Abstract
Soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE) protein interactions at the synaptic vesicle/plasma membrane interface play an essential role in neurotransmitter release. The membrane-proximal region (amino acids 77–90) of the v-SNARE vesicle-associated membrane protein 2 (VAMP 2, synaptobrevin) binds acidic phospholipids or Ca2+/calmodulin in a mutually exclusive manner, processes that are required for Ca2+-dependent exocytosis. To address the mechanisms involved, we asked whether this region of VAMP can interact with cis (outer vesicle leaflet) and/or trans (inner plasma membrane leaflet) lipids. To evaluate cis lipid binding, recombinant VAMP was reconstituted into liposomes and accessibility to site-directed antibodies was probed by surface plasmon resonance. Data indicated that the membrane-proximal domain of VAMP dips into the cis lipid bilayer, sequestering epitopes between the tetanus toxin cleavage site and the membrane anchor. These epitopes were unmasked by VAMP double mutation W89A, W90A, which abolishes lipid interactions. To evaluate trans lipid binding, VAMP was reconstituted in cis liposomes, which were then immobilized on beads. The ability of VAMP to capture protein-free 3H-labeled trans liposomes was then measured. When cis lipid interactions were eliminated by omitting negatively charged lipids, trans lipid binding to VAMP was revealed. In contrast, when cis and trans liposomes both contained acidic headgroups (i.e., approximating physiological conditions), cis lipid interactions totally occluded trans lipid binding. In these conditions Ca2+/calmodulin displaced cis inhibition, transferring the lipid-binding domain of VAMP from the cis to the trans bilayer. Our results suggest that calmodulin acts as a unidirectional Ca2+-activated shuttle that docks the juxtamembrane portion of the v-SNARE in the target membrane to prepare fusion.
Regulated exocytosis in neurons and neurosecretory cells requires Ca2+-dependent fusion of the membrane of a docked synaptic vesicle or secretory granule with the plasma membrane. At a molecular level this involves appropriate pairing between the vesicular soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (v-SNARE) [vesicle-associated membrane protein 2 (VAMP 2) or synaptobrevin] and plasmalemmal t-SNAREs [syntaxin 1 and synaptosomal associated protein of 25 kDa (SNAP-25)] (1, 2). Trans SNARE complex assembly may also provide the energy to overcome the forces that oppose fusion, including electrostatic repulsion between opposing bilayers and hydration of phospholipid head-groups (ref. 3; reviewed in refs. 4–7). Important issues to resolve are how cytoplasmic Ca2+ transients control this process by preparing, regulating, and ultimately triggering exocytosis. Ca2+ has been shown to promote SNARE complex assembly in the pheochromocytoma cell line PC12 (8). However, direct Ca2+ binding to SNARE proteins has not been documented. Ca2+-dependent regulation is thus probably conferred by distinct Ca2+ sensor proteins that interact with SNAREs, and abundant evidence points to a predominant role for synaptotagmin I (9–11).
Calmodulin has also been implicated in late steps of exocytosis in neuroendocrine cells (12–14), although in neurons it appears to regulate the refilling of readily releasable vesicle pools rather than the release process itself (15). It also plays a role in endosomal and intra-Golgi fusion (16, 17) and homotypic vacuole fusion in yeast (18). Thus calmodulin may provide one of the links between Ca2+ signaling and SNARE assembly. We have previously identified a Ca2+/calmodulin binding sequence on the v-SNARE VAMP 2. This consensus motif (VAMP77–90), located at the frontier between the t-SNARE interaction domain and the transmembrane anchor, is conserved in VAMP 2 homologues throughout evolution (19). Precisely the same domain binds to acidic phospholipids, and lipids can be competitively displaced by Ca2+/calmodulin. Approaches using both mimetic peptide injection and directed mutagenesis in neuroendocrine cells indicated that calmodulin and/or phospholipid interactions with VAMP77–90 are required for Ca2+-dependent exocytosis (20).
In the present report we address the mechanistic aspects of this process to understand how mutually exclusive calmodulin or phospholipid binding to the membrane proximal segment of a v-SNARE could promote fusion between opposing bilayers.
Materials and Methods
Liposomes. Dipalmitoyl phosphatidylcholine (DPPC), palmitoyl oleoyl phosphatidylcholine (POPC), and dioleoyl phosphatidylserine (DOPS) (all from Sigma) were dissolved in 2/1 (vol/vol) chloroform/methanol and mixed to obtain 100% PC or 75% PC/25% PS (wt/wt), stabilized with 50 nM butylated hydroxytoluene (BHT; Sigma). In certain experiments 0.5% [N-methyl-3H]DPPC (Amersham Pharmacia) was included as a tracer. After evaporation, lipids were heated to 60°C in 50 mM Hepes/150 mM NaCl adjusted to pH 7.2 with NaOH, to yield a lipid concentration of 1.4 mM. Samples were then sonicated on ice and for certain experiments extruded through a 100-nm filter (Avestin, Ottawa)
Bacterially expressed GST-VAMP or VAMP-His6-myc was produced as previously reported (19, 20). For protein reconstitution (DPPC, POPC, or DOPS) lipids were mixed to obtain 100% PC or 75% PC/25% PS. For immobilization by streptavidin, 1% N-biotinyl caproyl phosphatidylethanolamine (bPE) (Interchim, Montluçon, France) was substituted for PC. Lipids were evaporated, and then VAMP-His6-myc (0.7 mg/ml) was added in 1.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)/50 mM Hepes/150 mM NaCl adjusted to pH 7.2 with NaOH, and mixed for 5 min. Chromatography on Sephadex G50 (Amersham Pharmacia) then yielded proteoliposomes of ≈200-nm diameter and an estimated protein content of one VAMP molecule per 10 nm2 of vesicle surface area. In some experiments liposomes were treated with trypsin (2 μg/ml, Sigma) at 37°C for 2 h followed by addition of trypsin inhibitor (2 μg/ml, Sigma) or recombinant His6-tetanus toxin (TeNT) light chain (2 μg/ml, provided by the late H. Niemann, University of Hannover, Hannover, Germany) at 37°C for 2 h in 4 mM Hepes/100 mM NaCl/3.5 mM CaCl2/3.5 mM MgCl2, pH 7.4.
Surface Plasmon Resonance (SPR) Spectroscopy. SPR spectroscopy was performed at 25°C on a Biacore 3000 system (Pharmacia Biosensor, Uppsala, Sweden). GST-VAMP binding to immobilized lipids was evaluated by using an L1 sensor chip (Biacore); 25% DOPS/75% POPC liposomes were injected into the experimental flow cell and 100% POPC liposomes were injected into the control cell to yield 4,500 resonance units (RU) of bound lipids in each. Sensorgrams illustrate the difference between signals from experimental and control cells. Chips were regenerated with 16 mM NaOH between each injection. Rate constants were calculated by global fitting by using the BIA 3.1 evaluation program (Biacore) and a single-site interaction model. The software corrects for baseline drift that occurred during measurements. The quality of the fit was assessed by inspecting the statistical χ2 value and the residuals (observed - calculated). χ2 was in the range of 0.3–2.5, and the residuals did not exceed ±7% of the experimental curves.
SPR Measurements of Epitope Accessibility. An F1 sensor chip (Biacore) was activated and polyclonal rabbit anti-mouse Fcγ antibodies (Biacore) were applied to yield 3,500 RU of immobilized antibody. Residual matrix sites were blocked with 1 M ethanolamine. Five femtomoles (750 RU) of monoclonal anti-polyhistidine antibody (Sigma) was captured on anti-mouse antibodies in experimental flow cells. Experimental and control cells were then saturated with nonimmune mouse IgG (Interchim). Wild-type VAMP and double mutant W89A, W90A VAMP liposomes were immobilized by anti-polyhistidine antibodies, which bind to the C-terminal His6 tags of proteins reconstituted in an inside-out orientation. Staphylococcal protein A-purified IgG fractions (30 μg/ml) against two VAMP peptides (residues 1–20 or 60–88), and monoclonal 3D10, which recognizes an epitope between residues 20 and 60, were injected consecutively. Antibody binding was measured as the difference between the signal from the experimental cell and the control cell. Each experiment was repeated three times with different VAMP liposome preparations, and data were normalized to account for differences in amounts of immobilized liposomes and protein content.
3H-Liposome and Ca2+/Calmodulin Binding to VAMP Liposomes. VAMP liposomes were incubated with streptavidin-agarose beads (Sigma) for 1 h at 4°C and washed three times by centrifugation in the binding buffer. VAMP liposome beads (2.5 μM VAMP, 30 μl of beads) were suspended in 0.2 ml of 50 mM Hepes/150 mM NaCl/1 mM CaCl2/2 mM MgCl2/0.1% ovalbumin, pH 7.2. 3H-liposomes (150 μM) were added and incubated for 90 min at room temperature. Beads were washed three times, and bound lipid was quantified by scintillation counting. VAMP liposomes immobilized on beads were incubated with 15 μM calmodulin in the presence or absence of Ca2+ and trans liposomes as indicated. After washing and SDS/PAGE, Western blots were sequentially probed with anti-calmodulin mAb (Up-state Biotechnology, Lake Placid, NY) and anti-VAMP1–20 antibodies.
Results
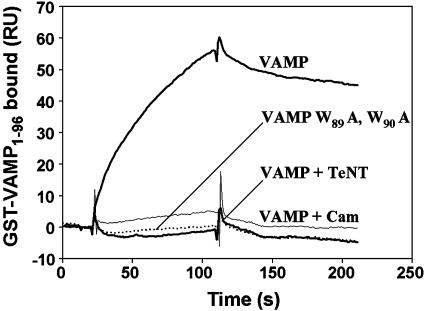
Lipid-Binding Properties of the Membrane-Proximal Region of VAMP. Ca2+/calmodulin or acidic phospholipids bind to a conserved membrane-proximal domain (residues 77–90) of VAMP 2 in a mutually exclusive manner. Fig. 1 illustrates the basic characteristics. Liposomes containing 25% DOPS/75% POPC were immobilized on a hydrophobic (L1) sensor chip of an SPR (Biacore) spectrometer. The cytoplasmic domain of VAMP 2 fused to GST (GST-VAMP1–96) was injected into the running buffer. Rapid association of VAMP 2 with lipids led to an increase in RU, followed by slow dissociation. Binding was abolished by coinjection with Ca2+/calmodulin, which interacts competitively with the lipid-binding domain, or by mutation of two tryptophan residues (W89A, W90A) required for calmodulin and phospholipid binding (19, 20). TeNT cleavage separates a 2-kDa lipid-binding fragment (VAMP77–96) from the 34-kDa (GST-VAMP1–76) N-terminal portion of the fusion protein, thus reducing the molecular mass of the ligand 18-fold. As the SPR signal is proportional to the molecular mass of the ligand, this cleavage led to an apparent loss of binding. Analysis of the binding constants (Fig. 6A, which is published as supporting information on the PNAS web site) for the interaction between GST-VAMP1–96 and DOPS/DOPC yielded Kd = 95 ± 29 nM; kon = 1.4 × 104 M-1·s-1; koff = 1.3 × 10-3·s-1. Binding was inhibited by increasing ionic strength (Ki for NaCl = 200 mM, data not shown) consistent with the contribution of electrostatic interactions between basic residues of VAMP77–90 and the negatively charged head groups of DOPS. Furthermore, fluorescence emission spectra of tryptophan displayed a blue shift when VAMP77–90 peptides were incubated with fluid phase but not gel phase PS/PC liposomes (Fig. 6B).
Fig. 1.
Binding of the membrane-proximal domain of VAMP to acidic phospholipids. Liposomes (25% DOPS/75% POPC) were immobilized on a hydrophobic sensor chip of an SPR (Biacore) apparatus. GST-VAMP1–96 (50 nM, thick upper trace) was injected in a running buffer containing 5 mM Hepes/NaOH, 100 mM NaCl, and 1 mM CaCl2, pH 7.4. Binding was inhibited by pretreatment with TeNT, 10 μg/ml, 1 h at 37°C, which cleaves the Q76–F77 peptide bond (thick lower trace), by introduction of the W89A, W90A mutation (dotted trace), and by coinjection of 10 μM calmodulin (thin trace).
Interactions of the Membrane-Proximal Region of VAMP with Cis Lipids. We then addressed the issue of topology to determine which membrane bilayers might be relevant targets for VAMP binding in the context of vesicular transport and docking. First, vesicle-inserted VAMP might fold to permit cis interactions with lipids in the outer leaflet of the vesicle membrane (see cartoon in Fig. 2). To evaluate cis interactions recombinant full-length VAMP-His6-myc (wild type or W89, W90A) was purified and reconstituted by its transmembrane anchor into liposomes. The orientation was analyzed by using antibodies against VAMP1–20 (an N-terminal cytoplasmic epitope) and myc (a C-terminal intravesicular epitope) and found to be ≈75% “cytoplasmic face out.” An antibody-binding strategy was then used to probe the accessibility of cytoplasmic VAMP domains. We hypothesized that cis interactions with lipids could mask epitopes for anti-VAMP antibodies located in proximity to residues 77–90, and consequently that mutations eliminating these interactions may expose epitopes. This hypothesis was tested (Figs. 2 and 6C) by using an antibody raised against VAMP60–88 (antibody III) that overlaps the putatively masked VAMP77–90 sequence. Negative controls included anti-VAMP1–20 and a monoclonal antibody against an epitope between residues 20 and 60 (antibodies I and II, respectively).
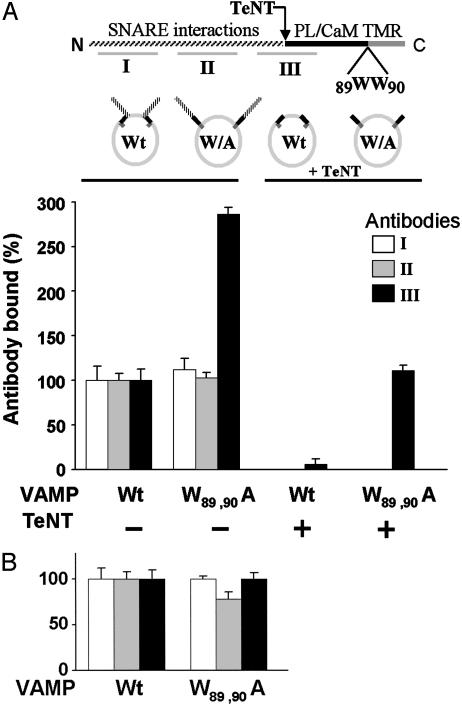
Fig. 2.
Cis lipid binding masks epitopes in the membrane-proximal domain of VAMP. Upper schema illustrates VAMP domains recognized by antibodies I, II, and III. The membrane-proximal region (black) is flanked N-terminally by the TeNT cleavage site (Q76–F77) and C-terminally by the transmembrane anchor (TMR). The mutation W89A, W90A inhibits lipid binding. PL, phospholipid; CaM, calmodulin. Lower schema represents the four VAMP liposome preparations. (A) Full-length recombinant wild type (Wt) or W89A, W90A mutant (W/A) VAMP was reconstituted into 25% DOPS/75% POPC liposomes (Left), and aliquots of each were treated with TeNT (Right). The four resulting liposome pools were immobilized on the sensor chip of an SPR apparatus and the binding of antibodies I (white bars), II (gray bars), and III (black bars) to each individual group was assayed. The data are presented as four groups of three bars. Bound antibody = mass (RU) of antibody bound/mass (RU) of VAMP liposome immobilized. Binding of each antibody to wild-type VAMP liposomes was normalized to 100%. Antibody III binding was corrected to account for direct effects of the W89A, W90A mutation (Fig. 6C). After TeNT treatment, binding of antibodies I and II was not detectable. Results are the means ± SD of three independent experiments, each in triplicate. (B) As in A Left, except that VAMP was reconstituted into 100% POPC. Results are the means ± SD of an experiment in quadruplicate.
Wild-type or W89A, W90A VAMP was reconstituted into 25% DOPS/75% POPC liposomes. SPR data demonstrated that the W89A, W90A mutation, which eliminates phospholipid binding, did not modify binding by antibodies I and II, but led to a 3-fold increment in recognition by antibody III (Fig. 2 A). When control experiments were performed in 100% POPC liposomes, no significant increase in antibody III binding was detected (Fig. 2B). To restrict analysis to epitopes located within the juxtamembrane domain (i.e., residues 77–96), proteoliposomes were treated with TeNT to cleave off the N-terminal VAMP1–76 domain. Binding of antibodies I and II was abolished. Binding of antibody III to wild-type VAMP was also reduced because of loss of epitopes N-terminal to the TeNT cleavage site. However, when cis lipid binding was abolished by the W89A, W90A mutation, antibody III binding increased 20-fold (Fig. 2 A). These results support the conclusion that membrane-anchored VAMP folds to allow cis lipid interactions with the juxtamembrane domain sequestering epitopes within this region.
Transfer of Lipid Interactions from Cis to Trans Bilayers by Ca2+/Calmodulin. During SNARE complex assembly the C-terminal region of VAMP would be pulled toward the plasma membrane, thus trans interactions with the inner leaflet of the plasma membrane might also occur. To address this question we reconstituted wild-type VAMP-His6-myc in liposomes of different lipid composition. VAMP liposomes contained 1% N-biotinyl caproyl phosphatidylethanolamine, allowing immobilization on streptavidin-agarose beads. VAMP liposome-beads were then incubated with protein-free liposomes, labeled with trace amounts of [3H]PC, to evaluate trans lipid binding.
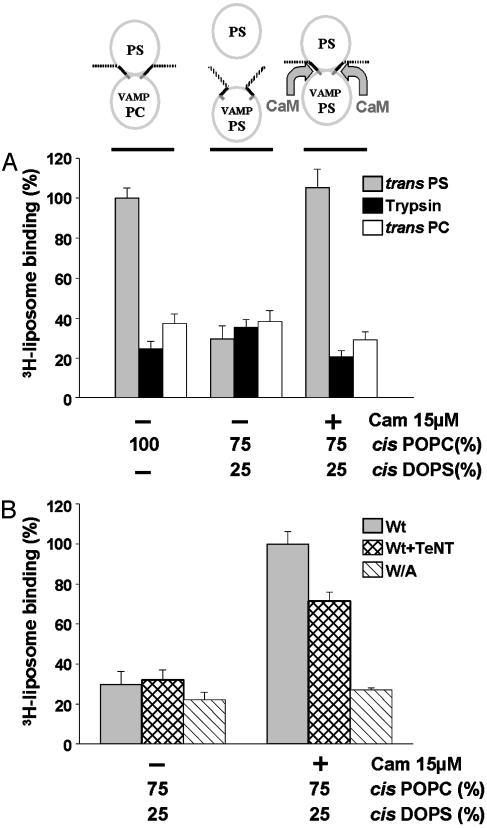
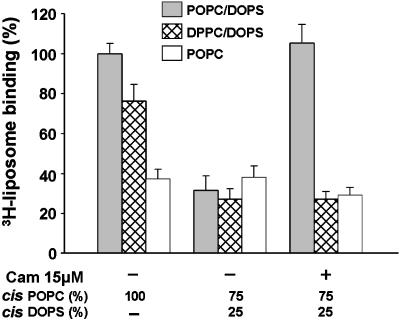
When VAMP is reconstituted in 100% PC liposomes (i.e., lacking acidic headgroups), cis interactions cannot occur, thus VAMP77–90 should in principle be optimally accessible for trans lipid binding (Fig. 3A Left). In these conditions robust trans binding of liposomes containing 25% PS/75% PC was measured (gray bar), compared with controls. Controls for nonspecific binding included trypsinized cis liposomes (black bars) and 100% PC trans liposomes (white bars). Thus trans lipid binding can occur, and it requires VAMP anchored in cis liposomes and acidic headgroups in trans liposomes. Parallel experiments were carried out with VAMP reconstituted in 25% PS/75% PC liposomes, which support cis lipid interactions (Fig. 3A Center). In this case, trans 3H-liposome binding (gray bar) was reduced to background levels (black and white bars). Thus in conditions that approximate the charge content of opposed vesicular and plasma membrane leaflets in vivo, binding of VAMP77–90 to the cis lipid bilayer totally precludes interactions with trans bilayers. Having shown that Ca2+/calmodulin can displace phospholipid binding, we asked whether it might provide a mechanism to remove cis inhibition. Fig. 3A Right (gray bar) shows that Ca2+/calmodulin strongly promoted trans lipid binding. In the presence of Ca2+/calmodulin, trans lipid binding to VAMP anchored in PS/PC liposomes attained the same level as that obtained when VAMP was reconstituted in 100% PC liposomes (Left, gray bar). These results suggest that the major fraction of VAMP is switched from cis interactions to trans interactions by Ca2+/calmodulin. Stimulation of trans lipid binding by Ca2+/calmodulin was totally suppressed when the W89A, W90A VAMP mutant was reconstituted into liposomes (Fig. 3B). In contrast, the effect of Ca2+/calmodulin persisted, albeit at a reduced level, after TeNT treatment of proteoliposomes containing wild-type VAMP (in conditions that totally removed epitopes for antibodies I and II in Fig. 2 A). These findings thus confirmed that the juxtamembrane domain of VAMP, on the C-terminal side of the TeNT cleavage site, mediated this process.
Fig. 3.
Ca2+/calmodulin transfers the lipid-binding domain of VAMP from cis to trans bilayers. (A) The schema at the top represents the three assay conditions that correspond to the gray bars in A, (black and white bars represent negative controls). Wild-type VAMP was reconstituted into liposomes of the indicated lipid composition. Cis VAMP liposomes were immobilized on beads and incubated with trans 3H-liposomes containing acidic headgroups (25% DOPS/75% POPC, gray bars) in the presence of 1 mM Ca2+ and in the presence or absence of calmodulin (CaM). After washing by centrifugation, bound radioactivity was measured. Nonspecific binding was evaluated with trypsin-treated cis liposomes (black bars) and 100% POPC trans 3H-liposomes (white bars). Trans 3H-liposome binding to VAMP reconstituted in 100% POPC cis liposomes was set at 100%. Results are the means ± SD of six independent experiments, each in triplicate. (B) Wild-type VAMP (gray and cross-hatched bars) or W89A, W90A VAMP (hatched bars) were reconstituted into cis liposomes (25% DOPS/75% POPC) and immobilized on beads. Wild-type VAMP liposomes were preincubated in the absence (gray bars) or presence (cross-hatched bars) of TeNT. Immobilized VAMP liposomes on beads were then incubated with trans 3H-liposomes (25% DOPS/75% POPC) in the presence and absence of calmodulin and processed as in A. 3H-liposome binding to native VAMP liposomes in the presence of calmodulin was set at 100%. Results are the means ± SD of two independent experiments, each in triplicate.
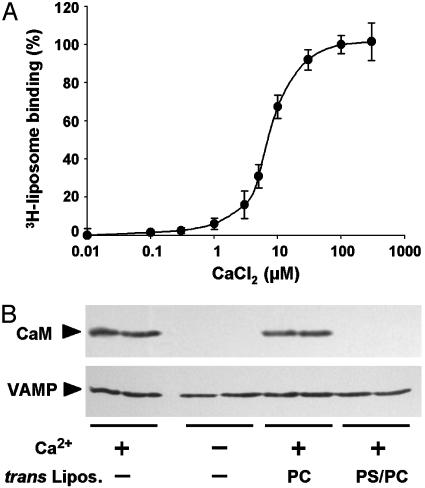
Finally we addressed mechanistic aspects of the cis to trans transfer of lipid binding by Ca2+/calmodulin. When free Ca2+ concentration was increased at a saturating calmodulin concentration (Fig. 4A), trans lipid binding was increased between 1 and 30 μM with an EC50 = 5 μM Ca2+. The concentration dependency for calmodulin at constant Ca2+ concentration (see Fig. 6D) also displayed an EC50 = 5 μM, consistent with the concentrations at which it inhibits 3H-liposome binding to VAMP (20). Our data suggest that Ca2+/calmodulin acts by binding to VAMP, dissociating cis interactions between VAMP and lipids. Ca2+/calmodulin would then in turn be displaced by trans lipids, thus promoting cis to trans switching. To examine whether these events occur, Ca2+/calmodulin binding to VAMP liposomes immobilized on beads was directly monitored by Western blotting (Fig. 4B). First, calmodulin interacted with VAMP reconstituted in 25% PS/75% PC liposomes in a Ca2+-dependent manner. The addition of 100% PC trans liposomes had no effect; however, no calmodulin binding was detected when 25% PS/75% PC liposomes were added (Fig. 4B). Similar results were obtained when VAMP was reconstituted in 100% PC liposomes (not shown). These results indicate that Ca2+/calmodulin displaces cis lipid interactions and binds to membrane-inserted VAMP, but subsequently dissociates upon addition of trans liposomes. The fact that no residual calmodulin binding was detected in the presence of trans liposomes is consistent with complete switching of VAMP to the trans bilayer.
Fig. 4.
Ca2+/calmodulin binding to cis liposomes mediates transfer of the lipid-binding domain of VAMP. (A) Wild-type VAMP liposomes (25% DOPS/75% POPC) immobilized on beads were incubated with 15 μM calmodulin and trans 3H-liposomes (25% DOPS/75% POPC) in the presence of a Ca2+/EGTA buffer to yield the indicated free Ca2+ concentrations. 3H-liposome binding was measured as in Fig. 3. Results are the means ± SD of two independent experiments, each in triplicate. (B) Wild-type VAMP liposomes (as in A) were incubated with 15 μM calmodulin in the presence or absence of 150 μMCa2+ and trans liposomes (100% POPC or 25% DOPS/75% POPC) as indicated. After washing, calmodulin binding and VAMP content were monitored by Western blotting. Each experimental condition was studied in duplicate, and the products were run on adjacent gel lanes. Results are representative of two independent experiments.
In these experiments cis and trans liposomes had the same phospholipid composition. Thus there is an apparent contradiction in the fact that calmodulin displaced cis phospholipids but was then in turn displaced by trans phospholipids. We hypothesized that VAMP may interact less strongly with the cis bilayer because of mechanical constraints imposed by bending of the juxtamembrane α-helix. In contrast, when VAMP unfolds, a greater degree of freedom might allow deeper penetration of tryptophan residues 89 and 90 into opposing bilayers and trans interactions might then become irreversible. It follows that if trans penetration is limited experimentally then cis to trans transfer should be reduced. This hypothesis was tested by modifying the phospholipid content of liposomes (fluid phase POPC versus gel phase DPPC; see Fig. 6B) to evaluate the consequences of changes in fluidity. First VAMP was reconstituted in 100% POPC (Fig. 5 Left). In these conditions in which cis inhibition is absent, VAMP bound to fluid- or gel-phase trans liposomes containing 25% PS. When cis lipid binding was conferred by the presence of POPS (Fig. 5 Center) trans lipid binding was blocked, again irrespective of trans liposome fluidity. However when Ca2+/calmodulin was added (Fig. 5 Right), fluid-phase (DOPS/POPC) but not gel-phase (DOPS/DPPC) trans liposomes were bound. Practically identical results were obtained (data not shown) when VAMP was reconstituted in cis 1iposomes containing DPPC (gel phase at room temperature) instead of POPC. To summarize these findings, reduced fluidity of the cis bilayer did not affect inhibition by cis lipid binding or reversal by calmodulin, suggesting that cis lipid interactions are relatively superficial and readily displaceable by calmodulin. In contrast, reduced fluidity of the trans bilayer completely suppressed calmodulin-activated trans liposome binding. Thus penetration of tryptophan residues into the bilayer of trans liposomes is required for these liposomes to displace calmodulin. These results are consistent with intrinsic differences in the nature of cis and trans lipid interactions and provide a basis for understanding how Ca2+/calmodulin can unidirectionally transfer a protein domain between two bilayers of identical phospholipid composition.
Fig. 5.
Unidirectional Ca2+/calmodulin-dependent transfer of the lipid-binding domain of VAMP requires fluid-phase trans bilayers. Wild-type VAMP was reconstituted into liposomes with the indicated cis lipid composition. Binding was assayed as in Fig. 3A using trans 3H-liposomes in fluid phase (25% DOPS/75% POPC, gray bars), gel phase (25% DOPS/75% DPPC, cross-hatched bars), or fluid phase lacking acidic headgroups (100% POPC, white bars). Results are the means ± SD of three independent experiments, each in triplicate.
Discussion
A common feature of SNARE proteins is the presence of clusters of basic residues in the linker regions that couple the coiled-coil domains to their membrane anchors (21). In the membrane-proximal region of the v-SNARE VAMP 2, these basic residues flank a triplet of aromatic residues, YWW at positions 88–90, exposed to the solvent at the surface of the SNARE complex (22). It has been suggested that this motif may contribute to fusion by destabilizing lipid bilayers (22, 23). Basic and hydrophobic residues in this region (VAMP77–90) are organized into a consensus Ca2+-dependent calmodulin-binding motif, and calmodulin or acidic phospholipids interact with this sequence in a mutually exclusive manner (20). Directed mutagenesis led to the conclusion that calmodulin and/or acidic phospholipid binding to this domain is required for Ca2+-dependent exocytosis by PC12 cells (20). The aim of our current study was to resolve how calmodulin binding to the membrane-proximal domain of VAMP might mediate interactions between vesicular and plasma membranes to prepare fusion.
Interactions between the membrane-proximal domain of VAMP and two topologically distinct lipid bilayers were envisaged: cis binding to the external leaflet of the vesicle and trans binding to the internal leaflet of the plasma membrane. Can cis interactions occur? It has been suggested that cis interactions between VAMP 2 and synaptic vesicle lipids intrinsically inhibit SNARE complex assembly (24). To evaluate cis lipid binding, we reconstituted VAMP into liposomes and analyzed its accessibility to antibodies. Membrane-proximal epitopes located on the C-terminal side of the TeNT cleavage site were indeed masked, but become available when cis lipid binding was eliminated by the W89A, W90A mutation. Thus membrane-anchored VAMP appears to fold back against the vesicle surface with the juxtamembrane region dipping into the cis lipid bilayer. Recent studies using fluorescence quenching and site-directed spin labeling with vesicle-inserted VAMP also support the conclusion that the membrane-proximal domain of VAMP penetrates obliquely into the cis bilayer (25, 26).
The idea that the same region of VAMP could also interact in trans with plasma membrane lipids has interesting implications for membrane fusion. It was tested by evaluating the capacity of VAMP reconstituted into lipid bilayers to pull down protein-free trans 3H-liposomes. Binding to trans bilayers was detected when cis interactions were abolished by omitting acidic phospholipids. However, when cis and trans liposomes both contained negatively charged phospholipids, trans binding was occluded. This composition approximates conditions relevant to vesicle docking in that the outer leaflet of the synaptic vesicle and the inner leaflet of the plasma membrane have a similar lipid composition and negative charge density. In these conditions the juxtamembrane domain dips into the cis bilayer and thus cis interactions inhibit binding to trans lipids. Strikingly the addition of Ca2+/calmodulin totally displaced cis inhibition, triggering trans lipid binding. Trans lipid binding was abolished by the W89A, W90A mutation but persisted after cleavage by TeNT, demonstrating that it is mediated by the juxtamembrane domain of VAMP. Thus calmodulin acts as a unidirectional Ca2+-activated shuttle conveying VAMP77–96 from cis to trans membranes. For such a mechanism to operate, relative affinities for binding to VAMP must be trans lipids > Ca2+/calmodulin > cis lipids. This rank order was initially counterintuitive because the lipid composition of cis and trans liposomes was identical. However, mechanical constraints in cis and trans are probably not equivalent. When the C-terminal region of VAMP dips obliquely into the cis bilayer, the α-helix close to the membrane anchor must bend (25, 26). Whereas bending would limit penetration of the juxtamembrane region of VAMP into the cis lipid bilayer, binding to trans bilayers may be less restricted, allowing deeper insertion. If VAMP interactions with cis lipids are mainly adsorptive and reversible, then manipulations that limit protein insertion into the cis bilayer would have limited consequences. In contrast, reducing penetration of VAMP into the trans bilayer might abolish cis to trans transfer by calmodulin. Protein/lipid interactions were manipulated by modifying lipid composition to reduce the fluidity of cis and/or trans bilayers. Whereas diminished cis bilayer fluidity had no effect, a reduction in trans bilayer fluidity eliminated cis to trans transfer by Ca2+/calmodulin. Thus VAMP interacts more superficially with cis lipids than trans lipids. Another factor that helps calmodulin to drive lipid binding from cis to trans is its multivalent nature. It is likely that several VAMP molecules anchor a trans to a cis liposome, providing a practically irreversible Velcro-type interaction. Thus when a monovalent reversible interaction is directly monitored, calmodulin blocks VAMP binding to membranes (Fig. 1). In contrast, in a system that measures the consequences of multivalent interactions, calmodulin blocks individual binding events to cis membranes, driving irreversible binding to trans membranes (Fig. 3). Additional mechanisms probably promote trans interactions in vivo, including VAMP binding to t-SNAREs and the presence in the plasma membrane of phosphoinositides conferring a higher density of negatively charged headgroups (27, 28).
Our results are consistent with observations that SNARE complex assembly is inhibited by interactions between VAMP and the cis lipid bilayer (24, 26). We may speculate that Ca2+-activated calmodulin can displace cis phospholipids, initiating two processes: (i) SNARE pairing and partial assembly and (ii) transfer of the juxtamembrane domain of VAMP into the plasma membrane. Calmodulin-driven insertion of VAMP into trans membranes occurred in vitro in the absence of syntaxin and SNAP-25, presumably in a spatially uncoordinated pattern. However pairing with a radial array of t-SNAREs could guide several v-SNARE juxtamembrane domains to converge and insert at a central point in the plasma membrane at which fusion could subsequently be initiated. Trans penetration of the juxtamembrane domain of VAMP would significantly reduce the distance between the opposed bilayers and might in itself induce hemifusion (mixing of the outer vesicle leaflet with the inner plasmalemmal leaflet). This hemifusion could diminish the energy required to subsequently achieve full fusion. A radial array of partially assembled trans SNARE complexes with the C-terminal domain of VAMP penetrating the plasma membrane might correspond to a readily releasable configuration in which a calcium sensor distinct from calmodulin then triggers fusion. It is unlikely that calmodulin itself is the calcium sensor in regulated exocytosis because Sr2+ ions trigger release in neurons and neuroendocrine cells (29) but do not activate calmodulin binding to VAMP (20). Thus synaptotagmin, which does bind Sr2+, remains the most likely candidate for the triggering step in neuroexocytosis. However, it is possible that the mechanism we propose is directly involved in calmodulin-dependent membrane fusion in other intracellular trafficking processes. In situ the majority of VAMP is complexed with synaptophysin and the c subunit of the V-ATPase (30). It has been proposed that the c subunit acts as a fusion pore, the assembly of which is promoted by Ca2+/calmodulin and SNAREs (31). It is tempting to speculate that Ca2+/calmodulin-driven cis to trans transfer of the juxtamembrane portion of the v-SNARE might contribute to this process.
Our findings may equally shed light on some of the consequences of clostridial toxin action. Synapses that have been functionally silenced by TeNT or botulinum neurotoxin treatment display an accumulation of docked synaptic vesicles that are unable to fuse. Thus intact SNARE proteins are not necessary for docking, leading to suggestions that other proteins must mediate this process. While it is probable that alternative anchoring partners do exist, C-terminal domains of VAMP could account for docking to plasma membrane lipids even after toxins have severed links with t-SNAREs.
Supplementary Material
Acknowledgments
We thank Drs. Masami Takahashi and Shunji Kozaki for supplying antibodies. Additional support was provided by the Association Française contre les Myopathies and the Assistance Publique des Hôpitaux de Marseille.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DOPS, dioleoyl phosphatidylserine; DPPC, dipalmitoyl phosphatidylcholine; PC, phosphatidylcholine; POPC, palmitoyl oleoyl phosphatidylcholine; PS, phosphatidylserine; RU, resonance units; SNAP-25, synaptosomal associated protein of 25 kDa; SNARE, soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor; SPR, surface plasmon resonance; TeNT, tetanus toxin; VAMP, vesicle-associated membrane protein.
References
- 1.Sollner, T., Whiteheart, S. W., Brunner, M., Erdjument-Bromage, H., Geromanos, S., Tempst, P. & Rothman, J. E. (1993) Nature 362, 318-324. [DOI] [PubMed] [Google Scholar]
- 2.McNew, J. A., Parlati, F., Fukuda, R., Johnston, R. J., Paz, K., Paumet, F., Sollner, T. H. & Rothman, J. E. (2000) Nature 407, 153-159. [DOI] [PubMed] [Google Scholar]
- 3.Weber, T., Zemelman, B. V., McNew, J. A., Westermann, B., Gmachl, M., Parlati, F., Sollner, T. H. & Rothman, J. E. (1998) Cell 92, 759-772. [DOI] [PubMed] [Google Scholar]
- 4.Jahn, R. & Südhof, T. C. (1999) Annu. Rev. Biochem. 68, 863-911. [DOI] [PubMed] [Google Scholar]
- 5.Mayer, A. (1999) Curr. Opin. Cell Biol. 11, 447-452. [DOI] [PubMed] [Google Scholar]
- 6.Lin, R. C. & Scheller, R. H. (2000) Annu. Rev. Cell Dev. Biol. 16, 19-49. [DOI] [PubMed] [Google Scholar]
- 7.Jahn, R., Lang, T. & Südhof, T. C. (2003) Cell 112, 519-533. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Y. A., Scales, S. J., Patel, S. M., Doung, Y. C. & Scheller, R. H. (1999) Cell 97, 165-174. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Chacon, R., Konigstorfer, A., Gerber, S. H., Garcia, J., Matos, M. F., Stevens, C. F., Brose, N., Rizo, J., Rosenmund, C. & Südhof, T. C. (2001) Nature 410, 41-49. [DOI] [PubMed] [Google Scholar]
- 10.Mackler, J. M., Drummond, J. A., Loewen, C. A., Robinson, I. M. & Reist, N. E. (2002) Nature 418, 340-344. [DOI] [PubMed] [Google Scholar]
- 11.Chapman, E. R. (2002) Nat. Rev. Mol. Cell. Biol. 3, 498-508. [DOI] [PubMed] [Google Scholar]
- 12.Kibble, A. V. & Burgoyne, R. D. (1996) Pflügers Arch. 431, 464-466. [DOI] [PubMed] [Google Scholar]
- 13.Chamberlain, L. H., Roth, D., Morgan, A. & Burgoyne R. D. (1995) J. Cell Biol. 130, 1063-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, Y. A., Duvvuri, V., Schulman, H. & Scheller R. H. (1999) J. Biol. Chem. 274, 26469-26476. [DOI] [PubMed] [Google Scholar]
- 15.Sakaba, T. & Neher, E. (2001) Neuron 20, 1119-1131. [DOI] [PubMed] [Google Scholar]
- 16.Columbo, M. I., Beron, W. & Stahl, P. D. (1997) J. Biol. Chem. 272, 7707-7712. [DOI] [PubMed] [Google Scholar]
- 17.Pryor, P. R., Mullock, B. M., Bright, N. A., Gray, S. R. & Luzio, J. P. (2000) J. Cell Biol. 149, 1053-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters, C. & Mayer, A. (1998) Nature 396, 575-580. [DOI] [PubMed] [Google Scholar]
- 19.Quetglas, S., Lévêque, C., Miquelis, R., Sato, K. & Seagar, M. (2000) Proc. Natl. Acad. Sci. USA 97, 9695-9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quetglas, S., Iborra, C., Sasakawa, N., de Haro, L., Kumakura, K., Sato, K., Lévêque, C. & Seagar, M. (2002) EMBO J. 21, 3970-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weimbs, T., Mostov, K., Low, S. H. & Hofmann, K. (1998) Trends Cell Biol. 8, 260-262. [DOI] [PubMed] [Google Scholar]
- 22.Sutton, R. B., Fasshauer, D., Jahn, R. & Brunger, A. T. (1998) Nature 395, 347-353. [DOI] [PubMed] [Google Scholar]
- 23.Montal, M. (1999) FEBS Lett. 447, 129-130. [DOI] [PubMed] [Google Scholar]
- 24.Hu, K. Caroll, J., Fedorovich, S., Rickman, C., Sukhodub, A. & Davletov, B. (2002) Nature 415, 646-650. [DOI] [PubMed] [Google Scholar]
- 25.Kweon, D. H., Kim, C. S. & Shin, Y. K. (2003) J. Biol. Chem. 278, 12367-12373. [DOI] [PubMed] [Google Scholar]
- 26.Kweon, D. H., Kim, C. S. & Shin, Y. K. (2003) Nat. Struct. Biol. 10, 440-447. [DOI] [PubMed] [Google Scholar]
- 27.Martin, T. F. (2001) Curr. Opin. Cell Biol. 13, 493-499. [DOI] [PubMed] [Google Scholar]
- 28.Sprong, H., Van der Sluijs, P. & Van Meer, G. (2001) Nature 2, 504-513. [DOI] [PubMed] [Google Scholar]
- 29.Sugita, S., Shin, O. H., Han, W., Lao, Y. & Südhof, T. C. (2002) EMBO J. 21, 270-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galli, T., McPherson, P. S. & De Camilli, P. (1996) J. Biol. Chem. 271, 2193-2198. [DOI] [PubMed] [Google Scholar]
- 31.Peters, C., Bayer, M. J., Bühler, S., Andersen, J. S., Mann, M. & Mayer, A. (2001) Nature 409, 581-588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.