Abstract
The envelope glycoproteins of herpes simplex virus 1 (HSV-1) and HSV-2, with the exception of glycoprotein G, elicit cross-reactive B- and T-cell responses. Human vaccine trials, using the cross-reactive glycoproteins B and D, have shown no protection against genital HSV-2 infection or disease. In this study, the mature form of glycoprotein G (mgG-2) of HSV-2 was used for immunization of mice, either alone or in combination with adjuvant CpG, followed by an intravaginal challenge with a lethal dose of a fully virulent HSV-2 strain. Mice immunized with mgG-2 plus CpG showed low disease scores and a significantly higher survival rate (73%) than mice immunized with mgG-2 alone (20%) or controls (0%). Accordingly, limited numbers of infectious HSV-2 particles were detected in the spinal cord of mice immunized with mgG-2 plus CpG. The observed protection was associated with a gamma interferon (IFN-γ) response by splenic CD4+ T cells upon antigen restimulation in vitro and in vaginal washes 1 day postinfection. The majority of sera collected from mice immunized with mgG-2 plus CpG showed macrophage-mediated antibody-dependent cellular cytotoxicity and antibody-dependent complement-mediated cytolysis, while no neutralization activity was observed. In conclusion, we have shown that immunization with the type-specific mgG-2 protein in combination with CpG could elicit protective immunity against an otherwise lethal vaginal HSV-2 challenge. The mgG-2 protein may therefore constitute a promising HSV-2 vaccine antigen to be considered for future human trials.
INTRODUCTION
Herpes simplex virus 2 (HSV-2) is one of the most common sexually transmitted infections worldwide (52). Epidemiological data from different countries support an increasing prevalence in the population (18). The first global estimate on HSV-2 infection, based on several studies from 12 regions, concluded that 536 million individuals were infected prior to 2003 and 23.6 million were infected during 2003 (35). HSV-2 infects the genital mucosa and establishes a latent infection in sensory dorsal root ganglia (DRG), from where the virus can reactivate, giving widespread genital lesions or, more commonly, no symptoms, i.e., asymptomatic shedding of virus. In newborns and in immunocompromised patients, HSV-2 can elicit severe and often fatal central nervous system (CNS) or disseminated infections. Further, genital HSV-2 infection is associated with a 3-fold increased risk of HIV acquisition (19). Thus, development of intervention approaches to counter genital HSV-2 infections is of major public health importance.
Great efforts have been made to develop a vaccine against genital HSV-2 infection or disease (28, 29). Human vaccine trials have been performed using the HSV-2 glycoprotein B (gB-2) and/or glycoprotein D (gD-2) as antigens. Results from randomized double-blind placebo-controlled multicenter trials including >13,000 subjects have been discouraging, showing no protection against HSV-2 infection or disease (5, 13, 14, 53). HSV-1 and HSV-2 are closely related viruses with a high degree of similarity at the protein level. For the immunogenic envelope glycoproteins, all but glycoprotein G contain immunogenic regions which elicit cross-reactive B- and T-cell responses. An interesting observation is that a previous HSV-1 infection reduces only the severity of the clinical symptoms but does not confer protection against acquisition of HSV-2 (8, 14, 32). Thus, HSV-2 can infect the individual despite the existence of cross-reactive immune responses elicited after a prior HSV-1 infection. Since HSV-2 is sexually transmitted and usually infects persons at an older age than does HSV-1, it is obvious that HSV-2 escapes cross-reactive immune responses elicited from the HSV-1 infection. Deduced from these observations, there is a rationale to evaluate an HSV-2 type-specific protein as a vaccine candidate.
Glycoprotein G of HSV-2 (gG-2) was first described in 1984 as an envelope protein which was lacking a counterpart in HSV-1-infected cells (36, 51). The gG-2 protein is expressed as an unglycosylated precursor which is further N-glycosylated, generating a high-mannose precursor. This precursor protein is cleaved into a secreted amino-terminal portion (sgG-2) and to a carboxy-terminal membrane-anchored portion. The latter protein is further O-glycosylated, generating the mature portion of gG-2 (mgG-2) (3, 54). As mgG-2 elicits a type-specific antibody response, such antibodies have been used for several years as a serological marker of HSV-2 infection in clinical settings. Subsequently, this antigen was also shown to elicit a type-specific CD4+ T cell response (9, 17). The function of mgG-2 in the genital HSV-2 infection, however, remains elusive. Although mgG-2 is nonessential for viral replication in cell culture, we have shown earlier that an intact mgG-2 in clinical HSV-2 isolates is of importance for human infection, as mgG-2-negative HSV-2 isolates are rarely detected (33). In cell culture, an mgG-2-negative HSV-2 mutant was recently shown to spread mostly from cell to cell with an impaired capacity to produce extracellular infectious HSV-2 particles (1). Altogether, these findings warrant studying the mgG-2 protein as a putative vaccine antigen to elicit protective immunity to genital HSV-2 infection or disease.
MATERIALS AND METHODS
Mice.
Six- to 8-week-old female C57BL/6 mice were purchased from Scanbur BK AB, Sweden. Gamma interferon (IFN-γ)-gene knockout mice on a C57BL/6 background (15) were a kind gift from Nils Lycke, MIVAC, University of Gothenburg. Mice were divided into groups (6 to 10 mice) and kept in separate cages for 1 week. Mice were anesthetized with 3% isoflurane (Baxter) throughout all injections, vaginal washings, and challenge with virus. All animal experiments were approved by the ethical board in Gothenburg (Dnr 46-2008).
Cells and viruses.
African green monkey kidney (GMK-AH1) cells were cultured in Eagle's minimal essential medium supplemented with 2% calf serum and antibiotics. Baby hamster kidney (BHK21) cells were propagated in Glasgow minimum essential medium (G-MEM) with 8% calf serum, 1% l-glutamine, and 8% tryptose phosphate broth. Wild-type (wt) HSV-2 strain 333 and a local wild-type HSV-2 isolate, B4327UR (26), were used.
Production of mgG-2 protein.
The mgG-2 protein is highly O-glycosylated, with coupling of N-acetylgalactosamine (GalNAc) residues to serine or threonine. This feature was utilized for purification by Helix pomatia lectin affinity chromatography as described earlier (42). Briefly, BHK21 virus-infected cell membranes (HSV-2 strain B4327UR) were solubilized in 1% NP-40 in 0.1 M glycine-NaOH, pH 8.8. Tris-buffered saline (TBS) was used as wash buffer, and 0.02 M GalNAc (Sigma-Aldrich) in TBS was used as elution buffer. The eluate was dialyzed at 4°C overnight to remove GalNAc before use. The protein concentration was measured by Bio-Rad protein assay.
Characterization of mgG-2 antigen.
For several years, we have utilized Helix pomatia lectin affinity chromatography-purified mgG-2 as antigen in an enzyme-linked immunosorbent assay (ELISA) format in our clinical laboratory for detection of human anti-mgG-2 antibodies. In the production of the antigen, exclusively type-specific reactivity of the mgG-2 antigen is a sensitive marker of a lack of other cross-reactive viral proteins. The purified mgG-2 protein was therefore tested in ELISA by using human sera from isolation-positive HSV-1- or HSV-2-infected individuals as well as from HSV-negative subjects. In addition, mgG-2 antigen was tested in ELISA and Western blotting (WB) as described previously (34) using cross-reactive HSV MAbs directed against gB, gC, and gD (6) and an anti-gC-2 monoclonal antibody (MAb; kindly provided by Edward Trybala) produced at our laboratory (unpublished data). The anti-mgG-2 MAbs O1.C5.B2 and O3.G11.H7 were used as positive controls (34). The mgG-2 protein was also subjected to SDS-PAGE using a 4 to 12% NuPAGE bis-Tris gel, followed by silver staining with a SilverXpress silver staining kit (Invitrogen) according to the manufacturer's description. Purified mgG-2 was tested for toxic effects on T cells derived from the spleens of three mice using the cell proliferation kit II {2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay; Roche Applied Science}. Potential endotoxin contamination was analyzed by use of Endochrome-K reagent (Charles River, Charleston, SC) at the Department of Bacteriology, University of Gothenburg.
Adjuvant.
Synthetic oligodeoxynucleotides with a complete phosphorothioate backbone containing two optimal mouse CpG motifs (ODN1826, TCC ATG ACG TTC CTG ACG TT; Operon Biotechnologies GmbH, Germany) were used as adjuvant (7).
Immunization scheme.
In this study, we first used a subcutaneous (s.c.) dose followed by two doses given intranasally (i.n.) with a 14-day interval. This immunization model was chosen to achieve systemic as well as mucosal immunity (20, 45). Four separate experiments were performed with, in total, 26 mice. The s.c. dose was given in a volume of 200 μl followed by two doses of 15 μl given i.n. Each dose contained 10 μg mgG-2 alone (mgG-2) or in combination with 20 μg CpG (mgG-2 plus CpG). A control group that received phosphate-buffered saline (PBS) was included in each experiment. A control group of 10 mice given CpG alone was included for the T-cell proliferation assay and for measurement of IFN-γ production in vaginal washes.
Serum samples.
Serum samples were collected 18 days after the third immunization. In addition, serum samples were obtained for HSV-2 DNA analysis from vaccinated and control mice at day 6 postinfection (p.i.).
Detection of IgG antibodies against mgG-2.
An indirect ELISA technique was used as described previously (55). Briefly, Helix pomatia lectin-purified mgG-2 (1 mg/ml) was coated at a 1:1,000 dilution in carbonate buffer (pH 9.6) on Maxisorp microtiter plates (Nalge Nunc Int.) and incubated overnight at 4°C. Plates were blocked with 2% bovine serum albumin (BSA) in PBS. After dilution in PBS with 1% BSA and 0.05% Tween 20, sera were incubated for 1 h at 37°C. Peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories) at a 1:1,000 dilution was used as a conjugate, and o-phenylenediamine was used as the substrate. The reaction was stopped with 1 M sulfuric acid, and the absorbance was read at 490 nm. Sera were titrated in 3-fold dilution steps starting at a 1:30 dilution. The antibody titer was defined as the reciprocal value of the highest serum dilution giving an optical density (OD) greater than that for the blank plus 0.3 OD unit. Subclass-specific ELISAs for detection of IgG1 and IgG2c were performed in a similar way by using subclass-specific peroxidase-conjugated goat anti-mouse IgG as the conjugate (Southern Biotech) at a 1:2,000 dilution.
NT assay.
The neutralization (NT) capacity of hyperimmune sera drawn from mice vaccinated with mgG-2 or mgG-2 plus CpG was tested in a microneutralization assay as described previously (2). Briefly, 50 PFU of wt 333 was mixed with 2-fold dilutions of serum at a 1:20 start dilution. The mixtures were incubated in a 96-well microtiter plate at 37°C for 2 h, followed by addition of GMK-AH1 cells in suspension. The NT titer was defined as the reciprocal value of the highest serum dilution for which no cytopathic effect was detected. Complement-dependent NT activity was evaluated with the same protocol described above with 2.5% guinea pig serum as the source of complement (Sigma-Aldrich). In both assays, HSV-2-positive human serum was used as a positive control.
ADCC.
The antibody-dependent cellular cytotoxicity (ADCC) assay was modified from the method described earlier (30). BHK21 target cells were infected with wt 333 at a multiplicity of infection of 4. The cells were labeled with 100 μCi Na251CrO4/million cells (Perkin Elmer) and dispersed by trypsinization. Cells were washed five times with 10 ml TBS supplemented with 2% inactivated fetal calf serum (IFS) and resuspended in G-MEM supplemented with 10% IFS, 1% l-glutamine, and 1% antibiotics (ADCCMEM). The ADCC assay was performed using two different sets of effector cells: (i) adherent macrophages from spleen cells (MP) or (ii) natural killer (NK) cells. Adherent MP were prepared by incubating spleen cells for 6 days (30), and mouse NK cells were enriched from spleen cells using a BD Imag NK cell enrichment set (BD Biosciences) according to the manufacturer's recommendation. Before use, cells were activated by preincubation for 24 h (MP) or 48 h (NK) in Iscove's medium supplemented with 10% IFS and 1% antibiotics (IMEM) containing 200 U/ml of recombinant human interleukin-2 (IL-2; Proleukin; Novartis). The IL-2 concentration was maintained throughout both assays. One hundred microliters of target cells (1 × 104) and serum samples, diluted in ADCCMEM, were added to a 96-well plate (Nuclon Surface; Nunc, Denmark) and incubated for 30 min at 37°C in a humid atmosphere with 5% CO2. Effector cells were added, followed by incubation for 18 h at 37°C in a humid atmosphere of 5% CO2. An effector/target (E/T) cell ratio of 100:1 was optimal for both assays. For detection of maximum release, 100 μl of 5% sodium dodecyl sulfate was added to wells containing 100 μl of target cells. Supernatants were collected by harvesting frames (Molecular Devices Corp.) and counted using a Cobra Auto-gamma counter (model 5002/5003; Packard Instrument Company). Results were presented as the mean value of tested triplicates. The percentage of specific release of 51Cr (ADCC%) was calculated by the following formula: [(counts per minute of immune serum − counts per minute of nonimmune serum)/(counts per minute of maximum release − counts per minute of nonimmune serum)] × 100.
Heat-inactivated serum from one mouse immunized with thymidine kinase-negative virus was used as a positive control, and serum from a control mouse immunized with PBS was used as nonimmune serum. All serum samples were tested at a 1:20 dilution. Sera presenting an ADCC% of >5 were considered ADCC positive.
Antibody-dependent complement-mediated cytolysis (ACMC).
HSV-2-infected (wt 333) BHK21 cells labeled as described previously (37) were washed and resuspended in TBS supplemented with 10% IFS and 1% antibiotics and used as target cells. Target cells (6 × 104) and 50 μl of heat-inactivated sera in 2-fold dilution steps starting at 1:25 were mixed into each well. After 1 h of incubation at 37°C, complement (serum from guinea pig) was added at a final 1:15 dilution. After an additional 2 h, supernatants were collected and specific release of 51Cr was measured. Maximum release from target cells was obtained by adding 100 μl sodium dodecyl sulfate to wells containing 100 μl of target cells. The percentage of cytolytic activity (ACMC%) of tested sera was expressed with the following formula: [(counts per minute of immune serum and complement − counts per minute of negative serum and complement)/(counts per minute of maximum release − counts per minute of negative serum and complement)] × 100.
The ACMC titer was defined as the reciprocal value of the serum dilution presenting an ACMC% of >10.
Proliferation assay.
Three weeks after the last immunization, spleens and genital lymph nodes (gLNs) were removed from three mice per group and pooled. Single-cell suspensions were produced, and an EasySep mouse CD4+ enrichment kit and EasySep Magnet (Stemcell Technologies Inc.) were used to purify CD4+ T cells from spleens according to the manufacturer's recommendations. A total of 2 × 105 spleen cells, gLN-derived cells, or enriched CD4+ T cells, including 20% splenocytes, were added per well in a 96-well plate (Nunc). Cells were added either alone with 2.5 μg/ml concanavalin A (Sigma-Aldrich) as a positive control or together with 3 μg/ml mgG-2 in Iscove's basal medium (Biochrom AG) containing 10% fetal bovine serum (Sigma-Aldrich) with 100 μg/ml gentamicin (Sigma-Aldrich), 50 μM 2-mercaptoethanol (Sigma-Aldrich), and 2 mM l-glutamine (Biochrome AG). Samples were added in triplicate. Cells were incubated at 37°C with 5% CO2 for 96 h, after which 1 μCi of [6-3H]thymidine (Amersham Biosciences) was added, followed by an additional incubation overnight. The cells were harvested and run in a β-counter (1450 MicroBeta; Trilux) to measure the cell proliferation as counts per minute. Data were expressed as the arithmetic mean stimulation index (SI), defined as the amount of [6-3H]thymidine incorporated into antigen-stimulated cultures divided by the mean amount incorporated into corresponding unstimulated control cultures.
Cytokine detection.
Supernatants from the cell proliferation assay collected at 96 h (triplicates were pooled) and vaginal washes collected at day 1 p.i. were run in a cytokine ELISA for IFN-γ (Duoset R&D kit). High-binding 96-well plates (Greiner) were coated with capture antibody overnight at room temperature. Plates were blocked with 1% BSA for 1 h at 37°C. The standard and supernatants from cell proliferation were diluted 1:3, and vaginal washes were diluted 1:2. Diluted samples were added, and the plates were incubated at room temperature for 3 h, after which biotinylated detection antibody was added. The plates were incubated overnight at 4°C, and streptavidin-horseradish peroxidase was added. Finally, the plates were developed with 3,3′,5,5′-tetramethylbenzidine, the reaction was stopped with H2SO4, and the plates were read at 490 nm in a spectrophotometer. In addition, supernatants from restimulated spleen cells and CD4+ T cells were assayed for the presence of IFN-γ, IL-2, IL-4, and IL-5 by a BD cytometric bead array (CBA) mouse Th1/Th2 cytokine kit (BD Biosciences).
IVAG challenge with HSV-2.
Four weeks after the third immunization, mice were challenged intravaginally (IVAG) with wt 333. Six days prior to challenge, mice were injected s.c. with 3 mg of medroxyprogesterone (DepoProvera; Pharmacia). In all vaccine experiments, mice were infected with 15 μl containing 5 × 104 PFU (12× 50% lethal dose).
Scoring of disease.
Mice were observed and scored daily after challenge. Vaginal inflammation and neurological illness were graded as follows: healthy (score, 0), genital erythema (score, 1), moderate genital inflammation with blisters (score, 2), severe and purulent genital lesions with loss of hair (score, 3), and hind-limb paralysis and/or general bad condition (score, 4). Mice presenting a score of 4 were euthanized.
Viral plaque assay.
To estimate the replication of the virus in the vagina, vaginal washes were collected at day 3 p.i. Washes were obtained by gently pipetting 40 μl of Hanks balanced salt solution (HBSS) in and out of the vagina until a clump of mucus was retrieved, followed by a second wash with an additional 40 μl. Both washes were pooled and collected in a total volume of 1 ml HBSS. DRG, whole spinal cord (including the medulla oblongata), and whole brain were removed at day 6 p.i. All samples were kept frozen at −70°C until analyzed. DRG, spinal cord, and brain samples were thawed and homogenized using a Dounce tissue grinder. The samples were diluted in HBSS, and infectious HSV-2 was estimated by a plaque assay. Briefly, samples were added to a monolayer of GMK-AH1 cells and incubated for 1 h at room temperature, before Iscove's medium containing 1% methylcellulose (Sigma-Aldrich), 2% new born calf serum (Sigma-Aldrich), and 1% antibiotics was added. The cells were incubated at 37°C with 5% CO2 for 72 h before viral plaques were stained with crystal violet (Sigma-Aldrich) and the number of PFU was counted using light microscopy.
Detection of HSV-2 DNA by PCR.
HSV-2 DNA was quantified in sera at day 6 p.i., as well as in DRG and spinal cord samples collected from surviving mice at day 21 p.i. Samples were analyzed by real-time PCR. Viral DNA was extracted by a Magnapure LC robot (Roche). Subsequent amplification of a segment of the gB-2 gene was performed utilizing TaqMan probes labeled with 6-carboxyfluorescein (FAM) and 6-carboxytetramethylrhodamine (TAMRA) and an ABI Prism 7000 PCR instrument (Applied Biosystems). This assay shows high specificity and a similar sensitivity as a nested PCR system (41). A plasmid (pUC57) containing the target sequence was constructed (GenScript) and amplified in Escherichia coli XL-1 Blue, purified by a HiSpeed plasmid maxikit (Qiagen), and quantified by spectrophotometer analysis. A standard curve was included in each run and was based on six 5-fold dilutions of the plasmid using an initial concentration of 1 × 106 HSV-2 genome copies per reaction. Due to inhibition of the PCR by the high content of cellular DNA, tissue samples from DRG, spinal cord, and brain were diluted 1:4 in PBS before extraction of HSV-2 DNA.
Statistics.
SPSS (version 16) software for Microsoft Windows was used for statistical calculations. Fisher's exact test was used for survival data, the Mann-Whitney (nonparametric) test was used for titer values, and the general linear model (univariate) was used for HSV-2 content (PFU and HSV-2 DNA genome copies). P values of <0.05 were considered statistically significant.
RESULTS
The mgG-2 antigen did not contain HSV-2 cross-reactive proteins.
In order to rule out the possibility that the mgG-2 antigen contained other HSV-2 proteins, sera obtained from HSV-negative, HSV-1-positive, and HSV-2-positive individuals were subjected to ELISA using purified mgG-2 as the coating antigen. As expected, anti-mgG-2 MAbs and HSV-2-positive sera were clearly reactive. Sera from HSV-negative and HSV-1-positive individuals were unreactive to mgG-2 antigen in the ELISA, indicating that no other immunogenic viral glycoproteins were present in the mgG-2 antigen preparation. Further, a lack of other major HSV-2 glycoproteins in the mgG-2 preparations was confirmed in Western blots and by ELISA using anti-HSV MAbs directed against gB, gC, and gD (data not shown). Purified mgG-2 presented a band in WBs with an apparent molecular mass of 115 kDa and a weaker band with a molecular mass of 70 kDa using anti-mgG-2 MAbs and HSV-2-positive sera, as described earlier (12, 34). SDS-PAGE followed by silver staining showed a marked band corresponding to the O-glycosylated mgG-2 (115 kDa) (Fig. 1). The mgG-2 protein showed no toxic properties in the XTT assay, and the level of endotoxin was below the detection limit (<0.5 EU/ml; data not shown).
Fig 1.
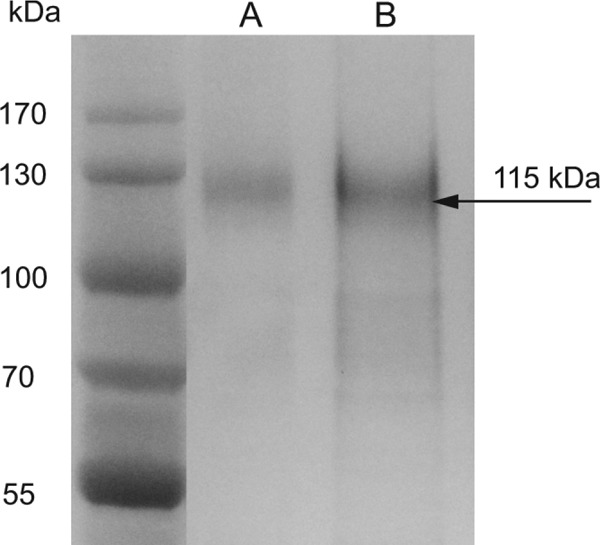
The mgG-2 protein, at a concentration of 1 μg (A) or 10 μg (B), is visualized by silver staining after separation by SDS-PAGE. The O-glycosylated mgG-2 protein (115 kDa) is indicated with an arrow.
mgG-2 induced antibody responses in vaccinated mice.
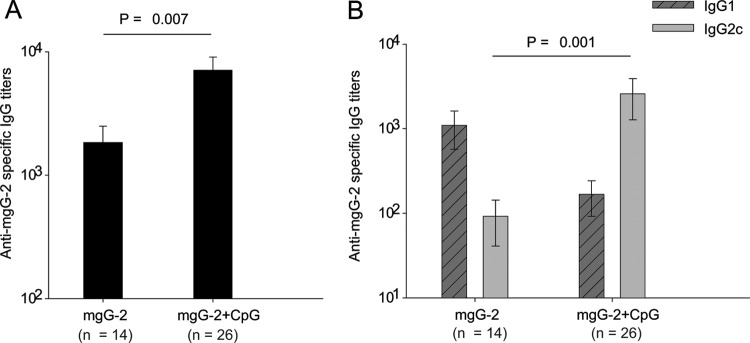
Sera collected from mice immunized with mgG-2 and mgG-2 plus CpG and from control mice 18 days after the third immunization were analyzed for anti-mgG-2 IgG, IgG1, and IgG2c antibodies. The mgG-2 antigen alone was immunogenic and elicited a marked antibody response. Mice immunized with mgG-2 plus CpG showed significantly higher IgG titers (P = 0.007) than the mgG-2 group (Fig. 2A). Serum samples were also analyzed for the presence of IgG1 and IgG2c subclass titers by using subclass-specific ELISAs. We found that mice immunized with mgG-2 alone presented generally higher IgG1 titers than IgG2c titers, while for sera from mice immunized with mgG-2 plus CpG, significantly higher IgG2c levels were detected (P = 0.001) (Fig. 2B).
Fig 2.
Immunization with mgG-2 elicits a B-cell response. Mice were immunized 3 times with mgG-2 or mgG-2 plus CpG. Three weeks after the last immunization, blood samples were collected. Sera were subjected to an mgG-2-specific IgG antibody ELISA (A) and a subclass ELISA for detection of IgG1 and IgG2c antibodies (B). Data are from four experiments and presented as mean ± SEM.
Immunization with mgG-2 plus CpG induced CD4+ T cell proliferation and enhanced IFN-γ production.
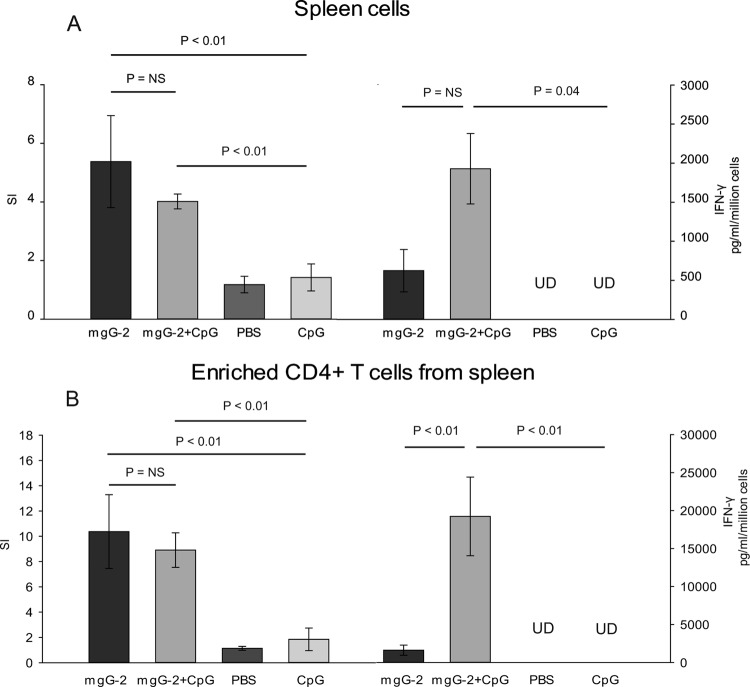
The role of cell-mediated immunity in HSV-2 protection has been investigated by others, showing that CD4+ as well as CD8+ T cells are of importance in HSV-2 clearance after a vaginal infection (38, 39, 47). In addition, the importance of IFN-γ in protection against genital herpes infection in mice is well documented (22, 49). A proliferation assay was used to examine if mgG-2 elicited a cellular immune response. Whole spleen cells, gLNs, and enriched splenic CD4+ T cells were collected from immunized mice and controls immunized with PBS. Spleen cells and enriched splenic CD4+ T cells were also collected from mice immunized with CpG alone. Collected cells were restimulated with mgG-2 in vitro. Spleen cells from control mice showed low SI values and undetectable levels of IFN-γ, while splenocytes from both mgG-2-immunized groups showed significantly higher SI values than the controls (P < 0.01) (Fig. 3A). The IFN-γ production in spleen cells for the mgG-2 plus CpG group was significantly higher than that in spleen cells for the controls (P = 0.04). Although the IFN-γ production was higher in the mgG-2 plus CpG group than the mgG-2 group, the difference was not statistically significant (P = 0.09). For both vaccinated groups, the SI was significantly higher than that for the controls, with no significant difference between mice given mgG-2 or mgG-2 plus CpG (Fig. 3A and B). However, the level of IFN-γ production for CD4+ T cells collected from mice immunized with mgG-2 plus CpG was significantly higher than that for cells from mice vaccinated with mgG-2 or controls (P < 0.01) (Fig. 3B). Proliferation from gLNs was low, with no IFN-γ production detected from any of the groups (data not shown). To confirm the enhanced IFN-γ production in restimulated spleen cells and enriched CD4+ T cells, all samples were reanalyzed using the CBA Th1/Th2 cytokine assay. Similar to what was described with the ELISA technique, significantly higher IFN-γ levels were detected for the mgG-2 plus CpG group than the mgG-2 group. In addition, for the mgG-2 group, IL-5 was detected in supernatant from both spleen cells and splenic CD4+ T cells, indicating a Th2-biased immune response. No significant levels of IL-2 or IL-4 could be detected for any of the groups (data not shown). Taken together, our results show that immunization with mgG-2 plus CpG can induce an antigen-specific Th1-type T-cell response.
Fig 3.
Protection against HSV-2 disease correlates with IFN-γ production in antigen-restimulated CD4+ cells. Female C57BL/6 mice were immunized with three doses of mgG-2 with or without the adjuvant CpG. Control mice which received PBS or CpG alone were also included. Three weeks after the last booster dose, three mice per group were sacrificed. Pooled single-cell suspensions from spleen cells (A) and CD4+ T cells derived from pooled spleen cells (B) were cultured in triplicate in 96-well plates and stimulated with 3 μg/ml of mgG-2 for 96 h, after which radioactively labeled thymidine was added. The proliferative response was measured and expressed as the mean SI (left). IFN-γ production from restimulated spleen cells and enriched CD4+ T cells was detected by ELISA in supernatants after 96 h of culture (right). Data for spleen cells are from four experiments, and for CD4+ T cells, data are from two experiments. Bars show mean ± SEM. UD, undetectable; NS, not significant.
Immunization with mgG-2 plus CpG induces protection in a mouse model of genital herpes.
Groups of 6 to 10 mice were immunized with one s.c. injection of mgG-2 or mgG-2 plus CpG, followed by two intranasal instillations as booster doses. As shown in Fig. 4A, nonimmune mice showed progressive disease starting at day 4 after challenge, and all mice were euthanized due to neurological disease at day 10. The mgG-2 group showed signs of vaginal infection at day 4. Although a delay in disease progression was observed, the survival rate was not significantly different from that for the controls (Fig. 4B). Mice vaccinated with mgG-2 plus CpG showed mild or no genital inflammation. Immunization with mgG-2 plus CpG conferred a protection rate (73%) significantly higher than that for the controls (0%, P < 0.0001) or that conferred by immunization with mgG-2 alone (20%, P = 0.0003) (Fig. 4B).
Fig 4.
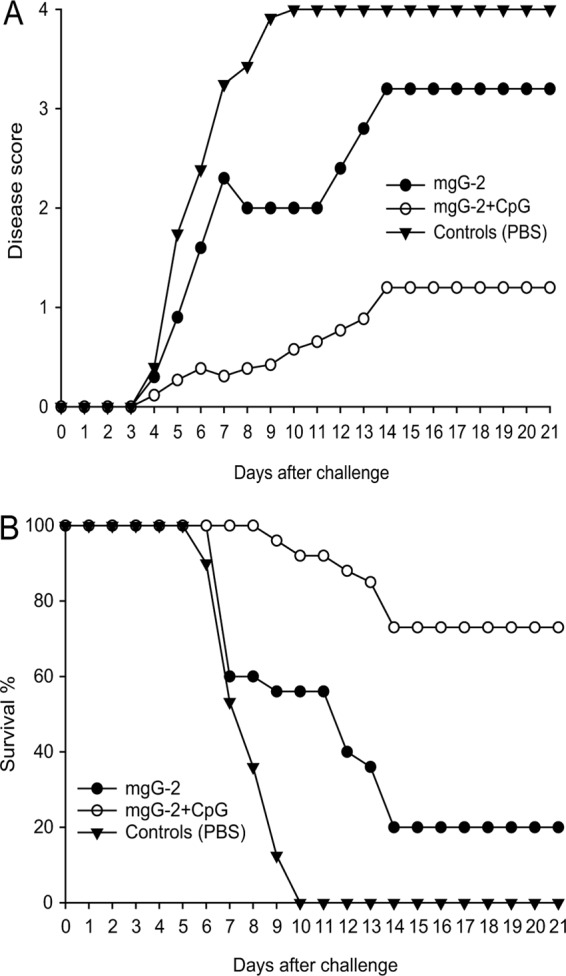
Vaccination with mgG-2 plus CpG confers protection against lethal HSV-2 disease. Mice were immunized three times with mgG-2 with or without adjuvant CpG. The disease score (A) and survival rate (B) after intravaginal challenge with 5 × 104 PFU of a fully virulent HSV-2 strain (wt 333) are illustrated. Data are pooled from four experiments with a total of 26 mice in each group. The differences in survival rate between the mgG-2 plus CpG group and the mgG-2 group or controls are highly significant (P < 0.0001 and P = 0.0003).
Immunization with mgG-2 plus CpG fails to generate protection in IFN-γ gene-knockout mice.
To confirm the importance of a Th1-type immune response in the protection, mice defective in the IFN-γ gene were immunized with mgG-2 plus CpG three times and challenged with a lethal dose of HSV-2 333, as described above. As shown in Fig. 5, no protection was observed and all mice developed severe disease and were euthanized at day 6 or 7 p.i. This result implies that IFN-γ plays an important role in the immunity induced following mgG-2 plus CpG immunization.
Fig 5.

No protection in immunized IFN-γ gene-knockout mice. Disease score (A) and survival rate (B) for IFN-γ gene-knockout mice immunized with mgG-2 plus CpG and unimmunized controls are illustrated. Mice were immunized three times and challenged with 5 × 104 PFU of wt 333. Data are from one experiment.
Reduction of HSV-2 load in vagina, spinal cord, and serum.
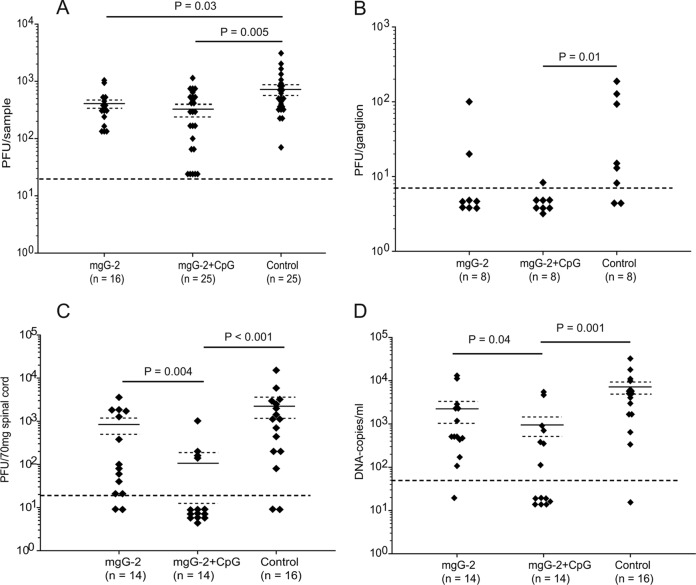
Vaginal washes were collected at 3 days p.i., and infectious virus was measured by a plaque assay. The mgG-2 and mgG-2 plus CpG groups presented significantly lower HSV-2 titers (mean values, 394 PFU/sample [P = 0.03] and 344 PFU/sample [P = 0.005], respectively) than the control group (mean value, 762 PFU/sample) (Fig. 6A). Although the difference in survival rates between mice vaccinated with mgG-2 and mgG-2 plus CpG was significant, no statistically significant differences in the viral load in the vagina were detected between the groups. Next, we investigated the presence of infectious virus in neuronal tissue. Results for the mgG-2, mgG-2 plus CpG, and control groups at day 6 p.i. are summarized in Fig. 6B and C. For the mgG-2 group, infectious HSV-2 was isolated from DRG in 2/8 mice. For the mgG-2 plus CpG group, 1 mouse of 8 was isolation positive, presenting a value close to the detection limit of 20 PFU/3 ganglia (∼7 PFU/ganglion). There was a significant difference (P = 0.01) between the mgG-2 plus CpG group and controls. For the spinal cords, significantly lower levels of infectious virus were detected for the mgG-2 plus CpG group (mean value, 109 PFU/70 mg spinal cord) than for the mgG-2 group (mean value, 821 PFU/70 mg spinal cord; P = 0.004) or controls (mean value, 2,314 PFU/70 mg spinal cord; P < 0.001) (Fig. 6C). HSV-2 could not be isolated from brain tissue collected from any of the groups (data not shown). Viral DNA was also quantified in DRG and spinal cord from six surviving mice in the mgG-2 and mgG-2 plus CpG groups at day 21 p.i. A low DNA copy number per ganglion (close to the detection limit) was found in DRG from 3/6 (mean value, 120) and 4/6 (mean value, 36) mice in the mgG-2 and mgG-2 plus CpG groups, respectively. For the spinal cord, 5/6 mice from the mgG-2 group were positive (mean value, 930), compared with 4/6 mice (mean value, 73) from the mgG-2 plus CpG group (data not shown).
Fig 6.
Significant reductions of viral load in vaginal washes, spinal cord, DRG, and serum. Viral loads are expressed as infectious HSV-2 (PFU) in vaginal washes at day 3 p.i. (data are from four experiments) (A). PFU were detected at day 6 p.i. in lumbosacral ganglia (data are from one experiment) (B) and spinal cord (two experiments) (C). HSV-2 DNA was detected in serum at day 6 p.i. by quantitative PCR (data are from two experiments) (D). Detection limits of 20 PFU/ml for vaginal washes, 7 PFU/ganglion, 20 PFU/70 mg spinal cord, and 50 DNA copies/ml for serum are indicated with dashed lines. All samples presenting negative results are plotted in groups below the detection limit. Arithmetic mean values (solid lines) ± SEM (dashed lines) are indicated in panels A, C, and D.
We also quantified the viral load in serum at day 6 p.i. by real-time PCR. Results are presented in Fig. 6D. The viral load in sera from the mgG-2 plus CpG group (mean value, 907 DNA copies/ml) was significantly lower than that in sera from the mgG-2 group (mean value, 2,554 DNA copies/ml; P = 0.04) or controls (mean value, 6,898 DNA copies/ml; P = 0.001).
Vaginal IFN-γ response in immunized mice after challenge.
Since protection in the mgG-2 plus CpG group correlated to IFN-γ production in CD4+ T cells, we also measured IFN-γ in vaginal washes collected at day 1 p.i. As shown in Fig. 7, the IFN-γ levels for the mgG-2 plus CpG group were significantly higher (mean value, 235; P = 0.05) than those for the mgG-2 group (mean value, 89) and controls immunized with PBS or CpG alone, in which the values for all except one of the mice were below the detection limit.
Fig 7.
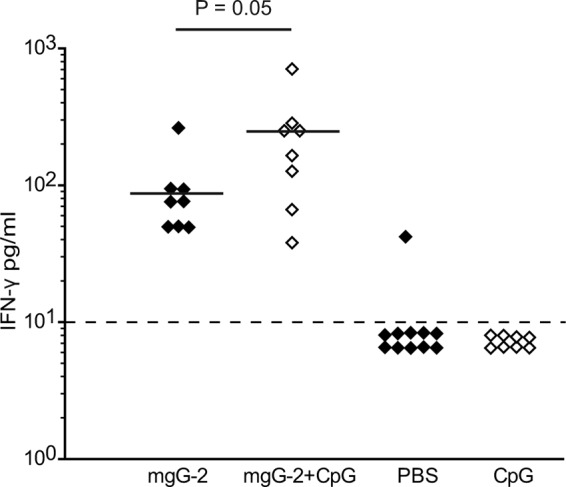
Higher levels of IFN-γ in vaginal washes from mice immunized with mgG-2 plus CpG. IFN-γ was analyzed in vaginal washes at day 1 p.i. Results are from one experiment and presented as pg/ml. The detection limit of 10 pg/ml is indicated with a dashed line.
Lack of neutralization capacity.
As antibody-mediated NT has been considered important for protection against certain viruses after natural infection or after immunization, we investigated NT activity. Immune sera collected from mice belonging to the mgG-2 (n = 5) and the mgG-2 plus CpG (n = 11) groups were tested. None of the immune sera presented NT activity alone or in the presence of complement (titers < 1/20) (Table 1).
Table 1.
Results from serological assays for sera from mice immunized with mgG-2, mgG-2 plus CpG, or PBS (negative sera)
| Method | No. of positive serum samples/total no. of serum samples from mice immunized with: |
||
|---|---|---|---|
| mgG-2 | mgG-2+CpG | PBS | |
| Neutralizationa | 0/5 | 0/11 | 0/3 |
| MP-ADCCb | NDd | 11/19 | 0/2 |
| NK-ADCCb | ND | 4/9 | 0/2 |
| ACMCc | 3/10 | 8/12 | 0/2 |
An NT titer of ≥20 was considered a positive result. Sera were tested alone and by addition of complement.
Macrophage-mediated ADCC (MP-ADCC) and NK cell-mediated ADCC (NK-ADCC) titers of 20 were considered positive.
Sera showing an antibody-dependent complement-mediated cytolysis (ACMC) titer of ≥25 were considered positive.
ND, not done.
Antibody-dependent cell-mediated cytotoxicity.
Since MP as well as NK cells can mediate ADCC, we used enriched fractions of these cells as effectors in ADCC assays (MP-ADCC and NK-ADCC). Eleven of 19 serum samples (58%) collected from mice immunized with mgG-2 plus CpG showed MP-ADCC, and 4 of 9 serum samples (44%) showed NK-ADCC (Table 1). We also investigated if there was a correlation between IgG antibody response and ADCC by comparison of the magnitude of anti-mgG-2-specific IgG, IgG1, and IgG2c titers for sera presenting positive or negative ADCC. Sera which presented a positive MP-ADCC showed significantly higher IgG titers (P = 0.02) and IgG2c titers (P = 0.012) than MP-ADCC-negative sera. There were no significant differences in IgG1 titers between MP-ADCC-positive and -negative sera or between total IgG or subclass titers and NK-ADCC (data not shown).
Antibody-dependent complement-mediated cytolysis.
We investigated if sera from immunized mice could mediate ACMC. Sera were tested at 2-fold dilutions starting at 1:25. Three of 10 serum samples collected from the mgG-2 group and 8/12 serum samples collected from the mgG-2 plus CpG group were positive (Table 1). All positive samples from both immunized groups showed titers of between ≥25 and ≤100.
DISCUSSION
We show that a combined systemic and mucosal immunization with mgG-2 plus CpG could elicit significant protection against vaginal challenge with an otherwise lethal dose of a fully virulent HSV-2 strain in mice. The observed protection induced by immunization with mgG-2 plus CpG was found to be associated with IFN-γ production from CD4+ T cells and by higher levels of IFN-γ in the vaginal washes at day 1 p.i. The importance of IFN-γ in the protection induced by immunization with mgG-2 plus CpG was further documented, in that immunization with mgG-2 plus CpG failed to elicit any appreciable level of protection in IFN-γ gene-knockout mice. Several studies using the murine model have shown that CD4+ T cells and IFN-γ production play important roles in protection against genital infection with HSV-2 (39, 47–49). Previous studies using a live attenuated thymidine kinase-deficient HSV-2 strain for immunization followed by a subsequent IVAG challenge have also shown that IFN-γ-secreting CD4+ T cells are crucial for protection (23, 24, 44, 49). Furthermore, vaccinated CD4+ T-cell-deficient mice, which rapidly die after a genital challenge, were shown to be rescued by treatment with exogenous IFN-γ (22).
Vaccination with mgG-2 alone or mgG-2 plus CpG significantly reduced the viral load in vaginal washes compared with that in controls. Although there was no significant difference in the numbers of infectious HSV-2 particles in the vaginal washes between the vaccinated groups, the significant reduction of HSV DNA in serum detected for mice immunized with mgG-2 plus CpG suggests that the overall viral replication was reduced. IFN-γ produced locally has been shown to be effective at reduction of the infection in the genital tract. It is therefore reasonable to assume that the higher levels of IFN-γ detected in the vaginal washes in the mgG-2 plus CpG group contributed to clearance of the infection more efficiently. An important issue in HSV-2 vaccine development is to achieve protection from infection of the DRG, spinal cord, and CNS. The increased survival rate and the lower disease scores for mice immunized with mgG-2 plus CpG were associated with significantly lower titers of HSV-2 in the spinal cord. In vitro studies using an mgG-2-negative HSV-2 mutant described that mgG-2 is involved in the extracellular release of infectious particles (1). Our data presented here suggest that the induced immune responses could suppress infectious HSV-2 from infecting neurons. However, IFN-γ has been shown to inhibit viral replication in the nervous system via induction of nitric oxide synthase, leading to production of nitric oxide (10), and IFN-γ-secreting CD4+ T cells are able to clear HSV-1 from sensory ganglia and spinal cord in a murine model (27). An alternative explanation to the reduced viral load in the nervous system tissue described here might be that locally produced IFN-γ can clear infectious HSV-2 particles of already infected neurons.
Several studies using B-cell-knockout mice challenged with a lethal dose of wild-type HSV-2 IVAG have shown that antibodies have a limiting effect in early stages of the vaginal infection (<24 h), with a reduced local viral load (16, 22, 40, 46). mgG-2 induced a high specific IgG antibody response both alone and when used together with CpG, albeit with an IgG2c subclass bias when mgG-2 was used together with CpG. The majority of sera from mice immunized with mgG-2 plus CpG exerted MP-ADCC activity, and the activity correlated with the IgG2c titers. It is well-known that the mouse IgG2 subclass binds to FcγRI more efficiently than IgG1 (50). Protection induced by passive transfer of anti-HSV MAbs or immune sera in mice was shown to correlate with Fcγ-dependent mechanisms, such as ADCC (2, 11, 25). As a single mgG-2 MAb was earlier shown to present low ADCC activity (2), four of our own produced anti-mgG-2 MAbs (34) were tested. They were all negative (unpublished observation). The discrepancy between the ADCC function of immune sera from vaccinated mice and the MAbs may be explained by the fact that all MAbs tested are of the IgG1 subclass. Furthermore, it was stated that the relatively poor efficacy observed in clinical HSV-2 vaccine trials, using gD and gB as immunization antigens, may have partially been the result of failure to elicit high levels of antibodies capable of mediating ADCC (31). Taken together, ADCC is a potentially important mechanism of antibodies in the control of the HSV infection.
Another interesting finding was that the immune sera presented no NT activity. Although there is no clear correlation between protection and levels of NT antibodies in either humans or animal models, this study is to our knowledge the first to report that it is possible to induce protection without NT activity of serum. Two anti-mgG-2 MAbs and a rabbit hyperimmune serum have also been shown earlier to be devoid of neutralizing activity (2, 4, 43). Furthermore, our own produced anti-mgG-2 MAbs exerted no NT activity with or without complement in GMK-AH1 cells (unpublished observation). The neutralizing antibody response has previously been used as the only surrogate of antibody-mediated protection in animal and human trials. The lack of NT activity of anti-mgG-2 antibodies may explain why mgG-2 has not been evaluated earlier as a vaccine candidate. However, clones from a random phage peptide display library obtained by using an anti-mgG-2 MAb (34) were used as immunogens and administered subcutaneously in a vaccination model in mice. The selected phage clones, which contained short stretches of mgG-2 (3 to 4 amino acids), induced partial protection (≤60%) against challenge with HSV-2 (21).
Taken together, we report for the first time that a combined systemic and mucosal immunization with mgG-2 plus CpG can generate considerable protective immunity to a lethal vaginal challenge with a fully virulent HSV-2 strain in mice. The protection was found to be associated with the IFN-γ response by CD4+ T cells and in the vagina. These observations encourage further evaluation of mgG-2 as a vaccine candidate for induction of protective immunity to HSV-2-induced infection or disease.
ACKNOWLEDGMENTS
This work was supported by grants from the ALF Foundation at Sahlgrenska University Hospital, the Gothenburg Medical Society (GLS), the Swedish Research Council, and the Magnus Bergvall Foundation. The study was also supported by the Swedish Foundation for International Cooperation in Research and Higher Education (STINT) and a European Commission Euronanomed iNanoDC grant.
We thank Kristina Broliden for critical review of the manuscript. The authors are also grateful to Mona Brantefjord, Carolina Gustafsson, and Karolina Thörn for excellent laboratory assistance.
Footnotes
Published ahead of print 2 May 2012
REFERENCES
- 1. Adamiak B, Ekblad M, Bergstrom T, Ferro V, Trybala E. 2007. Herpes simplex virus type 2 glycoprotein G is targeted by the sulfated oligo- and polysaccharide inhibitors of virus attachment to cells. J. Virol. 81:13424–13434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balachandran N, Bacchetti S, Rawls WE. 1982. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect. Immun. 37:1132–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balachandran N, Hutt-Fletcher LM. 1985. Synthesis and processing of glycoprotein gG of herpes simplex virus type 2. J. Virol. 54:825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balachandran N, Oba DE, Hutt-Fletcher LM. 1987. Antigenic cross-reactions among herpes simplex virus types 1 and 2, Epstein-Barr virus, and cytomegalovirus. J. Virol. 61:1125–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belshe RB, et al. 2012. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 366:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergstrom T, Sjogren-Jansson E, Jeansson S, Lycke E. 1992. Mapping neuroinvasiveness of the herpes simplex virus type 1 encephalitis-inducing strain 2762 by the use of monoclonal antibodies. Mol. Cell. Probes 6:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhat AA, Seth RK, Babu J, Biswas S, Rao DN. 2009. Induction of mucosal and systemic humoral immune responses in murine system by intranasal immunization with peptide antigens of P. vivax and CpG oligodeoxynucleotide (ODN) in microparticle delivery. Int. Immunopharmacol. 9:1197–1208 [DOI] [PubMed] [Google Scholar]
- 8. Brown ZA, et al. 1997. The acquisition of herpes simplex virus during pregnancy. N. Engl. J. Med. 337:509–515 [DOI] [PubMed] [Google Scholar]
- 9. Carmack MA, et al. 1996. T cell recognition and cytokine production elicited by common and type-specific glycoproteins of herpes simplex virus type 1 and type 2. J. Infect. Dis. 174:899–906 [DOI] [PubMed] [Google Scholar]
- 10. Chesler DA, Reiss CS. 2002. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 13:441–454 [DOI] [PubMed] [Google Scholar]
- 11. Chu CF, et al. 2008. Antibody-mediated protection against genital herpes simplex virus type 2 disease in mice by Fc gamma receptor-dependent and -independent mechanisms. J. Reprod. Immunol. 78:58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clavet CR, Margolin AB, Regan PM. 2004. Herpes simplex virus type-2 specific glycoprotein G-2 immunomagnetically captured from HEp-2 infected tissue culture extracts. J. Virol. Methods 119:121–128 [DOI] [PubMed] [Google Scholar]
- 13. Cohen J. 2010. Immunology. Painful failure of promising genital herpes vaccine. Science 330:304. [DOI] [PubMed] [Google Scholar]
- 14. Corey L, et al. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA 282:331–340 [DOI] [PubMed] [Google Scholar]
- 15. Dalton DK, et al. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739–1742 [DOI] [PubMed] [Google Scholar]
- 16. Dudley KL, Bourne N, Milligan GN. 2000. Immune protection against HSV-2 in B-cell-deficient mice. Virology 270:454–463 [DOI] [PubMed] [Google Scholar]
- 17. Eriksson K, et al. 2004. CD4(+) T-cell responses to herpes simplex virus type 2 (HSV-2) glycoprotein G are type specific and differ in symptomatic and asymptomatic HSV-2-infected individuals. J. Gen. Virol. 85:2139–2147 [DOI] [PubMed] [Google Scholar]
- 18. Fleming DT, et al. 1997. Herpes simplex virus type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 337:1105–1111 [DOI] [PubMed] [Google Scholar]
- 19. Freeman EE, et al. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83 [DOI] [PubMed] [Google Scholar]
- 20. Gallichan WS, et al. 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166:3451–3457 [DOI] [PubMed] [Google Scholar]
- 21. Grabowska AM, et al. 2000. Immunisation with phage displaying peptides representing single epitopes of the glycoprotein G can give rise to partial protective immunity to HSV-2. Virology 269:47–53 [DOI] [PubMed] [Google Scholar]
- 22. Harandi AM, Svennerholm B, Holmgren J, Eriksson K. 2001. Differential roles of B cells and IFN-gamma-secreting CD4(+) T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J. Gen. Virol. 82:845–853 [DOI] [PubMed] [Google Scholar]
- 23. Harandi AM, Svennerholm B, Holmgren J, Eriksson K. 2001. Protective vaccination against genital herpes simplex virus type 2 (HSV-2) infection in mice is associated with a rapid induction of local IFN-gamma-dependent RANTES production following a vaginal viral challenge. Am. J. Reprod. Immunol. 46:420–424 [DOI] [PubMed] [Google Scholar]
- 24. Iijima N, et al. 2008. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J. Exp. Med. 205:3041–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishizaka ST, Piacente P, Silva J, Mishkin EM. 1995. IgG subtype is correlated with efficiency of passive protection and effector function of anti-herpes simplex virus glycoprotein D monoclonal antibodies. J. Infect. Dis. 172:1108–1111 [DOI] [PubMed] [Google Scholar]
- 26. Jeansson S, Molin L. 1974. On the occurrence of genital herpes simplex virus infection. Clinical and virological findings and relation to gonorrhoea. Acta Derm. Venereol. 54:479–485 [PubMed] [Google Scholar]
- 27. Johnson AJ, Chu CF, Milligan GN. 2008. Effector CD4+ T-cell involvement in clearance of infectious herpes simplex virus type 1 from sensory ganglia and spinal cords. J. Virol. 82:9678–9688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnston C, Koelle DM, Wald A. 2011. HSV-2: in pursuit of a vaccine. J. Clin. Invest. 121:4600–4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koelle DM, Corey L. 2003. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin. Microbiol. Rev. 16:96–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kohl S, Cahall DL, Walters DL, Schaffner VE. 1979. Murine antibody-dependent cellular cytotoxicity to herpes simplex virus-infected target cells. J. Immunol. 123:25–30 [PubMed] [Google Scholar]
- 31. Kohl S, et al. 2000. Limited antibody-dependent cellular cytotoxicity antibody response induced by a herpes simplex virus type 2 subunit vaccine. J. Infect. Dis. 181:335–339 [DOI] [PubMed] [Google Scholar]
- 32. Langenberg AG, Corey L, Ashley RL, Leong WP, Straus SE. 1999. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N. Engl. J. Med. 341:1432–1438 [DOI] [PubMed] [Google Scholar]
- 33. Liljeqvist JA, Svennerholm B, Bergstrom T. 1999. Herpes simplex virus type 2 glycoprotein G-negative clinical isolates are generated by single frameshift mutations. J. Virol. 73:9796–9802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liljeqvist JA, et al. 1998. Localization of type-specific epitopes of herpes simplex virus type 2 glycoprotein G recognized by human and mouse antibodies. J. Gen. Virol. 79(Pt 5):1215–1224 [DOI] [PubMed] [Google Scholar]
- 35. Looker KJ, Garnett GP, Schmid GP. 2008. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull. World Health Organ. 86:805–812, A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marsden HS, Buckmaster A, Palfreyman JW, Hope RG, Minson AC. 1984. Characterization of the 92,000-dalton glycoprotein induced by herpes simplex virus type 2. J. Virol. 50:547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McClung H, Seth P, Rawls WE. 1976. Relative concentrations in human sera of antibodies to cross-reacting and specific antigens of herpes simplex virus types 1 and 2. Am. J. Epidemiol. 104:192–201 [DOI] [PubMed] [Google Scholar]
- 38. McDermott MR, Brais PL, PLoettsche GC, Evelegh MJ, Goldsmith CH. 1987. Expression of immunity to intravaginal herpes simplex virus type 2 infection in the genital tract and associated lymph nodes. Arch. Virol. 93:51–68 [DOI] [PubMed] [Google Scholar]
- 39. Milligan GN, Bernstein DI. 1997. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 229:259–268 [DOI] [PubMed] [Google Scholar]
- 40. Morrison LA, Zhu L, Thebeau LG. 2001. Vaccine-induced serum immunoglobin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. J. Virol. 75:1195–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Namvar L, Olofsson S, Bergstrom T, Lindh M. 2005. Detection and typing of herpes simplex virus (HSV) in mucocutaneous samples by TaqMan PCR targeting a gB segment homologous for HSV types 1 and 2. J. Clin. Microbiol. 43:2058–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olofsson S, Jeansson S, Lycke E. 1981. Unusual lectin-binding properties of a herpes simplex virus type 1-specific glycoprotein. J. Virol. 38:564–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Para MF, Parish ML, Noble AG, Spear PG. 1985. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J. Virol. 55:483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parr EL, Parr MB. 1999. Immune responses and protection against vaginal infection after nasal or vaginal immunization with attenuated herpes simplex virus type-2. Immunology 98:639–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parr EL, Parr MB. 1998. Immunoglobulin G, plasma cells, and lymphocytes in the murine vagina after vaginal or parenteral immunization with attenuated herpes simplex virus type 2. J. Virol. 72:5137–5145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parr MB, Parr EL. 2000. Immunity to vaginal herpes simplex virus-2 infection in B-cell knockout mice. Immunology 101:126–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parr MB, Parr EL. 1998. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J. Virol. 72:2677–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parr MB, Parr EL. 1999. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology 258:282–294 [DOI] [PubMed] [Google Scholar]
- 49. Parr MB, Parr EL. 2003. Vaginal immunity in the HSV-2 mouse model. Int. Rev. Immunol. 22:43–63 [DOI] [PubMed] [Google Scholar]
- 50. Ravetch JV, Kinet JP. 1991. Fc receptors. Annu. Rev. Immunol. 9:457–492 [DOI] [PubMed] [Google Scholar]
- 51. Roizman B, Norrild B, Chan C, Pereira L. 1984. Identification and preliminary mapping with monoclonal antibodies of a herpes simplex virus type 2 glycoprotein lacking a known type 1 counterpart. Virology 133:242–247 [DOI] [PubMed] [Google Scholar]
- 52. Smith JS, Robinson NJ. 2002. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J. Infect. Dis. 186(Suppl 1):S3–S28 [DOI] [PubMed] [Google Scholar]
- 53. Stanberry LR, et al. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652–1661 [DOI] [PubMed] [Google Scholar]
- 54. Su HK, Courtney RJ. 1988. Inducible expression of herpes simplex virus type 2 glycoprotein gene gG-2 in a mammalian cell line. J. Virol. 62:3668–3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Svennerholm B, Olofsson S, Jeansson S, Vahlne A, Lycke E. 1984. Herpes simplex virus type-selective enzyme-linked immunosorbent assay with Helix pomatia lectin-purified antigens. J. Clin. Microbiol. 19:235–239 [DOI] [PMC free article] [PubMed] [Google Scholar]