with both depression and anxiety scales.20 The summed overall GWB scores from 1992–1994 were placed into three categories representing levels of disorder: positive well being, moderate distress, and severe distress21. A Cochran-Armitage Trend Test was used to calculate odds ratios for associations with future psychiatric symptoms.
Results
Study population
MINI interviews were collected from 93 HTLV-I, 256 HTLV-II and 585 HTLV seronegative subjects, with demographic characteristics shown in Table 1. Almost three quarters of the study population was female. Approximately a quarter of the subjects were less than 51 years of age, half were between 52 and 64, and another quarter of the subjects were 65 or older. No single race or ethnicity dominated the cohort, with Whites representing just under half of the participants and Black subjects representing about another third of the group. The cohort had a relatively high level of educational attainment, with 30 percent having received a college or post graduate degree, and 45 percent having received at least some college or technical school education. More than two thirds of the subjects in the study reported having less than 2 drinks of alcohol per week – including 40 percent who reported no alcohol use, and almost 90 percent of the cohort reported either excellent, very good or good health at the time of the interview.
Table I.
Study Population
| Variable | HTLV NEG (n=585) | HTLV I (n=93) | HTLV II (n=256) |
|---|---|---|---|
| Gender | |||
| Male | 176 (30.09%) | 18 (19.35%) | 55 (21.48%) |
| Female | 409 (69.91%) | 75 (80.65%) | 201 (78.52%) |
| Age | |||
| <=51 | 153 (26.15%) | 19 (20.43%) | 73 (28.52%) |
| 52–64 | 277 (47.35%) | 51 (54.84%) | 141 (55.08%) |
| >64 | 155 (26.50%) | 23 (24.73%) | 42 (16.41%) |
| Race | |||
| White | 286 (48.89%) | 38 (40.86%) | 118 (46.09%) |
| Black | 166 (28.38%) | 37 (39.78%) | 80 (31.25%) |
| Hispanic | 67 (11.45%) | 2 (2.15%) | 48 (18.75%) |
| Asian | 35 (5.98%) | 13 (13.98%) | 4 (1.56%) |
| Other/Missing | 31 (5.30%) | 3 (3.23%) | 6 (2.34%) |
| Education | |||
| High school or less | 92 (15.73%) | 29 (31.18%) | 95 (37.11%) |
| Some college/Technical school | 256 (43.76%) | 38 (40.86%) | 121 (47.27%) |
| College graduate and higher | 228 (38.97%) | 23 (24.73%) | 36 (14.06%) |
| Missing | 9 (1.54%) | 3 (3.23%) | 4 (1.56%) |
| Drinks per week | |||
| Non-drinker | 221 (37.78%) | 44 (47.31%) | 115 (44.92%) |
| 0–1 drinks per week | 175 (29.91%) | 27 (29.03%) | 74 (28.91%) |
| > 1 drinks per week | 189 (32.31%) | 22 (23.66%) | 67 (26.17%) |
| Overall Health | |||
| Excellent | 139 (23.76%) | 19 (20.43%) | 35 (13.67%) |
| Very Good/Good | 411 (70.26%) | 62 (66.67%) | 174 (67.97%) |
| Fair/Poor | 35 (5.98%) | 12 (12.90%) | 47 (18.36%) |
Major depressive disorder and dysthymia
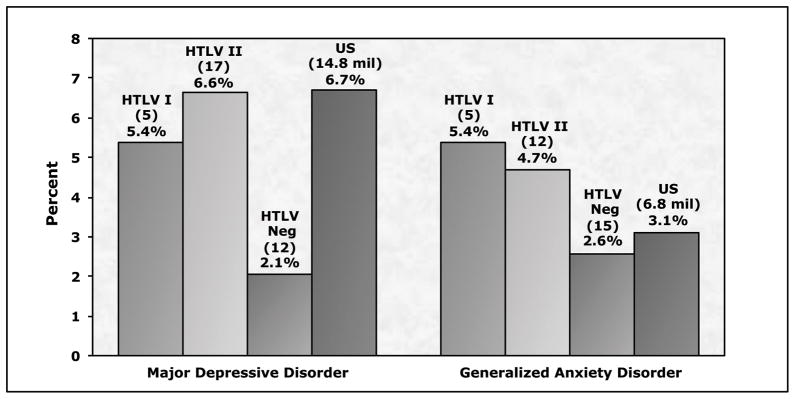
Major depression was diagnosed in 5 (5.4%) of HTLV-I and 18 (6.6%) of HTLV-II subjects, compared to 12 (2.1%) of seronegative subjects (Figure 1). The prevalence of major depression in HTLV-I and – II infected subjects was similar to current estimates for the general U.S. population (6.7%), while the prevalence of depression in our HTLV negative blood donors (2.1%) is much lower than population estimates. Anti-depression medication use was reported by 7 HTLV negatives (1.20%), 1 HTLV-I subject (1.03%) and 6 HTLV-II subjects (2.24%). Two HTLV negative subjects (0.35%) were diagnosed with dysthymia compared to no HTLV-I subjects and five HTLV-II subjects (2.0%), precluding further analysis of dysthymia.
Figure 1. Percentage of subjects with major depressive disorder or generalized anxiety disorder, by HTLV infection status.
US refers to United States population estimates from reference 18.
Generalized anxiety disorder
Generalized anxiety disorder was diagnosed in 5 (5.4%) of HTLV-I subjects and 12 (4.7%) of HTLV II positive subjects (Figure 1). The prevalence of generalized anxiety disorder in HTLV-I and II infected subjects was slightly higher than prevalence estimates for the adult U.S. population (3.1%), while the prevalence of generalized anxiety disorder we found in our HTLV negative subjects (2.6%) was slightly lower than the U.S. population prevalence estimate. Anti-anxiety medication use was reported by a total of 9 subjects, 3 negative controls and 6 HTLV II seropositive donors.
Multivariable analysis
In the final multivariable model in which overall health status was included (Table 2), neither HTLV-I nor HTLV-II was significantly associated with major depression or generalized anxiety disorder, although a trend was still observed. On the other hand, overall health status was significantly associated with both major depression (aOR = 27.31 in HTLV-I model and aOR =17.10 in HTLV-II model) and generalized anxiety disorder (aOR = 5.22 in HTLV-I model and aOR =4.25 in HTLV-II model).
Table 2.
Predictors of major depressive disorder (A) and generalized anxiety disorder (B)
| Major Depressive Disorder | OR (95% CI) | OR (95% CI) |
|---|---|---|
| HTLV Status | HTLV I | HTLV II |
| HTLV Negatives | 1 | 1 |
| HTLV Positives | 2.19 (0.63–7.55) | 1.61 (0.66–3.92) |
| Age | ||
| < 52 | 1 | 1 |
| 52–64 | 0.50 (0.15–1.65) | 0.75 (0.31–1.85) |
| >64 | 0.07 (<0.01–0.65) | 0.20 (0.04–1.00) |
| Education | ||
| High school diploma or less | 1 | 1 |
| Some college or technical school | 2.58 (0.58–11.48) | 1.44 (0.55–3.79) |
| College degree | 0.76 (0.13–4.63) | 0.44 (0.101.87) |
| Alcohol intake | ||
| Non- drinkers | 1 | 1 |
| 0–1 drinks per week | 0.80 (0.21–3.08) | 2.05 (0.73–5.78) |
| > 1 drinks per week | 0.36 (0.07–1.82) | 2.33 (0.79–6.83) |
| Overall Health | ||
| Excellent or very good | 1 | 1 |
| Good | 1.89 (0.45–7.99) | 2.73 (0.91–8.17) |
| Fair or poor | 27.31 (6.79–109.82)* | 17.1 (5.58–52.41)* |
| B. Generalized Anxiety Disorder | ||
| HTLV Status | ||
| HTLV Negatives | 1 | 1 |
| HTLV type | 2.32 (0.74–7.26) | 1.65 (0.68–4.01) |
| Age | ||
| < 52 | 1 | 1 |
| 52–64 | 0.28 (0.10–0.80) | 0.69 (0.29–1.66) |
| >64 | 0.14 (0.03–0.69) | 0.34 (0.09–1.29) |
| Education | ||
| High school diploma or less | 1 | 1 |
| Some college or technical school | 0.77 (0.21–2.89) | 2.19 (0.67–7.18) |
| College degree | 0.71 (0.18–2.79) | 1.95 (0.52–7.33) |
| Alcohol intake | ||
| Non- drinkers | 1 | 1 |
| 0–1 drinks per week | 1.35 (0.40–4.52) | 1.26 (0.40–3.94) |
| > 1 drinks per week | 1.26 (0.38–4.16) | 2.99 (1.09–8.21) |
| Overall Health | ||
| Excellent or very good | 1 | 1 |
| Good | 0.32 (0.07–1.53) | 1.50 (0.56–4.00) |
| Fair or poor | 5.22 (1.53–17.79)* | 4.25 (1.41–12.84)* |
General Well Being Scores
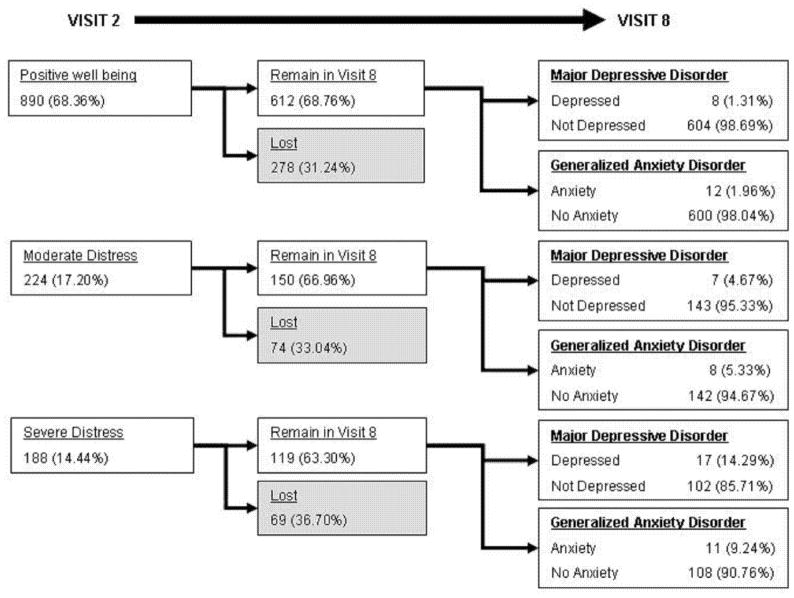
We compared subject scores on the General Well Being Scale administered during Visit 2 (1992–1994) to MINI results in Visit 8 and found a strong relationship between lower GWB score and diagnoses of major depressive disorder and generalized anxiety disorder in Visit 8 almost 15 years later (Figure 2). Prevalence of major depression increase from 1.31% in subjects with “Positive Well Being”, to 4.67% in subjects with “Moderate Distress”, to 13.51% in subjects with “Severe Distress” (p=<.0001). Similarly, prevalence of generalized anxiety disorder increased from 1.96% in subjects with “Positive Well Being” in Visit 2, to 5.33% of subjects with “Moderate Distress”, to 9.24% of subjects with “Severe Distress” (p=<.0001).
Figure 2. Diagnoses of Major Depression and Generalized Anxiety Disorder in Visit 8 according to General Well Being (GWB) Scores in Visit 2.
Subjects were classified into categories of “positive well being”, “moderate distress” and “severe distress” based upon their responses to questionnaires in Visit 2. Loss to follow-up, defined as non-completion of survey in Visit 8, was similar among GWB categories. The prevalence of subjects with major depressive disorder or generalized anxiety disorder during Visit 8 is inversely correlated with GWB score in Visit 2.
Discussion
We found that HTLV I and II positive subjects in our study had a two- to three-fold increased prevalence of major depression and generalized anxiety disorder when compared to HTLV seronegative, demographically similar blood donor controls. However the prevalence of depression and generalized anxiety disorder among these HTLV infected subjects was less than or similar to the expected population point prevalence for those disorders among U.S. adults. After controlling for overall health status and other confounders, the association between HTLV infection and psychiatric diagnoses did not remain significant, though a trend was observed. The strongest relationship we observed in our statistical modeling was between the presence of fair or poor overall health and both depression and anxiety.
We feel that much of the observed difference in the prevalence of psychiatric disorders between the HTLV negative and HTLV positive subjects in our study is likely due to what has been referred to as the “healthy donor effect” 22. Blood donors, selected for health and low risk of infectious disease, tend to have lower disease prevalence than the general population. The rates of psychiatric disorders found among our negative controls (all originally enrolled in the cohort as healthy blood donors) were more than three times lower than U.S. population norms for depression and comparable to (but still lower than) population norms for anxiety. These data reflect other published data comparing healthy blood donors to population norms in which donors have been found to have 70% of mortality compared to the general U.S. population23, 30% lower mortality and 4% lower cancer incidence lower than population rates in Denmark and Sweden24, and scored significantly higher on the General Well Being Scale, a measure of subjective feelings of psychological well being and distress, when compared to a large population sample from the first National Health and Nutrition Examination Study (NHANES1)21. In this context we feel that general population estimates are better comparisons for understanding the level of depression and anxiety in our subjects, and thus our study does not replicate results the Brazilian studies which found elevated rates of psychiatric disease in HTLV-I or HTLV-II infected subjects.
This conclusion raises an obvious question. Why would rates of depression found among Brazilian HTLV-I infected subjects be so much higher than the rates found in our study, and higher than published one-year population prevalence estimates for Brazil? That question will be impossible to answer with certainty, but there are some factors that should be considered. Socio-economic differences are likely to account for some of the difference. The Carvalho study analyzed a group of patients from Salvador, Brazil who were mostly female (68%), middle aged (mean = 46.9), unmarried (60%), non-white (88%), had low educational attainment (52%), and were from rural areas (54%). Stumpf studied blood donor candidates from Belo Horizonte, Brazil with similar socio-demographic characteristics who were mostly female (70%) with a median age of 37, low educational attainment (61%), and low income (43%). Soares et al25 also reported that black and mixed race and sexual and mother to child transmission were predominantly associated with positivity in their cohort. These demographics are in contrast to the subjects in our study who were older, more educated and of higher income. Numerous studies, including several from Brazil, have reported that people in lower socioeconomic groups are more likely to suffer from psychological disorders such as depression and anxiety, typically because of stress26,27,28,29, 30. McDowell describes some of the challenges specific to measuring depression, stating: “There is also a confounding effect whereby people in lower socioeconomic groups are likely to suffer great stress and more depression but also to exhibit different response styles and admit to more problems than the more educated.” 31
This confounding may be amplified further by cultural differences since it is well documented that cultural values and beliefs differ with regard to psychological disorders 32, 33, 34. Sheehan and Louie report on the “stigmatization of mental health problems within the Asian culture in which common, treatable depression may be equated with insanity.” 35 Many languages do not have words for depression and many cultures provide other explanations for depressive feelings. A direct translation for the word depression does not exist in Chinese, and in Filipino culture, which is predominantly Catholic depression is often viewed as a “test of one’s faith”.36 There is evidence that depression and other psychiatric disorders are not seen to be as pathological in Brazilian culture as they may be in other cultures, particularly the United States. Giosan et al 32 found that Brazilian participants judged only 20 of 68 conditions to be mental disorders, although 47 of the conditions represented DSM-IV disorders. In the same study Americans judged 36 and Romanians judged 28 conditions to be disorders. He concluded that elements of subjective distress and intra-psychic malfunction or conflict were not as frequently seen as mental disturbances (‘distúrbio mental’) among the Brazilians, possibly indicating more acceptance of and less stigma attached to disorders such as depression or anxiety. Des Courtis33 analyzed general attitudes about mental illness comparing responses by Brazilian and Swiss mental health professionals to a questionnaire and vignettes. The study found that Brazilian mental health professionals displayed a more positive attitude towards community psychiatry whereas the Swiss sample showed more stigmatization and social distance, and a more positive attitude towards psycho-pharmacology. Thus it seems possible that the differences between the results of the Brazilian and U.S. studies on depression and anxiety in HTLV infected subjects may be the result of cultural and socioeconomic differences between subjects.
There are at least four possible hypotheses for an association between HTLV-I and –II and psychiatric disease: i) viral infection results in a physiological cascade which increases vulnerability to depression and anxiety; or ii) depression and anxiety result from physical symptoms and illness accompanying HTLV-I and –II infection; or iii) stress related to the stigma and relationship difficulties inherent in having a sexually transmitted infection increases the probability of psychiatric disease; or iv) underlying psychological difficulties such as depression or anxiety increase the likelihood of risk behaviors lead which predispose to infection with HTLV (effect:cause). Our prospective data lend intriguing support to the second and third of these hypotheses. Because of the perspective follow-up of our subjects, we were able to assess the relationship between an earlier measure of general well-being and the occurrence of psychiatric disease 15 years later. If we assume that lower General Well Being Scale among HTLV subjects in Visit 2 reflected subjective experience of lessened vitality or lower overall health, it is very interesting that overt psychiatric disease occurring 15 years later could be predicted by the degree of earlier distress. This experience could be related to both the stress and difficulty of having a sexually transmitted infection as well as to the presence of physical symptoms, and we believe it underscores the importance of providing appropriate counseling and referral for blood donors who are found to be positive for HTLV during a donation visit.
Strengths of our study include its setting in a prospective cohort followed for many years. Controls were well matched to HTLV infected subjects, and data were collected using standardized instruments by trained research nurses. The MINI instrument which we utilized has been well validated. Finally, our cohort was free of HIV infection and had low rates of injection drug use, both of which may contribute co-morbidity.
One limitation of our study is potential selection bias due to the original recruitment of our subjects from blood donors. We have already described the “healthy donor effect” among our seronegative controls. Our HTLV positive former blood donors may also not be representative of other HTLV positive individuals in the general U.S. population. Additionally, though relationships between demographic or infection status variables can be observed and described, causal relationships are impossible to prove in a prevalent cohort study in which subjects were already infected with HTLV at enrollment.
In conclusion, we have observed moderately increased levels of psychiatric disease among HTLV-I and HTLV-II subjects that are most likely explained by a combination of selection bias, and viral-related symptoms and psychological distress. Our data do not support a biological role for HTLV in the pathogenesis of depression. Differences between our findings and those of previous Brazilian studies may be reconciled by further investigation of cultural differences by which psychiatric disease is experienced by patients and diagnosed by physicians.
Acknowledgments
The Authors would like to thank Daniel Arthur Hindes and Susan Yuen for their immeasurable contributions to the HOST study. We also thank the participants at all 5 blood centers for their ongoing participation in this very long term cohort.
Funding: This work was supported by National Institutes of Health [2R01-HL-62235, and K24-HL-75036] and by Blood Systems Research Institute.
APPENDIX
The HTLV Outcomes Study (HOST) is the responsibility of the following persons:
Study Headquarters:
University of California, San Francisco, CA: E.L.
Murphy (Principal Investigator), J.W. Engstrom, D. DeVita, S. Yuen.
Blood Centers:
American Red Cross Blood Services Greater Chesapeake and Potomac Region, Baltimore, MD: J.W. Gibble
American RedCross Blood Services Southeastern Michigan Region, Detroit, MI: B.H. Newman
American Red Cross Blood Services Southern California Region, Pomona, CA: G. Garratty, A. Ziman, S.T. Hutching
Blood Centers of the Pacific, San Francisco, CA: M.P. Busch
Sylvan N. Goldman Center, Oklahoma Blood Institute, Oklahoma City, OK: J.W. Smith.
Central Laboratory:
Blood Centers of the Pacific, San Francisco, CA:
M.P. Busch, L. Pitina, L.H. Tobler.
Diagnostic Review Panel:
E.L. Murphy, R.A. Sacher, J.L. Fridey.
Footnotes
There are no conflicts of interest among the authors.
References
- 1.Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5:163–171. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- 2.Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 3.Kunkel EJ, Kim JS, Hann HW, et al. Depression in Korean immigrants with hepatitis B and related liver diseases. Psychosomatics. 2000;41:472–480. doi: 10.1176/appi.psy.41.6.472. [DOI] [PubMed] [Google Scholar]
- 4.Arslan N, Buyukgebiz B, Ozturk Y, Akay AP. Depression and anxiety in chronic hepatitis B: effect of hepatitis B virus infection on psychological state in childhood. Turk J Pediatr. 2003;45:26–28. [PubMed] [Google Scholar]
- 5.Basseri B, Yamini D, Chee G, Enayati PD, Tran T, Poordad F. Comorbidities associated with the increasing burden of hepatitis C infection. Liver Int. 2010;7:1012–1018. doi: 10.1111/j.1478-3231.2010.02235.x. [DOI] [PubMed] [Google Scholar]
- 6.Ferenci P, Staufer K. Depression in chronic hepatitis: the virus, the drug, or the ethnic background? Liver Int. 2008;28:429–431. doi: 10.1111/j.1478-3231.2008.01703.x. [DOI] [PubMed] [Google Scholar]; Carta MG, Hardoy MC, Garofalo A, et al. Association of chronic hepatitis C with major depressive disorders: irrespective of interferon-alpha therapy. Clin Pract Epidemiol Ment Health. 2007;3:22. doi: 10.1186/1745-0179-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66:41–48. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 9.Beilke MA, Theall KP, O’Brien M, et al. Clinical outcomes and disease progression among patients coinfected with HIV and human T lymphotropic virus types 1 and 2. Clin Infect Dis. 2004;39:256–263. doi: 10.1086/422146. [DOI] [PubMed] [Google Scholar]
- 10.Roucoux DF, Murphy EL. The epidemiology and disease outcomes of human T-lymphotropic virus type II. AIDS Rev. 2004;6:144–154. [PubMed] [Google Scholar]
- 11.Carvalho AG, Galvao-Phileto AV, Lima NS, Jesus RS, Galvao-Castro B, Lima MG. Frequency of mental disturbances in HTLV-1 patients in the state of Bahia, Brazil. Braz J Infect Dis. 2009;13:5–8. doi: 10.1590/s1413-86702009000100003. [DOI] [PubMed] [Google Scholar]
- 12.Stumpf BP, Carneiro-Proietti AB, Proietti FA, Rocha FL. Higher rate of major depression among blood donor candidates infected with human t-cell lymphotropic virus type 1. Int J Psychiatry Med. 2008;38:345–355. doi: 10.2190/PM.38.3.i. [DOI] [PubMed] [Google Scholar]
- 13.Andrade L, Walters EE, Gentil V, Laurenti R. Prevalence of ICD-10 mental disorders in a catchment area in the city of Sao Paulo, Brazil. Soc Psychiatry Psychiatr Epidemiol. 2002;37:316–325. doi: 10.1007/s00127-002-0551-x. [DOI] [PubMed] [Google Scholar]
- 14.Almeida-Filho N, Mari J de J, Coutinho E, et al. Brazilian multicentric study of psychiatric morbidity. Methodological features and prevalence estimates. Br J Psychiatry. 1997;171:524–529. doi: 10.1192/bjp.171.6.524. [DOI] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. National Health and Nutrition Examination Survey III: Data Collection Forms. Hyattsville, Md: National Center for Health Statistics; 1991. [Google Scholar]
- 16.Murphy EL, Glynn SA, et al. Increased prevalence of infectious diseases and other adverse outcomes in human T lymphotropic virus types I- and II-infected blood donors. J Infect Dis. 1997;176(6):1468–1475. doi: 10.1086/514143. [DOI] [PubMed] [Google Scholar]
- 17.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 18.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan TT, Livieratos B. Interpreting a general index of subjective well-being. Milbank Mem Fund Q Health Soc. 1978;56(4):531–556. [PubMed] [Google Scholar]
- 20.McDowell Ian. Measuring Health: A Guide to Rating Scales and Questionnaires Third Edition. Oxford; New York: Oxford University Press; 2006. [Google Scholar]
- 21.Guiltinan AM, Murphy EL, Horton JA, Nass CC, McEntire RL, Watanabe K. Psychological distress in blood donors notified of HTLV-I/II infection. Retrovirus Epidemiology Donor Study. Transfusion. 1998;38:1056–1062. doi: 10.1046/j.1537-2995.1998.38111299056317.x. [DOI] [PubMed] [Google Scholar]
- 22.Atsma F, de Vegt F. The healthy donor effect: a matter of selection bias and confounding. Transfusion. 2011;51(9):1883–5. doi: 10.1111/j.1537-2995.2011.03270.x. [DOI] [PubMed] [Google Scholar]
- 23.Guiltinan AM, Kaidarova Z, Custer B, et al. Increased all-cause, liver, and cardiac mortality among hepatitis C virus-seropositive blood donors. Am J Epidemiol. 2008;167:743–750. doi: 10.1093/aje/kwm370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgren G, Tran TN, Hjalgrim H, et al. Improving health profile of blood donors as a consequence of transfusion safety efforts. Transfusion. 2007;47:2017–2024. doi: 10.1111/j.1537-2995.2007.01425.x. [DOI] [PubMed] [Google Scholar]
- 25.Soares BC, Proietti AB, Proietti FA. 2003 “HTLV-I/II and blood donors: determinants associated with seropositivity in a low risk population. Rev Saude Public. 2003;37(4):470–476. doi: 10.1590/s0034-89102003000400012. [DOI] [PubMed] [Google Scholar]
- 26.Carvalhais SM, Lima-Costa MF, Peixoto SV, Firmo JO, Castro-Costa E, Uchoa E. The influence of socio-economic conditions on the prevalence of depressive symptoms and its covariates in an elderly population with slight income differences: the Bambui Health and Aging Study (BHAS) Int J Soc Psychiatry. 2008;54:447–456. doi: 10.1177/0020764008090792. [DOI] [PubMed] [Google Scholar]
- 27.Anselmi L, Barros FC, Minten GC, Gigante DP, Horta BL, Victora CG. Prevalence and early determinants of common mental disorders in the 1982 birth cohort, Pelotas, Southern Brazil. Rev Saude Publica. 2008;42 (Suppl 2):26–33. doi: 10.1590/s0034-89102008000900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stansfeld SA, Clark C, Rodgers B, Caldwell T, Power C. Repeated exposure to socioeconomic disadvantage and health selection as life course pathways to mid-life depressive and anxiety disorders. Soc Psychiatry Psychiatr Epidemiol. 2010 doi: 10.1007/s00127-010-0221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aneshensel CS. Toward explaining mental health disparities. J Health Soc Behav. 2009;50:377–394. doi: 10.1177/002214650905000401. [DOI] [PubMed] [Google Scholar]
- 30.Almeida-Filho N, Lessa I, Magalhaes L, et al. Social inequality and depressive disorders in Bahia, Brazil: interactions of gender, ethnicity, and social class. Soc Sci Med. 2004;59:1339–1353. doi: 10.1016/j.socscimed.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 31.McDowell I. Measuring Health: A Guide to Rating Scales and Questionnaires. Oxford University Press; Oxford; New York: 2006. [Google Scholar]
- 32.Giosan CGV, Haslam N. The Lay Concept of ‘Mental Disorder’: A Cross-Cultural Study. Transcult Psychiatry. 2001;38:317–332. [Google Scholar]
- 33.Des Courtis N, Lauber C, Costa CT, Cattapan-Ludewig K. Beliefs about the mentally ill: a comparative study between healthcare professionals in Brazil and in Switzerland. Int Rev Psychiatry. 2008;20:503–509. doi: 10.1080/09540260802565125. [DOI] [PubMed] [Google Scholar]
- 34.Kleinman A. Culture and depression. N Engl J Med. 2004;351:951–953. doi: 10.1056/NEJMp048078. [DOI] [PubMed] [Google Scholar]
- 35.Sheehan V, Louie D. Depression, Smoking and Hepatitis B: The Cultural Context of Three Urgent Health Issues Facing Asian-Americans. San Francisco Medical Society; San Francisco, CA: 2010. [Google Scholar]
- 36.Ferenci P, Staufer K. Depression in chronic hepatitis: the virus, the drug, or the ethnic background? Liver Int. 2008;28:429–431. doi: 10.1111/j.1478-3231.2008.01703.x. [DOI] [PubMed] [Google Scholar]