Abstract
V1a vasopressin receptor (V1aR) and V2 vasopressin receptor (V2R) present distinct mechanisms of agonist-promoted trafficking. Although both receptors are endocytosed by way of β-arrestin-dependent processes, β-arrestin dissociates rapidly from V1aR, allowing its rapid recycling to the plasma membrane while β-arrestin remains associated with V2R in the endosomes, leading to their intracellular accumulation. Here, we demonstrate that, when coexpressed, the two receptors can be endocytosed as stable heterodimers. On activation with a nonselective agonist, both receptors cotrafficked with β-arrestin in endosomes where the stable interaction inhibited the recycling of V1aR to the plasma membrane, thus conferring a V2R-like endocytotic/recycling pattern to the V1aR/V2R heterodimer. Coexpression of the constitutively internalized R137HV2R mutant with V1aR was sufficient to promote cointernalization of V1aR in β-arrestin-positive vesicles even in the absence of agonist stimulation. This finding indicates that internalization of the heterodimer does not require activation of each of the protomers. Consistent with this notion, a V1aR-selective agonist led to the coendocytosis of V2R. In that case, however, the V1aR/V2R heterodimer was not stably associated with β-arrestin, and both receptors were recycled back to the cell surface, indicating that the complex followed the V1aR endocytotic/recycling path. Taken together, these results suggest that heterodimerization regulates the endocytotic processing of G protein-coupled receptors and that the identity of the activated protomer within the heterodimer determines the fate of the internalized receptors.
Keywords: endosomes, G protein-coupled receptors, internalization, oligomerization, recycling
G protein-coupled receptors (GPCR) represent the largest superfamily of plasma membrane proteins involved in signal transduction (1). They play major roles in regulating many physiological functions including vision, olfaction, taste, fluid and electrolyte balance, neurotransmission, and cardiovascular function. On stimulation, GPCR undergo conformational rearrangements that allow their coupling to G proteins and the activation of different intracellular effectors. In many cases, receptor activation also promotes the recruitment of β-arrestin to the receptor where it leads to signal termination by blocking G protein interaction and initiating receptor internalization through its interaction with clathrin and the adaptor protein AP-2 (2, 3). On the basis of their interaction with β-arrestin and their internalization patterns, GPCR can be divided into two distinct classes (4, 5). Class A receptors bind to β-arrestin 2 with higher affinity than β-arrestin 1 whereas class B GPCR bind to the two β-arrestins with the same affinity. After its initial recruitment, β-arrestin rapidly dissociates from class A GPCR during their internalization whereas it forms a stable complex with class B GPCR leading to their colocalization in endosomes. The stability of the receptor-β-arrestin interaction has been proposed to dictate the fate of the internalized receptor. After the dissociation from β-arrestin, class A GPCR can be rapidly dephosphorylated in the endosomes and recycled back to the plasma membrane. In contrast, class B GPCR are retained intracellularly, targeted to a perinuclear compartment, and recycle very slowly if at all (4, 6). An alternative β-arrestin-independent endocytotic pathway involving caveolae is also implicated in the internalization of some GPCR (7-10). Recently, homo- and heterodimerization have been recognized as common features of GPCR that can regulate several aspects of their function (11, 12). Although several studies have suggested that dimerization could influence agonist-promoted internalization (13-21), the consequence of heterodimerization between receptors that have distinct endocytosis/recycling profiles has not been investigated yet. We took advantage of our recent discovery that the class A V1a vasopressin receptor (V1aR) can heterodimerize with the class B V2 vasopressin receptor (V2R) (22) to determine the outcome of such oligomeric assembly on the pattern of receptor endocytosis/recycling.
Materials and Methods
Materials. Mouse anti-Myc (9E10) and anti-hemagglutinin (HA) (12CA5) antibodies were produced by our core facility as ascite fluids. Rabbit anti-Myc (A14), goat anti-rabbit coupled to Texas red and goat anti-mouse coupled to Alexa 633 antibodies were from Santa Cruz Biotechnology. All other reagents were of analytical grade and obtained from various commercial suppliers.
Expression Plasmids. Myc-V2R, Myc-V1aR, and HA-V1aR. The full-length cDNA encoding the human V2R and V1aR were subcloned into the eukaryotic expression vector pRK5. PCR-based mutagenesis was used to incorporate the Myc or the HA epitope after the initiating Met codon of the receptors as reported (22). HA-R137HV2R. The R137H mutant expressed in pEGFP-N3 (Clontech), with the receptor stop codon intact to prevent the enhanced green fluorescent protein (EGFP) expression, was a generous gift of M. Caron (23).
β-arrestin 2-yellow fluorescent protein (YFP). The rat β-arrestin 2 subcloned into pGFP-N1-Topaz (Perkin-Elmer) vector was constructed as described (24).
Myc-β2 adrenergic receptor (β2AR) and HA-β2AR. PCR-based mutagenesis was used to incorporate the Myc or the HA epitope at the amino terminus of the human β2AR coding sequence subcloned into pcDNA3 vector as reported (18, 24).
All constructs were verified by direct DNA sequencing.
Cell Culture and Transfection. Human embryonic kidney (HEK) 293T cells were grown in DMEM supplemented with 10% FBS, 100 units/ml penicillin/streptomycin, and 2 mM l-glutamine at 37°C in a humidified atmosphere at 95% air and 5% CO2. For immunofluorescence experiments, 3 × 105 cells were seeded in each well of a six-well plate, and transient transfections were performed by using the calcium phosphate precipitation method (25). For ELISA assays, 2 × 106 cells were plated per 100-mm Petri dishes and, to ensure a high efficiency of transfection, the indicated plasmids were transfected by using FuGENE6 (Roche Molecular Biochemicals) as described in the manufacturer's protocol. Cells were then transferred into polylysine-coated 12-well plates to perform the internalization assay.
Immunofluorescence Microscopy. Forty-eight hours after transfection, HEK 293T cells were incubated with rabbit polyclonal antibody A14 and/or mouse monoclonal antibody 12CA5 for 1 h at 4°C. After three washes at 4°C, cells were treated for 30 min at 37°C in the presence or absence of the appropriate agonist [100 nM arginine-vasopressin (AVP) or 600 nM F180]. Cells were then washed, fixed, and permeabilized before the addition of a secondary goat anti-rabbit antibody coupled to Texas red (for the Myc-tagged form) and/or a goat anti-mouse antibody coupled to Alexa 633 (for the HA-tagged form). The samples were analyzed by confocal laser-scanning microscopy by using a Leica TCS SP1 confocal microscope. Excitation and emission filters for the different labeled dyes were as follows: YFP (green): λex = 488 nm; λem = 540/25 nm; Texas red (red): λex = 568 nm; λem = 610/30 nm; Alexa 633 (blue): λex = 633 nm; λem = 705/45 nm. In the case of immunofluorescence experiments involving the R137HV2R mutant, cells were pretreated for 16 h with saturating concentration of the nonpeptidic vasopressin antagonist SR49059 to increase the cell surface expression of the mutant, thus facilitating the cell surface labeling of the receptor (26).
ELISA. Forty-eight hours posttransfection, cells were treated or not with the appropriate agonist (100 nM AVP or 600 nM F180) for 30 min at 37°C. After two washes, cells were fixed and incubated in blocking solution (PBS/0.2% BSA) for 15 min at room temperature. Cells were kept at room temperature for all subsequent steps. Cells were then incubated with anti-Myc (9E10) or anti-HA (12CA5) antibodies (1/500 dilution) for 30 min. After three PBS/0.2% BSA washes, cells were incubated with anti-mouse/horseradish peroxidase (HRP) conjugate 1/500 (Amersham Pharmacia Biotech). After extensive washing, the immunoreactivity was revealed by the addition of the HRP substrate according to manufacturer's instructions. For each experiment, mock conditions corresponding to cells transfected with empty vector were included. The percentage of internalization is defined as 100 × [(ODbasal - ODmock) - (ODstimulated - ODmock)]/(ODbasal - ODmock) where ODstimulated and ODbasal correspond to the OD obtained with agonist treated and nontreated cells, respectively. For kinetic analysis of receptor recycling, the agonist treatment was followed by extensive washes with PBS and acidic buffer (150 mM NaCl/5 mM acetic acid) at 4°C to remove all bound ligand. Cells were then transferred back to 37°C in DMEM for different times of recycling (40 or 120 min). The percentage of recycling is defined as 100 × [(ODrecycling - ODmock) - (ODstimulated - ODmock)]/[(ODbasal - ODmock) - (ODstimulated - ODmock)] where ODrecycling corresponds to the OD obtained after recycling. Triplicates were performed for each condition within an experiment.
Radio Ligand-Binding Assays. HEK 293T cells transfected with Myc-V2R or HA-V1aR were split in 12-well polylysine-coated plates 24 h after transfection. At 48 h posttransfection, cells were exposed to 20 nM [3H]AVP in 250 μl of binding medium (PBS, 1 mM tyrosine, 1 mM phenylalanine, 1 mg/ml glucose, 10 mg/ml BSA). Nonspecific binding was determined in the presence of 10 μM unlabeled AVP. Binding experiments were performed in triplicate. Radio ligand-binding assays were performed in parallel with ELISA experiments, on whole cells, to generate calibration curves between the OD measured in ELISA and the receptor density at the cell surface. The curves generated were used to estimate the number of receptors expressed in all endocytosis and recycling experiments.
Results and Discussion
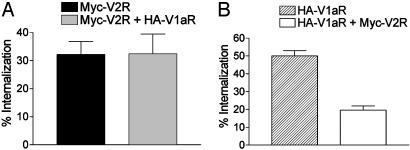
Heterologous Inhibition of V1aR Endocytosis by Activation of Coexpressed V2R. To study V1aR and V2R endocytosis, receptors were tagged to their N terminus with HA and Myc epitopes, respectively, and transiently transfected in HEK 293T cells. As reported (22), the presence of the tags did not significantly affect the binding properties of the receptors. Internalization was quantified by ELISA after treatment of the cells with saturating concentration of the agonist AVP (100 nM) or vehicle at 37°C during 30 min. As shown in Fig. 1, when the two receptors were expressed separately, agonist treatment induced a pronounced internalization of both V2R (32 ± 5%) and V1aR (50 ± 3%). On coexpression of the receptors, the extent of V2R internalization remained the same (32 ± 7%) whereas that of V1aR was reduced by 60% reaching only 20 ± 2%. This effect was not due to the relative expression levels of the two receptors because identical results were obtained for different V1aR/V2R ratios of expression (data not shown). Such nonreciprocal heterologous inhibition of V1aR internalization by V2R could be explained by the endocytotic properties of each receptor. Indeed, β-arrestin dissociates from V1aR at the plasma membrane before the internalization of the receptor and rapidly returns to the cytosol where it can be reused for the next round of endocytosis whereas β-arrestin traffics into the endosomes with V2R, thus decreasing its availability (27). Therefore, as already suggested for the inhibition of the β2AR endocytosis observed in the presence of V2R (28), the sequestration of β-arrestin by V2R most likely inhibits V1aR internalization by depleting the cytosol pool of β-arrestin.
Fig. 1.
Quantitative assessment of V2R and V1aR internalization induced by AVP. HEK 293T cells expressing Myc-V2R, HA-V1aR, or Myc-V2R plus HA-V1aR were treated or not with 100 nM AVP for 30 min at 37°C to promote internalization of the receptors. The cell surface Myc epitope-tagged V2R (A)orHA epitope-tagged V1aR (B) were detected by ELISA as described in Materials and Methods. The extent of internalization was determined by measuring the cell surface receptor before and after AVP stimulation and was expressed as the loss of cell surface expression (percentage of control). Experimental conditions were established so that V2R and V1aR were expressed at ≈200 fmol per well and 100 fmol per well, respectively, whether expressed alone or in combination. All values correspond to the mean ± SEM calculated from at least five independent experiments.
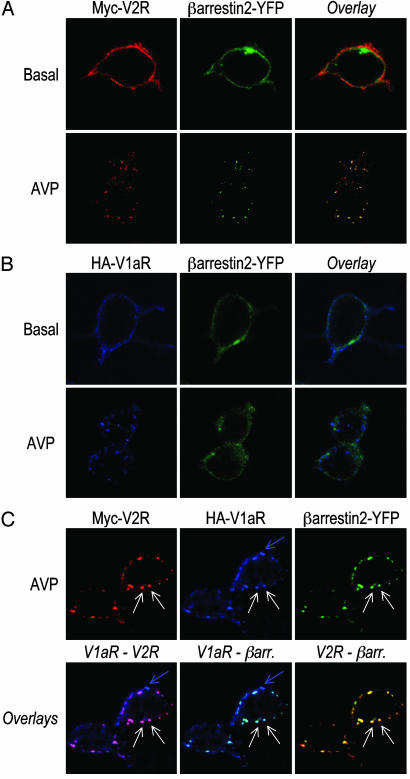
Coexpressed V1aR and V2R Enter the Class B GPCR Endocytotic Pathway After Nonselective Activation. To determine whether coexpression of V1aR and V2R would affect the endocytotic profile of each receptor, HEK 293T cells were transfected with Myc-V2R, HA-V1aR, or Myc-V2R plus HA-V1aR in the presence of a functional β-arrestin 2-YFP to simultaneously visualize the localization of each protein by confocal microscopy. Under basal conditions, individually expressed V1aR and V2R were visualized at the plasma membrane whereas β-arrestin 2 was distributed throughout the cytoplasm (Fig. 2 A and B). On exposure to the nonselective agonist AVP (30 min), both V1aR and V2R redistributed from the plasma membrane to endocytotic vesicles. However, whereas β-arrestin 2 extensively colocalized with V2R in the endosomes, it was excluded from the ones containing V1aR (Fig. 2 A and B). This result confirms that V2R belongs to class B GPCR showing high affinity for β-arrestin and internalization with β-arrestin in endosomes whereas V1aR belongs to class A GPCR forming a low-affinity unstable complex with β-arrestin that dissociates near or at the plasma membrane. After coexpression of V2R, V1aR, and β-arrestin 2, AVP treatment led to the apparition of two populations of endocytotic vesicles (Fig. 2C). V2R and V1aR were found to colocalize extensively in the same population of vesicles with β-arrestin 2 (see white arrows) whereas some intracellular vesicles contained V1aR alone without any V2R or β-arrestin 2 (see blue arrow). This result indicates that, after stimulation of the two receptors with a nonselective agonist, coexpression of V2R promoted an apparent change in the endocytotic behavior of a large fraction of V1aR that followed an endocytotic pathway typical of class B GPCR. Given the fact that V1aR and V2R are able to form constitutive homo- and heterodimers with the same propensity (22), two hypotheses could be formulated to explain these results: (i) the two receptors are internalized as separate entities but could use the same endosomes; and (ii) V1aR and V2R are internalized as a heterodimeric complex that belongs to the class B type whereas V1aR not engaged in dimerization with V2R still internalizes as a class A GPCR.
Fig. 2.
Cellular trafficking of internalized V2R and V1aR with coexpressed β-arrestin 2-YFP. HEK 293T cells transfected with Myc-V2R (A), HA-V1aR (B), or Myc-V2R plus HA-V1aR (C) in combination with β-arrestin 2-YFP were incubated with rabbit polyclonal antibody A14 and/or mouse monoclonal antibody 12CA5 for 1 h at 4°C. Next, cells were treated for 30 min at 37°C in the presence or absence of 100 nM AVP, fixed, permeabilized, and then labeled with goat anti-rabbit antibody coupled to Texas red (V2R) or goat anti-mouse antibody coupled to Alexa 633 (V1aR). The samples were analyzed by confocal laser-scanning microscopy as described in Material and Methods. In C, white arrows indicate endosome where both V2R and V1aR colocalize with β-arrestin 2, whereas the blue arrow indicates an intracellular vesicle that contains only V1aR without any V2R or β-arrestin 2.
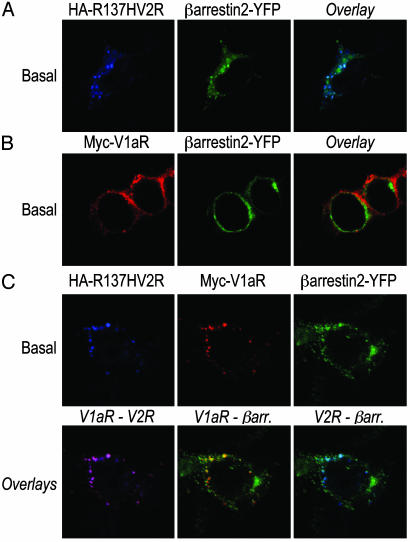
The Constitutively Internalized R137HV2R Mutant Brings V1aR Inside the Cell in Absence of Agonist. To determine whether V2R/V1aR heterodimerization could be responsible for the colocalization of the two receptors in β-arrestin-positive vesicles, we took advantage of a well characterized V2R mutant (R137HV2R) that is known to undergo constitutive internalization as a result of its spontaneous interaction with β-arrestin (23). In the absence of agonist, R137HV2R was found in endocytotic vesicles where it colocalized extensively with β-arrestin 2 (Fig. 3A). This markedly contrasts with the plasma membrane localization of V1aR expressed alone in the same conditions (Fig. 3B). However, coexpression of R137HV2R with V1aR led to the cointernalization of the two receptors in the same endosomes. Indeed, even in the absence of agonist stimulation, V1aR was found to colocalize with both R137HV2R and β-arrestin 2 (Fig. 3C).
Fig. 3.
Constitutive internalization of V1aR when coexpressed with R137HV2R. HEK 293T cells transfected with HA-R137HV2R (A), Myc-V1aR (B), or HA-R137HV2R plus Myc-V1aR (C) in combination with β-arrestin 2-YFP were incubated with mouse monoclonal antibody 12CA5 and/or rabbit polyclonal antibody A14 for 1 h at 4°C. After a 30-min incubation at 37°C in the absence of any agonist, cells were fixed, permeabilized, and labeled with Texas red-conjugated goat anti-rabbit and/or Alexa 633-conjugated goat anti-mouse to observe V1aR and R137HV2R, respectively.
The observation that coexpression of the constitutively internalized R137HV2R mutant is sufficient to promote endocytosis of V1aR in the absence of activation of the receptors discredits the hypothesis that V1aR and V2R are internalized as separate entities that then use the same endosomes. Rather, these results demonstrate that V1aR and V2R can be endocytosed as heterodimers by way of the class B GPCR endocytotic pathway. Taken with the observation that homo- and heterodimers between V2R and V1aR can form with the same propensity (22), coexpression of V1aR and V2R, in the same cell, most likely leads to three receptor subpopulations (V1aR homodimers, V2R homodimers, and V1aR/V2R heterodimers). After AVP treatment, the V1aR homodimers and the V1aR engaged in dimeric complex with V2R present opposite patterns of clathrin-mediated endocytosis in view of their association with β-arrestin. This finding can be clearly appreciated in the immunofluorescence experiments shown in Fig. 2C (see blue arrow vs. white arrows).
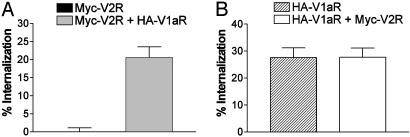
V1aR Selective Activation Leads to the Internalization of the V1aR-V2R Heterodimer. We then examine the effect of V1aR selective activation on the endocytosis of the two receptors by using the V1aR selective agonist, F180 (29). In cells expressing each of the receptor individually, F180 promoted a significant endocytosis of V1aR (28 ± 4%) but did not affect cell surface expression of V2R (Fig. 4), consistent with the selective action of the ligand. In contrast, coexpression of the two receptors led to a marked F180-promoted endocytosis of V2R (21 ± 3%) (Fig. 4A) whereas the extent of V1aR internalization remained identical to that observed in cells expressing this receptor alone (28 ± 3%) (Fig. 4B), suggesting that activation of V1aR is sufficient to bring V2R inside the cell. Although one cannot completely exclude that such internalization of V2R could result from heterologous desensitization, the absence of any phosphorylation site for PKC or PKA in the V2R sequence (30) rather suggests that the physical interaction between receptors underlies this cointernalization. The selectivity of this effect is further supported by the observation that activation of V1aR did not promote the endocytosis of the unrelated β2AR, coexpressed in the same cells. Similarly, activation of β2AR with the agonist isoproterenol led to its own endocytosis but was not accompanied by any change in the cell surface expression of coexpressed V2R (data not shown).
Fig. 4.
Quantitative assessment of V2R and V1aR internalization induced by F180. HEK 293T cells expressing Myc-V2R, HA-V1aR, or Myc-V2R plus HA-V1aR were treated or not with the V1aR selective agonist F180 (600 nM) at 37°C for 30 min to promote internalization of the receptors. The cell surface Myc epitope-tagged V2R (A) or HA epitope-tagged V1aR (B) were detected by ELISA as described under Materials and Methods. The extent of internalization was determined by measuring the cell surface receptor before and after F180 stimulation and was expressed as the loss of cell surface expression (percentage of control). Experimental conditions were established so that V2R and V1aR were expressed at ≈150 fmol per well whether expressed alone or in combination. All values correspond to the mean ± SEM calculated from at least seven independent experiments.
We previously reported that no cross-signaling occurs within the V1aR/V2R heterodimer. Indeed, selective activation of Gq-coupled V1aR with F180 did not induce any activation of V2R-coupled Gs because no change in adenylyl cyclase activity was detected despite a robust inositol phosphate production (22). This finding indicates that endocytosis of V2R, as part of the V1aR/V2R heterodimer, occurs in the absence of V2R activation, thus suggesting that activation of only one protomer is sufficient to promote internalization of the complex.
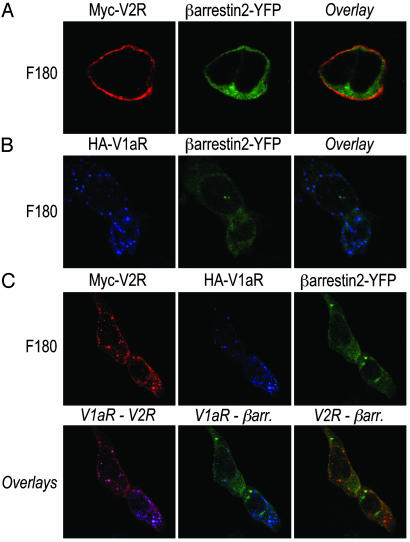
Coexpressed V1aR and V2R Enter the Class A GPCR Endocytotic Pathway After Selective Activation of V1aR. To precise the endocytotic pathway followed by the V1aR/V2R heterodimers after F180 stimulation, immunofluorescence experiments were performed. As expected, individually expressed V2R remained at the plasma membrane whereas β-arrestin 2 was distributed throughout the cytoplasm after F180 treatment (Fig. 5A). In contrast, F180 promoted V1aR trafficking from the plasma membrane to intracellular vesicles lacking β-arrestin 2 (Fig. 5B). When the two receptors were coexpressed in the same cells, two populations of V2R could be distinguished according to their subcellular localization after F180 treatment. Indeed, some V2R remained at the plasma membrane (V2R homodimers) whereas others were concentrated in vesicles containing V1aR but exempt of β-arrestin (V2R/V1aR heterodimers) (Fig. 5C). The observation that, on V1aR activation, V2R is cointernalized according to the class A endocytotic pattern clearly indicates that the identity of the activated protomer within the heterodimer determines the nature of the endocytotic path followed by the complex. One could also predict that, unlike what is observed after AVP stimulation, F180-promoted V2R endocytosis is not accompanied by a depletion of the cytoplasmic β-arrestin. Consistent with this notion, V2R coendocytosis did not decrease the extent of V1aR internalization promoted by F180 whereas it significantly inhibited that induced by AVP (compare Fig. 4 with Fig. 1).
Fig. 5.
Cellular trafficking of internalized V2R and V1aR with coexpressed β-arrestin 2-YFP after F180 treatment. HEK 293T cells transfected with β-arrestin 2-YFP in the presence of Myc-V2R (A), HA-V1aR (B), or Myc-V2R plus HA-V1aR (C) were incubated with rabbit polyclonal anti-Myc antibody A14 and/or mouse monoclonal anti-HA antibody 12CA5 for 1 h at 4°C. After treatment with 600 nM F180 for 30 min at 37°C, cells were fixed, permeabilized, and labeled with Texas red-conjugated goat anti-rabbit and/or Alexa 633-conjugated goat anti-mouse to visualize V2R and V1aR, respectively.
The difference in the β-arrestin redistribution patterns between class A and class B GPCR has been attributed to different strengths of interaction between the regulatory protein and the receptors. The lack of colocalization of class A GPCR with β-arrestin in endocytotic vesicles is believed to result from a rapid dissociation of β-arrestin from the receptor during internalization. Reciprocally, the ability of β-arrestin to remain associated with class B GPCR during endocytosis most likely reflects a stable high affinity interaction and slow dissociation (4, 5). Therefore, when considering the V1aR/V2R heterodimer, one could predict that the ability of the complex to stably interact with β-arrestin should determine whether a class A or class B endocytotic pattern is observed. On selective activation of V1aR, the lack of cross-activation of V2R leads to only the weak β-arrestin interaction characteristic of V1aR, thus resulting into class A endocytosis. In the case of the R137HV2R, the constitutive association with β-arrestin is sufficient to induce the internalization of the complex according to the class B phenotype. Finally, for the nonselective activation of the two receptors within the dimer, the stable interaction between β-arrestin and V2R would predominate over the transient one with V1aR, thus conferring a class B endocytosis to the V1aR/V2R heterodimer. Following this line of reasoning, the data suggest that the interaction of a single protomer with β-arrestin is sufficient to support dimer internalization.
The Recycling Efficiency of the V1aR/V2R Heterodimer Is Determined by the Trafficking Pattern of the Activated Protomer. After agonist-induced internalization, V1aR has been shown to recycle rapidly to the cell surface by direct sorting from the early endosomes whereas V2R accumulates intracellularly in the perinuclear recycling compartment for hours (27). Given that these distinct recycling behaviors are believed to reflect the type of interaction between the receptor and β-arrestin, the distinct cross-internalization profiles observed for the V1aR/V2R complex begs the question of the recycling path followed by the heterodimer. To determine the faith of the V1aR/V2R heterodimer after internalization, HEK 293T cells were transiently transfected with Myc-V2R, HA-V1aR, or Myc-V2R plus HA-V1aR and the cell surface reappearance of internalized receptors monitored by ELISA.
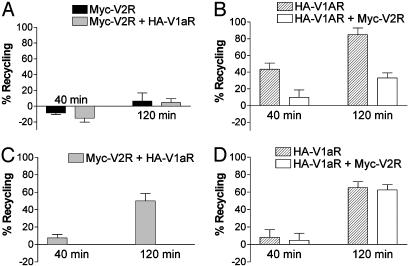
As expected, after AVP-promoted internalization, V2R expressed alone was unable to efficiently recycle to the cell surface (only 7% of recycling after 120 min) (Fig. 6A). In contrast, individually expressed V1aR recycled to the cell surface with high efficiency (44% of recycling after 40 min and 85% after 120 min) (Fig. 6B). When the two receptors were coexpressed, recycling of V2R after AVP stimulation was still very inefficient (4% of recycling at 120 min) (Fig. 6A) whereas recycling of V1aR was greatly impaired (10% and 33% of recycling at 40 min and 120 min respectively) (Fig. 6B). This observation indicates that, after the nonselective stimulation of the two receptors, the V1aR/V2R heterodimer, like V2R, is trapped inside the cell and fails to efficiently recycle back to the cell surface. The V1aR able to recycle to the cell surface probably corresponds to V1aR not engaged in heterodimerization with V2R (see blue arrow in Fig. 2C).
Fig. 6.
Recycling efficiency of V2R and V1aR after AVP- or F180-promoted internalization. HEK 293T cells transfected with Myc-V2R, HA-V1aR, or Myc-V2R plus HA-V1aR were treated with 100 nM AVP (A and B) or 600 nM F180 (C and D) at 37°C for 30 min to promote internalization of the receptors. The ligand remaining at the cell surface was removed by cold PBS and acidic washes; then, fresh medium was added, and cells were reincubated at 37°C for 40 or 120 min to allow recycling of the receptors. The differentially epitope-tagged receptors present at the cell surface before and after ligand treatment, as well as after incubation at 37°C, were then assessed by ELISA. (A and C) Assessment of Myc-V2R recycling after AVP-promoted (A) or F180-promoted (C) endocytosis in absence or presence of V1aR. (B and D) Assessment of HA-V1aR recycling after AVP-promoted (B) or F180-promoted (D) endocytosis in absence or presence of V2R. All values correspond to the mean ± SEM calculated from at least four independent experiments.
In contrast with its intracellular accumulation observed after AVP treatment, V2R efficiently recycled back to the plasma membrane when it had been cointernalized with V1aR after stimulation with the V1aR selective agonist F180 (50 ± 9% of recycling after 2 h compared with 4-7% with AVP) (Fig. 6C). Reciprocally, the recycling of V1aR after F180-promoted internalization was unaffected by the coexpression of V2R (65 ± 7% and 63 ± 6% of recycling after 2 h for V1aR expressed alone or with V2R, respectively) (Fig. 6D). Therefore, contrary to the intracellular retention of the V1aR/V2R heterodimer after AVP stimulation, the selective activation of V1aR within the heterodimer allows the efficient recycling of the complex to the plasma membrane. These results suggest that the recycling path used is not an independent intrinsic property of the receptor but depends on the nature of the interacting partners in the endocytotic vesicles, the strength of the association with β-arrestin most likely determining the fate of the receptor complex.
Such regulation of V1aR and V2R recycling efficiency due to their heterodimerization could have particular functional consequences in cells where these receptors are naturally coexpressed. Although coexpression in the same cells has not been confirmed yet, V1aR and V2R are both found in the collecting duct of the kidney (31), opening the possibility that heterodimerization could have an impact on the normal physiology of these receptors. For instance, the AVP-induced internalization and recycling of V1aR could be drastically different in cells coexpressing or not V2R. Also of potential significance is the fact that, in addition to AVP, oxytocin (OT) shows a good affinity for the vasopressin receptors. Whereas AVP nonselectively activates V1aR and V2R with the same affinity, OT displays a 10-fold higher affinity for V1aR than for V2R. It follows that, in cells coexpressing the two receptors, the endocytosis and recycling properties of the heterodimer could vary according to the activating hormone.
Our results clearly show that the V1aR/V2R heterodimerization plays a critical role in regulating the internalization and recycling properties of the receptors, the identity of the activated protomer determining the fate of the heterodimer. If such cross-regulation of the endocytotic pathways is a general trait of GPCR, heterodimerization could have dramatic implications for the regulation of hormone responsiveness in cells naturally coexpressing interacting GPCR.
Acknowledgments
We thank Dr. Pierre Rivière (Ferring Research Inc.) and Dr. Claudine Serradeil-Legal (Sanofi-Synthélabo) for kindly providing F180 and SR49059, respectively, and Dr. Marc Caron for the generous gift of the R137HV2R cDNA. Many thanks to Dr. Monique Lagacé for critical reading of the manuscript. This work was supported by a grant from the Canadian Institute for Health Research, the Kidney Foundation of Canada (to M.B.), and a Fonds de la Recherche en Santé du Québec-Institut National de la Santé et de la Recherche Médicale travel grant (to M.B. and C.B.). S.T. held a studentship from the Fondation pour la Recherche Médicale. M.B. is the holder of the Hans Selye Chair in Molecular and Cell Biology and holds a Canada Research Chair in Signal Transduction and Molecular Pharmacology.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AVP, arginine-vasopressin; β2AR, β2 adrenergic receptor; GPCR, G protein-coupled receptor; V1aR, V1a vasopressin receptor; V2R, V2 vasopressin receptor; HA, hemagglutinin; HEK, human embryonic kidney; YFP, yellow fluorescent protein.
References
- 1.Bockaert, J., Claeysen, S., Becamel, C., Pinloche, S. & Dumuis, A. (2002) Int. Rev. Cytol. 212, 63-132. [DOI] [PubMed] [Google Scholar]
- 2.Pierce, K. L., Premont, R. T. & Lefkowitz, R. J. (2002) Nat. Rev. Mol. Cell. Biol. 3, 639-650. [DOI] [PubMed] [Google Scholar]
- 3.Krupnick, J. G. & Benovic, J. L. (1998) Annu. Rev. Pharmacol. Toxicol. 38, 289-319. [DOI] [PubMed] [Google Scholar]
- 4.Oakley, R. H., Laporte, S. A., Holt, J. A., Barak, L. S. & Caron, M. G. (1999) J. Biol. Chem. 274, 32248-32257. [DOI] [PubMed] [Google Scholar]
- 5.Oakley, R. H., Laporte, S. A., Holt, J. A., Caron, M. G. & Barak, L. S. (2000) J. Biol. Chem. 275, 17201-17210. [DOI] [PubMed] [Google Scholar]
- 6.Anborgh, P. H., Seachrist, J. L., Dale, L. B. & Ferguson, S. S. (2000) Mol. Endocrinol. 14, 2040-2053. [DOI] [PubMed] [Google Scholar]
- 7.Gines, S., Ciruela, F., Burgueno, J., Casado, V., Canela, E. I., Mallol, J., Lluis, C. & Franco, R. (2001) Mol. Pharmacol. 59, 1314-1323. [PubMed] [Google Scholar]
- 8.Haasemann, M., Cartaud, J., Muller-Esterl, W. & Dunia, I. (1998) J. Cell Sci. 111, 917-928. [DOI] [PubMed] [Google Scholar]
- 9.Mentlein, R., Held-Feindt, J. & Krisch, B. (2001) Cell Tissue Res. 303, 27-34. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto, Y., Ninomiya, H., Miwa, S. & Masaki, T. (2000) J. Biol. Chem. 275, 6439-6446. [DOI] [PubMed] [Google Scholar]
- 11.Angers, S., Salahpour, A. & Bouvier, M. (2002) Annu. Rev. Pharmacol. Toxicol. 42, 409-435. [DOI] [PubMed] [Google Scholar]
- 12.George, S. R., O'Dowd, B. F. & Lee, S. P. (2002) Nat. Rev. Drug Discov. 1, 808-820. [DOI] [PubMed] [Google Scholar]
- 13.AbdAlla, S., Lother, H. & Quitterer, U. (2000) Nature 407, 94-98. [DOI] [PubMed] [Google Scholar]
- 14.Cvejic, S. & Devi, L. A. (1997) J. Biol. Chem. 272, 26959-26964. [DOI] [PubMed] [Google Scholar]
- 15.Hillion, J., Canals, M., Torvinen, M., Casado, V., Scott, R., Terasmaa, A., Hansson, A., Watson, S., Olah, M. E., Mallol, J., et al. (2002) J. Biol. Chem. 277, 18091-18097. [DOI] [PubMed] [Google Scholar]
- 16.Jordan, B. A. & Devi, L. A. (1999) Nature 399, 697-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan, B. A., Trapaidze, N., Gomes, I., Nivarthi, R. & Devi, L. A. (2001) Proc. Natl. Acad. Sci. USA 98, 343-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavoie, C., Mercier, J. F., Salahpour, A., Umapathy, D., Breit, A., Villeneuve, L. R., Zhu, W. Z., Xiao, R. P., Lakatta, E. G., Bouvier, M., et al. (2002) J. Biol. Chem. 277, 35402-35410. [DOI] [PubMed] [Google Scholar]
- 19.Overton, M. C. & Blumer, K. J. (2000) Curr. Biol. 10, 341-344. [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer, M., Koch, T., Schroder, H., Laugsch, M., Hollt, V. & Schulz, S. (2002) J. Biol. Chem. 277, 19762-19772. [DOI] [PubMed] [Google Scholar]
- 21.Rocheville, M., Lange, D. C., Kumar, U., Sasi, R., Patel, R. C. & Patel, Y. C. (2000) J. Biol. Chem. 275, 7862-7869. [DOI] [PubMed] [Google Scholar]
- 22.Terrillon, S., Durroux, T., Mouillac, B., Breit, A., Ayoub, M. A., Taulan, M., Jockers, R., Barberis, C. & Bouvier, M. (2003) Mol. Endocrinol. 17, 677-691. [DOI] [PubMed] [Google Scholar]
- 23.Barak, L. S., Oakley, R. H., Laporte, S. A. & Caron, M. G. (2001) Proc. Natl. Acad. Sci. USA 98, 93-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angers, S., Salahpour, A., Joly, E., Hilairet, S., Chelsky, D., Dennis, M. & Bouvier, M. (2000) Proc. Natl. Acad. Sci. USA 97, 3684-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mellon, P., Parker, V., Gluzman, Y. & Maniatis, T. (1981) Cell 27, 279-288. [DOI] [PubMed] [Google Scholar]
- 26.Morello, J. P., Salahpour, A., Laperrière, A., Bernier, V., Arthus, M. F., Lonergan, M., Petäjä-Repo, U., Angers, S., Morin, D., Bichet, D. G., et al. (2000) J. Clin. Invest. 105, 887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Innamorati, G., Le Gouill, C., Balamotis, M. & Birnbaumer, M. (2001) J. Biol. Chem. 276, 13096-13103. [DOI] [PubMed] [Google Scholar]
- 28.Klein, U., Muller, C., Chu, P., Birnbaumer, M. & von Zastrow, M. (2001) J. Biol. Chem. 276, 17442-17447. [DOI] [PubMed] [Google Scholar]
- 29.Andres, M., Trueba, M. & Guillon, G. (2002) Br. J. Pharmacol. 135, 1828-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Innamorati, G., Sadeghi, H., Eberle, A. N. & Birnbaumer, M. (1997) J. Biol. Chem. 272, 2486-2492. [DOI] [PubMed] [Google Scholar]
- 31.Arpin-Bott, M. P., Waltisperger, E., Freund-Mercier, M. J. & Stoeckel, M. E. (1999) Nephron 83, 74-84. [DOI] [PubMed] [Google Scholar]