Abstract
One of the main clinical features of obstructive sleep apnea (OSA) is sustained hypertension and elevated sympathetic activity during waking hours. Chronic intermittent hypoxia (CIH), animal model of the hypoxemia associated with OSA, produces a similar sustained increase in blood pressure. This study determined the role of ΔFosB in the median preoptic nucleus (MnPO) in the sustained increase in mean arterial pressure (MAP) associated with CIH. Rats were injected in the MnPO with viral vectors that expressed green fluorescent protein (GFP) alone or GFP plus a dominant negative construct that inhibits the transcriptional effects of ΔFosB. In GFP injected rats and uninjected controls, 7 day exposure to CIH increased MAP by 7–10 mmHg during both intermittent hypoxia exposure and normoxia. Dominant negative inhibition of MnPO ΔFosB did not affect changes in MAP during intermittent hypoxia exposure but significantly reduced the sustained component of the blood pressure response to CIH during the normoxic dark phase. Inhibition of MnPO ΔFosB reduced the FosB/ΔFosB staining in the paraventricular nucleus and rostral ventrolateral medulla but not the nucleus of the solitary tract. PCR-array analysis identified five AP-1 regulated genes expressed in the MnPO that were increased by CIH exposure: ace1, ace2, nos1, nos3, prdx2, and map3k3. Dominant negative inhibition of ΔFosB in the MnPO blocked increased expression of each of these genes in rats exposed to CIH except for Prdx2. ΔFosB may mediate transcriptional activity in MnPO necessary for sustained CIH hypertension suggesting that neural adaptations may contribute to diurnal hypertension in OSA.
Keywords: hypertension, sleep apnea, angiotensin, hypothalamus, sympathetic nervous system
INTRODUCTION
Sleep apnea commonly refers to a number of disorders that are characterized by repetitive bouts of disordered breathing leading to interruptions or reductions in airflow during sleep1, 2. The episodic hypoxemia resulting from sleep disordered breathing has been proposed to produce cardiovascular and metabolic disease in patients with sleep apnea and chronic activation of the sympathetic nervous system is central to the development of cardiovascular disease associated with these disorders1–3. Studies of chronic intermittent hypoxia (CIH) in animal models have identified several factors that contribute to this chronic activation of the sympathetic nervous system and resulting hypertension that include sensitization of carotid chemoreceptors and activation of the renin-angiotensin system4–7. Recent studies indicate a role for the central nervous system in the sustained hypertension and sympathetic activation associated with CIH8–10. However, the CNS regions and mechanisms involved have not been fully determined.
FosB and its more stable splice variant ΔFosB are members of the AP-1 transcription factor family that are expressed in the CNS following repeated activation of the CNS 11–13. In a previous study, we exposed adult male rats to CIH for seven days and used ΔFosB immunohistochemistry to identity regions that were chronically activated by this protocol. CIH increased ΔFosB staining in CNS regions that regulate sympathetic outflow including the lamina terminalis, paraventricular nucleus of the hypothalamus, the rostral ventrolateral medulla, and the nucleus of the solitary tract 14.
The lamina terminalis consists of three separate regions that lie along the anterior wall of the third ventricle: the subfornical organ (SFO), median preoptic nucleus (MnPO), and organum vasculosum of the lamina terminalis (OVLT). Both the SFO and OVLT are circumventricular organs that lack a functional blood-brain-barrier and contain neurons that are sensitive to changes in plasma osmolality and to circulating peptides including angiotensin II 15–17. Along with the MnPO, these regions are critically involved in central autonomic regulation and body fluid homeostasis 17–20. In animal models, electrolytic lesions of the ventral lamina terminalis (anteroventral region of the third ventricle or AV3V) reverse or prevent many forms of neurogenic hypertension 21, so we tested the effects of electrolytic AV3V lesions on CIH hypertension. Based on our results, we conducted additional experiments to further characterize the contribution of this region and the role of chronic transcriptional activation of the MnPO in CIH hypertension.
Several transcription factors have been identified as candidates for mediating the effects of intermittent hypoxia on gene expression related to changes in sympathetic outflow including the AP-1 family of transcription factors 22. Studies using virally mediated dominant negative inhibition of ΔFosB have shown that it regulates changes in gene expression that are necessary for several behavioral adaptations after repeated exposure to drugs of abuse, stress, or antidepressant treatments 23–27. We conducted experiments to determine if ΔFosB plays a similar role in the lamina terminalis during CIH. These experiments focused on the MnPO because it is highly interconnected with both SFO and OVLT and has a major efferent projection to the PVN 28. Also, ibotenic acid lesions of the MnPO have been shown to attenuate the sustained phase of angiotensin II induced hypertension in the rat 29. In order to test this hypothesis, an adeno-associated viral vector (AAV) that expresses ΔJunD, a dominant negative construct that antagonizes ΔFosB- and other AP-1-meidated transcription, was stereotaxically injected in the MnPO region prior to 7 d CIH exposure. Using radio telemetry recording to measure changes in blood pressure, heart rate, and respiratory rate, we observed that dominant negative inhibition of MnPO ΔFosB attenuated the sustained component of the CIH pressor response and altered the pattern of ΔFosB staining in CNS autonomic regulatory regions. We also combined virally induced dominant negative inhibition of MnPO ΔFosB with PCR-array analysis to identify several AP-1 regulated genes that could mediate the contribution of the MnPO to CIH-induced hypertension.
METHODS
Animals
Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, 250–350g) were individually housed and maintained on a 14/10 h light cycle and provided with ad libitum access to food and water. Animal procedures were conducted according to NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee.
Electrolytic AV3V Lesions
Rats were anesthetized with isofluorane (2%) and placed in a stereotaxic instrument equipped to deliver isofluorane anesthesia. A 23 gauge nichrome electrode (insulated except at the tip) was placed 0.2 mm posterior and 7.5 mm ventral from bregma at the midline and anodal current (2.5 mA) was applied for 20 s. The same electrode was used for sham lesions but it was placed 6.5 mm ventral to bregma. Each rat was monitored for adipsia following surgery. Lesioned rats that displayed adipsia were provided with 10% sucrose to drink and weaned to tap water as previously described30. Lesioned rats were only included in the study if they demonstrated adipsia immediately following surgery. Following 2 weeks of recovery, the rats were instrumented with radio telemetry transmitters.
Virally-mediated dominant negative inhibition of MnPO ΔFosB
For MnPO injections, rats were anesthetized with isofluorane (2%) and placed in a stereotaxic head frame equipped with an isoflurane delivery system (David Kopf Instruments, Tujunga, CA). A midline incision was made through the disinfected scalp and the skull exposed around bregma. Stereotaxic coordinates for each injection were 0 mm anterior, 0.9 mm lateral and −6.7 mm ventral to bregma 31 with the injector angled 8° from vertical. Each 400 nl injection was delivered through a 30 gauge stainless steel injection needle connected to a Hamilton 5 μl syringe. Rats were treated with constructs expressing GFP only (AAV-GFP) or expressing GFP and ΔJunD (AAV-GFP-ΔJunD). Vectors were constructed and packaged as previously described and titers of the vectors averaged 2.0 × 107 infectious units/ml23–27. Injections were made 2 weeks prior to telemetry surgery.
Telemetry monitoring of blood pressure and heart rate
A Dataquest IV radio telemetry system (Data Sciences Inc., St. Paul, MN) was used to continuously record mean arterial pressure (MAP) heart rate (HR), respiratory rate (RR) and activity. Using gas anesthesia (isoflurane 2%), rats were implanted with an abdominal aortic catheter attached to a TA11PA-C40 radio-telemetry transmitter. The transmitter was secured to the abdominal muscle and remained in the abdominal cavity for the duration of the experiment. Two weeks were allowed for recovery from surgery. Blood pressure measurements obtained during a 10-second sampling period (500 Hz) were averaged and recorded every 10 minutes. Signals from radio transmitters were measured at atmospheric pressure pre-operatively and post mortem to quantify and correct for any signal drift over the course of the protocol. In most cases, no post mortem offset adjustments were required.
Chronic intermittent hypoxia protocol
After post-surgical recovery, rats individually housed in their home cages were relocated into custom-built plexiglass chambers one week before beginning the treatment period as previously described 14, 32. Rats were allowed to acclimate to the chambers at normoxia (21% O2) for 4 days prior to recording baseline cardiovascular data for 3 days. Thereafter, rats were exposed to CIH for 7 days from 08:00–16:00 h. The O2 concentration in the chambers was regulated using custom built user-controlled timers that separately switched the flow of room air and nitrogen into each chamber. Flow rates of room air and nitrogen to each chamber were separately controlled using individual flow meters (University of Texas Health Science Center at San Antonio Instrumentation Services). The CIH protocol was as follows: 1) O2 was reduced from 21% to 10% in 105 s 2) O2 was held at 10% for 75 s, 3) O2 was returned to 21% in 105 s, and 4) O2 was held at 21% for 75 s. Each complete cycle lasted 6 minutes and the rats were exposed to 80 cycles per day. Chambers were maintained at 21% throughout the remainder of the light phase (4 h) and the dark phase (10 h).
Immunohistochemistry and histology
The morning after the last CIH exposure, rats were anesthetized with thiobutabarbital (Inactin, Sigma, 100 mg/kg ip) and were perfused transcardially with PBS followed by 4% paraformaldehyde. Brains were post-fixed for 1–2 h followed by cryoprotection in 20% sucrose (PBS) at 4°C. Three sets of coronal 40 μm sections from each brain were preserved in cryoprotectant and stored at −20°C to be processed at a later time.
Separate sets of serial sections from each rat were processed for FosB (goat anti-FosB, Santa Cruz Biotechnology, Santa Cruz, CA; 1:1000) immunohistochemistry as previously described 33 or used to verify the location of the injections sites. The anti-FosB antibody used in this study does not discriminate full length FosB from the splice variant ΔFosB33. Sections were then processed with a biotin-conjugated horse anti-goat IgG (Vector Laboratories, Burlingame, CA; 1:200) and reacted with an avidin-peroxidase conjugate (Vectastain ABC Kit; Vector Laboratories) and PBS containing 0.04% 3,3′–diaminobenzidine hydrochloride and 0.04% nickel ammonium sulfate for 10 to 11 minutes.
Sections were analyzed using an Olympus microscope (BX41) equipped for epifluorescence and an Olympus DP70 digital camera with DP manager software (v 2.2.1). Images were uniformly adjusted for brightness and contrast. Regions were identified using the rat brain stereotaxic atlas of Paxinos and Watson31. FosB/ΔFosB positive cells in each region were counted using ImageJ. Four to six sections were analyzed from each rat for each region.
Real-time quantitative PCR array
Each rat was anesthetized with Inactin (100mg/kg ip) and sacrificed. Their brains were removed and punch samples were collected containing the MnPO as previously described 34. RNA was isolated from the punch samples using Trizol reagent following the manufacturer’s protocol (Invitrogen, Carlsbad, CA) and dissolved in TE buffer. Reverse transcription of cDNA was performed from at least 400 ng of total RNA in a final volume of 20 μl using the ReactionReady First Strand cDNA synthesis kit according to the manufacturer’s protocol (SA Biosciences, Frederick, MD).
Data were generated and analyzed by SA Biosciences Profiler Array services. Newly synthesized cDNA was plated onto a custom designed PCR array manufactured by SA Biosciences to quantify mRNAs for a chosen list of 88 relevant genes that contain an AP-1 promoter binding site. The proprietary primers for this array are quality controlled for specificity, uniformity, and efficiency. The array also included 5 housekeeping control genes (rplp1, hprt1, rpl13a, ldha, and actb) for normalizing expression, negative controls (genomic DNA contamination), and 2 positive controls (for reverse transcription and PCR validation).
The qPCR was run using a HotStart Taq DNA polymerase contained in the RT2 qPCR Master mix. Sample and master mix were loaded into the PCR array according to manufacturer instructions (SA Biosciences). Data were collected at Qiagen using a Biorad iCycler according to the following recommended protocol: 1) 95°C for 10 m (hot start) and 2) 40 cycles of a) 95°C for 15 s; b) 55°C for 30–40 s; c)72°C for 30 s.
Data analysis and statistics
Effects of CIH on MAP, HR, RR, and activity during the dark phase and during the 8 h period of CIH exposure were analyzed separately by 2-way repeated measures ANOVA and Student-Neumann-Keuls post-hoc tests (SigmaPlot v. 12, Systat Software Inc., San Jose, CA). FosB/ΔFosB counts were analyzed by one-way analysis of variance with Student Newman-Keuls tests for post-hoc analysis. Data from the PCR array studies were analyzed RT2 Profiler PCR Array Analysis Template (v3.2, SA Biosciences). Significance was set at p < 0.05. All values are presented as mean ± SEM.
RESULTS
Effects of AV3V lesions on CIH Hypertension
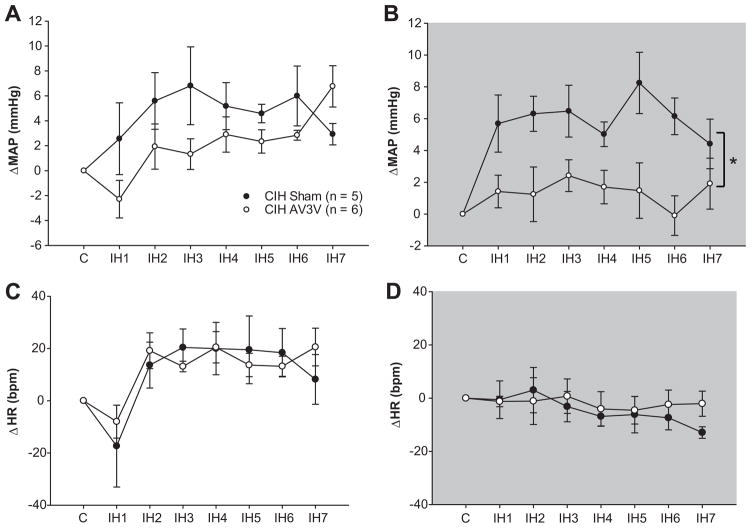
There were no differences between sham lesioned and AV3V lesioned rats observed during baseline recording of MAP, HR, or RR (please see http://hyper.ahajournals.org Table S1). During IH exposure between 0800–1600 h, changes in MAP were not different between sham and AV3V lesioned rats (Figure 1a). In sham lesioned rats, this increase in MAP was sustained during the dark phase in normoxic conditions (Figure 1b). This sustained increase in MAP was not observed in AV3V lesioned rats (Figure 1b). There were no differences between the groups for changes in HR recorded during CIH or the dark phase (Figures 1c and 1d) or for changes in RF either during intermittent hypoxia exposure or the dark phase (please see http://hyper.ahajournals.org Figure S1). The results suggest that the AV3V region is not required for the increases in MAP that occur during intermittent hypoxia exposure but may contribute to the CNS mechanisms that produce the sustained component of CIH hypertension.
Figure 1.
Electrolytic lesions of the AV3V region did not affect the increase in MAP during intermittent hypoxia exposure (a) but was associated with a significant decrease in MAP during the dark phase (b). Differences in heart rate between the two groups were not observed for either time period (c & d). * indicates a significant main effect of group across all days (two way repeated measure ANOVA).
Role of MnPO ΔFosB in CIH Hypertension
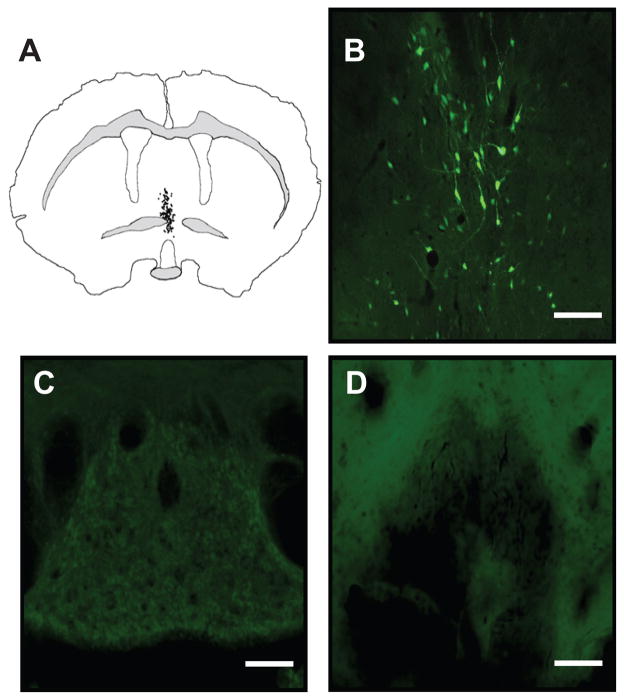
Next, we investigated the effects of dominant negative inhibition of ΔFosB in the MnPO on the cardiovascular effects of CIH by injecting AAV vectors into this region to determine if we would observe effects similar to the AV3V lesions. Histological examination of the injection sites showed that both viral vectors produced intense GFP labeling in the ventral and dorsal aspects of the MnPO (Figure 2a and 2b). GFP positive neurons were not observed in OVLT, SFO, or in the ependymal lining of the ventricles (Figure 2c and 2d). Injections of the viral vectors had no significant effects on baseline activity or respiratory rate (please see http://hyper.ahajournals.org Table S2). Baseline mean arterial pressure (MAP) was elevated in both groups injected with AAV vectors during the light and dark phases as compared to uninjected rats, while heart rate in AAV injected rats was increased only during the light phase (please see http://hyper.ahajournals.org Table S2).
Figure 2.
Injections of the viral vectors were largely confined to the MnPO. (a) A typical example of GFP labeling following of 400 nl injection of AAV-GFP-ΔJunD injection targeted at the MnPO is illustrated by a Neurolucida diagram depicting the distribution of individual GFP positive neurons in a single coronal section containing the MnPO which is located on the midline dorsal to the third ventricle and a (b) corresponding photomicrograph of GFP labeling in the MnPO. Examination of periventricular lamina terminalis regions dorsal (subfornical organ; c) and ventral (organum vasculosum of the lamina terminalis; d) indicated that the injection of the viral vector did not spread to adjacent regions of the lamina terminalis or access other regions via the ventricular system. Scale bar in (b) is 50 μm and in (c & d) is 100 μm.
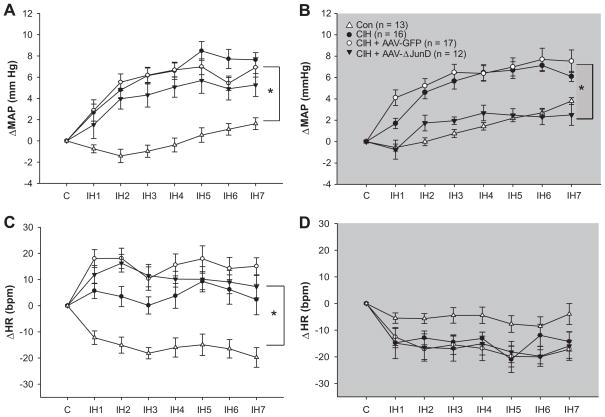
During the intermittent hypoxia exposure, significant increases in MAP were observed in uninjected rats and rats injected in the MnPO with either AAV-GFP or AAV-GFP-ΔJunD as compared to normoxic controls (Figure 3a). This significant increase in MAP was sustained throughout the dark phase in the absence of hypoxia in AAV-GFP and uninjected rats exposed to CIH (Figure 3b). In contrast, this sustained increase in MAP was not evident the rats injected in MnPO with AAV-GFP-ΔJunD (Figure 3b). During the normoxic dark phase, the MAPs of the AAV-GFP-ΔJunD treated rats were significant lower than the other groups exposed to CIH and not different from normoxic controls (Figure 3b). All rats exposed to CIH demonstrated comparable increases in heart rate during CIH exposure independent of AAV treatment (Figure 3c). During normoxia in the dark phase, there were no differences in heart rate among any of the treatment groups (Figure 3d). Similar results were observed for respiratory rate and activity (please see http://hyper.ahajournals.org Figure S2). Thus, inhibition of MnPO ΔFosB selectively affected the sustained component of CIH hypertension without influencing changes in MAP, HR or RR that occurred during the exposure to intermittent hypoxia. These results indicate that changes in MnPO gene expression mediated by ΔFosB are necessary for the persistent increase in MAP produced by CIH that occurs during normoxia which may be analogous to diurnal hypertension in patients with sleep apnea.
Figure 3.
Dominant negative inhibition of MnPO ΔFosB selectively attenuated the sustained component of CIH hypertension. In each graph changes in mean arterial pressure (ΔMAP) or heart rate (ΔHR) are expressed as differences from the daily average observed over a 3–4 day control period (C) for each day of intermittent hypoxia exposure (IH1–IH7). (a) Increases in mean arterial pressure observed during daily intermittent hypoxia exposures (0800h – 1600 h) were not affected by injections of AAV-GFP-ΔJunD or the control vector (AAV-GFP) as compared to uninjected rats exposed to CIH (CIH) but were increased compared to normoxic controls (CON). (b) Blood pressure remained elevated during the normoxic dark phase in uninjected controls and AAV-GFP injected rats exposed to intermittent hypoxia, however, this persistent increase in blood pressure was not evident in rats injected in the MnPO with AAV-GFP-ΔJunD. Blood pressure in the rats injected AAV-GFP-ΔJunD with were not different from normoxic control rats. (c) All groups of rats exposed to CIH had comparable increases in heart rate during daily intermittent hypoxia exposure. (d) There were no significant between groups differences for changes in HR recorded during the dark phase. Data are expressed as means ± s.e.m. * P < 0.05 between groups; analysis of variance followed by Student-Newman-Keuls test.
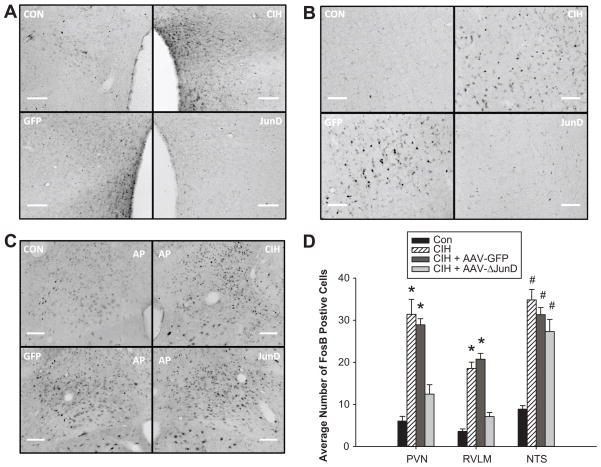
We examined ΔFosB staining in several autonomic regulatory regions of the CNS to further determine the effects of dominant negative inhibition of MnPO ΔFosB on the chronic transcriptional activation of these areas by CIH. Our previous work has demonstrated that CIH is associated with an increase in the numbers of ΔFosB positive neurons in the paraventricular nucleus of the hypothalamus (PVN, Figure 4a), the rostral ventrolateral medulla (RVLM, Figure 4b), and the nucleus of the solitary tract (NTS, Figure 4c). The PVN and RVLM are both sympathoexcitatory regions while the NTS is the primary termination point for visceral afferents including chemoreceptors and baroreceptors 20. Following CIH, the numbers of ΔFosB positive cells were significantly increased in each of these areas in uninjected and AAV-GFP rats (Figure 4d). Dominant negative inhibition of MnPO ΔFosB significantly reduced the numbers ΔFosB positive profiles in both the PVN and the RVLM (Figure 4d) suggesting that MnPO ΔFosB is necessary for the circuit-level activation of these other regions by CIH. Furthermore, decreased translational activation of the PVN and RVLM also may contribute to the lack of a sustained CIH-mediated increase in MAP associated with MnPO ΔFosB inhibition. In the NTS, ΔFosB staining associated with CIH was not influenced by dominant negative inhibition of ΔFosB in the MnPO (Figure 4c). In rats injected with AAV-GFP-ΔJunD in the MnPO, the numbers of ΔFosB positive profiles were significantly increased compared to normoxic controls and not different from the other CIH treated groups (Figure 4d). These findings are consistent with the observation that MnPO ΔFosB inhibition had no effect on the blood pressure or respiratory responses that occurred during CIH exposure and suggest that the MnPO does not contribute to translational activation of the NTS by CIH.
Figure 4.
Dominant negative inhibition of MnPO ΔFosB reduces translational activation in putative downstream autonomic regulatory regions. Digital images of ΔFosB staining in the paraventricular nucleus of the hypothalamus (a), rostral ventrolateral medulla (b), and subpostremal region of the nucleus of the solitary tract (c) from normoxic, uninjected rats (CON, n = 7), CIH treated uninjected rats (CIH, n = 8), CIH rats injected in the MnPO region with AAV-GFP (n = 8), and CIH rats injected in the MnPO region with AAV-GFP-ΔJunD (n = 8). (d) Summary data expressed as means ± s.e.m. * P < 0.05 from normoxic controls (CON) and AAV-GFP-ΔJunD treated rats and # P < 0.05 from CON; analysis of variance followed by Student-Newman-Keuls test.
Identification of Potential Downstream Targets of MnPO ΔFosB
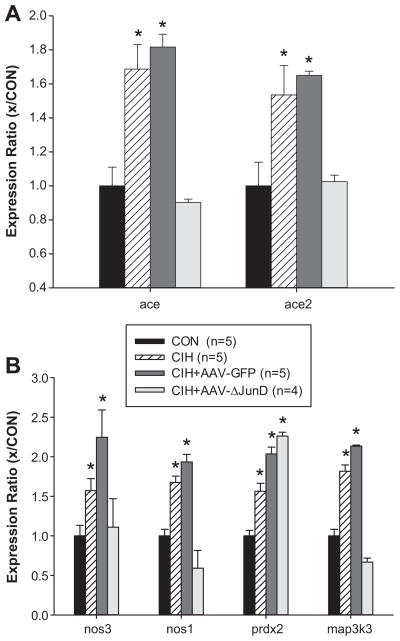
Based on these results, we investigated changes in MnPO gene expression associated with CIH focusing on candidate genes that could be regulated by ΔFosB and may contribute to the sustained component of CIH hypertension. Punch samples containing the MnPO were subjected to RT-qPCR analysis using a customized array (SABiosciences, Frederick, MD) that screened 86 genes with AP-1 promoter regions. Samples were collected from normoxic controls, uninjected rats exposed to CIH and rats injected in the MnPO with either AAV-GFP or AAV-GFP-ΔJunD prior to CIH. Of 86 genes analyzed, six were elevated following 7d CIH. Both angiotensin converting enzymes (ace, ace2) were up regulated by approximately 50% following CIH (Figure 5a). The neuronal and endothelial isoforms of nitric oxide synthase (nos1 and nos3), mitogen activated protein kinase kinase kinase 3 (map3k3), and peroxiredoxin 2 (prdx2) also were significantly elevated following CIH (Figure 5b). The same pattern of changes in MnPO gene expression was observed in rats injected with AAV-GFP prior to CIH. Dominant negative inhibition of MnPO ΔFosB significantly attenuated the CIH-mediated increases in ace, ace2, nos1, nos3, and map3k3 gene expression (Figure 5). The increase in MnPO prdx2 gene expression associated with CIH was not affected by AAV-GFP-ΔJunD injection. For genes whose expression pattern did not change following CIH, please see http://hyper.ahajournals.org Figure S3.
Figure 5.
Results of qRT-PCR array analyses (SA Bioscience) using punch samples that contained the MnPO from normoxic, uninjected rats (CON), CIH treated uninjected rats (CIH), CIH rats injected in the MnPO region with AAV-GFP (CIH + AAV-GFP), and CIH rats injected in the MnPO region with AAV-GFP-ΔJunD (CIH + AAV-ΔJunD) illustrating the effects of CIH and dominant negative inhibition of MnPO ΔFosB. Data are expressed as normalized gene expression ratios. The n’s indicated in the legend represent the number of rats used for the analysis. Extracted RNA was not pooled for qRT-PCR analyses. * P < 0.05 from normoxic controls (CON). Data were analyzed by one-way ANOVA and Student-Newman-Keuls tests.
DISCUSSION
The main results of these experiments indicate that increased ΔFosB expression in the MnPO is necessary for sustained hypertension associated with CIH. When rats injected in the MnPO with AAV-GFP-ΔJunD were exposed to intermittent hypoxia during the light phase between 0800–1600 h, their MAP significantly increased to levels comparable to those observed in the uninjected and AAV-GFP injected rats exposed to CIH. After the intermittent hypoxia was discontinued each day, MAP remained significantly elevated in both uninjected and AAV-GFP injected rats exposed to CIH. In AAV-GFP-ΔJunD injected rats, this component of the response to CIH was absent. During normoxia, their MAP was significantly lower than the other groups of rats that were exposed to CIH and not different from normoxic controls that were housed in similar conditions. These results indicate that changes in MnPO gene expression that are mediated by ΔFosB are necessary for CIH to produce a hypertensive response that is maintained in the absence of intermittent hypoxia stimulation. Dominant negative inhibition of MnPO ΔFosB significantly reduced CIH-mediated ΔFosB staining in both the PVN and the RVLM which indicates that this antihypertensive effect was due to a decrease in centrally generated sympathetic outflow20, 35. Transcriptional activation of the NTS by CIH was not influenced by blocking MnPO ΔFosB. The NTS receives direct innervation from chemoreceptor and baroreceptor afferents which likely drive activity and ΔFosB expression in this region during exposure to CIH independently of forebrain influences20, 35. This is consistent with the observation that rats injected in the MnPO with AAV-GFP-ΔJunD showed a significant increase in MAP during the daily exposures to intermittent hypoxia. It also indicates that activation of the NTS by intermittent hypoxia is not sufficient to drive transcriptional activation of the PVN and RVLM or the sustained increase in MAP that is observed during the normoxic periods between intermittent hypoxia exposures.
The transcription factor ΔFosB has been shown to contribute to neuroplasticity associated with depression, drug addiction, or other motived behaviors.13, 36, 37 The stability of ΔFosB 12, 38 appears to be a key feature allowing it to maintain plastic changes in neural function in the absence of continuous stimulation. The current findings suggest that ΔFosB makes a similar contribution to forebrain neural networks that regulate autonomic function by mediating changes in gene expression that are necessary for sustained CIH hypertension.
We identified 5 genes in the MnPO that appear to be regulated by ΔFosB during CIH. Expression of these genes increased following CIH exposure and virally mediated dominant negative inhibition of ΔFosB prevented these changes. MAP3K3 is a serine/threonine kinase that is reported to be an upstream regulator of AP-1 expression,39 angiotensin signaling,40 and several other signaling pathways41, 42 in some systems. Two of these genes, ACE1 and ACE2, mediate the generation of angiotensin related peptides that have reported to be either pro- or anti-hypertensive43. The expression of NOS1 and NOS3 was similarly effected by CIH and inhibition of MnPO ΔFosB. Decreased NOS activity in PVN is associated with elevated sympathetic outflow due to the influence of nitric oxide (NO) on local GABA release.44–46 Other studies have shown that increased NO in the lamina terminals region may be associated with increased blood pressure.47, 48 In other neural systems, changes in NO activity are associated with LTD and LTP, and, NO may participate in vasculoneuronal communication.49, 50 Together these changes in MnPO gene expression could represent a substrate for cellular adaptations that are mediated by ΔFosB during CIH and support the sustained increase in blood pressure during normoxia. Additional studies will be necessary to further characterize the role of these genes and their functions in MnPO which contribute to CIH mediated hypertension.
Previous studies have indicated that the hypertension associated with CIH is influenced by the renin-angiotensin system. CIH has been shown to increase plasma renin activity and treatment with an angiotensin receptor antagonist, delivered by daily gastric gavage, blocked the increase in blood pressure produced by CIH.51 Renal denervation attenuates both the increase in plasma renin activity as well as CIH-induced hypertension.51 Subsequently, it has been shown that chronic systemic angiotensin receptor blockade reduces sympathetic nerve activity in rats exposed to CIH and attenuates chemoreceptor sensitization.4 Local injection of angiotensin receptor antagonists into the PVN have been shown to attenuate CIH-induced hypertension.52 Angiotensin related peptides that are generated by ACE2 are reported to be antihypertensive and ACE2 over expression has been show to influence NOS expression.53–55 Our current results suggest that central angiotensin peptides associated with the MnPO may also contribute to elevated sympathetic outflow and sustained CIH hypertension.
Perspectives
The sustained elevation of MAP produced by CIH, which occurs in the dark phase of the light dark cycle when the rats are normoxic, is analogous to diurnal hypertension that is associated with sleep apnea. Diurnal hypertension, i.e., increased arterial pressure during waking hours, is an important pathophysiological feature of sleep apnea that is associated with an increased risk of adverse cardiovascular events. Increased MAP and sympathetic outflow in the absence of intermittent hypoxia stimulation likely requires some form of adaptation or plasticity in the neural circuits that regulate sympathetic outflow. Previous studies have demonstrated that CIH produces a sensitization of peripheral chemoreceptors which increases their activity during normoxia.56–58 Our results suggest that other CNS mechanisms also contribute specifically to the sustained component of CIH hypertension and that ΔFosB-mediated neuroplasticity in the MnPO is a critical contributing factor. A better understanding of the mechanisms that contribute to the pathogenesis and maintenance of the sustained component of the CIH hypertensive response may provide us with a better understanding of diurnal hypertension in sleep apnea patients.
Supplementary Material
Novelty and Significance.
1) What is New
Our study indicates that the central nervous system contributes to sustained hypertension associated with intermittent hypoxia.
This contribution involves activity dependent changes in gene expression that may change the way the neurons function as part of a network that controls the sympathetic nervous system.
Preventing these changes in gene expression selectively reduced the hypertension that is maintained in the absence of hypoxia which is similar to diurnal hypertension in patients with sleep apnea.
2) What is Relevant?
Our results indicate that activity dependent changes in gene expression in the central nervous system contribute to hypertension in animal model of the hypoxemia associated with sleep apnea.
The anti-hypertensive response occurred only during periods of the day when the animals were not being exposed to the hypoxia.
These findings could provide new insight to how hypertension develops in association with sleep apnea.
Summary
Our results show that FosB mediated changes in gene expression contributes to hypertension in an animal model of hypoxia related to sleep apnea. We identified 5 target genes and a specific regions of the brain that may be responsible for these effects.
Acknowledgments
The authors would like to acknowledge the technical assistance of Joel T. Little and Mayurika Dutta as well as Glenn M. Toney, PhD for his discussions about the work presented here.
Sources of Funding
This study was supported by NIH P01 HL-88052 to (JTC and SWM) and R01 MH-51399 (EJN).
Footnotes
Conflicts of Interest/Disclosures
None.
References
- 1.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolk R, Kara T, Somers VK. Sleep-disordered breathing and cardiovascular disease. Circulation. 2003;108:9–12. doi: 10.1161/01.CIR.0000072346.56728.E4. [DOI] [PubMed] [Google Scholar]
- 3.Smith ML, Niedermaier ONW, Hardy SM, Decker MJ, Strohl KP. Role of hypoxemia in sleep apnea-induced sympathoexcitation. Journal of the Autonomic Nervous System. 1996;56:184–190. doi: 10.1016/0165-1838(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 4.Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: Role of the angiotensin ii type 1 receptor. Respir Physiol Neurobiol. 2010;171:36–45. doi: 10.1016/j.resp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prabhakar NR, Peng YJ, Jacono FJ, Kumar GK, Dick TE. Cardiovascular alterations by chronic intermittent hypoxia: Importance of carotid body chemoreflexes. Clin Exp Pharmacol Physiol. 2005;32:447–449. doi: 10.1111/j.1440-1681.2005.04209.x. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher EC, Orolinova N, Bader M. Blood pressure response to chronic episodic hypoxia: The renin-angiotensin system. J Appl Physiol. 2002;92:627–633. doi: 10.1152/japplphysiol.00152.2001. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher EC, Lesske J, Behm R, Miller CC, 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol. 1992;72:1978–1984. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- 8.Kc P, Dick TE. Modulation of cardiorespiratory function mediated by the paraventricular nucleus. Respir Physiol Neurobiol. 2010;174:55–64. doi: 10.1016/j.resp.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kc P, Balan KV, Tjoe SS, Martin RJ, LaManna JC, Haxhiu MA, Dick TE. Increased vasopressin transmission from the paraventricular nucleus to the rostral medulla augments cardiorespiratory outflow in chronic intermittent hypoxia-conditioned rats. The Journal of Physiology. 2010;588:725–740. doi: 10.1113/jphysiol.2009.184580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg HE, Sica AL, Scharf SM, Ruggiero DA. Expression of c-fos in the rat brainstem after chronic intermittent hypoxia. Brain Res. 1999;816:638–645. doi: 10.1016/s0006-8993(98)01222-0. [DOI] [PubMed] [Google Scholar]
- 11.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: Control of gene expression by jun, fos and krox, and creb/atf proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic fos-related antigens: Stable variants of deltafosb induced in brain by chronic treatments. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. Deltafosb: A molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of fosb/δfosb in central autonomic regions. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2011;301:R131–R139. doi: 10.1152/ajpregu.00830.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ. The sensory circumventricular organs of the mammalian brain. Advances in Anatomy, Embryology & Cell Biology. 2003;172:III–XII. 1–122. doi: 10.1007/978-3-642-55532-9. back cover. [DOI] [PubMed] [Google Scholar]
- 16.Smith PM, Ferguson AV. Circulating signals as critical regulators of autonomic state—central roles for the subfornical organ. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2010;299:R405–R415. doi: 10.1152/ajpregu.00103.2010. [DOI] [PubMed] [Google Scholar]
- 17.Osborn J, Fink G, Sved A, Toney G, Raizada M. Circulating angiotensin ii and dietary salt: Converging signals for neurogenic hypertension. Current Hypertension Reports. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Morrison SF. Central efferent pathways for cold-defensive and febrile shivering. The Journal of Physiology. 2011;589:3641–3658. doi: 10.1113/jphysiol.2011.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKinley MJ, Gerstberger R, Mathai ML, Oldfield BJ, Schmid H. The lamina terminalis and its role in fluid and electrolyte homeostasis. J Clin Neurosci. 1999;6:289–301. doi: 10.1054/jocn.1998.0056. [DOI] [PubMed] [Google Scholar]
- 20.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 21.Brody MJ, Fink GD, Buggy J, Haywood JR, Gordon FJ, Johnson AK. The role of the anteroventral third ventricle (av3v) region in experimental hypertension. Circulation Research. 1978;43:I2–I13. [Google Scholar]
- 22.Nanduri J, Yuan G, Kumar GK, Semenza GL, Prabhakar NR. Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol. 2008;164:277–281. doi: 10.1016/j.resp.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, Iniguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolanos CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. Deltafosb in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winstanley CA, LaPlant Q, Theobald DEH, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. Δfosb induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. The Journal of Neuroscience. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A, Nestler EJ. An essential role for deltafosb in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]
- 26.Barrot M, Olivier JDA, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. Creb activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proceedings of the National Academy of Sciences. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berton O, Covington HE, III, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, Singewald N, Birnbaum S, Neve RL, Nestler EJ. Induction of deltafosb in the periaqueductal gray by stress promotes active coping responses. Neuron. 2007;55:289–300. doi: 10.1016/j.neuron.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 28.Saper CB, Levisohn D. Afferent connections of the median preoptic nucleus in the rat: Anatomical evidence for a cardiovascular integrative mechanism in the anteroventral third ventricular (av3v) region. Brain Research. 1983;288:21–31. doi: 10.1016/0006-8993(83)90078-1. [DOI] [PubMed] [Google Scholar]
- 29.Ployngam T, Collister JP. An intact median preoptic nucleus is necessary for chronic angiotensin ii-induced hypertension. Brain Res. 2007;1162:69–75. doi: 10.1016/j.brainres.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callahan MF, Cunningham JT, Kirby RF, Johnson AK, Gruber KA. Role of the anteroventral third ventricle (av3v) region of the rat brain in the pressor response to gamma 2-melanocyte-stimulating hormone (gamma 2-msh) Brain Res. 1988;444:177–180. doi: 10.1016/0006-8993(88)90925-0. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 32.Hinojosa-Laborde C, Mifflin SW. Sex differences in blood pressure response to intermittent hypoxia in rats. Hypertension. 2005;46:1016–1021. doi: 10.1161/01.HYP.0000175477.33816.f3. [DOI] [PubMed] [Google Scholar]
- 33.Ji LL, Fleming T, Penny ML, Toney GM, Cunningham JT. Effects of water deprivation and rehydration on c-fos and fosb staining in the rat supraoptic nucleus and lamina terminalis region. Am J Physiol Regul Integr Comp Physiol. 2005;288:R311–321. doi: 10.1152/ajpregu.00399.2004. [DOI] [PubMed] [Google Scholar]
- 34.Carreño FR, Walch JD, Dutta M, Nedungadi TP, Cunningham JT. Brain-derived neurotrophic factor-tyrosine kinase b pathway mediates nmda receptor nr2b subunit phosphorylation in the supraoptic nuclei following progressive dehydration. Journal of Neuroendocrinology. 2011;23:894–905. doi: 10.1111/j.1365-2826.2011.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dampney RA, Horiuchi J. Functional organisation of central cardiovascular pathways: Studies using c-fos gene expression. Prog Neurobiol. 2003;71:359–384. doi: 10.1016/j.pneurobio.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Hedges VL, Chakravarty S, Nestler EJ, Meisel RL. Δ fosb overexpression in the nucleus accumbens enhances sexual reward in female syrian hamsters. Genes, Brain and Behavior. 2009;8:442–449. doi: 10.1111/j.1601-183X.2009.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitchers KK, Frohmader KS, Vialou V, Mouzon E, Nestler EJ, Lehman MN, Coolen LM. Δfosb in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes, Brain and Behavior. 2010;9:831–840. doi: 10.1111/j.1601-183X.2010.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting ap-1 complex composed of altered fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 39.Xu L-G, Li L-Y, Shu H-B. Traf7 potentiates mekk3-induced ap1 and chop activation and induces apoptosis. Journal of Biological Chemistry. 2004;279:17278–17282. doi: 10.1074/jbc.C400063200. [DOI] [PubMed] [Google Scholar]
- 40.Abbasi S, Su B, Kellems RE, Yang J, Xia Y. The essential role of mekk3 signaling in angiotensin ii-induced calcineurin/nuclear factor of activated t-cells activation. Journal of Biological Chemistry. 2005;280:36737–36746. doi: 10.1074/jbc.M506493200. [DOI] [PubMed] [Google Scholar]
- 41.Sun W, Yang J. Molecular basis of lysophosphatidic acid-induced nf-κb activation. Cellular Signalling. 2010;22:1799–1803. doi: 10.1016/j.cellsig.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moscat J, Diaz-Meco MT, Albert A, Campuzano S. Cell signaling and function organized by pb1 domain interactions. Molecular Cell. 2006;23:631–640. doi: 10.1016/j.molcel.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: Properties and future directions. J Neurochem. 2008;107:1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li YFPKP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: The altered inhibitory mechanisms. Acta Physiologica Scandinavica. 2003;177:17–26. doi: 10.1046/j.1365-201X.2003.01043.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: Role of gaba. American Journal of Physiology - Regulatory Integrative and Comparative Physiology. 1998;275:R728–R734. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- 46.Latchford KJ, Ferguson AV. Angiotensin ii activates a nitric-oxide-driven inhibitory feedback in the rat paraventricular nucleus. Journal of Neurophysiology. 2003;89:1238–1244. doi: 10.1152/jn.00914.2002. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi K, Watanabe K, Yamaya K. Evaluation for roles of nitric oxide generated in the anteroventral third ventricular region in controlling vasopressin secretion and cardiovascular system of conscious rats. European Journal of Endocrinology. 2000;143:523–533. doi: 10.1530/eje.0.1430523. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi Ki, Hama H. A study on the mechanism by which sodium nitroprusside, a nitric oxide donor, applied to the anteroventral third ventricular region provokes facilitation of vasopressin secretion in conscious rats. Brain Research. 2003;968:35–43. doi: 10.1016/s0006-8993(02)04246-4. [DOI] [PubMed] [Google Scholar]
- 49.Collingridge GL, Isaac JTR, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 50.Garthwaite J. Concepts of neural nitric oxide-mediated transmission. European Journal of Neuroscience. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension. 1999;34:309–314. doi: 10.1161/01.hyp.34.2.309. [DOI] [PubMed] [Google Scholar]
- 52.da Silva AQG, Fontes MAP, Kanagy NL. Chronic infusion of angiotensin receptor antagonists in the hypothalamic paraventricular nucleus prevents hypertension in a rat model of sleep apnea. Brain Research. 2011;1368:231–238. doi: 10.1016/j.brainres.2010.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia H, Suda S, Bindom S, Feng Y, Gurley SB, Seth D, Navar LG, Lazartigues E. Ace2-mediated reduction of oxidative stress in the central nervous system is associated with improvement of autonomic function. PLoS One. 2011;6:e22682. doi: 10.1371/journal.pone.0022682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng H, Liu X, Patel KP. Angiotensin-converting enzyme 2 overexpression improves central nitric oxide-mediated sympathetic outflow in chronic heart failure. American Journal of Physiology - Heart and Circulatory Physiology. 2011;301:H2402–H2412. doi: 10.1152/ajpheart.00330.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia H, Lazartigues E. Angiotensin-converting enzyme 2: Central regulator for cardiovascular function. Current Hypertension Reports. 2010;12:170–175. doi: 10.1007/s11906-010-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iturriaga R, Rey S, Alcayaga J, Del Rio R. Chronic intermittent hypoxia enhances carotid body chemosensory responses to acute hypoxia. In: Hayashida Y, Gonzalez C, Kondo H, editors. The arterial chemoreceptors. Springer; US: 2006. pp. 227–232. [DOI] [PubMed] [Google Scholar]
- 57.Peng Y-J, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: Implications for recurrent apneas. PNAS. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sica AL, Greenberg HE, Ruggiero DA, Scharf SM. Chronic-intermittent hypoxia: A model of sympathetic activation in the rat. Respiration Physiology. 2000;121:173–184. doi: 10.1016/s0034-5687(00)00126-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.