Abstract
Replication-competent porcine endogenous retroviruses (PERVs) are either human cell tropic (PERV-A and PERV-B) or non-human cell tropic (PERV-C). We previously demonstrated that PERV in vitro cell tropism is modulated by 2 residues within the C terminus of SU and that the PERV receptor binding domain (RBD) extends beyond the variable regions A and B (VRA and VRB, respectively), to include the proline rich-region (PRR) of SU (M. Gemeniano et al., Virology 346:108–117, 2000; T. Argaw et al., J. Virol. 82:7483–7489, 2008). The present study aimed to identify the specific elements within the PERV RBD that interact with the C-terminal elements of SU to facilitate human cell infection. We constructed a series of chimeric and mutated envelopes between PERV-A and PERV-C and using pseudotyped retroviral vectors to map the human cell tropism-determining sequences within the PERV RBD. We show that the PRR from PERV-A is both necessary and sufficient to allow human cell infection when substituted into the homologous region of the PERV-C envelope carrying two C-terminal amino acid substitutions shown to influence human cell tropism, Q374R and I412V (PERV-Crv). Furthermore, substitution of a single amino acid residue in the PRR of the non-human-tropic PERV-Crv envelope allows vectors carrying this envelope to infect human cells. Receptor interference assays showed that these modified PERV-C envelopes do not bind either of the human PERV-A receptors, suggesting the presence of a distinct human PERV-C receptor. Finally, vectors carrying these modified PERV-C envelopes infect primary human endothelial cells, a cell type likely to be exposed to PERV in clinical use of certain porcine xenotransplantation products.
INTRODUCTION
Porcine endogenous retroviruses (PERVs) are gammaretroviruses presumably derived from an ancient infection of animals ancestral to the family Suidae. The germ line integration of the retrovirus in the genome and subsequent vertical transmission from generation to generation are thought to have occurred at least 3.5 million years ago (16, 25). Hence, today's swine all carry these genetic sequences in their genome (18). The findings that these retroviral sequences in the pig germ line give rise to infectious viruses and that two of the three receptor classes are able to infect human cells form the basis for concern that the clinical xenotransplantation of living pig cells into humans to treat disease may increase the risk of iatrogenic transmission of PERV to xenotransplantation product recipients.
The envelope (env) glycoprotein of gammaretroviruses is composed of two subunits, the surface (SU) and transmembrane (TM) units (19). The SU units of the envelope gene of most retroviruses have an amino-terminal domain designated the “receptor binding domain” (RBD) (2, 4, 10, 13, 17) and the carboxyl-terminal domain that stabilizes the viral envelope protein conformation and influences cell-to-cell fusion (11, 20). The proline rich-region (PRR) is thought to provide a flexible hinge between these two functional domains (8). For most gammaretroviruses, the RBD includes two variable regions, variable region A (VRA) and variable region B (VRB) (5, 23). Within the RBD, the host cell binding and receptor recognition activities of most gammaretroviruses have been mapped to the N terminus of SU with the primary determinant of receptor specificity localizing to VRA (5, 10, 14). In contrast, we have shown that the N-terminal 200 amino acids (aa) of the PERV SU comprising structural domains analogous to murine leukemia virus (MLV) gammaretroviral VRA and VRB lack cell binding activity and that binding requires additional C-terminal sequences, including the proline-rich region (PRR) (7). In addition, we found that 2 residues in the C terminus of the SU, R395 and V433 (residue positions based on PERV-A envelope [27]) impact PERV infection of human cells (3).
The purpose of the present study is to identify the specific elements within the PERV RBD, which includes the VRA, VRB, and PRR, that interact with the C-terminal elements of SU to facilitate human cell infection. Using the human-cell-tropic PERV-A and non-human-cell-tropic PERV-C, we generated a series of chimeric PERV envelopes and show that unlike other gammaretroviruses, the PRR of PERV SU and 2 aa in the C terminus of the SU provide functional complementarities to allow human cell infection. Therefore, study of PERV entry provides additional insights into the molecular mechanisms for host range and receptor recognition of gammaretroviruses because of distinct structural requirements for cell-specific entry compared to those of very closely related viruses.
MATERIALS AND METHODS
Cells.
Four cell lines were used in the study: 293HEK (ATCC, CRL-1573), 293T (a gift of Maribeth Eiden, NIMH, NIH, Bethesda, MD), ST (a cell line derived from swine testes, previously obtained from R. Fister, Tufts University, Boston, MA), and SIRC (ATCC CRL-60). In order to determine receptor or superinfection interference, 293HEK cells productively infected with the PERV-A isolate 14/220 were used, as kindly provided by Clive Patience (9), as well as ST cells chronically infected with PERV-C isolated from plasma of an NIH minipig (24). SIRC rabbit cells stably expressing the human PERV-A receptor type 2, HuPAR2 (6) (provided by Clive Patience), were also used to assess the in vitro host range in the infectivity assay. Primary human umbilical vein endothelial cells (HUVECs) were purchased from ATCC (ATCC PCS100-010) and grown in ATCC complete growth medium, F-12K medium (ATCC 30-22004) containing 10% fetal bovine serum (FBS) (HyClone, Logan, UT) and 1% penicillin-streptomycin, glutamine, and sodium pyruvate (BioWhittaker, Lonza, Walkersville, MD), and incubated at 37°C in 5% CO2. 293HEK, 293T, and ST cells were grown in Dulbecco's modified Eagle's medium (DMEM) (BioWhittaker, Lonza, Walkersville, MD) supplemented with 10% FBS and 1% streptomycin, penicillin antibiotics, glutamine, and sodium pyruvate, and incubated in 5% CO2 at 37°C. SIRC cells were cultured in Eagle's minimal essential medium (EMEM) (BioWhittaker, Lonza, Walkersville, MD) supplemented in the same manner and incubated under the same conditions.
Construction of mutant and chimeric envelope clones.
The PERV-A and PERV-C expression plasmids were derived according to methods previously reported (7). Two amino acids of the C terminus of the SU of PERV had been shown to be influential in human cell tropism of PERV (3). Hence, a plasmid construct encoding the PERV-C envelope, pC1neoPERV-C, and a plasmid construct encoding the PERV-C envelope carrying these two mutated amino acids, pC1neoPERV-C Q374R+I412V (referred to herein as PERV C-rv), served as backbones to substitute either singly or in combination for the homologous sequences of each putative structural domain of the PERV-A RBD, VRA, VRB, or PRR. To facilitate the cloning of the chimeric constructs, site-directed mutagenesis was used to introduce appropriate restriction endonuclease recognition sites, with silent mutations, into the cDNA sequences expressed in pC1neo vector plasmids of PERV-A env, PERV-C env, and PERV-Crv env, following the manufacturer's directions (Quick-Change mutagenesis kit; Stratagene, La Jolla, CA). The oligonucleotide sequences, related to PERV-A and PERV-C, are based on the reported sequences under GenBank accession no. Y12238 and AF038600, respectively (1, 12).
The sense oligonucleotides described below with the antisense complementary primers were used for introduction of the nucleotide changes at the desired position of the viral envelope sequences. In some cases in which a silent mutation was not possible, single or double amino acids were mutated to obtain a restriction enzyme recognition sequence and the mutated amino acids were subsequently restored by site-directed mutagenesis. The restriction enzyme-digested fragments were ligated, and the constructs were cloned following the manufacturer's instructions for the Rapid DNA ligation kit (Roche Diagnostics, Indianapolis, IN). All cloned chimeric envelope sequences and mutants were confirmed by DNA sequencing using BigDye chemistry on an ABI 310 genetic analyzer (Applied Biosystems, Foster City, CA) to confirm that site-directed mutations and chimeras were present and to verify that unscheduled mutations were absent.
To derive the chimeric PERV-C env carrying PERV-A VRA sequences, termed “PERV-CVRAa,” XcmI and Bsu361 sites were introduced upstream and downstream of VRA, respectively, within each cDNA encoding PERV-A or C envelope sequences, respectively. The sense primers CenvXcm1 (5′-ACTCAAGGGGAGGCTCCATTAGGAACCTGGT-3′) and CenvBsu361 (5′-GGAAATCCTCAGGATTTCTTTTGTAAACAAT-3′) with their complementary antisense primers were used to introduce these two restriction enzyme sequences by site-directed mutagenesis.
The Bsu361 restriction site, upstream of VRB, like the primer (CenvBSu361) described above, and the PshA1 site downstream of the VRB region were introduced in each cDNA encoding PERV-A or PERV-C envelopes to derive a chimeric PERV-C env with PERV-A-derived sequences for VRB, termed “PERV-CVRBa.” The primers CenvPshA1 (5′-AAGTGCTCTCCTTCAGACCTAGGTCACCTA-3′) and AenvPshA1 (5′-AGCTGTCATTCGTTAGACCTAGGTCACTTA-3′) with their complementary antisense primers were used to introduce the PshA1 restriction enzyme sequences in PERV-C envelope and PERV-A envelope cDNA-expressing vectors, respectively. For the chimeric PERV-C envelope carrying the PERV-A PRR region, termed “PERV-CPRRa,” a Bsu361 site was introduced upstream of the PRR region and the naturally existing ApaI restriction enzyme sequence 3′ to the end of the PRR coding region of both PERV-C and PERV-A envelope sequences were used to cleave and substitute the PRR region of PERV-C with the homologous PERV-A PRR. In order to introduce these Bsu361 restriction enzyme sequences, the primers CprrBsu361 (5′-ATTCTAACTATTCGCCTCAGGATAAACCAG-3′) and AprrBsu361 (5′-TTCTGACTATTCGCCTCAGGATAGAAACTC-3′) and their complementary antisense primers were used for PERV-C and PERV-A envelope-expressing vectors, respectively.
Derivation of viral vector pseudotypes, infectivity, and receptor interference assays.
Retroviral vector pseudotypes carrying wild-type or chimeric mutant envelopes were prepared as previously described (3). Briefly, 1.5 × 106 293T cells were seeded in 10-cm plates 1 day prior to DNA transfection. Three expression vectors were introduced into 293T cells by CaPO4-mediated transient transfection using the Profection kit (Promega, Madison WI) as specified by the manufacturer: (i) pRT43.2Tnslβgal encoding the β-galactosidase gene, (ii) pMLVgag-pol expressing the core and the enzymatic proteins derived from Moloney murine leukemia virus (MoMLV), and (iii) pCIneo expressing the PERV envelope cDNA or mutants. Eight to 12 h later, the transfection media were changed and replenished with fresh DMEM with 10% FBS. Seventy-two hours posttransfection, supernatant was collected, filtered through a 0.45-μm-pore membrane, and adjusted to a final concentration of 6 μg/ml Polybrene (Sigma, St. Louis, MO), and then target cells grown in a 12-well plate were exposed to supernatant (in triplicate wells). Seventy-two hours postexposure, infectivity or receptor interference was determined by assaying the β-galactosidase activity in both indicator and target cells, as previously described (26). In order to test receptor specificity and efficiency of receptor interference, viral vector pseudotypes carrying chimeric or wild-type envelopes were used to superinfect cells productively infected with the 14-220 strain of PERV-A (9) or PERV-C (24).
SU fusion protein preparation and binding assay.
In order to study the receptor binding activity of the mutant PERV-C envelopes, the full-length SU was fused in frame with rabbit IgG (rIgG) in the pCIneo vector as specified in our previous study (7). In order to facilitate the cloning of the SU, in frame with the rabbit immunoglobulin heavy chain, an SpeI restriction site was introduced by site-directed mutagenesis at the SU/TM junction of the respective pCIneo vector expressing the envelope cDNA, using the PCR primer combinations CenvSpeI and AenvSpeI. The primer CenvSpeI (5′-CAAAAGAAAGAAACCCACTAGTCTGACACTTG-3′) and the antisense complementary primer were used to introduce an SpeI restriction site into the vector expressing the PERV-C SU and mutant fusion proteins, whereas the primer Aenv-SpeI (5′-TCGGCCAAAAAGACTAGTCATATCCCTGAC-3′) and the complementary antisense primer were used to introduce the SpeI site into the plasmid vector expressing the wild-type PERV-A SU. Then the SU-encoding sequences were obtained by restriction digestion with SalI and SpeI. The pCIneo plasmid vector was cleaved with the enzymes SalI and NotI. Each restricted plasmid DNA was electrophoresed, and the bands corresponding to the SU and vector DNA sequences, respectively, were isolated from the agarose gel using the QIAquick gel extraction kit (Qiagen, Valencia, CA) as specified by the manufacturer.
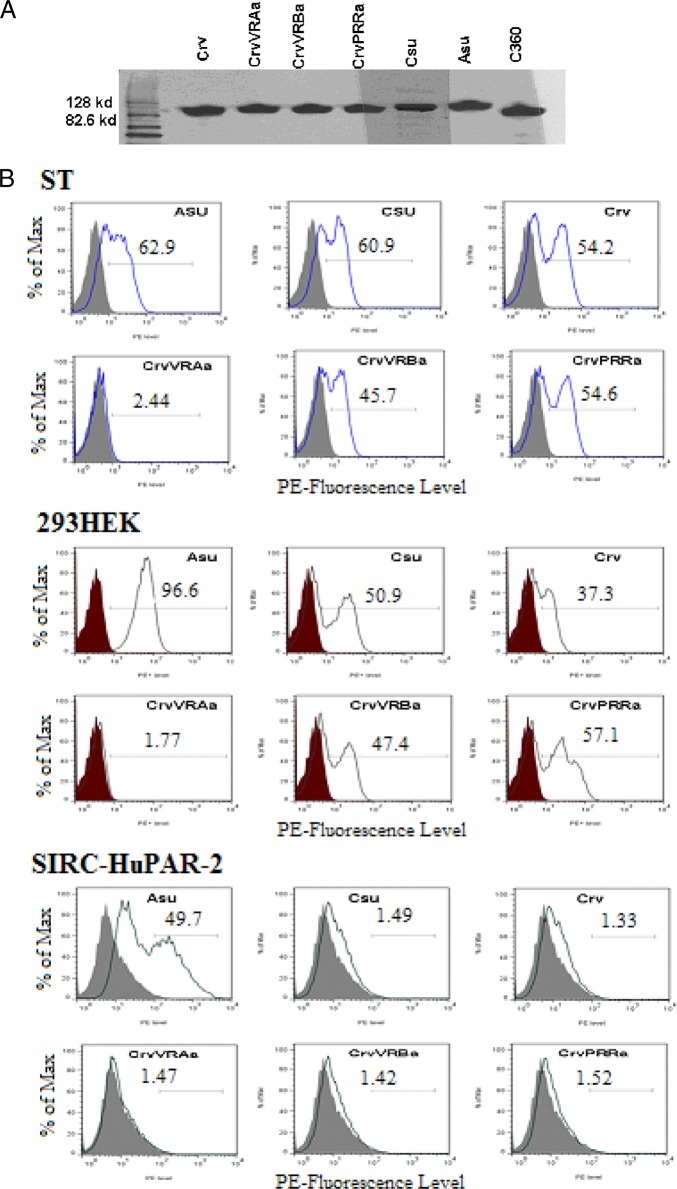
The plasmid vector pSK100, expressing the rabbit immunoglobulin (rIgG) heavy chain, as described previously (29), and the modified PERV envelope-expressing plasmid vectors were cleaved with SpeI and NotI enzymes to recover the plasmid DNA of rIgG and remove the transmembrane (TM) domain of the envelope gene, respectively. In order to clone the pCIneo vector expressing fusion protein, the vector, SU, and rIgG DNA sequences were ligated using the Rapid DNA ligation kit as specified by the manufacturer (Roche Diagnostics, Indianapolis, IN). The fusion protein was prepared, and expression was verified by Western blotting, as described previously (3). An anti-rabbit IgG enzyme-linked immunosorbent assay (ELISA) kit (Alpha Diagnostic International, San Antonio, TX) was used to standardize the amount of fusion protein used in subsequent assays, and the binding activity was measured by fluorescence-activated cell sorter (FACS) analysis as specified previously (7). Briefly, 1 million cells of the 293HEK, ST, and SIRC-HuPAR-2 lines were fixed separately in 4% formaldehyde, washed, and incubated with 500 ng of the indicated SU-IgG fusion protein for 1 h. Then the binding activity was determined by FACS analysis after the cells were washed and stained with R-phycoerythrin (PE)-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Controls for the binding assay, the A-460 and C-360 SU-IgG fusion proteins of PERV-A SU and PERV-C SU, respectively, were previously described (7). Supernatants collected from mock-transfected control 293T cells were processed in the same manner as the supernatants from transfected cells expressing the SU-rIG fusion proteins.
RESULTS
Retroviral vectors carrying chimeric PERV-C envelope with PERV-A substitutions have impaired infectivity and no detectable infection of human cells.
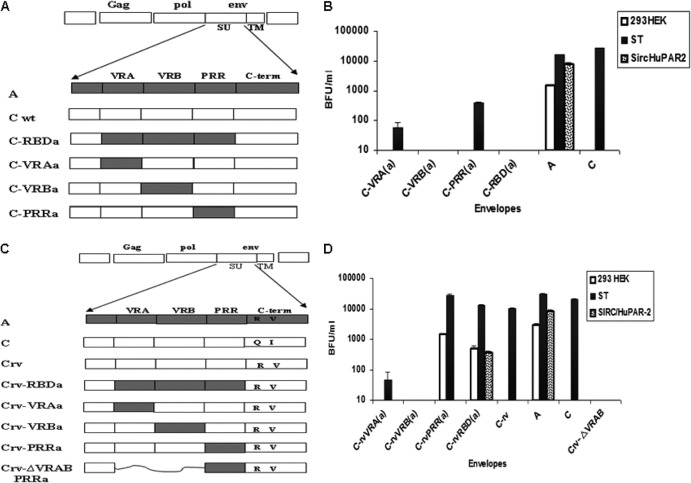
In order to evaluate the contribution of the SU variable regions in human cell infection, we first constructed chimeric PERV-C envelopes in which each of the three structural domains within the PERV-A SU, namely, variable region A (VRA), variable region B (VRB), or the proline-rich region (PRR), was substituted for the homologous region of PERV-C (schematically shown in Fig. 1A). Retroviral vectors carrying each of the chimeric envelopes were evaluated for infectivity as described in Materials and Methods, using three cell lines—human 293HEK, porcine ST, and rabbit SIRC cells expressing the human PERV-A receptor 2 (SIRC-HuPAR-2). All three cell lines were susceptible to infection by vectors carrying the control wild-type PERV-A envelope. As expected, vectors carrying the wild-type PERV-C envelope had detectable infectivity titers on pig ST cells only. None of the vectors carrying the chimeric PERV-C envelopes showed infection of human cells. In contrast, vectors carrying the PERV-C chimeric envelopes, including the VRA or PRR from human-tropic PERV-A showed infection of pig cells, albeit with reduced titers of 100-fold or 10-fold, respectively, compared to control PERV-C envelope-carrying vectors (Fig. 1B). However, no infection was detected in any of the cell lines exposed to vectors carrying either the chimeric envelope PERV-C-VRBa or PERV-C-RBDa. These results indicate that the PERV-A RBD is not sufficient to allow for human cell infection and that other regions of the envelope are required.
Fig 1.
Schematic and infectivity titers of retroviral vector pseudotypes carrying the PERV-A, PERV-C, or chimeric PERV envelope constructs. (A) regions derived from PERV-A env, shown in gray; Cwt, regions derived from PERV-C env, shown in white; the letter “a” (in “RBDa,” “VRAa,” etc.) denotes that the structural domain is derived from PERV-A envelope. (B) Infectivity titers of retroviral vector pseudotypes carrying chimeric PERV-C envelopes as shown in panel A. The titer is shown as blue-forming units (BFU)/ml of supernatant. White bars, human 293HEK cells; black bars, pig ST cells; patterned bars, rabbit SIRC-HuPAR-2 cells stably expressing PERV-A human cell receptor 2 (HuPAR-2). Panels C and D show schematic and infectivity titers of retroviral vector pseudotypes carrying the PERV-A, PERV-C, and chimeric PERV envelope constructs based on PERV-Crv. (C) Regions derived from PERV-A env are shown in gray; Crv, regions derived from PERV-C env with 2 residues, Q374 and I412, mutated to PERV-A sequences R395 and V433, respectively, within the C-terminal region of SU (Q374R and I412V), shown in white. The letter “a” denotes the structural domain is derived from PERV-A envelope, with substitution of corresponding sequences from PERV-A RBD, VRA, VRB or PRR of the SU. “ΔVRAB PRRa” denotes the deletion of VRA and VRB and substitution of PRR by PERV-A sequence. Panel D depicts infectivity titers of retroviral vector pseudotypes carrying chimeric PERV-C envelopes, as shown in panel C. Infectivity titers are shown as BFU/ml of supernatant. White bars, human 293HEK cells; black bars, pig ST cells; patterned bars, rabbit SIRC-HuPAR-2 cells stably expressing PERV-A human cell receptor 2 (HuPAR-2).
Two residues in the C terminus of PERV SU functionally complement the PERV-A PRR to confer human cell tropism to PERV-C mutant envelopes.
The lack of human cell infection by vectors pseudotyped with wild-type PERV-C envelope chimeras carrying VRA, VRB, or PRR of the human-tropic PERV-A led us to hypothesize that a mutant of PERV-C carrying 2 amino acid residues from PERV-A, Q374R, and I412V, termed “PERV-Crv,” might restore the infectivity of the chimeric envelopes. The basis for this hypothesis was our previous study, which showed that a chimeric PERV-C that carried the PERV-A RBD in addition to these 2 amino acid residues had infectivity titers on human cells comparable to those of PERV-A, while the analogous chimera without the two amino acid substitutions had titers 2 logs lower on human cells (3). To test our hypothesis, a second series of chimeric envelopes were derived using PERV-Crv as a backbone (Fig. 1C). As a control, we evaluated the infectivity of retroviral vector pseudotypes carrying these chimeric mutant PERV-C envelopes on pig ST cells (Fig. 1D). Titers comparable to those of PERV-A and PERV-C pseudotyped vectors were observed with vectors carrying PERV-CrvPRRa or PERV-CrvRBDa envelopes, while vectors carrying C-rvVRAa or C-rvVRBa envelopes had significantly reduced titers on ST cells (greater than 2 logs lower and no detectable infection, respectively) (Fig. 1D). Infection of human 293HEK cells was only observed when vectors carried control PERV-A, PERV-CrvRBDa, or PERV-CrvPRRa envelopes. To determine whether these vectors infect human 293HEK cells using the human PERV-A receptor, HuPAR-2, we also exposed rabbit SIRC cells expressing HuPAR-2 to vectors carrying the chimeric PERV-C envelopes. Only vectors carrying control PERV-A envelopes or the PERV-CrvRBDa were able to infect SIRC-HuPAR-2 cells (Fig. 1D). Notably, no detectable infection was observed on SIRC cells expressing the type 1 human PERVA receptor, HuPAR-1, with vectors carrying PERV-CrvPRRa (data not shown).
The surprising result that inclusion of PERV-A sequences encoding the PRR but not VRA or VRB altered the tropism of PERV-Crv prompted us to derive a chimeric PERV-Crv envelope without either VRA or VRB that retained the PRR from PERV-A, PERV-CrvΔVRAB-PRRa (Fig. 1C). However, vectors carrying PERV-CrvΔVRAB-PRRa were not infectious on any of the three cell lines tested (ST, 293HEK, or SIRC-HuPAR-2), demonstrating that these domains are required to at least maintain structural integrity of the envelope in order to remain infectious (Fig. 1D).
Binding of chimeric and mutant PERV-C SU fusion proteins to human cells is not via HuPAR-2.
Since the chimeric PERV-Crv envelopes and their mutants showed variable levels of infectivity, depending on cell type, we evaluated whether this variation corresponds with cell binding activity. As described in Materials and Methods, we derived SU-IgG fusion proteins corresponding to the panel of PERV-Crv chimeras that were evaluated for infectivity, as well as PERV-A and PERV-C (C360) SU-IgG as controls. The Western blot analysis shown in Fig. 2A demonstrates that all SU-IgG proteins were expressed and are of the expected size. Flow cytometry was used to detect SU-IgG binding to human 293HEK, pig ST, or rabbit SIRC-HuPAR-2 cells, as described in Materials and Methods (Fig. 2B). There was no detectable binding to SIRC-HuPAR-2 by PERV-C, PERV-Crv, and the three chimeric PERV-Crv SU-IgG proteins (Fig. 2B), correlating with the lack of infectivity observed on these cells by vectors carrying these envelopes (Fig. 1D). In contrast, all of the PERV-C and PERV-Crv derivative SU-IgG proteins tested, with the exception of PERV-CrvVRa, were able to bind both pig ST and human 293HEK cells (Fig. 2B). Interestingly, even PERV-CrvVRBa had comparable binding on these cells (Fig. 2B) in spite of showing no detectable infectivity (Fig. 1D), suggesting that other reasons may account for the lack of infectivity, such as poor incorporation of the envelope into virions.
Fig 2.
Detection of chimeric PERV envelope-derived SU-rIgG fusion proteins and their binding to pig ST, human 293HEK, and rabbit SIRC-HuPAR-2 PERV-A receptor-expressing cells. (A) Western blot analysis of the SU-rabbit IgG fusion proteins used in the binding assay, as described in Materials and Methods. The last lane (C360) contains a previously described SU-IgG fusion protein serving as a control (7), which is comprised of the N-terminal 360 aa from PERV-C envelope. (B) One million cells of each type were incubated with 500 ng of soluble SU-rabbit IgG fusion proteins, and binding was detected using PE-conjugated anti-rabbit IgG by flow cytometry. Unlike the ST and 293HEK cells, SIRC-HuPAR-2 cells overexpressing PERV-A receptor did not allow binding to the human cell-infecting PERV-C mutant PERV-CrvPRRa. Supernatants collected from normally growing 293HEK cells were treated by the same processes as the fusion proteins and used as a negative control (Neg). Csu, PERV-C envelope SU; Asu, PERV-A envelope SU.
PERV-A-infected human cells do not block superinfection of chimeric PERV-C envelope mutant, PERV-CrvPRRa.
To test whether PERV-CrvPRRa pseudotypes infect human cells using the same receptor(s) as PERV-A, human 293HEK and pig ST cells were chronically infected with PERV-A. As a control, we also used ST cells chronically infected with PERV-C. Corresponding uninfected and chronically infected cells were exposed to pseudotypes carrying the PERV-A or -C envelopes or derivative mutant chimeric envelopes, as indicated in Table 1. Unlike the control PERV-A pseudotyped vector, vectors carrying the PERV-CrvPRRa-infected 293HEK cells chronically infected with PERV-A at a titer comparable to that of uninfected 293HEK cells, suggesting entry must be via a receptor other than HuPAR-1 or −2. In contrast, the titer of PERV-CrvPRRa is reduced by greater than 2 logs in pig cells chronically infected with PERV-C compared to matched, uninfected ST cells (Table 1). Together, these data suggest that the PERV-CrvPRRa virus is using a receptor distinct from the PERV-A receptors to enter human cells. Likewise, productive infection of ST cells with PERV-C did not interfere with infection by the vectors carrying PERV-CrvRBDa (Table 1), confirming that this env does not use the PERV-C receptor to enter pig cells. Conversely, PERV-A did block infection of PERV-CrvRBDa vectors (Table 1), extending prior infectivity results of SIRC-HuPAR-2 (Fig. 1D) to show that this envelope uses the PERV-A receptor to infect human cells.
Table 1.
Receptor interference of retroviruses with pseudotypes carrying wild-type, chimeric, or mutated envelopes
| Superinfecting wild type or mutanta | Ratio of titer onb: |
||
|---|---|---|---|
| PERV-A-infected/uninfected 293 or ST cells |
PERV-C-infected/uninfected ST cells | ||
| 293 | ST | ||
| PERV-A | 0.037 | 0.007 | 1.31 ± 0.29 |
| PERV-Cwt | No infection | 0.89 ± 0.203 | 0.002 |
| PERV-C-rvPRRa | 0.92 ± 0.16 | 0.88 ± 0.105 | 0.002 |
| PERV-C-rvRBDac | 0.008 | 0.003 | 1.2 ± 0.19 |
The wild type or mutants were tested as retroviral vector pseudotypes carrying the indicated envelope. A schematic depiction of the indicated envelope is shown in Fig. 1C.
The data shown represent the mean ratio ± standard deviation from duplicate samples tested under each condition.
RBD, receptor binding domain including VRA, VRB, and PRR.
Vectors carrying PERV-Crv with a single amino acid change in PRR infect human cells, but not via the PERV-A receptors.
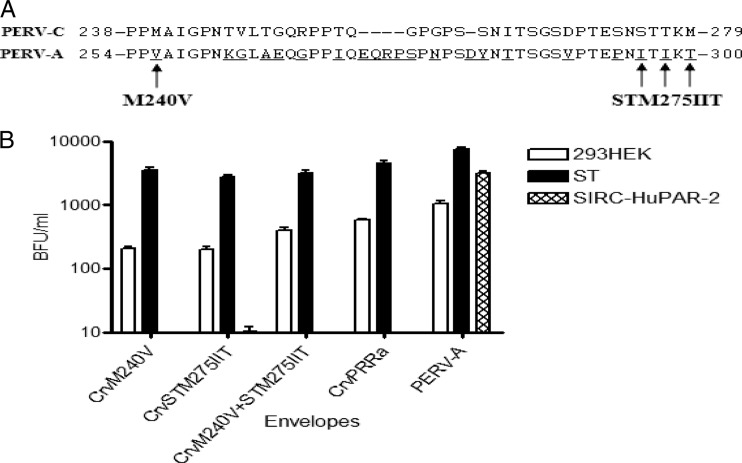
The PRR of PERV-A differs from the corresponding domain of PERV-C by 21 amino acid residues compared to PERV-C, including an insertion of 5 residues not found in PERV-C (Fig. 3A). In order to identify critical residues within the PERV-A PRR that correlate with human cell infection, we used site-directed mutagenesis to make amino acid substitutions within the PRR of the PERV-Crv envelope. For simplicity, we initiated our mutagenesis by first changing residues at either end of the PRR, namely, introducing a valine for methionine at position 240 (M240V) or replacing the serine, threonine, and methionine residues initiating at position 275 for the corresponding sequences of isoleucine, isoleucine, and threonine (STM275IIT). In addition, we created a mutant envelope that would carry mutations encoding all four amino acid residues, M240V+STM275IIT. As shown in Fig. 3B, vectors carrying PERV-Crv envelope with substitution of M240V or STM275IIT restored infectivity of 293HEK cells to approximately 30% that observed for PERV-CrvPRRa (titer of ∼200 versus ∼600) (Fig. 3B). In contrast, the combination of all four amino acid substitutions resulted in titers comparable to PERV-CrvPRRa on human cells (∼400 versus ∼600) (Fig. 3B). In addition, these mutants were assessed for receptor interference. Similar to vectors carrying wild-type PERV-C, the titers for the vectors carrying the mutant PERV-C envelopes were decreased by 2 to 3 logs in porcine ST cells productively infected with PERV-C, whereas in human 293HEK cells productively infected with PERV-A, the titers were not reduced by more than 30% (Table 2). Together, these data suggest that the mutants follow the phenotype of the PERV-CrvPRRa parent and use a receptor on human cells that is different from HuPAR2.
Fig 3.
Mutations of certain residues encoding amino acids within the PRR allow infection of human cells. (A) Sequence alignment of deduced amino acid residues in the PRR of PERV-C (amino acids 238 to 279) and PERV-A (amino acids 254 to 300) (27). The underlined letters denote the sequence differences, and gaps are denoted by dashes. Arrows show sites of mutated residues for M240V or STM275IIT. (B) Amino acid changes in the PRR and the C terminus of the SU impacted the infectivity of human cells by PERV-C. When the whole PRR of PERV-C was replaced by the corresponding PERV-A sequences resulting in the human cells' infectivity, then an infectivity study was conducted to gain insight into whether single amino acid changes at the PRR may impact the infectivity of human cells by PERV-C. The data shown represent the infectivities of the human 293HEK, pig ST, and rabbit SIRC cells expressing the PERV-A receptor by the vectors pseudotyped with PERV-Crv mutant envelopes. The PERV-C env mutant having 2 amino acid changes in the C terminus of the SU (PERV-Crv) was used as a backbone to introduce the amino acid changes in the PRR, as shown in panel A.
Table 2.
Infectivity and receptor interference of retroviral vectors carrying wild-type PERV or chimeric, mutant PERV-Crv envelopes
| Superinfecting wild type or mutanta | Ratio of titer onb: |
|
|---|---|---|
| PERV-A-infected/uninfected 293 cells | PERV-C infected/uninfected ST cells | |
| PERV-A | 0.002 ± 0.01 | 1.1 ± 0.045 |
| PERV-Cwt | No infection | 0.008 ± 0.001 |
| PERV-Crv-M240V | 0.67 ± 0.03 | 0.107 ± 0.023 |
| PERV-Crv-STM275IIT | 0.7 ± 0.05 | 0.07 ± 0.01 |
| PERV-Crv-M240V+STM275IIT | 0.73 ± 0.06 | 0.04 ± 0.007 |
Wild-type or mutant envelopes were tested as retroviral vector pseudotypes carrying the indicated envelope. Schematic depictions of the indicated envelope or mutants are shown in Fig. 1C or Fig. 3, respectively.
The data shown represent the mean ratio ± standard deviation from duplicate samples tested under each condition.
PERV-CrvPRRa infects primary HUVECs.
To determine whether the observed changes in species specificity of the PERV-CrvPRRa were relevant to human cell infection in vivo, we evaluated infection of vector pseudotypes on primary human umbilical vein endothelial cells (HUVECs). We chose these cells because endothelial cells are likely to be exposed to PERV in the context of clinical xenotransplantation. With infectivity titers comparable to those observed on human 293HEK cells, vectors carrying the PERV-Crv envelope with the PRR sequence from PERV-A infect primary human cells, HUVECs (Fig. 4).
Fig 4.
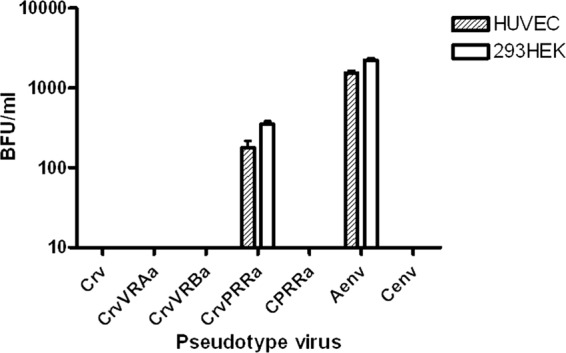
Infectivity titer of retroviral vector pseudotypes carrying PERV-A, PERV-C, or chimeric mutant PERV-C envelopes on human umbilical vein endothelial cells (HUVECs). Schematics of the envelopes used are shown in Fig. 1C. The infectivity titers are shown as mean blue-forming units (BFU)/ml of supernatant from triplicate samples. White bars, HUVECs; gray bars, 293HEK cells.
DISCUSSION
We show in the present study that the combination of residues R395 and V433 with the PRR of the PERV-A envelope confers human cell infectivity to vectors carrying the non-human-tropic PERV-C envelope. In addition, we show that human cell infection by this chimeric PERV occurs via a receptor that differs from either form of HuPAR used for PERV-A infection.
In order to identify the critical structural domains of the PERV RBD needed for human cell tropism, we substituted individually or together the VRA, VRB, or PRR from the human-tropic PERV-A SU for the homologous regions of the non-human-tropic PERV-C (Fig. 1A and C). The infectivity experiments of the pseudotyped vectors carrying the chimeric envelopes bearing these substitutions showed that when the backbone for the chimeric envelopes was wild-type PERV-C, none of the chimeric envelopes confers human cell tropism (Fig. 1B). In contrast, the data showed that among the different structural units of PERV SU, the substitution of the PRR is the only one that altered the host range of PERV-C (PERV-CrvPRRa) (Fig. 1D), suggesting that the sequences within the RBD that are critical for human cell infection are PRR, not VRA or VRB. Interestingly, vectors carrying envelopes with the VRA and VRB deleted were completely noninfectious on all cell lines tested. These observations could be due to poor incorporation or no incorporation into virions or lack of binding, but in either case, the results suggest that the VRA and VRB must be required to maintain the overall structural integrity of the envelope, to drive appropriate virus envelope function for infectivity.
To support the infectivity data and show the role the SU subdomains, VRA, VRB, and PRR play in receptor binding, we also prepared fusion proteins of the chimeric SUs and studied their binding activity in human cells by FACS analysis (Fig. 2A and B). Notably, of the envelopes tested, only the fusion protein including the PERV-A VRA lacks detectable binding activity on 293HEK human and ST pig cells, while the other envelopes tested demonstrated considerable binding to various degrees (Fig. 2B). Surprisingly, CrvVRBa showed binding on 293HEK and ST pig cells in spite of there being no detectable infection of any cell lines tested. This finding suggests that this form of the envelope is binding competent while not being infection competent, perhaps due to inability to undergo conformational changes associated with the second stages of viral entry. It is reminiscent of our earlier findings that PERV-C SU-Ig could bind human cells, but retroviral vectors carrying PERV-C envelopes were not infectious for human cells (7). Other results demonstrated that the binding activity did not strictly correlate with infectivity data. For example, PERV-CrvPRRa had infectivity titers comparable to those of PERV-A on 293HEK cells (Fig. 1D), while the binding was approximately 10-fold lower than PERV-A SU-IgG on the same cells. Thus, while the binding assay is informative to provide a “yes/no” result, it does not provide a quantitative prediction of infectivity. The lack of quantitative correlation between detectable binding and infectivity titers may be due to differences in binding affinity between monomeric SU-IgG versus the trimeric form of envelope found in multivalent viral particles or perhaps differences in efficiencies with regard to postbinding conformational changes that impact the overall infectivity titer. Further investigation is needed to identify the reason for these differences.
We also have investigated the impact of individual amino acid changes within the PRR in modifying the tropism of PERV-Crv. To our surprise, the first set of mutations we tested, either a single amino acid or 3 amino acids in the N terminus and C terminus of PRR, respectively, individually or together, were sufficient to allow PERV-Crv mutants to infect human cells (Fig. 3B). Therefore, we did not proceed with mutations of the additional residues that distinguish PERV-A and PERV-C in the PRR to determine if additional changes impact human cell infectivity (Fig. 3A).
We now have three lines of evidence to demonstrate that exchange of the PRR between PERV-C and PERV-A allows infection of human cells via a receptor that is distinct from the PERV-A receptors: (i) vectors carrying PERV-CrvPRRa do not infect SIRC cells expressing HuPAR-1 (data not shown) or HuPAR-2 (Fig. 1D), (ii) PERV-CrvPRRa SU-IgG binds human cells but not SIRC-HuPAR-2 (Fig. 2B), and (iii) PERV-C interferes with PERV-CrvPRRa vector infection of ST cells, but PERV-A does not block infection of PERV-CrvPRRa vectors in human cells (Table 1). We hypothesize that the role of the PRR is not directly related to receptor binding per se, as we had previously shown that the PERV-C SU-IgG is able to bind human cells (7) in the absence of being able to infect human cells. Furthermore, our prior results using a chimeric mutant envelope carrying the entire RBD of PERV-A in a backbone of PERV-C carrying mutations of the two residues Q374R and I412V clearly directs entry of vectors into human cells via the PERV-A receptor (3). Together our findings support a role for the PRR in postbinding steps, such as conformational changes and membrane fusion. We propose a model whereby the PERV-A PRR in combination with residues R395 and V433 in the context of the PERV-C envelope unlocks the envelope conformation after binding to the receptor to allow these postbinding steps to occur that are blocked when the wild-type PERV-C envelope binds the human form of the PERV-C receptor (3, 7) (Fig. 5). Interestingly, these observations mirror those previously reported for chimeric envelopes between Moloney and amphotropic murine leukemia viruses (MoMLV and A-MLV, respectively), whereby introduction of the PRR and the C terminus of the MoMLV SU into the A-MLV envelope resulted in altered receptor specificity as measured by lack of interference by A-MLV, even though the A-MLV RBD was intact on the envelope (11). Furthermore, Lavillette et al. demonstrated that the PRR sequences impact relative rates of fusogenicity of the envelopes and conclude that their results suggest a role for the PRR in facilitating the conformational change of the envelope for cell-to-cell or virus-to-cell fusion, while providing stability to the SU-TM association (11). Wu et al. developed a series of truncations of the MLV PRR and showed that the PRR impacts SU and TM incorporation as well as envelope processing. These findings further suggest a role in stabilizing the overall structure. In addition, Wu et al. demonstrated an impact on the fusogenic properties of the envelope, as certain deletions resulted in virus that was as infectious as virus carrying WT envelopes, although syncytium formation was severely reduced (28).
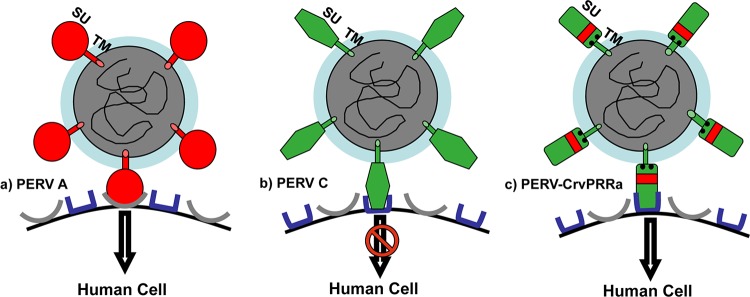
Fig 5.
Model of PERV-C and human cell infection. (a) The schematic depicts the retroviral vector carrying PERV-A envelope that is able to both bind and infect human cells expressing known human PERV-A receptors (HuPAR-1 or HuPAR-2). (b) The schematic depicts the retroviral vector carrying PERV-C envelope that is able to bind human cells via a receptor distinct from those of HuPAR-1 or HuPAR-2 but is not able to infect after binding. (c) The schematic depicts that when the retroviral vector carries a modified envelope, PERV-CrvPRRa, the binding is different, allowing viral entry to lead to infection.
In line with our results that the PRR is a critical determinant of human cell infection, a naturally occurring recombinant of PERV-A and PERV-C (strain 14/220) that was reported to have higher infectivity titers on human cells than other PERV-A isolates was shown to contain differences within the PRR that appeared to be important factors that determine the high-titer characteristic of the virus (9). Interestingly, the 14/220 strain of PERV-A has a PRR that is identical to PERV-C. In the case of a PERV-C backbone, with or without the additional two mutations in the C terminus Q374R and I412V, the presence of the PERV-C PRR does not confer human cell infection. However, introduction of the PERV-A PRR into the PERV-C backbone is sufficient to allow human cell infection, so long as the compensating Q374R and I412V mutations are also included. Similarly, the impact of the 14/220 PRR on human cell infectivity titers was greater in the context of the PERV-A envelopes with a compensating mutation in VRA, I140V (9). Together, these findings suggest that the PRR in the PERV envelope may provide a different function than in other gammaretroviruses, where it has been described as a “hinge” providing flexibility but not receptor specificity. Of note, insertion of tags tolerated in other gammaretroviruses into the PERV PRR renders vectors carrying the tagged envelopes noninfectious (C. Wilson, data not shown), further supporting the distinct structural impact of the PRR on envelope function. Recently, it was reported that a neutralizing antibody to PERV-B recognizes a peptide within the PRR (15), suggesting that the region is exposed to antibodies. Interestingly, the particular epitope identified is absent from both PERV-A and PERV-C (15).
Additionally, it is noteworthy that the 2 residues in the C-terminal region of SU play an indispensable role in human cell infectivity of the PERV-CrvPRRa, indicating cooperation of the C-terminal region of the SU with PRR to facilitate entry of the virus. While these two amino acids are necessary, they are not sufficient to allow human cell infection (Fig. 1D) (3). Several studies have demonstrated that the C-terminal region of gammaretroviral SU plays a role in receptor binding and viral entry. For instance, studies with chimeric feline leukemia virus (FeLV) envelopes have shown that sequences outside the RBD, near the C terminus of SU, expand the receptor specificity to permit use of human Pit2, in addition to Pit1 (22). A separate study showed that a subgroup of feline leukemia virus, FeLV-C, carries a second domain in the C-terminal region of the SU that is distinct from the N-terminal RBD and can independently bind to its host receptor, FLVCR1, in the absence of the N-terminal RBD (21).
In summary, our data demonstrate that sequences within the C terminus and the PRR together determine the host receptor interaction and tropism of PERV. This finding suggests that the PRR may provide a promising molecular target for antiviral strategies (e.g., the development of antiviral peptides or antibodies). Use of sequences that are conserved between PERV-A and PERV-C in this domain may allow for prevention of transmission of the naturally occurring high-titer recombinant viruses between PERV-A and PERV-C (9). Our results warrant further studies, such as characterization of the crystal structure of the PERV envelope and the search for a receptor for the PERV-C envelope in human cells.
ACKNOWLEDGMENTS
We are grateful for the technical assistance provided by Montgomery County, Maryland, Public Schools student interns Sheraddha Sheth and Evelina Cebatori and teacher-intern May Shlash and to Howard Hughes Medical Institute for supporting these internships. We thank Winston Colon-Moran for laboratory support and Clive Patience for providing us reagents. We are indebted to Maribeth Eiden and Jon Marsh for many critical discussions and Carol Weiss and Daniel Takefman for careful review of the manuscript.
Footnotes
Published ahead of print 13 June 2012
REFERENCES
- 1. Akiyoshi DE, et al. 1998. Identification of a full-length cDNA for an endogenous retrovirus of miniature swine. J. Virol. 72:4503–4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albritton LM, Kim JW, Tseng L, Cunninghan JM. 1993. Envelope-binding domain in the cationic amino acid transporter determines the host range of ecotropic murine retroviruses. J. Virol. 67:2091–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Argaw T, Figueroa M, Salomon DR, Wilson CA. 2008. Identification of residues outside of the receptor binding domain that influence the infectivity and tropism of porcine endogenous retrovirus. J. Virol. 82:7483–7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bae Y, Kingsman SM, Kingsman AJ. 1997. Functional dissection of the Moloney murine leukemia virus envelope protein gp70. J. Virol. 71:2092–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Battini J-L, Heard JM, Danos O. 1992. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J. Virol. 66:1468–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ericsson TA, et al. 2003. Identification of receptors for pig endogenous retrovirus. Proc. Natl. Acad. Sci. U. S. A. 100:6759–6764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gemeniano M, Mpanju O, Salomon DR, Eiden MV, Wilson CA. 2006. The infectivity and host range of the ecotropic porcine endogenous retrovirus, PERV-C, is modulated by residues in the C-terminal region of its surface envelope protein. Virology 346:108–117 [DOI] [PubMed] [Google Scholar]
- 8. Gray KD, Roth MJ. 1993. Mutational analysis of the envelope gene of Moloney murine leukemia virus. J. Virol. 67:3489–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harrison I, Takeuchi Y, Bartosch B, Stoye JP. 2004. Determinants of high titer in recombinant porcine endogenous retroviruses. J. Virol. 78:13871–13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heard JM, Danos O. 1991. An amino-terminal fragment of the Friend murine leukemia virus envelope glycoprotein binds the ecotropic receptor. J. Virol. 65:4026–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lavillette D, et al. 1998. A proline-rich motif downstream of the receptor binding domain modulates conformation and fusogenicity of murine retroviral envelopes. J. Virol. 72:9955–9965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le Tissier P, Stoye JP, Takeuchi Y, Patience C, Weiss RA. 1997. Two sets of human-tropic pig retroviruses. Nature 389:681–682 [DOI] [PubMed] [Google Scholar]
- 13. Lu CW, Roth MJ. 2001. Functional characterization of the N termini of murine leukemia virus envelope proteins. J. Virol. 75:4357–4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morgan RA, et al. 1993. Analysis of the functional and host range-determining regions of the murine ecotropic and amphotropic retrovirus envelope proteins. J. Virol. 67:4712–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakaya Y, Hoshino S, Yasuda J, Miyazawa T. 2011. Mapping of a neutralizing epitope in the surface envelope protein of porcine endogenous retrovirus subgroup B. J. Gen. Virol. 92:940–944 [DOI] [PubMed] [Google Scholar]
- 16. Niebert M, Tonjes RR. 2005. Evolutionary spread and recombination of porcine endogenous retroviruses in the Suiformes. J. Virol. 79:649–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ott D, Friedrich R, Rein A. 1990. Sequence analysis of amphotropic and 10A1 murine leukemia viruses: close relationship to mink cell focus-inducing viruses. J. Virol. 64:757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patience C, et al. 2001. Multiple groups of novel retroviral genomes in pigs and related species. J. Virol. 75:2771–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pinter A, Fleissner E. 1977. The presence of disulfide-linked gp70-pl5(E) complexes in AKR murine leukemia virus. Virology 83:417–422 [DOI] [PubMed] [Google Scholar]
- 20. Ragheb JA, Anderson WF. 1994. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry. J. Virol. 68:3220–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rey MA, Prasad R, Tailor CS. 2008. The C domain in the surface envelope glycoprotein of subgroup C feline leukemia virus is a second receptor-binding domain. Virology 370:273–284 [DOI] [PubMed] [Google Scholar]
- 22. Sugai J, et al. 2001. Identification of envelope determinants of feline leukemia virus subgroup B that permit infection and gene transfer to cells expressing human Pit1 or Pit2. J. Virol. 75:6841–6849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tailor C, Kabat D. 1997. Variable regions A and B in the envelope glycoproteins of feline leukemia virus subgroup B and amphotropic murine leukemia virus interact with discrete receptor domains. J. Virol. 71:9383–9391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takefman DM, Wong S, Maudru T, Peden K, Wilson CA. 2001. Detection and characterization of porcine endogenous retrovirus in porcine plasma and porcine factor VIII. J. Virol. 75:4551–4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tonjes RR, Niebert M. 2003. Relative age of proviral porcine endogenous retrovirus sequences in Sus scrofa based on the molecular clock hypothesis. J. Virol. 77:12363–12368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilson C, Eiden M. 1991. Viral and cellular factors governing hamster cell infection by murine and gibbon ape leukemia viruses. J. Virol. 65:5975–5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson CA, Wong S, VanBrocklin M, Federspiel MJ. 2000. Extended analysis of the in vitro tropism of porcine endogenous retrovirus. J. Virol. 74:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu BW, Cannon PM, Gordon EM, Hall FL, Anderson WF. 1998. Characterization of the proline-rich region of murine leukemia virus envelope protein. J. Virol. 72:5383–5391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zingler K, Young JA. 1996. Residue Trp-48 of Tva is critical for viral entry but not for high-affinity binding to the SU glycoprotein of subgroup A avian leukosis and sarcoma viruses. J. Virol. 70:7510–7516 [DOI] [PMC free article] [PubMed] [Google Scholar]