Abstract
Patients with asthma, a major public health problem, are at high risk for serious disease from influenza virus infection, but the pathogenic mechanisms by which influenza A causes airway disease and asthma are not fully known. We show here in a mouse model that influenza infection acutely induced airway hyper-reactivity (AHR), a cardinal feature of asthma, independently of T helper type 2 (TH2) cells and adaptive immunity. Instead, influenza infection induced AHR through a previously unknown pathway that required the interleukin 13 (IL-13)–IL-33 axis and cells of the non-T cell, non-B cell innate lymphoid type called ‘natural helper cells’. Infection with influenza A virus, which activates the NLRP3 inflammasome, resulted in much more production of IL-33 by alveolar macrophages, which in turn activated natural helper cells producing substantial IL-13.
Asthma is a major public health problem that affects nearly 10% of the general population in the USA and 300 million people world-wide. Airway hyper-reactivity (AHR) and airway inflammation are major components of the disease and are thought to be orchestrated by allergen-specific T helper type 2 (TH2) cells in combination with eosinophils and basophils. Such cells are present in the lungs of almost all patients with asthma1, particularly of those with allergic asthma, the most common form of asthma. TH2 cells contribute to the development of asthma by secreting TH2 cytokines, which enhance the production of allergen-specific immunoglobulin E (IL-4) and promote the growth of eosinophils (IL-5) and mast cells (IL-9), and by directly causing AHR (IL-13), a cardinal feature of asthma. However, although TH2 cells may be responsible for many of the classical features of asthma, several clinical and experimental observations suggest that the causes of asthma are more heterogeneous and complex than suggested by the TH2 paradigm.
For example, non-allergic forms of asthma, triggered by environmental factors, such as air pollutants (for example, smoke, diesel particles and ozone), stress, obesity and viral infection, seem to develop independently of TH2 cells2–5. In addition, non-TH2 factors, such as interferon-γ (IFN-γ), IL-17 and neutrophils, are frequently found in the lungs of patients with asthma, particularly in the lungs of patients with severe asthma or of patients with corticosteroid-resistant asthma6. Moreover, TH2-targeted therapies, including monoclonal antibody (mAb) to IL-4, mAb to IL-5 and IL-13 antagonists, have not been as effective as hoped in many clinical trials of asthma7. Such findings suggest that other cell types, in addition to TH2 cells, regulate the development of asthma. Indeed, subsets of natural killer T (NKT) cells that produce IL-4 and IL-13 or that produce IL-17, as well as IL-17-producing helper T cells, have been linked to the development of asthma8.
Although eosinophils and allergen-specific TH2 cells typify the inflammation observed in many patients with allergic asthma, viral respiratory infection precipitates asthma symptoms in almost all patients with asthma regardless of the presence of allergy. The asthma symptoms that occur with viral infection are often severe and frequently result in hospitalization because of a failure of conventional asthma therapies, such as corticosteroids, which effectively limit the function of eosinophils and TH2 cells. Rhinovirus is the most common cause of virus-associated asthma exacerbations, but infection with influenza virus is also extremely common and is associated with substantial morbidity and mortality in patients with asthma, as observed during the 2009 H1N1 influenza A virus pandemic9.
Precisely how viral infection, and in particular infection with influenza virus, causes acute asthma, and whether virus-induced asthma requires the presence of TH2 cells or cells of the innate immune response (‘innate cells’), are not known. To define the inflammatory cell types and processes involved in the development of acute virusinduced asthma, we established an experimental mouse model in which we infected mice with influenza A virus subtype H3N1 (called simply ‘H3N1’ here) and examined the development of acute AHR. Unexpectedly, infection with H3N1 acutely induced airway inflammation and AHR independently of TH2 cells or adaptive immunity. H3N1-induced AHR required the presence of an innate lymphoid cell population (natural helper cells) characterized by the absence of lineage markers (Lin−) and by expression of the membrane glycoprotein CD90.2 (Thy-1.2), the stem cell antigen Sca-1 and the IL-33 receptor ST2. These results suggest that infection with influenza virus causes acute AHR by activating an innate lymphoid cell population and the IL-13–IL-33 axis in the absence of adaptive immunity. Further study of this innate pathway may lead to improved therapies for acute severe exacerbations of asthma associated with viral infection.
RESULTS
H3N1 infection causes AHR independently of adaptive immunity
Infection of BALB/c mice with H3N1 resulted in rapid development of AHR that peaked on day 5 and decreased by day 15 of infection (Fig. 1a and Supplementary Fig. 1a–d). The AHR response after infection with H3N1 was associated with airway neutrophils and macrophages but not with airway eosinophils (Fig. 1b,c). C57BL/6 mice infected with H3N1 also developed AHR and neutrophilic airway inflammation (Fig. 1d,e and Supplementary Fig. 1c), which indicated that H3N1-induced AHR lacked strain ‘preference’, in contrast to Sendai virus–induced chronic asthma, which occurs only in C57BL/6 mice4.
Figure 1.
H3N1 infection causes AHR and inflammation. (a) Change in lung resistance (RL) in 8-week-old BALB/c mice (n = 7–9 per group; anesthetized, tracheotomized, intubated and mechanically ventilated) challenged with methacholine nebulized into the airways 5 d after infection with H3N1 or allantoic fluid (mock infection control (mock)). *P < 0.01 and **P < 0.001, compared with mock infection (two-way analysis of variance (ANOVA)). (b) Macrophages (MΦ), neutrophils (Neu), eosinophils (Eos) and lymphocytes (Lym) in BAL fluid 5 d after treatment as in a. ND, not detectable. *P < 0.001, compared with mock infection (Student’s two-tailed t-test). (c) Lung sections obtained from mock- or H3N1-infected BALB/c or C57BL/6 mice, stained with hematoxylin and eosin. Scale bars, 200 μm. (d) Change in lung resistance in 8-week-old C57BL/6 mice (n = 4 per group), treated and assessed as in a. *P ≤ 0.05 and **P ≤ 0.001. (e) Cells in BAL fluid 5 d after treatment as in d. *P < 0.001 (Student’s two-tailed t-test). (f,g) AHR in 8-week-old wild-type (WT) or Rag2−/− mice (n = 6–7 per group) 5 d after infection with H3N1 or allantoic fluid, assessed as change in lung resistance (f) or cells in BAL fluid (g), as in a,b. *P ≤ 0.01 and **P ≤ 0.001 (two-way ANOVA (f) or Student’s two-tailed t-test (g)). Data are representative of three independent experiments (mean and s.e.m.).
Furthermore, the speed with which H3N1 induced AHR (peaking within 5 d of infection; Supplementary Fig. 1a) suggested that innate immune mechanisms mediated the response. Indeed, in contrast to allergen-induced AHR, which requires sensitization of TH2 cells and priming of adaptive immunity over 7–14 d (ref. 10), AHR induced by H3N1 developed in mice deficient in recombination-activating gene 2 (Rag2−/− mice), which lack both T cells and B cells (Fig. 1f,g). Moreover, unlike AHR induced by allergens, ozone or Sendai virus, which requires NKT cells3,4,11, AHR induced by H3N1 occurred in NKT cell–deficient mice deficient in the antigen-presenting molecule CD1d (Supplementary Fig. 1e,f). These results indicated that the H3N1-induced AHR developed through innate immune pathways and did not require T cells, B cells or NKT cells.
ST2-deficient mice fail to develop H3N1-induced AHR
The induction of AHR by H3N1 infection was unique not only in being independent of T cells and NKT cells but also in its requirement for the ST2–IL-33 signaling pathway. ST2-deficient (Il1rl1−/−) mice infected with H3N1 failed to develop AHR or airway inflammation (Fig. 2a,b), and treatment of BALB/c mice with mAb to ST2 blocked the development of H3N1-induced AHR (Fig. 2c). Treatment of Rag2−/− mice with mAb to ST2 also blocked the development of H3N1-induced AHR (Fig. 2d), which indicated that the innate immune pathway that mediated the H3N1-induced AHR involved IL-33–ST2 signaling. In contrast, Il1rl1−/− mice sensitized and challenged with ovalbumin (OVA) developed severe AHR (Fig. 2e), which indicated that the ST2 pathway was required more for H3N1-induced AHR than for allergen-induced AHR.
Figure 2.
ST2-deficient mice fail to develop H3N1-induced AHR. (a,b) AHR in 8-week-old ST2-deficient (Il1rl1−/−) mice and their heterozygous (Il1rl1+/−) littermates (n = 4–6 per group) 5 d after infection with H3N1 or allantoic fluid, assessed (as in Fig. 1a,b) as change in lung resistance (a) and cells in BAL fluid (b). *P < 0.05, **P < 0.01 and ***P < 0.001 (two-way ANOVA (a) or Student’s two-tailed t-test (b)). (c,d) Change in lung resistance in 8-week-old wild-type mice (c) and Rag2−/− mice (d; n = 3–4 per group) treated with two injections of mAb to ST2 (0.5 mg) or control immunoglobulin G (IgG), on days −1 and day +4, and infected with H3N1 or allantoic fluid on day 0, assessed (as in Fig. 1a) on day +5. *P < 0.001, compared with H3N1 plus IgG (two-way ANOVA). (e) Change in lung resistance in 8-week-old ST2-deficient mice and their heterozygous littermates (n = 3 per group) sensitized with saline (control) or OVA-alum on day 0, then challenged with OVA on days 7–9 and assessed (as in Fig. 1a) on day 10. *P < 0.05 and **P < 0.001, compared with saline (two-way ANOVA). Data are representative of three independent experiments (mean and s.e.m.).
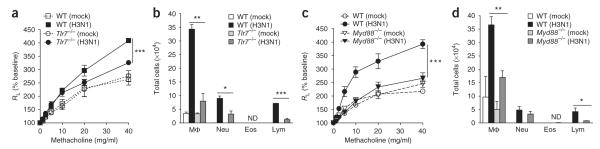
Because innate immune mechanisms mediated H3N1-induced AHR, we examined the role of Toll-like receptors (TLRs) in this response. Tlr7−/− mice developed a limited AHR response to H3N1 infection, similar to that observed for mice deficient in the adaptor MyD88 (Myd88−/− mice; Fig. 3). This indicated that TLR7 and MyD88 were also involved in the AHR response induced by H3N1, consistent with the idea that influenza A virus infects alveolar macrophages and activates TLR7 and the NLRP3 inflammasome12–15.
Figure 3.
H3N1-induced AHR requires TLR7 and MyD88. (a) Change in lung resistance in 8-week-old wild-type and Tlr7−/− mice (n = 5–6 per group) 5 d after infection with H3N1 or allantoic fluid (assessed as in Fig. 1a). (b) Cells in BAL fluid 5 d after treatment as in a. (c) Change in lung resistance in 8-week-old wild-type and Myd88−/− mice (n = 4–6 per group) treated and assessed as in a. (d) Cells in the BAL fluid 5 d after treatment as in c. *P < 0.05, **P < 0.01 and ***P < 0.001, compared with H3N1 (two-way ANOVA (a,c) or Student’s two-tailed t-test (b,d)). Data are representative of two independent experiments (mean and s.e.m.).
H3N1-induced IL-33 production in alveolar macrophages
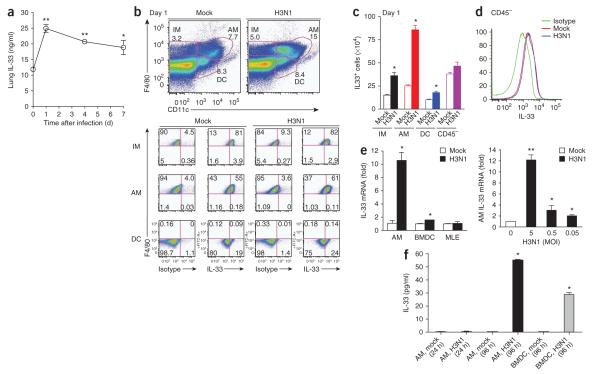
Infection with H3N1 resulted in much more production of IL-33 in the lungs, which doubled within 24 h of infection (Fig. 4a). The increase in IL-33 was due in part to more IL-33 production in alveolar macrophages in H3N1-infected mice, as demonstrated by intracellular cytokine staining of alveolar macrophages (Fig. 4b), and to greater absolute numbers of IL-33-producing alveolar macrophages in the lung (Fig. 4c). Although airway epithelial cells are thought to produce large quantities of IL-33, we found that infection with H3N1 did not affect IL-33 production in airway epithelial cells (CD45− cells). However, these cells had a high baseline concentration of IL-33 (Fig. 4d), which might have been a source of biologically active IL-33 in lung. Furthermore, infection of alveolar macrophages and bone marrow–derived dendritic cells (DCs) with H3N1 in vitro resulted in significantly more IL-33 mRNA expression (Fig. 4e) and more release of IL-33 (Fig. 4f), but infection of the mouse lung epithelial cell line MLE with H3N1 did not, which confirmed that infection with H3N1 resulted in more IL-33 production in alveolar macrophages. We also observed IL-33 production by alveolar macrophages after in vitro stimulation with an agonist of TLR7 and TLR8 (R-848) but not after stimulation with an agonist of TLR3 (poly(I:C); Supplementary Fig. 2). This suggested that activation of the NLRP3 inflammasome may not be required for IL-33 release, although we cannot rule out the possibility of the release of preformed IL-33 from dying macrophages.
Figure 4.
H3N1-induced production of IL-33 in alveolar macrophages. (a) Enzyme-linked immunosorbent assay of IL-33 in lungs of BALB/c mice (n = 3 per group) obtained on days 0, 1, 4 and 7 after infection with H3N1 or mock infection and homogenized in 1 ml PBS. *P < 0.05 and **P < 0.001, compared with mock infection (Student’s two-tailed t-test). (b) Flow cytometry sorting (top) of CD45+ interstitial macrophages (IM; F4/80+CD11c−), alveolar macrophages (AM; F4/80+CD11c+) and DCs (F4/80−CD11c+) from the lungs of BALB/c mice on day 1 after infection with H3N1 or mock infection, followed by staining with mAb to IL-33 or isotype-matched control antibody (below). Numbers adjacent to cell designation (top) or in quadrants (below) indicate percent cells in each. (c) Absolute number of IL-33+ interstitial macrophages, alveolar macrophages and DCs in the mice in b. *P < 0.001, compared with mock infection (Student’s two-tailed t-test). (d) IL-33 expression by CD45− nonhematopoietic lung cells from the mice in b. (e) Quantitative RT-PCR analysis of IL-33 mRNA in alveolar macrophages, bone marrow–derived DCs (BMDC) or the MLE mouse lung epithelial cell line infected for 24 h in vitro with H3N1 at a multiplicity of infection of 5 (top), or in alveolar macrophages infected for 24 h in vitro with H3N1 at various multiplicities of infection (bottom). *P < 0.05 and **P < 0.001, compared with mock infection (Student’s two-tailed t-test). (f) Enzyme-linked immunosorbent assay of IL-33 in supernatants of alveolar macrophages or bone marrow–derived DCs infected for 24 or 96 h in vitro with H3N1 (multiplicity of infection, 5; 5 × 105 cells per well, in 24 well plates) or given mock infection. *P < 0.001, compared with mock infection (Student’s two-tailed t-test). Data are representative of three independent experiments (a,e,f; mean and s.e.m.) or three experiments (b–d; mean and s.e.m. in c).
Natural helper cells in lungs of H3N1-infected mice
IL-33 activates a non-T cell, non-B cell, innate lymphoid cell type called natural helper cells16, nuocytes17 or multipotent progenitor cells18; these cells produce large quantities of IL-13 and IL-5 and are essential in intestinal responses to helminths. We found that such cells, which we call ‘natural helper cells’ here, were present in the lungs of H3N1-infected mice, as demonstrated by gating on lineagenegative (CD3−CD19−CD11b−CD11c−CD49b−F4/80−FcεR1−) ST2+c-Kit+Sca-1+ lymphocytes (Fig. 5a,b). These Lin−ST2+cells also expressed CD90.2 (Thy-1.2), CD25 (IL-2 receptor α-chain) and CD1d and had limited expression of major histocompatibility complex class II (Fig. 5c). Natural helper cells were present in both BALB/c and C57BL/6 mice, although Sca-1 expression by natural helper cells was higher in C57BL/6 mice than in BALB/c mice (data not shown). The number of natural helper cells present in the lungs peaked on day 6 of infection (Fig. 5d–f), when AHR was also at its maximum. The H3N1-induced population expansion of natural helper cells also occurred in the absence of adaptive immunity, as it occurred the lungs and in the bronchoalveolar lavage (BAL) fluid of H3N1-infected Rag2−/− mice (Fig. 5g).
Figure 5.
H3N1 infection results in a greater abundance of natural helper cells in the lungs. (a) Gating strategy for lung Lin−ST2+ cells among CD45+ cells. Numbers adjacent to outlined areas indicate percent cells in each gate. SSC, side scatter; FSC, forward scatter. (b,c) Expression of Sca-1 and c-Kit (b) and of c-Kit, CD90.2 (Thy-1.2), CD25, CD1d and major histocompatibility complex class II (MHCII; c) by lung cells from 8-week-old BALB/c mice 5 d after infection with H3N1 or mock infection, assessed after gating on the Lin−ST2+ subset. Numbers adjacent to outlined areas (b) indicate percent cells in each. FMO (c), fluorescence minus one (control). (d) Flow cytometry analysis of the expression of c-Kit and Sca-1 (bottom) by lung cells from 8-week-old BALB/c mice (n = 3 per group) 1, 3, 6, 9 or 15 d after infection as in b,c, assessed after gating on the Lin−ST2+ subset among CD45+ cells (top). Numbers adjacent to outlined areas (top) or in quadrants (bottom) indicate percent cells in each. (e,f) Frequency (e) and absolute number (f) of CD45+Lin−ST2+ c-Kit+Sca-1+ lung cells in d. *P < 0.05 and **P < 0.001, compared with day 0 (Student’s two-tailed t-test). (g) Flow cytometry analysis of the expression of c-Kit and Sca-1 (middle) and of CD90.2 (Thy-1.2) and Sca-1 (bottom) by CD45+ lung cells or BAL fluid cells from 8-week-old Rag2−/− mice (n = 3 per group) 5 d after infection as in b,c, assessed after gating on the Lin−ST2+ subset (top). Data are representative of or three experiments (mean and s.e.m. in e,f).
IL-13 production by natural helper cells in H3N1-infected mice
The H3N1 response depended on the production of IL-13, as Il13−/− mice failed to develop H3N1-induced AHR and airway inflammation (Fig. 6a,b). Production of IL-13 and IL-5 in the lungs peaked 4–5 d after H3N1 infection, around the time that AHR was greatest (Fig. 6c and Supplementary Fig. 3). Notably, the Lin−ST2+Sca-1+ natural helper cells, not the Lin+ST2+ cells (TH2 cells, NKT cells and basophils), produced the most IL-13 (Fig. 6d and Supplementary Fig. 4). As noted above, adaptive immunity was not required for this response, and there were more IL-13-producing natural helper cells in both wild-type and Rag2−/− mice after H3N1 infection (Fig. 6e,f), presumably in response to IL-33, which increases IL-13 production in natural helper cells. Although both natural helper cells and lung macrophages produced IL-13 on day 1 (Supplementary Fig. 5), by day 5 of infection, alveolar macrophages produced less IL-13 than did natural helper cells (Fig. 6d,g). Furthermore, infection with H3N1 did not induce T cells to produce IL-13 on day 5, although some IFN-γ secretion from T cells was induced (Supplementary Fig. 5d). Although IFN-γ production increased during H3N1 infection (Supplementary Fig. 6a), which could have limited airway eosinophilia, it was not required for H3N1-induced AHR, as both mice deficient in the transcription factor T-bet (which secrete less IFN-γ; Supplementary Fig. 6b,c) and IFN-γ-deficient mice developed robust H3N1-induced AHR (Supplementary Fig. 6d,e). Although some Lin− innate lymphoid cells (such as those associated with colitis) can produce IL-17A19, the natural helper cells in the lungs produced IL-13 and IL-5 but not IL-17A (Supplementary Fig. 7). Although clearance of H3N1 was somewhat delayed in Rag2−/− mice, viral titers in the lungs of Rag2−/− mice on day 5 of infection were similar to those in all other strains of mice (Supplementary Fig. 8). Furthermore, the development of H3N1-induced AHR did not correlate with the rapidity of viral clearance but instead correlated with the presence of IL-33, IL-13 and natural helper cells.
Figure 6.
IL-13 and natural helper cells cause AHR. (a,b) Change in lung resistance (a) and cells in BAL fluid (b) in 8-week-old wild-type and Il13−/− mice after infection with H3N1 or mock infection (n = 5–6 per group; assessed as in Fig. 1a,b). *P ≤ 0.05 and **P < 0.001 (two-way ANOVA (a) or Student’s two-tailed t-test (b)). (c) Quantitative RT-PCR analysis of IL-13 mRNA (top; relative to expression at day 0) and enzyme-linked immunosorbent assay of IL-13 protein (bottom) in homogenates of H3N1-infected BALB/c lungs (n = 3 per group). *P < 0.001, compared with day 0 (Student’s two-tailed t-test). (d) Flow cytometry analysis of intracellular IL-13 and Sca-1 in CD45+Lin−ST2+c-kit+Sca-1+ lung cells left unstimulated (US) or stimulated for 5 h with the phorbol DC ester PMA plus ionomycin (PMA + iono), assessed after gating (as in top row) on Lin+ST2+ cells (middle row) or Lin−ST2+ cells (bottom row). (e) Intracellular IL-13 and Sca-1 in lung cells obtained from wild-type or Rag2−/− mice IL-13 on day 5 d after infection with H3N1 or mock infection and stimulated for 5 h with PMA plus ionomycin (bottom), assessed after gating on Lin−ST2+c-Kit+ cells (top). (f) Absolute number of Lin−ST2+c-Kit+Sca-1+ cells (left) or Lin−ST2+c-Kit+Sca-1+IL-13+ cells (right) in the lungs of mice in e. *P ≤ 0.001 (Student’s two-tailed t-test). (g) Flow cytometry (top) of lung interstitial macrophages (F4/80+CD11c−), alveolar macrophages (F4/80+CD11c+) and DCs (F4/80−CD11c+) among lung leukocytes (CD45+) from the mice in e; below, intracellular IL-13 expression in single cells. Data are representative of three experiments (a–d; mean and s.e.m. in c) or three independent experiments (e–g; mean and s.e.m. in f).
Essential role for natural helper cells in H3N1-induced AHR
To more clearly demonstrate the critical role of natural helper cells in mediating H3N1-induced AHR, we did depletion and reconstitution experiments. First, depletion of natural helper cells by treatment of Rag2−/− mice with mAb to CD90.2 (Thy-1.2; Supplementary Fig. 9a,b) abolished the H3N1-induced AHR response (Fig. 7a,b). Second, we adoptively transferred highly purified (Lin−ST2+) natural helper cells (Supplementary Figs. 9c and 10a) to confirm the role of natural helper cells in AHR. These cells produced IL-5 and IL-13 but not IFN-γ, IL-4 or IL-17A after stimulation in vitro with IL-2 plus IL-33 (Supplementary Fig. 10b–d) and, when adoptively transferred (1 × 105 cells per recipient), fully reconstituted H3N1-induced AHR in Il13−/− recipient mice (Fig. 7c,d). Only the transferred natural helper cells could have produced IL-13 in the recipients; therefore, the IL-13-producing natural helper cells, but not other IL-13-producing lineages (such as basophils or mast cells), were sufficient for the development of H3N1-induced AHR. Together these results show the potency of natural helper cells in inducing AHR and demonstrate an essential role for the IL-13–IL-33 axis in the development of H3N1-induced AHR, which involves IL-33 produced by macrophages (and possibly by other cell types) and IL-13-producing natural helper cells (Supplementary Fig. 11).
Figure 7.
Natural helper cells are essential for H3N1-induced AHR in Rag2−/− mice. (a,b) Change in lung resistance (a) and cells in BAL fluid (b) in 8-week-old Rag2−/− mice (n = 4 per group) left undepleted (mock and H3N1) or depleted of CD90.2+ (Thy-1.2+) cells by three injections of mAb to CD90.2 (30-H12; 0.5 mg per mouse) on days −3, 0 and +3 (H3N1 + mAb to CD90.2), and infected with H3N1 or mock infected on day 0, analyzed 5 d after infection (as in Fig. 1a,b). *P < 0.05 and **P < 0.001, compared with H3N1 infection (two-way ANOVA (a) or Student’s two-tailed t-test (b)). (c,d) Change in lung resistance (c) and cells in BAL fluid (d) in Il13−/− recipients (n = 4 per group) given purified natural helper cells (Lin−ST2+ subsets) from Il13+/+ (Rag2−/−) donors or Il13−/− donors treated intranasally with IL-33 (1 μg) 5 d before adoptive transfer of cells (1× 105 cells/mouse) by intratracheal injection, followed by mock infection or infection of recipients with H3N1 and analysis 5 d after infection (as in Fig. 1a,b). *P ≤ 0.001 (two-way ANOVA (c) or Student’s two-tailed t-test (d)). Data are representative of two (a,b) or three (c,d) independent experiments (mean and s.e.m.).
DISCUSSION
Our studies have demonstrated a previously undescribed pathway by which infection with the H3N1 subtype of influenza A virus resulted in the development of AHR that was independent of TH2 cells and adaptive immunity and distinct from that involved in allergic asthma. We found that acute infection of mice with H3N1 rapidly induced AHR, which depended on IL-33, its receptor (ST2) and natural helper cells, which are a non-T, non-B lymphoid innate cell type expressing c-Kit, Sca-1, CD90.2 (Thy-1.2) and ST2. Infection with H3N1 activated this IL-13–IL-33 axis, characterized by the production of IL-33 in alveolar macrophages and the activation of IL-13-producing natural helper cells. Moreover, AHR induced by H3N1 occurred independently of NKT cells, as H3N1-induced AHR occurred in Rag2−/− mice and in NKT cell–deficient, CD1ddeficient mice.
The results of our studies are notable for several reasons. First, they have shown that natural helper cells, reported before as being present in the intestines in the context of helminth infection, were present in the lungs, and that natural helper cells were required for the development of AHR induced by influenza. Second, our studies have demonstrated a trigger (H3N1 infection) for the in vivo production of IL-33 during the development of AHR, which activated IL-33 production in alveolar macrophages, although it is possible that other cell types such as airway epithelial cells might also be activated by influenza A to release IL-33. Finally, our results have demonstrated that H3N1-induced AHR developed through an alternative pathway completely independently of TH2 cells and adaptive immunity that instead involved H3N1-induced IL-33 production associated with the population expansion of natural helper cells in the airways.
IL-33 is a member of the IL-1 cytokine family that is produced by endothelial cells, bronchial smooth muscle cells, fibroblasts, airway and intestinal epithelial cells, keratinocytes, adipocytes, DCs and macrophages20–23. Expression of IL-33 has been demonstrated in the lungs of patients with severe asthma24, and in genome-wide association studies, IL33 has been identified as a susceptibility gene for human asthma25,26. In addition, the administration of recombinant IL-33 to the lungs of mice induces the development of AHR27–30, and infection with respiratory syncytial virus in mice previously vaccinated with respiratory syncytial virus attachment protein G causes TH2-driven airway inflammation that depends on ST2 signaling31. However, neither the mechanisms for the induction of IL-33 nor the ST2-expressing target cells have been clarified, particularly as the development of allergen-induced AHR does not depend on ST2 signaling27–30, even though TH2 cells are known to express ST2 (refs. 32,33). IL-33 acts in synergy with stem cell factor and immunoglobulin E to activate primary human mast cells and basophils34 and enhances the survival and degranulation of eosinophils in humans35. However, although IL-33 can activate mast cells to produce IL-4 and IL-13, which might induce AHR, administration of IL-33 to mast cell–deficient mice still resulted in AHR, which suggests that mast cells are not the main target cell of IL-33 in asthma27. Instead, because natural helper cells, which have been shown to be present in fat-associated lymphoid clusters16 and in mesenteric lymph nodes of helminth-infected mice17,18, were required for the development of H3N1-induced AHR, we suggest that an important target cell of IL-33 in the lung is the natural helper cell, which responds to IL-33 by producing large quantities of IL-13.
The precise circumstances under which IL-33 is released from cells remain controversial. IL-33, along with IL-1β and IL-18, is thought to be released after cleavage by caspase-1 during apoptosis or pyroptosis36. Influenza A activates the inflammasome and pyroptosis by providing the following two required signals: double-stranded RNA to activate TLR7, and the M2 protein that activates NLRP3 and caspase-1 (refs. 12–15). However, IL-33 may not require caspase-1-mediated proteolysis for activation37,38, and other processing and release mechanisms might be involved39. For example, release of small amounts of preformed IL-33 from necrotic or pyroptotic virus–infected lung cells may occur in the absence of inflammasome activation, a possibility consistent with our results showing the development of modest AHR in Tlr7−/− mice and Myd88−/− mice. Nevertheless, we found that infection with H3N1 resulted in much more IL-33 production, particularly by alveolar macrophages, and that the development of AHR required IL-33, as well as ST2 and its target, natural helper cells.
Although the precise characteristics of natural helper cells (including nuocytes and multipotent progenitor cells) that respond to IL-33 are still being delineated, these cells do not express lineage markers but do express c-Kit, Sca-1 and the IL-7 receptor and respond to IL-33 and IL-25 (ref. 16). These cells have been shown to be important in the expulsion of nematodes from the intestines and may be related to a Lin− innate lymphoid cell that expresses Sca-1 and CD90.2 (Thy-1.2) and that responds to IL-23 by producing IL-17 and IFN-γ and is involved in the development of colitis19. Until now, however, Lin−Sca1+CD90.2+ innate lymphoid cells have not been studied in lung disease, to our knowledge. Our observations have demonstrated the presence of such cells in the lungs (in wild-type mice and also in Rag2−/− mice), their contribution to the development of AHR and their activation by H3N1 infection via an IL-33-dependent, fully innate pathway. These cells then produced large amounts of IL-13 and IL-5, which drove the development of AHR and resulted in more secretion of mucus from the airways. Consequently, during infection with influenza A virus, natural helper cells are necessary and sufficient for mediation of the development of AHR, although in other circumstances (such as in allergen-induced AHR), natural helper cells may be superfluous. Therefore, multiple pathways can indeed lead to asthma, some dependent on and some independent of TH2 cells8. It is likely, however, that many of these pathways to asthma coexist in patients, synergistically inducing airway inflammation, AHR and asthma, and that effective treatment for asthma may require attention to each of these pathways8.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureimmunology/.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Grusby (Harvard School of Public Health) for CD1d-deficient mice (backcrossed to the BALB/c strain) and Z. Luo for technical support. Supported by the US National Institutes of Health (R01 AI068085, R01 HL62348 and R01 051354).
Footnotes
Note: Supplementary information is available on the Nature Immunology website.
AUTHOR CONTRIBUTIONS Y.-J.C. designed the study, did experiments, analyzed the data and wrote the manuscript; H.Y.K. did experiments and analyzed the data; L.A.A. did experiments; N.B. provided the H3N1 virus and did experiments. A.N.J.M., D.E.S. and R.H.D. provided reagents and ST2-deficient (Il1rl1−/−) and IL-13 deficient (Il13−/−) mice; and D.T.U. designed the study and wrote the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/natureimmunology/.
reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Robinson DS, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 2.Johnston RA, et al. Allergic airway responses in obese mice. Am. J. Respir. Crit. Care Med. 2007;176:650–658. doi: 10.1164/rccm.200702-323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pichavant M, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J. Exp. Med. 2008;205:385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim EY, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat. Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright RJ. Stress and atopic disorders. J. Allergy Clin. Immunol. 2005;116:1301–1306. doi: 10.1016/j.jaci.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 6.Oboki K, Ohno T, Saito H, Nakae S. TH17 and allergy. Allergol. Int. 2008;57:121–134. doi: 10.2332/allergolint.R-07-160. [DOI] [PubMed] [Google Scholar]
- 7.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 8.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat. Immunol. 2010;11:577–584. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain S, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N. Engl. J. Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 10.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific TH1 cells fail to counterbalance TH2 cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Invest. 1999;103:175–183. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akbari O, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 12.Kanneganti TD, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 13.Thomas PG, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen IC, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat. Immunol. 2010;11:404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moro K, et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 17.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saenz SA, et al. IL-25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature. 2010;464:1371–1376. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buonocore S, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammad H, et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Wood IS, Wang B, Trayhurn P. IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem. Biophys. Res. Commun. 2009;384:105–109. doi: 10.1016/j.bbrc.2009.04.081. [DOI] [PubMed] [Google Scholar]
- 23.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS ONE. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prefontaine D, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J. Allergy Clin. Immunol. 2010;125:752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 25.Moffatt MF, et al. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gudbjartsson DF, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat. Genet. 2009;41:342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 27.Kondo Y, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int. Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 28.Kurowska-Stolarska M, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- 29.Hoshino K, et al. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J. Exp. Med. 1999;190:1541–1548. doi: 10.1084/jem.190.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangan NE, Dasvarma A, McKenzie AN, Fallon PG. T1/ST2 expression on TH2 cells negatively regulates allergic pulmonary inflammation. Eur. J. Immunol. 2007;37:1302–1312. doi: 10.1002/eji.200636520. [DOI] [PubMed] [Google Scholar]
- 31.Walzl G, et al. Inhibition of T1/ST2 during respiratory syncytial virus infection prevents T helper cell type 2 (TH2)-but not TH1-driven immunopathology. J. Exp. Med. 2001;193:785–792. doi: 10.1084/jem.193.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu D, et al. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not TH2 cells. J. Exp. Med. 1998;188:1485–1492. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silver MR, et al. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflamm. Res. 2010;59:207–218. doi: 10.1007/s00011-009-0088-5. [DOI] [PubMed] [Google Scholar]
- 35.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bortoluci KR, Medzhitov R. Control of infection by pyroptosis and autophagy: role of TLR and NLR. Cell. Mol. Life Sci. 2010;67:1643–1651. doi: 10.1007/s00018-010-0335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luthi AU, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. USA. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao W, Hu Z. The enigmatic processing and secretion of interleukin-33. Cell. Mol. Immunol. 2010;7:260–262. doi: 10.1038/cmi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.