Abstract
The Rieske nonheme mononuclear iron oxygenase MdpJ of the fuel oxygenate-degrading bacterial strain Aquincola tertiaricarbonis L108 has been described to attack short-chain tertiary alcohols via hydroxylation and desaturation reactions. Here, we demonstrate that also short-chain secondary alcohols can be transformed by MdpJ. Wild-type cells of strain L108 converted 2-propanol and 2-butanol to 1,2-propanediol and 3-buten-2-ol, respectively, whereas an mdpJ knockout mutant did not show such activity. In addition, wild-type cells converted 3-methyl-2-butanol and 3-pentanol to the corresponding desaturation products 3-methyl-3-buten-2-ol and 1-penten-3-ol, respectively. The enzymatic hydroxylation of 2-propanol resulted in an enantiomeric excess of about 70% for the (R)-enantiomer, indicating that this reaction was favored. Likewise, desaturation of (R)-2-butanol to 3-buten-2-ol was about 2.3-fold faster than conversion of the (S)-enantiomer. The biotechnological potential of MdpJ for the synthesis of enantiopure short-chain alcohols and diols as building block chemicals is discussed.
INTRODUCTION
Enzymes catalyzing the hydroxylation and desaturation of aliphatic tertiary alcohols seem to be rare and have not been well characterized thus far. In previous studies of the biodegradation of the fuel oxygenates methyl tert-butyl ether (MTBE) and tert-amyl methyl ether (TAME), at least one tertiary alcohol-hydroxylating enzymatic reaction has been proposed for the transformation of the central ether metabolites tert-butyl and tert-amyl alcohol (TBA and TAA, respectively) (9, 38). Though this reaction can be expected in several bacterial strains known to completely degrade MTBE and TAME under aerobic conditions (11, 13, 21, 27, 37), only in the fuel oxygenate-degrading bacterial strain Aquincola tertiaricarbonis L108 has the identity of the tertiary alcohol-attacking enzyme recently been revealed by gene knockout experiments (35). In this bacterium, the oxygenase MdpJ catalyzes not only the hydroxylation of TBA to 2-methylpropane-1,2-diol (MPD) but also the desaturation of TAA to 2-methyl-3-buten-2-ol (35).
MdpJ and its corresponding reductase, MdpK, have been described for the first time by Hristova and coworkers as MTBE-induced proteins and subunits of the postulated TBA-hydroxylating enzyme in Methylibium petroleiphilum PM1 (14). MdpJK belongs to the family of Rieske nonheme iron aromatic ring-hydroxylating oxygenases (RHO), such as the well-characterized phthalate and naphthalene dioxygenases (5, 20, 28, 36). These are multicomponent enzymes which use reduced pyridine nucleotide as the electron donor. The electrons are transported via a flavin cofactor of the reductase subunit to a [2Fe-2S] iron-sulfur cluster located on the same protein component as in MdpK (32) or on a separate ferredoxin subunit. The electrons are further passed to a [2Fe-2S] Rieske cluster and a mononuclear iron center of the oxygenase subunit (23, 40). The multifunctionality of MdpJ to catalyze hydroxylation as well as desaturation reactions (35) has likewise been found for other RHO enzymes, e.g., naphthalene dioxygenase catalyzes mono- and dihydroxylations as well as desaturations (22). Practically all known RHO enzymes, however, are described to exclusively attack aromatic compounds (19). Thus, the use of aliphatic substrates underlines the uniqueness of MdpJ among the RHO family.
The natural substrates of MdpJ-catalyzed hydroxylations and desaturations might be tertiary alcohols, e.g., the fuel oxygenate intermediates TBA and TAA. Accordingly, it has already been shown that MdpJ also catalyzes the desaturation of the tertiary alcohol 3-methyl-3-pentanol to 3-methyl-1-penten-3-ol (35). Here, we demonstrate that MdpJ can also attack the secondary alcohols 2-propanol, 2-butanol, 3-methyl-2-butanol, and 3-pentanol. In 1,2-propanediol formation from 2-propanol via hydroxylation and 2-butanol desaturation to 3-buten-2-ol, a preference for the (R)-enantiomer was found, underpinning the potential of MdpJ for the synthesis of enantiopure short-chain diols and unsaturated alcohols from secondary alcoholic precursors.
MATERIALS AND METHODS
Chemicals.
(S)-(+)-2-Phenylbutyryl chloride was synthesized as described by Hammarström and Hamberg (12) using (S)-(+)-2-phenylbutyric acid (99% pure; Sigma-Aldrich Chemie GmbH, Steinheim, Germany) and thionyl chloride (99% pure; Merck Schuchardt, Hohenbrunn, Germany). Suppliers and purities of other chemicals used in this study are listed in the supplemental material.
Bacterial strains and growth media.
Aquincola tertiaricarbonis L108, isolated from an MTBE-contaminated aquifer (Leuna, Germany) (21, 31), was cultivated in liquid mineral salt medium (MSM; see the supplemental material) containing MTBE at a concentration of 0.3 g liter−1. The previously generated knockout mutant strain A. tertiaricarbonis L108 (ΔmdpJ) K24 (35) was cultivated on 2-methylpropane-1,2-diol (MPD) at 0.5 g liter−1 in MSM supplemented with kanamycin (50 mg liter−1).
Resting-cell experiments.
Cultures were incubated at 30°C on rotary shakers. Bacterial cells were pregrown on MPD (0.5 g liter−1) and then incubated on TBA (0.5 g liter−1) overnight. Afterwards, cells were harvested by centrifugation at 13,000 × g and 4°C for 10 min. After being washed twice with MSM, cells were immediately used, and biomass was adjusted to values of 1.4 g (dry weight) per liter by dilution with MSM for the 2-butanol experiments and 2.2 g per liter when cells were incubated with 2-propanol, 3-methyl-2-butanol, and 3-pentanol. During the experiments, bacteria were incubated at 30°C in 25 ml MSM in glass serum bottles, sealed gas tight with butyl rubber stoppers. Liquid and gas samples were taken as described before (33) by puncturing the butyl rubber stoppers with syringes equipped with 0.6- by 30-mm Luer Lock needles. For chiral analysis of 1,2-propanediol, the complete culture liquid was harvested by centrifugation at 13,000 × g and 4°C for 10 min in order to obtain a clear supernatant. The shown data represent mean values and standard deviations of results from four replicate experiments.
Chiral analysis of 1,2-propanediol.
Methods of Powers et al. for chiral analysis of short-chain diols and hydroxy carboxylic acids (30) and Jenske and Vetter for chiral analysis of hydroxy fatty acids (15) were modified for determining the ratios of different 1,2-propanediol enantiomers formed in bacterial cultures. Samples from resting-cell experiments (200 ml) were saturated with NaCl and extracted two times with 100 ml diethyl ether. The diethyl ether phase was dried with Na2SO4 and evaporated completely. Standards of the 1,2-propanediol racemate and (S)-enantiomer were applied directly (3 μl). Pyridine (400 μl) and (S)-(+)-2-phenylbutyryl chloride (100 μl) were added for derivatization. After 2 h of incubation on a slow shaker at room temperature, H2O (5 ml) and one spatula tip of K2CO3 were added. Then, the solutions were extracted with MTBE (5 ml) via shaking for 1 h at room temperature. The MTBE phase was dried with Na2SO4 and evaporated to 0.5 ml. Analysis was performed using gas chromatography (GC) (model 7890A chromatograph; Agilent) coupled to mass spectrometry (MS) (mass selective detector [MSD] model 5975C; Agilent) on an RTX-5Sil MS column (30 m, 250 μm, 0.25 μm; Restek) with an Integra-Guard column (5 m; Restek). GC conditions were as follows: the carrier gas was helium, constant flow was nominally 0.8 ml min−1, the injector temperature was 230°C, the split injection ratio was 20:1, programmed oven temperatures were 70°C for 1 min, with an increase of 1°C min−1 to 76°C and then 6°C min−1 to 350°C, after which the temperature was held for 1 min, and the MSD transfer line temperature was 250°C. MSD conditions were as follows: full-scan mode (m/z 40 to 600) was used, the ion source temperature was 230°C, and the quadrupole temperature was 150°C. Compounds were identified by comparison with calibrated retention times and mass spectra of authentic standards.
Other analytics.
Volatile compounds (TBA, isobutene, 2-propanol, acetone, 2-butanol, 3-buten-2-ol, 2-butanone, 3-buten-2-one, 3-methyl-2-butanol, 3-methyl-2-butanone, 3-pentanol) were quantified by headspace GC using flame ionization detection (FID) (33). Compounds were assigned according to retention times of pure GC standards. Additionally, ketonic metabolites of 2-butanol as well as metabolites of 3-methyl-2-butanol and 3-pentanol were identified by GC-MS analysis (see Fig. S3 to S6 in the supplemental material). Quantitative analysis of 1,2-propanediol was performed using high-performance liquid chromatography (HPLC) with refractive index detection (RID) as described elsewhere (24, 25).
RESULTS
Whole-cell assay for analyzing MdpJ substrate specificity.
For studying the substrate and catalysis specificity of MdpJ, a test system employing the isolated enzyme is recommended. However, purification of the active enzyme from cells of strain L108 or after heterologous expression in Escherichia coli was not successful (see the supplemental material). Therefore, we developed a whole-cell assay comparing the substrate usage and product formation of wild-type strain L108 with that of the mdpJ knockout strain L108 (ΔmdpJ) K24 (35). MPD-grown cells of both strains were incubated in the presence of TBA for 10 to 16 h. This procedure was sufficient to induce MdpJ and MdpK in the wild-type strain (see Fig. S1 in the supplemental material); however, the mutant strain K24 did not synthesize these enzymes due to the insertion mutation in the mdpJ gene. In line with this, subsequent resting-cell experiments revealed that only the wild-type cells were able to degrade TBA (see Fig. S2 in the supplemental material). However, the formation of small amounts of the alkene isobutene from TBA was observed with both strains. This dehydration activity is a side reaction of MTBE metabolism in strains L108 and PM1 and has previously been ascribed to MdpJ (33). The dehydration activity of the mutant strain clearly shows now that an enzyme other than MdpJ must be involved. Likely, the same dehydratase that has recently been considered for the conversion of the tertiary alcohols TAA and 2-methyl-3-buten-2-ol to isoamylene and isoprene, respectively (35), forms isobutene from TBA. Nevertheless, knockout cells formed only less than 30% of the alkene amount of wild-type cells, indicating a higher dehydration activity during complete TBA metabolization.
Hydroxylation of 2-propanol to 1,2-propanediol.
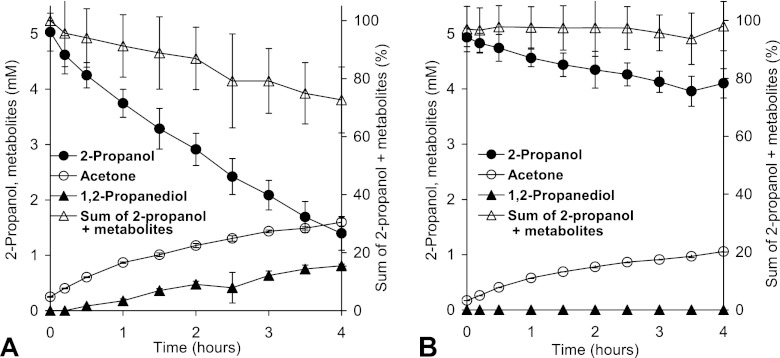
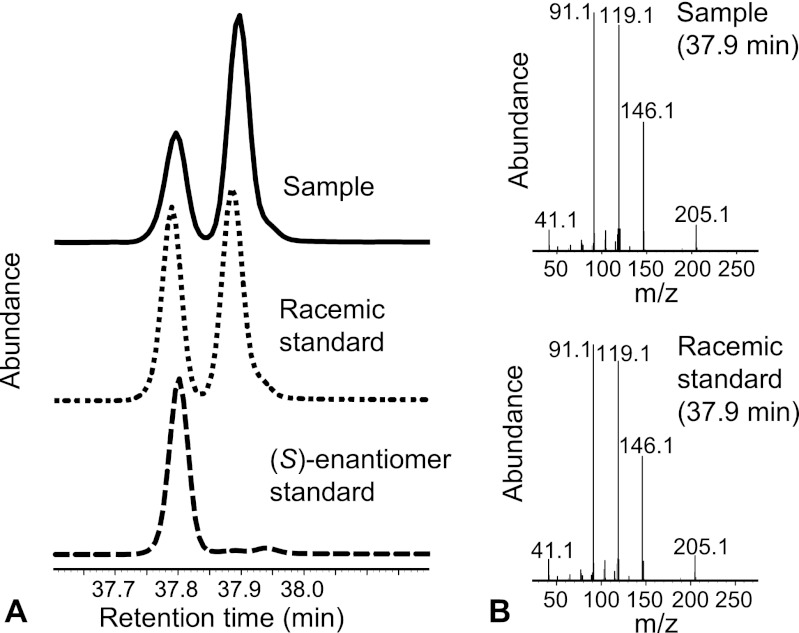
During the whole-cell assay, wild-type cells of strain L108 formed 1,2-propanediol from the secondary alcohol 2-propanol (Fig. 1), by analogy with the hydroxylation of TBA to MPD. In addition, the dehydrogenation product acetone accumulated. In contrast, the mdpJ knockout cells converted 2-propanol exclusively to acetone, providing evidence that MdpJ catalyzes the hydroxylation of 2-propanol in the wild-type strain L108. Acetone formation, on the other hand, is probably caused by an unspecific secondary alcohol dehydrogenase expressed in both strains under the experimental conditions. Formation of 1,2-propanediol was about 7 times slower than TBA conversion (1.5 and 10 nmol min−1 mg−1 [dry biomass], respectively), indicating that TBA is a much better substrate for MdpJ. As 1,2-propanediol is asymmetric, the stereospecificity of the hydroxylation reaction was investigated. After derivatization to the corresponding (S)-phenylbutyryl diester, the (S)- and (R)-enantiomers of 1,2-propanediol could be distinguished by GC analysis. Both enantiomers were formed by MdpJ. However, the hydroxylation to (R)-1,2-propanediol was slightly favored, resulting in an enantiomeric excess (EE) of about 70% (Fig. 2). During resting-cell experiments with the wild-type strain L108, substrate recovery of the conversion products 1,2-propanediol and acetone was below three-quarters, as these metabolites represented only less than 25 and 50% of the converted substrate, respectively. In contrast, knockout cells, which converted only one-third of the 2-propanol amount of wild-type cells, accumulated exclusively acetone, with close to 100% substrate recovery. This confirms that acetone is not degraded by strain L108 (21) and that 1,2-propanediol may be converted slowly to other metabolites not accumulating under the experimental conditions. By analogy to TBA and MPD metabolism, likely degradation products of 1,2-propanediol are lactaldehyde and lactic acid.
Fig 1.
Degradation of 2-propanol and accumulation of metabolites in resting-cell experiments of A. tertiaricarbonis wild-type strain L108 (A) and mdpJ knockout strain L108 (ΔmdpJ) K24 (B). In the latter case, 1,2-propanediol was below the detection limit (10 μM) throughout the experiment. The sum of 2-propanol and metabolites represents the percentage of all analyzed 2-propanol-derived compounds (2-propanol, acetone, and 1,2-propanediol) relative to the initial substrate concentration.
Fig 2.
GC-MS analysis of the (S)-2-phenylbutyryl derivatives of a racemic (RS)-1,2-propanediol standard, a pure (S)-1,2-propanediol standard, and a sample from a resting-cell experiment of A. tertiaricarbonis wild-type strain L108 incubated on 2-propanol. (A) Total ion chromatogram signals; (B) mass spectra of peaks occurring in the total ion chromatograms of the sample and the racemic standard at 37.9 min, representing the (S)-2-phenylbutyryl derivative of (R)-1,2-propanediol.
Desaturation of 2-butanol, 3-methyl-2-butanol, and 3-pentanol.
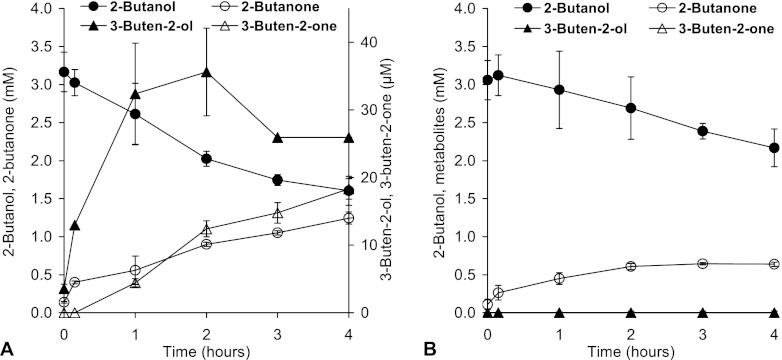
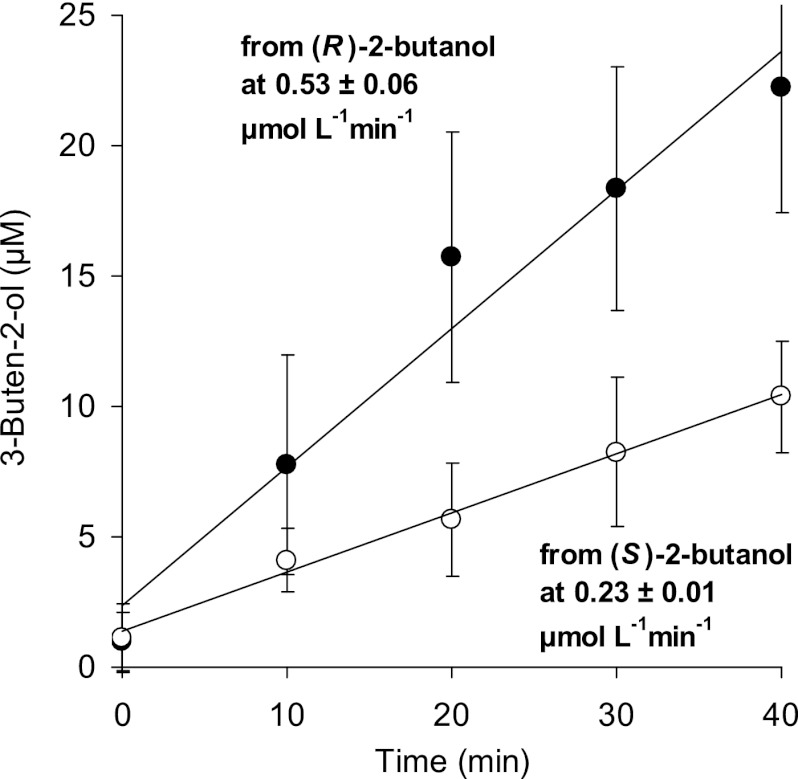
As 2-propanol turned out to be a substrate for MdpJ, the higher homologue 2-butanol was also tested. Surprisingly, hydroxylation products were not obtained with wild-type cells; only the desaturation product 3-buten-2-ol was formed from racemic 2-butanol (Fig. 3). Both secondary alcohols, 2-butanol and 3-buten-2-ol, were oxidized to their corresponding ketones, 2-butanone and 3-buten-2-one, respectively (Fig. 3 and see Fig. S3 and S4 in the supplemental material). In line with the assumption that MdpJ is responsible for 2-butanol desaturation, neither 3-buten-2-ol nor 3-buten-2-one was formed by the knockout mutant strain. Likely due to the high dehydrogenase activity, resulting in a nearly complete substrate conversion to 2-butanone, only very small amounts of 2-butanol of maximally 2% and 1% were recovered as the desaturation product 3-buten-2-ol and its corresponding ketone, 3-buten-2-one, respectively. In experiments with enantiopure substrates, conversion of (R)-2-butanol to 3-buten-2-ol was about 2.3 times faster than desaturation of the (S)-enantiomer (Fig. 4).
Fig 3.
Degradation of racemic 2-butanol and accumulation of metabolites in resting-cell experiments of A. tertiaricarbonis wild-type strain L108 (A) and mdpJ knockout strain L108 (ΔmdpJ) K24 (B). In the latter case, 3-buten-2-ol and 3-buten-2-one were below the detection limit (2 μM) throughout the experiment.
Fig 4.
Formation of 3-buten-2-ol from 2-butanol by resting cells of A. tertiaricarbonis wild-type strain L108 after application of pure enantiomers as the substrate.
In line with the observed transformation of 2-butanol to 3-buten-2-ol, 3-methyl-2-butanol and 3-pentanol were also converted to the corresponding desaturation products 3-methyl-3-buten-2-ol and 1-penten-3-ol, respectively, by wild-type cells of strain L108 (see Fig. S5 and S6 in the supplemental material). In addition, both wild-type and knockout mutant cells oxidized the secondary alcoholic substrates to the corresponding ketones. In the case of incubation with 3-methyl-2-butanol, the oxidation to the unsaturated ketone 3-methyl-3-buten-2-one by the wild-type cells was also observed (see Fig. S5 in the supplemental material). Generally, desaturation activities were lower than with 2-butanol, and prolonged incubation periods of up to 8 h were necessary to accumulate sufficient amounts of desaturation products for GC-MS analysis.
DISCUSSION
The Rieske nonheme mononuclear iron oxygenase MdpJ of Aquincola tertiaricarbonis L108 has previously been found to convert the tertiary alcohols TBA, TAA, and 3-methyl-3-pentanol to the corresponding diols and unsaturated alcohols (35). By comparing the product formations of wild-type and mdpJ knockout mutant cells, this study now demonstrates that short-chain secondary alcohols can also be attacked by MdpJ.
As indicated recently by Schuster and coworkers in the context of TAA and 3-methyl-3-pentanol metabolism (35), the mode of MdpJ catalysis obviously depends strongly on the molecule structure of the substrate, allowing either hydroxylation or desaturation reactions. An overview of the products obtained from the thus-far-tested substrates (C3 to C5 secondary alcohols and C4 to C6 tertiary alcohols) is given in Fig. S7 in the supplemental material. The structures of 2-propanol and TBA do not contain an ethyl group that can be desaturated. Accordingly, only hydroxylation to diols has been observed. On the other hand, 2-butanol, 3-methyl-2-butanol, 3-pentanol, TAA, and also 3-methyl-3-pentanol are desaturated mainly by MdpJ, as they all possess at least one pair of vicinal carbon atoms which can form a double bond. It can also be speculated whether the unsaturated alcohols 3-buten-2-ol and 2-methyl-3-buten-2-ol, considering their structural similarity with the MdpJ substrates 2-butanol and TAA, can be used by MdpJ. As these compounds are already desaturated, it appears likely that hydroxylation products would be formed (see Fig. S7 in the supplemental material). However, when testing both unsaturated alcohols in the whole-cell assay, we were not able to detect the formation of 1,2-diols, but HPLC analysis indicated their conversion to other products, likely unsaturated 2-hydroxy carboxylic acids (data not shown). Thus far, these compounds have not been unambiguously assigned by GC-MS measurements, and additional identification work is needed.
The capacity of MdpJ for hydroxylation and desaturation of small aliphatic alcohols as found in strain L108 seems to be quite unique. In the context of MTBE metabolism, a few other strains which should possess enzymes with a similar substrate spectrum have been described. Accordingly, the conversion of TBA to MPD has also been shown for the strains Mycobacterium vaccae JOB5 (37), Hydrogenophaga flava ENV 735 (13), Mycobacterium austroafricanum IFP 2012 (11), and Methylibium petroleiphilum PM1 (27). However, only the MdpJ of strain L108 has been characterized for oxidation of TBA in MTBE metabolism. In addition, strain PM1 possesses a nearly identical enzyme, showing 97% sequence identity to MdpJ of strain L108, which has been proposed to be involved in tertiary alcohol metabolism (14). Moreover, MdpJ has been detected in environmental samples. The mdpJ gene is present in MTBE-degrading enrichment cultures from a contaminated groundwater treatment plant in Leuna, Germany (33), and the enzyme has been detected in oxygenate-degrading mixed cultures by 13C metagenomic and metaproteomic stable-isotope probing (SIP) experiments (2, 4). These findings underline the important role of MdpJ in bacterial MTBE degradation.
Short-chain chiral alcohols and diols are interesting building blocks for the synthesis of pharmaceuticals and other active compounds (3, 10, 18, 39). Enantiopure alcohols, for example, are key intermediates in the side chain synthesis of serum cholesterol-reducing drugs, i.e., 3-hydroxy-3-methylglutaryl-coenzyme A (CoA) reductase inhibitors (29). In addition, 1,2-propanediol is a chiral building block for the synthesis of antiviral drugs like tenofovir or efavirenz (26, 17). Thus far, the most important enzyme-based method to synthesize enantiopure alcohols is lipase-catalyzed kinetic resolution of alcoholic and diolic racemates (6, 18, 34). In addition, enantioselective reduction of ketones (29) or carboxylic acids (6) with specific dehydrogenases can be applied. Enantiopure 1,2-propanediol for commercial purposes, on the other hand, is currently synthesized only via chemical routes (1, 6). A more straightforward approach for the synthesis of enantiopure unsaturated alcohols and, particularly, diols may be the stereospecific desaturation and hydroxylation of the corresponding saturated alcoholic compounds. However, a one-step synthesis employing MdpJ would require a high stereospecificity. In our preliminary experiments on MdpJ catalysis, only an EE value of about 70% of that of (R)-1,2-propanediol and a 2.3-fold preference for (R)-2-butanol as a desaturation substrate were achieved. This indicates that at least for the secondary alcohols tested, the same orientation in the reaction center of the enzyme is favored (Fig. 5). On the basis of this finding, the poor stereospecificity could be improved by site-directed mutagenesis or novel approaches like directed enzyme evolution (7, 8). The stereospecificity of the MdpJ-catalyzed desaturation of 3-methyl-2-butanol and 3-pentanol to the hemiterpenic 3-methyl-3-buten-2-ol and to 1-penten-3-ol, respectively, has not yet been analyzed. Nevertheless, a preference similar to that with (R)-2-butanol could be expected. Hence, an improved MdpJ enzyme showing higher-than-normal stereospecificity could be employed for the synthesis of chiral allylic alcohols and related compounds currently available only through multistep chemical synthesis (3, 16). At the present stage of our research, however, we exclude the possibility of applying pure MdpJ, because preparation of the cell-free enzyme always resulted in a complete loss of enzymatic activity. Alternatively, application in whole-cell systems is quite promising but requires either metabolic manipulation of the wild-type strain L108 in order to avoid the ketone-forming side reaction or the transformation of the mdpJK genes into a more appropriate host strain lacking such side activities.
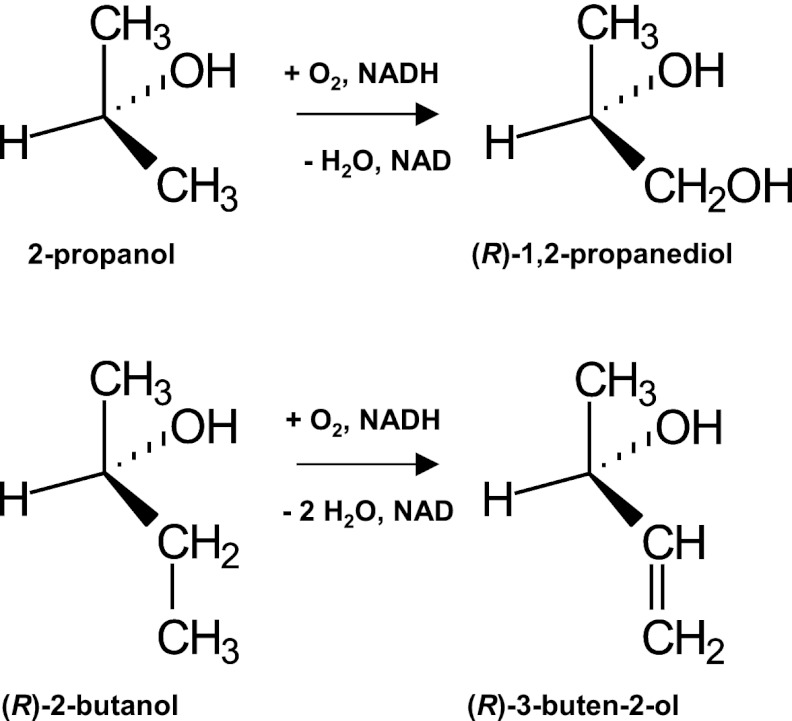
Fig 5.
Favored substrate and corresponding product enantiomers of MdpJ-catalyzed hydroxylation of 2-propanol to 1,2-propanediol and desaturation of 2-butanol to 3-buten-2-ol.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to the Deutsche Bundesstiftung Umwelt (DBU) for financial support of F.S. (grant AZ 20008/994).
Footnotes
Published ahead of print 29 June 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Altaras NE, Cameron D. 1999. Metabolic engineering of 1,2-propanediol pathway in Escherichia coli. Appl. Environ. Microbiol. 65: 1180–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aslett D, Haas J, Hyman M. 2011. Identification of tertiary butyl alcohol (TBA)-utilizing organisms in BioGAC reactors using 13C-DNA stable isotope probing. Biodegradation 22: 961–972 [DOI] [PubMed] [Google Scholar]
- 3. Balmer E, Germain A, Jackson WP, Lygo B. 1993. Larger scale preparation of optically-active allylic alcohols. J. Chem. Soc. Perkin Trans. 1: 399–400 [Google Scholar]
- 4. Bastida F, et al. 2010. Elucidating MTBE degradation in a mixed consortium using a multidisciplinary approach. FEMS Microbiol. Ecol. 73: 370–384 [DOI] [PubMed] [Google Scholar]
- 5. Batie C, La Haie JE, Ballou DP. 1987. Purification and characterization of phthalate oxygenase and phthalate oxygenase reductase from Pseudomonas cepacia. J. Biol. Chem. 262: 1510–1518 [PubMed] [Google Scholar]
- 6. Breuer M, et al. 2004. Industrial methods for the production of optically active intermediates. Angew. Chem. Int. Ed. Engl. 43: 788–824 [DOI] [PubMed] [Google Scholar]
- 7. Chen R. 2001. Enzyme engineering: rational redesign versus directed evolution. Trends Biotechnol. 19: 13–14 [DOI] [PubMed] [Google Scholar]
- 8. Dalby PA. 2003. Optimising enzyme function by directed evolution. Curr. Opin. Struct. Biol. 13: 500–505 [DOI] [PubMed] [Google Scholar]
- 9. Fayolle F, Vandecasteele JP, Monot F. 2001. Microbial degradation and fate in the environment of methyl tert-butyl ether and related fuel oxygenates. Appl. Microbiol. Biotechnol. 56: 339–349 [DOI] [PubMed] [Google Scholar]
- 10. FDA 1992. FDA's statement for the development of new stereoisomeric drugs. Chirality 4: 338–340 [DOI] [PubMed] [Google Scholar]
- 11. François A, et al. 2002. Biodegradation of methyl tert-butyl ether and other fuel oxygenates by a new strain, Mycobacterium austroafricanum IFP 2012. Appl. Environ. Microbiol. 68: 2754–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hammarström S, Hamberg M. 1973. Steric analysis of 3-, ω4-, ω3- and ω2-hydroxy acids and various alkanols by gas-liquid chromatography. Anal. Biochem. 52: 169–179 [DOI] [PubMed] [Google Scholar]
- 13. Hatzinger PB, et al. 2001. Biodegradation of methyl tert-butyl ether by a pure bacterial culture. Appl. Environ. Microbiol. 63: 5601–5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hristova KR, et al. 2007. Comparative transcriptome analysis of Methylibium petroleiphilum PM1exposed to the fuel oxygenates methyl tert-butyl ether and ethanol. Appl. Environ. Microbiol. 73: 7347–7357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jenske R, Vetter W. 2007. Highly selective gas-chromatography-electron-capture negative-ion mass spectrometry method for the indirect enantioselective identification of 2- and 3-hydroxy fatty acids in food and biological samples. J. Chromatogr. A 1146: 225–231 [DOI] [PubMed] [Google Scholar]
- 16. Jones S, Valette D. 2009. Enantioselective synthesis of allylic alcohols via an oxazaborolidinium ion catalyzed Diels-Alder/Retro-Diels-Alder sequence. Org. Lett. 11: 5358–5361 [DOI] [PubMed] [Google Scholar]
- 17. Kadyrov R, Koenigs RM, Brinkmann C, Voigtlaender D, Rueping M. 2009. Efficient enantioselective synthesis of optically active diols by asymmetric hydrogenation with modular chiral metal catalysts. Angew. Chem. Int. Ed. Engl. 48: 7556–7559 [DOI] [PubMed] [Google Scholar]
- 18. Kourist R, Bornscheuer UT. 2011. Biocatalytic synthesis of optically active tertiary alcohols. Appl. Microbiol. Biotechnol. 91: 505–517 [DOI] [PubMed] [Google Scholar]
- 19. Kweon O, et al. 2008. A new classification system for bacterial Rieske non-heme iron aromatic ring-hydroxylating oxygenases. BMC Biochem. 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larkin MJ, Allen CCR, Kulakov LA, Lipscomb DA. 1999. Purification and characterization of a novel naphthalene dioxygenase from Rhodococcus sp. NCIMB12038. J. Bacteriol. 181: 6200–6204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lechner U, et al. 2007. Aquincola tertiaricarbonis gen. nov., sp. nov., a tertiary butyl moiety-degrading bacterium. Int. J. Syst. Evol. Microbiol. 57: 1295–1303 [DOI] [PubMed] [Google Scholar]
- 22. Lee K, Gibson DT. 1996. Toluene and ethylbenzene oxidation by purified naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816-4. Appl. Environ. Mic robiol. 62: 3101–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mason JR, Cammak R. 1992. The electron-transport proteins of hydroxylating bacterial dioxygenases. Annu. Rev. Microbiol. 46: 277–305 [DOI] [PubMed] [Google Scholar]
- 24. Müller RH, Rohwerder T, Harms H. 2007. Carbon conversion efficiency and limits of productive bacterial degradation of methyl tert-butyl ether and related compounds. Appl. Environ. Microbiol. 73: 1783–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Müller RH, Rohwerder T, Harms H. 2008. Degradation of fuel oxygenates and their main intermediates by Aquincola tertiaricarbonis L108. Microbiology 154: 1414–1421 [DOI] [PubMed] [Google Scholar]
- 26. Murakami H. 2007. From racemates to single enantiomers—chiral synthetic drugs over the last 20 years. Top. Curr. Chem. 269: 273–299 [DOI] [PubMed] [Google Scholar]
- 27. Nakatsu CH, et al. 2006. Methylibium petroleiphilum PM1T gen. nov., sp. nov., a new methyl tert-butyl ether (MTBE) degrading methylotroph of the beta-Proteobacteria. Int. J. Syst. Evol. Microbiol. 56: 983–989 [DOI] [PubMed] [Google Scholar]
- 28. Nomura Y, Nakagawa M, Ogawa N, Harashima S, Oshima Y. 1992. Genes in PHT plasmid encoding the initial degradation pathway of phthalate in Pseudomonas putida. J. Ferment. Bioeng. 74: 333–344 [Google Scholar]
- 29. Panke S, Held M, Wubbolts M. 2004. Trends and innovations in industrial biocatalysis for the production of fine chemicals. Curr. Opin. Biotechnol. 15: 272–279 [DOI] [PubMed] [Google Scholar]
- 30. Powers L, et al. 1994. Assay of the enantiomers of 1,2-propanediol, 1,3-butanediol, 1,3-pentanediol and the corresponding hydroxyacids by gas chromatography-mass spectrometry. Anal. Biochem. 221: 323–328 [DOI] [PubMed] [Google Scholar]
- 31. Rohwerder T, Breuer U, Benndorf D, Lechner U, Müller RH. 2006. The alkyl tert-butyl ether intermediate 2-hydroxyisobutyrate is degraded via a novel cobalamin-dependent mutase pathway. Appl. Environ. Microbiol. 72: 4128–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schäfer F, et al. 2007. Growth of Aquincola tertiaricarbonis L108 on tert-butyl alcohol leads to the induction of a phthalate dioxygenase-related protein and its associated oxidoreductase subunit. Eng. Life Sci. 7: 512–519 [Google Scholar]
- 33. Schäfer F, et al. 2011. Alkene formation from tertiary alkyl ether and alcohol degradation by Aquincola tertiaricarbonis L108 and Methylibium spp. Appl. Environ. Microbiol. 77: 5981–5987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmid A, Hollmann F, Byung Park J, Bühler B. 2002. The use of enzymes in the chemical industry in Europe. Curr. Opin. Biotechnol. 13: 359–366 [DOI] [PubMed] [Google Scholar]
- 35. Schuster J, et al. 2012. Bacterial degradation of tert-amyl alcohol proceeds via hemiterpene 2-methyl-3-buten-2-ol by employing the tertiary alcohol desaturase function of the Rieske nonheme mononuclear iron oxygenase MdpJ. J. Bacteriol. 194: 972–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simon MJ, et al. 1993. Sequences of genes encoding naphthalene dioxygenase in Pseudomonas putida strains G7 and NCIB 98 16-4. Gene 127: 31–37 [DOI] [PubMed] [Google Scholar]
- 37. Smith CA, O'Reilly T, Hyman MR. 2003. Characterization of the initial reactions during the cometabolic oxidation of methyl tert-butyl ether by propane-grown Mycobacterium vaccae JOB5. Appl. Environ. Microbiol. 69: 796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steffan RJ, McClay K, Vainberg S, Condee CW, Zhang D. 1997. Biodegradation of the gasoline oxygenates methyl tert-butyl ether, ethyl tert-butyl ether and tert-amyl methyl ether by propane-oxidizing bacteria. Appl. Environ. Microbiol. 63: 4216–4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vanhessche KPM, Sharpless KB. 1997. Catalytic asymmetric synthesis of new halogenated chiral synthons. Chem. Eur. J. 3: 517–522 [Google Scholar]
- 40. Wackett LP. 2002. Mechanism and applications of Rieske non-heme iron dioxygenases. Enzyme Microbial Technol. 31: 577–587 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.