Abstract
Cronobacter spp. are emerging pathogens that cause severe infantile meningitis, septicemia, or necrotizing enterocolitis. Contaminated powdered infant formula has been implicated as the source of Cronobacter spp. in most cases, but questions still remain regarding the natural habitat and virulence potential for each strain. The iron acquisition systems in 231 Cronobacter strains isolated from different sources were identified and characterized. All Cronobacter spp. have both the Feo and Efe systems for acquisition of ferrous iron, and all plasmid-harboring strains (98%) have the aerobactin-like siderophore, cronobactin, for transport of ferric iron. All Cronobacter spp. have the genes encoding an enterobactin-like siderophore, although it was not functional under the conditions tested. Furthermore, all Cronobacter spp. have genes encoding five receptors for heterologous siderophores. A ferric dicitrate transport system (fec system) is encoded specifically by a subset of Cronobacter sakazakii and C. malonaticus strains, of which a high percentage were isolated from clinical samples. Phylogenetic analysis confirmed that the fec system is most closely related to orthologous genes present in human-pathogenic bacterial strains. Moreover, all strains of C. dublinensis and C. muytjensii encode two receptors, FcuA and Fct, for heterologous siderophores produced by plant pathogens. Identification of putative Fur boxes and expression of the genes under iron-depleted conditions revealed which genes and operons are components of the Fur regulon. Taken together, these results support the proposition that C. sakazakii and C. malonaticus may be more associated with the human host and C. dublinensis and C. muytjensii with plants.
INTRODUCTION
Cronobacter spp. are Gram-negative, rod-shaped bacteria within the family Enterobacteriaceae. The genus Cronobacter has been shown to be phenotypically and genetically diverse (45) and has been proposed to comprise seven species: C. sakazakii, C. malonaticus, C. turicensis, C. muytjensii, C. dublinensis (C. dublinensis subsp. dublinensis, C. dublinensis subsp. lausannensis, and C. dublinensis subsp. lactaridi), C. universalis, and C. condimenti (34, 36). These emerging pathogens cause severe meningitis, septicemia, or necrotizing enterocolitis in neonates and infants (40, 57). Although the disease frequency is very low, the mortality rate ranges from 40% to as high as 80% (29, 57). Meningitis caused by Cronobacter spp. occurs both as sporadic cases and as outbreaks, and contaminated powdered infant formula (PIF) has been epidemiologically implicated as the source of the pathogen in most cases (14, 44, 71, 79). However, extrinsic contamination of opened PIF cans and bottled water supplies has also been reported (59). Cronobacter spp. have been also isolated from a wide spectrum of environmental sources and food products (25, 38), but their natural habitat and whether all strains have the capacity to produce disease are unclear. An environmental niche of eukaryotic plant material has been proposed for Cronobacter spp. due to the ability to produce a yellow pigment that protects the cell against the effects of UV radiation from sunlight, and expression of capsules and fimbriae to aid in adherence to surfaces and promote survival under high osmotic and desiccated stressful growth conditions (35, 69).
Identification of virulence markers to distinguish pathogenic from nonpathogenic strains will improve our understanding of the epidemiology of Cronobacter spp., which will, in turn, help elucidate potential contamination risks associated with this food-borne pathogen. Recently, we reported that 98% of 229 Cronobacter isolates possessed a plasmid that was closely related or identical to pESA3 and pCTU1 (pESA3-/pCTU1-like plasmid) (23), plasmids harbored by C. sakazakii ATCC BAA-894 and C. turicensis z3032, respectively (43, 74). Furthermore, we found that pESA3-/pCTU1-like plasmids encode common virulence factors, including an aerobactin-like siderophore and an ABC ferric-iron transporter eitABCD (23).
Iron is an essential cofactor for many enzymes involved in cellular respiration, electron transfer, and superoxide metabolism (28). Iron is also an important factor for bacterial pathogenesis (9, 77, 82). Although the concentration of iron in the environment is sufficient to sustain the viability of microbes, under aerobic conditions, most iron is present as ferric hydroxide (Fe3+), which is insoluble and biologically inaccessible to bacteria (58). Under iron starvation conditions, bacteria produce small iron-chelating molecules termed siderophores (54). Siderophores bind to the six coordinate sites of ferric ions by forming water-soluble hexadentate ferric complexes. Siderophores are usually classified by the ligands used to chelate the ferric iron. The major groups of siderophores include the catecholates-phenolates (e.g., enterobactin), hydroxamates (e.g., aerobactin and ferrichrome), and carboxylates (e.g., citric acid and derivatives) (54). In Gram-negative bacteria, the Fe3+-siderophore complex is recognized and transported into the periplasm via TonB-dependent receptors and is transferred into the cytoplasm by ABC transporters formed by a permease and an ATPase protein (18, 54). Once in the cytoplasm of the cell, the Fe3+-siderophore complex is usually reduced to Fe2+ to release the iron, especially in the case of “weaker” siderophore ligands such as hydroxamates and carboxylates (53, 81). Siderophore decomposition or other biological mechanisms can also release iron, especially in the case of catecholates such as ferric enterobactin, whose reduction potential is too low for reducing agents (48). Under anaerobiosis or low-pH growth conditions, the iron equilibrium shifts from the ferric (Fe3+) to ferrous (Fe2+) form, which is more easily accessible to microorganisms. This allows several permeases of different protein families to also contribute to overall iron uptake (11, 64).
The presence of iron acquisition systems is advantageous for the growth of bacteria under low-iron-availability stress conditions. Pathogens, in particular, require efficient iron acquisition mechanisms to enable them to compete successfully for iron in the highly iron-restricted environment of the human host. There is considerable variation in the type of iron transporters and iron sources utilized by different microbial species. This may reflect the diversity of various niches occupied by particular strains and the nature of the source of iron available in a specific environment. In this study, we performed a comparative in silico analysis of putative iron acquisition systems found in the genomes of nine strains of Cronobacter, representing six species. Furthermore, we identified the iron acquisition systems profile of a collection of 231 Cronobacter strains isolated from clinical, food, and environmental sources and from diverse geographical locations. Expression of the putative iron acquisition genes under different iron concentrations and functionality of identified siderophores were also determined.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in the present study are described in Table 1. The Cronobacter strains screened for iron acquisition system gene targets consisted of 180 C. sakazakii, 25 C. malonaticus, 12 C. muytjensii, 6 C. turicensis, 6 C. dublinensis, and 2 C. universalis strains from our laboratory culture collection; these strains represent isolates obtained from clinical (50 [22%]), food (48 [21%]), and environmental (122 [53%]) sources (4% unknown) and from diverse geographical locations. Assignment of the Cronobacter species nomenclature to the strains was performed according to the proposed classification scheme suggested by Iversen et al. (34), and identification was confirmed using the species-specific rpoB PCR assay described by Stoop et al. (75). Fosmid clones ESA-C01 and ESA-M04, containing and lacking the cronobactin gene, respectively, were obtained from a C. sakazakii BAA-894 fosmid library (43). Presence and absence of viuB, shiF, and iucABCD-iutA in ESA-C01 and ESA-M04 were confirmed by PCR using primers derived from each gene. Cronobacter and E. coli strains were grown at 37°C in Luria-Bertani (LB) broth with shaking (175 rpm) or on LB agar. Antibiotics were added, when required, at the following concentrations: 40 μg/ml for chloramphenicol and 100 μg/ml for ampicillin. Results were submitted to the Pathogen-Annotated Tracking Resource Network (PATRN) system, which is located at http://www.patrn.net and is accessible to users after a free registration process.
Table 1.
Bacterial strains used for in silico analysis in this studya
| Species | Strain ID | Biological source | Geographical source | Relevant characteristic | Reference |
|---|---|---|---|---|---|
| Bacterial strains used in in silico analysis | |||||
| C. sakazakii | ATCC BAA-894 | Infant | Tennessee, USA | 32 | |
| C. sakazakii | 2151 | CSF | United States | This study | |
| C. turicensis | z3032 | Blood | Zurich, Switzerland | 74 | |
| C. malonaticus | LMG 23826 | Breast abscess | United States | 34 | |
| C. dublinensis subsp. dublinensis | LMG 23823 | Milk powder production facility | Dublin, Ireland | 34 | |
| C. dublinensis subsp. lactaridi | LMG 23825 | Milk powder production facility | Zimbabwe | 34 | |
| C. dublinensis subsp. lausennensis | LMG 23824 | Water fountain | Lausanne, Switzerland | 34 | |
| C. universalis | NCTC 9529 | Water | London, England | 34 | |
| C. muytjensii | ATCC 51329 | Unknown | Unknown | 34 | |
| Additional bacterial strains used for experimental analysis | |||||
| C. turicensis | 3032.2A | C. turicensis z3032 cured of pCTU1 | 23 | ||
| C. sakazakii | BAA-894.3 | C. sakazakii ATCC BAA-894 cured of pESA3 | 23 | ||
| C. muytjensii | E488 | Unknown | Lausanne, Switzerland | Lacks pESA3-/pCTU1-like plasmid | 23, 75 |
| C. muytjensii | E456 | Unknown | Lausanne, Switzerland | Lacks pESA3-/pCTU1-like plasmid | 23, 75 |
| C. dublinensis subsp. dublinensis | CDC 0743-75 | Human, clinical | Wisconsin, USA | Lacks pESA3-/pCTU1-like plasmid | 23 |
| C. sakazakii | CDC 9363-75 | Unknown | New York, USA | Lacks pESA3-/pCTU1-like plasmid | 23 |
| C. muytjensii | CDC 3523-75 | Human, clinical | Arizona, USA | Harbors pESA3-/pCTU1-like plasmid | 23 |
| Vibrio vulnificus | UNCC913 | Unknown | Environmental | Hydroxamate/catechol siderophores | 73 |
| E. coli | ESA-C01 | E. coli EPI300 fosmid clone containing viuB-shiF and iucABCD-iutA from pESA3 | 43 | ||
| E. coli | ESA-M04 | E. coli EPI300 fosmid clone lacking viuB-shiF and iucABCD-iutA | 43 | ||
Strain metadata for these and those screened by PCR can be found in PATRN (http://www.patrn.net). ID, identity; CSF, cerebrospinal fluid.
In silico analysis.
The genomes of all nine Cronobacter strains used in this study were annotated using the RAST server (4). They include C. sakazakii ATCC BAA-894, CP000783; the genome of C. turicensis z3032, FN543093; C. sakazakii 2151, AJKT01000000; C. universalis NCTC 9529, AJKW01000000; C. malonaticus LMG 23826, AJKV01000000; C. dublinensis subsp. dublinensis LMG 23823, AJKZ01000000; C. dublinensis subsp. lactaridi LMG 23825, AJKX01000000; C. dublinensis subsp. lausanensis LMG 23824, AJKY01000000; and C. muytjensii ATCC 51329, AJKU01000000. Additionally, plasmid sequences for pESA3 (NC_009780) and pCTU1 (NC_013383) were used in this study. Iron acquisition genes and gene clusters were identified by intrinsic RAST subsystem profiling for each genome as well as manual gene homologue BLAST searches. Comparative genomics in the SEED viewer (60) was used to confirm identification and conservation of putative iron acquisition genes within Cronobacter genome sequences.
Phylogenetic analysis.
Phylogenetic analyses of iron acquisition system nucleotide sequences, a total of 16, were conducted in MEGA5 (76), using the entire iron acquisition gene or gene cluster for each system, retrieved from the SEED viewer and NCBI (see supplemental material for those not shown in Fig. 3). The evolutionary history was inferred by using the neighbor-joining method (65). The bootstrap consensus tree inferred from 1,000 replicates is taken to represent the evolutionary history of the taxa analyzed (20). The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to each branch (20). Trees are drawn to scale, with branch lengths in units similar to those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed by using the Poisson correction method (84) and are in the units of the number of amino acid substitutions per site. All positions containing gaps and missing data were eliminated from the data set.
Fig 3.
Evolutionary history of iron acquisition system genes. (A) Enterobactin gene cluster in Cronobacter spp., entHABEC-fepB and entS-fepDGC; (B) the TonB receptor-encoding gene foxA; (C) the TonB receptor-encoding gene fcuA; (D) the ferric reductase gene, viuB.
PCR assays.
PCR primers were designed that targeted the different putative iron acquisition genes and gene clusters. Except for the primers for cronobactin genes, fecR, fecB, fecE, fcuA, and fct, all primers were derived from common sequences of the nine sequenced Cronobacter strains tested in this study. Primers for the cronobactin genes were derived from common sequences of pESA3 and pCTU1, primers for fecR, fecB, and fecE were designed from C. sakazakii 2151, and primers for fcuA and fct were derived from common sequences of C. dublinensis strains LMG 23823, LMG 23825, and LMG 23824 and C. muytjensii ATCC 51329. The sequences of the primers, targeted genes, and the amplification parameters used for each set of primers are shown in Table 2. In each PCR, the positive control consisted of DNA preparations of the nine strains sequenced; the negative controls were DNA preparations of the closely related sister species, Enterobacter helveticus z513 and Enterobacter turicensis z508, as well as water (no-template control). All PCR mixtures were prepared using the GoTaq Green master mix (Promega Corp., Madison, WI) according to the manufacturer's instructions, using 1 μl of the plasmid preparation (approximately 90 ng of DNA/25-μl reaction mixture) or 5 μl of boiled genomic DNA sample (approximately 50 ng of DNA/25-μl reaction mixture) as the DNA template. In all PCRs, the polymerase was activated by using a 3-min incubation step at 94°C, followed by 30 cycles of denaturation at 94°C for 30 s and annealing and extension steps according to the PCR parameters described in Table 2. For each reaction, a final extension step of 7 min at the cycle extension temperature, as described for each PCR, was used (Table 2).
Table 2.
PCR primers used in this study
| Target | Primer pairsa | Sequence (5′–3′) | Amplicon size (bp) | Annealing/extension cycle parametersb |
|---|---|---|---|---|
| viuB | viuB_108F | RCATGAAGCGCCCGATCAGCG | 445 | 58°C for 30 s/72°C for 45 s |
| viuB_552R | CGCCAGCGGCACTTCCAGAAA | |||
| shif | ShiF_761R | CGGAGATCGCCATGAAACAG | 490 | 54°C for 30 s/72°C for 60 sc |
| ShiF_272F | TGCTGAGTTTCGCCGTGATG | |||
| iucA | IucA_352F | GAGAGCCACCGCCATACCTG | 657 | 58°C for 30 s/72°C for 60 s |
| IucA_1008R | CACCCAGCCATCTTCCTGCA | |||
| iucB | IucB_319F | CGTGCGGGAATACAGTTTGACG | 574 | 55°C for 30 s/72°C for 60 s |
| IucB_892R | GCTTGTGCGGGAAATCGAACTC | |||
| iucC | IucC_389F | TGCAGTGCCTGATGTCAGGCCAT | 660 | 58°C for 30 s/72°C for 60 s |
| IucC_1049R | ACGCCAAACATCTCCTGATAGCG | |||
| iucD | IucD_327F | GAGCAATCTGTCGTTCAGCC | 484 | 52°C for 30 s/72°C for 60 s |
| IucD_810R | GATAGCGAGCAGCGATTCGC | |||
| iutA | IutA_943F | CGCGATGAGAGCCTGACCTA | 598 | 55°C for 30 s/72°C for 60 s |
| IutA_1540R | CAAGACGATAGGTGCCGGAG | |||
| eitA | EitA_904R | CCTTTTTCACGGCGTCGAGCTG | 281 | 60°C for 30 s/72°C for 30 s |
| EitA_624F | TCTCTTCTGGTTCTCCAGCGCG | |||
| eitD | EitD_358F | CCGTCGATTGAATCGCTGCTG | 536 | 56°C for 30 s/72°C for 60 s |
| EitD_893R | GCCACGCTGACAAACGAGGC | |||
| fepA | FepA_840F | GTTTGAAGCGGGCTACAGCC | 597 | 55°C for 30 s/72°C for 60 s |
| FepA_1436R | GGGCTCCAGTTGTTGCCAAC | |||
| entF | EntF_1936F | AACCGGCTGCTGTGGATGCAAA | 280 | 58°C for 30 s/72°C for 30 s |
| EntF_2215R | TACAGAACACCTGRCGCAGCGA | |||
| entE | EntE_1496R | AGTTTGAAATCGGCGACGCCCAG | 244 | 58°C for 30 s/72°C for 30 s |
| EntE_1253F | CASGGCTACATCACCGTTCAGGG | |||
| fepE | FepE_272F | GCTGCCGCAGAAATGGACCAG | 339 | 58°C for 30 s/72°C for 30 s |
| FepE_610R | AGCGTCCAGGAGCTGTAAGGC | |||
| entC | EntC_1149R | GTGGAGCATGGTGGAGAGCT | 711 | 55°C for 30 s/72°C for 60 s |
| EntC_439F | TCCCGCCTTATCGACATCAC | |||
| fepG | FepG_230F | GCGCGATTTTCCAGTCGCTGATG | 263 | 58°C for 30 s/72°C for 30 s |
| FepG_492R | CCASGTATTGAACGCCACCAGC | |||
| entS | Ents_127F | CAGATCCAGACGCTCACCGG | 369 | 56°C for 30 s/72°C for 30 s |
| Ents_495R | GAGCAGCGGCGAAATYACCG | |||
| fepB | FepG_36F | CGCCAGCCTGCTGSTTTTAGG | 600 | 58°C for 30 s/72°C for 60 s |
| FepG_635R | GCGAATCTCTGGACCGCCGA | |||
| fhuA | Fhua_4F | GCGCGTTCYACTCACACTCAG | 275 | 55°C for 30 s/72°C for 30 s |
| Fhua_278R | TCCATCTCTTCSCGCGTCAC | |||
| fhuB | Fhub_1313F | TGATGCTGCTGTTCGTGCCG | 493 | 58°C for 30 s/72°C for 45 s |
| Fhub_1805R | AGCATSCGCGTAATGTGCGG | |||
| fecR | FecR_108F | GCGCTGGCAACAGTGGTATG | 487 | 56°C for 30 s/72°C for 60 s |
| FecR_594R | CTGCACGTCAAGCTGCGTGA | |||
| fecB | FecB_286F | GCTGCCCTGAAACCAGACCT | 324 | 56°C for 30 s/72°C for 60 s |
| FecB_609R | CAGAGAAGCCAGCACGCTGC | |||
| fecE | FecE_283F | GAACTGGTTTCCTACGGCCG | 435 | 56°C for 30 s/72°C for 60 s |
| FecE_717R | CGCTTCTACGCTGAACACCG | |||
| feoB | Feob_895F | CTGCTCGCCATTAACATCGGCG | 296 | 58°C for 30 s/72°C for 30 s |
| Feob_1190R | ACGAACGATTTGCCCGGCAG | |||
| efeB | Efeb_167F | AGACGCAGCCGTTTTACGGCG | 431 | 58°C for 30 s/72°C for 45 s |
| Efeb_597R | GTCGCGCAGCGCATGGATMAC | |||
| efeO | Efeo_26F | CGCTTTCCGTGACGCTGCTG | 312 | 58°C for 30 s/72°C for 30 s |
| Efeo_337R | GCTTGCCTTTCGGGTTGCTCAG | |||
| fhuF | FhuF_270F | GCTGAAATCGCTCTGGGCGCAG | 351 | 58°C for 30 s/72°C for 30 s |
| FhuF_620R | ACGGTCTCKTCGCCGAGCCA | |||
| fhuE | FhuE_156F | CACGCCTGACGAGTCRCAGGA | 291 | 58°C for 30 s/72°C for 30 s |
| FhuE_446R | GCTGGAACTTTGGCGATACCGC | |||
| pfeA | PfeA_286F | CGCCAGATTGACATTCGCGG | 494 | 56°C for 30 s/72°C for 45 s |
| PfeA_779R | AATACCGCGCAGAACGGCTC | |||
| foxA | FoxA_10F | GCTTTAACCCTGAAACGCTCCGC | 400 | 58°C for 30 s/72°C for 45 s |
| FoxA_409R | GAGCGACGGCGGCAGCTATA | |||
| yncD | YncD_1726F | CTTGACGCCACCTACCGCRC | 243 | 58°C for 30 s/72°C for 30 s |
| YncD_1968R | AAATTCCAGCGCGGCAACTGG | |||
| btuB | BtuB_1953F | GTCAGCCTGTGGGATGTCGC | 156 | 58°C for 30 s/72°C for 30 s |
| BtuB_2108R | TGTCAGGCAGCTACACCTTCTGA | |||
| fcuA | fcuA_356F | CCTACCGCATTCGCGGCTTTG | 198 | 58°C for 30 s/72°C for 30 s |
| fcuA_553R | CGTCGGCATGTTTCGGCTCAAG | |||
| fct | fct_311F | GCTCCAACCGCAACGACGAAG | 554 | 58°C for 30 s/72°C for 60 s |
| fct_864R | GTCGCTGACGTTGAAATCGCGC | |||
| yfeX | YfeX_125F | TAGCGACCTTTCAGGCGCAGT | 281 | 58°C for 30 s/72°C for 30 s |
| YfeX_405R | CACGGTTTTCGCTGGGTGGAA | |||
| repAd | repA_185F | CAGACGCGACTGAGGAGCTTG | 784 | 56°C for 30 s/72°C for 60 s |
| repA_968R | AGAGGATCGATGCCAGCAGCC | |||
| repEe | repE_46F | CTGCAGGAACATGACGGCATC | 380 | 56°C for 30 s/72°C for 60 s |
| repE_425R | CCTGAGCCATCAGGTTTACGG | |||
| 16S rRNA | P0 | AGAGTTTGATCCTGGCTCAG | 1,503 | 55°C for 30 s/72°C for 90 s |
| P6 | GTACGGCTACCTTGTTACGA |
Number in primer name corresponds to 5′ nucleotide position of the ClustalW alignments for each gene (see Table S1 in the supplemental material).
All PCRs were performed with 30 cycles, except for that for Cronobacter 16S rRNA, which was performed with 25 cycles.
Dimethyl sulfoxide (DMSO) at a 7% (final concentration) was added to the PCR mix.
The repA gene of plasmid pCS2151.
The repE gene of plasmid pCS2151.
RT-PCR.
Expression of the iron acquisition genes was determined by reverse transcription (RT)-PCR. Total RNA of Cronobacter strains was obtained using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. Trace levels of genomic DNA were removed by treatment with DNase I using a DNA-free kit (Applied Biosystems), and cDNA was synthesized from 1 μg of total RNA using the SuperScript III First-Strand Synthesis System for RT-PCR kit (Invitrogen) according to the manufacturer's instructions with the primers listed in Table 2. RNA samples lacking reverse transcriptase were used as controls to detect DNA contamination. PCR conditions were selected to permit detection of the PCR products in the linear range of the reaction, and PCRs were performed using the same conditions as described above to screen for detection of the various iron acquisition systems (Table 2). The intensity of the PCR products was quantified using ImageJ, version 1.45s (1). Differences in the intensities of the PCR products are interpreted as differences in transcription and/or stability of the iron acquisition gene mRNA. The level of expression of Cronobacter 16S rRNA using primers P0 and P6 (12) served as an internal control.
Siderophore detection.
Siderophore production was determined using the chrome azurol S (CAS) agar diffusion (CASAD) assay as described previously (23). Briefly, the CAS agar plate was punched with 5-mm-diameter holes by using a gel puncher. Each hole was filled in a two-step process with 70 μl (35 μl twice) of cell-free culture supernatant of the test bacteria grown for 18 h in LB broth containing 300 μM 2,2′-dipyridyl (Sigma-Aldrich). After incubation at 37°C for 4 to 8 h, the presence of an orange halo around a hole indicated that a culture was positive for siderophore production.
The presence of phenolic-type and/or hydroxamate-type siderophores was detected in cell-free culture supernatants and whole-cell lysate preparations obtained from iron-depleted cultures using the colorimetric assays described by Arnow (3) and Csáky (15), respectively. Cell-free culture supernatants were lyophilized and concentrated 10-fold for the Arnow test. The Csáky assay was carried out with and without the sulfuric acid digestion step. Cell-free culture supernatants of Vibrio vulnificus UNCC913 (73) and catechol (6 μg) were used as positive controls in the Csáky and Arnow tests, respectively.
Statistical analysis.
Data were analyzed by the Student t test (paired); a P value of <0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Identification of Cronobacter iron acquisition systems.
Targeted in silico sequence analysis of the genomes of nine Cronobacter strains, including three C. dublinensis strains, two C. sakazakii strains, and single strains of C. malonaticus, C. turicensis, C. muytjensii, and C. universalis, revealed the presence of shared iron acquisition systems, with additional systems in some Cronobacter strains. The identified iron acquisition systems and their locations in the genome of the nine Cronobacter strains are listed in Table S1 in the supplemental material. These systems include genes encoding ferric and ferrous transporters and heme-iron extractors, as well as putative TonB-dependent iron receptors and ferric reductases.
(i) Ferric iron transporters.
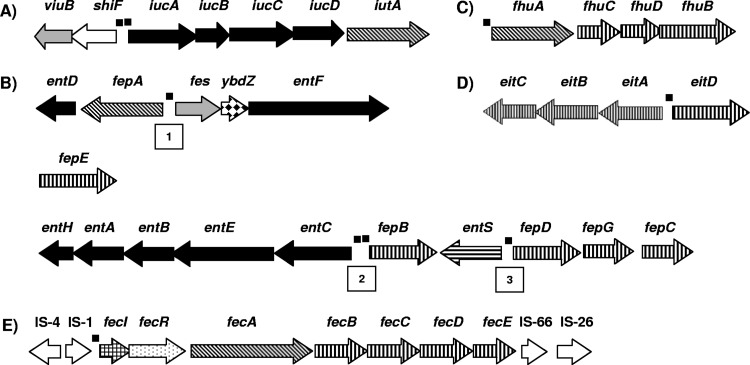
For acquisition of ferric iron (Fe3+), all nine Cronobacter genomes contain genes homologous to the hydroxamate-type siderophore aerobactin (named cronobactin in this study) and catechol-type siderophore enterobactin genes, except for C. muytjensii 51329, which did not have the cronobactin gene cluster because it does not harbor the common virulence plasmid. The cronobactin locus consists of five genes homologous to biosynthetic genes iucABCD and the receptor gene iutA (Fig. 1A). In agreement with a previous report (23), this gene cluster is localized on a pESA3-/pCTU1-like plasmid.
Fig 1.
Ferric iron transporters encoded by Cronobacter spp. (A) Cronobactin siderophore; (B) enterobactin-like siderophore; (C) hydroxamate ABC transporter encoded by fhuACDB; (D) ferric iron/siderophore/heme ABC transporter encoded by eitCBAD; (E) ferric dicitrate transport system. Arrows show the direction of transcription, and arrow fills identify genes encoding synthesis of siderophores (black), TonB-dependent outer membrane receptors (diagonal lines), ABC transporters (vertical lines), export of enterobactin (horizontal lines), intracellular release of the iron from siderophore-iron complex (gray), sigma factor (small grids), transmembrane signal transducer (dots), IS transposases (white), and unknown function (diamonds). Numbers in boxes shown in the enterobactin-like siderophore diagrams show locations of the three putative bidirectional promoter-operator regions. The small filled boxes upstream of some genes or operons show locations of putative Fur boxes.
In contrast to the single enterobactin locus carried by Escherichia coli and other bacteria, the genes encoding the enterobactin-like siderophore in seven of the nine Cronobacter genomes analyzed are localized in three different loci in the chromosome (Fig. 1B). The first locus contains a cluster of five genes, entD, fepA, fes, ybdZ, and entF, the second locus contains only fepE, and the third locus comprises 10 genes, entH, entA, entB, entE, entC, fepB, entS, fepD, fepG, and fepC. C. dublinensis subsp. lactaridi LMG 23825 and C. dublinensis subsp. lausennensis LMG 23824 contain loci one and three but lack the second locus containing fepE. In E. coli, entABCDEFH are involved in enterobactin biosynthesis, entS is involved in enterobactin export, fepA encodes the ferric enterobactin receptor, fepBCDEG allow the transport of the ferric enterobactin complex inside the cell, and fes encodes an esterase that catalyzes hydrolytic cleavage of the ferric enterobactin backbone, leading to the intracellular release of iron (26, 48, 62).
All nine Cronobacter genomes harbor genes homologous to the fhuACDB operon (Fig. 1C). The fhuA gene encodes the TonB-dependent outer membrane receptor specific for hydroxamate-type siderophore ferrichrome (19), suggesting that Cronobacter spp. are able to incorporate ferrichrome produced by other species. The fhuCDB genes encode an ABC transporter for a range of hydroxamate siderophores (19, 63). It has been determined that mutations in the fhuCDB operon abolish the ability of bacteria to use ferrichrome, aerobactin, and coprogen (22, 42). Thus, the FhuCDB transport system must be involved in the import of the iron-cronobactin complex into the cell. Furthermore, the eight Cronobacter genomes harboring pESA-3/pCTU1-like plasmids contain an eitABCD operon with homology to ABC transporters that mediate translocation of ferric iron, siderophores, and heme (Fig. 1D), which was previously reported (23).
Genes homologous to the ferric dicitrate transport genes fecIRABCDE (fec genes) were found solely in the genome of C. sakazakii strain 2151 (Fig. 1E). This iron transport system has been well characterized in E. coli K-12 and other bacteria (50, 51, 80). The fec system is capable of maintaining bacterial growth under iron-limited conditions in the absence of other iron uptake systems (50, 51, 80). Like the E. coli fec system, the C. sakazakii 2151 fec locus consists of genes located within two operons carrying the regulatory genes fecI and fecR and the downstream structural genes fecABCDE. The fecA gene encodes a TonB-dependent receptor for the ferric dicitrate complex, and fecBCDE encodes the transport system (50, 51, 80). In C. sakazakii 2151, the fec genes are contained in a plasmid, named pCSA2151, not previously identified in Cronobacter spp. Similar to the case with other bacteria (50, 51, 80), the fec genes of C. sakazakii 2151 are flanked by insertion sequences (IS), indicating their mobility (Fig. 1E).
(ii) Ferrous iron transporters.
All nine Cronobacter genomes analyzed have both the genes encoding the Feo and Efe systems to acquire the ferrous (Fe2+) form of iron from their environment. The Feo system is the major ferrous transporter in E. coli and is widely distributed among bacteria (37). This system operates anaerobically at pH ≥7. Like for all Enterobacteriaceae, the Cronobacter Feo system is comprised of three genes, feoABC (Fig. 2). In E. coli, FeoA is a small soluble SH3 domain protein probably located in the cytosol (11). FeoA of Cronobacter spp. conserves the FeoA-SH3 domain of E. coli, suggesting that the Cronobacter and E. coli Feo systems are regulated by the same mechanisms. FeoB is most likely a permease, and FeoC is a small protein apparently functioning as an [Fe-S]-dependent transcriptional repressor (11).
Fig 2.
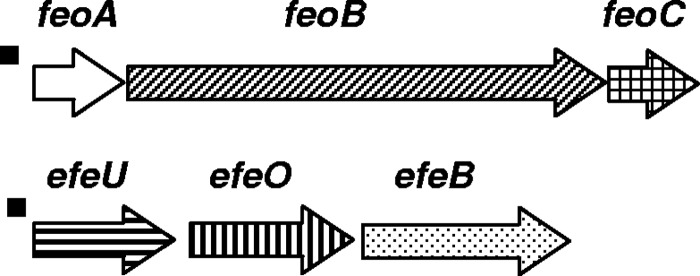
Genes for ferrous iron transporters, feoABC and efeUOB, carried by the chromosome of Cronobacter spp. Arrows show the direction of transcription, and arrow fills identify genes encoding GTP-binding protein (probably permease) (diagonal lines), Fe-S-dependent transcriptional regulator of FeoABC expression (square), high-affinity iron permease (horizontal lines), transporter periplasmic protein (vertical lines), periplasmatic peroxidase protein (dots), and unknown function (white). The small filled boxes upstream of the operons show location of putative Fur boxes.
The Efe system is a ferrous transport system that operates aerobically under low pH, conditions in which the ferrous iron remains stable (10). The bacterial Efe system in general has similarities to the well-studied high-affinity Fe2+ transporter (Ftr1p) of Saccharomyces cerevisiae (10). Like the S. cerevisiae Fe2+ transporter, the Efe system consists of three genes, efeUOB (Fig. 2). EfeU is homologous to the high-affinity iron permease, Ftr1p, of S. cerevisiae; EfeO is periplasmic, with a cupredoxin N-terminal domain; and EfeB is also periplasmic and is a heme peroxidase-like protein (10). The Efe system in E. coli K-12 is not functional due to a frameshift mutation in efeU (10). The efeU gene of Cronobacter spp. lacks any frameshift, suggesting that the Efe system is functional in this genus.
(iii) Heme iron extractors.
In the mammalian host, most of the iron is sequestered and contained within heme proteins. Many pathogens have the ability to use these host compounds directly, and heme utilization genes have been identified in numerous pathogens (55, 56, 78). No genes homologous to heme receptor-encoding genes were identified in any of the nine Cronobacter genomes analyzed; however, all nine Cronobacter spp. have a common gene whose predicted encoded protein has significant identity with E. coli YfeX. It has been reported that YfeX and EfeB of the Efe system in E. coli promote iron extraction from heme (46). Both YfeX and EfeB are widespread and highly conserved in bacteria.
(iv) Putative TonB-dependent iron receptors.
In addition to genes for the TonB-dependent receptors IutA, FepA, FhuA, and FecA, specific for cronobactin, enterobactin, ferrichrome, and ferric dicitrate, respectively, the nine Cronobacter genomes contain five common genes whose predicted encoded proteins have significant identity with TonB-dependent iron receptors. These outer membrane receptors include the siderophore receptor YncD (83), the vitamin B12/cobalamin outer membrane transporter BtuB (30), the ferroxamine receptor FoxA (5), the ferric rhodotorulic acid/coprogen receptor FhuE (67), and the ferric enterobactin receptor PfeA. All of these TonB-dependent receptors are required for the virulence of different bacteria, with the exception of BtuB (39, 61, 66, 83). PfeA is 60% identical to the ferric enterobactin receptor FepA encoded by the enterobactin gene cluster (Fig. 1B), and the presence of two putative ferric enterobactin receptors in Cronobacter spp. suggests that there may be siderophore receptor redundancy.
The chromosomes of the C. muytjensii and the three C. dublinensis strains analyzed encode TonB-dependent iron receptors Fct and FcuA, not encoded by the other Cronobacter strains. Fct has significant homology with the ferrichrysobactin receptor (Fct) encoded by the plant pathogen Erwinia chrysanthemi and other Gram-negative bacteria (68). Siderophore chrysobactin is an important virulence factor of E. chrysanthemi (16). FcuA is also a hydroxamate receptor encoded by Yersinia enterocolitica and other Gram-negative bacteria (41). The presence of these TonB-dependent outer membrane receptors suggests that Cronobacter spp. can incorporate many heterologous siderophores of both bacterial and fungal origins.
(v) Ferric reductase.
Upstream of the cronobactin gene iucA in all of the pESA3-/pCTU1-like plasmid-harboring Cronobacter genomes analyzed are two open reading frames (ORFs), named shiF and viuB due to their similarity to shiF and viuB of Shigella and Vibrio spp., respectively (Fig. 1A). Similar shiF-like genes lie upstream of the aerobactin locus in a number of bacteria (22), suggesting a possible role in the aerobactin system. Annotations of shiF-like genes indicate that it is a member of the COG0477 permeases of the major facilitator superfamily (MFS). The role of ShiF in the aerobactin system has not been determined, but similar MFS systems have been involved in the export of siderophores (26, 54). The putative protein encoded by viuB has significant identity with ViuB and YqjH encoded by Vibrio cholerae and E. coli (8, 81). These two proteins reduce the bound iron from the ferric state to the ferrous state, resulting in the loss of affinity of the ferrous iron for the siderophore. Results reported by Wang et al. (81) suggest that the function of YqjH is to aid in the release of the iron from the siderophore into the cytosol.
Furthermore, the chromosomes of all Cronobacter genomes analyzed have a common gene encoding the ferric reductase FhuF. The homologous ferric reductase proteins found in E. coli and described by Matzanke et al. (53) are thought to promote the release of iron from hydroxamate siderophores, specifically coprogen, ferrichrome, and ferroxamine B.
Phylogenetic analysis of Cronobacter iron acquisition systems.
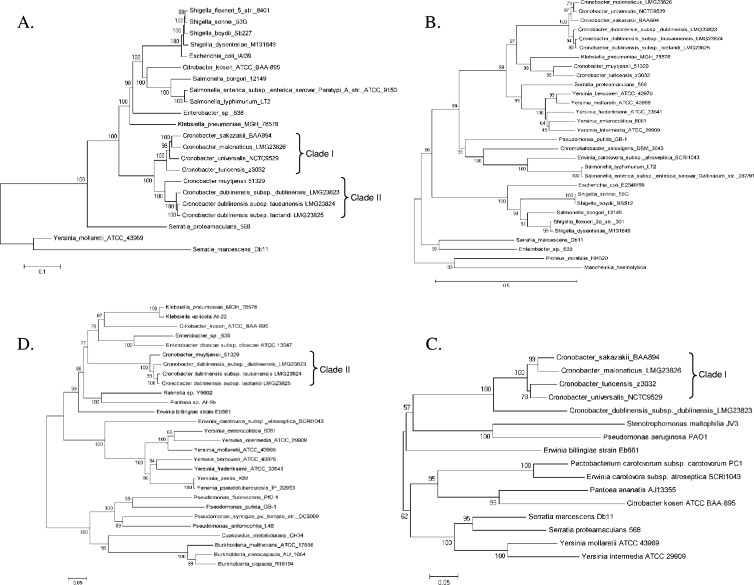
To infer evolutionary relationships, phylogenetic analysis was performed on the nucleotide sequence of each putative iron acquisition operon or gene identified in the in silico analysis. Phylogenetic analyses of 16S rRNA genes and housekeeping genes typically place the group consisting of Citrobacter, Klebsiella, Escherichia, Shigella, Salmonella, and Enterobacter spp. as the nearest neighbors to Cronobacter spp., followed by Erwinia, Serratia, and Yersinia. This general trend was also observed for the majority of iron acquisition genes in Cronobacter spp. In the majority of instances, homologues in the genome of Klebsiella pneumoniae strain MGH 78578 were the most closely related by nucleotide sequence, for example, to the three-enterobactin gene cluster containing entH to fepC (Fig. 3A). After Klebsiella, typically, homologues in the genomes of Enterobacter spp., such as strain 638, several Salmonella enterica serovar strains, and Citrobacter koseri ATCC BAA-895 were the next most closely related sequences, followed by homologous sequences present in various strains of E. coli and Shigella spp. Typically, homologous genes in the genomes of Serratia, Yersinia, Erwinia, and Edwardsiella spp. and other enterics formed a cluster separate from that containing Cronobacter spp. This is indeed true for the observed phylogenetic evolutionary reconstruction of both ferric transporters, enterobactin (large and small clusters) and the fhu operon, the fhuF ferric reductase, the feo ferrous transporter, and the putative heme iron extractor-encoding gene, yfeX (Fig. 3A; see also Fig. S1 in the supplemental material).
In two instances, homologues in E. coli and Shigella spp. were more closely related than those in Salmonella spp.: the TonB receptor-encoding genes, pfeA and yncD (see Fig. S1). In a few cases, homologues were not present in all members of this enteric clade, which contained Cronobacter spp., such as Salmonella and Citrobacter spp. for the ferrous transporter, efe (see Fig. S1), also for which genes of Klebsiella pneumoniae MGH 78578 are the most distantly related in the cluster, and the TonB receptor-encoding gene, fhuE (see Fig. S1), for which there is no apparent homologue in Klebsiella, and a homologue in the genome of Erwinia carotovora strain SCRI1043 is most closely related to Cronobacter spp.
Two of the iron acquisition genes that are present in all genomes of Cronobacter spp. analyzed present a phylogenetic evolutionary history quite different from the conserved enteric features described above. For the TonB receptor-encoding gene, foxA, again the homologue of K. pneumoniae MGH 78578 is most closely related; in fact, this homologue clusters among Cronobacter spp. (Fig. 3B). The next closest homologues are contained in a cluster comprised of Serratia proteamaculans strain 568 and various Yersinia species other than Y. pestis. For btuB, it is clear that this gene is under considerably different evolutionary pressure within the Enterobacteriaceae (see Fig. S1 in the supplemental material). The btuB genes of Cronobacter spp. form three clusters, in contrast to other conserved iron acquisition genes, which typically display a two-clade phylogeny within Cronobacter genomes. Likewise, other enterics demonstrate a mixture of species clustering and monophyletic divergence.
Of the iron acquisition elements analyzed, six are not conserved among all species of Cronobacter. Previously, we performed an in silico analysis on the cronobactin (aerobactin) gene cluster, iucABCD-iutA, and the eit ferric transporter, eitCBAD, which are harbored on a nonmobile F-type plasmid (23). The reconstructed evolutionary history of shiF-viuB is considerably different from that of the genes downstream, namely, the cronobactin operon. The cronobactin operon clusters with homologous sequences from two Enterobacter spp. and that of Escherichia fergusonii, and this cluster is closely related to homologous systems found in Serratia and Yersinia spp. (23). The Cronobacter viuB gene is also similar to the same gene in Serratia and Yersinia spp.; however, it is more closely related to homologues in several plant-associated species, including Erwinia spp., Stenotrophomonas maltophilia, Pectobacterium carotovorum, and Pantoea ananatis (Fig. 3C). Differences in G+C content between viuB and shiF (66%) and between iucABCD and iutA (59.7%), together with differences in inferred phylogenetic history, strongly suggest operon evolution in which the genomes of Cronobacter spp., as well as Serratia and Yersinia spp., have acquired these two accessory genes, most likely in two separate events.
For the two TonB receptor-encoding genes, fct and fcuA, present in the genomes of C. dublinenisis and C. muytjensii, inferred phylogenetic history reveals that, as for the majority of conserved iron acquisition genes, these genes are most closely related to homologues present in the genomes of Enterobacter spp., Klebsiella spp., and Citrobacter koseri. For fcuA, this cluster containing Cronobacter spp. is then most similar to homologues in water- and plant-associated species, Rahnella, Pantoea, and Erwinia, followed by Yersinia spp. (Fig. 3D). For fct, the cluster containing Cronobacter spp. is most similar to homologues in water- and plant-associated species, Serratia, Pantoea, and Erwinia chrysanthemi (see Fig. S1 in the supplemental material).
The plasmid-encoded ferric dicitrate system present in the genome of C. sakazakii strain 2151 is almost identical in sequence to the same found on plasmid pKPN-IT of K. pneumoniae strain ST258 and in the chromosome of Enterobacter cloacae subsp. cloacae NCTC 9394, and it is highly similar to homologous sequences in several pathogenic strains of E. coli and Shigella spp. Distantly related are homologues from the plant-associated species Erwinia carotovora and Photorhabdus spp. (see Fig. S1).
Within the genus Cronobacter, phylogenetic analysis revealed two subclades for most iron acquisition genes and systems analyzed (Fig. 3A; see also Fig. S1 in the supplemental material). One subclade is comprised of the species C. sakazakii, C. malonaticus, C. universalis, and C. turicensis; the other subclade is comprised of C. muytjensii and C. dublinensis. This phylogenetic relationship is suggestive of the fact that these two groups of Cronobacter spp. are under different evolutionary pressure and most likely reflects differences in ecological niches. This clustering was in agreement with that reported for the rpoB sequence (47), virulence markers on pESA3-/pCTU1-like plasmids (23), and whole-genome phylogeny (C. J. Grim and B. D. Tall, unpublished data).
Detection of Cronobacter species iron acquisition systems by PCR.
In order to determine the distribution of iron acquisition genes and systems identified by the in silico analysis, a total of 231 strains of Cronobacter spp. isolated from different sources were screened by PCR (Table 3). Previously, it was reported that 98% (226) of the same 231 Cronobacter isolates harbor a pESA3-/pCTU-1-like plasmid that has the genes encoding the cronobactin siderophore and EitABCD transporter (23). In this study, PCR results showed that all 226 Cronobacter strains containing the plasmid-encoded iucABCD-iutA operon also have the viuB gene, suggesting that this gene has a possible role in cronobactin activity. PCR analysis of the enterobactin genes entF (locus 1), fepE (locus 2), and entE and fepG (locus 3) show that all 231 Cronobacter strains are positive for locus 1 and locus 3, while only 98% are positive for locus 2 due to only two of the six C. dublinensis isolates being positive for the fepE gene (Table 3).
Table 3.
Distribution of iron acquisition systems in 231 Cronobacter strains
| Iron acquisition system system | Gene | No. (%) of isolates PCR positive for target gene |
|||||
|---|---|---|---|---|---|---|---|
| C. sakazakii (n = 180) | C. malonaticus (n = 25) | C. turicensis (n = 6) | C. muytjensii (n = 12) | C. dublinensis (n = 6) | C. universalis (n = 2) | ||
| Ferric transporter | |||||||
| Cronobactin | iucC | 179 (99) | 25 (100) | 6 (100) | 9 (75) | 5 (83) | 2 (100) |
| Enterobactin | entF | 180 (100) | 25 (100) | 6 (100) | 12 (100) | 6 (100) | 2 (100) |
| fepE | 180 (100) | 25 (100) | 6 (100) | 12 (100) | 2 (33) | 2 (100) | |
| entE | 180 (100) | 25 (100) | 6 (100) | 12 (100) | 6 (100) | 2 (100) | |
| fepG | 180 (100) | 25 (100) | 6 (100) | 12 (100) | 6 (100) | 2 (100) | |
| FhuABCD | fhuA | 180 (100) | 25 (100) | 6 (100) | 12 (100) | 6 (100) | 2 (100) |
| fhuB | 180 (100) | 25 (100) | 6 (100) | 12 (100) | 6 (100) | 2 (100) | |
| EitABCD | eitA | 179 (99) | 25 (100) | 6 (100) | 9 (75) | 5 (83) | 2 (100) |
| Ferric dicitrate | fecR | 26 (14) | 4 (16) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| fecE | 26 (14) | 4 (16) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Ferrous transporter | |||||||
| FeoABC | feoB | 180 (100) | 25 (100) | 6 (100) | 12 (100) | 6 (100) | 2 (100) |
| EfeUOB | efeB | 180 (100) | 25 (100) | 6 (100) | 12 (100) | 6 (100) | 2 (100) |
| efeO | 180 (100) | 25 (100) | 6 (100) | 12 (100) | 6 (100) | 2 (100) | |
| Ferric reductase | viuB | 179 (99) | 25 (100) | 6 (100) | 9 (75) | 5 (83) | 2 (100) |
| fhuF | 180 (100) | 25 (100) | 6 (100) | 12 (100) | 6 (100) | 2 (100) | |
| TonB receptor | fcuA | 0 (0) | 0 (0) | 0 (0) | 12 (100) | 6 (100) | 0 (0) |
| fct | 0 (0) | 0 (0) | 3 (50) | 12 (100) | 6 (100) | 0 (0) | |
| yncD | 179 (99) | 25 (100) | 6 (100) | 12 (100) | 5 (83) | 2 (100) | |
| btuB | 180 (100) | 25 (100) | 6 (100) | 12 (100) | 6 (100) | 2 (100) | |
| foxA | 175 (97) | 25 (100) | 6 (100) | 12 (100) | 6 (100) | 2 (100) | |
| fhuE | 180 (100) | 25 (100) | 6 (100) | 12 (100) | 6 (100) | 2 (100) | |
| pfeA | 180 (100) | 25 (100) | 6 (100) | 12 (100) | 6 (100) | 2 (100) | |
| Heme iron extractor | yfeX | 180 (100) | 25 (100) | 6 (100) | 12 (100) | 6 (100) | 2 (100) |
PCR results also showed that all 231 Cronobacter isolates possess the genes encoding the ferric transporter FhuACDB and the ferrous transporters FeoABC and EfeUOB, as well as the heme iron extractor, YfeX, and ferric reductase, FhuF (Table 3). The ferric dicitrate genes fecR and fecE were found in only14% of the C. sakazakii and 16% of the C. malonaticus strains tested. These genes were not found in any of the other Cronobacter species (Table 3). Interestingly, of the 30 Cronobacter strains containing the fec transport system, 19 were isolated from clinical samples, suggesting that this iron system may be a virulence marker and play a role in the virulence of Cronobacter spp. Further analysis of this locus using repA and repB origin-of-replication genes found that three of the C. sakazakii strains and one C. malonaticus strain harbor the fec genes on a pCSA2151-like plasmid, and the remaining 26 strains have these genes on their chromosomes (Table 4). The presence of insertion sequences flanking the fec genes suggests that this transport system is mobilizable and possibly integrated into the chromosome at different regions of the Cronobacter genome.
Table 4.
Distribution of ferric dicitrate transport system and plasmid pCSA2151 replication genes among 231 strains of Cronobacter spp.a
| Species | No. of isolates | No. (%) PCR positive |
|||
|---|---|---|---|---|---|
| fecR | fecE | repA | repE | ||
| C. sakazakii | 180 | 3 (1.7) | 3 (1.7) | 3 (1.7) | 3 (1.7) |
| 23 (13) | 23 (13) | 0 (0) | 0 (0) | ||
| C. malonaticus | 25 | 1 (4) | 1 (4) | 1 (4) | 1 (4) |
| 3 (12) | 3 (12) | 0 (0) | 0 (0) | ||
| 0 (0) | 0(0) | 1 (4) | 1 (4) | ||
| C. turicensis | 6 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| C. muytjensii | 12 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| C. dublinensis | 6 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| C. universalis | 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Multiple profiles were found among isolates of C. sakazakii and C. malonaticus.
Independent of the source of isolation, all Cronobacter spp. tested have the putative genes encoding the TonB-dependent iron receptors YncD, BtuB, FoxA, FhuE, and PfeA; however, the genes encoding the TonB-dependent receptor FcuA are unique to the C. dublinensis and C. muytjensii strains tested (Table 3). Furthermore, the gene encoding the TonB-dependent receptor Fct was specific to all six C. dublinensis and 12 C. muytjensii strains as well as three of six C. turicensis strains tested (Table 3). It is known that most TonB-dependent iron receptors are substrate specific (54). Even though Cronobacter spp. do not produce the siderophores specific for some of their TonB-dependent receptors, in polymicrobial communities, Cronobacter strains may interact with ferric siderophores produced by other bacteria or fungi. This suggests that the presence of FcuA and/or Fct in C. dublinensis, C. muytjensii, and some C. turicensis strains may give them an advantage to compete more successfully for iron in certain ecological niches where they may encounter the specific siderophores of FcuA and Fct, such as chrysobactin, which is produced by plant pathogen E. chrysanthemi.
Expression of iron acquisition systems.
The expression of most iron transport systems is repressed by iron via interaction of the Fur protein at the promoter site. Their promoters contain the consensus Fur binding site, or Fur box. In the presence of iron, Fur binds to the Fur box and blocks transcription (17). In silico analysis identified potential Fur boxes matching the E. coli Fur box consensus sequence 5′-GATAATGATAATCATTATC-3′ in the upstream region of all putative Cronobacter iron acquisition systems, except in the genes for the heme iron extractor, yfeX, and btuB, which encodes a predicted vitamin B12/cobalamin receptor (Table 5). In order to confirm if the Cronobacter iron acquisition systems are regulated by Fur, their expression was determined after the bacterial cells were grown for 16 h in LB medium (iron-replete conditions) and LB supplemented with the ferrous iron chelator 2,2′-dipyridyl (DIP) at 300 μM (iron-depleted conditions). Of 25 genes tested, 18 exhibited higher expression under iron-depleted conditions than under iron-replete conditions, indicating derepression of these genes (Table 5). On average, their expression increased 2.7-fold when cells were grown in LB medium amended with DIP (Table 5).
Table 5.
Putative Fur boxes identified in Cronobacter iron acquisition systems and fold increase in expression under iron-depleted conditions
| Gene or operon(s) | Putative phenotype | Sequence (5′–3′)a | No. of identical bases | Locationb | Fold increase in expressionc |
|---|---|---|---|---|---|
| iucABCD-iutA | Cronobactin biosynthesis and transport | CATAACGATAATCATTATC | 17 | 19 bp of iucA | 4.1 ± 0.2 (iucC), 2.9 ± 0.1 (iutA) |
| shiF-viuB | Ferric reductase and MFS permease | AATATTTGTTTTCATTAAT | 11 | 44 bp of shiF | 2.2 ± 0.2 (viuB) |
| fepA-entD-fes-ybdZ-entF | Enterobactin biosynthesis, receptor and iron release | GATAATAACTATCATTATC | 17 | 18 bp of fepA, 71 bp of fes | 1.5 ± 0.05 (fepA), 4.4 ± 0.3 (entF) |
| fepE | Enterobactin transport | Not present | 0.8 ± 0.03 (fepE) | ||
| entCEBAH-fepB | Enterobactin transport and biosynthesis | GAAAATGAGAAGCATTATT ACAAATGATAACAATTATC | 16, 14 | 26 bp of fepB, 29 bp of entC | 1.5 ± 0.08 (fepB), 1.7 ± 0.3 (entC) |
| entS-fepDGC | Enterobactin transport and biosynthesis | GATAATAACTATCATTATC | 16 | 19 bp of fepD, 107 bp of entS | No expression (fepG), 1.0 ± 0.04 (entS) |
| fhuACDB | Ferrichrome receptor and hydroxamate transport | GCGCATAATAATAATTCTC | 13 | 42 bp of fhuA | 1.8 ± 0.2 (fhuA) |
| fecIRABCDE | Ferric dicitrate receptor and transport | TGTAATGATAACCATTCTC | 16 | 45 bp of fecI | 3.4 ± 0.2 (fecB)d |
| eitABC-eitD | ABC ferric transporter | AAGAATGATTTTCATTTGC | 13 | 75 bp of eitA, 141 bp of eitD | 0.7 ± 0.05 (eitA), no expression (eitD) |
| feoABC | Ferrous transporter | AAAAACCATTCTCATTACC | 12 | 103 bp of feoA | 1.8 ± 0.3 (feoB) |
| efeUOB | Ferrous transporter | GGTAATGATAATCACTTTC | 16 | 59 bp of efeU | 1.9 ± 0.3 (efeO) |
| yncD | Siderophore receptor | GAGAATAATAATCATTATT | 16 | 164 bp of yncD | 3.4 ± 0.4 (yncD) |
| foxA | Ferroxamine receptor | GATAATAATTCGCATTCTT | 13 | 60 bp of foxA | 3.0 ± 0.4 (foxA) |
| fhuE | Ferric rhodotorulic acid/coprogen receptor | ACAAATGATTATATTTCTC | 12 | 138 bp of fhuE | 2.7 ± 0.3 (fhuE) |
| pfeA | Ferric enterobactin receptor | GATAATTATTATCATTATC | 17 | 47 bp of pfeA | 4.3 ± 0.4 (pfeA) |
| fcuA | Hydroxamate receptor | AATAATGACAGGACAACCT | 8 | 6 bp of fcuA | 2.8 ± 0.3 (fcuA) |
| GCAAATGATTATTAGTAAC | 14 | 116 bp of yncE | |||
| fct | Ferrichrysobactin receptor | GGTAATGATTTTCAATATC | 16 | 63 bp of fct, 189 bp of fesA | 2.8 ± 0.2 (fct) |
| fhuF | Ferric reductase | AGGATTGGCAATCATTATC | 13 | 28 bp of fhuF | 2.1 ± 0.4 (fhuF) |
| btuB | Vitamin B12/cobalamin receptor | Not present | 1.0 ± 0.04 (btuB) | ||
| yfeX | Heme iron extractor | Not present | 0.76 ± 0.2 (yfeX) |
Comparison with E. coli consensus 5′-GATAATGATAATCATTATC-3′ (17). Identical nucleotides are in boldface type.
Number of nucleotides upstream of the start codon.
Average ± standard deviation of three RT-PCRs to determine fold change in the expression of the gene under iron-depleted conditions (LB broth supplemented with 300 μM DIP). All RT-PCRs were performed in C. turicensis z3032, except for those for fhuA, fct, and fcuA as well as fecB, which were performed in C. dublinensis LMG23825 and C. sakazakii 2151, respectively.
Growth in LB broth supplemented with 300 μM DIP and 1 mM citrate.
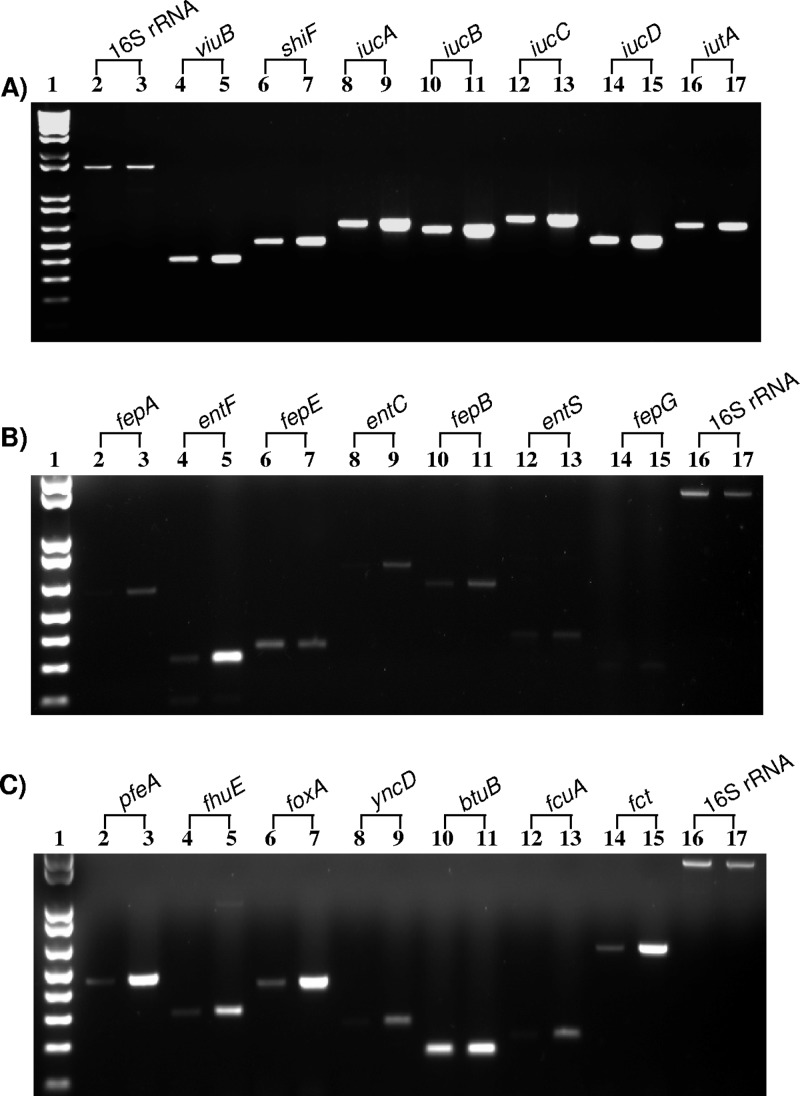
RT-PCR results of C. turicensis z3032 showed that all four biosynthetic cronobactin genes, iucABCD, and the ferric-cronobactin receptor gene, iutA, were expressed at higher levels under iron-depleted growth conditions (Fig. 4A). Expression of iucC and iutA increased 4.1- and 2.9-fold, respectively, under iron-depleted conditions (Table 5). A potential Fur-binding site is located 19 bp upstream of the putative start of iucA (Table 5). This Fur box matches the Fur box consensus sequence in 17 out of 19 bases (17). Furthermore, supernatants of C. turicensis z3032 had siderophore activity in the CASAD assay only when it was grown in the presence of DIP (data not shown), which confirms that production of cronobactin siderophore is inducible and expressed only under iron-depleted growth conditions. The genes viuB and shiF were also expressed at higher levels under iron-depleted conditions, confirming that these genes are also components of the Fur regulon (Table 5; Fig. 4A). The stop codon of shiF overlaps the start codon of viuB, suggesting that these two genes are transcribed together. These two genes are located 136 bp upstream of iucA and transcribed in directions opposite to that of the iucABCD-iutA operon (Fig. 1A). This sequence structure suggests that similar to the E. coli enterobactin operons, transcription of the shiF-viuB and iucABCD-iutA operons may be Fur controlled by a bidirectional promoter-operator region (7). In addition to the putative Fur box located 19 bp upstream of iucA, there is a potential Fur-binding site located 43 bp upstream of the putative start of shiF (Table 5). The presence of two Fur boxes between iucA and shiF suggests that the promoters of each operon may be independently expressed and controlled by Fur from distinct operator sites. This sequence displays a resemblance to the E. coli bidirectional intercistronic region controlling the expression of enterobactin fepB-entC genes (7). In contrast to Cronobacter spp., a single Fur box is located between iucA and shiF in Y. pestis and likely serves as an iron- and Fur-regulated promoter for expression of aerobactin and shiF (22).
Fig 4.
Representative RT-PCR of Cronobacter iron acquisition systems under iron-replete (even-numbered lanes) and iron-depleted (odd-numbered lanes) conditions. (A) Cronobactin and shiF-viuB operons. Lane 1, 1 kb plus DNA ladder; lanes 2 and 3, 16S rRNA; lanes 4 and 5, viuB; lanes 6 and 7, shiF; lanes 8 and 9, iucA; lanes 10 and 11, iucB; lanes 12 and 13, iucC; lanes 14 and 15, iucD; lanes 16 and 17, iutA. (B) Enterobactin genes. Lane 1, 1 kb plus DNA ladder; lanes 2 and 3, fepA; lanes 4 and 5, entF; lanes 6 and 7, fepE; lanes 8 and 9, entC; lanes 10 and 11, fepB; lanes 12 and 13, entS; lanes 14 and 15, fepG; lanes 16 and 17, 16S rRNA. (C) TonB-dependent iron receptors. Lane 1, 1 kb plus DNA ladder; lanes 2 and 3, pfeA; lanes 4 and 5, fhuE; lanes 6 and 7, foxA; lanes 8 and 9, yncD; lanes 10 and 11, btuB; lanes 12 and 13, fcuA; lanes 14 and 15, fct; lanes 16 and 17, 16S rRNA.
The Cronobacter eitD and eitA genes, which are part of the operon encoding the ABC transporter EitABCD localized on pCTU1 in C. turicensis z3032 (Fig. 1D), were not derepressed under iron-depleted conditions (Table 5). The eitA gene was poorly expressed and eitD was not expressed under both iron-replete and iron-depleted growth conditions, despite the presence of putative Fur boxes 73 bp upstream of eitA and 150 bp upstream of eitD (Table 5). Similar RT-PCR results were obtained for C. dublinensis LMG23825 (data not shown). One explanation for the lack of expression in Cronobacter spp. could be the gene arrangement of the cluster eitABC-eitD, which is different from the typical arrangement in other Enterobacteriaceae (Fig. 1D) (23).
In E. coli, the enterobactin gene cluster includes 14 genes organized into six contiguous operons originating from three Fur-controlled bidirectional promoter-operator regions (7, 33, 70). The bidirectional control regions include fepA-entD and fes to entF plus fepE, entCEBAH and fepB, and entS and fepDGC. These three control regions possess distinct regulatory architectures (7, 33, 70), suggesting that control by the Fur repressor is manifested through different regulatory strategies. In Cronobacter spp., with the exception of fepE, which forms a single locus separate from the fes operon, we found the same gene organization as in E. coli even though the enterobactin genes are found in three distinct chromosomal loci (Fig. 1B). Potential Fur boxes were identified in all three putative bidirectional promoter-operator regions but not upstream of fepE (Table 5). As in E. coli, single putative Fur boxes were identified in the first and third putative bidirectional promoter regions between fepA and fes and between entS and fepD, respectively (Table 5). In E. coli, the operons controlled by these two bidirectional promoter regions transcribe from overlapping promoters regulated by the binding of Fur to a single binding site (13, 33). In contrast, two putative Fur boxes were identified in the second regulatory region between entC and fepB (Table 5). In E. coli, the promoters for fepB and the entCEBA-ybdB operon are situated back to back, and each one is controlled by Fur from distinct operator sites (7).
To test the expression of the Cronobacter enterobactin-like system, we determined the expression of representative genes from each promoter region in C. turicensis z3032 (Table 5; Fig. 4B). We found that fepA and entF, comprising the two operons of the first putative bidirectional promoter region (Fig. 1B), were expressed at higher levels, 1.5- and 4.4-fold, respectively, under iron-depleted conditions. Expression of fepE was higher under iron-replete conditions, most likely due to its genomic rearrangement to a distant locus without a putative Fur box in the promoter region. For genes of the second bidirectional promoter region (Fig. 1B), fepB and entC were expressed at higher levels under iron-limiting conditions; however, entS and fepG of the third regulatory region (Fig. 1B) were poorly and not expressed, respectively, when grown under both iron-depleted and iron-replete conditions (Table 5; Fig. 4B).
Expression of the fec transport genes starts with the binding of diferric citrate to the FecA protein (21), which causes substantial structural changes in FecA, triggering a signal cascade (6). FecA interacts with FecR in the periplasm, which, in turn, transmits the signal across the cytoplasmic membrane into the cytoplasm and activates the FecI sigma factor, which binds to the RNA polymerase core enzyme and directs the RNA polymerase to the promoter upstream of the fecABCDE transport genes to initiate transcription (52). Promoters of the fecIR regulatory genes and fecABCDE transport genes are repressed by the Fur protein, loaded with Fe2+ (2). Therefore, transcription of the fec transport genes is subjected to a doubly controlled regulation scheme. In silico analysis in C. sakazakii 2151 identified a putative Fur box 44 bp upstream of the fecI start codon (Table 5). Accordingly, fecB and fecE were expressed at higher levels under iron-depleted growth conditions and in the presence of 1 mM citrate than under iron-replete growth conditions lacking citrate, confirming that expression of the fec transport genes is induced by citrate and derepressed by low-iron growth conditions (Table 5).
Putative Fur boxes were identified in the upstream region of the ferrous transporter system genes feo and efe (Table 5). Even though in E. coli, the expression of the feoABC operon is active under anaerobic conditions (37), we observed that genes of these iron transport systems were expressed at higher levels under aerobic iron-limiting conditions (Table 5).
Putative Fur binding sites were identified upstream of the putative TonB-dependent iron receptors fhuA, yncD, foxA, fhuE, and pfeA (Table 5; Fig. 4C), suggesting that these receptors are repressed by iron via the interaction of Fur protein. Expression of these genes was repressed when C. turicensis z3032 was grown in the presence of iron, confirming that they are regulated by iron concentration (Table 5). It has been reported that in addition to iron concentration, some of these genes are also positively regulated by the presence of their cognate siderophore (49). Furthermore, it has recently been determined that YncD plays an important role in survival inside the host and that the gene is overexpressed under stress conditions such as heat and acid (83). In contrast to the TonB-dependent iron receptors mentioned above, no putative Fur boxes were found upstream of btuB and yfeX, and levels of expression of these genes were similar under iron-replete and iron-depleted conditions (Table 5; Fig. 4C, lanes 10 and 11), indicating that these genes are constitutively expressed in Cronobacter spp.
Expression of fct and fcuA was determined in C. dublinensis LMG23825. RT-PCR results showed that expression of both genes was repressed under iron-replete conditions, suggesting that these two genes are also part of the Fur regulon (Table 5; Fig. 4C, lanes 12 to 15). Interestingly, a gene encoding an enterochelin esterase, named fesA in this study, is located 269 bp upstream of the fct start codon. The two genes are oppositely transcribed, and a potential Fur box is located intergenically, 62 bp upstream of the fct start codon (Table 5). This organizational structure is similar to that described for the E. coli fepA-fes bidirectional promoter, where fepA encodes the enterobactin receptor and fes encodes an esterase involved in the release of iron from ferric enterobactin inside the cell. Furthermore, the same organization is found for the fct promoter region of E. chrysanthemi, where overlapping promoters are controlled by the binding of Fur to a single binding site (68).
A putative Fur box was found 5 bp upstream of the fcuA start codon, but the sequence is only identical to 8 of the 19 nucleotides of the Fur box consensus sequence (Table 5). Sixty-three base pairs upstream of fcuA, there is an uncharacterized gene, yncE, which is transcribed in the same orientation as fcuA. A potential Fur box, matching the consensus sequence at 14 of the 19 bp, is located upstream of yncD (Table 5). It is unclear if fcuA is cotranscribed with yncD under the control of one promoter-Fur box region or, alternatively, both genes transcribe independently under the control of its own promoter-Fur box region.
Cronobactin promotes the growth of Cronobacter spp. under iron-limiting conditions.
Previously, we found that wild-type C. sakazakii BAA-894 and C. turicensis z3032, but not their plasmid-cured derivatives (strains BAA-894.3 and 3032.2A, respectively) produce active siderophores (23). To further confirm that the siderophore activity was due to the production of cronobactin encoded by the plasmids, we assayed for siderophore production in the cell-free culture supernatant of an E. coli strain, ESA-C01, harboring a fosmid containing the complete pESA3 iucABCD-iutA operon. Using the CASAD assay, siderophore production was detected in the cell-free culture supernatant of ESA-C01 but not in the supernatant of a fosmid clone control strain, ESA-M04, containing a different region of pESA3 (Fig. 5), indicating that the iucABDCD synthesis genes do encode active siderophores. However, larger orange halos were produced by wild-type C. sakazakii BAA-894 harboring pESA3 compared to E. coli ESA-C01, suggesting that C. sakazakii BAA-894 has additional factors that may increase the cronobactin activity or result in a greater amount of cronobactin being secreted.
Fig 5.
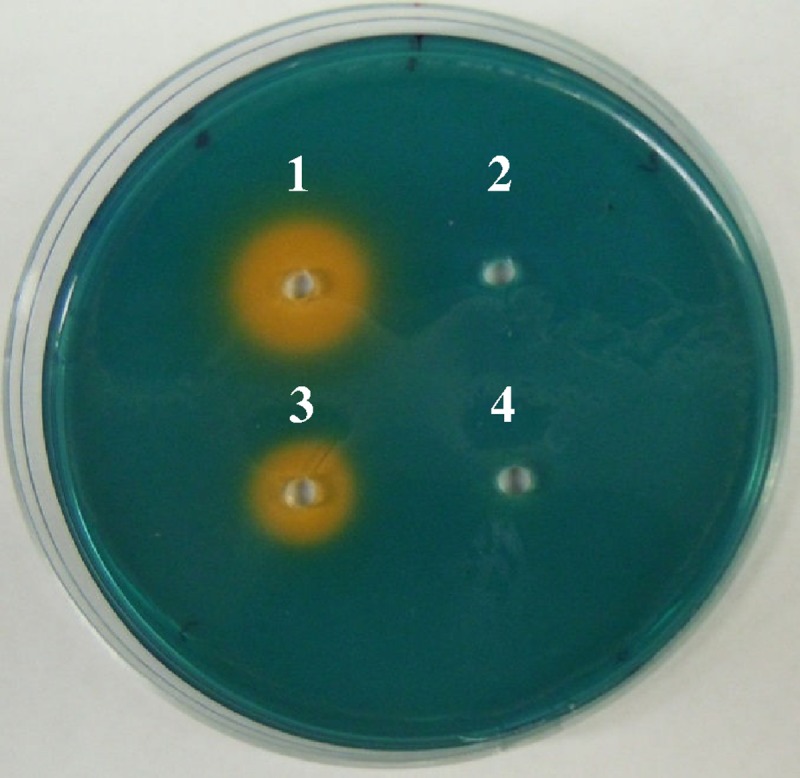
Siderophore activity using the CASAD assay. Wells were filled with cell-free culture supernatants of wild-type C. sakazakii BAA-894 (1), plasmid-cured derivative BAA-894.3 (2), fosmid clone ESA-C01 containing the cronobactin genes (3), and fosmid clone ESA-M04 lacking the cronobactin genes (4).
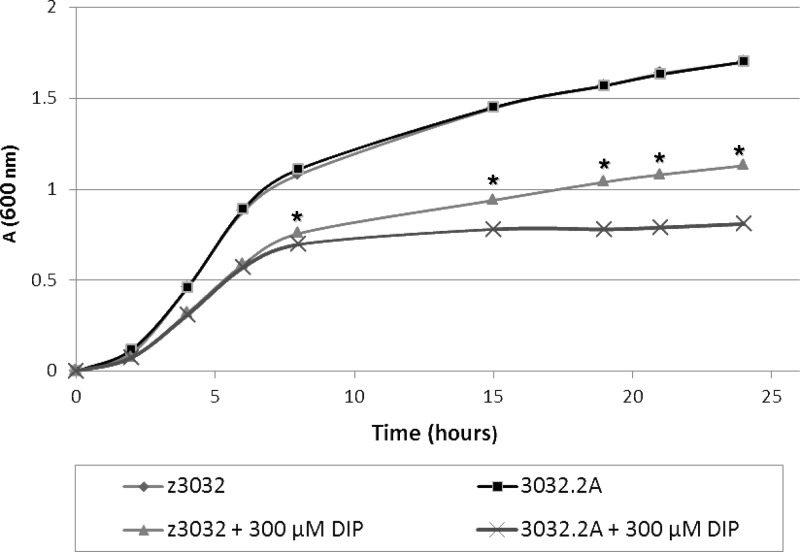
In order to determine whether the iucABDCD-iutA operon enhances growth of Cronobacter spp. under iron-depleted growth conditions, C. turicensis z3032 harboring plasmid pCTU1 and its plasmid-cured derivative strain 3032.2A were grown in LB medium and LB medium supplemented with 300 μM DIP. While no difference in growth was observed when both strains were grown in LB medium alone, 3032.2A grew significantly (P < 0.001) slower than the wild-type strain between 7 and 24 h in LB medium supplemented with DIP (Fig. 6). Similar results were obtained when wild-type C. sakazakii BAA-894 and its plasmid-cured derivative, BAA-894.3, were grown under low-iron conditions (data not shown). RT-PCR analysis showed no difference in the expression of iucC after 5 h and 18 h of growth in LB medium supplemented with DIP, but a larger halo was produced after 18 h than after 5 h of growth, suggesting that a greater amount of siderophore accumulates in the cell-free culture supernatant during the stationary phase (data not shown). Overall, these results strongly suggest that cronobactin encoded by pESA3-/pCTI-1-like plasmids plays a critical role in promoting growth of Cronobacter spp. under iron-limiting conditions.
Fig 6.
Growth of wild-type C. turicensis z3032 harboring pCTU1 and its plasmid-cured derivative, 3032.2A, in LB broth and iron-depleted LB broth containing 300 μM DIP. The data were obtained from 3 independent experiments. *, P < 0.001.
Enterobactin-like siderophore is not functional.
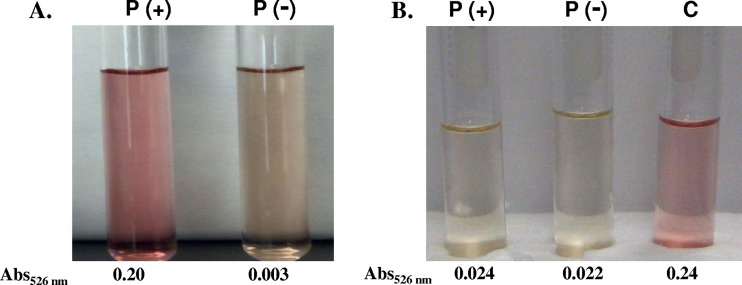
Even though the chromosomes of most of the Cronobacter isolates tested have the enterobactin genes, we were not able to detect siderophore activity in C. sakazakii BAA-894 and C. turicensis z3032 lacking pESA3 and pCTU-1, respectively (Fig. 5) (23). These results suggest that the bacteria do not encode active enterobactin siderophores and the activity identified by the CASAD assay is solely due to the cronobactin siderophore encoded by the virulence plasmids. To determine whether the enterobactin inactivity encoded by the chromosome of C. sakazakii BAA-894 and C. turicensis z3032 was defective only in these strains, we performed a CASAD assay of the cell-free culture supernatants of C. muytjensii ATCC 51329 and the other four Cronobacter strains, from our culture collection, lacking pESA3-/pCTU1-like plasmids (strains listed in Table 1). Siderophore activity was detected in the cell-free culture supernatants of two control Cronobacter strains harboring pESA3-/pCTU1-like plasmids (strains listed in Table 1), but not in the supernatants of any of the five Cronobacter strains lacking the plasmids (data not shown). These results suggest that Cronobacter spp. do not produce active enterobactin-like siderophores. To confirm these results, the colorimetric tests described by Arnow (3) and Csáky (15) were used to identify catechol-type (such as enterobactin) and hydroxamate-type (aerobactin) siderophores, respectively. The results showed that cell-free culture supernatants of C. turicensis z3032 harboring pCTU1 produce hydroxamate-type but not catechol-type siderophores, while its plasmid-cured derivative does not produce any type of siderophore (Fig. 7), confirming that C. turicensis z3032 solely produces the hydroxamate-type cronobactin siderophore encoded by pCTU1.
Fig 7.
Results of the Csáky (A) and Arnow (B) tests used to identify hydroxamate-type and catechol-type siderophore activity, respectively, using cell-free culture supernatants of wild-type C. turicensis z3032 harboring pCTU1[P(+)] and its plasmid-cured derivative, 3032.2A [P(−)]. Cell-free culture supernatants of Vibrio vulnificus UNCC913 (C) and catechol (6 μg) were used as positive controls in the Csáky and Arnow tests, respectively.
Closely related plasmids pESA3 and pCTU1 encode some common transport genes, including permeases of the MFS that may be involved in the secretion of siderophores and some transcriptional regulators that may influence the expression of enterobactin synthesis genes. To test whether siderophore inactivity identified in plasmidless strains was due to defects in secretion, we tested siderophore activity in whole-cell lysate preparations of wild-type C. turicensis z3032 and its plasmid-cured derivative, 3032.2A. Using the CASAD assay, we did not find siderophore activity in either of the whole-cell lysate preparations of C. turicensis z3032 or 3032.A (data not shown). Furthermore, we did not observe catechol-type siderophore activity in any of the lysate preparations using the Arnow test (data not shown), indicating that the enterobactin inactivity is not due to a secretion defect in the wild-type or plasmid-cured derivative strain. In order to determine whether siderophore inactivity identified in plasmidless strains was due to defects in the expression of enterobactin, we compared the expression of enterobactin genes in strains z3032 and 3032.2A. RT-PCR of representative enterobactin synthesis genes, including entC and entE, showed no difference in the expression between strains z3032 and 3032.2A (data not shown), indicating that pCTU1 is not involved in the regulation of expression of enterobactin synthesis genes.
Sequence analysis of the enterobactin cluster genes revealed that the enterobactin inactivity was not due to an obvious gene truncation; however, in contrast to the case with other bacteria, the enterobactin genes in Cronobacter spp. are localized in three separate loci in the chromosome (Fig. 1B). Our expression analysis showed that expression of fepE was not repressed by iron (Fig. 4B; Table 5). In addition, entS and fepG localized in the third regulatory region were poorly or not expressed, and the level of expression was not modified under conditions of restricted iron availability (Fig. 4B; Table 5). In E. coli, fepG is involved in ferric enterobactin transport and entS plays a role in the export of enterobactin outside the cell (26, 62). However, our results, presented above, indicate that the enterobactin inactivity is not due to a secretion defect. Taken together, these results indicate that the rearrangement of enterobactin in three different regions of the chromosome is the most likely cause for the defect in expression of the enterobactin genes.
We do not rule out the possibility that Cronobacter spp. produce active enterobactin in the host. It has been determined that enterobactin and other iron acquisition systems are upregulated 2- to 5-fold in vivo in comparison to growth in LB broth (72). It is possible that the expression of enterobactin genes under laboratory conditions is not sufficient to be detected by the CASAD or Arnow assay, i.e., is below the level of detection. In support of this, we observed that the cronobactin genes were more highly expressed than enterobactin genes by RT-PCR (Fig. 4). It is also possible that the inactivity of enterobactin expression may be due to amino acid variation in the biosynthetic enzymes compared to known functional enzymes from E. coli and other Gram-negative bacteria. Alternately, enterobactin genes may have posttranscriptional modifications that affect the activity of the siderophore.
We observed that the ferric enterobactin receptor FepA was expressed and its expression was upregulated under iron-depleted conditions (Table 2), suggesting that this receptor is functional. However, the presence and expression of specific siderophore receptor genes do not reliably predict activity of the complex protein assemblies involved in synthesis of siderophores. For example, it has been reported that uropathogenic E. coli strains contain the genes encoding enterobactin, aerobactin, yersinabactin, and salmochelin siderophores, but only yersinabactin and salmochelin are produced (31). In polymicrobial communities, pathogenic bacteria may benefit from inactivated siderophore production if they retain the ability to sense and “steal” ferric siderophore complexes in which the siderophore is produced by a neighboring competing cell, thereby avoiding the metabolic cost of siderophore biosynthesis (27).
Conclusions.
Cronobacter spp. have transport systems for both ferric and ferrous iron. For acquisition of ferrous iron, all Cronobacter spp. have both the Feo and Efe systems, and for transport of ferric iron, all plasmid-harboring strains (97%) have the aerobactin-like siderophore cronobactin. All Cronobacter spp. have the genes encoding the enterobactin-like siderophore, but this siderophore was not functional under the conditions tested in this study. In addition to receptors for cronobactin and enterobactin, all Cronobacter spp. have five common receptors (FhuA, YncD, FoxA, FhuE, and PfeA) for siderophores produced by other organisms. The ferric dicitrate transport system was found specifically in a small subset of C. sakazakii and C. malonaticus strains, most of which were isolated from clinical samples, suggesting that this iron acquisition system plays a role in the virulence of Cronobacter spp. Furthermore, C. dublinensis and C. muytjensii have two receptors, Fct and FcuA, for heterologous siderophores produced by plant pathogens, which may give an advantage to those Cronobacter spp. to compete more successfully for iron in a plant niche. In silico identification of putative Fur boxes and expression of the genes under iron-depleted conditions suggest that most of these iron transport systems form part of the Fur regulon. Phylogenetic analysis of TonB-dependent iron receptors showed that fcuA and fct are closely related to homologues from water- and plant-associated species. In contrast, the ferric dicitrate transport genes specific to C. sakazakii and C. malonaticus are more closely related to orthologous genes in several pathogenic strains of E. coli and Shigella spp. but distantly related to orthologous genes from plant-associated species. Moreover, phylogenetic analysis of most of the iron acquisition genes and systems separate the genus Cronobacter into two subclades. One subclade includes the species C. sakazakii, C. malonaticus, C. universalis, and C. turicensis, and the other subclade comprises C. muytjensii and C. dublinensis. This clustering was in agreement with virulence markers on pESA3-/pCTU1-like plasmids (23), where the Cronobacter plasminogen activator (Cpa) apparently involved in invasion and serum resistance (24) was found specifically in C. sakazakii strains. Overall, these results suggest that C. dublinensis and C. muytjensii are more likely inhabitants of an environmental niche related to eukaryotic plant material; in contrast, C. sakazakii and C. malonaticus may be more closely associated with the human host, which may explain why most Cronobacter-related disease in humans is caused by C. sakazakii and C. malonaticus.
Supplementary Material
ACKNOWLEDGMENTS
C. J. Grim and K. G. Jarvis are Oak Ridge Institute for Science and Education fellows.
We thank the Department of Energy for financial support.
Footnotes
Published ahead of print 15 June 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Abramoff MD, Magalhaes PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics Int. 11: 36–42 [Google Scholar]
- 2. Angerer A, Braun V. 1998. Iron regulates transcription of the Escherichia coli ferric citrate transport genes directly and through the transcription initiation proteins. Arch. Microbiol. 169: 483–490 [DOI] [PubMed] [Google Scholar]
- 3. Arnow LE. 1937. Colorimetric determination of the components of 3,4-dihydroxy-phenylalanine-tyrosine mixture. J. Biol. Chem. 118: 531–541 [Google Scholar]
- 4. Aziz RK, et al. 2008. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9: 75–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bäumler AJ, Hantke K. 1992. Ferrioxamine uptake in Yersinia enterocolitica: characterization of the receptor protein FoxA. Mol. Microbiol. 6: 1309–1321 [DOI] [PubMed] [Google Scholar]
- 6. Braun V, Mahren S, Ogierman M. 2003. Regulation of the FecI-type ECF sigma factor by transmembrane signalling. Curr. Opin. Microbiol. 6: 173–180 [DOI] [PubMed] [Google Scholar]
- 7. Brickman TJ, Ozenberger BA, McIntosh MA. 1990. Regulation of divergent transcription from the iron-responsive fepB-entC promoter-operator regions in Escherichia coli. J. Mol. Biol. 212: 669–682 [DOI] [PubMed] [Google Scholar]
- 8. Butterton JR, Calderwood SB. 1994. Identification, cloning, and sequencing of a gene required for ferric vibriobactin utilization by Vibrio cholerae. J. Bacteriol. 176: 5631–5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calderwood SB, Mekalanos JJ. 1987. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J. Bacteriol. 169: 4759–4764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao J, Woodhall MR, Alvarez J, Cartron ML, Andrews SC. 2007. EfeUOB (YcdNOB) is a tripartite, acid-induced and CpxAR-regulated, low-pH Fe2+ transporter that is cryptic in Escherichia coli K-12 but functional in E. coli O157:H7. Mol. Microbiol. 65: 857–875 [DOI] [PubMed] [Google Scholar]
- 11. Cartron ML, Maddocks S, Gillingham P, Craven CJ, Andrews SC. 2006. Feo—transport of ferrous iron into bacteria. Biometals 19: 143–147 [DOI] [PubMed] [Google Scholar]
- 12. Cawthorn DM, Botha S, Witthuhn RC. 2008. Evaluation of different methods for the detection and identification of Enterobacter sakazakii isolated from South African infant formula milks and the processing environment. Int. J. Food Microbiol. 127: 129–138 [DOI] [PubMed] [Google Scholar]
- 13. Christoffersen CA, Brickman TJ, Hook-Barnard I, McIntosh MA. 2001. Regulatory architecture of the iron-regulated fepD-ybdA bidirectional promoter region in Escherichia coli. J. Bacteriol. 183: 2059–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark NC, Hill BC, O'Hara CM, Steingrimsson O, Cooksey RC. 1990. Epidemiologic typing of Enterobacter sakazakii in two neonatal nosocomial outbreaks. Diagn. Microbiol. Infect. Dis. 13: 467–472 [DOI] [PubMed] [Google Scholar]
- 15. Csáky TZ. 1948. On the estimation of bound hydroxylamine in biological materials. Acta Chem. Scand. 2: 450–454 [Google Scholar]
- 16. Dellagi A, et al. 2005. Siderophore-mediated upregulation of Arabidopsis ferritin expression in response to Erwinia chrysanthemi infection. Plant J. 43: 262–272 [DOI] [PubMed] [Google Scholar]
- 17. Escolar L, Pérz-Martin J, de Lorenzo V. 1999. Opening the iron box: transcriptional metalloregulation by the Fur protein. J. Bacteriol. 181: 6223–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faraldo-Gómez JD, Sansom MS. 2003. Acquisition of siderophores in Gram-negative bacteria. Nat. Rev. Mol. Cell Biol. 4: 105–116 [DOI] [PubMed] [Google Scholar]
- 19. Fecker L, Braun V. 1983. Cloning and expression of the fhu genes involved in iron(III)-hydroxamate uptake by Escherichia coli. J. Bacteriol. 156: 1301–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791 [DOI] [PubMed] [Google Scholar]
- 21. Ferguson AD, et al. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295: 1715–1719 [DOI] [PubMed] [Google Scholar]
- 22. Forman S, Nagiec MJ, Abney J, Perry RD, Fetherston JD. 2007. Analysis of the aerobactin and ferric hydroxamate uptake systems of Yersinia pestis. Microbiology 153: 2332–2341 [DOI] [PubMed] [Google Scholar]
- 23. Franco AA, et al. 2011. Characterization of putative virulence genes on the related RepFIB plasmids harbored by Cronobacter spp. Appl. Environ. Microbiol. 77: 3255–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franco AA, et al. 2011. Cpa, the outer membrane protease of Cronobacter sakazakii, activates plasminogen and mediates resistance to serum bactericidal activity. Infect. Immun. 79: 1578–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedemann M. 2007. Enterobacter sakazakii in food and beverages (other than infant formula and milk powder). Int. J. Food Microbiol. 16: 1–10 [DOI] [PubMed] [Google Scholar]
- 26. Furrer JL, Sanders DN, Hook-Barnard IG, McIntosh MA. 2002. Export of the siderophore enterobactin in Escherichia coli: involvement of a 43 kDa membrane exporter. Mol. Microbiol. 44: 1225–1234 [DOI] [PubMed] [Google Scholar]
- 27. Griffin AS, West SA, Buckling A. 2004. Cooperation and competition in pathogenic bacteria. Nature 430: 1024–1027 [DOI] [PubMed] [Google Scholar]
- 28. Griffiths E. 1999. Iron in biological systems, p 1–26 In Bullen J, Griffiths E. (ed), Iron and infection. Wiley, Chichester, United Kingdom [Google Scholar]
- 29. Gurtler B, Kornacki JL, Beuchat LR. 2005. Enterobacter sakazakii: a coliform of increased concern to infant health. Int. J. Food Microbiol. 104: 1–34 [DOI] [PubMed] [Google Scholar]
- 30. Heller K, Mann BJ, Kadner RJ. 1985. Cloning and expression of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J. Bacteriol. 161: 896–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Henderson JP, et al. 2009. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 5(2):e1000305 doi:10.1371/journal.ppat.1000305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Himelright I, Harris E, Lorch V, Anderson M. 2002. Enterobacter sakazakii infections associated with the use of powdered infant formula—Tennessee, 2001. JAMA 287: 2204–2205 [PubMed] [Google Scholar]
- 33. Hunt MD, Pettis GS, McIntosh MA. 1994. Promoter and operator determinants for Fur-mediated iron regulation in the bidirectional fepA-fes control region of the Escherichia coli enterobactin gene system. J. Bacteriol. 176: 3944–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iversen C, et al. 2008. Cronobacter gen. nov., a new genus to accommodate the biogroups of Enterobacter sakazakii, and proposal of Cronobacter sakazakii gen. nov., comb. nov., Cronobacter malonaticus sp. nov., Cronobacter turicensis sp. nov., Cronobacter muytjensii sp. nov., Cronobacter dublinensis sp. nov., Cronobacter genomospecies 1, and of three subspecies, Cronobacter dublinensis subsp. dublinensis subsp. nov., Cronobacter dublinensis subsp. lausannensis subsp. nov. and Cronobacter dublinensis subsp. lactaridi subsp. nov. Int. J. Syst. Evol. Microbiol. 58: 1442–1447 [DOI] [PubMed] [Google Scholar]
- 35. Iversen C, Lane M, Forsythe SJ. 2004. The growth profile, thermotolerance and biofilm formation of Enterobacter sakazakii grown in infant formula milk. Lett. Appl. Microbiol. 38: 378–382 [DOI] [PubMed] [Google Scholar]
- 36. Joseph S, et al. 22 July 2011, posting date Cronobacter condimenti sp. nov., isolated from spiced meat and Cronobacter universalis sp. nov., a novel species designation for Cronobacter sp. genomospecies 1, recovered from a leg infection, water, and food ingredients. Int. J. Syst. Evol. Microbiol. doi:10.1099/ijs.0.032292-0 [DOI] [PubMed] [Google Scholar]
- 37. Kammler M, Schon C, Hantke K. 1993. Characterization of the ferrous uptake system of Escherichia coli. J. Bacteriol. 175: 6212–6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kandhai MC, Reij MW, Gorris LGM, Guillaume-Gentil O, van Schothorst M. 2004. Occurrence of Enterobacter sakazakii in food production environments and households. Lancet 363: 39–40 [DOI] [PubMed] [Google Scholar]
- 39. Kingsley RA, et al. 1999. Ferrioxamine-mediated iron(III) utilization by Salmonella enterica. Appl. Environ. Microbiol. 65: 1610–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kleiman MB, Allen SD, Neal P, Reynolds J. 1981. Meningoencephalitis and compartmentalization of the cerebral ventricles caused by Enterobacter sakazakii. J. Clin. Microbiol. 14: 352–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koebnik R, Hantke K, Braun V. 1993. The TonB-dependent ferrichrome receptor FcuA of Yersinia enterocolitica: evidence against a strict co-evolution of receptor structure and substrate specificity. Mol. Microbiol. 7: 383–393 [DOI] [PubMed] [Google Scholar]
- 42. Köster W, Braun V. 1990. Iron(III) hydroxamate transport into Escherichia coli. Substrate binding to the periplasmic FhuD protein. J. Biol. Chem. 265: 21407–21410 [PubMed] [Google Scholar]
- 43. Kucerova E, et al. 2010. Genome sequence of Cronobacter sakazakii BAA-894 and comparative genomic hybridization analysis with other Cronobacter species. PLoS One 5: e9556 doi:10.1371/journal.pone.0009556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lehner A, Stephan R. 2004. Microbiological, epidemiological, and food safety aspects of Enterobacter sakazakii. J. Food Prot. 67: 2850–2857 [DOI] [PubMed] [Google Scholar]
- 45. Lehner A, Tasara T, Stephan R. 2004. 16S rRNA gene based analysis of Enterobacter sakazakii strains from different sources and development of a PCR assay for identification. BMC Microbiol. 4: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Létoffé S, Heuck G, Delepelaire P, Lange N, Wandersman C. 2009. Bacteria capture iron from heme by keeping tetrapyrrol skeleton intact. Proc. Natl. Acad. Sci. U. S. A. 106: 11719–11724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li Y, et al. 2012. Use of rpoB gene sequence analysis for phylogenetic identification of Cronobacter species. J. Microbiol. Methods 88: 316–318 [DOI] [PubMed] [Google Scholar]
- 48. Lin H, Fischbach MA, Liu DR, Walsh CT. 2005. In vitro characterization of salmochelin and enterobactin trilactone hydrolases IroD, IroE, and Fes. J. Am. Chem. Soc. 127: 11075–11084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Llamas MA, et al. 2006. The heterologous siderophores ferrioxamine B and ferrichrome activate signaling pathways in Pseudomonas aeruginosa. J. Bacteriol. 188: 1882–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luck SN, Turner SA, Rajakumar K, Sakellaris H, Adler B. 2001. Ferric dicitrate transport system (Fec) of Shigella flexneri 2a YSH6000 is encoded on a novel pathogenicity island carrying multiple antibiotic resistance genes. Infect. Immun. 69: 6012–6021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mahren S, Schnell H, Braun V. 2005. Occurrence and regulation of the ferric citrate transport system in Escherichia coli B, Klebsiella pneumoniae, Enterobacter aerogenes, and Photorhabdus luminescens. Arch. Microbiol. 184: 175–186 [DOI] [PubMed] [Google Scholar]
- 52. Mahren S, Enz S, Braun V. 2002. Functional interaction of region 4 of the extracytoplasmic function sigma factor FecI with the cytoplasmic portion of the FecR transmembrane protein of the Escherichia coli ferric citrate transport system. J. Bacteriol. 184: 3704–3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Matzanke BF, Anemuller S, Schunemann V, Trautwein AX, Hantke K. 2004. FhuF, part of a siderophore-reductase system. Biochemistry 43: 1386–1392 [DOI] [PubMed] [Google Scholar]
- 54. Miethke M, Marahiel MA. 2007. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 71: 413–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mills M, Payne SM. 1997. Analysis of shuA, the gene encoding the heme receptor of Shigella dysenteriae, and analysis of invasion and intracellular multiplication of a shuA mutant. Infect. Immun. 65: 5358–5363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mills M, Payne SM. 1995. Genetics and regulation of heme iron transport in Shigella dysenteriae and detection of an analogous system in Escherichia coli O157: H7. J. Bacteriol. 177: 3004–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nazarowec-White M, Farber JM. 1997. Enterobacter sakazakii: a review. Int. J. Food Microbiol. 34: 103–113 [DOI] [PubMed] [Google Scholar]
- 58. Neilands JB. 1981. Iron absorption and transport in microorganisms. Annu. Rev. Nutr. 1: 27–46 [DOI] [PubMed] [Google Scholar]
- 59. Noriega FR, Kotloff KL, Martin MA, Schwalbe RS. 1990. Nosocomial bacteremia caused by Enterobacter sakazakii and Leuconostoc mesenteroides resulting from extrinsic contamination of infant formula. Pediatr. Infect. Dis. J. 9: 447–449 [PubMed] [Google Scholar]
- 60. Overbeek R, et al. 2005. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 33: 5691–5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rabsch W, et al. 2003. Role of receptor proteins for enterobactin and 2,3-dihydroxybenzoylserine in virulence of Salmonella enterica. Infect. Immun. 71: 6953–6961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Raymond KN, Dertz EA, Kim SS. 2003. Enterobactin: an archetype for microbial iron transport. Proc. Natl. Acad. Sci. U. S. A. 100: 3584–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rogers MB, Sexton JA, DeCastro GJ, Calderwood SB. 2000. Identification of an operon required for ferrichrome iron utilization in Vibrio cholerae. J. Bacteriol. 182: 2350–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Runyen-Janecky LJ, Reeves SA, Gonzales EG, Payne SM. 2003. Contribution of the Shigella flexneri Sit, Iuc, and Feo iron acquisition systems to iron acquisition in vitro and in cultured cells. Infect. Immun. 71: 1919–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425 [DOI] [PubMed] [Google Scholar]
- 66. Sampson BA, Gotschlich EC. 1992. Elimination of the vitamin B12 uptake or synthesis pathway does not diminish the virulence of Escherichia coli K1 or Salmonella typhimurium in three model systems. Infect. Immun. 60: 3518–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sauer M, Hantke K, Braun V. 1987. Ferric-coprogen receptor FhuE of Escherichia coli: processing and sequence common to all TonB-dependent outer membrane receptor proteins. J. Bacteriol. 169: 2044–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sauvage C, Franza T, Expert D. 1996. Analysis of the Erwinia chrysanthemi ferrichrysobactin receptor gene: resemblance to the Escherichia coli fepA-fes bidirectional promoter region and homology with hydroxamate receptors. J. Bacteriol. 178: 1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schmid M, et al. 2009. Evidence for a plant-associated natural habitat for Cronobacter spp. Res. Microbiol. 160: 608–614 [DOI] [PubMed] [Google Scholar]
- 70. Shea CM, McIntosh MA. 1991. Nucleotide sequence and genetic organization of the ferric enterobactin transport system: homology to other periplasmic binding protein-dependent systems in Escherichia coli. Mol. Microbiol. 5: 1415–1428 [DOI] [PubMed] [Google Scholar]
- 71. Simmons BP, Gelfans MS, Haas M, Metts L, Ferguson J. 1989. Enterobacter sakazakii infections in neonates associated with intrinsic contamination of a powdered infant formula. Infect. Control Hosp. Epidemiol. 10: 398–401 [DOI] [PubMed] [Google Scholar]
- 72. Snyder JA, et al. 2004. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 72: 6373–6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stelma GN, Jr, Reyes AL, Peeler JT, Johnson CH, Spaulding PL. 1992. Virulence characteristics of clinical and environmental isolates of Vibrio vulnificus. Appl. Environ. Microbiol. 58: 2776–2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stephan R, Lehner A, Tischler P, Rattei T. 2011. Complete genome sequence of Cronobacter turicensis LMG 23827, a food-borne pathogen causing deaths in neonates. J. Bacteriol. 193: 309–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stoop B, Lehner A, Iversen C, Fanning S, Stephan R. 2009. Development and evaluation of rpoB based PCR systems to differentiate the six proposed species within the genus Cronobacter. Int. J. Food Microbiol. 136: 165–168 [DOI] [PubMed] [Google Scholar]
- 76. Tamura K, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Torres AG, Redford P, Welch RA, Payne SM. 2001. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect. Immun. 69: 6179–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Torres AG, Payne SM. 1997. Haem iron transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 23: 825–833 [DOI] [PubMed] [Google Scholar]
- 79. van Acker J, et al. 2001. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J. Clin. Microbiol. 39: 293–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Veitinger S, Braun V. 1992. Localization of the entire fec region at 97.3 minutes on the Escherichia coli K-12 chromosome. J. Bacteriol. 174: 3838–3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang S, Wu Y, Outten FW. 2011. Fur and the novel regulator YqjI control transcription of the ferric reductase gene yqjH in Escherichia coli. J. Bacteriol. 193: 563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wyckoff EE, Boulette ML, Payne SM. 2009. Genetics and environmental regulation of Shigella iron transport systems. Biometals 22: 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xiong K, et al. 2012. Deletion of yncD gene in Salmonella enterica ssp. enterica serovar Typhi leads to attenuation in mouse model. FEMS Microbiol. Lett. 328: 70–77 [DOI] [PubMed] [Google Scholar]
- 84. Zuckerkandl E, Pauling L. 1965. Evolutionary divergence and convergence in proteins, p 97–166 In Bryson V, Vogel HJ. (ed), Evolving genes and proteins. Academic Press, Inc., New York, NY [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.