Abstract
The protein kinase A (PKA) signaling pathway plays a role in regulating growth and differentiation in the dimorphic fungus Mucor circinelloides. PKA holoenzyme is comprised of two catalytic (C) and two regulatory (R) subunits. In M. circinelloides, four genes encode the PKAR1, PKAR2, PKAR3, and PKAR4 isoforms of R subunits. We have constructed null mutants and demonstrate that each isoform has a different role in growth and differentiation. The most striking finding is that pkaR4 is an essential gene, because only heterokaryons were obtained in knockout experiments. Heterokaryons with low levels of wild-type nuclei showed an impediment in the emission of the germ tube, suggesting a pivotal role of this gene in germ tube emergence. The remaining null strains showed different alterations in germ tube emergence, sporulation, and volume of the mother cell. The pkaR2 null mutant showed an accelerated germ tube emission and was the only mutant that germinated under anaerobic conditions when glycine was used as a nitrogen source, suggesting that pkaR2 participates in germ tube emergence by repressing it. From the measurement of the mRNA and protein levels of each isoform in the wild-type and knockout strains, it can be concluded that the expression of each subunit has its own mechanism of differential regulation. The PKAR1 and PKAR2 isoforms are posttranslationally modified by ubiquitylation, suggesting another regulation point in the specificity of the signal transduction. The results indicate that each R isoform has a different role in M. circinelloides physiology, controlling the dimorphism and contributing to the specificity of cyclic AMP (cAMP)-PKA pathway.
INTRODUCTION
Mucor circinelloides is a dimorphic fungus from the new subphylum Mucormycotina (formerly classified as Zygomycetes) that displays yeast or filamentous morphology as a consequence of different environmental conditions, including gas atmosphere and level of nutrients (33). Fungi from this subphylum are organized in multinucleated cells. M. circinelloides yeasts display multipolar budding and each cell harbors more than one nucleus, while mycelium is aseptate and has distributed nuclei along the hyphae.
Fungal dimorphism is particularly important since genetic evidence indicates that in a number of pathogenic fungi in animals and plants, the morphogenetic transition is directly associated with virulence and pathogenesis (4, 21, 29). M. circinelloides is a causal agent of the lethal fungal infectious disease mucormycosis (6, 10), which has been reported in patients with impaired immunity (22, 27, 38, 40, 49). Recently, it has been reported that the spore size dimorphism is linked to virulence in M. circinelloides (23). The functional analysis of genes involved in the control of dimorphism in M. circinelloides is a contribution to advance the understanding of pathogenic zygomycetes.
One of the key regulators of polarity in fungi, as well as of other processes such as development, mating, and virulence, is the cyclic AMP (cAMP)-dependent protein kinase A (PKA) (5, 16). The participation of cAMP in the morphogenetic process of Mucor has been shown for both Mucor rouxii (35, 36) and M. circinelloides (53).
The PKA from M. circinelloides is a tetrameric holoenzyme that resembles its mammalian counterparts, although with a higher affinity in the interaction between regulatory (R) and catalytic (C) subunits. An acidic cluster present in the N terminus of the M. circinelloides R subunit (linker I region) is involved in determining the high affinity of this holoenzyme (32, 39).
We have studied the role of PKA in the regulation of morphological and cellular development in M. circinelloides. Four genes encoding PKA regulatory subunits (R) were identified and characterized; we have also predicted a similar multiplicity of isoforms from other sequenced genomes from zygomycetes, i.e., Phycomces blakesleeanus and Rhizopus oryzae (31). All of the M. circinelloides isoforms are expressed differentially during aerobic and anaerobic development (31). pkaR1 was the first gene cloned, and its role in the regulation of morphology was recently reported (53). A pkaR1Δ mutant (ΔR1) showed a reduction in growth and alterations in germination rates, cell volume, germ tube length, and asexual sporulation. The lack of the pkaR1 gene resulted in a decreased cAMP binding activity and in a protein kinase activity that was still dependent on cAMP, although with a higher −/+ cAMP activity ratio, due to the existence of other cAMP binding activities (31).
In this work we studied the functions of the other three pkaR genes. We generated different M. circinelloides mutant strains which have a disruption in the pkaR2, pkaR3, or pkaR4 gene and analyzed the role of each pkaR gene in growth and differentiation. We could establish that the multiple PKAR isoforms, evolutionarily retained, may have acquired different specificities in a subfunctionalization process (17), which is supported by the existence of different expression patterns and different effects on growth, sporulation, and differentiation under aerobic and anaerobic growth conditions. Among all the isoforms, PKAR4 is shown to have a key role in the regulation of germ tube emission in aerobiosis and after the shift from anaerobiosis, while the remaining PKAR isoforms affect in different ways and degrees growth and differentiation. We also show the ubiquitylation of some of the PKAR isoforms under different growth conditions, suggesting that this modification could also contribute to the specificity of PKA signaling. These results indicate that PKA is involved in multiple and differential processes in M. circinelloides physiology.
MATERIALS AND METHODS
Strains and growth and transformation conditions.
Strain MU402 (30), a uracil and leucine auxotroph derived from R7B (41), was used as the recipient strain in transformation experiments to knock out the pkaR3 and pkaR4 genes, giving rise to MU359 and MU365, named in this work the ΔR3 and ΔR4 strains, respectively. The leucine auxotroph R7B (wild type [wt]) is a leuA mutant strain derived from M. circinelloides forma lusitanicus CBS277.49 (41). This strain was used as the recipient strain in transformation experiments to knock out the pkaR2 gene, giving rise to BA201, named the ΔR2 strain (ΔpkaR2). The R7B strain was used as a control in all the experiments performed with ΔR3 and ΔR4 because it has the same auxotrophy as the mutants. The KFA89 strain (53) was used as a control in all the experiments performed with ΔR2 because it has the same genetic background. Both R7B and KFA89 showed the same behavior in all the analyses, and therefore, data or pictures for only one of them are shown in the figures for simplicity.
Cultures were grown at 30°C in YPG, MMC, or YNB medium (29), which were supplemented with uridine (200 μg · ml−1) or leucine (20 mg · ml−1) when required. The selective medium was MMC for the ΔR3 and ΔR4 strains and YNB for the ΔR2 strain. The pH was adjusted to 4.5 and 3.2 for mycelial and colonial growth, respectively. Transformation was carried out as described previously (15).
Deletion of pkaR genes.
Plasmid pUC19R2, harboring the pkaR2 gene from position 718 to position 1730 (GenBank accession no. BankIt1191938 FJ800364), was obtained by cloning the corresponding PCR fragment in the HindIII/EcoRI sites of pUC19.
Plasmid pR2leuA, which contains the M. circinelloides leuA gene flanked by pkaR2 sequences, was used to disrupt pkaR2. This plasmid was constructed by PCR amplification of pUC19R2 with primers s1n (′5-TGTAGCTGCAGCACACTGTTCA GCTCTTGC-3′) and s1c (5′-ATGTACTGCAGGCCTCGTGCTGCCTCC-3′), which contained PstI restriction sites for cloning purposes (in italics). The amplified fragment had a 0.594-kb deletion of the pkaR2 coding region and was digested with PstI and ligated with a leuA 4.3-kb PstI fragment from pLEU4. The plasmid generated was pR2leuA, which was linearized with PvuII to transform R7B by the electroporation protocol (15).
Plasmid pUC18R3 harbors the pkaR3 gene from position −956 to position 2709 (GenBank accession no. BankIt1476716 JN624777). A 3,665-bp PCR fragment was cloned in the EcoRI/XbaI sites of pUC18.
Plasmid pR3pyrG, which contains the M. circinelloides pyrG gene (37) flanked by pkaR3 sequences, was used to disrupt pkaR3. It was constructed from a PCR fragment amplified from pUC18R3 with the primers PKAR3-1537 BamHI-R (5′-GGATCGGATCCTGGTAGTCCTGGGATAAAGC-3′) and PKAR3-2741BamHI-F (5′-CCATGGGATCCTTCAAGAAGCCAGATCC-3′), which included BamHI restriction sites (in italics). This PCR fragment, containing a 1.184-kb deletion of the pkaR3 coding region, was digested with BamHI and ligated with a pyrG 3.2-kb BamHI fragment from pEMP1 (37). The plasmid linearized with EcoRI was introduced into MU402 by electroporation.
Plasmid pGEM-T pkaR4, containing a fragment from position −1248 to 2480 of the pkaR4 gene (GenBank accession no. BankIt1476724 JN624778), was constructed by cloning a 3,728-bp DNA PCR fragment in the pGEM-T vector.
Plasmid pR4pyrG, which contains the M. circinelloides pyrG gene flanked by pkaR4 sequences, was constructed from pGEMT-R4 to disrupt pkaR4. PGEMT-R4 was PCR amplified using primers PKAR4-2925BamHI-F (5′-AGAAAGGATCCATGCCATTATCAATCAGC-3′) and PKAR4-1505BamHI-R (5′-TCCTGGGATCCGGGTTCGCCGAACGTAAAGG-3′), which contained BamHI restriction sites (in italics). The PCR fragment with a 1.409-kb deletion of the pkaR4 coding region was digested with BamHI and ligated to a pyrG 3.2 kb BamHI fragment from pEMP1 (37) to produce plasmid pR3pyrG. The plasmid was linearized with EcoRI and introduced into MU402 by electroporation.
Nucleic acid isolation and analysis.
General procedures for plasmid DNA purification and cloning transformation of Escherichia coli and standard manipulations for hybridization analyses were performed as described in standard manuals (44). DNA from M. circinelloides was prepared as described previously (43).
For Southern blot analysis, restriction-digested chromosomal DNA (1 μg) was blotted onto positively charged nylon filters (Gene Screen hybridization transfer membrane; Perkin-Elmer Life Science, Inc.) and hybridized at 65°C to radioactively labeled probes in Church buffer. Probes were labeled with [γ-32P]dCTP using a NEBlot kit (New England BioLabs).
Plasmids containing the M. circinelloides leuA gene flanked by pkaR2 sequence (GenBank accession no. BankIt1191938 FJ800364) or the pyrG gene flanked by pkaR3 or pkaR4 sequence (GenBank accession no. BankIt1476716 JN624777 and BankIt1476724 JN624778, respectively) were used to knock out the corresponding genes in the recipient strains (see the supplemental material for details).
cAMP binding assay.
cAMP binding was measured by nitrocellulose filter assay. Crude extracts from the wild-type, ΔR2, ΔR3, and ΔR4 strains, grown aerobically for 4 h in YPG medium or in MMC medium after anaerobic growth overnight and shifted to aerobic conditions for 4 h, were incubated for 30 min at 30°C in a final volume of 70 μl with 0.3 μM [3H]cAMP (62,000 dpm · pmol−1) and 0.5 M NaCl in 10 mM Tris-HCl buffer (pH 8). At this concentration, cAMP was saturating. An aliquot was spotted on nitrocellulose membrane filters under vacuum and washed with 20 mM Tris-HCl buffer (pH 7.5) (13). R activity was estimated by calculating the amount of cAMP binding in femtomoles.
Standard PKA assay.
The PKA C subunit activity was determined by assay of its phosphotransferase activity with kemptide as a substrate. The phosphorylation of kemptide was performed by adding an aliquot of PKA, semipurified with DEAE-cellulose, to a standard incubation mixture containing 15 mM MgCl2, 0.1 mM [γ-32P]ATP (700 dpm · pmol−1), 200 μM kemptide, and 10 μM cAMP (when added). After 15 min at 30°C, aliquots were processed by the phosphocellulose paper method (42). PKA activity was expressed in units defined as picomoles of phosphate incorporated into substrate · min−1 at 30°C.
R subunit purification.
Wild-type strain R7B and ΔR mutants (106 spores/ml) were grown in liquid medium for 3 to 4 h until germ tube emission under aerobic conditions, overnight under anaerobic conditions, or under anaerobic conditions and further shifted to aerobic conditions for 3 h until tube germ emission (shift). The R subunit was isolated using N6cAMP-agarose from Biolog. Briefly, equal amounts of protein of crude extracts from both strains were loaded onto the resin; the resins were washed exhaustively with 0.5 M NaCl. Finally SDS-PAGE cracking buffer was added to the resin to elute proteins bound to the resin.
Western blotting.
Samples of purified R preparations or crude extracts were analyzed by SDS-PAGE and blotted onto nitrocellulose membranes using 25 mM Tris–192 mM glycine–20% (vol · vol−1) methanol buffer. Blots were blocked with 5% nonfat milk and 0.05% Tween 20 in Tris-buffered saline. Primary anti-R antibody was prepared in our laboratory against purified R subunit from M. rouxii prepared in rabbit. Primary antiubiquitin antibody was from Santa Cruz Biotechnology Inc. Secondary antibody (peroxidase-conjugated anti-rabbit immunoglobulin G) was from Sigma. The blots were developed with Luminol chemiluminescence reagent.
Immunoprecipitation assay.
Crude extracts prepared from strain R7B grown in YPG medium under aerobic conditions for 8 h were incubated with anti-R antibody for 1 h at 4°C, followed by addition of A/G agarose and incubation for 16 h at 4°C. The immunoprecipitates were washed with 5 ml of buffer (10 mM Tris-HCl [pH 7.5], 5 mM EDTA, 2 mM EGTA, 2 mM β-mercaptoethanol, and protease inhibitors) plus 0.1 M NaCl. The immunoprecipitates were subjected to Western blot analysis with anti-R and antiubiquitin antibodies.
Radial growth assay and sporulation.
Portions (10 μl) of a spore suspension (103 spores · ml−1) were spotted onto YNB (ΔR2 strain) or pH 4.5 MMC (ΔR3 and ΔR4 strains) plates in triplicate. Radial colony growth was monitored at 30°C every 12 h from 24 to 72 h by colony diameter measurement. Growth rates are expressed in μm · h−1. All experiments were performed three times, and the results from representative experiments are presented.
For sporulation determination, 100 spores of each strain were spread on selective medium plates and incubated at 30°C for 5 days, and then a plug of agar (1 cm2) was removed for spore calculation. The results shown are averages for three replicates.
Microscopic analysis.
For microscopic assays, the cells were fixed with 8% glutaraldehyde and analyzed by using a Nikon E-600 microscope. Pictures were taken with a Nikon Cool Pix-5000 camera. The cell volume and hyphal length were calculated by analyzing 500 cells from each strain and using Science Lab 98, Image Gauge version 3.12. The values are expressed in arbitrary units (a.u.), and each value represents the mean ± the standard error of the mean (SEM) for three independent experiments.
Semiquantitative RT-PCR.
RNA was prepared from samples of wild-type, ΔR1, ΔR2, ΔR3, and ΔR4 M. circinelloides strains grown up to different stages using standard procedures. Semiquantitative reverse transcription-PCR (RT-PCR) of each pkaR RNA was performed using the elongation factor EF-1a gene (tef-1) as an internal standard. The tef-1 gene has been demonstrated to be constitutively expressed throughout germination in M. circinelloides (47). The RT-PCR amplification reaction, using Superscript II reverse transcriptase (Invitrogen), was calibrated in order to determine the optimal number of cycles that would allow detection of the appropriate mRNA transcripts while still keeping amplification for these genes in the log phase. Specific oligonucleotide sense and antisense primers were designed so as to identify specifically the mRNA of each gene. These primers included Tef-1f (5′-TCACGTCGATTCCGGTAAGTC-3′), Tef-1r (5′-TATCACCGTGCCAGCCAGA-3′), pkaR1F (5′-CTTGTTTGATACCAATGATACCAGTAATG-3′), pkaR1R (5′-CTCCTGTCCATCTCCAAAATAAACT-3′), pkaR2F (5′-AGACAAGTAGTTGAACATCAGCCTG-3′), pkaR2R (5′-TTGCTGAGAGCCGTCAGGCAGCTTCTT-3′), pkaR3F (5′-CATCCCATGTGGGCGGCATTGGCCC-3′), pkaR3R (5′-CCTTGCTTCATTTCATTGACCAACTG-3′), pkaR4F (5′-GATGTGTACTGCAAAGATCAGCCTCA-3′), and pkaR4R (5′-CTGCAAGATAACATGCTCTCCATCATTG-3′). The expected PCR product lengths were 775, 955, 817, and 994 bp for pkaR1, pkaR2, pkaR3, and pkaR4, respectively, and 550 bp for tef-1. The PCR bands were analyzed and quantified by digital imaging (Bio-Imaging Analyzer Bas-1800II and Image Gauge 3.12; Fujifilm), with the pixel intensities expressed in arbitrary units.
PCR screening.
Genomic DNA was prepared from samples of wild-type, ΔR2, ΔR3, and ΔR4 M. circinelloides strains grown up in selective solid medium for 2 days or in YPG liquid medium (pH 4.5) overnight. PCRs were performed with the primers S1s (5′-ATCTCGCCAATACGACCAGCACCCTC-3′), S1f (5′-CGATCCACACCCACGTATACTTCGG-3′), S1t (5′-GACGTCTGTGTACTGCTCTTCGTC-3′), Lr (5′-AGTACAGTGCAACGAATGCAGGTC-3′), Pf (5′-TCGTTGAGCTGCCTGTTGTTGTTG-3′), S3f (5′-CACTTGTGTGTTTTCGCCACGCTCC-3′), P3F1 (5′-CACTTGTGTGTTTTCGCCACGCTCC-3′), PF2 (5′-GGCAAGTAACACCACATTCAGAGC-3′), S3f (5′-ATCCATGACTTGAGCCAGCAGG-3′), S3r (5′-TCCATGACAGGACCTAATAAACG-3′), p3R (5′-ACGAGAGCCTTTGCCGAAGC-3′), PR2 (5′-ATCCCACCAGAAGGAGTACATGG-3′), Pr (5′-GATGCTTGACAGTGTTGCTAGC-3′), S4f (5′-AA TGACCTTTACGTTCGGCGAAC-3′), and S4r (5′-CATGGTGATGAGTGTCTCTGTCG-3′), as indicated in Fig. 1.
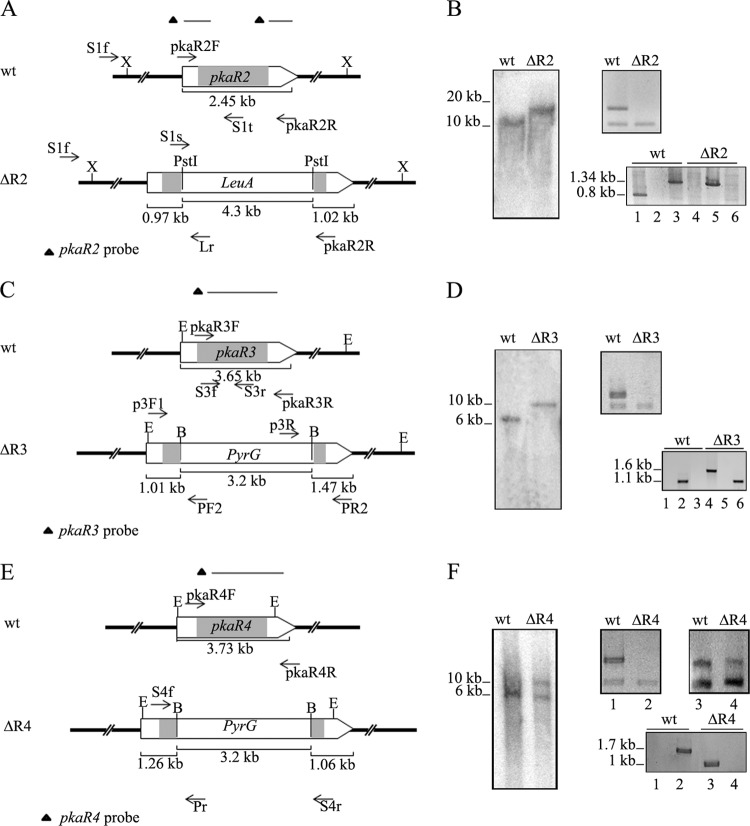
Fig 1.
Disruption of pkaR genes. (A) Genomic structures of the wild-type (wt) pkaR2 locus and of the locus after homologous recombination with the replacement fragment. The primers (arrows) and the two probes used are indicated. X, XhoI. (B) Genomic DNAs from the wild-type strain (MU402) and the pkaR2 knockout mutant (ΔR2) were digested with XhoI and hybridized with the pkaR2 probe (left panel). RT-PCR was assessed to evaluate mRNA from overnight cultures using primers pkaR2F and pkaR2R, with Tef-1f/Tef-1r as an internal standard (top right panel). PCR from genomic DNA was performed with primers S1s-pkaR2R (lanes 1 and 4), S1f-Lr (lanes 2 and 5), or S1f-S1t (lanes 3 and 6) (bottom right panel). Note that an expected 4.7-kb fragment in lane 4 was not amplified due to its large size. (C) Genomic structures of the wild-type (wt) pkaR3 locus and of the locus after homologous recombination with the replacement fragment. The primers (arrows) and probes used are indicated. E, EcoRI; B, BamHI (mutant strain).(D) Genomic DNAs from the wild-type strain (MU402) and the pkaR3 knockout mutant (ΔR3) were digested with EcoRI and hybridized with the pkaR3 probe (left panel). RT-PCR was performed to evaluate mRNA from overnight cultures with primers pkaR3F and pkaR3Rr, with Tef-1/Tef-1r as an internal standard (top right panel). PCR was performed with primers p3R-PR2 (lanes 1 and 4), S3f-S3r (lanes 2 and 5), or P3F1-PF2 (lanes 3 and 6) (bottom right panel). Note that an expected 5-kb fragment in lane 4 was not amplified due to its large size. (E) Genomic structures of the wild-type (wt) pkaR4 locus and of the locus after homologous recombination with the replacement fragment. The primers (arrows) and probes used are indicated. E, EcoRI; B, BamHI (mutant strain). (F) Genomic DNAs from the wild-type strain (MU402) and the pkaR4 heterokaryotic transformant (ΔR4) were digested with EcoRI and hybridized with the pkaR4 probe (left panel). RT-PCR was performed with RNA from overnight cultures from the initial transformants (lanes 1 and 2) and after a few vegetative cycles (lanes 3 and 4) with primers pkaR4F and pKAR4R, with Tef-f1/Tef-1r as an internal standard (top right panel). PCR was performed with primers S4f-Pr (lanes 1 and 3) or S4f-S4r (lanes 2 and 4) (bottom right panel). The positions and sizes of the DNA fragments are indicated at the left of the panels. The arrows represent the cloned region in every case, and the coding region is indicated in gray.
RESULTS
Construction of knockout strains for the pkaR genes.
We have already shown that the R subunit genes (pkaR1, pkaR2, pkaR3, and pkaR4) display differential expression patterns during aerobic and anaerobic development of M. circinelloides, suggesting that they have acquired different specificities retained by evolutionary mechanisms (31). Through the use of a mutant strain with a disrupted pkaR1 gene (ΔR1), we could demonstrate that this isoform is involved in several developmental processes (31). Here, the roles of the remaining three isoforms of the R subunit in the morphology and differentiation of the fungus were studied by constructing mutants with deletions of the pkaR2 (ΔR2), pkaR3 (ΔR3), and pkaR4 (ΔR4) genes.
pkaR null mutants were generated by gene replacement using knockout vectors containing a selective marker gene, i.e., the pyrG gene for the construction of the ΔR3 and ΔR4 strains and the leuA gene for the ΔR2 strain, flanked by sequences of the respective pkaR gene. Restriction fragments from the knockout vectors containing sufficient sequence of the pkaR genes to allow homologous recombination (28, 37) were used to transform a wild-type PKA strain. Strain MU402, which is wild type for PKA but auxotrophic for uracil and leucine, was used to disrupt the pkaR3 and pkaR4 genes, while strain R7B, which is wild type for PKA but auxotrophic for leucine, was used to disrupt the pkaR2 gene. Initial transformants were grown in selective medium for several vegetative cycles to finally obtain transformants showing 100% of colonies with the selective marker, which were assumed to be homokaryons. They were named ΔR2, ΔR3, and ΔR4. The disruption of each pkaR gene was confirmed by PCR, RT-PCR, and Southern blot analysis.
PCRs were performed using a primer that hybridized in a pkaR genomic region outside the replacement fragments and another that hybridized inside the selective marker sequence (Fig. 1A, C, and E). The expected DNA fragments were obtained only in PCRs in which DNA from the mutants was used as the template, confirming the insertion of leuA or pyrG sequence in the corresponding pkaR gene (Fig. 1B, D, and F, bottom right panels). Deletion of pkaR2 and pkaR3 was confirmed by PCR using internal primers that hybridized with genomic regions deleted in the corresponding mutants, because the expected product was obtained only in the wild-type strain (Fig. 1B). In the case of pkaR4, PCR was performed using two primers that hybridized outside the replaced fragment. The expected product was obtained only in wild-type strains because the fragment from the mutant was too large to be amplified (Fig. 1F, bottom right panel).
The disruption of the pkaR genes was further confirmed by Southern blot analysis using pkaR2-, pkaR3-, or pkaR4-specific probes that detected both the wild-type and the disrupted pkaR alleles, although with different expected sizes due to the replacement of wild-type alleles with a 4.3-kb DNA fragment containing the leuA sequence in ΔR2 or a 3.2-kb fragment containing the pyrG sequence in ΔR3 and ΔR4 (Fig. 1A, C, and E). The ΔR2 and ΔR3 transformants showed the expected DNA fragments with an increase in size of 3.7 kb and 2 kb, respectively, compared to the fragments of each wild-type strain, indicating that the pkaR2 and pkaR3 wild-type alleles had been correctly replaced (Fig. 1B and D, left panel). Moreover, lack of pkaR2 and pkaR3 mRNA in the corresponding mutants was assessed by RT-PCR (Fig. 1B and D, top right panels). Strain ΔR4 showed a special behavior in Southern blotting because the transformant isolated as a homokaryon showed the expected DNA fragment with an expected increase of 1.8 kb compared to the fragment of the wild-type strain (data not shown). However, when DNA from transformants grown for several vegetative cycles was used, the fragment with the same size as the wild-type pkaR4 allele was observed in addition to the disrupted allele (Fig. 1F, left panel).
These results suggested that the transformant of ΔR4 was a heterokaryon, containing a small proportion of wild-type nuclei that should increase by successive transfers onto new selective medium. This was further confirmed by analysis of pkaR4 mRNA accumulation by RT-PCR. The pkaR4 mRNA was not detected in a ΔR4 mutant strain analyzed immediately after it was isolated, but significant pkaR4 mRNA levels were detected when the strain was subjected to successive growth cultures (Fig. 1F, top right panel, lanes 3 and 4). This finding suggested that ΔR4 strains are viable only as heterokaryons and that pkaR4 should be an essential gene, because it was not possible to isolate a stable ΔR4 mutant after several attempts.
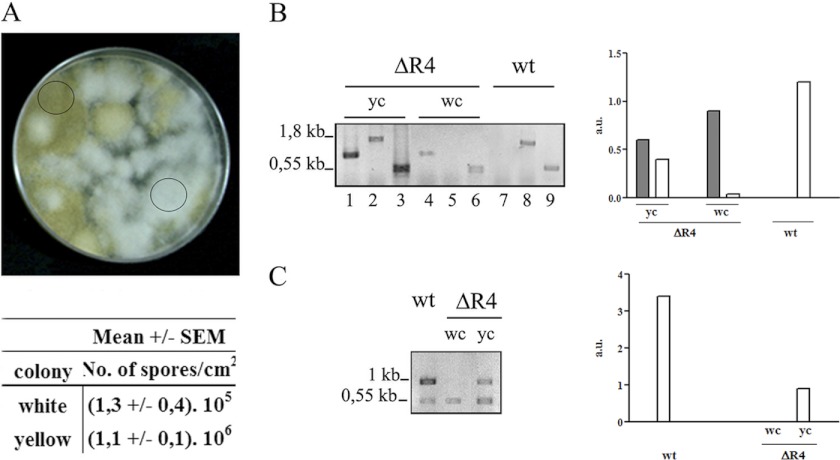
Remarkably, the morphology of the putative ΔR4 did not resemble that of the wild-type strain, since when grown in solid medium it displayed predominantly white colonies, which showed a reduced growth in a selective medium at pH 4.5 (Fig. 2A and data not shown). The sporulation capacity was much lower in white than in yellow colonies, and as a consequence, after several subcultures colonies were mostly yellow (Fig. 2A, bottom panel). PCR and RT-PCR were used analyze the presence and the expression of the pkaR4 gene in both types of colonies. PCR was performed with primer sets specifically designed so as to detect the pkaR4 gene whether or not it was interrupted with the pyrG sequence (Fig. 2B). We obtained the expected products from both types of colonies, i.e., one fragment from white colonies and two fragments corresponding to interrupted and wt nuclei from yellow colonies. By RT-PCR, performed as described in Materials and Methods, pkaR4 mRNA was detected in the wild-type strain and in the yellow colonies but not in the white ones (Fig. 2C). The tef-1 gene was used as an internal control in both assays.
Fig 2.
Heterokaryotic ΔR4 strain. (A) ΔR4 strain grown in the selective medium MMC (pH 4.5) in triplicate and incubated at 30°C for 5 days. The circles indicate a white colony and a yellow colony. The numbers of spores per cm2 from white and yellow colonies were determined. Plugs of agar (1 cm2) were removed in triplicate for spore calculation. (B) PCR performed with genomic DNA from the wild-type strain (wt), a yellow colony (yc), and a white colony (wc) with the primers pkaR4F-Pr (lanes 1, 4, and 7) or pkaR4F-pkaR4R (lanes 2, 5, and 8). Tef-1f-Tef-1r was used as an internal control (lanes 3, 6 and 9). The primers are shown in Fig. 1E. (C) RT-PCR performed with RNA from wt, dc and wc with primers pkaR4F-pkaR4R, with Tef-1f/Tef-1r as internal control. The positions and sizes of the DNA fragments are indicated at the left of the panels. The quantification of the PCR products, after normalization with the tef-1 signal, is shown in the right panel. The dark bars represent the mutant allele, and the white bars represent the wt allele. The primers used are shown in Fig. 1E.
All these results confirm the heterokaryon nature of the ΔR4 strain. Signals corresponding to both the presence and absence of the pkaR4 allele were observed, and their relative intensities suggested an unbalanced proportion of both types of nuclei. We thus conclude that a pkaR4 deletion mutant is viable only as a heterokaryon containing wild-type nuclei and that therefore pkaR4 is essential for viability in M. circinelloides.
Biochemical characterization of the pkaR mutant strains.
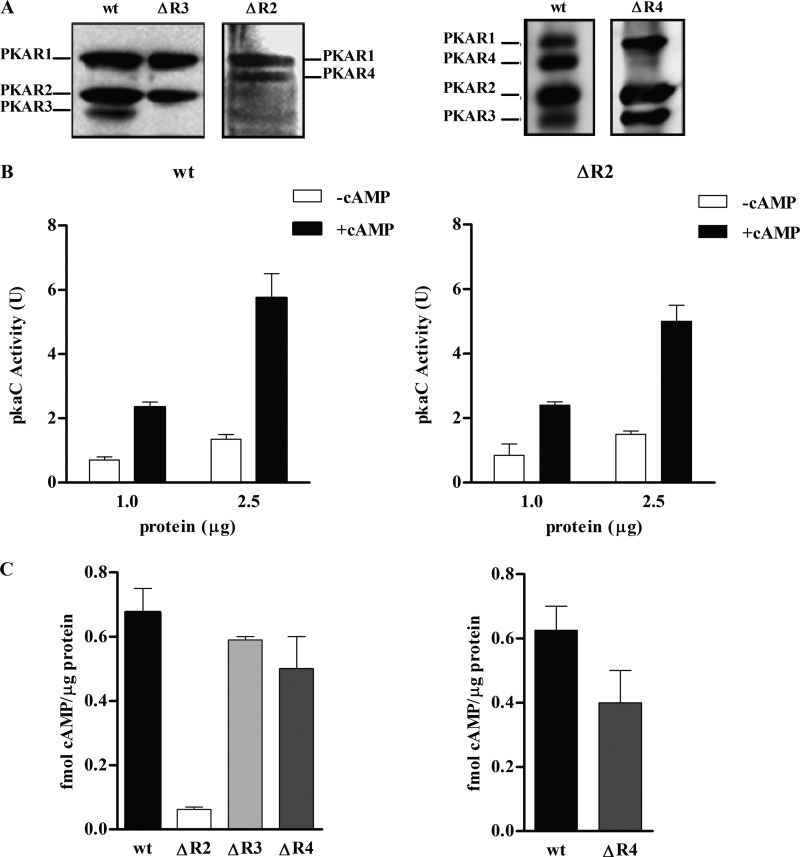
The presence or the absence of the PKAR proteins in each mutant was evaluated by analyzing samples purified through cAMP-agarose from wild-type, ΔR2, and ΔR3 strains grown under aerobic conditions by Western blotting using anti-R. The identification of the PKAR proteins purified by cAMP-agarose and visualized by Western blotting using anti-R antibody has been previously validated by matrix-assisted laser desorption ionization–tandem time of flight (MALDI TOF-TOF) mass spectrometry (31). The absence of the specific PKAR isoform in the mutant strains was confirmed (Fig. 3A). It is worth noting that the ΔR2 strain showed higher levels of PKAR4 and lower levels of PKAR3 than the wild-type strain. Cultures of the ΔR4 strain subjected to an anaerobic-aerobic shift were analyzed, since in a wild-type strain the highest pkaR4 expression level was detected under these growth conditions (31). A reduced, but not null, expression of PKAR4 protein relative to that in the wild-type strain was detected. The presence of PKAR4 protein is in agreement with the heterokaryon nature of the mutant. The relative expression among the protein isoforms changed in each mutant (see below in expression analysis).
Fig 3.
Biochemical characterization of pkaR mutant strains. (A) Western blot analysis using a polyclonal antibody raised against M. rouxii PKAR (anti-R) of samples semipurified with cAMP-agarose from wt, ΔR2, and ΔR3 strains grown under aerobic conditions (left panel) and from wt and ΔR4 heterokaryotic strains from shift conditions (overnight growth in anaerobiosis and a 3-h shift to aerobiosis) (right panel). (B) PKAC activity was measured in the absence (□) or in the presence (■) of cAMP in semipurified samples from wild-type (wt) and ΔR2 strains grown under aerobic conditions. The data shown correspond to five assays, performed with independent preparations. Values are represented as means ± SEM from four replicates for each assay. (C) Binding activity measured in crude extracts from the wt, ΔR2, ΔR3, and ΔR4 strains grown under aerobic conditions (left panel) and from wt and ΔR4 strains after a shift from anaerobic to aerobic conditions (right panel).
For the biochemical characterization, the levels of total cAMP-dependent kinase (PKAC) and cAMP binding activities in ΔR2 were analyzed and the results were compared with the results previously published for ΔR1 (31). The rationale was the following. (i) Under aerobic growth conditions, PKAR1 and PKAR2 are the most highly expressed isoforms. (ii) The linker region and the acidic nature of the residues in the linker have a key role in the interaction of R and C subunits and therefore in cAMP kinase dependence (32). (iii) Each PKAR isoform has different number of acidic residues in the linker region, and PKAR1 and PKAR4 have a higher number of acidic residues than PKAR2 and PKAR3; therefore, the interaction affinity of the latter isoforms with C should be lower than that of PKAR1 and PKAR4. Thus, the measure of the kinase activity in ΔR2 and ΔR1 is considered to model how the lack of one type of PKAR can affect the total PKAC or cAMP dependence of the kinase activity.
The lack of the pkaR1 gene resulted in a decrease of PKAC, which was still dependent on cAMP although with a higher −/+ cAMP activity ratio (31). In the PKAR2 null mutant neither the total PKAC activity nor the kinase cAMP dependence was significantly different from that in the wild-type strain (Fig. 3B). We have already shown that PKAR3 and PKAR4 isoforms are both poorly expressed under aerobic conditions; PKAR3 is expressed mainly in spores but immediately decreased upon germination, and PKAR4 is almost not expressed until the emission of the germ tube in aerobiosis and is highly expressed in the shift from anaerobiosis to aerobiosis. Furthermore, PKAR3 has the same low number of acidic residues in the linker region as PKAR2, in contrast to the isoforms PKAR1 and PKAR4, which have a higher number of acidic residues (31). The lack of one PKAR isoform with a high or low number of acidic residues did not have the same effect on the kinase activity. We therefore think that the existence of more than one regulatory subunit isoform should affect the activation of PKA by cAMP and in this way contribute to the specificity of the signaling.
The cAMP binding activity in pkaR mutants from cultures grown under aerobic conditions for 4.5 h was assessed. We observed, as expected according to their known levels of expression, a slight decrease in the cAMP binding activity in ΔR3 and ΔR4 mutant strains and a striking decrease in that in ΔR2 compared with the wild type (Fig. 3C, left panel). cAMP binding activity was also measured in the ΔR4 strain grown overnight under anaerobic conditions and then shifted to aerobic conditions for 4 h, conditions under which pkaR4 has the highest expression, reaching levels similar to those of pkaR1 and pkaR2. A slight decrease in binding activity compared to that in the wild-type strain was observed in this case (Fig. 3C, right panel).
These results corroborate the lack of each PKAR protein in the mutants and that there is not a direct correlation between the absence of one PKAR subunit and a change in the kinase activity.
Growth, sporulation, and morphology of the pkaR mutants.
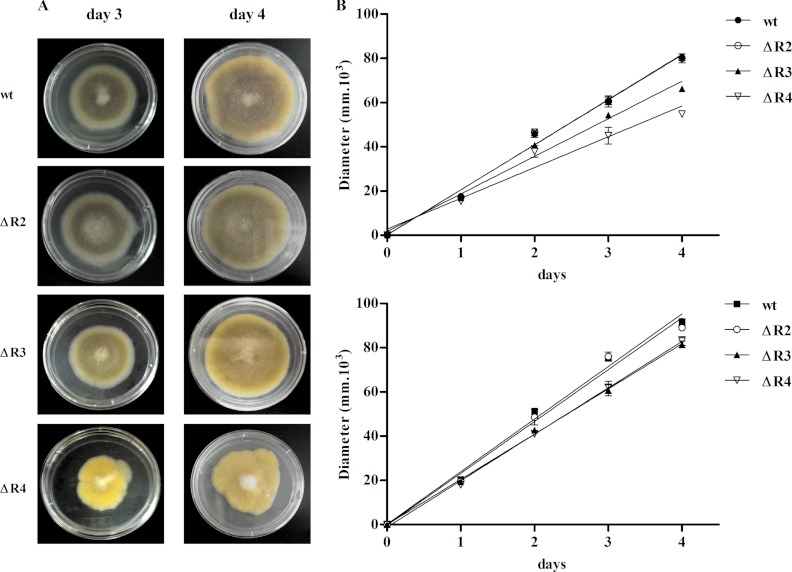
The roles of the PKAR isoforms in macroscopic morphology were monitored by analyzing the radial growth and sporulation in solid medium. The radial growth rate on solid medium and the sporulation capacity of the ΔR2 strain were similar to those of the wild-type strain (Fig. 4 and Table 1, whereas the ΔR3 mutant strain showed a slight reduction) in radial growth rate and spore formation (Fig. 4 and Table 1).
Fig 4.
Growth rates of the pkaR mutants. (A) Aliquots containing the same number of spores of each strain were spotted onto minimal medium plates (pH 4.5) in triplicate and incubated at 30°C for 4 days. Pictures from day 3 (left panels) and day 4 (right panels) are shown. (B) Radial growth was monitored for 4 days. A graph of the calculated radial growth rates from three assays is shown. Top panel, graphic representation of three experiments performed with initial transformants; bottom panel, graphic representation of three experiments performed after several vegetative cycles. The wild-type strains used were strain R7B to compare with ΔR3 and ΔR4 and strain KFA89 to compare with ΔR2; as both wild-type strains showed the same behavior, a representative curve is shown.
Table 1.
Sporulation capacities of the pkaR mutantsa
| Strain | No. of spores/cm2, mean ± SEM |
|---|---|
| wt | (1.2 ± 0.2) · 107 |
| ΔR2 | (1.0 ± 0.1) · 107 |
| ΔR3 | (8.1 ± 0.2) · 106 |
| ΔR4 | (3.0 ± 0.1) · 105 |
Spores (n = 100) of each strain were spread onto minimal medium plates in triplicate and incubated at 30°C for 5 days. Plugs of agar (1 cm2) were removed for spore counting.
The radial growth rate in the ΔR4 mutant strain was analyzed using spores from initial transformants and spores harvested after several subcultures. When using spores from initial transformants, the radial growth rate and the sporulation capacity were reduced compared to those of wild-type strain (Fig. 4A and B [top panel] and Table 1). The colony shape of the mutant was also altered, showing jagged edges (Fig. 4A). However, when the experiments were performed with spores harvested after several vegetative cycles, the phenotypic differences between ΔR4 and the wild-type strain were lessened (Fig. 4B, bottom panel). The loss of the phenotypic differences of ΔR4 correlated with the fact that spores with more wild-type nuclei are generated after every asexual cycle.
The mutants ΔR1, ΔR2, and ΔR3 showed hyphae with a morphology highly similar to that of the wild-type strain when grown on solid medium, whereas the ΔR4 heterokaryon mutant exhibited a series of striking morphological alterations. Its hyphae were shorter and swollen in a bulbous manner, with curves or undulations, and the frequency of subapical branching was reduced, suggesting that pkaR4 is involved in regulation of hyphal cell polarity (see Fig. S1 in the supplemental material).
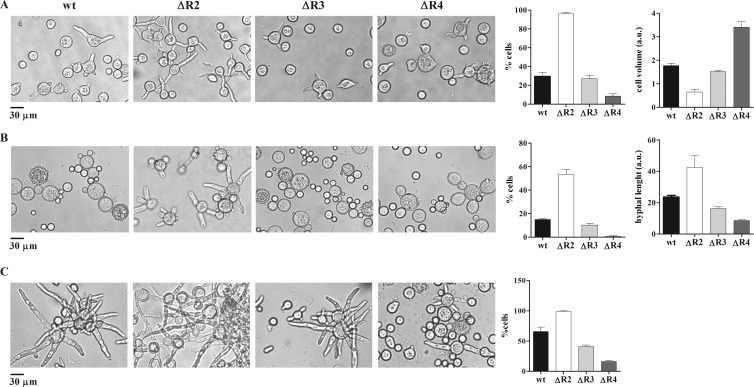
The microscopic morphologies of the mutant strains were also investigated by measuring the germination kinetics, the mother cell volume, and the number and length of germ tubes of spores grown in submerged cultures under aerobic and anaerobic conditions and after the transition from anaerobic to aerobic conditions (Fig. 5; see Fig. S2 in the supplemental material). The ΔR3 strain showed only slight differences from the wild-type strain in all the parameters analyzed and under all the growth conditions. These results correlated with the low expression level of the PKAR3 isoform. Cultures of ΔR2 spores continuously grown under aerobic conditions showed important differences compared to the wild-type strain. The germ tube emission was earlier than in the wild-type strain, and the mother cell volume was reduced (Fig. 5A; see Fig. S2 in the supplemental material). The heterokaryon ΔR4 strain showed the most remarkable differences from the wild-type strain, since it had a severe defect in germ tube emission and an increase in the mother cell volume (Fig. 5A). The measurement of the mother cell volume in the ΔR2, ΔR3, and ΔR4 strains grown under anaerobic conditions showed no differences compared to the wild-type cell volume (not shown).
Fig 5.
Microscopic morphology of pkaR mutants. Microscopic examination of cells of the indicated strains grown at 30°C under different culture conditions is shown. (A) Cells from cultures grown under aerobic conditions for 3.5 h in YPG medium, pH 4.5. Average volumes of the mother cell are shown; each value represents the mean ± SEM for three independent experiments. The volumes were estimated from the diameters, measured in arbitrary units (a.u.). (B) Cells from cultures grown for 2 h under aerobic conditions after a shift from overnight anaerobic growth in minimal medium. The average length of the germ tubes (expressed in arbitrary units) is represented on the bar graph, with each value representing the mean ± SEM for three independent experiments. (C) Cells from cultures grown for 4 h under aerobic conditions after a shift from overnight anaerobic growth in minimal medium. In all cases the numbers of germinated cells were determined and are expressed as the percentage of the total cells (right graph). Each value represents the mean ± SEM for three independent experiments.
These results were also different from those observed in the ΔR1 strain, which showed a reduced mother cell volume, an earlier emergence of the germ tubes, and a reduction in the hyphal extension rate after its emission. Marking a difference from the other three R subunits deletions, ΔR1 showed a reduce cell volume compared to the wild-type strain under anaerobic growth conditions (31).
The morphological changes observed after the shift from anaerobiosis to aerobiosis in the mutants were similar to those observed under aerobic conditions. The germ tube emission in ΔR2 was earlier and the hyphal length was longer than in the wild-type strain. ΔR3 was similar to the wild-type strain, and ΔR4 showed the most critical differences, i.e., a severe defect in the emission of the germ tube. The morphological alterations shown by ΔR4 were of different degrees depending on whether the spore had come from initial heterokaryon transformants or from spores harvested after several subcultivations.
The microscopic morphologies of the mutant strains were also investigated in the presence of different carbon sources and concentrations (glucose, fructose, and glycerol) and in the presence of a poor nitrogen source, such as glycine, in both aerobiosis and anaerobiosis, since for dimorphic species such as M. rouxii and M. circinelloides the main factors influencing morphology are the presence (and concentration) of a fermentable hexose and nitrogen availability (3). It has been reported (55) that a Neurospora crassa temperature-sensitive (mcb) mutant which is defective in the one gene encoding the regulatory subunit of PKA has an apolar growth phenotype in glucose, while the growth of the wt is polarized. When the hexose used as carbon source in the culture medium was fructose, this phenotype was lost and both mutant and wt strains grew alike. Under all the aerobic conditions studied, the differences observed between wt and mutant strains were similar to the ones already detected using the standard YPD growth medium (data not shown). These results indicate that unlike for the mcb mutant, nutritional conditions are not determinant in the differential behavior of each mutant.
The only new interesting phenotype that could be observed was under anaerobic conditions in the presence of glucose as a carbon source and glycine as a nitrogen source. Under those culture conditions, the wt, ΔR1, and ΔR3 strains showed a reduced isodiametric growth, whereas ΔR2 grew with germ tube emergence that was predominantly unipolar (see Fig. S3 in the supplemental material). Therefore, when using glycine as a nitrogen source in anaerobiosis, the growth of ΔR2 is switched to hyphal growth and that of the rest of the strains is impaired.
From these results it can be concluded that each PKAR isoform has a distinct role during M. circinelloides growth and differentiation, that PKAR4 is essential for emission of the germ tube, and that PKAR2 seems to be necessary for an accurate control of germ tube emission.
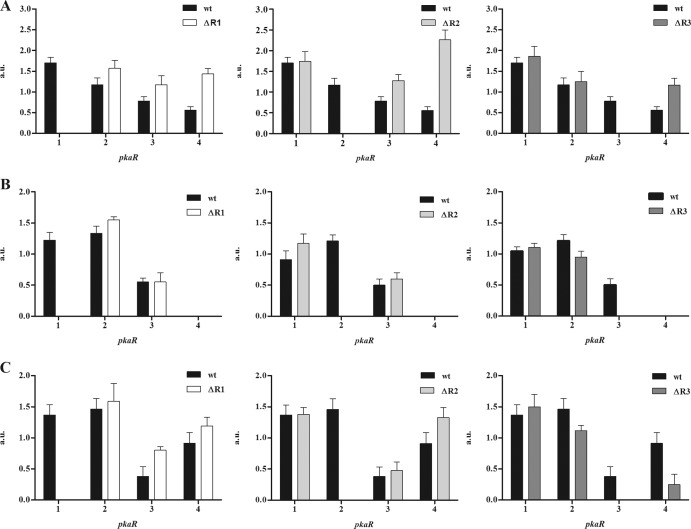
Expression of pkaR genes.
The expression at the mRNA level of each pkaR gene in a wild-type strain showed a differential expression at different developmental stages (31). We now measured mRNA levels of these genes in the different pkaR mutant backgrounds (Fig. 6). In ΔR1 and under aerobic conditions (Fig. 6A) the levels of the other three pkaR transcripts were slightly increased; pkaR4 mRNA showed the greatest increase. As stated above, we have observed that in the ΔR1 strain the emergence of germ tubes under aerobic and anaerobic growth conditions was earlier than that in the wild-type strain and, on the other hand, that the ΔR4 strain had an impediment in the emission of germ tubes. Taking into account the expression level of pkaR4 in the ΔR1 strain and the alteration in the germination rate, these results are in agreement with a key role for pkaR4 in germ tube emission. In ΔR2, the pkaR4 mRNA level was increased even more than in the case of ΔR1; the phenotypic analysis showed that the ΔR2 strain had the earliest germ tube emission under aerobic growth conditions among the mutants, and in the ΔR3 strain there was not a significant change in the pattern of mRNA levels in comparison with those of the wild-type strain (Fig. 6A).
Fig 6.
Expression of pkaR genes. Semiquantitative RT-PCR was used to analyze the relative abundance of mRNA from each pkaR gene using tef-1 mRNA to normalize the data. Total RNA was extracted from cell cultures of the wild-type, ΔR1, ΔR2, and ΔR3 strains grown for 4.5 h under aerobic conditions (A) and from cell cultures under anaerobic conditions (B) or shifted to aerobiosis for 4 h (C). PCR bands were analyzed and quantified by digital imaging, expressing the pixel intensities in arbitrary units (a.u.) relative to the tef band intensity. Each value represents the mean ± SEM for four independent experiments.
Under anaerobic growth conditions in the wild-type strain, the levels of pkaR1 and pkaR2 mRNAs were similar and were higher than those of pkaR3; pkaR4 mRNA was undetectable. This pattern of expression did not change in any of the deletion mutants (Fig. 6B).
The levels of mRNAs were also measured in the mutant strains during the shift from yeast to filamentous growth. Under this condition in the wild-type strain, there was an increase in pkaR4 mRNA levels compared with those under the anaerobic condition (31). The ΔR1 and ΔR2 strains showed an increase in pkaR3 and pkaR4 mRNA levels, whereas ΔR3 showed a decrease in pkaR4 mRNA level in comparison with that of the wild-type strain (Fig. 6C).
These results suggest that the expression of each pkaR gene is highly regulated and coordinated in response to the environment, allowing the regulation of morphogenesis.
Posttranslational modification of PKAR isoforms.
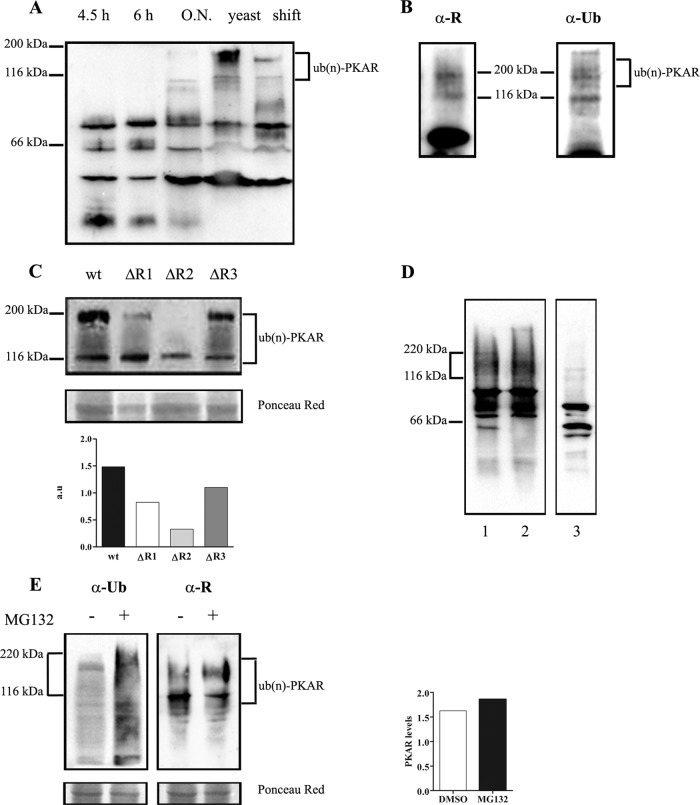
A previous study from our laboratory described that anti-R antibody specifically detected an abundant protein with the expected apparent molecular mass corresponding to R subunits but that it also detected polypeptides with a higher molecular mass that possibly arose from covalent modification of the isoforms (25). The pkaR genes encode four proteins (PKAR1, PKAR2, PKAR3, and PKAR4) with predicted molecular masses of 44, 44, 47, and 49 kDa, respectively. However, Western blot analysis with anti-R antibody of extracts prepared from a wild-type strain grown at different times under aerobic conditions, extracts from cultures grown under anaerobic conditions, and extracts from cultures after a shift to aerobic conditions showed forms with higher molecular masses. The detection of these high-molecular-mass forms was more evident in extracts from an overnight aerobic or yeast anaerobic culture, and they were visualized even 4 h after the shift from yeast to filamentous growth (Fig. 7A).
Fig 7.
Posttranslational modification of PKAR isoforms. (A) Western blot analysis developed with anti-R antibody of crude extracts from the R7B wild-type strain grown under aerobic conditions in YPG medium (pH 4.5) for 4.5 h or 6 h or overnight (O.N.) (15 h) or in MMC medium under anaerobic conditions (yeast) or 4 h after the shift to aerobic conditions as indicated in each lane. (B) Immunoprecipitation of a crude extract of the R7B strain grown under aerobic conditions in YPG medium (pH 4.5) overnight, analyzed by Western blotting with anti-R (α-R) and antiubiquitin (α-Ub) antibodies. (C) Western blot analysis developed with antiubiquitin antibody of crude extracts from R7B (wt), ΔR1, ΔR2, and ΔR3 grown under aerobic conditions in YPG medium (pH 4.5) overnight. Quantification of the higher-molecular-mass band (of around 200 kDa) is shown. Intensities are expressed in arbitrary units (a.u.) relative to the total protein intensity. (D) Western blot analysis using anti-R antibody of samples from the wt strain grown under aerobic conditions for 5 h. Lane 1, crude extract; lane 2, flowthrough; lane 3, semipurification by cAMP-agarose. (E) Western blot revealed with anti-R and antiubiquitin antibodies of samples from the wild-type strain grown for 15 h and then incubated for an additional 4 h in the presence of 50 μMMG-132 in 0.2% dimethyl sulfoxide (DMSO) (+) or in 0.2% DMSO alone (−). Quantification of the higher-molecular-mass band is shown. Intensities are expressed in arbitrary units (a.u.) relative to the total protein intensity.
Since the extra bands showed an important increase of the apparent molecular mass, it appeared conceivable that the isoforms were modified by attachment of multiple ubiquitin moieties. Glycosylation and phosphorylation modifications were ruled out because the extracts were subjected to treatment with agarose-immobilized lectin wheat germ agglutinin (WGA) and to alkaline phosphatase treatment and neither of the treatments removed the high-molecular-mass bands detected by the anti-R antibody (data not shown). To verify the ubiquitylation hypothesis, the PKAR isoforms were immunoprecipitated using anti-R antibody in extracts from the wild-type strain grown overnight and analyzed for the presence of ubiquitylated isoforms by subsequent immunoblotting using an antiubiquitin antibody. High-molecular-mass ubiquitin forms, between 116 and 200 kDa, were detectable in PKAR immunoprecipitates (Fig. 7B). The data reveal modification of endogenous PKAR isoforms by multiple ubiquitin attachment.
With the aim of knowing whether the four PKAR isoforms could be polyubiquitylated, we analyzed extracts from the ΔR1, ΔR2, and ΔR3 strains grown under aerobic conditions overnight. The higher-molecular-mass forms around and closer to 200 kDa decreased in the ΔR1 and ΔR2 mutant strains but did not significantly decrease in the ΔR3 strain (Fig. 7C). These results suggest that the PKAR1 and PKAR2 isoforms could be modified by ubiquitylation and that the longer the culture growth, the larger the amount of ubiquitylated PKAR isoforms.
Polyubiquitylated proteins are usually targeted for degradation in the 26S proteasome, causing a reduction in protein levels; however, polyubiquitylation does not always result in protein degradation, and in this case it could regulate the protein activity. During the purification of R isoforms with cAMP-agarose, we could observe that the polyubiquitylated forms of high molecular mass were not retained in the cAMP-agarose resin but were present in the column flowthrough, indicating that these modified forms could not bind cAMP (Fig. 7D). Moreover, when cultures of the wild-type strain were incubated with the proteasome inhibitor MG132, no changes in the polyubiquitylated forms of R were observed (Fig. 7E), although the treatment increased significantly the total ubiquitylated proteins as revealed by the analysis of the same samples with antiubiquitin antibodies, suggesting that they are not intermediate forms of degradation by the 26S proteasome.
Although more experiments are necessary to confirm the polyubiquitylation of PKAR isoforms in M. circinelloides, these results lead us to think that this modification somehow inhibits the cAMP binding activity and therefore modulates the protein kinase activity of holoenzymes that contain PKAR1 and PKAR2 isoforms.
DISCUSSION
Previous results from our laboratory showed that PKA is involved in the morphogenetic process of Mucor (35) and that it has a role in hyphal branching during filamentous growth in M. circinelloides (25). Recently, we have shown the role of the PKA pathway in growth and differentiation in M. circinelloides by generating a pkaR1 gene deletion mutant. This mutant showed a reduction in growth and asexual sporulation, an earlier germ tube emergence in aerobic cultures, and a reduced cell volume (31). From the biochemical and morphological results obtained for ΔR1, we predicted and confirmed the existence of three additional genes encoding the regulatory subunit of PKA.
The aim of the present work was to analyze the participation of every PKAR isoform in M. circinelloides differentiation and establish whether each isoform had a different role in this process. We thus disrupted the three pkaR genes pkaR2, pkaR3, and pkaR4 and characterized each mutant biochemically and phenotypically.
One of the most important observations in this work was the definition of the ΔR4 strain as a heterokaryon strain, indicating that a pkaR4 deletion mutant is not viable without containing wild-type nuclei and consequently that pkaR4 is essential for viability in M. circinelloides. Spores harvested after several vegetative cycles of growth under different conditions gradually lost the phenotypic differences from the wild type. This correlates with the fact that the heterokaryotic state is broken during sporulation, and since spores of M. circinelloides are multinucleated, spores containing more wild-type nuclei are generated during the sporulation process. It has been described that if an essential gene is deleted, rather than causing death, the null allele will be rescued by spontaneous generation of a heterokaryon (34). An example had been described for the filamentous fungus Aspergillus nidulans, in which the deletion of the protein kinase C-encoding gene (pkcA) was lethal and only heterokaryotic strains were obtained (18).
Samples from each mutant strain semipurified with cAMP-agarose and analyzed by Western blotting showed the absence of the specific PKAR isoform (Fig. 3A). Interestingly, the levels of PKAR3 and PKAR4 were altered in the ΔR2 strain under aerobic conditions, suggesting that pkaR2 directly or indirectly controls the levels of both isoforms. However, this control should take place at different levels, because the increase of the PKAR4 protein level was consistent with an increase in mRNA levels (Fig. 6A), suggesting a transcriptional control, whereas the great decrease in PKAR3 protein levels were not accompanied by a decrease in the pkaR3 mRNA levels and could be a consequence of translational or posttranslational regulation.
The absence of one PKAR isoform did not have the same effect on the kinase activity. The ΔR1 strain showed a decreased in total PKAC activity, and this activity showed a lower dependence on cAMP for catalytic activity. However, the ΔR2 strain showed almost no differences from the wild-type strain in PKAC activity. This difference could be explained by considering the sequences and biochemical characteristics of the remaining PKAR isoforms in each mutant strain. PKAR isoforms differ in the amino-terminal region and in the linker region (31). The R-C affinity interaction of PKA in M. circinelloides is higher than that in other species, and this is due to the presence of an acidic region in linker I (32), a region present between the N terminus and the cAMP binding domains. PKAR1 and PKAR4 have a higher number of acidic residues in the linker I region than PKAR2 and PKAR3. A higher-affinity interaction between PKAC and PKAR1 or PKAR4 than between PKAC and PKAR2 or PKAR3 is expected. The decreased cAMP dependence of the PKAC activity in the ΔR1 strain could therefore be a consequence of the lower interaction affinity between PKAC and the remaining PKAR2 or PKAR3 isoforms expressed in this mutant. In ΔR1, as a consequence of a decreased −/+ cAMP ratio, the C subunit is more easily dissociable; a C subunit that is not part of a holoenzyme is more unstable than when it is associated with an R subunit (35). Thus, this could explain the decrease in total PKAC in this mutant. The opposite situation would occur in the ΔR2 mutant strain, considering that the isoforms expressed in this mutant are PKAR1 and an increased PKAR4; therefore, a higher interaction affinity between PKAC and these PKAR isoforms as a result of cAMP dependence would not be altered, explaining the lack of a difference from the wild-type strain.
Therefore, the existence of more than one regulatory subunit isoform should affect the degree of activation of PKA by cAMP due to differences in the linker domain of each R isoform, and this degree of activation will depend on the concentration of each of the isoforms at a given time. Besides the multiple isoforms of the R subunit, we have detected the existence of 10 catalytic subunit isoforms for Mucor circinelloides and also have predicted a similar high number of isoforms from the genomes of the other two sequenced zygomycetes, Rhizopus oryzae and Phycomyces blakesleeanus (17; L. Fernandez Nuñez et al., unpublished results). Therefore, a multiplicity of possible different PKA holoenzymes due to the combination of these C and R subunits isoforms could give different responses to a burst in cAMP.
Until now, the gene encoding the regulatory subunit of PKA has been found to be present in a single copy in the fungi Saccharomyces cerevisiae, Ustilago maydis, Neurospora crassa, Magnaporthe grisea, Cryptococcus neoformans, Candida albicans, Schizosaccharomyces pombe, Aspergillus fumigatus, Aspergillus niger, Colletotricum lagenarium, Yarrowia lipolytica, and Blastocladiella emersonii. Thus, zygomycetes seem to be the only class of fungi that have more than one isoform for the R subunit (1, 2, 8, 9, 11, 14, 26, 46, 50, 51, 52, 54). The corresponding mutants with mutations in the genes encoding the PKA regulatory subunit have been obtained from several of these fungi, and there does not seem to be a unique way in how fungi respond to the deletion of the R subunit, since some of them, such as A. niger (46), have the same level of PKA activity as in the wild-type cell, whereas in others, such as C. neoformans or A. fumigatus (11, 54), the PKA activity is increased. However, the different mutants are different from the wt in both microscopic and macroscopic phenotypic features. A characteristic that seems to be a common feature of R mutants of filamentous fungi is a slower radial growth, since besides our own results, this has also been reported for N. crassa, C. lagenarium, A. fumigatus, and A. niger (2, 46, 51, 54). Germination rates of the fungal R mutant strains are lower than those of the wt strains. Sporulation or conidiation defects also appear to be common to these mutants in filamentous fungi, such as A. niger, A. fumigatus, or C. lagenarium (46, 51, 54). Some R subunit mutants also show a loss of growth polarity, as in A. niger, C. albicans, and N. crassa (2, 8, 46). In plant- and human-pathogenic fungi such as U. maydis, C. albicans, and C. neoformans, R subunits are also required for filamentous growth (8, 11, 12, 14). Contrary to what occurs in the mutants detailed above, homozygous R subunit mutants of C. albicans and Y. lipolytica are not viable (8, 9).
In M. circinelloides the germination kinetics of mutants and wild-type strains were different in submerged cultures, although ΔR3 showed only slight or no differences from the wild type under all the conditions analyzed. PKAR3 is the least-expressed isoform under aerobic and anaerobic growth conditions, and thus PKAR3 could be redundant in function with some other PKAR isoform or could carry out a particular function unrelated to the analyzed phenotypes. ΔR2 and ΔR1 (31) showed similar phenotypes, which were opposite to those of ΔR4 under aerobic growth conditions. ΔR2 showed a smaller mother cell volume, earlier germ tube emergence, and higher hyphal extension rate than the wild-type strain. ΔR1 also showed a reduced cell volume and early germ tube emergence (31). On the other hand, ΔR4 showed a severe reduction in the germ tube emission, and the mother cell volume was significantly increased compared to that of the wild type. Therefore, these results suggest that pkaR1 and pkaR2 display roles antagonistic to those of pkaR4 in the fungus dimorphism. However, many data support that pkaR4 may play a pivotal role in germ tube emission. It is expressed only in the transition from yeast to filamentous growth (31) (Fig. 3A and 6), its deletion affects germ tube emission and seems to be lethal as expected for a gene with an important function in germ tube emission, and finally, mutants with increased pkaR4 levels (e.g., ΔR2 and ΔR1) show early germ tube emission (Fig. 5) (31).
Other data are in agreement with the main role of pkaR4 in germ tube emission. Thus, under anaerobic conditions, no differences in either mother cell volume or number of buds were observed among ΔR2, ΔR3, ΔR4, and the wild-type strain, but after the shift from anaerobiosis to aerobiosis, the phenotypes of mutant strains resembled those observed under aerobic growth conditions, with ΔR4 showing the most significant alteration, since it emitted fewer germ tubes than the wild-type strain. Moreover, heterokaryon ΔR4 in aerobiosis showed a decrease in both sporulation and radial growth compared to the wild type, and its hyphae were shorter and swollen in a bulbous manner, with curves or undulations and a reduced subapical branching frequency (see Fig. S1 in the supplemental material). All these phenotypes could be a consequence of a defect in germ tube emergence. In addition to pkaR4, pkaR2 also participates in controlling germ tube emergence, because ΔR2 had an earlier emission under aerobic conditions and in the shift from anaerobiosis to aerobiosis and grew as a strongly polarized mycelium, with only one germ tube, under anaerobiosis in the presence of a poor nitrogen source, conditions under which the growth of the rest of the strains was impaired. Therefore, the absence of pkaR2 facilitated germ tube emergence under different environmental conditions, suggesting that PKAR2 represses this cellular process.
The measurement of the mRNA levels of each isoform in the wild-type and knockout strains indicates that the expression of each subunit has its own mechanism of differential regulation, suggesting that each PKAR isoform has a differential role during M. circinelloides growth and differentiation. We can therefore envision that the multiplicity of holoenzymes that could be formed within this organism should have a functional significance.
The results show that the phenotype displayed by each PKAR mutant is a consequence of the lack of a gene that results in a physiological response. Each of the multiple PKAR isoforms may have acquired different specificity, as supported by the existence of different expression patterns. Different isoforms of the holoenzyme may show differences in the activation by cAMP due to differences in the linker domain of the R subunit in each holoenzymatic isoform, as well as possible different subcellular localization through the N-terminal anchoring domain of each R (19, 20, 45), thus providing specificity in both substrate phosphorylation and signaling pathway.
PKAR isoforms are posttranslationally modified by ubiquitylation, and the most affected isoforms are PKAR1 and PKAR2. The ubiquitylation can regulate the switching on or off of a signal transduction pathway regulating the half-life of its active components (24). However, there are examples in which the polyubiquitylation does not seem to be implicated in protein degradation. The MCWC-1b protein (white collar 1-b isoform, implicated in the regulation of carotenogenesis in M. circinelloides) is oligoubiquitylated, and these forms do not seem to be intermediate forms of degradation by the 26S proteasome and could result in an inactive form of this transcription factor (48).
We have observed that the PKAR isoforms are differentially ubiquitylated, and this could mean that the polyubiquitylation of PKARs could contribute to the specificity in cAMP signaling. Our results suggest that this modification regulates the cAMP binding activity of the regulatory subunits PKAR1 and PKAR2, and therefore it could modulate the holoenzyme kinase activities that are conformed by these PKAR isoforms. This modification occurs mainly in two of the four isoforms, suggesting another regulation point in the specificity of the signal transduction. The fact that this broad-specificity protein kinase mediates a number of discrete physiological responses following cAMP engagement has raised a major question of how specificity is maintained in the cAMP/PKA system. In this work we have characterized the unique features of each regulatory subunit isoform that may contribute to explain how differential effects of cAMP may orchestrate the PKA signal transduction pathway that regulates dimorphism in M. circinelloides.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica, Universidad de Buenos Aires, and the Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina. The work carried out at the University of Murcia was funded by the Fundación Séneca de la Comunidad Autónoma de la Región de Murcia (grant 08802/PI/08).
We thank Cristina Paveto for technical advice.
Footnotes
Published ahead of print 25 May 2012
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1. Adachi K, Hamer JE. 1998. Divergent cAMP signaling pathways regulate growth and pathogenesis in the rice blast fungus Magnaporthe grisea. Plant Cell 10: 1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banno S, et al. 2005. A catalytic subunit of cyclic AMP dependent protein kinase, PKAC-1, regulates asexual differentiation in Neurospora crassa. Genes Genet. Syst. 80: 25–34 [DOI] [PubMed] [Google Scholar]
- 3. Bartnicki-García S, Nickerson WJ. 1962. Nutrition, growth and morphogenesis of Mucor rouxii. J. Bacteriol. 84: 841–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biswas S, Van Dijck P, Datta A. 2007. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol. Mol. Biol. Rev. 71: 348–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borges-Walmsley MI, Walmsley AR. 2000. cAMP signalling in pathogenic fungi: control of dimorphic switching and pathogenicity. Trends Microbiol. 8: 133–141 [DOI] [PubMed] [Google Scholar]
- 6. Brown J. 2005. Zygomycosis: an emerging fungal infection. Am. J. Health Syst. Pharm. 62: 2593–2596 [DOI] [PubMed] [Google Scholar]
- 7. Bruno KS, Aramayo R, Minke PF, Metzenber RL, Plamann M. 1996. Loss of growth polarity and mislocalization of septa in a Neurospora mutant altered in the regulatory subunit of cAMP-dependent protein kinase. EMBO J. 15: 5772–5782 [PMC free article] [PubMed] [Google Scholar]
- 8. Cassola A, et al. 2004. Candida albicans lacking the gene encoding the regulatory subunit of protein kinase A displays a defect in hyphal formation and an altered localization of the catalytic subunit. Eukaryot. Cell 3: 190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cervantes-Chávez JA, Ruiz-Herrera J. 2007. The regulatory subunit of protein kinase A promotes hyphal growth and plays an essential role in Yarrowia lipolytica. FEMS Yeast Res. 7: 929–940 [DOI] [PubMed] [Google Scholar]
- 10. Chayakulkeeree M, Ghannoum MA, Perfect JR. 2006. Zygomycosis: the re-emerging fungal infection. Eur. J. Clin. Microbiol. Infect. Dis. 25: 215–229 [DOI] [PubMed] [Google Scholar]
- 11. D'Souza CA, et al. 2001. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell. Biol. 21: 3179–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Durrenberger F, Wong K, Kronstad JW. 1998. Identification of a cAMP-dependent protein kinase catalytic subunit required for virulence and morphogenesis in Ustilago maydis. Proc. Natl. Acad. Sci. U. S. A. 95: 5684–5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gilman A. 1970. Presentation of the Academy Medal to Harry Eagle, M. D. Bull. N. Y. Acad. Med. 46: 666–669 [PMC free article] [PubMed] [Google Scholar]
- 14. Gold SE, Duncan G, Barrett K, Kronstad J. 1994. cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 8: 2805–2816 [DOI] [PubMed] [Google Scholar]
- 15. Gutierrez A, Lopez-Garcia S, Garre V. 2011. High reliability transformation of the basal fungus Mucor circinelloides by electroporation. J. Microbiol. Methods 84: 442–446 [DOI] [PubMed] [Google Scholar]
- 16. Harris SD. 2006. Cell polarity in filamentous fungi: shaping the mold. Int. Rev. Cytol. 251: 41–77 [DOI] [PubMed] [Google Scholar]
- 17. He X, Zhang J. 2006. Higher duplicability of less important genes in yeast genomes. Mol. Biol. Evol. 23: 144–151 [DOI] [PubMed] [Google Scholar]
- 18. Ichinomiya M, Uchida H, Koshi Y, Ohta A, Horiuchi H. 2007. A protein kinase C-encoding gene, pkcA, is essential to the viability of the filamentous fungus Aspergillus nidulans. Biosci. Biotechnol. Biochem. 71: 2787–2799 [DOI] [PubMed] [Google Scholar]
- 19. Jarnaess E, Tasken K. 2007. Spatiotemporal control of cAMP signalling processes by anchored signalling complexes. Biochem. Soc. Trans. 35: 931–937 [DOI] [PubMed] [Google Scholar]
- 20. Kinderman FS, et al. 2006. A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Mol. Cell 24: 397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klein BS, Tebbets B. 2007. Dimorphism and virulence in fungi. Curr. Opin. Microbiol. 10: 314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kontoyiannis DP, et al. 2005. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J. Infect. Dis. 191: 1350–1360 [DOI] [PubMed] [Google Scholar]
- 23. Li CH, et al. 2011. Sporangiospore size dimorphism is linked to virulence of Mucor circinelloides. PLoS Pathog. 7: e1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu Z, Hunter T. 2009. Degradation of activated protein kinases by ubiquitination. Annu. Review Biochem. 78: 435–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lübbehusen T, et al. 2004. Protein kinase A is involved in the control of morphology and branching during aerobic growth of Mucor circinelloides. Microbiology 150: 143–150 [DOI] [PubMed] [Google Scholar]
- 26. Marques MV, Gomes LS. 1992. Cloning and structural analysis of the gene for the regulatory subunit of cAMP-dependent protein kinase in Blastocladiella emersonii. J. Biol. Chem. 267: 17201–17207 [PubMed] [Google Scholar]
- 27. Marr KA, Carter RA, Crippa F, Wald A, Corey L. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34: 909–917 [DOI] [PubMed] [Google Scholar]
- 28. Navarro E, et al. 2001. A negative regulator of light-inducible carotenogenesis in Mucor circinelloides. Mol. Genet. Genomics 266: 463–470 [DOI] [PubMed] [Google Scholar]
- 29. Nemecek JC, Wuthrich M, Klein BS. 2006. Global control of dimorphism and virulence in fungi. Science 312: 583–58816645097 [Google Scholar]
- 30. Nicolas FE, de Haro JP, Torres-Martinez S, Ruiz-Vazquez RM. 2007. Mutants defective in a Mucor circinelloides dicer-like gene are not compromised in siRNA silencing but display developmental defects. Fungal Genet. Biol. 44: 504–516 [DOI] [PubMed] [Google Scholar]
- 31. Ocampo J, et al. 2009. A subunit of protein kinase A regulates growth and differentiation in the fungus Mucor circinelloides. Eukaryot. Cell 8: 933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ocampo J, Moreno S, Rossi S. 2007. PKA from Mucor circinelloides: model to study the role of linker I in the interaction between R and C subunits. Biochem. Biophys. Res. Commun. 362: 721–726 [DOI] [PubMed] [Google Scholar]
- 33. Orlowski M. 1991. Mucor dimorphism. Microbiol. Rev. 55: 234–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Osmani AH, Oakley BR, Osmani SA. 2006. Identification and analysis of essential Aspergillus nidulans genes using the heterokaryon rescue technique. Nat. Protoc. 1: 2517–2526 [DOI] [PubMed] [Google Scholar]
- 35. Pereyra E, Mizyrycki C, Moreno S. 2000. Threshold level of protein kinase A activity and polarized growth in Mucor rouxii. Microbiology 146: 1949–1958 [DOI] [PubMed] [Google Scholar]
- 36. Pereyra E, Zaremberg V, Moreno S. 1992. Effect of dibutyryl cAMP on growth and morphology of germinating Mucor rouxii sporangiospores. Exp. Mycol. 16: 93–101 [Google Scholar]
- 37. Quiles-Rosillo MD, Ruiz-Vazquez RM, Torres-Martinez S, Garre V. 2003. Cloning, characterization and heterologous expression of the Blakesleatrispora gene encoding orotidine-5′-monophosphate decarboxylase. FEMS Microbiol. Lett. 222: 229–236 [DOI] [PubMed] [Google Scholar]
- 38. Ribes JA, Vanover-Sams CL, Baker DJ. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13: 236–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rinaldi J, Ocampo J, Rossi S, Moreno S. 2008. A novel activating effect of the regulatory subunit of protein kinase A on catalytic subunit activity. Arch. Biochem. Biophys. 480: 95–103 [DOI] [PubMed] [Google Scholar]
- 40. Roden MM, et al. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41: 634–653 [DOI] [PubMed] [Google Scholar]
- 41. Roncero MI, Zabala C, Cerda-Olmedo E. 1984. Mutagenesis in multinucleate cells: the effects of N-methyl-N′-nitro-N-nitrosoguanidine on Phycomyces spores. Mutat. Res. 125: 195–204 [DOI] [PubMed] [Google Scholar]
- 42. Roskoski R. 1983. Assays of protein kinase. Methods Enzymol. 99: 3–6 [DOI] [PubMed] [Google Scholar]
- 43. Ruiz-Perez VL, Murillo FJ, Torres-Martinez S. 1995. PkpA, a novel Phycomyces blakesleeanus serine/threonine protein kinase. Curr. Genet. 28: 309–316 [DOI] [PubMed] [Google Scholar]
- 44. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 45. Sarma GN, et al. 2010. Structure of D-AKAP2:PKA RI complex: insights into AKAP specificity and selectivity. Structure 18: 155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saudohar M, et al. 2002. Cyclic AMP-dependent protein kinase is involved in morphogenesis in Aspergillus niger. Microbiology 148: 2635–2645 [DOI] [PubMed] [Google Scholar]
- 47. Schipper MA. 1976. Induced azygospore formation in Mucor (Rhizomucor) pusillus by Absidia corymbifera. Antonie Van Leeuwenhoek 42: 141–144 [DOI] [PubMed] [Google Scholar]
- 48. Silva F, et al. 2008. A RING-finger protein regulates carotenogenesis via proteolysis-independent ubiquitylation of a white collar-1-like activator. Mol. Microbiol. 70: 1026–1036 [DOI] [PubMed] [Google Scholar]
- 49. Spellberg B, Edwards J, Jr, Ibrahim A. 2005. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 18: 556–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stiefel J, et al. 2004. Suppressors of an adenylate cyclase deletion in the fission yeast Schizosaccharomyces pombe. Eukaryot. Cell 3: 610–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Takano Y, Komeda K, Kojima K, Okuno T. 2001. Proper regulation of cyclic AMP-dependent protein kinase is required for growth, conidiation, and appressorium function in the anthracnose fungus Colletotrichum lagenarium. Mol. Plant Microbe Interact. 14: 1149–1157 [DOI] [PubMed] [Google Scholar]
- 52. Toda T, et al. 1987. Cloning and characterization of BCY1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol. Cell. Biol. 7: 1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wolff AM, Appel KF, Petersen JB, Poulsen U, Arnau J. 2002. Identification and analysis of genes involved in the control of dimorphism in Mucor circinelloides (syn. racemosus). FEMS Yeast. Res. 2: 203–213 [DOI] [PubMed] [Google Scholar]
- 54. Zhao W, et al. 2006. Deletion of the regulatory subunit of protein kinase A in Aspergillus fumigatus alters morphology, sensitivity to oxidative damage, and virulence. Infect. Immun. 74: 4865–4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ziv C, Gorovits R, Yarden O. 2008. Carbon source affects PKA-dependent polarity of Neurospora crassa in a CRE-1-dependent and independent manner. Fungal Genet. Biol. 45: 103–116 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.