Abstract
The study of brain evolution, particularly that of the neocortex, is of primary interest because it directly relates to how behavioural variations arose both between and within mammalian groups. Artiodactyla is one of the most diverse mammalian clades. However, the first 10 Myr of their brain evolution has remained undocumented so far. Here, we used high-resolution X-ray computed tomography to investigate the endocranial cast of Diacodexis ilicis of earliest Eocene age. Its virtual reconstruction provides unprecedented access to both metric parameters and fine anatomy of the most complete endocast of the earliest artiodactyl. This picture is assessed in a broad comparative context by reconstructing endocasts of 14 other Early and Middle Eocene representatives of basal artiodactyls, allowing the tracking of the neocortical structure of artiodactyls back to its simplest pattern. We show that the earliest artiodactyls share a simple neocortical pattern, so far never observed in other ungulates, with an almond-shaped gyrus instead of parallel sulci as previously hypothesized. Our results demonstrate that artiodactyls experienced a tardy pulse of encephalization during the Late Neogene, well after the onset of cortical complexity increase. Comparisons with Eocene perissodactyls show that the latter reached a high level of cortical complexity earlier than the artiodactyls.
Keywords: neocortex, encephalization quotient, cortical fissurization, ungulates, perissodactyls
1. Introduction
By collecting and processing sensory information, the brain ensures optimal behavioural responses in a given environment. Accordingly, brain size and pattern diversified dramatically as mammals evolved to fill an extensive variety of ecological niches. Artiodactyla has achieved one of the most successful diversifications among mammals and is today by far the predominant group within ungulates (or hoofed mammals). The diversity of modern artiodactyls is also perceptible in their brain pattern. They show a wide array of brain morphologies [1–3], including some of the biggest and most convoluted mammalian brains in those of delphinid cetaceans (e.g. Tursiops truncatus and Delphinus delphis), where brain size expressed as a function of the body mass (‘encephalization quotient’, or EQ [4]) ranks second after that of humans [5]. Accordingly, recent studies have focused on the evolutionary increase of brain size in Artiodactyla, with a special attention to cetaceans [6,7]. Yet only a few studies have dealt with the issue of morphological early evolutionary history of the brain of non-cetacean artiodactyls.
Artiodactyla suddenly appears in the fossil record at the earliest Eocene (≈55.8 Ma) with Diacodexis, a deer-like small artiodactyl retaining tribosphenic molars [8,9]. The oldest known artiodactyl endocast described so far originates from the early Middle Eocene of Pakistan (Diacodexis pakistanensis, ≈48 Ma), and it consists of a composite reconstruction based on nine skull fragments lacking crucial features such as the extent and precise structure of the neocortex (isocortex) or volume of the braincase [10]. The evolutionary history of the artiodactyl brain was mainly studied during the 1960s and early 1970s, based on natural or plaster endocranial casts (endocasts) of either endemic Late Eocene/Oligocene European artiodactyls [11–13] or Middle Eocene to Recent North American camelids [3]. Overall, the first 10 Myr of artiodactyl brain evolution remains unknown, and endocranial morphology of Eocene artiodactyl groups is still scantily documented.
Two main pulses of generic diversity are recorded in the terrestrial artiodactyl fossil record [14]; one during the Eocene epoch, with the emergence of modern groups [15], and a second major one during the Pliocene epoch, resulting from the recent explosive radiation in bovids [16]. Here we examine the early evolution of the Artiodactyla brain in terms of both volume increase and neocortical external surface complexity during the Eocene first diversification pulse of the group. We used high-resolution X-ray computed tomography to fill the temporal gap in our knowledge of artiodactyl endocast by investigating the endocranial cast of the North American diacodexeid Diacodexis ilicis of earliest Wasatchian age (≈55 Ma; Sand Coulee beds, Clark Fork Basin [17]). Its virtual reconstruction provides unprecedented access to both metric parameters and fine anatomy of the earliest artiodactyl endocast. This picture is assessed in a broad comparative context by reconstructing endocasts of Early and Middle Eocene representatives of five dichobunoid families (Diacodexeidae, Dichobunidae, Homacodontidae, Helohyidae and Cebochoeridae), and of 10 other taxa representing basal members of most Palaeogene artiodactyl families (Agriochoeridae, Mericoidodontidae, Protoceratidae, Choeropotamidae, Cainotheriidae, Entelodontidae, Leptomerycidae and Hypertragulidae), comprising putative stem taxa to each crown group of terrestrial artiodactyl subclades [18–20]. We therefore document for the first time the pattern and diversity of early artiodactyl brain volume and external morphology through an almost exhaustive sampling at the family level. We aim to illustrate the structure of the ancestral artiodactyl brain and to identify the first steps of artiodactyl brain evolution, in terms of both volume increase and neocortical external surface complexity. Additionally, this approach gives us the opportunity to evaluate differences in the brain of early artiodactyls compared with coeval perissodactyl ungulates. The Diacodexis specimens described here are stored in the American Museum of Natural History in New York, USA (AMNH).
2. Results and discussion
(a). The oldest artiodactyl endocast
The specimen we investigated (AMNH VP 16141) consists of a nearly complete cranium from the earliest Wasatchian (≈55 Ma; Sand Coulee beds, Willwood Formation, Clark Fork Basin, Wyoming [17]), referred to D. ilicis [9]. A detailed description of the basicranium of this specimen was previously published by Coombs & Coombs [21]. The cranium is crushed dorsoventrally and its occipital region is ventrally shifted. This implies that volume estimates of the braincase are drastically underestimated. An isolated natural endocast (AMNH FM 143933) from younger deposits of the same area (≈50 Ma; Lost Cabin Member, Wyoming [22]) is here tentatively reported to the genus Diacodexis based on compatible measurements and shared morphological characters [23]. Proposed minimal volume estimations are therefore based on the two available specimens. Dynamic three-dimensional reconstructions of the two endocasts of Diacodexis, as well as dental measurements and justification for specific and generic attribution of the specimens (figure S1 and table S1) are provided in the electronic supplementary material. Complete full-resolution raw datasets are archived and available upon request by the different institutions where the specimens are curated. Phylogenetic relationships of Eocene artiodactyls remain a current topic of investigation. However, recent studies show that stem taxa to extant artiodactyl groups are found in the paraphyletic assemblage of dichobunoid artiodactyls [18,19,24]. Among them, Diacodexis appears either as the first offshoot at the base of the artiodactyl clade [18,25] or more highly nested in the Artiodactyla tree and closely related to cetaceans [19,26]. Accordingly, while being the oldest artiodactyl brain known so far, it might not represent the basalmost artiodactyl morphology.
Our reconstruction of D. ilicis endocast reveals a very simple brain pattern with huge olfactory bulbs located between the orbits and covering the whole interorbital distance (figure 1). The estimated volume of the olfactory bulbs represents 8.65 per cent of the overall brain volume of the D. ilicis deformed cranium and 6.80 per cent when overall brain minimal volume estimations are considered. Within Artiodactyla, D. ilicis has among the largest olfactory bulb volume expressed as a percentage of the total braincase volume—bulb volume of Middle Eocene artiodactyl taxa ranges between 3.1 (Oxacron) and 7.6% (Dichobune; see the electronic supplementary material, table S3). The bulbs are only separated from the cerebral hemispheres by a short circular fissure and are not pedunculate (figure 2). The presence of large olfactory bulbs is congruent with the huge development of the pyriform lobe (‘olfactory cortex’) of the cerebrum and the presence of large olfactory tubercles (which receive inputs from the olfactory bulbs). Large rounded casts of olfactory tubercles have been described in oreodontids [23] and are also observed in other dichobunoids of this study for which this character can be assessed (Homacodon, Cebochoerus and Dichobune), as well as in the early cainotheriid Robiacina and basal Suinamorpha (Entelodon).
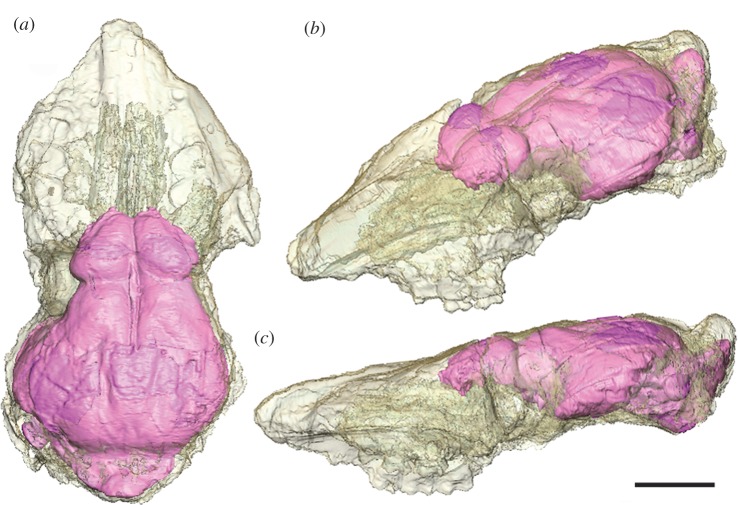
Figure 1.
In situ endocast of the earliest Eocene artiodactyl D. ilicis (AMNH VP 16141), visible through a translucent rendering of the cranium in (a) lateral and (b) dorsal views. Scale bar, 1 cm.
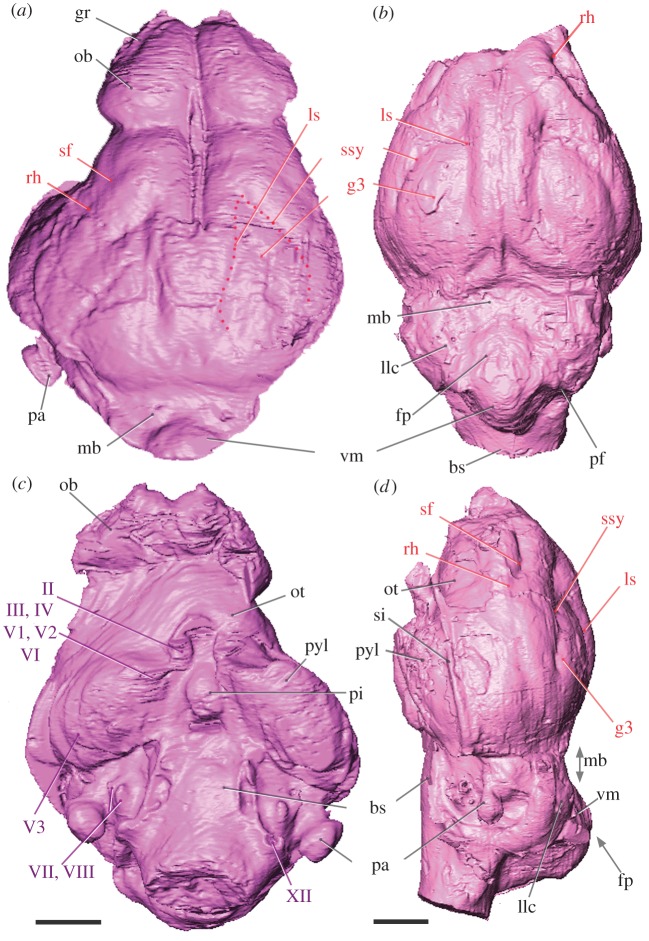
Figure 2.
Labelled endocasts of the dichobunoid artiodactyls (a,c) D. ilicis (AMNH VP 16141) and (b,d) Homacodon vagans (USNM 482369) in the (a,b) dorsal, (c) ventral and (d) lateral view (scale bar, 5 mm). Abbreviations: bs, brain stem; fp, fissura prima; gr, groove; g3, gyrus 3; llc, lateral lobe of the cerebellum; ls, lateral sulcus; mb, midbrain; ob, olfactory bulb; ot, olfactory tubercle; pa, paraflocculus; pf, paramedian fissure; pi, pituitary; pyl, pyriform lobe; rh, rhinal fissure; sf, sylvian fossa; si, sinus; ssy, suprasylvia; vm, vermis; II–XII refer to cranial nerves. Institutional abbreviation: USNM, National Museum of Natural History, Smithsonian Institution, Washington.
Dorsal exposure of the olfactory peduncles in Diacodexis was hypothesized in the previous composite reconstruction of D. pakistanensis [10]. Nonetheless, the virtual reconstruction of D. ilicis shows instead that the anterior development of the neocortex was sufficiently expansive to cover the olfactory peduncles in this earliest Eocene Diacodexis. Indeed, D. ilicis presents a small anterior neocortical lobe delimited posteriorly by a clear sylvian fossa and by a dorsal inflection of the neocortex. Noteworthy and in contrast to D. ilicis, the neocortex does not abut the olfactory bulbs in a number of Palaeogene artiodactyls (North American Agriochoerus and Leptauchenia; European Cebochoerus and Tapirulus). This suggests that the earliest Diacodexis brain might be already advanced in terms of differentiation and development of the anterior part of the neocortex, especially when compared with some stratigraphically younger artiodactyls. A key feature to assess the degree of neocorticalization in mammals is the position of the rhinal fissure that marks the boundary between the neocortex (isocortex) and the paleocortex (‘olfactory cortex’). This feature could not be determined in the D. pakistanensis reconstruction [10], but both Diacodexis specimens of this study, as well as other dichobunoids investigated here (such as Homacodon vagans, illustrated in figure 2b,d) show a clear and continuous rhinal fissure that delineates a short but inflated cap of the neocortex. Underlining the early evolutionary stage of the neocortex of Early Eocene artiodactyls, the lateral extent of the neocortex in Diacodexis, Homacodon and Helohyus does not completely cover the dorsal part of the hemisphere (figures 2 and 3). The neocortex of D. ilicis bears two deep neocortical sulci anteriorly fused to form an almond-shaped gyrus (figure 2a). In early artiodactyls, the latter is interpreted as the gyrus 3 and is formed medially by the lateral sulcus, and ventrally by the suprasylvian sulcus [11,12]. Contrary to the composite reconstruction of the somewhat younger D. pakistanensis [10], none of the Diacodexis endocasts in this study shows a coronal sulcus, and the lateral and suprasylvian sulci join at their anterior tip. While the midbrain is deep to other structures in living artiodactyls, the caudal part of the cerebrum of D. ilicis did not abut the cerebellum, and a portion of the midbrain is dorsally exposed. The colliculi are not visible. Midbrain exposure is observed in all dichobunoids of this study, and in most other early artiodactyls (as illustrated here in figure 3). By contrast, and owing to the posterior extension of the neocortex, the midbrain is completely covered in earliest Ruminantia, such as Leptomeryx and Hypertragulus, as well as in Entelodon, one of the earliest Suinamorpha, and the stem camelid Protylopus.
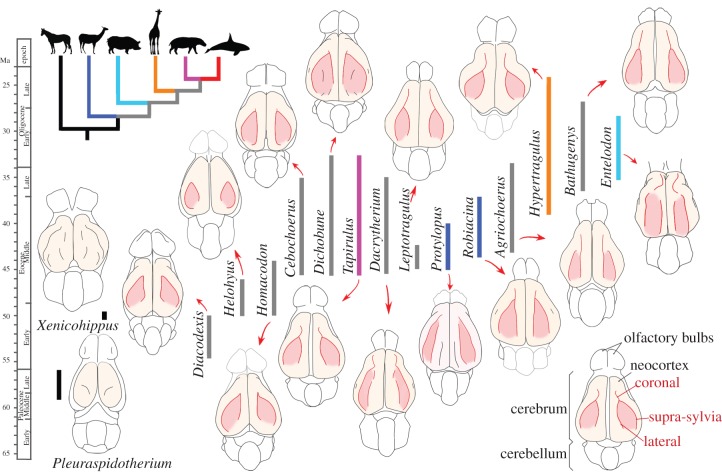
Figure 3.
Morphology and temporal distribution of early artiodactyl endocasts. Endocasts are figured in dorsal view, anterior tip (olfactory bulbs) pointing upwards. Colour codes for stratigraphical ranges of each taxon coincide with their phylogenetic affinities among non-artiodactyls (black), stem artiodactyls (grey) and crown artiodactyls (see the phylogenetic tree for colour codes; phylogenetic relationships according to Asher & Helgen [35] and Meredith et al. [36]). The main neocortical sulci are highlighted in red; accessory sulci are figured in black. Each genus typifies an artiodactyl family: Diacodexeidae (Diacodexis), Homacodontidae (Homacodon), Helohyidae (Helohyus), Cebochoeridae (Cebochoerus), Dichobunidae (Dichobune), Choeropotamidae (Tapirulus), Dacrytheriidae (Dacrytherium), Protoceratidae (Leptotragulus), Oromerycidae (Protylopus [3]), Cainotheriidae (Robiacina), Agriochoeridae (Agriochoerus), Hypertragulidae (Hypertragulus), Mericoidodontidae (Bathygenys [23], fig. 4) and Entelodontidae (Entelodon). Pleuraspidotherium illustrates endocast morphology of an archaic ungulate (‘condylarth’) closely related to Artiodactyla [51]; Xenicohippus illustrates early equid endocast. The parts reconstructed after comparison to closely related taxa appear in light grey. Endocasts not to scale.
Most of the cerebellum of AMNH VP 16141 is not accessible, owing to postmortem deformation. Both Diacodexis specimens show a broad and rounded paraflocculus (lobe of the cerebellar hemisphere associated with coordination, balance and vestibular sensory acquisition [27,28]), a plesiomorphic character within therian mammals [29]. Diacodexis shows the biggest parafloccular lobes among our sample, including Early Eocene Homacodon (figure 2c,d) and Helohyus. On the ventral aspect of the endocast, the hypophyseal fossa of Diacodexis is an ovoid deep depression, observed in all early artiodactyls of this study, except Leptotragulus, Agriochoerus and Leptauchenia. The nerve pattern of Diacodexis is similar to that previously described by Sigogneau-Russell & Russell [10] and to that described so far for other early artiodactyls [11].
(b). Evolution of terrestrial artiodactyl neocortex during Eocene times
The mammalian neocortex is the six-layered roof of the cerebral hemispheres responsible for superior cognitive functions such as sensory perception, spatial reasoning and voluntary movement. As such, the neocortex is a major feature we focused on to assess brain evolution. Given the lack of resolution at the base of the Artiodactyla tree and the current instability of the phylogenetic position of the basalmost artiodactyl taxa [19,25], the evolution of terrestrial artiodactyl endocast features is here described chronologically, faute de mieux.
The temporal arrangement of the endocast morphology of basal members of terrestrial artiodactyl families documented in this study shows that the Early Eocene to early Middle Eocene taxa Diacodexis, Homacodon and Helohyus share a very simple neocortical pattern, with two coronal sulci forming the almond-shaped gyrus (figure 3). This gyrus, already described from natural endocasts of Middle and Late Eocene artiodactyls from Europe [11–13], is identified here in all Eocene artiodactyl groups documented so far (figure 3). By contrast, it is completely obliterated in modern artiodactyls, owing to a widely increased cortical complexity (‘gyrification’). Throughout the last 200 Myr of mammalian evolution, the neocortex has undergone marked variations in size, shape and convolutional pattern complexity from a lissencephalic ancestral condition [30–32], and a convoluted neocortex evolved independently several times in placentals [28,33]. Artiodactyla, Perissodactyla and Carnivora brains are hypothesized to derive from the same ancestral pattern of convolution composed of two longitudinal dorsal fissures [28,34], which is congruent with the hypothesis of close phylogenetic relationships between ungulates and Ferae within Laurasiatheria [35,36]. However, the primary arrangement of these two sulci differs by their orientation in early representatives of the three groups [11,12]. Previous hypotheses of an ancestral fissurization pattern of artiodactyls, consisting of two longitudinal sulci, were either based on a more restricted fossil record [34], or on the brain morphology of the smallest extant artiodactyls (Hyemoschus) considered to retain the plesiomorphic condition for the order [28]. However, our results show that the almond-shaped gyrus is not only present in the earliest artiodactyl but also in all putative basal artiodactyl groups (figure 3), which indicates that it might instead represent the ancestral fissurization pattern of the artiodactyl brain. This neocortical pattern differs from that of the early diverging equid perissodactyl, Xenicohippus [37] (figure 3) and those of other Palaeogene ungulate groups [38], as well as from that of published early Carnivora (e.g. Vulpavus [39]). This almond-shaped gyrus pattern is so far unique to artiodactyls within Laurasiatheria. Very interestingly, a similar almond-shaped pattern was independently acquired in the macroscelid afrotherian mammal Rhynchocyon cirnei [40], with a comparable expansion of the neocortex. In other words, the endocast of Early Eocene artiodactyls is seemingly showing a stage of brain evolution equivalent to that of R. cirnei. Other macroscelids are either lissencephalic (Elephantulus and Petrodromus) or lacking a suprasylvian fissure (Rhynchocyon petersi) [41]. The almond-shaped gyrus of R. cirnei is thus interpreted here as a convergence.
For most Middle Eocene taxa, increase in the brain pattern complexity is restricted to the addition of a third sulcus to the pre-existing almond gyrus, recognized as the coronal sulcus [11] (figure 3). From our sample, it seems that this coronal sulcus, originally thought to join either the lateral sulcus or the suprasylvia [12], is more likely to have originated from the anterior junction between the two pre-existing neocortical sulci. Dichobune (Dichobunidae), Tapirulus (Choeropotamidae) and Cebochoerus (Cebochoeridae) show a reduced development of the coronal sulcus. Eocene taxa show a lengthening of the coronal sulcus and a concurrent development of the frontal lobe. This is accompanied by a narrowing of the gyrus 3 (figure 3) and by a concomitant shift to a more parallel arrangement of the sulci [11], except in basal Ruminantia, as exemplified by Hypertragulus, which retain a wide gyrus 3 (figure 3). The earliest record of additional accessory neocortical sulci occurs during the Middle Eocene in various families: Dacrytheriidae (Dacrytherium) and Cainotheriidae (Robiacina) have a small additional postero-lateral sulcus, referred to as ‘δ-sulcus’ [11], whereas Oromerycidae (Protylopus) have an additional longitudinal dorsal sulcus [3] (figure 3). Dichobune (Dichobunidae) presents both the δ-sulcus and additional sulci within the gyrus 3. Variation in the presence of accessory sulci and in the confluence of coronal, suprasylvian and lateral sulci has been described in Middle to Late Eocene artiodactyls, notably in oreodontids [23].
Dechaseaux [11] mentioned the apparent absence of morphological changes in artiodactyl neocortical complexity during the Late Eocene (≈37–34 Ma). Our study shows instead that neocortical complexity of artiodactyls remained simple and generalized among early artiodactyls throughout the Early Eocene period (55.8–48.6 Ma), before a more complicated pattern is documented in early Middle Eocene artiodactyls from Europe and North America. Following the increase in generic and familial diversity, the endocast of Middle and Late Eocene artiodactyls shows a wider range of morphologies. This first increase in Eocene artiodactyl endocast diversity includes stem taxa or putative stem taxa to modern artiodactyl groups: Choeropotamidae, proposed as sister taxa to Hippopotamoidea [20], as represented by Tapirulus; stem Camelidae [3,18,19], as represented by the oromerycid Protylopus and the cainotheriid Robiacina; and stem Ruminantia, as represented by Hypertragulus [18]. These taxa show simple brain patterns compared with their modern counterparts, but already display somewhat distinct neocortical fissurization. This implies that the complex gyrification observed in modern artiodactyls would have resulted from independent convergent processes of neocortical complexity increase. Notably, camelids, which are the first offshoot within modern artiodactyls according to molecular data [35,36], are also the first ones to show a marked diverging neocortical fissurization pattern, as observed with Protylopus.
(c). Evolution of brain size and encephalization quotients
The estimated minimal endocranial volume of D. ilicis equals 4.7 cm3. Depending on the equation (m1 length [42]; skull length [43]; astragalus size [44]), body mass estimates of D. ilicis range from 554 to 935 g. Details of estimated body mass calculation, endocast volumes and corresponding EQs are given in the electronic supplementary material (tables S4–S6). The EQ of D. ilicis ranges from 0.54 to 0.79 using Eisenberg's [4] equation and from 0.40 to 0.57 using Jerison's [34] equation, which falls within the range of EQs of small archaic ungulates (0.2–0.7 using Eisenberg's equation). Integration of the new fossil data to pre-existing fossil EQ datasets and to a combination of modern EQ dataset sources confirms that EQs of extinct artiodactyls are significantly smaller than those of modern artiodactyls (figure 4). However, there is no significant increase in brain size relative to body size between Palaeogene and Neogene terrestrial artiodactyl assemblages. These results contrast with the general idea of brain size increase among ungulates between the Palaeogene and the Neogene periods [34,45,46], and support more recent results based on a smaller fossil sample, suggesting that there was no significant increase in EQ throughout the Cenozoic in artiodactyls [7].
Figure 4.
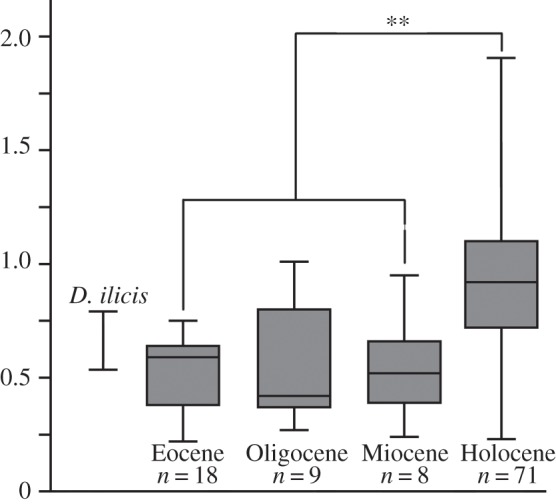
Box plot of EQs, based on Eisenberg's equation [4], of Eocene, Oligocene, Miocene and modern terrestrial artiodactyls, as calculated from intracranial volume. The range of estimates for D. ilicis reflects the array of body mass estimates (see the electronic supplementary material, table S5). Overlain brackets and lines indicate significant pairwise differences in the EQ values. **p < 0.05. See the electronic supplementary material for EQ calculation, corresponding body mass estimates (tables S4–S7) and statistic tests (table S8).
We demonstrated above that terrestrial artiodactyls documented in this study showed little morphological change in their brain pattern during the Early Eocene interval and that a higher complexity is observed in artiodactyl endocasts from the Middle Eocene interval. Generalized high complexity of artiodactyl neocortex is achieved during the Oligocene epoch [3,12,47]. However, significant increase in EQs probably occurred during the Pliocene–Pleistocene. This indicates that increase in cortical complexity preceded brain size increase in terrestrial artiodactyls. However, the sample of Miocene endocasts would need to be augmented. This observation contrasts with cetaceans, the encephalization pulse of which occurred during the Oligocene epoch [48]. Disunity in brain evolutionary history of terrestrial and aquatic artiodactyl is not surprising given the great eco-physiological discrepancies between both groups.
(d). Artiodactyla versus Perissodactyla
Ungulates have traditionally been treated together in EQ studies [34,46]. However, the early history of artiodactyl brain evolution, characterized by a relative stability and simplicity throughout the Early and Middle Eocene, contrasts greatly with what is observed in coeval early diverging hippomorph and tapiromorph perissodactyls from North America and Europe. One of the earliest equid hippomorphs, Xenicohippus osborni (Early Eocene of North America, ≈53 Ma), shows a slightly more complex fissurization pattern than Diacodexis, with three or four sulci [37] (figure 3), while another early equid, Orohippus pumilus (≈52–46 Ma, North America), already displays a complex neocortical pattern with additional sulci lateral to the sylvia (i.e. the presylvian sulcus and the sulcus posticus) [49]. Endocasts of Middle Eocene hippomorphs also present an increased complexity with multiplication of sulci and gyri, as seen in Anchilophus desmaresti (42–37.2 Ma, Europe [50]). The earliest rhinocerotoid tapiromorph Hyrachyus modestus from North America shows a complicated neocortical pattern with three longitudinal sulci and a well-differentiated frontal lobe by Early Eocene times [50]. These examples show that Early and Middle Eocene perissodactyls presented a more complex neocortical fissurization than their artiodactyl counterparts. Perissodactyls radiated in a wide array of body sizes from the earliest Eocene onward, whereas artiodactyls remained small until the Late Middle Eocene. Therefore, increased neocortical complexity in perissodactyls and retention of low complexity in artiodactyls could be related to body-size allometry [28].
3. Conclusion
The direct study of mammalian brain evolution is limited to the information provided by the fossil record: brain size, brain shape and sulcal pattern. In this framework, the endocast of D. ilicis provides detailed anatomical information on the brain of earliest artiodactyls. Its comparison with a wide familial sample of Eocene artiodactyl endocasts provides an unprecedented picture of early artiodactyl brain morphology and reveals that they share a common basic neocortical pattern, so far only observed in Artiodactyla among ungulates. Based on our sample, the neocortex of early artiodactyls retained this simple fissurization pattern during the Early Eocene, before a growing cortical diversity is observed during the Middle and Late Eocene, as generic diversity increases. Contrary to the traditional scheme for ungulates, there is no significant increase in brain mass over body mass until the latest Neogene in artiodactyls. A comparison with their Eocene perissodactyl counterparts demonstrates that artiodactyls and perissodactyls have non-simultaneous complexity increase pulses and that perissodactyls reached a high level of cortical complexity earlier than the artiodactyls.
Acknowledgements
Thanks to R. O'Leary (AMNH), Meng Jin (AMNH), C. Argot (Muséum National d'Histoire Naturelle) and B. Marandat (Université Montpellier2) for having granted access to the collections they are in charge of; to J. Thostenson, R. Rudolph and M. Hill for acquisition of the computed tomography scan raw data; we are grateful to P.-O. Antoine, A. Ramdarshan and M. A. O'Leary for constructive comments on earlier versions of the manuscript. This work has been possible thanks to the high-resolution computed tomography scanner facility of the AMNH funded by the NSF MR1–R2 0959384 granted to N. Landman, co-principal investigators D. Ebel and D. Frost, and to the Montpellier Rio Imaging platform (Montpellier, France). This is ISE-M publication 2012-067. This research was supported by the ANR funding project Palasiafrica, headed by L. Marivaux.
References
- 1.Anthony R., de Grzybowski J. 1931. Le neopallium des Suides. Etude de son développement et interprétation de ses plissements. Arch. Zool. Exp. Gen. 74, 1–24 [Google Scholar]
- 2.Anthony R., de Grzybowski J. 1936. Le neopallium du mouton. Etude de son développement et interprétation de ses plissements. J. Anat. Lond. 71, 41–53 [PMC free article] [PubMed] [Google Scholar]
- 3.Edinger T. 1966. Brains from 40 million years of camelid history. In Evolution of the forebrain, phylogenesis and ontogenesis of the forebrain (eds Hassler R., Stephan H.), pp. 153–162 New York, NY: Plenum Press [Google Scholar]
- 4.Eisenberg J. F. 1981. The mammalian radiations. Chicago, IL: University of Chicago Press [Google Scholar]
- 5.Marino L. 1998. A comparison of encephalization between odontocete cetaceans and anthropoid primates. Brain Behav. Evol. 51, 230–238 10.1159/000006540 (doi:10.1159/000006540) [DOI] [PubMed] [Google Scholar]
- 6.Marino L., Uhen M. D., Pyenson N. D., Frohlich B. 2003. Reconstruction cetacean brain evolution using computed tomography. Anat. Rec. 272, 107–117 10.1002/ar.b.10018 (doi:10.1002/ar.b.10018) [DOI] [PubMed] [Google Scholar]
- 7.Schultz S., Dunbar R. 2010. Encephalization is not a universal macroevolutionary phenomenon in mammals but is associated with sociality. Proc. Natl Acad. Sci. USA 107, 21 582–21 586 10.1073/pnas.1005246107 (doi:10.1073/pnas.1005246107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose K. D. 2006. The beginning of the age of mammals. Baltimore, MD: The John Hopkins University Press [Google Scholar]
- 9.Rose K. D., Chew A. E., Dunn R. H., Kraus M. J., Fricke H. C., Zack S. P. 2012. Earliest Eocene mammalian fauna from the Paleocene–Eocene thermal maximum at Sand Creek Divide, Southern Big Horn Basin, Wyoming. Univ. Michigan Pap. Paleontol. 36, 1–122 [Google Scholar]
- 10.Sigogneau-Russell D., Russell D. E. 1983. A new dichobunoid artiodactyl (Mammalia) from the Eocene of North-West Pakistan—III Reconstitution du moulage endocrânien. Proc. K. Ned. Akad. B Phys. 3, 319–330 [Google Scholar]
- 11.Dechaseaux C. 1969. Moulages endocrâniens d'artiodactyles primitifs, essai sur l'histoire du néopallium. Ann. Paleontol. (Vert.) 55, 195–248 [Google Scholar]
- 12.Dechaseaux C. 1970. Les grandes lignes de l'histoire de la fissuration du néopallium des artiodactyles. Comptes Rendus Acad. Sci. Paris D 268, 653–655 [PubMed] [Google Scholar]
- 13.Dechaseaux C. 1973. Essais de paléoneurologie. Ann. Paleontol. (Vert.) 59, 115–132 [Google Scholar]
- 14.Cifelli R. L. 1981. Patterns of evolution among Artiodactyla and Perissodactyla (Mammalia). Evolution 35, 433–440 10.2307/2408192 (doi:10.2307/2408192) [DOI] [PubMed] [Google Scholar]
- 15.Gatesy J. 2009. Whales and even toad ungulates. In The timetree of life (eds Hedges S. B., Kumar S.), pp. 511–515 Oxford, UK: Oxford Biology [Google Scholar]
- 16.Janis C. 2007. Artiodactyla paleoecology and evolutionary trends. In The evolution of artiodactyls (eds Prothero D. R., Foss S.), pp. 292–315 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 17.Gingerich P. D. 1989. New earliest Wasatchian mammalian fauna from Eocene of northwestern Wyoming, composition and diversity in a rarely sampled high-floodplain assemblage. Univ. Michigan Pap. Paleontol. 28, 1–97 [Google Scholar]
- 18.Geisler J. H., Theodor J. M., Uhen M. D., Foss S. E. 2007. Phylogenetic relationships of cetaceans to terrestrial artiodactyls. In The evolution of artiodactyls (eds Prothero D. R., Foss S.), pp. 19–31 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 19.Spaulding M., O'Leary M. A., Gatesy J. 2009. Relationships of Cetacea (Artiodactyla) among mammals, increased taxon sampling alters interpretations of key fossils and character evolution. PLoS ONE 4, 1–14 10.1371/journal.pone.0007062 (doi:10.1371/journal.pone.0007062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orliac M. J., Boisserie J.-R., MacLatchy L., Lihoreau F. 2010. Earliest Miocene hippopotamids constrain phylogenetic and spaciotemporal settings of hippopotamid origin. Proc. Natl Acad. Sci. USA 107, 11 871–11 876 10.1073/pnas.1001373107 (doi:10.1073/pnas.1001373107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coombs M. C., Coombs W. P. 1982. Anatomy of the ear region of four Eocene artiodactyls, Gobiohyus, ?Helohyus, Diacodexis and Homacodon. J. Vert. Paleontol. 2, 219–236 10.1080/02724634.1982.10011931 (doi:10.1080/02724634.1982.10011931) [DOI] [Google Scholar]
- 22.Gunnell G. F., Murphey P. C., Stucky R. K., Townsend K. E. B., Robinson P., Zonneveld J.-P., Bartels W. S. 2009. Biostratigraphy and biochronology of the latest Wasatchian, Bridgerian, and Uintan North American land mammal ‘ages’. Mus. Northern Arizona Bull. 65, 279–330 [Google Scholar]
- 23.Macrini T. E. 2009. Description of a digital cranial endocast of Bathygenys reevesi (Merocoidodontidae, Oreodontoidea) and implications for apomorphy based diagnosis of isolated, natural endocasts. J. Vert. Paleontol. 29, 1199–1211 10.1671/039.029.0413 (doi:10.1671/039.029.0413) [DOI] [Google Scholar]
- 24.Theodor J. M., Erfurt J., Métais G. 2007. The earliest artiodactyls. In The evolution of Artiodactyla (eds Prothero D. R., Foss S.), pp. 32–58 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 25.Geisler J. G. M., Theodor J. M. 2009. Hippopotamus and whale phylogeny. Nature 458, 1190–1194 10.1038/nature00776 (doi:10.1038/nature00776) [DOI] [PubMed] [Google Scholar]
- 26.Orliac M. J., Ducrocq S. 2011. Eocene raoellids (Mammalia, Cetartiodactyla) outside the Indian subcontinent, palaeogeographical implications. Geol. Mag. 149, 80–92 10.1017/S0016756811000586 (doi:10.1017/S0016756811000586) [DOI] [Google Scholar]
- 27.Butler A. B., Hodos W. 1996. Comparative vertebrate neuroanatomy, evolution and adaptation. New York, NY: Wiley-Liss [Google Scholar]
- 28.Nieuwenhuys R., Ten Donkelaar H. J., Nicholson C. 1998. The central nervous system of vertebrates. Berlin, Germany: Springer [Google Scholar]
- 29.Macrini T. E., Rougier G. W., Rowe T. 2007. Description of a cranial endocast from the fossil mammal Vincelestes neuquenianus (Theriiformes) and its relevance to the evolution of endocranial characters in therians. Anat. Rec. 290, 875–892 10.1002/ar.20551 (doi:10.1002/ar.20551) [DOI] [PubMed] [Google Scholar]
- 30.Welker W. 1990. Why does cerebral cortex fissure and fold? A review of determinants of gyri and sulci. In Comparative structure and evolution of cerebral cortex part II (eds Jones E. G., Peters A.), pp. 3–136 New York, NY: Plenum Press [Google Scholar]
- 31.Kielan-Jaworowska Z., Cifelli R. L., Luo Z. -X. 2004. Mammals from the age of dinosaurs, origins, evolution, and structure. New York, NY: Columbia University Press [Google Scholar]
- 32.Rowe T. B., Macrini T. E., Luo Z. X. 2011. Fossil evidence on origin of the mammalian brain. Science 332, 955–957 10.1126/science.1203117 (doi:10.1126/science.1203117) [DOI] [PubMed] [Google Scholar]
- 33.Striedter G. F. 2005. Principles of brain evolution. Sunderland, MA: Sinauer Associates [Google Scholar]
- 34.Jerison H. J. 1973. Evolution of brain and intelligence. New York, NY: Academic Press [Google Scholar]
- 35.Asher R. J., Helgen K. M. 2010. Nomenclature and placental mammal phylogeny. BMC Evol. Biol. 10, 102. 10.1186/1471-2148-10-102 (doi:10.1186/1471-2148-10-102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meredith R. W., et al. 2011. Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science 334, 521–524 10.1126/science.1211028 (doi:10.1126/science.1211028) [DOI] [PubMed] [Google Scholar]
- 37.Radinsky L. B. 1976. Oldest horse brains: more advanced than previously realized. Science 194, 626–627 10.1126/science.790567 (doi:10.1126/science.790567) [DOI] [PubMed] [Google Scholar]
- 38.Orliac M. J., Gilissen E., Argot C. 2012. Digital cranial endocast of Hyopsodus (Mammalia, ‘Condylarthra’): a case of Paleogene terrestrial echolocation? PLoS ONE 7, e30000. 10.1371/journal.pone.0030000 (doi:10.1371/journal.pone.0030000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radinsky L. 1977. Brains of early carnivores. Paleobiology 3, 333–349 [Google Scholar]
- 40.Bauchot R., Stephan H. 1967. Encéphales et moulages endocrâniens de quelques insectivores et primates actuels. Coll. Int. Centre Natl Rech. Sci. 163, 575–586 [Google Scholar]
- 41.Sherwood C. C., Stimpson C. D., Butti C., Bonar C. J., Newton A. L., Allman J. M., Hof P. R. 2009. Neocortical neuron types in Xenarthra and Afrotheria: implications for brain evolution in mammals. Brain Struct. Funct. 213, 301–328 10.1007/s00429-008-0198-9 (doi:10.1007/s00429-008-0198-9) [DOI] [PubMed] [Google Scholar]
- 42.Damuth J. 1990. Problems in estimating body masses of archaic ungulates using dental measurements. In Body size in mammalian paleobiology (eds Damuth J., MacFadden B. J.), pp. 229–253 Cambridge, UK: Cambridge University Press [Google Scholar]
- 43.Janis C. 1990. Correlation of cranial and dental variables with body size in ungulates and macropodoids. In Body size in mammalian paleobiology (eds Damuth J., MacFadden B. J.), pp. 255–299 Cambridge, UK: Cambridge University Press [Google Scholar]
- 44.Martinez J.-N., Sudre J. 1995. The astragalus of Paleogene artiodactyls, comparative morphology, variability and prediction of body mass. Lethaia 28, 187–209 10.1111/j.1502-3931.1995.tb01612.x (doi:10.1111/j.1502-3931.1995.tb01612.x) [DOI] [Google Scholar]
- 45.Jerison H. J. 1970. Brain evolution: new light on old principles. Science 170, 1224–1225 10.1126/science.170.3963.1224 (doi:10.1126/science.170.3963.1224) [DOI] [PubMed] [Google Scholar]
- 46.Radinsky L. B. 1978. Evolution of brain size in carnivorous and ungulates. Am. Nat. 112, 815–831 10.1086/283325 (doi:10.1086/283325) [DOI] [Google Scholar]
- 47.Sigogneau D. 1968. Le genre Dremotherium (Cervoidea) Anatomie du crâne, denture et moulage endocrânien. Ann. Paleontol. 54, 3–64 [Google Scholar]
- 48.Marino L., McShea D., Uhen M. D. 2004. The origin and evolution of large brains in toothed whales. Anat. Rec. 281A, 1247–1255 10.1002/ar.a.20128 (doi:10.1002/ar.a.20128) [DOI] [PubMed] [Google Scholar]
- 49.Edinger T. 1948. Evolution of the horse brain. Geol. Soc. Am. Mem. 25, 1–177 [Google Scholar]
- 50.Edinger T. 1929. Die fossilen Gehirne. Zeits. Gesam. Anat. Abt. III 28, 1–249 [Google Scholar]
- 51.Ladevèze S., Missiaen P., Smith T. 2010. First skull of Orthaspidotherium edwardsi (Mammalia, ‘Condylarthra’) from the late Paleocene of Berru (France) and phylogenetic affinities of the enigmatic European family Pleuraspidotheriidae. J. Vert. Paleontol. 30, 1559–1578 10.1080/02724634.2010.501440 (doi:10.1080/02724634.2010.501440) [DOI] [Google Scholar]