Abstract
The nitrogen-fixing rhizobial symbiont Sinorhizobium meliloti 1021 produces acidic symbiotic exopolysaccharides that enable it to initiate and maintain infection thread formation on host legume plants. The exopolysaccharide that is most efficient in mediating this process is succinoglycan (exopolysaccharide I [EPSI]), a polysaccharide composed of octasaccharide repeating units of 1 galactose and 7 glucose residues, modified with succinyl, acetyl, and pyruvyl substituents. Previous studies had shown that S. meliloti 1021 mutants that produce increased levels of succinoglycan, such as exoR mutants, are defective in symbiosis with host plants, leading to the hypothesis that high levels of succinoglycan production might be detrimental to symbiotic development. This study demonstrates that increased succinoglycan production itself is not detrimental to symbiotic development and, in fact, enhances the symbiotic productivity of S. meliloti 1021 with the host plant Medicago truncatula cv. Jemalong A17. Increased succinoglycan production was engineered by overexpression of the exoY gene, which encodes the enzyme responsible for the first step in succinoglycan biosynthesis. These results suggest that the level of symbiotic exopolysaccharide produced by a rhizobial species is one of the factors involved in optimizing the interaction with plant hosts.
INTRODUCTION
Sinorhizobium meliloti 1021 is a soil bacterium that establishes a nitrogen-fixing symbiosis with the host plants Medicago sativa (alfalfa) and Medicago truncatula cv. Jemalong A17 (reviewed in references 17 and 21). These plants are not only agriculturally important but are also key model organisms for studying the symbiotic interaction between rhizobial bacteria and their legume plant hosts. S. meliloti fixes dinitrogen gas to ammonia in root nodules formed by the host plant. Nodule development requires that several signals be exchanged between the plant and the rhizobial bacteria. Host plant flavonoid compounds signal S. meliloti to produce lipochitooligosaccharides called Nod factors (NFs) (39). NF activates multiple host plant responses, including tight curling of root hairs that traps bacterial cells within the curl and the induction of cell divisions in the root cortex which establish the nodule primordium (14, 36). The bacteria invade and colonize the roots through “infection threads,” which originate from microcolonies of bacteria trapped in the curled root hair cells (21, 48). Infection threads are progressive ingrowths of plant cell membrane that contain a matrix composed of bacterial exopolysaccharides (EPSs) and plant cell wall material (14). Infection thread initiation and development require that S. meliloti propagate in the infection thread and produce both NF and the exopolysaccharide succinoglycan (25). New infection threads initiate at each cell layer, eventually delivering the bacteria to cells of the inner root cortex (48). There, the rhizobial bacteria are endocytosed by host cells within individual compartments of host cell membrane origin (6, 17). Within these compartments, the low-oxygen environment and signals provided by the plant induce the bacteria to differentiate into a form called a “bacteroid” and to begin symbiotic nitrogen fixation (33, 40).
Succinoglycan production by S. meliloti 1021 has been studied extensively (reviewed in reference 21). This exopolysaccharide is required for this strain to induce infection thread development on plant hosts, since it is the only symbiotically active exopolysaccharide that this strain produces in large quantities (18, 28). The reasons that rhizobial exopolysaccharides are required for host plant invasion are not fully understood. S. meliloti 1021 strains that do not produce succinoglycan, such as the exoY mutant, are able to colonize plant surfaces and are trapped within curled root hairs, but they fail to initiate infection thread formation (8). These aborted infections are associated with gene expression responses (22) and cytological responses (34) characteristic of plant defense reactions. Several possible roles for succinoglycan in infection thread initiation and development have been proposed, including modulating factors such as pH, Ca2+ ion concentration, or reactive oxygen species (ROS) that affect root hair elongation and/or plant defense responses (1, 23) by influencing cross-linking of infection thread matrix components and generating turgor pressure against the root hair cell membrane (4) or serving as a signal to the host plant through a plant receptor (37).
Although succinoglycan is required for efficient S. meliloti 1021 invasion of its plant hosts, there has also been speculation that the production of higher than normal amounts of succinoglycan is detrimental in the invasion process (51). This was based on the observation that overproduction of succinoglycan (known as a “calcofluor-bright phenotype”) is found in several mutant strains with symbiotic defects, including strains with mutations of exoR (12), bluB (5), cbrA (16), and relA (52). However, most of these mutations have been found to have pleiotropic effects on S. meliloti, in addition to succinoglycan overproduction (5, 7, 15, 52, 54). Therefore, a specific role of succinoglycan overproduction in the host invasion defects of these strains could not be determined. Other S. meliloti 1021 mutants that produce higher levels of succinoglycan than the reference strain are those that carry mutations in the exoX gene (41, 57). These strains are able to induce the formation of nitrogen-fixing nodules on the host plant alfalfa (41, 57). However, ExoX appears to exert its inhibitory effect on succinoglycan biosynthesis at the posttranslational level (41), and it is unknown whether mutations in exoX have other effects on the interactions of S. meliloti with host plants.
The goal of this study was to determine whether increased succinoglycan production by S. meliloti 1021 has a positive or negative effect on its symbiotic proficiency on host plants. Increased succinoglycan production was achieved by overexpression in the S. meliloti 1021 reference strain of the exoY gene in the absence of other mutations that might have pleiotropic effects. The exoY gene encodes an undecaprenyl-phosphate galactose phosphotransferase that performs the first step in succinoglycan biosynthesis (43). The expression of exoY under the control of the constitutive Smb21651 promoter has been shown to be sufficient to restore succinoglycan production in an exoY mutant, but the amount of succinoglycan produced by these strains was not quantified (58). In this study, S. meliloti 1021 strains that express exoY under the control of this promoter have been constructed. We find that succinoglycan levels are elevated in these strains and that the symbiotic productivity of the host plant M. truncatula A17 inoculated with these strains is enhanced.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. meliloti 1021 strains (Table 1) were grown at 30°C in the following media: LBMC (Luria-Bertani Miller medium supplemented with 2.5 mM MgSO4 and 2.5 mM CaCl2); modified M9 salts supplemented with 1 μg/ml biotin (29) and either 0.12 mM sucrose and 9.35 mM NH4Cl (M9) or 12 mM mannitol, 5 mM glutamic acid, and sodium salt (M9GM); GMS (glutamate mannitol salts) medium (55, 56) containing 27.5 mM mannitol, 0.01 μg/ml biotin, and 0.1 μg/ml thiamine; and Jensen's plant growth medium (29, 49) supplemented with 5 mM glutamic acid sodium salt, 27.5 mM mannitol, and 1 μg/ml biotin (Jensen's GM). (The compositions of M9, M9GM, GMS, and Jensen's GM media are detailed in Table S1 in the supplemental material.) Bacterial plates contained 1.5% Bacto agar (BD, Franklin Lakes, NJ). Calcofluor polysaccharide indicator plates contained 0.02% calcofluor white M2R (fluorescent brightener 28; Sigma, St. Louis, MO) (29). S. meliloti 1021 was grown with 500 μg/ml streptomycin, and S. meliloti strains carrying pLAFR5-based plasmids were grown with 10 μg/ml tetracycline. S. meliloti strains carrying transposon Tn5 were grown with neomycin at 200 μg/ml. Sinorhizobium medicae WSM419 and ABS7 were grown on LBMC or LBMC calcofluor plates. Escherichia coli strains were grown at 37°C in LB medium (44), with appropriate antibiotics used at the following concentrations: 50 μg/ml kanamycin, 10 μg/ml chloramphenicol, 10 μg/ml tetracycline.
Table 1.
Strains, plasmids, and primers
| Strain, plasmid, or primer | Description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli DH5α | F− ϕ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 (rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1 | Life Technologies |
| E. coli MM294A | pro-82 thi-1 endA hsdR17 supE44 | 2 |
| E. coli MT616 | MM294A recA56 carrying plasmid pRK600, Cmr | 13 |
| S. meliloti 1021 | SU47 Smr | 31 |
| 1021/pstb-LAFR5.1a | 1021 carrying plasmid pstb-LAFR5, isolate from conjugation 1, Smr Tcr | This study |
| 1021/pstb-LAFR5.2s | 1021 carrying plasmid pstb-LAFR5, isolate from conjugation 2, Smr Tcr | This study |
| 1021/pstb-LAFR5-exoY.1a | 1021 carrying plasmid pstb-LAFR5-exoY from conjugation 1, Smr Tcr | This study |
| 1021/pstb-LAFR5-exoY.2s | 1021 carrying plasmid pstb-LAFR5-exoY from conjugation 2, Smr Tcr | This study |
| 1021/pstb-LAFR5-exoY.1.3 | 1021 carrying plasmid pstb-LAFR5-exoY from conjugation 3, Smr Tcr | This study |
| S. medicae ABS7 | Rhizobial isolate from saline Algerian soil | 3 |
| S. medicae WSM419 | Rhizobial isolate from Sardinia, Italy | 42 |
| Plasmids | ||
| pLAFR5 | Broad-host-range cosmid vector, Tcr | 24 |
| pstb-LAFR5 | pLAFR5 carrying the SMb21651 promoter fragment, cloned into HindIII/PstI the restriction sites, Tcr | This study |
| pstb-LAFR5-exoY | pstb-LAFR5 carrying the exoY ORF fragment + ribosome binding site, cloned into PstI/BamHI restriction sites, Tcr | This study |
| Primers | ||
| b21651pro.fw.Hind | 5′ GCAAGCTTGGCACGCGTCTGAATGTCCATTGT, SMb21651 promoter forward primer | This study |
| b21651pro.rev.Pst | 5′ GCCTGCAGGTCGATGTCCGTGCCGCAACT, SMb21651 promoter reverse primer | This study |
| Pst.stprbs.exoYfw | 5′ GCCTGCAGTAACGTAACGTAAATGGAGTCACCTCTATGAAGTCCGCGACGCGCTC, exoY forward primer with stop codons, followed by ribosome-binding site | This study |
| Bam.exoY.ORF.rev | 5′ GCGGATCCTCAGTAGCTGCCGCGGGAGAGG, exoY reverse primer | This study |
Construction of the pstb-LAFR5 vector, the exoY overexpression vector pstb-LAFR5-exoY, and S. meliloti 1021 strains carrying these plasmids.
A 725-bp fragment upstream of the putative plasmid stability ORF SMb21651 of S. meliloti 1021 was amplified from the genome using Phusion polymerase (New England BioLabs, Ipswich, MA) and primers from Eurofins MWG Operon (Huntsville, AL). This fragment has been demonstrated to have strong constitutive promoter activity in S. meliloti (58). It was cloned into the HindIII and PstI restriction sites in the broad-host-range plasmid pLAFR5 (24). The resulting plasmid was named pstb-LAFR5 (stb is an abbreviation for “stability protein”). The exoY ORF was amplified from the S. meliloti genome and the 5′ end of the ORF was fused with the sequence 5′ CTGCAGTAACGTAACGTAAATGGAGTCACCTCT 3′, which contains a PstI site followed by 3 stop codons, as well as a GGAGTC ribosome-binding site (35, 58). This fragment was cloned into the PstI and BamHI restriction sites in pstb-LAFR5 to generate plasmid pstb-LAFR5-exoY.
Plasmids were mobilized into S. meliloti by triparental mating as described previously, using E. coli strain MM294A (19). S. meliloti exconjugants were selected on LBMC medium containing 10 μg/ml tetracycline and 1 mg/ml streptomycin. Each exconjugant strain that was used in these studies was isolated from a separate conjugation reaction.
Plant nodulation assays.
The host plant Medicago sativa (alfalfa) cv. Iroquois was prepared for inoculation with S. meliloti as described by Leigh et al. (29) with modifications: seeds were sterilized for 5 min in 50% bleach, rinsed in sterile water, and germinated for 3 days on plant cell culture-tested 1% (wt/vol) agar–water (Sigma, St. Louis, MO) (29). Seedlings were then moved to individual 100-mm by 15-mm Jensen's medium plates (49) and inoculated with 100 μl of S. meliloti or S. medicae of the appropriate strain at an optical density at 600 nm (OD600) of 0.05. Plants were grown in a Percival AR-36L incubator (Perry, IA) at 21°C with 60 to 70% relative humidity and 100 to 175 μmol m−2 s−1 light for 5 weeks.
The host plant Medicago truncatula (barrel medic) cv. Jemalong A17 was prepared for inoculation with S. meliloti by scarifying seeds in concentrated sulfuric acid for 10 min, rinsing 5 times in sterile water, soaking overnight at 4°C, and germinating for 3 days on plant cell culture-tested 1% (wt/vol) agar–water (38). Seedlings were then treated, inoculated, and maintained as for alfalfa (see above) for 7 weeks.
Bacterial growth curves.
Each strain to be tested was streaked to LBMC with 500 μg/ml streptomycin and 10 μg/ml tetracycline, and 3 single colonies of each were inoculated into precultures of the same medium and grown for 2 to 3 days. Cells from the LBMC preculture were washed in 0.85% saline and inoculated into a second preculture of the growth medium to be tested. After 2 to 3 days growth, cells from the second preculture were used to inoculate the growth curve cultures to an OD600 of 0.01, except for Jensen's glutamate-mannitol medium, which was inoculated to an OD600 of 0.05. All media for growth curves contained streptomycin at 500 μg/ml and tetracycline at 10 μg/ml.
Anthrone-sulfuric acid assays of polysaccharide production.
Total polysaccharide in supernatants from cultures grown for 6 days in media lacking hexose sugars (GMS; M9 salts plus 12 mM mannitol, 5 mM glutamic acid, and sodium salt; or Jensen's medium plus 12 mM mannitol, 5 mM glutamic acid, and sodium salt) were quantified as follows: culture supernatants were collected from cultures by centrifugation and frozen at −80°C. Frozen culture supernatants were diluted 1/30 in Milli-Q H2O, and 0.325 ml of each diluted supernatant was mixed with 0.65 ml of 0.2% anthrone (wt/vol) in concentrated sulfuric acid (30) and boiled for 5 min. The OD620 values of the boiled samples were measured and compared to a 2-fold dilution series of glucose (from 20 μg to 0.31 μg) dissolved in 1/30 of the appropriate culture medium. Anthrone assays performed on a 1/30 dilution of the appropriate culture medium served as the blanks. The microgram equivalents of glucose for each sample were normalized to the OD600 of the cell pellet from that sample after resuspension of the pellet in 0.85% saline.
Calcofluor fluorescence quantitation of culture supernatants.
Frozen culture supernatants were diluted 1/2 for LBMC and Jensen's GM media and 1/4 for GMS medium and were left undiluted for M9 and M9GM. For each culture supernatant, 175 μl of the appropriate dilution (or of the appropriate dilution of uninoculated blank medium) was pipetted into a 96-well plate. Twenty-five microliters of a 0.14-mg/ml solution of calcofluor white M2R (dissolved in 0.04% Triton X-100–400 mM Tris-HCl, pH 8.0) was added to each well. Fluorescence was read on a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA) at low sensitivity with excitation at 365 nm, emission at 435 nm, and cutoff at 435 nm. The appropriate blank was subtracted from each raw fluorescence reading, and the values were normalized to the OD600 of the cell pellet from that sample after resuspension in 0.85% saline.
RESULTS AND DISCUSSION
Sinorhizobium meliloti strains with increased expression of the succinoglycan biosynthesis enzyme encoded by exoY produce elevated levels of the exopolysaccharide succinoglycan.
The exoY gene, which encodes an undecaprenyl phosphate galactose phosphotransferase that attaches galactose to the lipid carrier on which succinoglycan is synthesized (43), was cloned into the plasmid pstb-LAFR5 (this study). pstb-LAFR5 carries a strong constitutive promoter from the region upstream of the SMb21651 ORF (58). Both the exoY overexpression construct pstb-LAFR5-exoY and the negative-control construct pstb-LAFR5 were conjugated into S. meliloti 1021 and tested for calcofluor fluorescence. Calcofluor white M2R fluoresces with maxima at ∼420 nm and ∼440 nm when bound to β-glucans (53). Succinoglycan is the only polysaccharide produced by S. meliloti 1021 that exhibits calcofluor fluorescence (18). Therefore, differences in calcofluor fluorescence between strains streaked onto this indicator are expected to be due to differences in succinoglycan production.
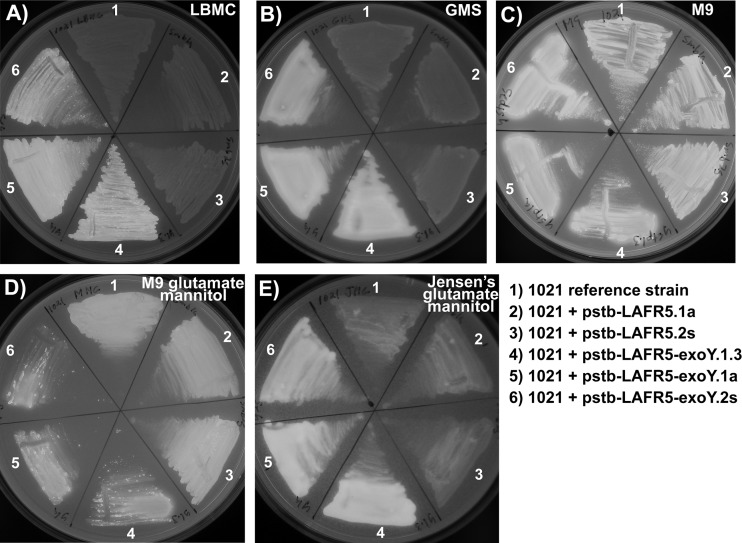
The results in Figure 1 show that strains carrying the exoY overexpression construct have much brighter calcofluor fluorescence (calcofluor-bright phenotype) than the S. meliloti 1021 reference strain or this strain carrying the negative-control construct pstb-LAFR5 on the rich medium LBMC (Fig. 1A). (A comparison of the calcofluor fluorescence of S. meliloti 1021 with that of the exoY::Tn5 mutant, which cannot make succinoglycan, is shown in Fig. S1 in the supplemental material.) The exoY overexpression strains are also brighter on glutamate-mannitol salts medium (GMS), which is a low-osmolarity medium that is commonly used in studies of succinoglycan production (55, 56) (Fig. 1B), and on Jensen's medium (49) supplemented with glutamate and mannitol (Jensen's GM) as the nitrogen and carbon source, respectively. Jensen's is the plant growth medium used for host plant nodulation assays (29). On either M9 medium containing NH4Cl as the nitrogen source and sucrose as the carbon source or M9 containing glutamate as the nitrogen source and mannitol as the carbon source (M9GM), the calcofluor fluorescence of the exoY overexpression strains is not brighter than that of the reference strain or the negative-control strains (Fig. 1C and D). Also, the growth of the exoY overexpression strains on M9 and M9GM media was somewhat reduced relative to the growth of the reference strain and the negative-control strains, regardless of which carbon source and nitrogen source were used (Fig. 1C and D; also see Fig. 3 and 4). (The compositions of M9, M9GM, GMS, and Jensen's GM media are listed in Table S1 in the supplemental material.) The overall increase in calcofluor fluorescence of the reference strain and the negative-control strains seen in M9 medium versus their calcofluor fluorescence levels in other media probably reflects the high (∼200 mM) phosphate concentration of this medium. High levels of phosphate have been shown to lead to an increase in succinoglycan production in S. meliloti (32). Most importantly, however, the calcofluor fluorescence assay demonstrates a strong calcofluor-bright phenotype of exoY overexpression strains grown on Jensen's plant medium, indicating that these strains overproduce succinoglycan under conditions that most closely approximate those used for host plant nodulation assays.
Fig 1.
Overexpression of the exoY glycosyltransferase in S. meliloti 1021 produces a calcofluor-bright phenotype. Binding of succinoglycan to the dye calcofluor white M2R produces fluorescence when excited with UV light. The stronger calcofluor fluorescence in the exoY overexpression strains (numbers 4, 5, and 6 in each panel) than in the control strains indicates increased succinoglycan production. The exoY overexpression strains are calcofluor bright on (A) LBMC medium after 2 days growth, (B) GMS (glutamate mannitol salts medium) after 3 days growth, and (E) Jensen's medium supplemented with glutamate and mannitol after 3 days growth. On (C) M9 minimal salts medium with ammonium chloride and sucrose as the nitrogen and carbon source, respectively, and on (D) M9 with glutamate and mannitol, growth of the exoY overexpression strains is slowed relative to growth of the control strains, and the cells do not appear to exhibit a stronger overall calcofluor signal than the control strains. All exposures were 0.5 s on a 302-nm UV light box.
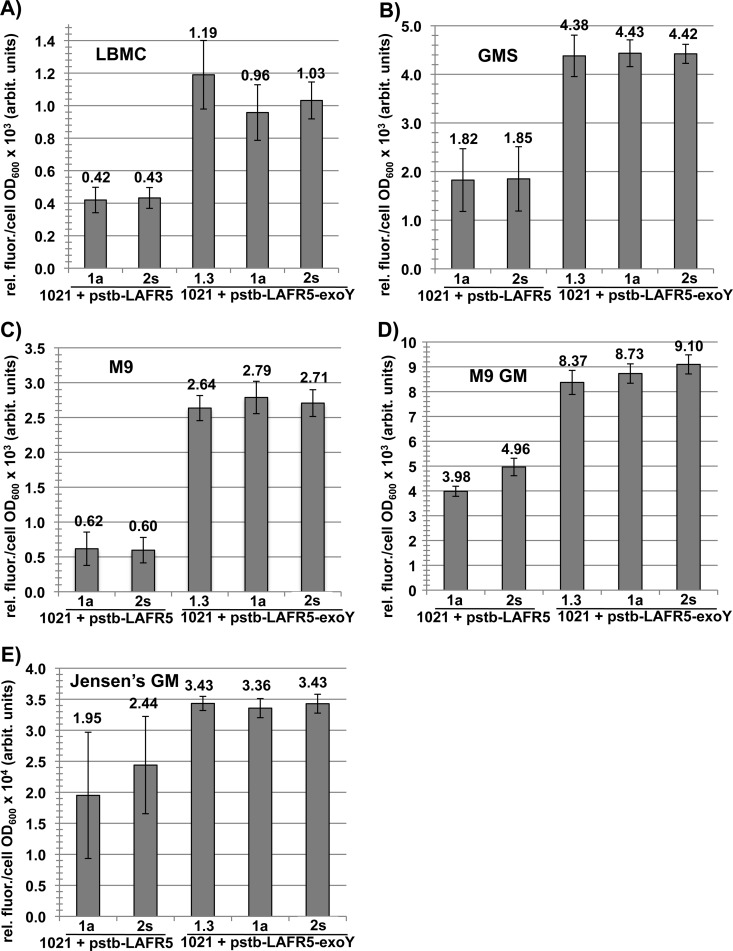
Fig 3.
Production of calcofluor-fluorescent material by the exoY-overexpressing strains is increased in all growth media tested. The relative levels of calcofluor-fluorescent material in the culture supernatants from exoY overexpression strains and control strains were assayed for cells grown in the indicated media. There was an increase in calcofluor-fluorescent material in the exoY overexpression strains under all of these growth conditions. Relative fluorescence units from each culture supernatant were normalized to cell culture OD600. Arbit., arbitrary. Error bars show standard errors of the means.
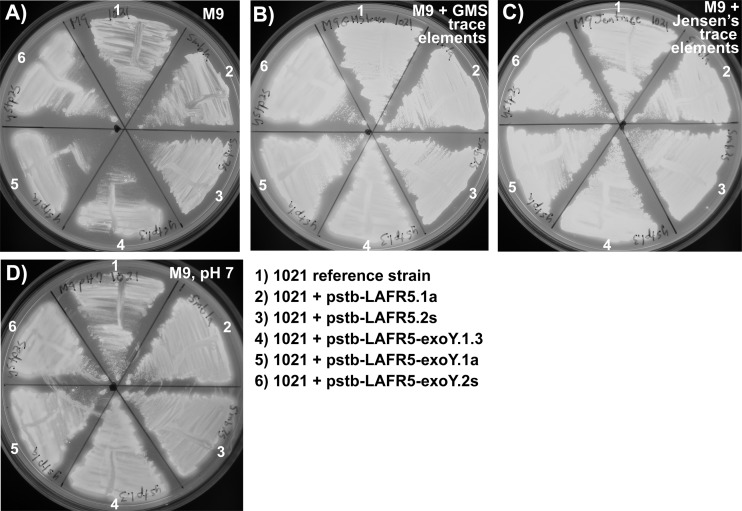
Fig 4.
Adding the GMS medium trace elements or Jensen's medium trace elements to M9 medium or adjusting the pH of M9 medium to pH 7 alleviates the growth defect of the exoY overexpression strains. When grown on M9 medium (A), the exoY overexpression strains had reduced growth on solid medium relative to the growth of the S. meliloti 1021 reference strain and the negative-control strains. In contrast, when the trace element mixture from GMS medium (B) or the trace element mixture from Jensen's medium (C) was added to M9 medium, the growth of the exoY overexpression strains was similar to the growth of the negative-control strains and the S. meliloti 1021 reference strain. Also, when M9 medium, which is normally buffered to pH ∼7.3, is buffered to pH 7 (D), the growth defect of the exoY overexpression strains is eliminated. Panel A in this figure is the same image as panel C in Fig. 1. All exposures were 0.5 s on a 302-nm UV light box.
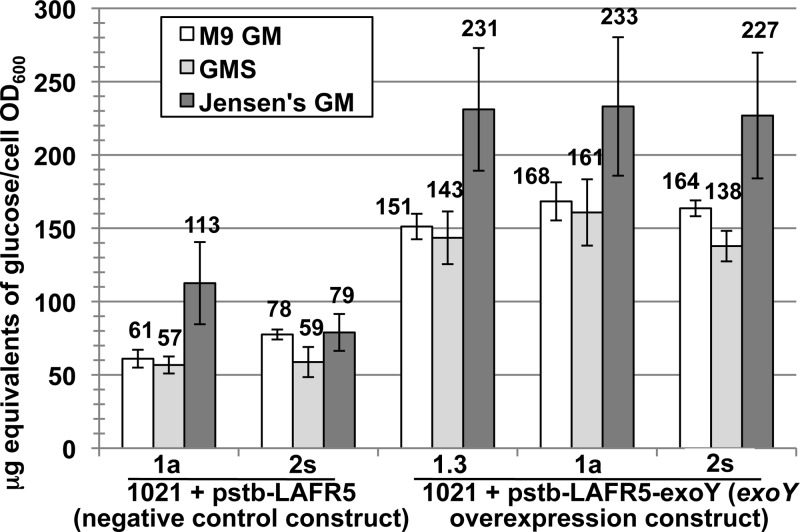
The exopolysaccharide production of the exoY overexpression strains grown in liquid medium was measured using the anthrone-sulfuric acid assay for hexose sugars (Fig. 2) (30). Since the carbon source mannitol is not detected by this assay, all the hexose sugars quantified in supernatants of cultures grown in M9GM, GMS, and Jensen's GM should be of bacterial origin. Since succinoglycan is the most abundant exopolysaccharide produced by the S. meliloti 1021 reference strain (18, 28), most of the sugars detected by the anthrone-sulfuric acid assay are from succinoglycan. A standard curve of glucose (Fig. 2) was used to calculate the quantity of polysaccharide in each culture supernatant, and these values were normalized to the cell OD600 of the cultures. In M9GM, GMS, and Jensen's GM media, the exoY overexpression strains produced, respectively, 2.3-fold, 2.5-fold, and 2.4-fold more exopolysaccharide than strains carrying the negative-control constructs (Fig. 2).
Fig 2.
Production of exopolysaccharide by the exoY-overexpressing strains is increased in all growth media tested. Overexpression of exoY under the control of the SMb21651 promoter results in an average of ≥2.3-fold increased production of exopolysaccharide relative to that of strains carrying the negative-control construct. When S. meliloti 1021 is grown in liquid medium, an increase in exopolysaccharide production is observed in all media tested: M9 GM, GMS, and Jensen's GM. Anthrone assay data are plotted relative to a standard dilution series of glucose. Microgram equivalents of polysaccharide produced by each strain are normalized to cell culture OD600. Error bars show standard errors of the means.
To confirm that the increase in exopolysaccharide production detected by the anthrone method was due to an increase in succinoglycan production, a quantitative calcofluor fluorescence microplate assay was devised based on that of Schmitt and Budde for wort β-glucan (45). The calcofluor fluorescence of culture supernatants was quantified in a fluorescence plate reader with excitation at 365 nm and emission at 435 nm and blanked against uninoculated media (see Materials and Methods for details). The fluorescence value for each culture supernatant was normalized to the cell OD600 of the culture. The average relative fluorescence for the exoY overexpression strains and the negative-control strains for each growth condition is presented in Fig. 3. The calcofluor fluorescence of the exoY overexpression strains is increased relative to that of the negative-control strains in all media tested. In LBMC and M9 media, which could not be tested for exopolysaccharide production by the anthrone-sulfuric acid method due to the presence of hexose sugars, the exoY overexpression strains have, respectively, a 2.5-fold (Fig. 3A) and a 4.4-fold (Fig. 3C) increase in calcofluor fluorescence. The 4.4-fold increase in calcofluor fluorescence of exoY overexpression strains grown in M9 medium is higher than expected, given that very little difference in calcofluor fluorescence between these strains and the negative-control strains was observed on M9 agar plates. It is possible that more succinoglycan is produced by negative-control strains grown on M9 solid medium than in liquid medium or that the presence of calcofluor in the plates during growth on M9 solid medium affects succinoglycan production.
The calcofluor fluorescence of the exoY overexpression strains grown in GMS is 2.4-fold greater than that of the negative-control strains (Fig. 3B). This value is very close to the 2.5-fold increase in exopolysaccharide production in GMS medium measured by the anthrone-sulfuric acid method (Fig. 2). The calcofluor fluorescence of the exoY overexpression strains grown in M9GM medium (Fig. 3D) and Jensen's GM medium (Fig. 3E) is also greater than those of the negative-control strains. In M9GM medium, the exoY overexpression strains have 2-fold greater calcofluor fluorescence than the negative-control strains (Fig. 3D), while in Jensen's GM medium, the exoY overexpression strains have 1.6-fold greater calcofluor fluorescence than the negative-control strains (Fig. 3E). The calcofluor differences in M9GM and Jensen's GM are not as great as the difference in total polysaccharide production between these strains measured by the anthrone-sulfuric acid method (Fig. 2). It is possible that some of the difference in total exopolysaccharide production (Fig. 2) is due to production of non-calcofluor-fluorescent exopolysaccharide such as EPSII (18) or cyclic β-glucans (50) or that succinoglycan produced in M9GM medium and Jensen's medium is more refractory to calcofluor binding and thus is not apparent in the calcofluor fluorescence microplate assay.
The exoY overexpression strains clearly produce higher levels of exopolysaccharide and more calcofluor-fluorescent material than the negative-control strains, indicating the production of more succinoglycan. Most importantly, the exoY overexpression strains grown in Jensen's GM plant medium produce culture supernatants with higher levels of exopolysaccharide and greater calcofluor fluorescence than the negative-control strains, indicating that these strains overproduce succinoglycan under conditions that approximate those used for host plant nodulation assays.
Exopolysaccharide overproduction is not deleterious to S. meliloti growth in most media tested.
To determine the physiological effects of increased succinoglycan production on free-living cells, the growth rates of the strains described were compared. The exoY overexpression strains, which have been shown as described above to produce an excess of exopolysaccharide, have a growth pattern that is indistinguishable from the growth of the negative-control strains in LBMC (see Fig. S2A in the supplemental material), GMS (see Fig. S2B), or Jensen's GM plant growth medium (see Fig. S2E). Therefore, it is not expected that any symbiotic plant phenotypes conferred by exoY overexpression would be due to effects on cell growth. In M9 medium (see Fig. S2C) or M9GM medium (see Fig. S2D), the exoY overexpression strains had slightly reduced growth, similar to the reduced growth on M9 medium observed on agar plates (Fig. 1C and D). (The detailed compositions of M9, GMS, and Jensen's GM media are listed in Table S1 in the supplemental material.) The addition of the trace element mixture from GMS medium (Fig. 4B) or from Jensen's medium (Fig. 4C) to M9 agar medium alleviates the growth defect of the exoY overexpression strains, suggesting that one or more of these trace elements is limiting in the presence of increased levels of the ExoY protein, increased activity of enzymes of the succinoglycan biosynthesis pathway, or increased levels of succinoglycan.
Medium additives that M9 lacks that are provided by the addition of either GMS trace elements or Jensen's trace elements are Fe3+, Zn2+, Cu2+, Mn2+, MoO42−, and BO33− (see Table S1 in the supplemental material). Among these, the addition of MnSO4 has been shown to increase the growth rate of S. meliloti 1021 in M9 medium (10), and Mn2+ is known to function as a glycosyltransferase cofactor (26), although the concentrations of Mn2+ that might be required by ExoY or the other enzymes of the succinoglycan biosynthesis pathway are unknown. Also, anionic bacterial exopolysaccharides are known to chelate divalent cations (46), so one possibility is that high levels of these polysaccharides could interfere with bacterial growth when one or more divalent cations are limiting.
Another thing that differentiates M9 medium from GMS and Jensen's GM is that M9 is phosphate buffered to pH ∼7.3, while both GMS and Jensen's GM have a final pH of 7 (see Table S1 in the supplemental material). We found that buffering of M9 medium to pH 7 instead of 7.3 also alleviates the relative growth defect of the succinoglycan-overproducing strains, even without the addition of trace elements (Fig. 4D). One possible explanation for this is a decrease in exopolysaccharide chelation of cations at lower pH. Recent studies suggest that exopolysaccharides with ionizable functional groups bind lower levels of some positively charged metals as a function of decreasing pH (9, 20, 27). Growth on lower-pH medium might make higher concentrations of positively charged metals available for use by bacterial cells.
exoY overproduction in Sinorhizobium meliloti 1021 enhances symbiotic proficiency with the host plant M. truncatula cv. Jemalong A17.
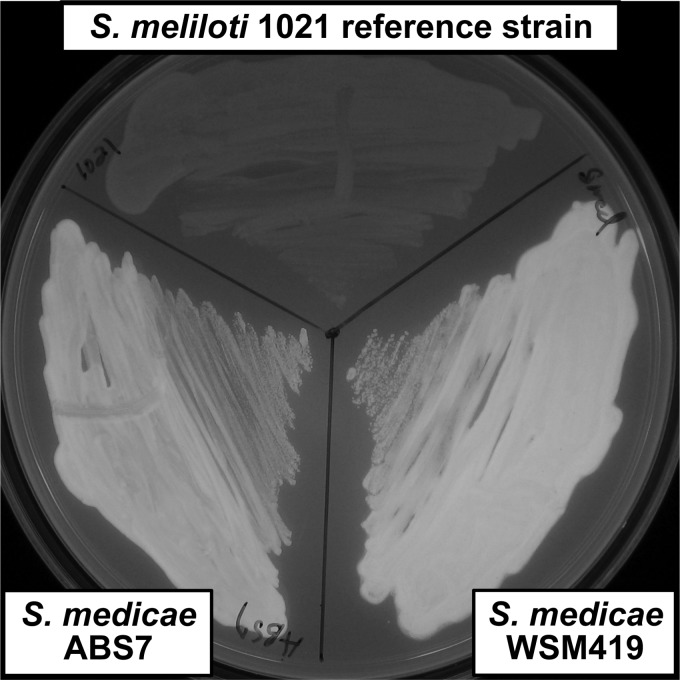
It has previously been shown that on the plant host Medicago truncatula cv. Jemalong A17, the S. meliloti 1021 reference strain is not as effective a symbiont as wild-type S. medicae ABS7 or S. medicae WSM419 (47). One possible reason for this is a difference in succinoglycan production. Both S. medicae ABS7 and S. medicae WSM419 produce more calcofluor-fluorescent material than the S. meliloti 1021 reference strain (Fig. 5). Exopolysaccharide from S. medicae WSM419 has been demonstrated by proton nuclear magnetic resonance to contain succinyl, acetyl, and pyruvyl substituents, which suggests it may be similar in structure to succinoglycan produced by S. meliloti 1021 (11).
Fig 5.
S. medicae strains have stronger calcofluor fluorescence than S. meliloti 1021. This indicates that S. medicae ABS7 and S. medicae WSM419 produce more calcofluor-fluorescent exopolysaccharide than S. meliloti 1021 on LBMC medium.
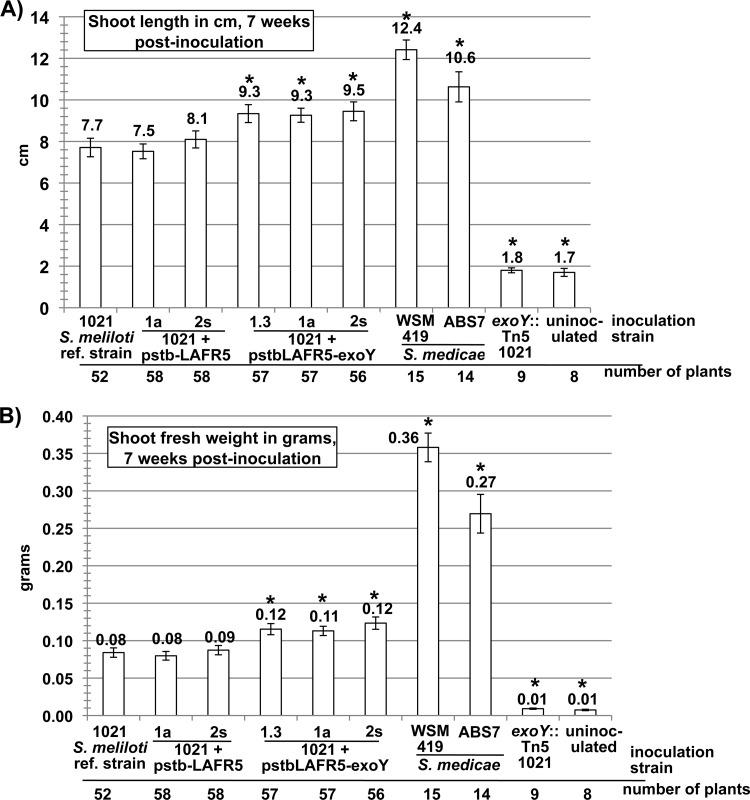
To determine whether increased succinoglycan production could enhance the symbiotic effectiveness of S. meliloti 1021 with M. truncatula A17, the symbiotic proficiency of the exoY overexpression strains was compared with that of the S. meliloti 1021 reference strain, the negative-control strains, S. medicae ABS7 wild type, and S. medicae WSM419 wild type. On this plant host, exoY overexpression was sufficient to confer a significant symbiotic enhancement to S. meliloti 1021, as measured by shoot length (Fig. 6A) and shoot fresh weight (Fig. 6B). However, exoY overexpression did not increase the symbiotic productivity of S. meliloti 1021 on this plant host to the level of either S. medicae ABS7 or S. medicae WSM419. This indicates that there are probably additional factors that make the S. medicae strains more effective symbionts than S. meliloti 1021 on M. truncatula A17.
Fig 6.
exoY-overexpressing strains of S. meliloti 1021 have enhanced symbiosis on the host plant Medicago truncatula A17. The symbiotic productivity of S. meliloti 1021 on the host plant M. truncatula A17 is improved slightly, but statistically significantly, by exoY overexpression. Symbiotic productivity is measured as (A) shoot length in cm and (B) shoot fresh weight in grams. However, the symbiotic improvement due to exoY overexpression does not bring the S. meliloti 1021 symbiotic productivity on this plant host up to the level of that of S. medicae strains WSM419 and ABS7. This indicates that factors other than the level of succinoglycan production are responsible for the superior symbiotic efficiency of S. medicae strains on M. truncatula A17. Asterisks over graph bars denote a statistically significant difference from the reference strain in an unpaired t test. Plants inoculated with an exoY::Tn5 null mutant that does not produce any succinoglycan had similar shoot lengths and fresh weight to uninoculated plants. ref., reference. Error bars show standard errors of the means.
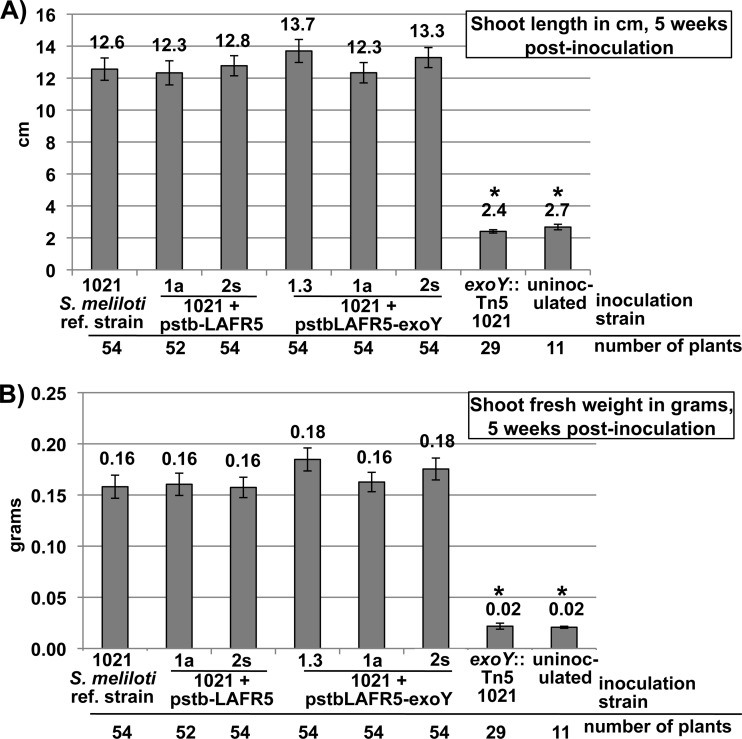
On the host plant alfalfa, the shoot length (Fig. 7A) and shoot fresh weight (Fig. 7B) of plants inoculated with the exoY overexpression strains were not significantly different from those of plants inoculated with the S. meliloti 1021 reference strain or with the negative-control strains. This demonstrates that overproduction of succinoglycan in itself is not detrimental to the symbiosis with alfalfa and, therefore, is unlikely to be one of the factors that contribute to symbiotic failure of S. meliloti mutants, such as relA and exoR. This also demonstrates that the level of succinoglycan production of the S. meliloti 1021 reference strain is not a limiting factor for symbiosis on the plant host alfalfa.
Fig 7.
The symbiotic productivity of alfalfa plants inoculated with S. meliloti 1021 strains overexpressing exoY is not significantly different from that of plants inoculated with control strains. exoY-overexpressing strains of S. meliloti 1021 were inoculated onto the host plant alfalfa. Symbiotic productivity is measured as (A) shoot length in cm and (B) shoot fresh weight in grams. This indicates that an increased level of succinoglycan production by the S. meliloti 1021 reference strain does not reduce the efficiency of the symbiosis with the host plant alfalfa. Also, since there is not a significant improvement in symbiotic productivity in exoY overexpression strains, it indicates that succinoglycan production is not a limiting factor for the S. meliloti 1021 reference strain in establishing a functional symbiosis on the host plant alfalfa. Plants inoculated with an exoY::Tn5 null mutant that does not produce any succinoglycan had similar shoot lengths and fresh weight to uninoculated plants. Asterisks over graph bars denote a statistically significant difference from the reference strain in an unpaired t test. ref., reference. Error bars show standard errors of the means.
Our results also show that increased production of succinoglycan is sufficient to increase the productivity of the symbiosis between S. meliloti 1021 and the host plant M. truncatula A17. This suggests that the native level of succinoglycan produced by S. meliloti 1021 is a limiting factor for invasion of this plant host. In the future, it might be beneficial to modify rhizobial strains that are used in the inoculation of legume host crop plants so that they produce higher levels of symbiotic exopolysaccharides and to determine if this can increase crop yields.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the USDA National Institute of Food and Agriculture, Agriculture and Food Research Initiative grant 2010-65108-20582 to K.M.J.
I thank Michiko E. Taga and Brian K. Washburn and the peer reviewers for critical review of the manuscript.
Footnotes
Published ahead of print 8 June 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Aslam SN, et al. 2008. Bacterial polysaccharides suppress induced innate immunity by calcium chelation. Curr. Biol. 18: 1078–1083 [DOI] [PubMed] [Google Scholar]
- 2. Backman K, Boyer HW. 1983. Tetracycline resistance determined by pBR322 is mediated by one polypeptide. Gene 26: 197–203 [DOI] [PubMed] [Google Scholar]
- 3. Bekki A, Trinchant J-C, Rigaud J. 1987. Nitrogen fixation (C2H2 reduction) by Medicago nodules and bacteroids under sodium chloride stress. Physiol. Plant. 71: 61–67 [Google Scholar]
- 4. Brewin NJ. 2004. Plant cell wall remodelling in the rhizobium-legume symbiosis. Crit. Rev. Plant Sci. 23: 293–316 [Google Scholar]
- 5. Campbell GR, et al. 2006. Sinorhizobium meliloti bluB is necessary for production of 5,6-dimethylbenzimidazole, the lower ligand of B12. Proc. Natl. Acad. Sci. U. S. A. 103: 4634–4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Catalano CM, Czymmek KJ, Gann JG, Sherrier DJ. 2007. Medicago truncatula syntaxin SYP132 defines the symbiosome membrane and infection droplet membrane in root nodules. Planta 225: 541–550 [DOI] [PubMed] [Google Scholar]
- 7. Chen EJ, Sabio EA, Long SR. 2008. The periplasmic regulator ExoR inhibits ExoS/ChvI two-component signalling in Sinorhizobium meliloti. Mol. Microbiol. 69: 1290–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng HP, Walker GC. 1998. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 180: 5183–5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Comte S, Guibaud G, Baudu M. 2008. Biosorption properties of extracellular polymeric substances (EPS) towards Cd, Cu and Pb for different pH values. J. Hazard. Mater. 151: 185–193 [DOI] [PubMed] [Google Scholar]
- 10. Davies BW, Walker GC. 2007. Disruption of sitA compromises Sinorhizobium meliloti for manganese uptake required for protection against oxidative stress. J. Bacteriol. 189: 2101–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dilworth MJ, Rynne FG, Castelli JM, Vivas-Marfisi AI, Glenn AR. 1999. Survival and exopolysaccharide production in Sinorhizobium meliloti WSM419 are affected by calcium and low pH. Microbiology 145( Pt 7): 1585–1593 [DOI] [PubMed] [Google Scholar]
- 12. Doherty D, Leigh JA, Glazebrook J, Walker GC. 1988. Rhizobium meliloti mutants that overproduce the R. meliloti acidic calcofluor-binding exopolysaccharide. J. Bacteriol. 170: 4249–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finan TM, Kunkel B, de Vos GF, Signer ER. 1986. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J. Bacteriol. 167: 66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gage DJ. 2004. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev. 68: 280–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gibson KE, Barnett MJ, Toman CJ, Long SR, Walker GC. 2007. The symbiosis regulator CbrA modulates a complex regulatory network affecting the flagellar apparatus and cell envelope proteins. J. Bacteriol. 189: 3591–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gibson KE, Campbell GR, Lloret J, Walker GC. 2006. CbrA is a stationary-phase regulator of cell surface physiology and legume symbiosis in Sinorhizobium meliloti. J. Bacteriol. 188: 4508–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gibson KE, Kobayashi H, Walker GC. 2008. Molecular determinants of a symbiotic chronic infection. Annu. Rev. Genet. 42: 413–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Glazebrook J, Walker GC. 1989. A novel exopolysaccharide can function in place of the calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell 56: 661–672 [DOI] [PubMed] [Google Scholar]
- 19. Glazebrook J, Walker GC. 1991. Genetic techniques in Rhizobium meliloti. Methods Enzymol. 204: 398–418 [DOI] [PubMed] [Google Scholar]
- 20. Guibaud G, Bordas F, Saaid A, D'Abzac P, Van Hullebusch E. 2008. Effect of pH on cadmium and lead binding by extracellular polymeric substances (EPS) extracted from environmental bacterial strains. Colloids Surf. B Biointerfaces 63: 48–54 [DOI] [PubMed] [Google Scholar]
- 21. Jones KM, Kobayashi H, Davies BW, Taga ME, Walker GC. 2007. How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model. Nat. Rev. Microbiol. 5: 619–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones KM, et al. 2008. Differential response of the plant Medicago truncatula to its symbiont Sinorhizobium meliloti or an exopolysaccharide-deficient mutant. Proc. Natl. Acad. Sci. U. S. A. 105: 704–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones KM, Walker GC. 2008. Responses of the model legume Medicago truncatula to the rhizobial exopolysaccharide succinoglycan. Plant Signal. Behav. 3: 888–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keen NT, Tamaki S, Kobayashi D, Trollinger D. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70: 191–197 [DOI] [PubMed] [Google Scholar]
- 25. Klein S, Hirsch AM, Smith CA, Signer ER. 1988. Interaction of nod and exo Rhizobium meliloti in alfalfa nodulation. Mol. Plant Microbe Interact. 1: 94–100 [DOI] [PubMed] [Google Scholar]
- 26. Lairson LL, Henrissat B, Davies GJ, Withers SG. 2008. Glycosyltransferases: structures, functions, and mechanisms. Annu. Rev. Biochem. 77: 521–555 [DOI] [PubMed] [Google Scholar]
- 27. Lamelas C, Benedetti M, Wilkinson KJ, Slaveykova VI. 2006. Characterization of H+ and Cd2+ binding properties of the bacterial exopolysaccharides. Chemosphere 65: 1362–1370 [DOI] [PubMed] [Google Scholar]
- 28. Leigh JA, Lee CC. 1988. Characterization of polysaccharides of Rhizobium meliloti exo mutants that form ineffective nodules. J. Bacteriol. 170: 3327–3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leigh JA, Signer ER, Walker GC. 1985. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl. Acad. Sci. U. S. A. 82: 6231–6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loewus FA. 1952. Improvement in anthrone method for determination of carbohydrates. Anal. Chem. 24: 219 [Google Scholar]
- 31. Meade HM, Long SR, Ruvkun GB, Brown SE, Ausubel FM. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149: 114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mendrygal KE, González JE. 2000. Environmental regulation of exopolysaccharide production in Sinorhizobium meliloti. J. Bacteriol. 182: 599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mergaert P, et al. 2006. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium-legume symbiosis. Proc. Natl. Acad. Sci. U. S. A. 103: 5230–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Niehaus K, Kapp D, Puhler A. 1993. Plant defence and delayed infection of alfalfa pseudonodules induced by an exopolysaccharide (EPS I)-deficient Rhizobium meliloti mutant. Planta 190: 415–425 [Google Scholar]
- 35. Oke V, Long SR. 1999. Bacterial genes induced within the nodule during the rhizobium-legume symbiosis. Mol. Microbiol. 32: 837–849 [DOI] [PubMed] [Google Scholar]
- 36. Oldroyd GE, Downie JA. 2006. Nuclear calcium changes at the core of symbiosis signalling. Curr. Opin. Plant Biol. 9: 351–357 [DOI] [PubMed] [Google Scholar]
- 37. Pellock BJ, Cheng HP, Walker GC. 2000. Alfalfa root nodule invasion efficiency is dependent on Sinorhizobium meliloti polysaccharides. J. Bacteriol. 182: 4310–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Penmetsa RV, Cook DR. 2000. Production and characterization of diverse developmental mutants of Medicago truncatula. Plant Physiol. 123: 1387–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peters NK, Frost JW, Long SR. 1986. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 233: 977–980 [DOI] [PubMed] [Google Scholar]
- 40. Prell J, Poole P. 2006. Metabolic changes of rhizobia in legume nodules. Trends Microbiol. 14: 161–168 [DOI] [PubMed] [Google Scholar]
- 41. Reed JW, Capage M, Walker GC. 1991. Rhizobium meliloti exoG and exoJ mutations affect the ExoX-ExoY system for modulation of exopolysaccharide production. J. Bacteriol. 173: 3776–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reeve WG, et al. 2010. Complete genome sequence of the Medicago microsymbiont Ensifer (Sinorhizobium) medicae strain WSM419. Stand. Genomic Sci. 2: 77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reuber TL, Walker GC. 1993. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell 74: 269–280 [DOI] [PubMed] [Google Scholar]
- 44. Sambrook J, Fritsch EF, Maniatis T. 1982. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 45. Schmitt MR, Budde AD. 2009. Calcofluor fluorescence assay for wort β-glucan in a microplate format. Cereal Chem. 86: 33–36 [Google Scholar]
- 46. Sutherland I. 1997. Microbial exopolysaccharides—structural subtleties and their consequences. Pure Appl. Chem. 69: 1911–1917 [Google Scholar]
- 47. Terpolilli JJ, O'Hara GW, Tiwari RP, Dilworth MJ, Howieson JG. 2008. The model legume Medicago truncatula A17 is poorly matched for N2 fixation with the sequenced microsymbiont Sinorhizobium meliloti 1021. New Phytol. 179: 62–66 [DOI] [PubMed] [Google Scholar]
- 48. Timmers AC, Auriac MC, Truchet G. 1999. Refined analysis of early symbiotic steps of the rhizobium-medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development 126: 3617–3628 [DOI] [PubMed] [Google Scholar]
- 49. Vincent JM. 1970. A manual for the practical study of the root-nodule bacteria. Blackwell, Oxford, England [Google Scholar]
- 50. Wang LX, Wang Y, Pellock B, Walker GC. 1999. Structural characterization of the symbiotically important low-molecular-weight succinoglycan of Sinorhizobium meliloti. J. Bacteriol. 181: 6788–6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wells DH, Chen EJ, Fisher RF, Long SR. 2007. ExoR is genetically coupled to the ExoS-ChvI two-component system and located in the periplasm of Sinorhizobium meliloti. Mol. Microbiol. 64: 647–664 [DOI] [PubMed] [Google Scholar]
- 52. Wells DH, Long SR. 2002. The Sinorhizobium meliloti stringent response affects multiple aspects of symbiosis. Mol. Microbiol. 43: 1115–1127 [DOI] [PubMed] [Google Scholar]
- 53. Wood PJ. 1980. The interaction of direct dyes with water soluble substituted celluloses and cereal beta-glucans. Ind. Eng. Chem. Prod. Res. Dev. 19: 19–23 [Google Scholar]
- 54. Yao SY, et al. 2004. Sinorhizobium meliloti ExoR and ExoS proteins regulate both succinoglycan and flagellum production. J. Bacteriol. 186: 6042–6049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. York GM, Walker GC. 1997. The Rhizobium meliloti exoK gene and prsD/prsE/exsH genes are components of independent degradative pathways which contribute to production of low-molecular-weight succinoglycan. Mol. Microbiol. 25: 117–134 [DOI] [PubMed] [Google Scholar]
- 56. Zevenhuizen LPTM, van Neerven ARW. 1983. (1–2)-b-D-Glucan and acidic oligosaccharides produced by Rhizobium meliloti. Carbohydr. Res. 118: 127–134 [Google Scholar]
- 57. Zhan HJ, Leigh JA. 1990. Two genes that regulate exopolysaccharide production in Rhizobium meliloti. J. Bacteriol. 172: 5254–5259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang XS, Cheng HP. 2006. Identification of Sinorhizobium meliloti early symbiotic genes by use of a positive functional screen. Appl. Environ. Microbiol. 72: 2738–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.