Abstract
Objective
To understand the etiology and resolution of unanticipated events in the operating room (OR).
Background
The majority of surgical adverse events occur intra-operatively. The OR represents a complex, high-risk system. The influence of different human, team, and organizational/environmental factors on safety and performance is unknown.
Methods
We video-recorded and transcribed 10 high-acuity operations, representing 43.7 hours of patient care. Deviations, defined as delays and/or episodes of decreased patient safety, were identified by majority consensus of a multidisciplinary team. Factors that contributed to each event and/or mitigated its impact were determined and attributed to the patient, providers, or environment/organization.
Results
Thirty-three deviations (10 delays, 17 safety compromises, 6 both) occurred – with a mean of one every 79.4 minutes. These deviations were multifactorial (mean 3.1 factors). Problems with communication and organizational structure appeared repeatedly at the root of both types of deviations. Delays tended to be resolved with vigilance, communication, coordination, and cooperation, while mediation of safety compromises was most frequently accomplished with vigilance, leadership, communication, and/or coordination. The organization/environment was not found to play a direct role in compensation.
Conclusions
Unanticipated events are common in the OR. Deviations result from poor organizational/environmental design and suboptimal team dynamics, with caregivers compensating to avoid patient harm. While recognized in other high risk domains, such human resilience has not yet been described in surgery and has major implications for the design of safety interventions.
Introduction
Operating is an inherently precarious task that requires complex coordination between individuals and teams of disparate experience levels and disciplines, within the context of intricately organized hospital systems, under constraints posed by time and uncertainty. For this reason, the OR is a prime candidate for human factors analysis. As a field of engineering concerned with “the interaction among humans and other elements of a system…physical, cognitive, organizational, environmental, and other,”1 human factors has been responsible for safety and reliability advances in other high-risk work domains – nuclear reactor control and aeronautics, for example. It posits that error stems from multiple, heterogeneous sources; effective interventions, therefore, must address all etiologies, at all levels – individual, team, and organizational/environmental.
A growing body of literature addressing human factors in the OR exists. This research has begun to describe the relationship between surgical outcomes and the performance of the OR as a complex system2–4, but the relative contributions of the key components of this system are unknown. Much of what we know about intra-operative adverse events comes from malpractice claims analysis 5–7, self-reporting systems, and root cause analyses8–9 – methodologies based upon retrospective reconstruction of events that therefore suffer from recall bias. In hindsight, many significant intra-operative factors and events are forgotten, particularly those that are recovered and do not result in a discrete adverse outcome. Prospective analyses have generally entailed live observation in the OR3–4, 10–12, which, while yielding rich data, is limited by the time and space it requires. Few observers may reasonably be present during an operation, and their data is inevitably restricted by their physical and mental capacity to observe, process, and remember.
Video offers several advantages to human factors researchers in the OR: its ability to record prospectively and undergo review retrospectively minimizes the bias against partially or fully compensated events, while its amenability to replay improves reproducibility, facilitates the resolution of inter-observer discrepancies, and allows input to be gathered from multiple observers without requiring their presence at the time of data collection. We sought to 1) develop a methodology of audio-video recording operations, and 2) identify the individual, team, and organization/environment factors that play a role in =the etiology and/or recovery of unanticipated events in the OR, using a human factors approach and a multidisciplinary team.
Methods
Audiovisual Technology
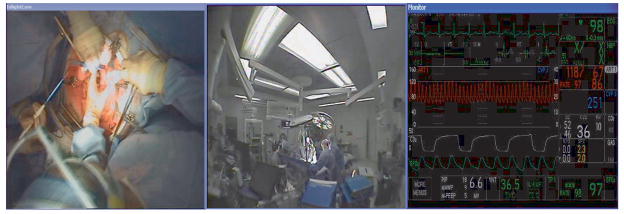
Over a two year period, we piloted and refined the use of audiovisual recording technology in the operating rooms at our institution. Our configuration (Figure 1) includes a camera incorporated into the operative lights, a wide-angle (270°) camera, a feed from the anesthesiologists’ monitor, 5 high-fidelity microphones, and synchronization hardware and software. With it, we are able to capture a view of the operative field in detail, a view of the entire operating room (OR), a dynamic record of the patient’s vital signs, as well as various conversations occurring throughout the OR, in synchrony.
Figure 1.
Video Recording Configuration. Left, view from camera installed in operative lights. Center, view from 270° camera. Right, anesthesia monitor feed.
Data Security and Protection of Human Subjects
In collaboration with Risk Management at our institution, we developed the following data security policies. In order to minimize identification of individuals, the wide-angle camera is purposely low-resolution and the in-light camera capture is restricted to the operative field. After recording, each video is assigned a unique study identification number and stored on our firewall-protected research server, which is backed-up via mirrored array. Identifiers linking the videos to the patient are maintained for 30 days to facilitate chart review; no data is recorded on the providers in the room. Video files are deleted within 90 days of recording as per protocol. This study was approved by the Partners Human Research Committee. A Certificate of Confidentiality was issued by the Department of Health & Human Services to protect this sensitive research material against involuntary disclosure.
Subject Recruitment and Consent
The study was presented to the departments of surgery, anesthesiology, and nursing during their weekly conferences. To minimize workflow disruptions, consent was obtained via an opt-out process, whereby those who did not indicate a desire to “opt out” were assumed to be willing to participate. Verbal assent was confirmed with participating OR staff members immediately prior to each recording to the extent possible; if any person dissented, the recording was cancelled.
To maximize our potential for capturing unanticipated events in the OR, we selected for operations within general surgery and surgical oncology that had published expected complication rates of >20%. We then identified these cases on review of the pre-admission testing center and OR schedules. Prior to each, we contacted the operative attending surgeon to ascertain his willingness to enroll a given patient in the study. Formal written consent was obtained from the patient during the pre-admission testing appointment.
Among 17 surgeons with eligible cases, only one categorically opted out of the study, stating that s/he would be willing to participate in future studies, after we demonstrated that our recording processes would not affect OR turnover time. Sixteen assented to participation at some point during our study period. We identified 389 cases meeting our inclusion/exclusion criteria that were ultimately not recorded. The majority of these losses (59%) were attributable to lack of a functioning in-light camera in the scheduled OR; only a subset of ORs at our institution are installed with this piece of equipment, and 4 were under repair when we began the study. A small minority of recording losses were due to lack of assent from the attending surgeon (6%, usually citing an underlying mental or emotional vulnerability in the patient) or other OR staff (i.e. anesthesiologists or nurses, 5%), and as such we feel that any selection bias towards the capture of teams with safer attitudes or behaviors is unlikely.
Coding
Ten complex surgical procedures were audio and video-recorded from room set-up (marked by the opening of the sterile kits) through patient exit. The videos were analyzed using a modified version of RATE, open access software developed by Guerlain et al13 at the University of Virginia for playing and annotating multiple video and audio streams in synchrony. Two surgical research fellows (AFA, YYH) independently generated transcripts of the videos, and these transcripts were reviewed by a surgeon (CCG), a cognitive psychologist (EMR), and an educational psychologist (SEP). Transcript review was supplemented by review of the primary video data as needed.
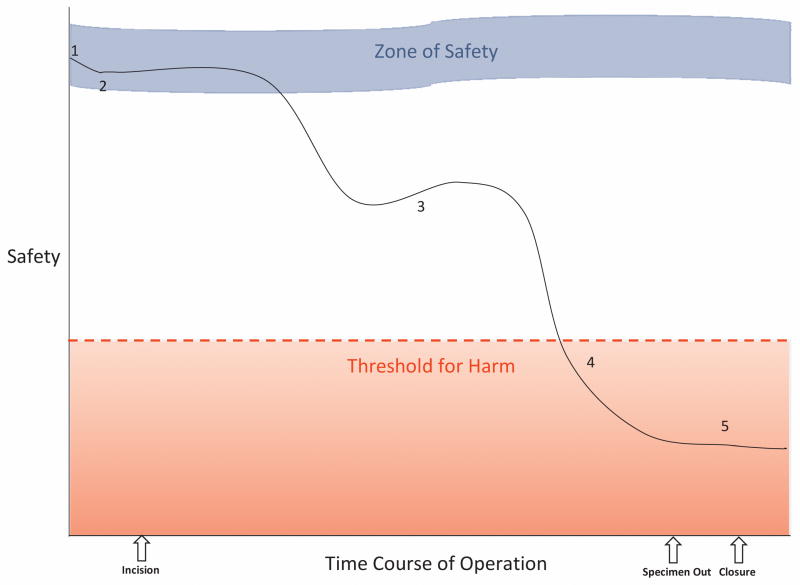
This core research team identified deviations by consensus, using two distinct definitions. Delays consisted of complete halts in forward progress for the entire team lasting over 2 minutes, a threshold set by our international advisory panel of surgeons and intra-operative human factors experts that is more conservative than those reported by others 14. Safety compromises represented episodes of increased risk of harm to the patient. According to our conceptual model (Figure 2), safety compromises may be partially or fully recoverable; however, their effects may also be additive, accumulating until a threshold for harm is reached.
Figure 2.
Conceptual Model of Safety Compromise. The course of the operation is represented by the solid black line. 1. At the beginning of the case, the patient’s level of safety is determined largely by baseline patient factors. 2. A safety compromise occurs, decreasing the level of safety, but the case remains within the Zone of Safety; buffering by other factors maintains a safe environment for the patient. 3. Another safety compromises decreases the level of safety beyond the Zone of Safety, but partial recovery (upward slope) prevents the patient from progressing further towards harm. 4. A final safety compromise decreases the level of safety below the Threshold for Harm; negative patient outcomes are manifested. 5. Stabilization (flattened slope) is achieved; the patient’s harm is not reversed, but no further harm is done.
We asked our clinical domain experts (two surgeons: RSS, RTO; an anesthesiologist: AMB; and an OR nurse: PS) to independently categorize these deviations according to the following schema: delay, safety compromise, both, or neither. The final designation of an episode as a deviation and its type was based on a majority ruling among the clinical domain experts and the core research team.
Members of the core research team then reviewed the final deviations to identify (again, by consensus) all factors that played a role in the causality, as well as the mitigation or recovery (if applicable), of each. These contributing and compensatory factors were attributed to the patient, an individual, the team, or the organization/environment. Because we found significant overlap between individual and team factors, a single providers category was used to encompass both. Additionally, the time from inception until resolution of each deviation was noted.
Summary statistics were calculated for the number of deviations per case, the number of contributing and compensatory factors per deviation, and the time to resolution. The frequency of appearance of contributing and compensatory factors is expressed as a proportion of deviations.
Results
General descriptions of the cases under study are given in Table 1. As planned, a variety of complex general surgical procedures were recorded, with individual case durations ranging from 78 minutes to over 7 hours. In total, we obtained a total of 43.7 hours on video, including 38.8 hours of patient-in-room time and 28.3 hours of operative time. Thirty-three deviations – 10 delays, 17 safety compromises, and 6 both – were captured. As shown in Table 1, every case had at least one deviation; each typically demonstrated more than 3 (mean 3.3, median 3.5). Eighty percent of cases had at least 1 delay (mean 2, median 1.5), while 90% had at least 1 safety compromise (mean 2.6, median 2).
Table 1.
Recorded Cases
| Case Description | Scheduled Duration (min) | Actual Duration (min) | Number of Delays | Number of Safety Compromises | Total Number of Deviations |
|---|---|---|---|---|---|
| Upper gastrointestinal | 350 | 291 | 4 | 3 | 6 |
| Upper gastrointestinal | 150 | 118 | 1 | - | 1 |
| Retroperitoneal sarcoma | 300 | 422 | - | 3 | 3 |
| Lower gastrointestinal | 230 | 263 | 3 | 3 | 4 |
| Hepatopancreaticobiliary | 240 | 152 | 1 | 5 | 5 |
| Retroperitoneal sarcoma | 240 | 185 | - | 1 | 1 |
| Lower gastrointestinal | 230 | 146 | 2 | 2 | 4 |
| Lower gastrointestinal | 180 | 78 | 1 | 2 | 3 |
| Lower gastrointestinal | 350 | 240 | 3 | 2 | 4 |
| Hepatopancreaticobiliary | 480 | 430 | 1 | 2 | 2 |
| Total | 2750 | 2325* | 16† | 23† | 33† |
Note: Actual duration represents the amount of time the patient is in the room. We started our analysis with nursing set-up; thus the amount of patient care time analyzed is greater than this number.
Note: 6 deviations were both delays and safety compromises.
Etiology
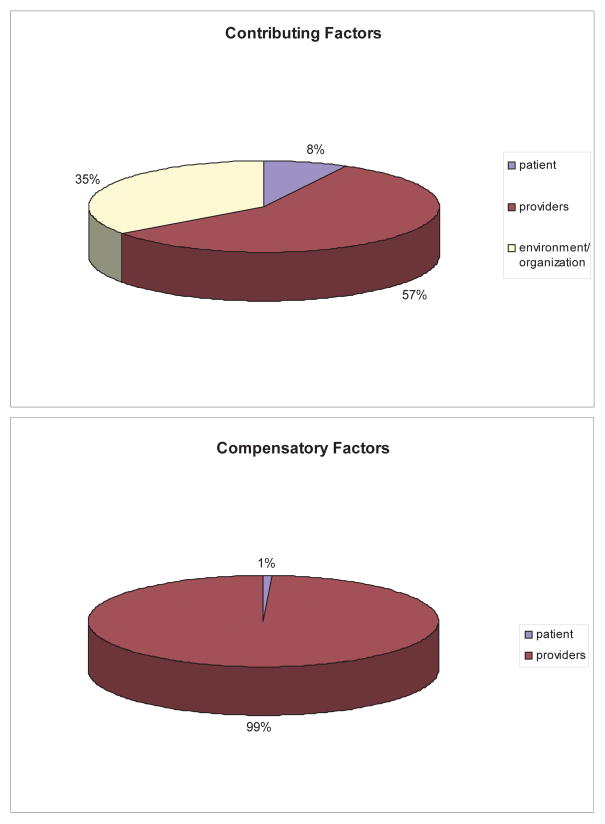
The factors found to contribute to each type of deviation are displayed on the left side of Table 2. The distribution of these factors to patient, provider, or environment/organization is seen in the top panel of Figure 3. An example illustrating each of the factors is provided in Table 3. Only 24% of all deviations could be traced to patient factors. In contrast, provider factors contributed to 70% of all deviations. Among these, failures in communication (33.3%), coordination (30.3%), leadership (24.2%), and knowledge/training (24.2%) were most frequently observed. Over half (52%) of all deviations were at least in part attributable to the organization/environment, most commonly problems with organization (39.4%), system-level communication (24.2%), system-wide coordination (21.2%), and equipment (21.2%). To illustrate the distinction between factors that appear both at the level of the provider and at the level of the organization/environment, notable instances of communication and coordination failures are described below.
Table 2.
Contributing and Compensatory Factors for Deviations
| Factor | Contributed To: | Compensated For: | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Number of Delays | Number of Safety Compromises | Number of All Deviations | Number of Delays | Number of Safety Compromises | Number of All Deviations | |
|
| ||||||
| Patient | 2 (12.5%) | 7 (30.4%) | 8 (24.0%) | - | 1 (4.4%) | 1 (3.0%) |
| Anatomy | 1 (6.3%) | 3 (13.0%) | 3 (9.1%) | - | - | - |
| Physiology | 1 (6.3%) | 4 (17.4%) | 5 (15.2%) | - | 1 (4.4%) | 1 (3.0%) |
|
| ||||||
| Providers | 11 (68.8%) | 18 (78.3%) | 23 (70.0%) | 16 (100%) | 22 (95.7%) | 32 (97.0%) |
| Monitoring/Vigilance | 2 (12.5%) | 4 (17.4%) | 6 (18.2%) | 9 (56.3%) | 14 (60.9%) | 19 (57.6%) |
| Knowledge/Training | 4 (25.0%) | 6 (26.1%) | 8 (24.2%) | 1 (6.3%) | 6 (26.1%) | 6 (18.2%) |
| Decision Making | 1 (6.3%) | 2 (8.7%) | 2 (6.1%) | 4 (25.0%) | 7 (30.4%) | 9 (27.3%) |
| Leadership | 6 (37.5%) | 6 (26.1%) | 8 (24.2%) | 4 (25.0%) | 11 (47.8%) | 13 (39.4%) |
| Temperament | 1 (6.3%) | 2 (8.7%) | 2 (6.1%) | - | 2 (8.7%) | 2 (6.1%) |
| Adaptability | - | - | - | 3 (18.8%) | 3 (13.0%) | 5 (15.2%) |
| Mistakes/Slips | 2 (12.5%) | 4 (17.4%) | 4 (12.1%) | - | - | - |
| Communication | 7 (43.8%) | 7 (30.4%) | 11 (33.3%) | 10 (62.5%) | 12 (52.2%) | 20 (60.6%) |
| Coordination | 7 (43.8%) | 5 (21.7%) | 10 (30.3%) | 6 (37.5%) | 10 (43.5%) | 15 (45.5%) |
| Cooperation | - | 1 (4.4%) | 1 (3.0%) | 6 (37.5%) | 6 (26.1%) | 10 (30.3%) |
| Distraction | 1 (6.3%) | 1 (4.4%) | 1 (3.0%) | - | - | - |
| Competing Priorities | 1 (6.3%) | 2 (8.7%) | 2 (6.1%) | - | - | - |
| Status Asymmetry | 2 (12.5%) | 3 (13.0%) | 4 (12.1%) | - | - | - |
| Contingency Planning | - | - | - | 5 (31.3%) | 5 (21.7%) | 8 (24.2%) |
|
| ||||||
| Environment/Organization | 9 (56.3%) | 11 (47.8%) | 17 (52.0%) | - | - | - |
| Equipment | 3 (18.8%) | 5 (21.7%) | 7 (21.2%) | - | - | - |
| Organization | 8 (50.0%) | 8 (34.8%) | 13 (39.4%) | - | - | - |
| Communication | 7 (43.8%) | 3 (13.0%) | 8 (24.2%) | - | - | - |
| Coordination | 3 (18.8%) | 6 (26.1%) | 7 (21.2%) | - | - | - |
| Decision Making | - | 1 (4.4%) | 1 (3.0%) | - | - | - |
Figure 3.
Attribution of Factors to Patient, Providers, or Environment/Organization. Top, distribution of factors that contributed to deviations among patient, providers, or environment/organization. Bottom, distribution of factors that aided in compensation and/or mitigation of deviations among patient, providers, or environment/organization.
Table 3.
Illustrative Examples of Factors
| A. Contributing Factors | ||
|---|---|---|
| Factor | Example | |
| Patient | Anatomy | Patient’s obesity prolongs entry into the abdomen and hinders pelvic exposure. |
| Physiology | Patient has a vagal response to intra-abdominal manipulation. | |
| Providers | Monitoring/Vigilance | Surgeons do not notice that anesthesiologists are troubleshooting the ventilator with Biomedical Engineering; they ask for the volume of their music to be increased. |
| Knowledge/Training | Trainee inexperienced at central line placement punctures the carotid artery. | |
| Leadership | Surgical resident contacts attending 3 times for help; he does not scrub until 28 minutes after incision. | |
| Communication | Anesthesiology resident misinterprets surgeons’ claims of “no surgical bleeding” to mean there is no bleeding; resuscitation lags. | |
| Coordination | Attending surgeon’s absence stalls progress; team is unsure about supplies and/or surgical approach and is unable to prepare and/or start. | |
| Cooperation | Surgeons are unresponsive to anesthesiologists’ announcement that the patient’s blood pressure has dropped; they do not engage in problem-solving. | |
| Environment/Organization | Equipment | Equipment malfunction and patient monitors share the same alert signal; users cannot distinguish between malfunction and patient instability based on the sound of the alarm. |
| Organization | During a case complicated by patient obesity, a scrub technician notes that longer instruments were requested 6 months ago by another surgeon and never acquired. | |
| Communication | Laboratory work drawn in pre-admission testing returns with abnormal results, but is not seen or addressed until the day of surgery because there is no system by which such results are checked and/or communicated. The case is delayed while the tests are repeated and specialists consulted. | |
| Coordination | The number of ongoing oncology cases is greater than the number of available oncology nurses or oncology kits. A delay results, as unfamiliar nurses attempt to assemble a kit from the pieces of other kits. | |
| B. Compensatory Factors | ||
|---|---|---|
| Factor | Example | |
| Patient | Physiology | Patient weans off of his nitroglycerin drip before leaving the room. |
| Providers | Monitoring/Vigilance | Pod nurse enters the room during induction and realizes the scrub technician is missing. He scrubs in his place to prevent a delay. |
| Knowledge/Training | Attending anesthesiologist recognizes the critical nature of the case and accelerates resuscitation. | |
| Leadership | An alarm sounds, and the nursing and anesthesiology teams become absorbed in searching for its source, leaving their posts. The circulator sends everyone back to work and contacts Biomedical Engineering. | |
| Adaptability | The urology team is unaware that a patient was booked for stents prior to his colorectal resection, but, upon being contacted, agrees to come immediately. The nurses, anesthesiology resident, and surgical resident halt their tasks to help him. | |
| Communication | Surgeon goes to Pathology himself to orient them to the specimen. His insistence on synchronous communication reduces the length of time required for pathological determination of the margins. | |
| Coordination | Plastic surgery is scheduled to close the abdomen. Primary surgeon gives the plastic surgery team ample advance notice, both pre- and intra-operatively. | |
| Cooperation | When asked by the anesthesiology resident, the surgeons remove all instruments from the abdomen until the patient stabilizes. | |
| Contingency Planning | After an episode of bleeding during which a scrub technician had difficulty finding the requested instruments, the surgeon instructs him as to which ones to have ready in the future. | |
Communication
Problems with communication may result from inefficient delivery and/or incomplete comprehension on the part of individuals. The following episode can be used to demonstrate this more classic type of communication failure – occurring at the provider-level. About one hour after incision, the anesthesiology resident noted low urine output and significant volume in the suction canister. The surgeons, who were finding the case more technically challenging than they had anticipated and initially indicated, had been actively discussing the complexity of the case amongst themselves; however, they had not notified the anesthesiology team about any altered expectations with regards to operative time or blood loss. The anesthesiology resident asked the surgeons if irrigation had been used (no) and if there was oozing (yes), but received no further insight. Throughout the remainder of the case, the anesthesiology resident continued to ask the surgeons about bleeding, and each time, they denied it. Resuscitation lagged, and the patient developed cardiac ischemia. Towards the end of the case, the surgeons clarified that they had meant that there had been no “surgical bleeding” – that they had only encountered continuous oozing, as they had originally indicated. The lack of a shared understanding about the expected course of the operation, as well as the differing perceptions each held about the meaning of the term “bleeding” contributed to this deviation.
As this case demonstrates, deviations tended to be multifactorial in etiology; on average, each was attributable to at least 3 factors (mean 3.1, median 3.3). Here, under-resuscitation of a high-risk patient with a pre-existing heart condition led to cardiac ischemia and arose from problems with several factors including 1) communication, as described above; 2) monitoring/vigilance (surgeons did not notice the patient’s hemodynamic instability or seek physiologic information; anesthesiology resident did not hear the surgeons’ discussion about the changing demands of the case); 3) coordination (anesthesiology attending was unable to fully supervise his resident due to his responsibilities in other ORs); 4) knowledge/training (the anesthesiology resident did not recognize the urgency of the situation when the patient became volume depleted), and possibly 5) status asymmetry (the anesthesiology resident felt uncomfortable pushing the attending surgeon to explain or predict the blood loss).
Failures in communication may also result from a feature of the larger organization/environment. We observed several instances in which the structure of the organization that was built to facilitate communication in fact hindered it. In one case, we observed extensive and redundant discussions about the potential need for available blood as different providers – nurses, anesthesiologists, and the surgical fellow – arrived preoperatively. Blood had not been ordered with the booking, whereas all of these providers had expected it would be; hence, they ordered it. When the attending surgeon arrived, he announced that blood was not necessary for this case – that he had intentionally not ordered it. The booking sheets, which varied by surgeon, all had space for the surgeons to make requests for such needs, but some lacked corresponding ones in which to explicitly deny them. To the recipients of these forms, the purposeful act of not ordering blood appeared exactly the same as forgetting to do so. In relying on teams to surmise the attending surgeons’ intentions, this poorly designed vehicle for communication contributed to wasted resources, time, and energy.
Coordination
Coordination was similarly observed to contribute to deviations at both a provider and an organizational/environmental level. Attending surgeons were frequently absent at the beginning of their cases, causing delays ranging from 8 to 28 minutes. Even teams familiar enough with their attendings’ preferences to begin without them ultimately reached a point at which progress halted and they were forced to idly wait. These attending surgeons often arrived to find that their preferences had been inaccurately approximated – the positioning suboptimal or the proper supplies unavailable – and the delays persisted while the team reconciled the discrepancies.
In comparison, among nurses and anesthesiologists, coordination failures tended to be induced by organizational factors such as scheduling policies. For example, poorly timed hand-offs were generally the product of an OR scheduling mechanism insensitive to the demands and/or progress of individual cases, rather than the actions of discrete providers; therefore, these were attributed to the organization. In one case, a second attending anesthesiologist arrived in the midst of induction to send the original attending home and the resident to assist in another OR. This unexpected hand-off prompted an extensive discussion of ongoing staffing issues throughout the OR and necessitated an immediate, but unplanned, transfer of information, increasing the team’s workload during its highest-intensity phase of care – a set-up conducive to lapses in memory. Furthermore, the providers responsible for the remainder of the patient’s anesthetic care, including intra-operative management and extubation, were not those who were the most knowledgeable about him; they had not experienced his difficult airway or hemodynamic instability. Following the completion of the induction and the hand-off, a replacement resident also unexpectedly arrived, requiring yet another hand-off – again increasing workload as well as the potential for information loss. Finally, because the switch had been wholly unanticipated, the original team indicated they were unprepared for the hand-off; the outgoing attending and resident noted and apologized for various tasks that remained to be done, which, with more notice, they would have completed prior to hand-off in order to streamline the workload for their successors. In similar fashion, inopportune hand-offs were noted to prolong surgical bleeding (when a scrub technician’s scheduled break occurred during a critical portion of the case, his substitute could not find the appropriate instruments) and complicate the count (when the scrub technicians and circulators handed-off immediately prior to closure, the new team, unfamiliar with the case, was unable to reconcile the other’s count).
Recovery
Some form of compensation was observed in every deviation except one, in which the patient left the OR before the safety compromise was recognized. Only 1 of the remaining 22 safety compromises concluded with measurable patient harm. On average, each deviation lasted 37 minutes from beginning to end, and while safety compromise and delays tended to be similar in duration (34 and 31 minutes, respectively), the simultaneous occurrence of the two took nearly twice as long to resolve (54 minutes). Like contributing factors, compensatory factors were rarely patient-related (3.0%). In stark contrast, however, we were unable to ascribe any compensatory factors directly to the environment/organization. Overwhelmingly (97.0%), providers were the source of recovery.
As in their genesis, the mitigation of deviations tended to be complex, typically requiring 3 factors each to resolve (mean 3.3, median 3). To compensate for deviations, providers most frequently intensified teamwork efforts, including communication (60.6%), coordination (45.5%), leadership (39.4%), and cooperation (30.3%). More individual attributes such as monitoring/vigilance (57.6%), decision-making (27.3%), and contingency planning (24.2%) commonly emerged as well.
In one exemplary episode, the patient desaturated and became hypotensive about 30 minutes after incision. The anesthesiology resident recognized these changes immediately (monitoring/vigilance) and clearly alerted the surgeons (communication), explaining that he had already given phenylephrine without response. He instructed them to remove their retractors, followed by any packing (decision-making, leadership, coordination); they complied readily (cooperation). The surgical attending then requested a fluid bolus (decision-making, leadership), to which the anesthesiology resident agreed (cooperation). While waiting for the patient to recover, both the anesthesiology resident and the surgical attending taught the surgical resident about troubleshooting hemodynamic instability with an open abdomen (knowledge/training). Four minutes later, the anesthesiology resident notified the surgeons that it was possible to proceed (coordination, leadership).
Interestingly, we observed episodes of hemodynamic instability often, but without such smooth engagement from the team. In another case, upon noting bradycardia and hypotension, the anesthesiology attending alerted the surgical attending. He, however, responded, “Well, we’re not really doing anything,” and proceeded to operate. The previous team is thus worth highlighting first for its ability to cooperate, but also for its exchange. Leadership and decision-making roles were shared among team members, and such flexibility permitted an interplay of expertise that improved the team’s ability to provide care.
Discussion
Intra-operative deviations in care occur routinely – by our estimates, once every 79 minutes during complex procedures. At the root of these deviations, both provider and organizational/environmental factors predominate; we observed suboptimal team dynamics and poorly designed systems causing delays and compromising patient safety. These findings are in keeping with previous work on human factors, both in and outside of medicine2, 10–12, 15–16.
In surgery, the contribution of various provider factors to deviations is well-established. Communication, for example, is an often-cited provider factor in both safety compromises and delays. Based upon intra-operative observations, Lingard17 estimated that 31% of all procedurally-relevant communications in the OR fail, with 36% of these failures impacting case flow (18% cause inefficiency, 8% delays, 2% workarounds, 2% resource waste) and/or patients (2% inconvenience, 1% procedural error). Mazzocco4 demonstrated an increased odds of complications or death with low intra-operative information sharing. Higher levels of positive communication were correlated with lower risk-adjusted mortality rates across Veterans Affairs Medical Centers18. Similarly, absence of the attending surgeon at the beginning of the operation has been highlighted in a number of studies of OR delays14, 19.
The impact of organizational/environmental factors on intra-operative processes has also been recognized. Many have published their stories of OR efficiency improvements based upon changes in infrastructure and/or policy – for example, those regarding the allocation of human resources19, block time release20, and standardization of booking processes21 or instrument trays20, Equipment problems are particularly common in the OR. In their intra-operative observations, Healey22 noted that unavailable or non-functional equipment was the distraction/interruption most likely to interfere with the case, while Wiegmann11 traced 11% of all surgical flow disruptions to difficulties with equipment or technology.
To our knowledge, no previous studies have attempted to determine the relative importance of specific provider or organizational/environmental factors in precipitating deviations or characterized their role in mitigation. Nearly all of our deviations (97%) were salvaged by providers; the organization and environment played no direct role in recovery in the cases that we observed. Such data seems to directly contradict the prevailing dogma. Our understanding of error in medicine has paralleled that in the human factors world, albeit with several years’ lag time. Initially, in their field, as in ours currently, adverse events were blamed on human fallibility, and emphasis was placed on the construction of standardized systems with safeguards to protect us from our own inconsistent performance 23.
Although this assumption became the impetus for many successful safety advances such as checklists24–25 and technological adjuncts for tracking sponges26, the human factors field has since experienced a sea change. To follow-up his 1990 treatise on failure and the limitations of human cognition, “Human Error,”27 James Reason recently published “The Human Contribution: Unsafe Acts, Accidents, and Heroic Recoveries”28. Human factors experts like Reason now recognize the power embedded within human variability: our ability to adapt. The concept of human resilience is beginning to emerge in healthcare29.
In their observational study of 243 neonatal arterial switch operations, deLeval et al3 found that compensation reduced the risk of death in the event of major (potentially life-threatening) intra-operative errors. In a case study of patient load increases in the emergency department, Anders et al30 described individual personnel as the source of resilience; they alone recognized strain on the system and recruited additional resources to buffer it. Patterson et al31 detailed the role of collaborative cross-checking in detecting and recovering medication error incidents. Protocol-driven cross-checks were noted to be relatively ineffective; resilience was largely induced by people outside of their official roles and responsibilities.
System redesign is unquestionably an important tool in preventing and mitigating deviations. Some of the deviations we captured in our study could have been prevented through system modifications – particularly those that were attributable to the organization or environment, like the unnecessary blood order or the inopportune shift changes – thus obviating the need for provider-driven rescue. However, deviations are, to some degree, inevitable in the practice of medicine, where the potential knowledge base is limitless and presentations and circumstances constantly change. Academic tertiary care centers, for example, are bound to encounter physiologic fragility or provider inexperience, no matter how thoughtfully their support systems are designed. Therefore, as we continue our work to improve safety and efficiency in health care, we must simultaneously build systems that will help us avert deviations and train providers to anticipate and deal with those that are unavoidable.
We posit that the best-designed systems incorporate a degree of flexibility to accommodate positive fluctuations in the performance of its providers. Indeed, attempts at system redesign may have unintended consequences. Standardization, for example, so often the product of systematic change, may prove constricting in certain situations when the opposite is required. We have previously reported on the deleterious impact of disabling protocols32, and suggest that hospitals examine the downstream impact of all new procedures post-implementation, with particular focus on changes in provider functionality33–34. In order for providers to flexibly adapt as deviations arise, the environment must be conducive to exploration. The importance of a culture of safety in this regard cannot be understated35–38. For any safety initiative to gain traction and achieve meaningful, lasting results, it must have, at its foundation, support. Safety must be institutionalized – valued by hospital leadership and administration, as well as individual providers. As has been said repeatedly by human factors experts, safe is not something organizations are, but something they do. Safety comes with an ongoing commitment and willingness to change39.
Limitations
As in any observational study, the Hawthorne effect may be a concern. However, we believe the use of video, with equipment placed as non-obtrusively as possible, minimized this risk. We suspect that its impact on our findings was smaller than that of a similar study using field observations, in which the presence of live personnel in the OR may serve as a constant reminder of the ongoing study. The operations we recorded were long and often arduous, absorbing the full attention of the teams. As the discussion in the room occasionally touched upon confidential topics, it became apparent that participants forgot about the recording in the course of the case. Thus, we believe that our capture is naturalistic. However, even if the teams remained aware of the study throughout the case and adjusted their actions accordingly, then our estimate of deviations – our primary outcome – is a conservative one.
Because all recordings were performed at a single institution, generalizability may be a concern. As an academic, tertiary-care hospital, we may treat more complex patients; however, as patient factors contributed a relatively small amount to the evolution or the recovery of deviations, this difference in case mix is likely immaterial. While we have a strong safety culture, our hospital does not employ the system innovations reported by other groups20–21, 40, and team training efforts41 here are not yet widespread; thus, we doubt our results would differ significantly from those of a typical academic tertiary care hospital. As others in the surgical literature have previously reported the importance of various provider and system factors – communication4, 6, 17, 42 or leadership12, for example – in the OR, we suspect the themes highlighted by our data are universal.
We developed a novel coding system for identifying and classifying deviations. Benefiting from the input of a multidisciplinary team, we based our decisions upon discussion and consensus rather than attempting to achieve concordance between two trained coders. Existing global rating scales for provider performance43–45 and environmental distractions22, 46, while seemingly easier to report and/or replicate, were considered, but ultimately discarded for their insufficient inter-rater reliability, which we surmise stems from their ambiguity. Likert-type rating scales cannot encompass the nuances in human interactions with each other or their environment. Furthermore, our study teams (both the core research team and the clinical domain consultant team) were constructed with the goal of representing multiple, equally valuable perspectives. The discussion that preceded consensus allowed us to achieve a nuanced understanding of each deviation. If we had instead required the agreement of independent coders to designate deviations, the highly informative, domain-specific data generated by any single expert’s reading of an event (e.g. human factors, nursing, anesthesiology) may have been lost. Finally, as consensus was readily achieved despite the disparate backgrounds of our study team members, we expect that the results are indeed reproducible by others.
As Jeffcott et al47 state, tools for measurement in resilience engineering are currently lacking. Sheridan48 further explains that because resilience is in its early stages of development, the approach to it is more likely to be qualitative than quantitative. As such, there were no existing methodologies to aid us in our characterization of error mitigation in the OR. We feel that our methods, which combined qualitative and quantitative techniques and allowed us to retain a rich level of detail in our data, were best suited to our purposes.
Implications
Using video, we captured and deconstructed episodes of safety compromise and delay, as well as their recoveries, in the operating room. These events are common, and, contrary to popular belief, are not purely the consequence of human imperfections allayed by protective systems. While providers contributed to deviations (along with system and organizational factors), they were also an important source of recovery. Such human resilience has been recognized outside of surgery and will be a critical component of surgical safety in the future.
To date, the approach to improving surgical safety has focused primarily on error prevention. While such interventions have led to marked improvements in safety, our results suggest that increased standardization is only part of the solution. Error mitigation is a complementary approach to patient safety; in this model, practitioners, with the uniquely human capacity for adaptation, are a primary source of resilience in an imperfect system. To cultivate this quality, we must design systems and promote a culture able to accommodate it48–49, taking care not to restrict providers’ ability to improvise with over-protocolization. In doing so, we will not only minimize deviations in care, but we will also learn to recover from them when they inevitably occur.
Acknowledgments
This work was supported by grants from the Risk Management Foundation of the Harvard Medical Institutions, The Rx Foundation, and the National Institutes of Health (Research Training in Alimentary Tract Surgery, #2T32DK00754-12; Loan Repayment Program #L30RR031458-01 and #L30CA123695-03). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Meetings: Presented at the Quality, Outcomes, and Costs II session of the Surgical Forum at the 2011 American College of Surgeons Clinical Congress.
Conflicts of Interest: No conflicts of interest to declare.
Justification for Authorship
Our manuscript lists 11 authors, all of whom have contributed significantly and meet the criteria set forth by Annals of Surgery. The specific contributions of each author are listed as follows:
1) Study Conception & Design: Yue-Yung Hu, Alexander Arriaga, Emilie Roth, Sarah Peyre, Caprice Greenberg
Acquisition of Data: Yue-Yung Hu, Alexander Arriaga, Katherine Corso, Richard Swanson, Angela Bader, Michael Zinner, Caprice Greenberg
Analysis and Interpretation of Data: Yue-Yung Hu, Alexander Arriaga, Emilie Roth, Sarah Peyre, Katherine Corso, Richard Swanson, Robert Osteen, Pamela Schmitt, Angela Bader, Caprice Greenberg
2) Article Drafting: Yue-Yung Hu, Caprice Greenberg
Critical Revisions: Yue-Yung Hu, Alexander Arriaga, Emilie Roth, Sarah Peyre, Katherine Corso, Richard Swanson, Robert Osteen, Pamela Schmitt, Angela Bader, Michael Zinner, Caprice Greenberg
3) Final Approval: Yue-Yung Hu, Alexander Arriaga, Emilie Roth, Sarah Peyre, Katherine Corso, Richard Swanson, Robert Osteen, Pamela Schmitt, Angela Bader, Michael Zinner, Caprice Greenberg
References
- 1. [Accessed 28 April, 2011];What is Ergonomics? at http://www.iea.cc/01_what/What%20is%20Ergonomics.html.
- 2.Carthey J, de Leval MR, Reason JT. The human factor in cardiac surgery: errors and near misses in a high technology medical domain. Ann Thorac Surg. 2001;72:300–5. doi: 10.1016/s0003-4975(00)02592-3. [DOI] [PubMed] [Google Scholar]
- 3.de Leval MR, Carthey J, Wright DJ, et al. Human factors and cardiac surgery: a multicenter study. J Thorac Cardiovasc Surg. 2000;119:661–72. doi: 10.1016/S0022-5223(00)70006-7. [DOI] [PubMed] [Google Scholar]
- 4.Mazzocco K, Petitti DB, Fong KT, et al. Surgical team behaviors and patient outcomes. Am J Surg. 2009;197:678–85. doi: 10.1016/j.amjsurg.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Rogers SO, Jr, Gawande AA, Kwaan M, et al. Analysis of surgical errors in closed malpractice claims at 4 liability insurers. Surgery. 2006;140:25–33. doi: 10.1016/j.surg.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg CC, Regenbogen SE, Studdert DM, et al. Patterns of communication breakdowns resulting in injury to surgical patients. J Am Coll Surg. 2007;204:533–40. doi: 10.1016/j.jamcollsurg.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Regenbogen SE, Greenberg CC, Studdert DM, et al. Patterns of technical error among surgical malpractice claims: an analysis of strategies to prevent injury to surgical patients. Ann Surg. 2007;246:705–11. doi: 10.1097/SLA.0b013e31815865f8. [DOI] [PubMed] [Google Scholar]
- 8.Faltz LL, Morley JN, Flink E, et al. The New York Model: Root Cause Analysis Driving Patient Safety Initiative to Ensure Correct Surgical and Invasive Procedures. Assessment. 2008;1 [PubMed] [Google Scholar]
- 9.Mallett R, Conroy M, Saslaw LZ, et al. Preventing Wrong Site, Procedure, and Patient Events Using a Common Cause Analysis. Am J Med Qual. 2011 doi: 10.1177/1062860611412066. [DOI] [PubMed] [Google Scholar]
- 10.Christian CK, Gustafson ML, Roth EM, et al. A prospective study of patient safety in the operating room. Surgery. 2006;139:159–73. doi: 10.1016/j.surg.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 11.Wiegmann DA, ElBardissi AW, Dearani JA, et al. Disruptions in surgical flow and their relationship to surgical errors: an exploratory investigation. Surgery. 2007;142:658–65. doi: 10.1016/j.surg.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 12.Catchpole K, Mishra A, Handa A, et al. Teamwork and error in the operating room: analysis of skills and roles. Ann Surg. 2008;247:699–706. doi: 10.1097/SLA.0b013e3181642ec8. [DOI] [PubMed] [Google Scholar]
- 13.Guerlain S, Adams RB, Turrentine FB, et al. Assessing team performance in the operating room: development and use of a “black-box” recorder and other tools for the intraoperative environment. J Am Coll Surg. 2005;200:29–37. doi: 10.1016/j.jamcollsurg.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Weinbroum AA, Ekstein P, Ezri T. Efficiency of the operating room suite. Am J Surg. 2003;185:244–50. doi: 10.1016/s0002-9610(02)01362-4. [DOI] [PubMed] [Google Scholar]
- 15.Calland JF, Guerlain S, Adams RB, et al. A systems approach to surgical safety. Surg Endosc. 2002;16:1005–14. doi: 10.1007/s00464-002-8509-3. discussion 1015. [DOI] [PubMed] [Google Scholar]
- 16.Vincent C, Moorthy K, Sarker SK, et al. Systems approaches to surgical quality and safety: from concept to measurement. Ann Surg. 2004;239:475–82. doi: 10.1097/01.sla.0000118753.22830.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lingard L, Espin S, Whyte S, et al. Communication failures in the operating room: an observational classification of recurrent types and effects. Qual Saf Health Care. 2004;13:330–4. doi: 10.1136/qshc.2003.008425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davenport DL, Henderson WG, Mosca CL, et al. Risk-adjusted morbidity in teaching hospitals correlates with reported levels of communication and collaboration on surgical teams but not with scale measures of teamwork climate, safety climate, or working conditions. J Am Coll Surg. 2007;205:778–84. doi: 10.1016/j.jamcollsurg.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 19.Overdyk FJ, Harvey SC, Fishman RL, et al. Successful strategies for improving operating room efficiency at academic institutions. Anesth Analg. 1998;86:896–906. doi: 10.1097/00000539-199804000-00039. [DOI] [PubMed] [Google Scholar]
- 20.Heslin MJ, Doster BE, Daily SL, et al. Durable improvements in efficiency, safety, and satisfaction in the operating room. J Am Coll Surg. 2008;206:1083–9. doi: 10.1016/j.jamcollsurg.2008.02.006. discussion 1089-90. [DOI] [PubMed] [Google Scholar]
- 21.Cima RR, Brown MJ, Hebl JR, et al. Use of Lean Six Sigma Methodology to Improve Operating Room Efficiency in a High-Volume Tertiary-Care Academic Medical Center. J Am Coll Surg. 2011 doi: 10.1016/j.jamcollsurg.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Healey AN, Sevdalis N, Vincent CA. Measuring intra-operative interference from distraction and interruption observed in the operating theatre. Ergonomics. 2006;49:589–604. doi: 10.1080/00140130600568899. [DOI] [PubMed] [Google Scholar]
- 23.Leape LL. Error in medicine. JAMA. 1994;272:1851–7. [PubMed] [Google Scholar]
- 24.de Vries EN, Hollmann MW, Smorenburg SM, et al. Development and validation of the SURgical PAtient Safety System (SURPASS) checklist. Qual Saf Health Care. 2009;18:121–6. doi: 10.1136/qshc.2008.027524. [DOI] [PubMed] [Google Scholar]
- 25.Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–9. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg CC, Diaz-Flores R, Lipsitz SR, et al. Bar-coding surgical sponges to improve safety: a randomized controlled trial. Ann Surg. 2008;247:612–6. doi: 10.1097/SLA.0b013e3181656cd5. [DOI] [PubMed] [Google Scholar]
- 27.Reason JT. Human Error. 1. Cambridge University Press; 1990. [Google Scholar]
- 28.Reason JT. The Human Contribution. 1. Arena; 2008. [Google Scholar]
- 29.Nemeth C, Wears RL, Woods DD, et al. Minding the Gaps: Creating Resilience in Health Care. In: Henriksen K, Battles JB, Keyes MA, Grady ML, editors. Advances in Patient Safety: New Directions and Alternative Approaches (Vol 3: Performance and Tools) Rockville, MD: Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- 30.Anders S, Woods DD, Wears RL, et al. Limits on Adaptation: Modeling Resilience and Brittleness in Hospital Emergency Departments. Symposium on Resilience Engineering; 2006 Nov 8–10; Juan-les-Pins, France. 2006. [Google Scholar]
- 31.Patterson ES, Woods DD, Cook RI, et al. Collaborative Cross-Checking to Enhance Resilience. Cogn Tech Work. 2007;9:155–162. [Google Scholar]
- 32.Dierks MM, Christian CK, Roth EM, et al. Healthcare Safety: The Impact of Disabling Safety Protocols. IEEE Transactions on Systems, Man, and Cybernetics - Part A: Systems and Humans. 2004;34:693–698. [Google Scholar]
- 33.Patterson ES, Cook RI, Render ML. Improving patient safety by identifying side effects from introducing bar coding in medication administration. J Am Med Inform Assoc. 2002;9:540–53. doi: 10.1197/jamia.M1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pew RW, Mavor AS, et al., editors. Technology CoH-SDSfC. Human-System Integration in the System Development Process: A New Look. Washington, DC: The National Academies Press; 2007. [Google Scholar]
- 35.Flin R, Yule S, McKenzie L, et al. Attitudes to teamwork and safety in the operating theatre. Surgeon. 2006;4:145–51. doi: 10.1016/s1479-666x(06)80084-3. [DOI] [PubMed] [Google Scholar]
- 36.Sexton JB, Thomas EJ, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ. 2000;320:745–9. doi: 10.1136/bmj.320.7237.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sax HC, Browne P, Mayewski RJ, et al. Can aviation-based team training elicit sustainable behavioral change? Arch Surg. 2009;144:1133–7. doi: 10.1001/archsurg.2009.207. [DOI] [PubMed] [Google Scholar]
- 38.McCulloch P, Mishra A, Handa A, et al. The effects of aviation-style non-technical skills training on technical performance and outcome in the operating theatre. Qual Saf Health Care. 2009;18:109–15. doi: 10.1136/qshc.2008.032045. [DOI] [PubMed] [Google Scholar]
- 39.Reason JT. Managing the Risks of Organizational Accidents. Brookfield, VT: Ashgate Publishing Company; 1997. [Google Scholar]
- 40.Stahl JE, Sandberg WS, Daily B, et al. Reorganizing patient care and workflow in the operating room: a cost-effectiveness study. Surgery. 2006;139:717–28. doi: 10.1016/j.surg.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Wolf FA, Way LW, Stewart L. The efficacy of medical team training: improved team performance and decreased operating room delays: a detailed analysis of 4863 cases. Ann Surg. 2010;252:477–83. doi: 10.1097/SLA.0b013e3181f1c091. discussion 483-5. [DOI] [PubMed] [Google Scholar]
- 42.Griffen FD, Stephens LS, Alexander JB, et al. Violations of behavioral practices revealed in closed claims reviews. Ann Surg. 2008;248:468–74. doi: 10.1097/SLA.0b013e318185e196. [DOI] [PubMed] [Google Scholar]
- 43.Hull L, Arora S, Kassab E, et al. Observational teamwork assessment for surgery: content validation and tool refinement. J Am Coll Surg. 2011;212:234–243. e1–5. doi: 10.1016/j.jamcollsurg.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Sevdalis N, Lyons M, Healey AN, et al. Observational teamwork assessment for surgery: construct validation with expert versus novice raters. Ann Surg. 2009;249:1047–51. doi: 10.1097/SLA.0b013e3181a50220. [DOI] [PubMed] [Google Scholar]
- 45.Mishra A, Catchpole K, McCulloch P. The Oxford NOTECHS System: reliability and validity of a tool for measuring teamwork behaviour in the operating theatre. Qual Saf Health Care. 2009;18:104–8. doi: 10.1136/qshc.2007.024760. [DOI] [PubMed] [Google Scholar]
- 46.Sevdalis N, Forrest D, Undre S, et al. Annoyances, disruptions, and interruptions in surgery: the Disruptions in Surgery Index (DiSI) World J Surg. 2008;32:1643–50. doi: 10.1007/s00268-008-9624-7. [DOI] [PubMed] [Google Scholar]
- 47.Jeffcott SA, Ibrahim JE, Cameron PA. Resilience in healthcare and clinical handover. Qual Saf Health Care. 2009;18:256–60. doi: 10.1136/qshc.2008.030163. [DOI] [PubMed] [Google Scholar]
- 48.Sheridan TB. Risk, human error, and system resilience: fundamental ideas. Hum Factors. 2008;50:418–26. doi: 10.1518/001872008X250773. [DOI] [PubMed] [Google Scholar]
- 49.Patterson ES, Woods DD, Roth EM, et al. Three Key Levers for Achieving Resilience in Medication Delivery with Information Technology. J Patient Saf. 2006;2:33–38. [Google Scholar]