Abstract
Cryptococcus neoformans, the etiologic agent of cryptococcosis, is an obligately aerobic yeast that inhabits an environmental niche exposed to ambient air. The cell doubling time was significantly prolonged under 1% O2 relative to that under normoxic conditions. No apparent cell cycle arrest occurred following a shift from ambient air to 1% O2. However, yeast cells became hypersensitive to the actin monomer-sequestering agent latrunculin A at 1% O2, indicating that proper actin function is critical for growth at low oxygen concentrations. We showed that Sac6, an actin-binding protein, played an important role in cell growth under low oxygen conditions. Sac6 colocalized with cortical actin patches and with the ring structures between mother cells and buds. Under low oxygen conditions, the sac6 deletion mutant grew poorly, and accumulation of the actin capping protein Cap1 was observed in the vacuole of the sac6Δ strain. Furthermore, endocytic processes were hampered in the sac6Δ mutant, but cell polarity and cytokinesis were not visibly disturbed. The deficiency of endocytosis in the sac6Δ strain could be rescued by 1 M sorbitol under 1% O2, but growth remained retarded. These results suggest an absence of a direct link in C. neoformans between endocytosis and coping with the stress of low oxygen conditions. This interpretation is further supported by the observation that deletion of three conserved genes, ABP1, CRN1, and SLA2, which play important roles in endocytosis, had no effect on growth under 1% O2. Interestingly, deletion of SAC6 in C. neoformans had no effect on virulence in mice.
INTRODUCTION
The human fungal pathogen Cryptococcus neoformans exists in ambient-air (20 to 21% oxygen) natural environments, such as soil surfaces, dehydrated pigeon droppings, and decaying tree barks (23). Airborne cells of C. neoformans inhaled by the host reach the central nervous system by hematogenous dissemination and cause life-threatening meningoencephalitis. The oxygen concentration in the human brain is considerably lower than that in ambient air and differs significantly between anatomical sites (15). In the laboratory, the atmospheric oxygen level is more important for optimum growth of C. neoformans than the level of glucose in the culture medium (28). Since the oxygen level in the host brain is suboptimal for growth, C. neoformans would have to initiate specific cellular processes in order to adapt to the new environment.
The importance of exposure to oxygen-limited environments during fungal pathogenesis has gained appreciation in the past few years (for a review, see reference 18). Recently, several studies have begun to unravel the cellular machinery that enables C. neoformans to handle the challenge of growth in a low-oxygen environment. Sre1, the mammalian sterol regulatory element-binding protein (SREBP) homolog, is required for sterol homeostasis, oxygen sensing, and virulence in mice (8, 11). Low oxygen concentrations trigger activation of the membrane-bound Sre1 by the cleavage-activating protein Scp1 (8). Cleavage of Sre1 within its predicted first transmembrane segment is executed by Stp1, a functionally conserved ortholog of the mammalian Site-2 protease (7). After the cleavage, Sre1 is further modified by phosphorylation. Several mutants exhibiting high susceptibility to low oxygen concentrations and alteration in Sre1 production have been identified in C. neoformans (9). Like mammalian SREBP, Sre1 regulates many genes in the sterol biosynthetic pathway that are required for normal sterol synthesis under normoxic conditions as well as under low oxygen conditions (8). In a low-oxygen environment, Sre1 also regulates the activation of the genes that are involved in high-affinity uptake of iron and copper, including genes for both siderophore-mediated and reductive iron transport. Furthermore, a genetic screen using the hypoxia-mimetic cobalt chloride (CoCl2) revealed that mutants compromised in mitochondrial function are defective in survival under both high concentrations of CoCl2 and low oxygen conditions (19). Expression and functional analysis of the genes revealed that susceptibility to low oxygen conditions is linked to mitochondrial function, sterol and iron homeostasis, ubiquitination, and the ability of cells to respond to reactive oxygen species (19). These findings imply that multiple pathways are involved in oxygen sensing in C. neoformans.
We used a genetic approach to identify genes that are important for the growth of C. neoformans under low-oxygen stress. By employing Agrobacterium tumefaciens-mediated transformation, we have previously identified C. neoformans clones containing mutations in various genes that function in the Sre1 pathway, important for growth under low oxygen conditions at 37°C (9). We have also identified several clones containing mutations in genes that are unrelated to the Sre1 pathway and share high homology with actin-binding proteins (see below). Actin is a highly conserved protein found in all eukaryotes; it functions in fundamental processes such as cell polarity, cytokinesis, and endocytosis. These events are driven by the coordinated activities of a set of more than 20 highly conserved actin-binding proteins (for reviews, see references 4 and 26). The actin-binding proteins function in driving the polymerization of actin, organizing actin filaments into higher-order structures, such as bundles, severing actin filaments, or depolymerizing filaments to their monomeric state. The combined activities of these proteins control the spatial and temporal assembly of actin structures and ensure a dynamic turnover of actin structures in response to internal and external cues.
In this report, we characterize the growth behavior of C. neoformans under 1% O2 and demonstrate that C. neoformans cells are hypersensitive to the actin-depolymerizing agent latrunculin A under low oxygen conditions. The importance of normal actin function for growth at low oxygen concentrations was supported by the observation that deletion of two actin-binding protein homologs, Sac6 and Tpm1, caused a more pronounced growth defect under low oxygen conditions than under normoxic conditions. Sac6 shares homology with fimbrin, an actin-bundling protein, which functions in the organization and maintenance of the actin cytoskeleton (1, 2, 17). Tpm1 shares homology with tropomyosin, which binds to and stabilizes actin cables and filaments that direct polarized cell growth and the distribution of several organelles (24, 32). Furthermore, the actin cytoskeleton is known to play a direct role in endocytosis (4, 13). However, we provide evidence that endocytosis is not directly involved in the growth of C. neoformans in a low-oxygen environment.
MATERIALS AND METHODS
Strains, media, and growth conditions.
All strains used in this study are listed in Table 1. IFM 49144 was obtained from S. Kawamoto at Chiba University in Japan and is of the VNI molecular type, like the serotype A strain H99 (data not shown). All other strains were derived from B-3501A, a serotype D strain for which the genome has been sequenced and annotated. YEPD contained 1% yeast extract, 2% Bacto peptone, and 2% dextrose. YPG contained 1% each glucose, yeast extract, and polypeptone. Low oxygen conditions were maintained using an Invivo2 400 workstation at the temperatures indicated in the text and figure legends (Ruskinn, United Kingdom). To mimic the host environment of the animal, cryptococcal cells in all experiments under low O2 conditions were grown in the presence of 5% CO2. To simplify, we described only the oxygen concentration without mentioning the CO2 concentration in most of our study. To calculate the solubility of oxygen in water under 1% O2 at 30°C, we used Henry's law and the equation recommended by Benson et al. to obtain the mole fraction oxygen solubility (6). The mole fraction of oxygen was then converted to the amount of dissolved oxygen, 0.3795 mg/liter (35). Latrunculin A (LatA) was dissolved in dimethyl sulfoxide (DMSO) and was incorporated into the medium at the indicated concentrations. For the analysis of growth phenotypes, serial dilutions of cultures were spotted onto culture plates, incubated under the indicated conditions for 3 to 4 days, and photographed.
Table 1.
List of strains relevant to this study
| Strain | Genotype |
|---|---|
| C747 | sac6-T DNA-NEO |
| C784 | tpm1-T DNA-NEO |
| C960 | bni1-T DNA-NEO |
| C1412 | sac6Δ::NEO |
| C1435 | GPF-SAC6-NAT |
| C1454 | CAP1-RFP-HYG sac6Δ::NEO |
| C1456 | CAP1-RFP-HYG |
| C1458 | sac6Δ::NEO cap1Δ::HYG |
| C1460 | cap1Δ::HYG GPF-SAC6-NAT |
| C1468 | cap2Δ::NEO |
| C1469 | sac6Δ::NEO cap2Δ::HYG |
| C1476 | bni1Δ::NEO |
| C1478 | tpm1Δ::NEO |
| C1482 | cap1Δ::HYG |
| C1484 | cap1Δ::HYG cap2Δ::NEO |
| C1488 | CAP1-RFP-NEO GPF-SAC6-NAT |
| C1497 | abp1Δ::NEO |
| C1503 | crn1Δ::NEO |
| C1509 | sla2Δ::NEO |
| C1506 | SAC6-NAT |
| C1538 | GPF-SAC6-NAT GPDH(p)-Lifeact-RFP-NEO |
Synchronization of yeast growth.
IFM 49144 cells were synchronized as described previously (30). Briefly, cells were grown at 30°C in YPG medium in a 250 ml flask on a shaker with moderate aeration (100 rpm) to an approximate optical density of 4 at 600 nm. Cells were diluted 2.5 times with fresh YPG medium to a final volume of 125 ml and were cultivated with limited aeration. After 5 h of incubation, most cells were arrested at the unbudded G2 phase. The cultures were diluted and were incubated at 30°C with shaking at 250 rpm. The cells resumed growth by synchronous budding, followed by synchronous nuclear division.
Flow cytometry.
Fluorescence-activated cell sorter (FACS) analysis was performed as described previously (37). Briefly, cells were harvested at the indicated time points and were fixed in ice-cold 70% ethanol overnight at 4°C. The cells were washed, resuspended in NS buffer (10 mM Tris-HCl [pH 7.2], 0.25 M sucrose, 1 mM EDTA, 1 mM MgCl2, 0.1 mM ZnCl2, 0.4 mM phenylmethylsulfonyl fluoride, 7 mM β-mercaptoethanol), and digested with 0.5 mg/ml RNase A for 2 h. Propidium iodide (10 μg/ml) was added, and cells were incubated for an additional 2 h at 37°C in the dark. Before being subjected to FACS analysis, the cells were sonicated with a homogenizer for 10 s. Fluorescence was measured with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA), and data were acquired using CellQuest software. At least 30,000 cells of each strain were analyzed for their DNA contents.
Microscopy.
Differential interference contrast (DIC) microscopy and fluorescent images were viewed with a Zeiss Axiovert fluorescence microscope equipped with an AxioCam MRm digital camera. The image was captured with Axiovision (version 4.0) and was processed by Adobe Photoshop CS4 software. Hoechst 33342, trihydrochloride trihydrate (Invitrogen, CA), was used at 10 μg/ml to visualize the nuclei in live cells. For vesicle and endosome staining, log-phase cells grown in YEPD medium at 30°C were shifted to 37°C for 1 h under 20% O2 or for 2 h under 1% O2 with or without 1 M sorbitol. Cultures were centrifuged at 300 × g, and cells were incubated in the indicated media containing 10 μg/ml of N-(3-triethylammoniumpropyl)-4-(p-diethylaminophenyl-hexatrienyl)pyridinium dibromide (FM4-64) for 20 min under the indicated conditions. FM4-64 was removed by centrifugation. After washing with YEPD medium, cells were incubated in a medium with or without the presence of 1 M sorbitol for 60 to 70 min under the indicated conditions. The FM4-64-stained cells were pelleted and stored on ice until ready for microscopy. For samples grown under 1% O2, the spinning, staining, and poststaining incubation procedures were carried out in the hypoxia chamber.
Gene deletion and complementation.
Annotated genes were selected from the B-3501A genome database (http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=283643). Genes of interest were disrupted via homologous recombination by biolistic transformation (38). Disruption constructs were created by an overlapping PCR technique (22). Homologous integrations were confirmed by PCR and Southern hybridization. Wild-type genes from strain B-3501A were PCR amplified, cloned, and sequenced. All the original mutants and the deletion strains were complemented with their respective wild-type genes using biolistic transformation. The gene used for complementation was inserted into the multiple cloning site of pYCC744, which contained the NAT gene as a selectable marker. Stable transformants were selected after repeated transfer on media without antibiotics. To complement the sac6Δ mutant, a genomic clone of SAC6 was cloned into a plasmid containing NAT as the selectable marker, and the resulting plasmid was transformed into C1142. Transformant C1506, containing a homologous integration of SAC6 at the deleted sac6 locus, was identified as described above.
Construction of strains.
Briefly, to tag Sac6, green fluorescent protein (GFP) was cloned into the N terminus of Sac6 to yield plasmid pYCC957. This plasmid, containing NAT as the selectable marker, was transformed into strain C1412 (sac6Δ). Transformant C1435, containing a homologous integration of GFP-Sac6 at the SAC6 locus in the sac6Δ strain, was identified as described above. Lifeact-red fluorescent protein (RFP) was cloned by PCR using N1-Lifeact (a gift from Tamas Balla, NIH, MD) and TagRFP (Evrogen, Moscow, Russia) as the templates and was placed under the control of the glyceraldehyde-3-phosphate dehydrogenase (GPDH) promoter. The Lifeact-RFP construct was cloned into a NEO-containing plasmid to yield pYCC996 and was transformed into C1435 to yield C1538. To tag Cap1, TagRFP was cloned into the C terminus of Cap1 to construct plasmid pYCC974. This plasmid, containing NEO as the selectable marker, was transformed into C1460, and transformant C1488, containing a homologous integration of Cap1RFP into the deleted cap1 locus, was identified as described above.
Virulence studies.
The animal experiments were carried out with the approval and oversight of the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Strains B-3501A, C1412 (sac6Δ), and C1506 (SAC6) were used in the virulence study. Female BALB/c mice (6 to 8 weeks old) were injected via the lateral tail vein with 0.2 ml of a suspension of the indicated yeast strain (2.5 × 106 cells/ml in 0.9% NaCl), and mortality was monitored as described previously (10). Kaplan-Meier analysis of survival was performed using JMP software for Macintosh (SAS Institute, Cary, NC).
RESULTS
Growth of C. neoformans under low oxygen conditions.
We chose two C. neoformans strains, B-3501A (serotype D) and IFM 49144 (serotype A), for study of their growth behavior under 1% O2. The genome-sequenced strain, B-3501A (25), had been consistently used in our previous studies on the cryptococcal response to low oxygen concentrations (8, 9, 19). The cell cycle progression and growth behavior of C. neoformans under low oxygen conditions can be best studied in strains where growth can be synchronized. No parameters for synchronization of the cell cycle, however, have been achieved yet for B-3501A. Synchronization has been reported in the C. neoformans strain IFM 49144 when grown under specific conditions (30). We found that the doubling times of strains B-3501A and IFM 49144 were comparable at 28°C in ambient air (20% oxygen) (107.8 ± 1.3 min versus 104.4 ± 2.6 min) (see Fig. S1 in the supplemental material). To mimic the host environment of the animal, cryptococcal cells in all experiments under low O2 conditions were grown in the presence of 5% CO2. The growth rates of both strains were considerably retarded under 1% O2 compared to 20% O2. The doubling time of B-3501A at 28°C was faster than that of IFM 49144 under 1% O2 (250.0 ± 8.5 min versus 303.8 ± 30.7 min). In contrast, the growth rate of IFM 49144 at 37°C under 20% O2 was higher than that of B-3501A (117.7 ± 11.8 min versus 156.3 ± 5.0 min). The growth rates of both strains, however, were markedly reduced at 1% O2 (10.9 ± 0.4 h for IFM 49144 versus 18.3 ± 3.8 h for B-3501A). These results show that the growth rate of C. neoformans is significantly retarded under low oxygen conditions regardless of the strain background.
It has been reported that a deficit of oxygen causes G2 cell cycle arrest in C. neoformans (29). However, shifting nonsynchronized log-phase cultures of B-3501A and IFM 49144 from 20% to 1% O2 at 28°C in a hypoxia chamber did not result in complete cell cycle arrest following 6 h of incubation (Fig. 1). Fluorescence-activated cell sorter (FACS) analysis revealed that for strain B-3501A, the population of cells containing 2 copies of DNA (2C) increased when the cultures were shifted to 1% O2 (53% at 0 h versus 65% at 6 h [Fig. 1A]). In strain IFM 49144, however, an increase was observed in the population of cells containing 1 copy of DNA (1C) (33% at 0 h versus 52% at 6 h [Fig. 1B]). When the log-phase cells of B-3501A and IFM 49144 grown under 20% oxygen were viewed under a microscope, no clear difference was observed between the two strains. However, there were higher numbers of unbudded cells in the culture of IFM 49144 than in that of B-3501A following a shift to 1% O2 for 6 h (Fig. 1A and B, lower panels).
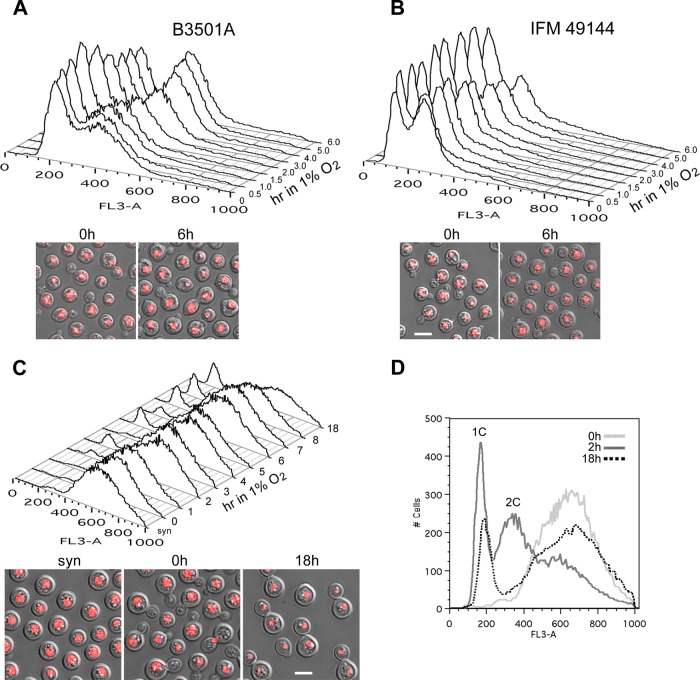
Fig 1.
Low oxygen conditions affect the growth of C. neoformans. (A and B) Log-phase cells of B-3501A (A) or IFM 49144 (B) were grown under 20% O2 at 28°C and were then transferred to 1% O2 with 5% CO2. Cells were examined at the indicated time points. (Top) FACS analysis; (bottom) morphology of cells in the start culture at the log phase (0 h) and at 6 h of culture under 1% O2 (right). (C) IFM 49144 cells were grown under limited aeration conditions for 5 h (syn) and were released to 20% O2 for 60 min (0 h). The culture was transferred to 1% O2 with 5% CO2, and cells were examined at the indicated time points. (Top) FACS analysis; (bottom) cell morphology of cultures with limited aeration (left), 20% O2 for 60 min (center), and 1% O2 for 18 h (right). (D) FACS analysis of IFM 49144 cells. 0 h, cells were grown at 20% O2 for 60 min after release from arrest; 2 h, cells from 0 h were continuously grown for an additional 2 h under 20% O2; 18 h, synchronized cells were grown under 1% O2 for 18 h. Bars, 5 μm.
To further investigate the response of C. neoformans to 1% O2, cells of strain IFM 49144 were first arrested at the G2 phase by limiting aeration (see Materials and Methods) and were then released into 20% oxygen and shaken vigorously for 60 min before shifting to 1% O2. The arrested IFM 49144 cultures contained mostly unbudded cells, which were generally larger than the log-phase cells (Fig. 1C, bottom left). FACS analysis of the arrested cells showed a broad peak with a median fluorescence intensity about twice that of the 2C DNA peak (Fig. 1C). This observation was similar to that reported in a previous study where the IFM 49144 cells had been arrested (29). After release of the IFM 49144 cells into 20% oxygen for 60 min, most of the cells were synchronized as budding cells (Fig. 1C, bottom center), producing a FACS pattern similar to that of the prerelease culture. FACS analysis showed that the synchronized cells of IFM 49144 proceeded through the cell cycle in 20% oxygen after 60 min and showed the typical 1C and 2C DNA peaks while gradually diminishing the high fluorescence peak (Fig. 1D, 2 h; also data not shown). However, when the synchronized 60-min cultures were shifted to 1% O2, only a small portion of the cells entered the nuclear 1C stage, while a majority of the cells remained at the higher fluorescence stage (Fig. 1C, top, and D, 18 h). When cells from the 18-h culture at 1% O2 were viewed under a microscope, two major types of cells were observed: one type comprised uninucleate cells in doublets, which indicated completion of mitosis without cytokinesis, and the other type comprised cells that were singlets, which corresponded to the 1C peak of the FACS and accounted for 18% of the population (Fig. 1C, bottom right, and D). These two types of cells were detectable in cultures that had been shifted to 1% O2 for 2 h, although the 1C peak represented only 4.5% of the population at that time point (Fig. 1C, top; also data not shown). These data indicate that under low oxygen conditions, a majority of the synchronized cells stayed at the postmitosis stage, and only a minor population proceeded to the G1 stage. Further studies on the cellular machinery involved in cryptococcal behavior at low oxygen concentrations were carried out using strain B-3501A.
Fig 2.
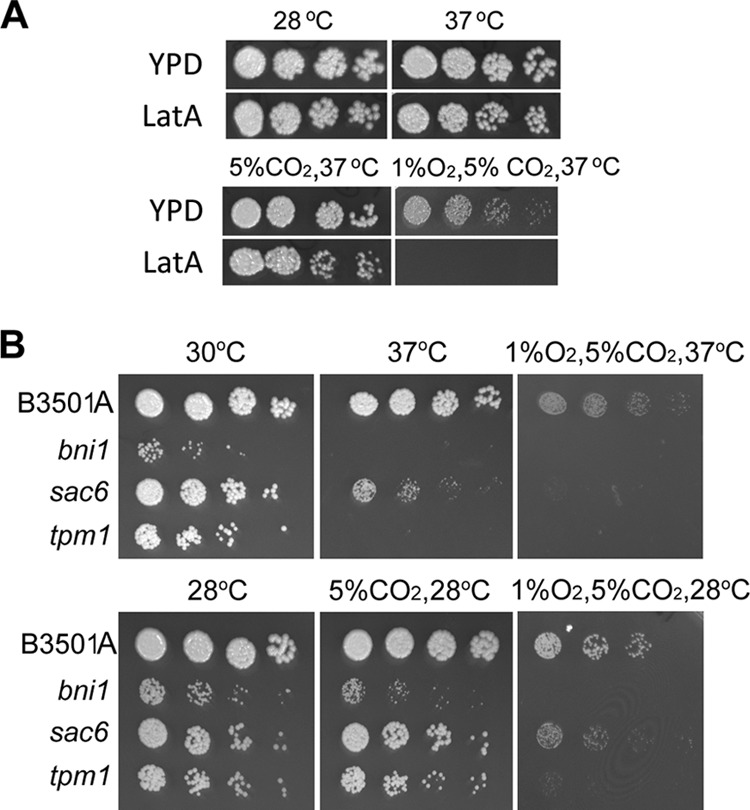
Spot assay. (A) Threefold serial dilutions of B-3501A (starting with approximately 500 cells) were spotted onto YEPD alone or YEPD containing 0.5 μM latrunculin A. Cultures were incubated under the conditions indicated for 3 days, and photographs were taken. (B) Threefold serial dilutions of the T-DNA insertional mutants were spotted onto YEPD medium and were incubated under the conditions indicated for 3 days. The mutations are given on the left.
Actin-related proteins are involved in the growth of C. neoformans at low oxygen concentrations.
Actin is known to be important for many cellular functions, including cell cycle progression. During the analysis of cryptococcal cell behavior under 1% O2, we tested a few compounds that are known to interfere with actin function. B-3501A was observed to be hypersensitive to 1% O2 in the presence 0.5 μM latrunculin A (LatA) at 37°C (Fig. 2A). LatA is a toxic natural product of Latrunculia species that causes disruption of the actin cytoskeleton at submicromolar concentrations in many eukaryotic cells (36). In a screen of our T-DNA insertion mutant collection, three strains showed severe growth defects in a low-oxygen environment. These mutants contained a T-DNA insertion in the genes that are known to be associated with the function of actin. Figure 2B shows that the growth of the strain containing a T-DNA insertion in the SAC6 homolog (CNJ01680) is affected under 20% oxygen at 37°C and is barely detectable under 1% O2 at 37°C. Insertional mutations in homologs of BNI1 (CNJ02400) and TPM1 (CNF02720) resulted in a temperature-sensitive (TS) phenotype at 37°C (Fig. 2B, top). Since bni1 and tpm1 were TS at 37°C, we tested their growth at 28°C. The growth of all three mutants was retarded under 1% O2 at 28°C (Fig. 2B, bottom). The functions of Sac6, Tpm1, and Bni1 have been characterized in other organisms. For instance, Sac6, a homolog of fimbrin, is one of the two well-documented actin-bundling proteins in Saccharomyces cerevisiae, Sac6 (yeast fimbrin) and Scp1 (yeast Sm22/transgelin) (2, 17, 40). However, only the Sac6 homolog was detected in the C. neoformans genome. BNI1 encodes a protein similar to formin which nucleates the formation of linear actin filaments involved in budding and mitotic spindle orientation, requiring the formation of polarized actin cables (33, 42). TPM1 is a homolog of tropomyosin, which in S. cerevisiae contains two isoforms, TPM1 and TPM2, that primarily stabilize the filaments in actin cables and the actomyosin ring. Tpm1 and Tpm2 are the only proteins known to localize exclusively to cables (24). C. neoformans contains only TPM1 (CNF02720), which is closely related to tropomyosin.
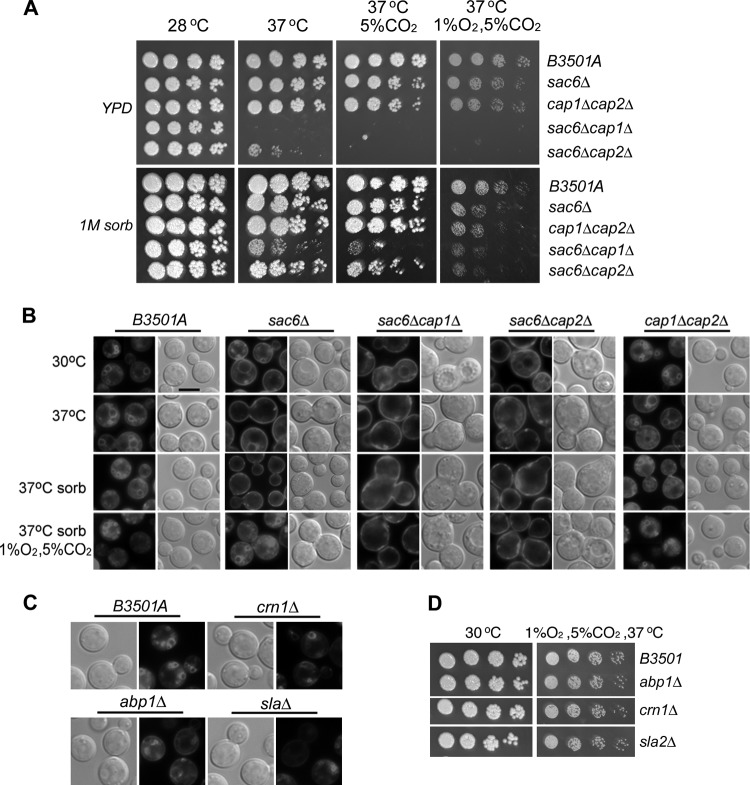
To further confirm the importance of BNI1, SAC6, and TPM1 to the growth of C. neoformans at low oxygen concentrations, the three genes were independently deleted in B-3501A. As in the original T-DNA insertion mutants, deletion of BNI1 and TPM1 exhibited aberrant cell morphology at 30°C, suggesting cytoskeletal abnormality (see Fig. S2 in the supplemental material), and resulted in a TS phenotype at 37°C (Fig. 3A, bottom). The growth of bni1Δ and tpm1Δ strains was also retarded at 28°C with or without 5% CO2, and the strains were hypersensitive to 1% O2 at 28°C. However, the degree of growth retardation of the bni1Δ strain relative to the growth of B-3501A under 1% O2 was similar to what was observed under 5% CO2. Therefore, only the tpm1Δ strain was considered sensitive to low oxygen conditions. The growth of the sac6Δ strain at both 28°C and 37°C under ambient conditions was comparable to that of B-3501A, differing from the phenotype of the original T-DNA insertional mutant (compare Fig. 3A with Fig. 2B). This observation suggests that an additional hidden mutation(s) causes growth reduction at 37°C in the sac6 T-DNA insertional mutant or that the insertion allele generates a toxic fragment. The reduction in the growth of the sac6Δ strain under 1% O2 was more severe at 37°C than at 28°C. Compared to the growth of B-3501A, LatA treatment drastically affected the growth of the three deletants under all conditions, suggesting a possible impairment of actin function in the deletants (Fig. 3B). We noted, however, that the sac6Δ strain grew nearly as well as B-3501A in the presence of LatA under 5% CO2 at ambient oxygen concentrations (Fig. 3B).
Fig 3.
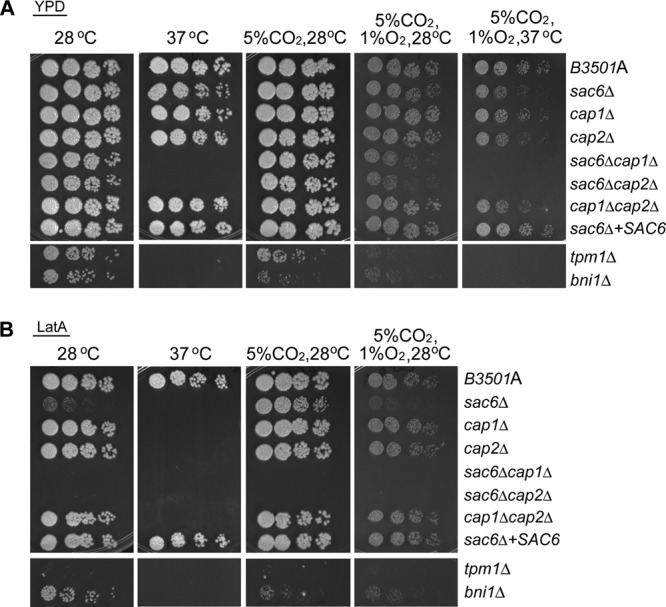
Spot assay. Threefold serial dilutions of each strain were spotted onto YEPD medium alone (A) or YEPD medium containing 0.5 μM latrunculin A (B). Cultures were incubated under the conditions indicated for 3 days and were photographed. The mutations are given on the right.
Sac6 and Tpm1 in yeast are actin-binding proteins that decorate the sides of filaments as well as bundles and/or stabilize actin filaments (26). Deletion of genes encoding actin-binding proteins that target the ends of actin filaments would presumably affect the growth of C. neoformans in a low-oxygen environment. The yeast capping proteins Cap1 and Cap2 are tightly associated heterodimeric proteins that cap the barbed ends of actin filaments (39). We deleted homologs of capping proteins, CAP1 (CNB04560) and CAP2 (CNB03880), in B-3501A. Deletion of CAP1 did not show a clear growth defect under any of the conditions tested. Compared to that of B-3501A, however, the growth of the cap2Δ strain was slightly reduced under 1% O2 at 37°C but not at 28°C (Fig. 3A). The cap1Δ cap2Δ double deletion strain had a phenotype similar to that of the cap2Δ strain. In addition, the cap1Δ, cap2Δ, and cap1Δ cap2Δ double deletion strains were hypersensitive to LatA at 37°C but not at 28°C (Fig. 3B). In contrast, the sac6Δ cap1Δ and sac6Δ cap2Δ double deletion strains were TS at 37°C, and their growth was clearly reduced at 28°C under 1% O2 (Fig. 3A). In addition, the sac6Δ cap1Δ and sac6Δ cap2Δ double deletants were hypersensitive to LatA under all conditions (Fig. 3B). These data suggest that the factors related to bundling, cross-linking, and/or stabilizing actin filaments are important for the growth of C. neoformans under low oxygen conditions.
Involvement of Sac6 in response to low oxygen concentrations revealed by its localization.
Since the strain with a deletion of SAC6 had a growth defect under low oxygen conditions but grew normally under other conditions, we focused our attention on SAC6. To explore the cellular function of Sac6 in C. neoformans, we tagged the Sac6 protein with GFP. Homologous insertion of GFP-Sac6 into the sac6Δ locus complemented the sac6Δ phenotype, suggesting that tagging with GFP did not interfere with Sac6 function (data not shown). In addition, we fused the Lifeact sequence to the monomer of TagRFP (Lifeact-RFP) and transformed the Lifeact-RFP construct into the strain expressing GFP-Sac6. Lifeact contains a 17-amino-acid peptide of Abp140 from S. cerevisiae, which is a versatile marker for the visualization of actin (34). Figure 4A shows the typical patches and cables stained with Lifeact-RFP (red). GFP-Sac6 did not stain the actin cable as did Lifeact-RFP, but GFP-Sac6 localized at the cortical actin patch as well as in the emerging bud and at the actin ring in the bud neck area, a pattern similar to that of S. cerevisiae Sac6 (Fig. 4A and C, arrows) (12). Furthermore, GFP-Sac6 also localized at the actin patch but not at the actin cable in rhodamine-phalloidin-stained C. neoformans cells (data not shown). The GFP-Sac6 and Lifeact-RFP signals disappeared rapidly after cells were treated with 100 μM LatA for 5 min, indicating that filamentous actin is required for GFP-Sac6 localization (Fig. 4B).
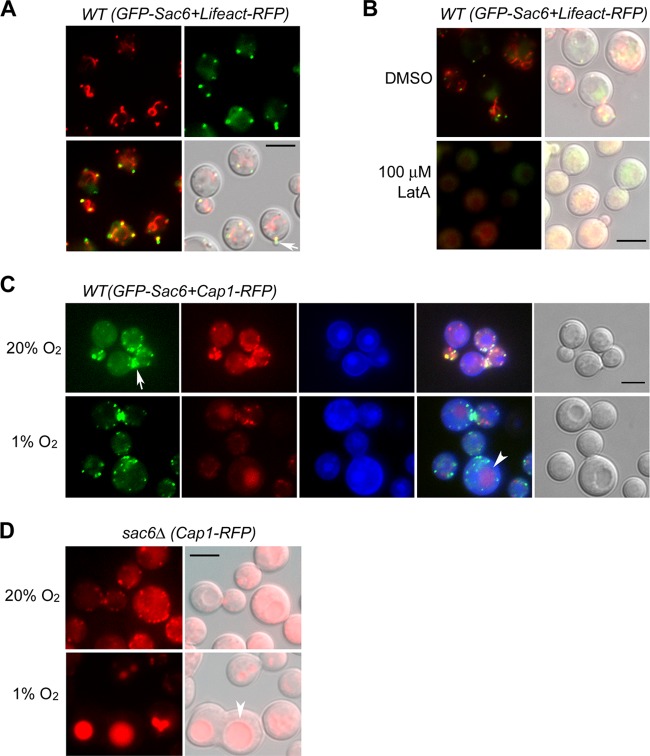
Fig 4.
Localization of GFP-Sac6. (A) Fluorescent images of GFP-Sac6 and Lifeact-RFP. Logarithmically growing cells expressing Lifeact-RFP (red) and GFP-Sac6 (green) were observed by fluorescence microscopy. (Lower left) Merged fluorescent image; (lower right) merged DIC microscopy image. An arrow indicates the emerging bud. (B) Brief treatment with LatA causes diminishing of the fluorescence signals of GFP-Sac6 and Lifeact-RFP. Log-phase cells were treated either with DMSO or with 100 μM LatA for 5 min and were observed under a microscope. (C) Localization of GFP-Sac6 and Cap1-RFP under 20% O2 (top) or 1% O2 (bottom) at 28°C for 18 h. The nuclei were stained blue by live staining with the dye Hoechst 33342. The arrow and arrowhead indicate the locations of the bud neck and vacuole, respectively. (D) Localization of Cap1-RFP in the sac6Δ strain under 20% O2 (top) or 1% O2 (bottom) at 28°C for 18 h. The arrowhead indicates the location of the vacuole. Bars, 5 μm.
To investigate the distribution of Sac6 further, Cap1 was tagged with TagRFP and was expressed in the GFP-Sac6 strain. Figure 4C shows that Cap1-RFP (red) colocalized predominantly with GFP-Sac6 (green) at the actin patches and at the actin ring in the bud neck area under ambient oxygen conditions (top). When the cryptococcal cells were shifted from 20% to 1% O2, the intensity of GFP-Sac6 dots was slightly reduced, but the distribution was not significantly different (Fig. 4C, bottom), suggesting that low oxygen conditions did not alter the location of Sac6. However, the red dots of Cap1-RFP gradually disappeared from the cytoplasm and accumulated in the vacuole by 18 h under 1% O2 (Fig. 4C, bottom, arrowhead). The accumulation of Cap1-RFP in the vacuole was more prominent in the sac6Δ cells incubated under 1% O2 (Fig. 4D, arrowhead), suggesting that low oxygen conditions and the deletion of SAC6 affect Cap1 turnover.
Endocytosis is not important in C. neoformans for growth under low oxygen conditions.
It has been reported that sorbitol appears to protect cap2 and sac6 mutants of S. cerevisiae from lysis (21). We tested whether sorbitol could rescue the growth defect in our mutants. Figure 5A shows that in the presence of 1 M sorbitol, the TS phenotype was restored in the sac6Δ cap2Δ strain and was partially rescued in the sac6Δ cap1Δ strain. However, the growth of both double deletants was reduced compared to that of B-3501A under 1% O2 at 37°C, despite the presence of 1 M sorbitol. Furthermore, 1 M sorbitol did not improve the growth defects of the sac6Δ and cap1Δ cap2Δ strains under 1% O2.
Fig 5.
(A) Spot assay. Threefold serial dilutions of each strain were spotted onto YEPD medium alone (upper panels) or YEPD medium containing 1 M sorbitol (bottom). The plates were incubated under the indicated conditions for 3 to 4 days, and photographs were taken. The mutations are given on the right. (B) FM4-64 internalization assay for endocytosis. Representative fluorescent images of each strain stained with the membrane marker FM4-64 are shown. Cells were grown under the conditions indicated on the left. “37°C sorb” means that cells were grown in YEPD medium containing 1 M sorbitol at 37°C; “1% O2 sorb” means that cells were grown in YEPD medium containing 1 M sorbitol under 1% O2 and 5% CO2 at 37°C. Bar, 5 μm. (C) Endosomal staining of deletants with FM4-64. (D) Spot assay. Threefold serial dilutions of each strain were spotted onto YEPD medium and were incubated at 30°C (left) or under 1% O2 and 5% CO2 at 37°C (right). No growth defect is seen for any of the three mutants.
Actin functions in the fundamental processes of cell polarity, cytokinesis, and endocytosis. In order to detect the possible effects on endocytosis in our mutants, cells were stained with FM4-64, a styryl dye that binds to the vacuolar membrane after internalization through endocytosis. In B-3501A, FM4-64 stained various bright ring-like structures in the cell, representing endosomes, under all conditions (Fig. 5B). The patterns of FM4-64 staining in the cap1Δ and cap2Δ strains were similar to that for B-3501A (data not shown). Endosome staining was reduced in the sac6Δ strain, and large vacuoles were often observed at 30°C. This is similar to the finding for the S. cerevisiae sac6Δ strain, where endocytic patches are impaired in moving inward from the cell cortex (20). Endosome staining was infrequently found in C. neoformans sac6Δ at 37°C under both 20% and 1% O2 (Fig. 5B; also data not shown). The presence of 1 M sorbitol did not improve the endosome staining of the sac6Δ strain under 20% O2 at 37°C. However, the endosome staining pattern in the sac6Δ strain was comparable to that for B-3501A under 1% O2 at 37°C in 1 M sorbitol (Fig. 5B).
On the other hand, the cell morphology of the sac6Δ cap1Δ and sac6Δ cap2Δ double deletants was aberrant at 30°C, and the cells were unusually large at 37°C (Fig. 5B). Endosome staining was rarely positive in the sac6Δ cap1Δ and sac6Δ cap2Δ strains, regardless of the incubation condition. However, no clear differences were observed in the FM4-64 staining pattern between the cap1Δ cap2Δ strain and B-3501A under any growth condition (Fig. 5B). These results suggest that there is no direct relationship between the ability of C. neoformans to grow at low oxygen concentrations and the pattern of FM4-64 staining, an indicator of endocytosis function. To further investigate the role of endocytosis in cryptococcal growth under low oxygen conditions, we deleted three genes, ABP1 (CND04880), CRN1 (CNH03300), and SLA2 (CNE03040), known to be involved in endocytosis in other organisms (for a review, see reference 39a). Endosomal staining with FM4-64 was abnormal in all three deletants (Fig. 5C), suggesting their involvement in the endocytic process. However, none of the three gene deletions affected cell growth under 1% O2 at 37°C (Fig. 5D). These data suggest that the ability of C. neoformans cells to grow under low oxygen conditions is independent of endocytosis.
SAC6 is not required for virulence.
Since the sac6Δ strain was the only mutant that showed a reduction in growth under 1% O2 without an obvious defect at 37°C in normoxia, we used a mouse model to study the importance of SAC6 in virulence. As shown in Fig. 6, deletion of SAC6 had no effect on virulence in mice.
Fig 6.
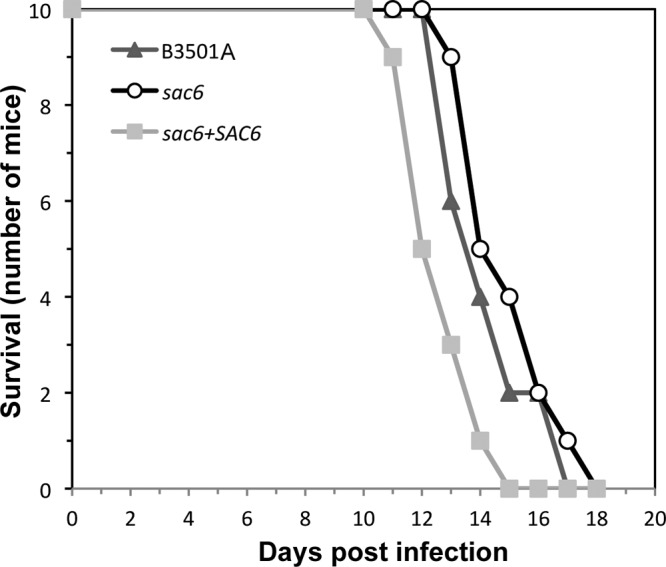
SAC6 is not important for virulence in mice. Female BALB/c mice (10 per group) were injected with C. neoformans strains via the tail vein, and mortality was monitored. Symbols represent the survival records of mice challenged with the wild type (triangles), the sac6Δ strain (circles), or the SAC6-complemented strain (squares).
DISCUSSION
C. neoformans is an obligately aerobic fungus, and its growth is reduced at oxygen levels lower than those in ambient air (28). We show that the growth rates for both serotype A (IFM 49144) and D (B-3501) strains of C. neoformans were significantly lower under 1% O2. The doubling time under 1% O2 was 2- to 3-fold longer than that under normoxic conditions at 28°C, and the delay in growth was even more pronounced at 37°C. When log-phase cells were shifted from ambient air to 1% O2, no apparent cell cycle arrest was observed in either strain. Furthermore, when synchronized IFM 49144 cells were grown in ambient air and were then shifted to 1% O2, no cell cycle arrest at G2 was observed. Instead, a majority of the synchronized IFM 49144 cells proceeded from early budding to the postmitotic stage without cytokinesis. A small portion of the cells, however, was able to complete cytokinesis but stayed at the 1C nuclear stage. On the other hand, it is clear that IFM 49144 cells are arrested at the G2 stage after 5 h of incubation under limited aeration (Fig. 1) (29, 30). The starting cultures before limited aeration were in transition between the late-log phase and the stationary phase. After 5 h of growth with limited aeration, IFM 49144 cells were arrested at G2, and the dissolved oxygen concentration was 1.5 mg/liter after 5 h of treatment (29, 30). In contrast, the cells in our IFM 49144 cultures were in an active budding stage at the time of the shift to 1% O2 (Fig. 1C, bottom center), and the calculated saturable oxygen concentration was 0.38 mg/liter at 1% O2 (see Materials and Methods). Therefore, it appears that the cell cycle behavior of C. neoformans is influenced not only by low oxygen conditions but also by other variables in the culture environment.
Interestingly, C. neoformans was hypersensitive to latrunculin A in a low-oxygen environment, suggesting the importance of normal actin function under these conditions. Actin exists in two forms, the monomeric (globular actin [G-actin]) and the filamentous (F-actin) form. With the aid of actin-binding proteins, G-actin can assemble into F-actin. The dynamic assembly and disassembly of the filaments are central to actin function in the cell (for reviews, see references 26 and 31). We found that Tpm1 and Sac6, two actin-binding proteins, are important for the growth of C. neoformans under low oxygen conditions. In S. cerevisiae, Sac6/fimbrin localizes at the cortical actin patches and is also found within the actin bundles. In Schizosaccharomyces pombe, fimbrin localizes at the medial ring and functions during cytokinesis (41). C. neoformans GFP-Sac6 localized at both the cortical actin patches and the ring structures in the bud neck, suggesting a similar function for Sac6 in C. neoformans.
Although actin function is important for the growth of C. neoformans under low oxygen conditions, its requirement does not depend on a fully functional endocytosis process. Endocytosis is a process in eukaryotic cells by which the plasma membrane becomes invaginated, resulting in the production of vesicles. These vesicles fuse with the endosome and enter the endolysosomal membrane system. Actin plays a pivotal role in the entire process of endocytosis (4). Sac6/fimbrin is associated with both actin organization and endocytosis in yeast (13, 26). The yeast Sac6 and Scp1 normally allow cells to cross-link actin and generate a strong meshwork during endocytic invagination. In cells that lack these proteins, most endocytic assembly events fail, with reduced invagination as well as postscission movement (16). We found that a homolog of Sac6 but not of Scp1 exists in the C. neoformans genome, and the deletion of sac6 affected endocytosis in C. neoformans. Interestingly, addition of 1 M sorbitol was able to rescue the endocytic defect of the sac6Δ strain under low oxygen conditions but not the growth defect. We also show that deletion of three conserved genes involved in endocytosis did not affect the growth of cryptococcal cells under 1% O2 at 37°C (Fig. 5D). In addition to endocytosis, cell polarity and cytokinesis are the two major functions involving actin. However, neither cell polarity nor cytokinesis in the sac6Δ strain appears to be affected by low oxygen conditions (Fig. 4 and 5B). It is not clear how deletion of SAC6, coding for an actin bundle protein, affects growth under low oxygen conditions but not at ambient levels.
Some differences were observed in the phenotypes of cap1Δ, cap2Δ, and sac6Δ mutants between S. cerevisiae and C. neoformans. In S. cerevisiae, deletions of CAP1 and CAP2 separately or in combination did not affect cell viability, and they resulted in similar phenotypes (5). However, these null mutants had a severe deficit in the actin cables and an increased number of actin spots in the mother cells. Cells were round and relatively large. In contrast, the morphology of the C. neoformans cap1Δ, cap2Δ, and cap1Δ cap2Δ double deletion cells was not different from that of the wild type (Fig. 5B; see also Fig S2 in the supplemental material). Double mutations of SAC6 and CAP1, as well as of SAC6 and CAP2, are synthetic-lethal mutations in S. cerevisiae (3). In C. neoformans, however, double deletions of these homologs only showed a TS phenotype, and the cells were enlarged under all the conditions tested. Since the mechanism of genetic interaction between capping proteins and fimbrin has yet to be elucidated, it is difficult to explain the differences observed between these homologous mutants in S. cerevisiae and C. neoformans.
Although the sac6Δ strain showed reduced growth under 1% O2, its virulence in mice was similar to that of the wild-type strains. We tested the virulence of 13 strains where each strain carried a different gene deletion and showed a growth defect under 1% O2. A majority of them (11 of 13) had reduced virulence (9) (data not shown). These results suggest that the ability of C. neoformans to grow under low oxygen conditions is associated with the ability to cause fatal infection in mice. Oxygen concentrations in the brain differ significantly among anatomical sites (15), and the mean tissue oxygen tension in the brain has been reported to range from 5 mm Hg to 40 mm Hg (14, 27). We chose the very stringent 1% O2 condition with which to examine the growth phenotypes of all the mutants. In fact, the growth retardation of the sac6Δ strain under 5% O2 was minimal compared to that under 1% O2 at 37°C (see Fig. S3 in the supplemental material). It is likely that sac6Δ cells are able to establish themselves in tissues where oxygen tensions are high enough for them to thrive and cause fulminating infection. The relationship between the distribution of cryptococcal cells and tissue oxygen tension merits further study.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Varma for critical reading of the manuscript.
This study was supported by funds from the intramural program of the National Institute of Allergy and Infectious Diseases, NIH. The funders had no role in the study design, the data collection and analysis, the decision to publish, or the preparation of the manuscript.
Footnotes
Published ahead of print 4 May 2012
REFERENCES
- 1. Adams AE, Botstein D. 1989. Dominant suppressors of yeast actin mutations that are reciprocally suppressed. Genetics 121:675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams AE, Botstein D, Drubin DG. 1989. A yeast actin-binding protein is encoded by SAC6, a gene found by suppression of an actin mutation. Science 243:231–233 [DOI] [PubMed] [Google Scholar]
- 3. Adams AE, Cooper JA, Drubin DG. 1993. Unexpected combinations of null mutations in genes encoding the actin cytoskeleton are lethal in yeast. Mol. Biol. Cell 4:459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aghamohammadzadeh S, Ayscough KR. 2010. The yeast actin cytoskeleton and its function in endocytosis. Fungal Biol. Rev. 24:37–46 [Google Scholar]
- 5. Amatruda JF, Gattermeir DJ, Karpova TS, Cooper JA. 1992. Effects of null mutations and overexpression of capping protein on morphogenesis, actin distribution and polarized secretion in yeast. J. Cell Biol. 119:1151–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benson BB, Krause D, Peterson MA. 1979. Solubility and isotopic fractionation of gases in dilute aqueous-solution. 1. Oxygen. J. Solut. Chem. 8:655–690 [Google Scholar]
- 7. Bien CM, Chang YC, Nes WD, Kwon-Chung KJ, Espenshade PJ. 2009. Cryptococcus neoformans Site-2 protease is required for virulence and survival in the presence of azole drugs. Mol. Microbiol. 74:672–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang YC, Bien CM, Lee H, Espenshade PJ, Kwon-Chung KJ. 2007. Sre1p, a regulator of oxygen sensing and sterol homeostasis, is required for virulence in Cryptococcus neoformans. Mol. Microbiol. 64:614–629 [DOI] [PubMed] [Google Scholar]
- 9. Chang YC, Ingavale SS, Bien C, Espenshade P, Kwon-Chung KJ. 2009. Conservation of the sterol regulatory element-binding protein pathway and its pathobiological importance in Cryptococcus neoformans. Eukaryot. Cell 8:1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang YC, Penoyer LA, Kwon-Chung KJ. 2001. The second STE12 homologue of Cryptococcus neoformans is MATa-specific and plays an important role in virulence. Proc. Natl. Acad. Sci. U. S. A. 98:3258–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chun CD, Liu OW, Madhani HD. 2007. A link between virulence and homeostatic responses to hypoxia during infection by the human fungal pathogen Cryptococcus neoformans. PLoS Pathog. 3:e22 doi:10.1371/journal.ppat.0030022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drubin DG, Miller KG, Botstein D. 1988. Yeast actin-binding proteins: evidence for a role in morphogenesis. J. Cell Biol. 107:2551–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Engqvist-Goldstein AE, Drubin DG. 2003. Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 19:287–332 [DOI] [PubMed] [Google Scholar]
- 14. Erdmann W, Kunke S, Krell W. 1973. Tissue pO2 and cell function—an experimental study with multimicroelectrodes in the rat brain, p 167–174 In Kesler M, Lübbers DW. (ed), Oxygen supply: theoretical and practical aspects of oxygen supply and microcirculation of tissue. Urban & Schwarzenberg, Munich, Germany [Google Scholar]
- 15. Erecinska M, Silver IA. 2001. Tissue oxygen tension and brain sensitivity to hypoxia. Respir. Physiol. 128:263–276 [DOI] [PubMed] [Google Scholar]
- 16. Gheorghe DM, et al. 2008. Interactions between the yeast SM22 homologue Scp1 and actin demonstrate the importance of actin bundling in endocytosis. J. Biol. Chem. 283:15037–15046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goodman A, Goode BL, Matsudaira P, Fink GR. 2003. The Saccharomyces cerevisiae calponin/transgelin homolog Scp1 functions with fimbrin to regulate stability and organization of the actin cytoskeleton. Mol. Biol. Cell 14:2617–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grahl N, Shepardson KM, Chung D, Cramer RA. Hypoxia and fungal pathogenesis: to air or not to air? Eukaryot. Cell. 2012 doi: 10.1128/EC.00031-12. doi: 10.1128/EC.00031-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ingavale S, et al. 2008. Importance of mitochondria in survival of Cryptococcus neoformans under low oxygen conditions and tolerance to cobalt chloride. PLoS Pathog. 4:e1000155 doi:10.1371/journal.ppat.1000155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaksonen M, Toret CP, Drubin DG. 2005. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123:305–320 [DOI] [PubMed] [Google Scholar]
- 21. Karpova TS, Lepetit MM, Cooper JA. 1993. Mutations that enhance the cap2 null mutant phenotype in Saccharomyces cerevisiae affect the actin cytoskeleton, morphogenesis and pattern of growth. Genetics 135:693–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuwayama H, et al. 2002. PCR-mediated generation of a gene disruption construct without the use of DNA ligase and plasmid vectors. Nucleic Acids Res. 30:E2 doi:10.1093/nar/30.2.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwon-Chung KJ, Bennett JE. 1992. Medical mycology. Lea & Febiger, Philadelphia, PA [Google Scholar]
- 24. Liu HP, Bretscher A. 1989. Disruption of the single tropomyosin gene in yeast results in the disappearance of actin cables from the cytoskeleton. Cell 57:233–242 [DOI] [PubMed] [Google Scholar]
- 25. Loftus BJ, et al. 2005. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307:1321–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moseley JB, Goode BL. 2006. The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiol. Mol. Biol. Rev. 70:605–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nair P, Whalen WJ, Buerk D. 1975. pO2 of cat cerebral cortex: response to breathing N2 and 100 per cent O21. Microvasc. Res. 9:158–165 [DOI] [PubMed] [Google Scholar]
- 28. Odds FC, De Backer T, Dams G, Vranckx L, Woestenborghs F. 1995. Oxygen as limiting nutrient for growth of Cryptococcus neoformans. J. Clin. Microbiol. 33:995–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohkusu M, Raclavsky V, Takeo K. 2001. Deficit in oxygen causes G2 budding and unbudded G2 arrest in Cryptococcus neoformans. FEMS Microbiol. Lett. 204:29–32 [DOI] [PubMed] [Google Scholar]
- 30. Ohkusu M, Raclavsky V, Takeo K. 2004. Induced synchrony in Cryptococcus neoformans after release from G2-arrest. Antonie Van Leeuwenhoek 85:37–44 [DOI] [PubMed] [Google Scholar]
- 31. Pollard TD. 2007. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu. Rev. Biophys. Biomol. Struct. 36:451–477 [DOI] [PubMed] [Google Scholar]
- 32. Pruyne D, Bretscher A. 2000. Polarization of cell growth in yeast. J. Cell Sci. 113(Pt 4):571–585 [DOI] [PubMed] [Google Scholar]
- 33. Pruyne D, et al. 2002. Role of formins in actin assembly: nucleation and barbed-end association. Science 297:612–615 [DOI] [PubMed] [Google Scholar]
- 34. Riedl J, et al. 2008. Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5:605–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rooney PC, Daniels DD. 1998. Oxygen solubility in various alkanolamine/water mixtures. Petroleum Technol. Q. 1998:97–101 [Google Scholar]
- 36. Spector I, Shochet NR, Kashman Y, Groweiss A. 1983. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science 219:493–495 [DOI] [PubMed] [Google Scholar]
- 37. Tanaka R, Taguchi H, Takeo K, Miyaji M, Nishimura K. 1996. Determination of ploidy in Cryptococcus neoformans by flow cytometry. J. Med. Vet. Mycol. 34:299–301 [PubMed] [Google Scholar]
- 38. Toffaletti DL, Rude TH, Johnston SA, Durack DT, Perfect JR. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wear MA, Cooper JA. 2004. Capping protein: new insights into mechanism and regulation. Trends Biochem. Sci. 29:418–428 [DOI] [PubMed] [Google Scholar]
- 39a. Weinberg J, Drubin DG. 2012. Clathrin-mediated endocytosis in budding yeast. Trends Cell Biol. 22:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Winder SJ, Jess T, Ayscough KR. 2003. SCP1 encodes an actin-bundling protein in yeast. Biochem. J. 375:287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu JQ, Bahler J, Pringle JR. 2001. Roles of a fimbrin and an alpha-actinin-like protein in fission yeast cell polarization and cytokinesis. Mol. Biol. Cell 12:1061–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zahner JE, Harkins HA, Pringle JR. 1996. Genetic analysis of the bipolar pattern of bud site selection in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 16:1857–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.