Abstract
Peptide neurotransmitters function as key intercellular signaling molecules in the nervous system. These peptides are generated in secretory vesicles from proneuropeptides by proteolytic processing at dibasic residues, followed by removal of N- and/or C-terminal basic residues to form active peptides. Enkephalin biosynthesis from proenkephalin utilizes the cysteine protease cathepsin L and the subtilisin-like prohormone convertase 2 (PC2). Cathepsin L generates peptide intermediates with N-terminal basic residue extensions, which must be removed by an aminopeptidase. In this study, we identified cathepsin H as an aminopeptidase in secretory vesicles that produces (Met)enkephalin (ME) by sequential removal of basic residues from KR-ME and KK-ME, supported by in vivo knockout of the cathepsin H gene. Localization of cathepsin H in secretory vesicles was demonstrated by immunoelectron microscopy and confocal immunofluorescence microscopy. Purified human cathepsin H sequentially removes N-terminal basic residues to generate ME, with peptide products characterized by nano-LC-MS/MS tandem mass spectrometry. Cathepsin H shows highest activities for cleaving N-terminal basic residues (Arg and Lys) among amino acid fluorogenic substrates. Notably, knockout of the cathepsin H gene results in reduction of ME in mouse brain. Cathepsin H deficient mice also show a substantial decrease in galanin peptide neurotransmitter levels in brain. These results illustrate a role for cathepsin H as an aminopeptidase for enkephalin and galanin peptide neurotransmitter production.
Keywords: aminopeptidase, cathepsin H, peptide neurotransmitter, enkephalin, galanin, secretory vesicle, neuropeptide, protease
Introduction
Peptide neurotransmitters are required for cell-cell signaling among neurons to transmit commands among neural circuits to regulate behavior and physiological functions. Such peptide neurotransmitters, also known as neuropeptides, mediate key functions of the nervous system. As examples, the enkephalin-endorphin neuropeptide family regulates pain relief and analgesia (Gustein and Akil, 2006; Law et al., 2000). Galanin in brain participates in cognition for learning and memory (Steiner et al., 2001; Robinson, 2004). CRF (corticotropin-releasing factor) and dynorphin mediate stress responses related to addiction (Koob, 2008; Logrip et al., 2011). NPY (neuropeptide Y) participates in brain regulation of feeding behavior and obesity (Ramos et al., 2005; Zhang et al., 2011). These and numerous other neuropeptides are required for health and are involved in human diseases (Kastin, 2006; Kim et al., 2011).
Neuropeptides are generated from inactive precursor proteins by proteolytic processing (Seidah et al., 2008; Hook et al., 2008; Mbikay and Seidah, 2011) in secretory vesicles, which store the neuropeptides for activity-dependent, regulated secretion for their function in neurotransmission. Endoproteolytic processing of proneuropeptides occurs at paired basic residues, as well as monobasic and multibasic residues. Proneuropeptide processing in secretory vesicles is achieved by the cysteine protease cathepsin L (Hook et al., 2008; Funkelstein et al., 2010) and the subtilisin-like prohormone convertases 1 and 2 (PC1/3 and PC2) (Seidah et al., 2008; Mbikay and Seidah, 2011). Protease gene knockout mouse studies have demonstrated the prominent roles of the cathepsin L and prohormone convertase protease pathways in the biosynthesis of peptide neurotransmitters (Miller et al., 2003a, b: Scamuffa et al., 2006; Hook et al., 2008; Mbikay and Seidah, 2011). The well-established PC1/3 and PC2 proteases cleave dibasic residues at their COOH-terminal side, resulting in peptide intermediates with basic residues at their COOH-termini which are removed by carboxypeptidase E (Fricker, 1988). The more recently discovered cathepsin L cysteine protease cleaves dibasic residues at their N-termini, as well as between the dibasic residues, that flank neuropeptide sequences within their precursor proteins (Hook et al., 2008; Funkelstein et al., 2010). Peptide products generated by cathepsin L will then require removal of NH2- and COOH-terminal basic residues by aminopeptidase activity and carboxypeptidase E.
Because of the requirement for aminopeptidase activity to remove basic residues from peptide intermediates for neuropeptide production, this study investigated the hypothesis that cathepsin H, a cysteine protease, participates in removing basic residues from peptides to form the active enkephalin opioid neuropeptide. The rationale for this hypothesis is the knowledge that cathepsin H possesses aminopeptidase activity for NH2-terminal basic residues (Rothe and Dodt, 1992; Kirschke, 2004), combined with the finding in this study that cathepsin H is present in neuropeptide-producing secretory vesicles by immunoelectron microscopy and immunofluorescence confocal microscopy. Neuropeptide-generating aminopeptidase activity of cathepsin H was demonstrated by its sequential removal of NH2-terminal Lys-Arg and Lys-Lys residues from peptide substrates to generate the active (Met)enkephalin neuropeptide, illustrated by HPLC and mass spectrometry. Notably, cathepsin H knockout mice show significant reduction in brain levels of (Met)enkephalin and galanin neuropeptides, indicating the functional role of cathepsin H to produce peptide neurotransmitters. These findings support the hypothesis for participation of secretory vesicle cathepsin H in neuropeptide biosynthesis.
Materials and Methods
Bovine adrenal medulla tissue
Bovine adrenal medullary chromaffin cell tissue was utilized for preparation of purified secretory vesicles (also known as chromaffin granules) and primary cultures of chromaffin cells for studies of cathepsin H. The bovine adrenal medulla tissue was obtained from fresh bovine adrenal glands (from the company Sierra Medical Science, Whittier, CA) using procedures approved by the Biohazard Use Authorization of the University of California Institutional Biosafety Committee. This authorization includes implementation of ethical research guidelines.
Cathepsin H in secretory vesicles of bovine adrenal medullary chromaffin cells demonstrated by western blots and immunoelectron microscopy
Bovine adrenal medulla tissue was utilized for purification of secretory vesicles (also known as chromaffin granules) by sucrose density gradient centrifugation, performed as we have described previously (Wegrzyn et al., 2010). The high purity of the isolated secretory vesicles has been established by enzyme markers of subcellular organelles and by electron microscopy that demonstrates the integrity of intact secretory vesicles.
The presence of cathepsin H enzyme was assessed by anti-cathepsin H western blots using 12% NuPAGE Bis-Tris gel electrophoresis (Novex-Life Technologies, Grand Island, NY ) of the isolated secretory vesicles and anti-cathepsin H (rabbit) (1:1000, from R & D Systems, Minneapolis, MN). Western blots were developed with secondary HRP-linked anti-rabbit IgG (donkey) (1:40,000 dilution, Amersham-GE Healthcare, Piscataway, NJ) using the ECL Plus western blot detection reagent (Amersham-GE Healthcare, Piscataway, NJ), conducted as we have described previously (Wegrzyn et al., 2010).
In addition, the specificity of the anti-cathepsin H was evaluated by western blotting with purified cathepsin H (50 ng, Athens Research & Technology, Athens, GA) and the related cysteine cathepsins B, L, and V (50 ng each of cathepsins B and L from Athens Research & Technology, and 50 ng cathepsin V was from R & D Systems, Minneapolis, MN). The anti-cathepsin H recognizes cathepsin H, but not cathepsins B, L, and V.
Immunoelectron microscopy of cathepsin H in secretory vesicles
Immunoelectron microscopy was conducted (as we have described previously (Yasothornsrikul et al., 2003) to assess the presence of cathepsin H in secretory vesicles that produce enkephalin and numerous neuropeptides. Secretory vesicles were isolated from adrenal medulla by differential sucrose density centrifugation as described in the previous paragraph (Wegrzyn et al., 2010), and were fixed in 0.2% glutaraldehyde, 2% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.2. Samples were osmicated in 2% osmium tetroxide in 0.1 M cacodylate and embedded in Epon 812. Ultrathin sections were partially deosmicated through 1% periodic acid/9% sodium periodate, and incubated in 3% normal goat serum in 1X TBS. Cathepsin H was visualized by anti-cathepsin H (rabbit) detected by 6 nm gold conjugated to anti-rabbit IgGs. Controls without primary antibodies resulted in the absence of immunostaining. Sections were examined in a Tecnai-12 transmission electron microscope using a CCD camera and Digital Micrograph Software (Gatan Inc., CA).
Immunofluorescence confocal microscopy of cathepsin H in neuropeptide secretory vesicles of neuronal-like chromaffin cells in primary culture
Primary cultures of neuronal-like chromaffin cells were prepared from fresh bovine adrenal medulla as we have described previously (Yasothornsrikul et al., 2003). Cells were fixed in 4% paraformaldehyde (PFA), permeabilized with 0.1% Triton X-100, and cells were incubated with primary antibodies anti-(Met)enkephalin-rabbit (1:50 dilution, from Millipore, Billerica, MA) or anti-cathepsin H-goat (0.5 μg/μl, R & D Systems, Minneapolis, MN) in PBS containing 3% bovine serum albumin (PBS-BSA 3%) for 2 hours at room temperature. After washing with PBS, cells were then incubated with secondary donkey anti-rabbit Alexa Fluor 594 and secondary donkey anti-goat Alexa Fluor 488 (1:200, red and green fluorescence labels, respectively, Molecular Probes, OR), respectively, in PBS-BSA 3%. Colocalization of cathepsin H and (Met)enkephalin was observed by merged images, utilizing the Olympus IX70 microscope or Nikon Eclipse 800 microscope coupled to a BCM confocal system. Images were analyzed with Delta Vision Spectris Image Deconvolution System and SIMPLE PCI software.
Quantitation of the localization of cellular cathepsin H with (Met)enkephalin was conducted by measuring the Pearson’s correlation coefficient, Rr, using the Veolocity software (version 5.5.2 from Perkin-Elmer) (Manders et al., 1993). An Rr value of ‘1’ indicates complete colocalization, and a Rr value of ‘0’ indicates no specific colocalization. The Rr value (0.77) obtained for the observed colocalization of cathepsin H and (Met)enkephalin indicate their partial colocalization.
Control immunofluorescence staining procedures with only secondary antisera (no primary antibody incubation), resulted in lack of immunofluorescence; these controls indicate immunofluorescence signals resulting from anti-cathepsin H and from anti-(Met)enkephalin.
Cathepsin H processing of Lys-Arg-(Met)enkephalin (KR-ME) and Lys-Lys-(Met)enkephalin (KK-ME) analyzed by HPLC and mass spectrometry
Purified human cathepsin H (20 ng/assay, Athens Research and Technology, Athens, GA) was incubated (14 μl volume) with enkephalin peptides (1 μg for each peptide) with basic residues at their NH2-terminus, consisting of Lys-Arg-(Met)enkephalin (KR-ME) and Lys-Lys-(Met)enkephalin (KK-ME) (peptides were synthesized by the Tufts University Core Facility, Boston, MA) in 20 mM potassium-phosphate, pH 6.8, 1 mM EDTA, 3 mM cysteine at 37° C. Aliquots (2 μl) of the assay were removed at time points of 15 and 30 minutes, and at 1, 2, 6, and 18 hours incubation. Samples were then quenched by addition of formic acid to 5% and frozen at −70° C. For LC-MS/MS tandem mass spectrometry analyses, samples were heated at 80° C for 2 minutes, and analysed on the Agilent XCT Ultra ion trap with Chip Cube module, electrospray ionization (ESI) ion source, and thermostated autosampler, as we have described previously (Gupta et al., 2010; Wegrzyn et al., 2010). The HPLC Chip utilized an enrichment column of 4 mm, 40 nl, Zorbax 300SB-C18, 5 μm, and an analytical column of 43 mm x 75 μm, Zorbax 300SB-C18, 5 μm. Samples were delivered to the enrichment column at 2 μl/min in 0.25% formic acid (buffer A), follwed by elution and analysis on the analytical column starting at 100% buffer A for 5 minutes, with elution by a gradient of 100% buffer A to 55% buffer A and 45% buffer B (acetonitrile in 0.25% formic acid) in 10 minutes at 0.4 μl/min. The HPLC peaks (monitored by total ion chromatogram) were analyzed for their areas using the Agilent DataAnalysis 3.3 software. Using (Met)enkephalin as standard peptide, replicate LC peptide peak integrations vary by less than 1%, as we have previously demonstrated (Hwang et al. 2007). Peptide sequences were determined from MS/MS spectra analyzed by Spectrum Mill (Agilent) or InsPecT at high levels of statistical confidence, as we have described previously (Gupta et al., 2010; Wegrzyn et al., 2010).
Cathepsin H activity characterization with amino acid-MCA substrates and inhibitors
Cathepsin H was assayed with fluorogenic amino acid substrates linked to AMC (aminomethylcoumarin amide) to compare aminopeptidase cleavage of the 20 different amino acids. These substrates consisted of Ala-MCA, Asp-MCA, and Thr-MCA were from Bachem (Torrance, CA); Arg-MCA, Asn-MCA, S-benzyl-Cys-MCA, Glu-MCA, Gln-MCA, Gly-MCA, His-MCA, Ile-MCA, Lys-MCA, Met-MCA, Pro-MCA, Ser-MCA, and Trp-MCA were from Chem-Impex International (Wood-Dale, IL). Leu-MCA, Phe-MCA, Tyr-MCA, and Val-MCA were from Sigma Aldrich (St. Louis, MO). Each amino acid substrate (200 μM final concentration) was incubated with purified human cathepsin H (20 ng) in 75 mM potassium phosphate, pH 6.8, 1 mm EDTA, 3 mM cysteine at 37° C and the free fluorescent AMC (aminomethylcoumarin amide) group was measured in a fluorimeter as we have previously described (Hwang et al., 2007).
In addition, the effects of several inhibitors – amastatin, arphamenine A, and bestatin (from Calbiochem, San Diego, CA) – that typically inhibit aminopeptidases were assessed for effects on cathepsin H activity assayed with Arg-MCA and Lys-MCA.
Cathepsin H knockout mice, and ethics statement
Cathepsin H gene knockout mice were grown and genotyped in the laboratory of Dr. Christoph Peters at the Albert Ludgwigs University, Freiburg, Germany, as we have described (Buhling et al., 2011). (These mice are not from a commercial source) Genotyping established WT (+/+) and cathepsin H deficient (−/−) mice (C57BL/6 strain, 15-16 weeks of age). The WT and cathepsin H deficient groups each contained 8 mice (4 males and 4 females).
The generation and phenotype analyses of cathepsin H knockout mice for this study was conducted in accordance to the German law of animal protection. According to this law, the animal work was reviewed and approved by the ethics committee of the governmental regional board, Freiburg.
Brain levels of (Met)enkephalin in cathepsin H knockout mice
The absence of cathepsin H in the cathepsin H deficient mice has been confirmed by the lack of cathepsin H activity, mRNA, and protein (Buhling et al., 2011). Brain tissues from adult mice homogenized in 1 M acetic acid, heated at 95° C for 10 min., centrifuged (15,000 x g for 15 min), and the supernatant was collected for analyses of (Met)enkephalin by HPLC and radioimmunoassay (RIA) (RIA from Phoenix Pharmaceuticals, Burlingame, CA). Samples were subjected to HPLC fractionation and fractions corresponding to the elution position of standard (Met)enkephalin were collected for RIA (radioimmunoassay) measurement of the peptide. The HPLC fractionation (using the Agilent 1200 HPLC) utilized a 50 mm x 1 mm Vydac C18 column (Grace Davison, Deerfield, IL) with a gradient consisting of 100% buffer A (0.25% formic acid in H20) for 5 minutes, followed by a gradient to 70% buffer A and 30% buffer B (100% acetonitrile in 0.25% formic acid) over 20 minutes, with a flow rate of 50 μl/min. Fractions corresponding to the elution position of standard (Met)enkephalin peptide were collected for quantitation of (Met)enkephalin by radioimmunoassay. (Met)enkephalin levels in brain cortex of wild-type and cathepsin H deficient mice were expressed as pg enkephalin per μg brain cortex extract, with the average mean + s.e.m (standard error of the mean) calculated for each group.
The neuropeptide galanin was also measured in the extracts of brain cortex from wild-type and cathepsin H deficient mice, achieved by radioimmunoassay to quantitate galanin (galanin RIA from Phoenix Pharmaceuticals, Burlingame, CA). Results are expressed as pg galanin per μg brain cortex extract, with the mean ± s.e.m (standard error of the mean) calculated for each group. In addition, levels of the neuropeptides dynorphin A and β-endorphin were measured in brains of wild-type and cathepsin H knockout by radioimmunoassays (from Phoenix Pharmaceuticals, Inc., Burlingame, CA) as we have previously conducted (Minokadeh et al., 2010; Funkelstein et al., 2008).
Statistical Analyses
Statistical analyses of neuropeptide levels in brain extracts of cathepsin H knockout mice compared to wild-type mice was conducted by the student’s t-test, with significance level of p < 0.05. Neuropeptide results are expressed as the mean + s.e.m. (standard error of the mean).
Results
Cathepsin H is present in neuropeptide-producing secretory vesicles
The presence of cathepsin H in neuropeptide-containing secretory vesicles was analyzed to implicate a possible role of cathepsin H as one of several protease steps utilized to produce neuropeptides in secretory vesicles. Secretory vesicles purified from bovine adrenal medulla of the sympathetic nervous system contain numerous neuropeptides (enkephalin, galanin, NPY, VIP, and others). This model secretory vesicle system has been shown to contain proteolytic processing proteases for producing peptide neurotransmitters (neuropeptides) (Hook et al., 2008). Therefore, the presence of the cysteine protease cathepsin H in these purified secretory vesicles was analyzed by western blot, immunoelectron microscopy, and immunofluorescence confocal microscopy.
Western blots with anti-cathepsin H of isolated secretory vesicles from bovine adrenal medulla showed the presence of cathepsin H of ~28 kDa (Fig. 1a). This molecular weight of the detected cathepsin H likely corresponds to the single chain form of this cysteine protease (Kirschke, 2004). The specificity of the anti-cathepsin H to detect purified cathepsin H, with detection of other related cysteine cathepsins (cathepsins B, L, and V) was illustrated by western blot (supplemental figure A). Thus, the cathepsin H western blot data illustrates the presence of cathepsin H in isolated adrenal medullary secretory vesicles.
Figure 1. Cathepsin H in secretory vesicles illustrated by western blot and immunoelectron microscopy.
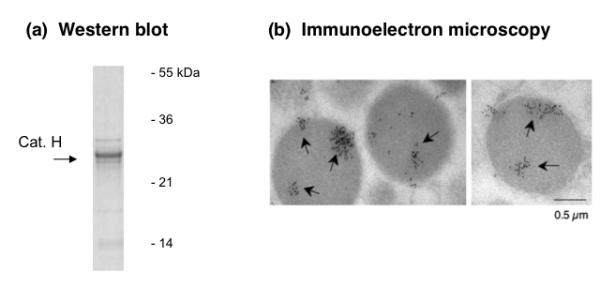
(a) Western blot of cathepsin H in secretory vesicles isolated from bovine adrenal medulla. Secretory vesicles isolated from adrenal medulla were subjected to anti-cathepsin H western blots. Results indicate the presence of a 26-29 kDa band that likely corresponds to the single chain form of cathepsin H with calculated molecular weight of 28 kDa (Kirschke, 2004).
(b) Immunoelectron microscopy of cathepsin H in isolated secretory vesicles. Cathepsin H in isolated secretory vesicles from bovine adrenal medulla was illustrated by immunoelectron microscopy. Cathepsin H is indicated by the 6 nm gold labeled particles conjugated to anti-rabbit to detect anti-cathepsin H rabbit (see methods).
Immunoelectron microscopy demonstrated the presence of cathepsin H in neuropeptide secretory vesicles isolated from adrenal medulla (Fig. 1b). Immunogold labeling with anti-rabbit conjugated with 6 nm gold particles detected cathepsin H within the secretory secretory vesicle. Controls with only secondary antibody labeled with gold particles (no anti-cathepsin H) resulted in absence of immunogold labeling (supplemental figure B); this control supports the immunogold detection of cathepsin H. Electron microscopy also illustrated the integrity of the purifed secretory vesicle preparation.
Neuronal-like chromaffin cells in primary culture (from bovine adrenal medulla) were analyzed for colocalization of cathepsin H and the (Met)enkephalin (ME) neuropeptide by immunofluorescence confocal microscopy (Fig. 2). ME is illustrated by immunofluorescence (red fluorescence) signal in the cells, showing a punctate pattern of subcellular localization that is consistent with the known secretory vesicle location of ME. Cathepsin H was also shown to be present in the chromaffin cells by immunofluorescence (green fluorescence) confocal microscopy (Fig. 2). Moreover, partial colocalization of cathepsin H and ME is illustrated by the merged image, with areas of yellow immunofluorescence illustrating colocalization (Fig. 2). Controls showed lack of immunofluorescence when only secondary antibodies (no primary ant-cathepsin H or anti-enkephalin) (supplemental figure C), thus, supporting the immunodetection of cathepsin H and enkephalin in secretory vesicles.
Figure 2. Cathepsin H localization with (Met)enkephalin (ME) in secretory vesicles illustrated by immunofluorescence confocal microscopy of neuronal-like chromaffin cells.
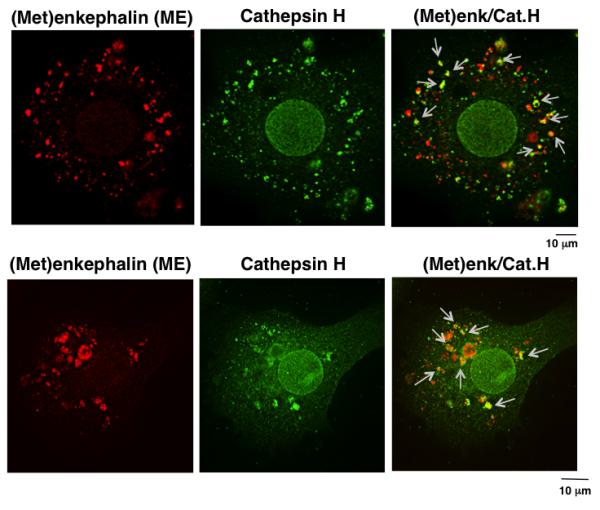
The colocalization of cathepsin H (Cat.H, green immunofluorescence) with ME ((Met)enk, red immunofluorescence) in secretory vesicles is shown by the merged yellow immunofluorescence areas (indicated by arrows) within neuronal-like chromaffin cells. The merged yellow immunofluorescence shows partial localization of cathepsin H with ME. Quantitation of the cathepsin H colocalized with (Met)enkephalin was assessed by measurement of the Pearson’s correlation coefficient (Rr value) of 0.77 + 0.024 (n = 12 cells), which indicates partial colocalization. An Rr value of ‘1’ indicates complete colocalization and a value of ‘0’ indicates no specific colocalization.
Quantitation of the relative cellular cathepsin H colocalized with (Met)enkephalin in a population of cells was assessed by measurement of the Pearson’s correlation coefficient (Rr value) of 0.77 (n = 12 cells), which indicates partial colocalization. An Rr value of ‘1’ indicates complete colocalization and a value of ‘0’ indicates no specific colocalization.
Overall, cathepsin H was found to be present in neuropeptide secretory vesicles by multiple approaches of western blots, immunoelectron microscopy, and immunofluorescence confocal microscopy.
Cathepsin H removes NH2-terminal basic residues to generate active (Met)enkephalin
The presence of cathepsin H in secretory vesicles suggested that this protease may be involved in enkephalin neuropeptide production. Therefore, aminopeptidase activity of cathepsin H was evaluated for removal Lys or Arg residues from the NH2-termini of peptide intermediates produced by cathepsin L from proneuropeptides in secretory vesicles (Hook et al., 2008; Funkelstein et al., 2010). (Met)enkephalin is an abundant neuropeptide produced in secretory vesicles of adrenal medulla. Thus, Lys-Arg-ME (KR-ME) and Lys-Lys-ME (KK-ME) peptide substrates were utilized to assess the aminopeptidase activity of cathepsin H to generate active ME.
Cathepsin H incubation of KR-ME resulted in the sequential removal of the NH2-terminal Lys and Arg residues to generate ME. After incubation of KR-ME with cathepsin H, the sample was subjected to nano-LC-MS/MS for identification of products. The total extracted ion chromatogram (Fig. 3a), shows the presence of KR-ME, and its products R-ME and ME. The molecular masses of KR-ME, R-ME, and ME observed by mass spectrometry are shown in supplemental Table 1. The identification of the R-ME intermediate by MS/MS tandem mass spectrometry data is illustrated (Fig. 3b). Analyses of the KR-ME and its peptide products R-ME and ME by tandem mass spectrometry confirmed their identification (supplemental figure D). The time course studies showed that cathepsin H first generates R-ME from KR-ME (Fig. 3c, as early as 15 minutes incubation), with appearance of ME starting at longer incubationtimes. The continued time course study shows time-dependent increases in formation of R-ME (1-6 hrs), concomitant with increases in the final ME product (1-6 hrs, and 18 hrs).
Figure 3. Cathepsin H removes NH2-terminal Lys and Arg from Lys-Arg-(Met)enkephalin (KR-ME).
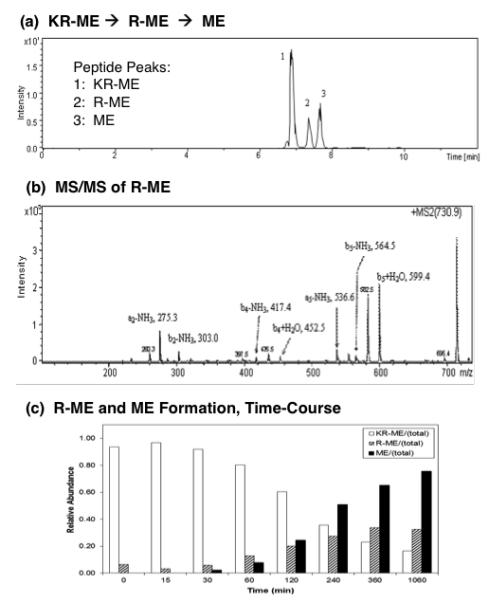
(a) KR-ME substrate conversion to R-ME and M: total ion chromatogram. Cathepsin H was incubated with KR-ME and nano-LC-MS/MS was conducted to identify peptide cleavage products. The total ion chromatogram (TIC) shows the presence of KR-ME substrate, and the products R-ME and ME, based on their masses (shown in supplemental Table 1).
(b) R-ME identified by tandem mass spectrometry. R-ME peptide (RYGGFM) was identified by MS/MS tandem mass spectrometry. The MS/MS spectra is illustrated here; those for KR-ME and ME are shown in supplemental Figure A.
(c) Time-course of cathepsin H production of R-ME and ME from KR-ME. The time course of cathepsin H conversion of KR-ME to R-ME and ME is illustrated by the open bars, hatched bars, and black solid bars, respectively. Using (Met)enkephalin standard peptide, LC peptide peak integrations vary by less than 1%, which we have previously demonstrated (Hwang et al., 2007).
Cathepsin H also converted the peptide substrate KK-ME to K-ME and ME, illustrated by the total extracted ion chromatogram of the nano-LC-MS/MS analyses (Fig. 4a). The identification of the intermediate K-ME by MS/MS tandem mass spectrometry is illustrated (Fig. 4b). The MS/MS identification of KK-ME combined with the products K-ME and ME are illustrated in supplemental information (supplemental figure E). Time course studies confirmed the formation of K-ME and ME occuring with continued incubation (Fig. 4c). K-ME production increases during upt to 2 hours incubation, and ME production increases from 30 minutes to 4 hours incubation. These data demonstrate that cathepsin H possesses aminopeptidase activity to remove NH2-terminal basic residues for the formation of the (Met)enkephalin peptide neurotransmitter.
Figure 4. Cathepsin H removes NH2-terminal Lys from Lys-Lys-(Met)enkephalin (KK-ME).
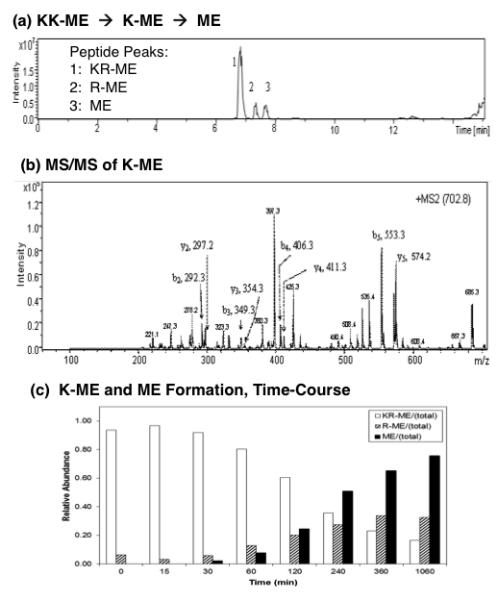
(a) KK-ME substrate conversion to K-ME and M: total ion chromatogram. Cathepsin H was incubated with KK-ME and nano-LC-MS/MS was conducted to identify peptide cleavage products. The total ion chromatogram (TIC) shows the presence of KK-ME substrate, and the products K-ME and ME, based on their masses (shown in supplemental Table 1).
(b) K-ME identified by tandem mass spectrometry. R-ME peptide (RYGGFM) was identified by MS/MS tandem mass spectrometry. The MS/MS spectra is illustrated here; those for KK-ME and ME are shown in supplemental Figure A.
(c) Time-course of producing K-ME and ME from KK-ME by cathepsin H. The time course of cathepsin H conversion of KK-ME to K-ME and ME is illustrated by the open bars, hatched bars, and black solid bars, respectively. Using (Met)enkephalin standard peptide, LC peptide peak integrations vary by less than 1%, which we have previously demonstrated (Hwang et al., 2007).
Analyses of cathepsin H aminopeptidase for different amino acid fluorescent substrates showed its high preference for cleaving NH2-terminal Lys and Arg residues, as well as Met and Asn residues (supplemental Table 2).
Inhibitor analyses of cathepsin H activity assayed with Arg-MCA and Lys-MCA substrates showed that inhibitors that inhibit aminopeptidase B (AP-B) (Hwang et al. 2007; Pham et al., 2007) – bestatin and arphamenine A – had no effects on cathepsin H (supplemental Table 3). These data distinguish cathepsin H from AP-B. In addition, amastatin, an aminopeptidase inhibitor, had no effect on cathepsin H.
Cathepsin H knockout mice show reduced brain levels of (Met)enkephalin
Production of (Met)enkephalin from proenkephalin utilizes cathepsin L which cleaves at the N-terminal side or between dibasic residue processing sites (Yasothornsrikul et al., 2003; Hook et al., 2008). Such peptide intermediates need a Lys/Arg aminopeptidase to generate mature enkephalin. Therefore, to asssess the in vivo aminopeptidase function of cathepsin H for production of (Met)enkephalin (ME), cathepsin H gene knockout mice were evaluated for changes in ME. Key findings show that knockout of the cathepsin H gene resulted in a significant 50% decrease of (Met)enkephalin in brain cortex (Fig. 5).
Figure 5. Knockout of the cathepsin H gene results in decreased brain levels of (Met)enkephalin.
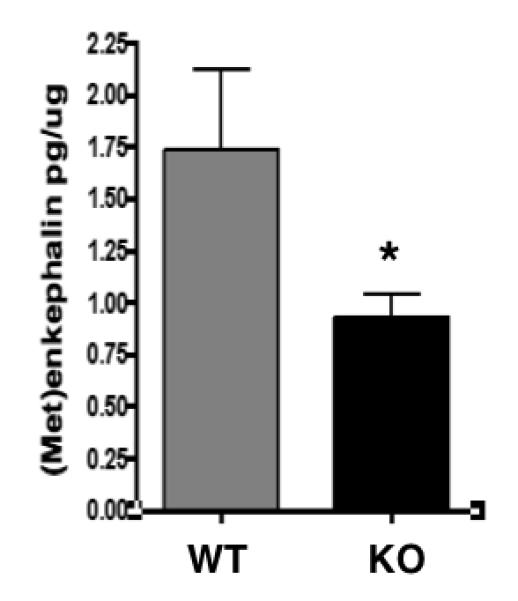
(Met)enkephalin in brain cortex of cathepsin H knockout (KO) and wild-type (WT) mice were measured by radioimmunoassay (RIA) of brain extracted fractions. Brain tissue was prepared as an acetic acid extract, fractionated by RP-HPLC, and fractions corresponding to the elution position of (Met)enkephalin were assayed for content of (Met)enkephalin by RIA. Brain (Met)enkephalin is expressed as the pg per μg brain extract protein, calculated as the mean + s.e.m (standard error of the mean). *Statististically significant (p < 0.05, n = 8 per group).
The effects of cathepsin H gene knockout were also assessed on brain levels of galanin, another peptide neurotransmitter. Data show that cathepsin H knockout mouse brain cortex show a substantial decrease in galanin that is reduced by ~80% compared to wild-type controls (Fig. 6). These findings indicate a significant role for cathepsin H for regulating galanin levels in brain cortex. Thus, the protease gene knockout data show that cathepsin H is involved in galanin production.
Figure 6. Cathepsin H knockout results in a substantial decrease in galanin.
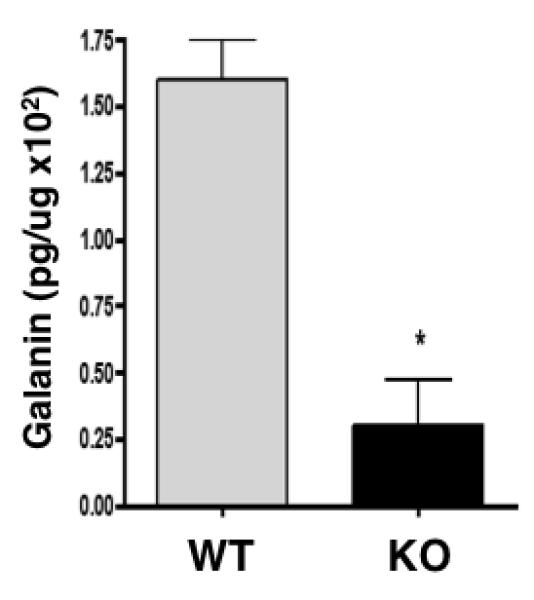
Galanin in brain cortex extracts of cathepsin H knockout (KO) and wild-type (WT) mice were measured by radioimmunoassay (RIA). Galanin is expressed as pg per μg brain extract protein, calculated as the mean + s.e.m (standard error of the mean). *Statististically significant (p < 0.05, n = 8 per group).
Interestingly, the cathepsin H knockout mouse brains show no change in dynorphin A or β-endorphin neuropeptides (Table 1). These data demonstrate the selective role of cathepsin H for production of (Met)enkephalin and galanin.
Table 1.
Cathepsin H Knockout Selectively Decreases (Met)enkephalin and Galanin Compared to Other Neuropeptides in Mouse Brains.
| Neuropeptide | Wild-Type (pg/mg) | Cathepsin H KO (pg/mg) | CatH KO/WT |
|---|---|---|---|
| (Met)enkephalin | 864 ± 189 | 498 ± 34 * | 0.57 * |
| Galanin | 16 ± 1.5 | 3 ± 1.7 * | 0.19 * |
| Dynorphin A | 76 ± 7.0 | 92 ± 8.0 | 1.21 |
| Beta-Endorphin | 98 ± 16 | 80 ± 16 | 0.82 |
Brain cortex extracts were subjected to radioimmunoassays to measure levels of (Met)enkephalin, galanin, dynorphin A, and beta-endorphin. Neuropeptide levels are expressed as pg/mg protein.
Statistically significant for cathepsin H knockout (KO) compared to wild-type levels of neuropeptides in brain (p < 0.05 by student’s test).
Overall, data from the cathepsin H knockout mice demonstrate the in vivo function of cathepsin H for production of (Met)enkephalin and galanin peptide neurotransmitters.
Discussion
Results of this study show that cathepsin H participates in the in vivo production of the peptide neurotransmitter (Met)enkephalin, by functioning as an aminopeptidase to remove NH2-terminal basic residues from peptide intermediates to generate active ME in neuropeptide secretory vesicles. Cathepsin H knockout mouse brains contain reduced levels of ME, that were approximately 50% lower than wild-type control mouse brains. Cathepsin H removes basic residues (Lys, Arg) from the NH2-termini of neuropeptide intermediates, to generate mature ME. Cathepsin H is colocalized with ME in secretory vesicles, the primary subcellular site of neuropeptide production. Western blots showed the presence of cathepsin H in secretory vesicles from adrenal medulla; immunoelectron microscopy demonstrated localization of cathepsin H within neuropeptide secretory vesicles, and confocal microscopy of neuronal-like chromaffin cells illustrated the colocalization of cathepsin H and ME. Furthermore, cathepsin H knockout mice showed a substantial decrease in brain levels of galanin, another peptide neurotransmitter. Interestingly, cathepsin H knockout mouse brains showed no change in dynorphin A or β-endorphin neuropeptides, indicating the selectivity of cathepsin H for biosynthesis of enkephalin and galanin. These findings support the hypothesis that cathepsin H in secretory vesicles particpates in the production of selected peptide neurotransmitters.
The findings of this study support the hypothesis that cathepsin H provide Lys/Arg aminopeptidase activity for removal of NH2-terminal basic residues from peptide intermediates generated from the proneuropeptide by secretory vesicle cathepsin L, which cleaves at the NH2-terminal side of dibasic residues, as well as between dibasic residues (Hook et al., 2008; Funkelstein, 2010) (Fig. 7). Subsequent to cathepsin L, both Lys/Arg aminopeptidase and carboxypeptidase E (CPE) (Fricker, 1988) to remove basic residues from NH2- and COOH-termini of peptide intermediates, respectively. Cathepsin L functions jointly with the prohormone convertases 1 and 2 (PC1/3 and PC2), subtilisin-like proteases, which prefer to cleave at the COOH-terminal side of dibasic residues (Fig. 7). Peptide products generated by these PC enzymes then require CPE to remove COOH-terminal basic residues to produce the active neuropeptide.
Figure 7. Cathepsin H functions with the cathepsin L and prohormone convertase protease pathways for producing enkephalin and galanin peptide neurotransmitters.
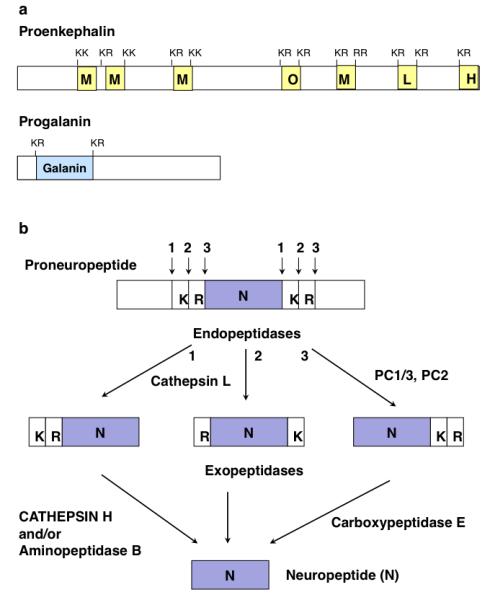
(a) Proenkephalin and progalanin proneuropeptides. The proneuropeptide precursors are schematically illustrated for proenkephalin and progalanin that undergo proteolytic processing to generate active enkephalin and galanin peptide neurotransmitters (neuropeptides). Active neuropeptides are typically flanked by dibasic residue processing sites within the precursor proteins.
(b) Protease pathways for neuropeptide production: cathepsin L and prohormone convertase pathways. It is proposed that cathepsin H functions as an aminopeptidase, subsequent to the endoproteolytic action of secretory vesicle cathepsin L. Cathepsin L cleaves proneuropeptides at dibasic residues, at the NH2-terminal side or between the dibasic residues. Cathepsin H participates as an exopeptidase to remove NH2-terminal basic residues from peptide intermediates; aminopeptidase B (AP-B) also functions as a Lys/Arg aminopeptidase (Hwang et al., 2007) with cathepsin H. The carboxypeptidase E (CPE) exopeptidase removes COOH-terminal basic residues from peptide intermediates generated by cathepsin L, as well as by the subtilisin-like prohormone convertases (PC1/3 and PC2). Thus, cathepsin H participates in proneuropeptide processing achieved by the cathepsin L and prohormone convertase protease pathways.
Production of (Met)enkephalin from the proenkephalin precursor utilizes cathepsin L and PC2, based on data from protease gene knockout mice (Yasothornsrikul et al., 2003; Miller et al., 2003a). Participation of cathepsin L, generating peptide intermediates with basic residue extensions at their N-termini, implicates roles for aminopeptidases to generate mature (Met)enkephalin. Such aminopeptidase activity of cathepsin H may be involved in enkephalin production. Notably, brain levels of (Met)enkephalin were reduced by 50% in the cathepsin H knockout mice compared to wild-type control mice. This data indicates that cathepsin H participates in enkephalin production. However, there is likely another aminopeptidase that can generate the 50% (Met)enkephalin remaining in the cathepsin H knockout mouse brain. Our previous studies have demonstrated aminopeptidase B (AP-B) as a processing protease in secretory vesicles for production of (Met)enkephalin and NPY peptide neurotransmitters (Hwang et al., 2007). Results show that AP-B and cathepsin H have similar time-courses for cleaving basic residues to generate (Met)enkephalin (Hwang et al., 2007). These findings indicate the presence of two Lys/Arg aminopeptidase in secretory vesicles for neuropeptide production, cathepsin H and AP-B (illustrated in Fig. 7). It will be of interest in future studies to investigate the distribution of cathepsin H and AP-B among brain and neuroendocrine tissue regions to compare their utilization among neuroendocrine cells.
The peptide neurotransmitter galanin is substantially reduced in cathepsin H knockout mouse brain cortex. These results implicate cathepsin L in processing progalanin. Indeed, cathepsin L knockout mice show significant reduction of galanin in brain that is decreased by about 60% compared to wild-type controls (supplemental figure F). These data support the hypothesis that cathepsin H participates in galanin production.
It is of interest that dynorphin A and β-endorphin peptide neurotransmitter levels in cathepsin H knockout mouse brains were not altered, indicating the selectivity of cathepsin H for producing galanin and (Met)enkephalin. While dynorphin A and β-endorphin utilize the endoprotease cathepsin L in their biosynthesis (Minokadeh et al., 2010; Funkelstein et al., 2008), it is hypothesized that subsequent removal of N-terminal basic residues may be achieved by aminopeptidase B or cathepsin H (Fig. 7). But since the cathepsin H knockout mice show no changes in brain levels of dynorphin A or β-endorphin, it is likely that AP-B may be involved in dynorphin and β-endorphin production. Results of this study indicate the selective nature of cathepsin H for producing (Met)enkephalin and galanin neuropeptides.
The biochemical properties of cathepsin H are compatible with its proposed function in secretory vesicles for neuropeptide production. The pH optimum of cathepsin H (pH 5.5 to 7.0) indicates that this enzyme is active within the internal internal pH environment of secretory vesicles of pH 5.5 to 6.5 (Hook et al., 2008). Cathepsin H activity occurs under reducing conditions (Kirschke, 2004) which is compatible with the in vivo reducing conditions within secretory vesicles that contain reducing factors including ascorbic acid and glutathione (Yasothornsrikul et al., 1999). These pH and reducing conditions for cathepsin H resembles that of aminopeptidase B (AP-B) that also possesses Lys/Arg aminopeptidase activity. However, inhibitors distinguish cathepsin H from AP-B, since inhibitors of AP-B (bestatin and arphemenine A) have no effect on cathepsin H. Cathepsin H and AP-B, thus, represent distinct Arg/Lys aminopeptidases in secretory vesicles for neuropeptide production. It will be of interest in future studies to learn how natural occuring inhibitors of cathepsin H, the cystatins (Kirschke, 2004), may be regulated in neuropeptide biosynthesis.
Results from this study illustrate a novel biological function for cathepsin H aminopeptidase activity in secretory vesicles, for production of an active peptide neurotransmitter. In addition to data of this study showing the presence of cathepsin H in secretory vesicles, an earlier investigation also demonstrated the presence of cathepsin H in secretory vesicles of neuroendocrine GH4C1 pituitary cells (Waguri et al., 1995). This biological function of cathepsin H contrasts with its known role in lysosomes for protein degradation (Turk et al., 1997; Kirschke, 2004). In fact, human cathepsin H purified from brain has been shown to degrade mature neuropeptide substrates (Brguljan et al., 2003). It is apparent that the function of cathepsin H depends on its secretory vesicle or lysosomal location.
Additional new biological functions of cathepsin H have been illustrated in the cathepsin H knockout mice. Cathepsin H null mice, achieved by gene targeting in embryonic stem cells, results in impairment of lung surfactant based on the role of cathepsin H in production of the pulmonary surfactant protein B (SP-B) (Buhling et al., 2011). Cathepsin H can act as an additional progranzyme B convertase, in addition to cathepsin C (D’Angelo et al., 2010). Also, deletion of the cathepsin H gene perturbs angiogneic switching, vascularization and growth of tumors in a mouse model of pancreatic islet cell cancer (Gocheva et al., 2010). These findings show that cathepsin H possesses biological functions in different physiological systems.
In summary, this study shows that cathepsin H functions as an aminopeptidase in secretory vesicles for the production of a peptide neurotransmitter, (Met)enkephalin. Cathepsin H is, thus, a new protease member of the cathepsin L pathway for processing proneuropeptides, together with the prohormone convertases (PC1/3 and PC2) for production of active peptide neurotransmitters and hormones.
Supplementary Material
Acknowledgments
The authors appreciate support of this research by grants to V. Hook consisting of R01DA04271, R01NS24553, and R01MH077305 from the NIH, and P01HL58120 (D. O’Connor, PI; V. Hook, leader for project 3) from NIH. W.D. Lu was supported by NIH T32 DA07315 training grant. Technical assistance by Charles Mosier for the research is appreciated. There are no conflicts of interest.
References
- Brguljan PM, Turk V, Nina C, Brzin J, Krizaj I, Popovic T. Human brain cathepsin H as a neuropeptide and bradykinin metabolizing enzyme. Peptides. 2003;24:1977–1984. doi: 10.1016/j.peptides.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Bühling F, Kouadio M, Chwieralski CE, Kern U, Hohlfeld JM, Klemm N, Friedrichs N, Roth W, Deussing JM, Peters C, Reinheckel T. Gene targeting of the cysteine peptidase cathepsin H impairs lung surfactant in mice. PLoS One. 2011;6:e26247. doi: 10.1371/journal.pone.0026247. Epub 2011 Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo ME, Bird PI, Peters C, Reinheckel T, Trapani JA, Sutton VR. Cathepsin H is an additional convertase of pro-granzyme B. J. Biol. Chem. 2010;285:20514–20519. doi: 10.1074/jbc.M109.094573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulon T, Cadel S, Piesse C, Cohen P. Aminopeptidase B. In: Barrett AJ, Rawlings ND, Woessner JF, editors. Handbook of Proteolytic Enzymes. 2nd edition 2004. pp. 328–330. [Google Scholar]
- Fricker LD. Carboxypeptidase E. Annu. Rev. Physiol. 1988;50:309–21. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- Funkelstein L, Beinfeld M, Minokadeh A, Zadina J, Hook V. Unique biological function of cathepsin L in secretory vesicles for biosynthesis of neuropeptides. Neuropeptides. 2010;44:457–66. doi: 10.1016/j.npep.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkelstein L, Toneff T, Hwang SR, Beuschlein F, Lichtenauer UD, Reinheckel T, Peters C, Hook V. Major role of cathepsin L for producing the peptide hormones ACTH, β-endorphin, and α-MSH, illustrated by protease gene knockout and expression. J. Biol. Chem. 2008;83:35652–35659. doi: 10.1074/jbc.M709010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocheva V, Chen X, Peters C, Reinheckel T, Joyce JA. Deletion of cathepsin H perturbs angiogenic switching, vascularization and growth of tumors in a mouse model of pancreatic islet cell cancer. Biol. Chem. 2010;391:937–945. doi: 10.1515/BC.2010.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Bark SJ, Lu WD, Taupenot L, O’Connor DT, Pevzner P, Hook V. Mass spectrometry-based neuropeptidomics of secretory vesicles from human adrenal medullary pheochromocytoma reveals novel peptide products of prohormone processing. J. Proteome Res. 2010;9:5065–75. doi: 10.1021/pr100358b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustein HB, Akil H. Opioid analgesics. In: Brunton LL, Lazo JS, Parker KL, editors. The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 2006. pp. 547–590. [Google Scholar]
- Hook V, Funkelstein L, Lu D, Bark S, Wegrzyn J, Hwang SR. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu. Rev. Pharmacol. Toxicol. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SR, O’Neill A, Bark S, Foulon T, Hook V. Secretory vesicle aminopeptidase B related to neuropeptide processing: molecular identification and subcellular localization to enkephalin- and NPY-containing chromaffin granules. J. Neurochem. 2007;100:1340–50. doi: 10.1111/j.1471-4159.2006.04325.x. [DOI] [PubMed] [Google Scholar]
- Kastin A. Handbook of Biologically Active Peptides. Academic Press; San Diego: 2006. [Google Scholar]
- Kim Y, Bark S, Hook V, Bandeira N. NeuroPedia: neuropeptide database and spectral library. Bioinformatics. 2011;27:2772–3. doi: 10.1093/bioinformatics/btr445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H. Cathepsin H. In: Barrett AJ, Rawlings ND, Woessner JF, editors. Handbook of Proteolytic Enzymes. Elsevier Academic Press; Amersterdam: 2004. pp. 1089–1092. [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu. Rev. Pharmacol. Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Koob GF, Zorrila EP. Role of corticotropin-releasing factor in drug addiction: potential for pharmacological intervention. CNS Drugs. 2011;25:271–287. doi: 10.2165/11587790-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manders EMM, Verbeek FJ, Aten JA. Measurement of co-localization of objects in dual-colour confocal images. J. Microscopy. 1993;169(Pt 3):373–382. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- Mbikay M, Seidah NG. Proprotein convertases. Human Press Inc.; New York: 2011. [Google Scholar]
- Miller R, Toneff T, Vishnuvardhan D, Beinfeld M, Hook VY. Selective roles for the PC2 processing enzyme in the regulation of peptide neurotransmitter levels in brain and peripheral neuroendocrine tissues of PC2 deficient mice. Neuropeptides. 2003a;37:140–8. doi: 10.1016/s0143-4179(03)00027-1. [DOI] [PubMed] [Google Scholar]
- Miller R, Aaron W, Toneff T, Vishnuvardhan D, Beinfeld MC, Hook VY. Obliteration of alpha-melanocyte-stimulating hormone derived from POMC in pituitary and brains of PC2-deficient mice. J. Neurochem. 2003b;86:556–63. doi: 10.1046/j.1471-4159.2003.01856.x. [DOI] [PubMed] [Google Scholar]
- Minokadeh A, Funkelstein L, Toneff T, Hwang SR, Beinfeld M, Reinheckel T, Peters C, Zadina J, Hook V. Cathepsin L participates in dynorphin production in brain cortex, illustrated by protease gene knockout and expression. Mol Cell Neurosci. 2010;43:98–107. doi: 10.1016/j.mcn.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Nomura S, Okada M, Ito T, Katsumata Y, Kikkawa F, Hattori A, Tsujimoto M, Mizutani S. Immunoaffinity purification and characterization of native placental leucine aminopeptidase/oxytocinase from human placenta. Placenta. 2000;21:528–634. doi: 10.1053/plac.2000.0564. [DOI] [PubMed] [Google Scholar]
- Pham VL, Cadel MS, Gouzy-Darmon G, Hanquez C, Beinfeld MC, Nicolas P, Etchebest C, Foulon T. Aminopeptidase B, a glucagon-processing enzyme: siste directed mutagenesis of the Zn2+-binding motif anmd molecular modelling. BMC Biochemistry. 2007;8:21. doi: 10.1186/1471-2091-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos EJ, Meguid MM, Campos AC, Coelho JC. Neuropeptide Y, alpha-melanocyte-stimulating hormone, and monoamines in food intake regulation. Nutrition. 2005;21:69–79. doi: 10.1016/j.nut.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Robinson JK. Galanin and cognition. Behav. Cogn. Neurosci. Rev. 2004;3:222–242. doi: 10.1177/1534582305274711. [DOI] [PubMed] [Google Scholar]
- Rothe M, Dodt J. Studies on the aminopeptidase activity of rat cathepsin H. Eur. J. Biochem. 1992;210:759–764. doi: 10.1111/j.1432-1033.1992.tb17478.x. [DOI] [PubMed] [Google Scholar]
- Scamuffa N, Calvo F, Chrétien M, Seidah NG, Khatib AM. Proprotein convertases: lessons from knockouts. FASEB J. 2006;20:1954–63. doi: 10.1096/fj.05-5491rev. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Mayer G, Zaid A, Rousselet E, Nassoury N, Poirier S, Essalmani R, Prat A. The activation and physiological functions of the proprotein convertases. Int. J. Biochem. Cell Biol. 2008;40:1111–1125. doi: 10.1016/j.biocel.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Steiner RA, Hohmann JG, Holmes A, Wrenn CC, Cadd G, Jureus A, Clifton DK, Luo M, Gutshall M, Ma SY, Mufson EJ, Crawley JN. Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2001;98:4184–4189. doi: 10.1073/pnas.061445598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk B, Turk V, Turk D. Structural and functional aspects of papain-like cysteine proteinases and their protein inhibitors. Biol. Chem. 1997;378:141–150. [PubMed] [Google Scholar]
- Waguri S, Sato N, Watanabe T, Ishidoh K, Kominami E, Sato K, Uchiyama Y. Cysteine proteinases in GH4C1 cells, a rat pituitary tumor cell line, are secreted by the constitutive and regulated secretory pathways. Eur. J. Cell Biol. 1995;67:308–18. [PubMed] [Google Scholar]
- Wardman J, Fricker LD. Quantitative peptidomics of mice lacking peptide-processing enzymes. Methods Mol. Biol. 2011;768:307–23. doi: 10.1007/978-1-61779-204-5_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegrzyn JL, Bark SJ, Funkelstein L, Mosier C, Yap A, Kazemi-Esfarjani P, La Spada AR, Sigurdson C, O’Connor DT, Hook V. Proteomics of dense core secretory vesicles reveal distinct protein categories for secretion of neuroeffectors for cell-cell communication. J Proteome Res. 2010;9:5002–24. doi: 10.1021/pr1003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasothornsrikul S, Aaron W, Toneff T, Hook VYH. Evidence for the proenkephalin processing enzyme prohormone thiol protease (PTP) as a multicatalytic cysteine protease complex: activation by glutathione localized to secretory vesicles. Biochemistry. 1999;38:7421–7430. doi: 10.1021/bi990239w. [DOI] [PubMed] [Google Scholar]
- Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Toneff T, Bundey R, Miller R, Schilling B, Petermann I, Dehnert J, Logvinova A, Goldsmith P, Neveu JM, Lane WS, Gibson B, Reinheckel T, Peters C, Bogyo M, Hook V. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc. Natl. Acad. Sci. U S A. 2003;100:9590–9595. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bijker MS, Herzog H. The neuropeptide Y system: pathophysiological and therapeutic implications in obesity and cancer. Pharmacol. Ther. 2011;131:91–113. doi: 10.1016/j.pharmthera.2011.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


