Abstract
A role for Notch in the elaboration of existing neural processes is emerging that is distinct from the increasingly well understood function of this gene in binary cell-fate decisions. Several research groups, by using a variety of organisms, have shown that Notch is important in the development of neural ultrastructure. Simultaneously, Presenilin (Psn) was identified both as a key mediator of Notch signaling and as a site of genetic lesions that cause early-onset Alzheimer's disease. Here we demonstrate that Notch loss of function produces memory deficits in Drosophila melanogaster. The effects are specific to long-term memory, which is thought to depend on ultrastructural remodeling. We propose that Notch plays an important role in the neural plasticity underlying consolidated memory.
Whereas the Notch protein plays an important role in binary cell-fate decisions during development (1), it is also present in the adult brain (2, 3). This finding is particularly interesting in adult Drosophila because the CNS is established by eclosion (4). Given that Notch is not needed for cell-fate decisions in the adult brain, its role in this tissue is unclear.
Several groups have shown that Notch is important in the arborization of neuritic processes in development. In mammals, Notch regulates arborization of cortical neurons in vivo (5, 6) and in tissue culture (7, 8). In Drosophila, the Notch pathway is required for the elaboration of processes in the CNS in the developing third-instar larva (9) and in the embryo (10, 11). Recently, this work has been extended to the analysis of the developing neuromuscular junction, a structure that serves as an accessible model for CNS plasticity. In addition, a Psn-mediated role for Notch is required in the development of neural projections mediating learned thermo-taxis in Caenorhabditis elegans (12). Recently (3), we demonstrated that prolonged disruptions in Notch function produced an early lethality and an impairment in the coordinated neuromuscular activity of flight. Accordingly, because Notch clearly is involved in the regulation of neural ultrastructure during development, we investigated the possibility that Notch is also required for memory consolidation, a process believed to require remodeling of existing neurons in adults (13).
Drosophila is an ideal organism for studying genes influencing behavioral phenotypes. Advances in our understanding of learning and memory mechanisms have been achieved through genetic, transgenic, and genomic studies in the fly (14). To investigate Notch function in adults, it is necessary to use conditional reagents to avoid developmental phenotypes that may kill the fly or compromise behavior. We used a Notch temperature-sensitive allele (Nts1) (15) and RNA interference (RNAi) derived from an inducible transgene (16), in combination with two independent behavioral assays for memory. We show that short-term memory is not impaired by conditional manipulations of Notch, but that Notch is required in adults for long-term memory.
Methods
Drosophila Strains. Flies were reared in standard cornmeal molasses medium at 18°C or 25°C. Typically, flies studied were products of crosses between two separately maintained lines to reduce effects of potential modifiers. We used Nts1, Canton Special (CS) (17), P[w+,hsΔEGF1–18] (hs-ΔEgf-N) (18), P[w+,hsN](hs-N+) (19), c772 (20), UAS-Ni (16), and UAS-LacZi (21). Working Nts1 parental strains were generated from a line bearing a duplication of the Notch locus to limit the accumulation of Notch modifiers. Isolines were chosen based on permissive temperature flight and nonpermissive temperature impairments in flight and longevity, which is consistent with our previously published phenotypes for adult Notch dysfunction (16).
Conditioned Courtship. For tests of short-term memory, male flies of the indicated genotypes were collected within 4 h of eclosion. Flies were either maintained at permissive conditions (18°C) until training and testing, or were reared at 18°C until 3 days after eclosion and then transferred to the nonpermissive conditions (29°C) for an additional 2 days. Before training, all males were placed individually into empty 12 × 75 mm glass tubes. Female flies that had been directly observed copulating the previous evening were placed with males in the tubes for 1 h. Female flies were then removed from the tubes and males were isolated for 30 min. Flies were coded and the observing investigator was blind as to both the genotype and experience of flies at testing. After being allowed to explore their new environment for 30 sec, male behavior was observed in a clear rectangular plastic examination chamber (20 × 20 × 5 mm) with a virgin female. During the subsequent 5-min test interval, the percentage of time the male spent engaged in any courtship behavior (tapping, singing, following, etc.; 22) was recorded. Males that succeeded in copulating with a female in <2 min were excluded from the experiment, even though the frequency of this event occurred in <10% of the pairings (23). Time spent actively courting was divided by the examination time to obtain a courtship index. In tests of long-term memory, flies were collected and prepared as in short-term testing; however, all males were placed into small glass tubes with ≈0.5 ml of culture medium, and the training interval was extended to 5 h. Female flies were removed from the tubes and males were returned to the appropriate incubators. Two days later, flies were coded and a blind investigator examined courtship behavior of males in pairings with virgin females as described above. Behaviors from at least 15 flies (a typical number for this assay) were included in the analysis of every cohort shown. Memory was assessed by comparing the courtship of trained and naïve flies. Behavioral differences were tested for significance by using a Student's t test.
Pavlovian Olfactory Conditioning. In temperature-sensitive experiments, flies were maintained at 18°C for at least 3 days and were then shifted to nonpermissive conditions for 4 days in short-term tests, or 2 days in long-term tests. RNAi experiments were conducted in flies maintained at 25°C for at least 4 days after eclosion. Training and testing were carried out following protocols described (24, 25). Briefly, ≈100 flies were transferred to copper grids and exposed to air bubbled through concentrations of benzaldehyde (7.5 × 10-5) or 3-octanol (10-4) diluted in mineral oil, and were temporally paired with 12 electric shocks (90 volts direct current for 1.25 sec) over the course of 1 min. After a 45-sec rest interval, the flies were then exposed to the other odor without shock. To test short-term memory retention, flies were allowed to choose between the two odors in a T-maze 30 min after training. Avoidance of each shock-paired odor was recorded, and those two values were averaged to generate a performance index (PI). A PI of 100 indicates a perfect avoidance of the shock-paired odor, whereas 0 indicates no detectable odor preference. For long-term memory tests, flies were subjected to 10 rounds of single-cycle training described above, with a 15-min rest interval between each cycle. Flies were then returned to the appropriate temperature (18°C, 25°C, or 29°C) on culture medium and then tested 1 day later. Comparisons were made by using either Student's t test (for two groups) or analysis of ANOVA and Student–Newman–Keuls multiple range tests (for more than two groups).
Results
Temperature-Sensitive Notch Mutants Display Intact Short-Term Memory and Impaired Long-Term Memory in Courtship Conditioning. Of the several protocols used to train and test memory in Drosophila, we began with conditioned courtship, because the assay is robust and employs cues and behavioral assessments within the normal range of experience of adult flies (23). In conditioned-courtship training, the vigor of a male's courtship activity, as determined by the time spent courting, is assayed after prolonged rejection by a nonvirgin fly. Once exposed to the unresponsive female, the subsequent courtship activity of the male (measured as a courtship index) is usually reduced compared to a naïve male. Thus, flies were tested 30 min after training to see whether their short-term memory is impaired when Notch is compromised. We observed that after shifting to 29°C (the nonpermissive temperature for the Nts1 mutant), both CS-control and Nts1 males spent significantly less time courting after training (Fig. 1a). Presumably, this change occurs because the short-term memory of the rejected attempts at copulation is intact. These results indicate that Notch dysfunction does not cause generalized developmental defects or problems with the sensory, processing, or motor apparatus.
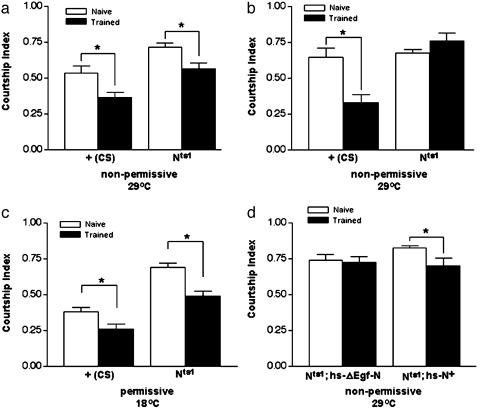
Fig. 1.
Conditioned courtship indicates that Notch dysfunction impairs long-term memory. (a) Short-term memory was examined 30 min after training. Wild-type (CS) and Nts1 flies display intact short-term memory after 2 days in nonpermissive conditions.*, Statistical differences between naïve and trained cohorts indicating memory of training (P = 0.0087 for CS and P = 0.0088 for Nts1). (b) Long-term memory was examined 2 days after training. CS males show intact long-term memory (P = 0.0007 for CS) after 2 days in nonpermissive conditions, whereas Nts1 flies do not demonstrate long-term memory. (c) Long-term memory was examined in flies maintained at permissive temperatures to test for developmental enfeeblement. Under permissive conditions, both CS and Nts1 flies display statistically significant differences in naïve versus trained data sets, demonstrating the presence of long-term memory (P = 0.0188 for CS and P = 0.0003 for Nts1). (d) Two-day long-term memory was examined in Nts1 flies bearing heat shock-driven wild-type Notch transgene (Nts1;hs-N+/+) or a phenotypically nonfunctional control Notch missing ligand-interaction domains (Nts1;hs-ΔEgf-Ni/+). Only flies carrying functional Notch displayed statistically significant rescue of long-term memory (P = 0.0430 for Nts1;hs-N+/+). These results indicate that requirements for long-term memory are specific for functional Notch. At least 15 flies were examined in every cohort shown.
We next investigated long-term memory under nonpermissive conditions by testing flies 2 days after training. We found that Nts1 (but not CS) males spent as much time courting as naïve flies (Fig. 1b), indicating that long-term memory is impaired in Nts1 flies shifted to 29°C.
To address the possibility that unspecified developmental enfeeblement of Nts1 flies causes long-term memory defects, we tested flies that were maintained at the permissive temperature. Under these conditions, we observed significant courtship differences between naïve and trained cohorts, indicating that long-term memory is intact in Nts1 flies if they area kept at 18°C (Fig. 1c). Thus, the neuroanatomical structures that may be specific for long-term memory (26) are spared by maintaining Nts1 flies under permissive conditions. These results further demonstrate that there is no “developmental” enfeeblement of Nts1 flies.
We confirmed that the above effects on long-term memory are specific for the Notch locus by testing Nts1 flies carrying separate inducible transgenes in a rescue experiment. The hs-N+ transgene (19) carries a wild-type copy of the Notch gene under control of the hsp 70 promoter. The hs-ΔEgf-N transgene (driven by the same promoter as hs-N+) encodes a nonfunctional Notch protein lacking important ligand-interaction domains (27). Behavior of naïve and trained flies was not significantly different in hs-ΔEgf-N flies, indicating that the nonfunctional Notch control did not rescue the memory defects of Nts1 (Fig. 1d). However, trained Nts1 flies bearing hs-N+ spent significantly less time courting than did naïve flies, suggesting that the memory impairment rescued in these flies is specific to the Notch locus.
Temperature-Sensitive Notch Mutants Display Intact Short-Term Memory and Impaired Long-Term Memory in Pavlovian Olfactory Conditioning. We sought to confirm our findings by using a second, more quantitative method. In our Pavlovian olfactory conditioning assay, an unconditioned stimulus (electric shock) is paired temporally with a conditioned stimulus (odor). Subsequent aversion to the odor demonstrates a memory of the pairing with the shock (24, 25). We used Pavlovian olfactory conditioning to examine the effects of Notch loss of function on short-term and long-term memory.
Nts1 flies tested under nonpermissive conditions showed intact short-term memory of odor-shock pairing as demonstrated by normal performance indices (Fig. 2a). This finding further strengthens the observation that Nts1 flies are not developmentally impaired or defective in the neural systems mediating sensory, motor, and short-term memory functions. However, Nts1 flies under nonpermissive conditions showed impaired long-term memory compared to controls (Fig. 2b), whereas, Nts1 flies maintained at permissive conditions showed normal memory (Fig. 2c). These results are all in agreement with our data from the conditioned courtship assay presented above. They confirm that Notch loss of function, mediated through the Nts1 allele, disturbs long-term memory specifically.
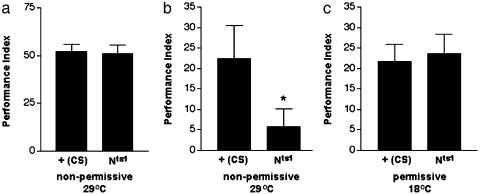
Fig. 2.
Nts1-mediated Notch dysfunction impairs long-term memory, as demonstrated by Pavlovian olfactory conditioning. (a) Short-term memory was examined 30 min after training in flies kept in nonpermissive conditions for 4 days. Flies displayed strong avoidance of the odor paired with footshock, demonstrating intact short-term memory (P < 0.0001 for both). There were also no statistically significant differences between behavior of CS and Nts1 flies. (b) Flies were kept in nonpermissive conditions for 2 days, were trained, and were then tested for long-term memory 1 day after training. Under nonpermissive conditions, CS flies displayed statistically significant odor avoidance (P = 0.0182 for CS), whereas Nts1 fly performance was impaired and did not differ significantly from 0 (indicated by an asterisk). These results indicate that Nts1 flies have an impaired long-term memory. (c) Long-term memory was examined in flies maintained at permissive conditions. Both CS and Nts1 flies displayed significant odor avoidance, indicating that long-term memory is intact for both (P = 0.0003 for CS and P = 0.0005 for Nts1). Data for female flies are shown above. We found that females performed slightly better than males under these nonoptimal conditions for memory testing, although males showed the same trends. Data from six PIs for short-term (representing ≈1,200 flies) and 12 PIs for long-term memory are shown.
RNAi-mediated Notch Dysfunction Spares Short-Term Memory and Impairs Long-Term Memory in Pavlovian Olfactory Conditioning. To further test the requirement of Notch in long-term memory, we used another reagent capable of silencing Notch function in a conditional manner. We have developed an inducible Notch RNAi transgene (Ni) that dramatically reduces Notch protein levels to produce a loss-of-function phenotype when assayed in a developmental context (16). This reagent can be used with the upstream activating sequence (UAS)-GAL4 binary expression system, which allows tissue-specific targeting (28) of the RNAi. We tested several Drosophila enhancer-Gal4 lines known to be expressed in the adult CNS and/or mushroom bodies (MBs), which are the neuroanatomical structures known to mediate olfactory associations (29). Many of these combinations (CNS-Gal4; UAS-Ni) caused prepupal lethality, or displayed no phenotypic effects (data not shown). However one line (c772), which has previously been used to target gene expression to the MBs (30), enabled us to study Notch dysfunction in adult flies.
We found that all three genotypes tested for short-term memory (UAS-Ni with no driver, c772 driving UAS-Ni, and c772 driving RNAi for the LacZ gene) were not significantly different (Fig. 3a). This result also indicates that when Notch is silenced in the c772 expression pattern, neural structures mediating sensory acuity and motor behavior are spared. However, when these flies are tested for long-term memory, they are impaired in comparison to control flies bearing Ni without the driver or control flies expressing an irrelevant RNAi (LacZi) under control of c772 (Fig. 3b). These observations further confirm the results from Nts1 experiments through an entirely different mechanism for inhibiting Notch. They strengthen the conclusion that Notch is required for the generation of long-term memory and they agree with correlative work suggesting that long-term memory is processed in the MBs (26).
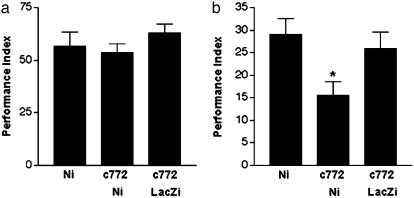
Fig. 3.
RNAi-mediated Notch silencing impairs long-term memory of Pavlovian olfactory conditioning. (a) Flies were examined for short-term memory 30 min after training at 25°C. We tested flies containing the responder only [UAS-Ni/+ (Ni)] as a control for insertion-site effects, flies expressing Notch RNAi in adult CNS including MBs [c772/+; UAS-Ni/+ (c772-Ni)], and flies bearing a control RNAi-producing transgene driven by the c772-Gal4 line [c772/+; UAS-LacZi/+ (c772-LacZi)]. All groups showed statistically significant short-term memory with no differences among groups (ANOVA, F(33) = 0.92, P = 0.4187). (b) Long-term memory was tested in Ni, c772-Ni, and c772-LacZi flies 1 day after training. Statistical analysis demonstrated long-term memory in all cohorts, but significant impairment of long-term memory in c772-Ni flies was identified by a Student–Newman–Keuls test (ANOVA, F(33) = 4.20, P = 0.0207, shown by an asterisk). Data from 6 PIs in short-term memory and at least 16 PIs in long-term memory are shown.
Discussion
We have shown that Notch dysfunction in the adult CNS impairs long-term memory in Drosophila through two different mechanisms: a temperature-sensitive allele and RNAi. The results were confirmed by using two different behavioral assays: conditioned courtship and Pavlovian odor avoidance training. Similar deficits in long-term memory have been observed by using heat shock-driven dominant-negative protein constructs (Y. Zhong, personal communication). Our interventions do not affect the structure of the Drosophila CNS at the gross anatomical level as determined by planimetric measurements of their autofluorescent profiles (data not shown), and because they are conditional, we are able to examine behavioral phenotypes that would be masked by developmental defects. Furthermore, because we can exercise temporal control, and because there is no neurogenesis in the adult Drosophila CNS (4), we can examine Notch functions independent of cell-fate decisions. The mechanism through which Notch facilitates long-term memory is unclear. However, we speculate that Notch may function in the regulation of structural neuroplasticity (neuronal arborization and synaptogenesis), which is essential to support long-term memory consolidation.
Prior studies in mammals (31, 32) have identified memory defects in Notch pathway mutants. Conditional Psn-defective mice showed subtle perturbations in memory, but because Psn function is integral in several pathways, it was not clear which might have been responsible for the impairment. Recently, a complementary study in mice (33) has shown that heterozygotes deficient for Notch1 or the downstream effector, Su(H)/CBF1/RBP-Jκ, showed similar memory defects. In agreement with our findings, this impairment in mammals appears to preferentially affect the generation of long-term memory in the Morris water maze test, while sparing short-term spatial memories.
The finding that Notch is required for long-term memory is exciting in the context of our understanding of learning, memory, and behavior. The role we have demonstrated for Notch in the generation of long-term memory provides a means to genetically manipulate long-term memory, and is consistent with previous studies (14) indicating that memory phases are mechanistically distinct. For example, much of the progress in understanding and dissecting pathways involved in learning and short-term memory has been aided by the careful analysis of behavior in mutants affecting the cAMP pathway (e.g., dunce and rutabaga; ref. 34). In contrast, only recently has a screen for long-term memory consolidation been completed, which has identified a key gene in the processes, stuafen (35). It is possible that both staufen and Notch will be the pioneering members of new class of memory genes that regulate synaptic connectivity, just as the cAMP/cyclic AMP response element-binding protein (CREB) pathway regulates synaptic activity (34). Thus, conditional strategies that allow for the gene functions to be separated (developmental requirements from adult function) will continue to be important tools for addressing such questions. Perhaps by understanding the role of Notch in ultrastructural remodeling and behavioral plasticity, we may open new avenues for understanding the mechanisms of memory consolidation and integrated signaling necessary to establish long-term memory. In addition, our use of tissue-restricted expression of an interfering RNA against Notch suggests that MBs are the anatomical structures where long-term memory is consolidated. These results are consistent with the recent study of ala (α-lobes absent)-mutant flies. Such flies have long-term memory impairments, and they also display specific defects in MB substructures (26).
Finally, these findings are also exciting because they may be relevant to human disease processes. In its earliest phase, Alzheimer's disease (AD) is best characterized by the loss of the ability to encode new memories because of synapse dysfunction (36). Research into the causes of AD has profited enormously from the identification of the genetic lesions that cause early onset familial AD. The two loci most frequently identified are the two Psn genes (37). Interestingly, in the circumstances in which AD-producing mutations have been analyzed for Notch activity, all show loss-of-function Notch phenotypes (12, 38–40). Because we have demonstrated here that compromising Notch damages memory, it is possible that this dysfunction may also contribute to some cases of AD pathology.
Whether Notch contributes to AD pathology remains an exciting open question for future experiments. However, the results presented above clearly demonstrate that Notch is necessary for important functions of the adult nervous system. Elucidating the molecular mechanism responsible for this observation should offer new insight into the cellular basis of learning and memory in this genetically tractable organism.
Acknowledgments
We thank Y. Zhong for the generous sharing of data before publication; E. Giniger, T. Leiber, M. Young, S. Artavanis-Tsakonas, U. Heberlein, and H. Bellen for reagents; and R. Zwacka and J. S. Nye for helpful discussion and comments on the manuscript. This work was supported by National Science Foundation Grants MCB-0230186 (to A.J.A.) and IBN-0237395 (to J.S.d.B.).
Abbreviations: RNAi, RNA interference; PI, performance index; CS, Canton Special; UAS, upstream activating sequence; MB, mushroom body; AD, Alzheimer's disease.
References
- 1.Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. (1999) Science 284, 770-776. [DOI] [PubMed] [Google Scholar]
- 2.Kidd, S., Baylies, M. K., Gasic, G. P. & Young, M. W. (1989) Genes Dev. 3, 1113-1129. [DOI] [PubMed] [Google Scholar]
- 3.Presente, A., Andres, A. & Nye, J. S. (2001) NeuroReport 12, 3321-3325. [DOI] [PubMed] [Google Scholar]
- 4.Ito, K. & Hotta, Y. (1992) Dev. Biol. 149, 134-148. [DOI] [PubMed] [Google Scholar]
- 5.Redmond, L., Oh, S. R., Hicks, C., Weinmaster, G. & Ghosh, A. (2000) Nat. Neurosci. 3, 30-40. [DOI] [PubMed] [Google Scholar]
- 6.Sestan, N., Artavanis-Tsakonas, S. & Rakic, P. (1999) Science 286, 741-746. [DOI] [PubMed] [Google Scholar]
- 7.Franklin, J. L., Berechid, B. E., Cutting, F. B., Presente, A., Chambers, C. B., Foltz, D. R., Ferreira, A. & Nye, J. S. (1999) Curr. Biol. 9, 1448-1457. [DOI] [PubMed] [Google Scholar]
- 8.Berezovska, O., Frosch, M., McLean, P., Knowles, R., Koo, E., Kang, D., Shen, J., Lu, F. M., Lux, S. E., Tonegawa, S. & Hyman, B. T. (1999) Brain Res. 69, 273-280. [DOI] [PubMed] [Google Scholar]
- 9.Hassan, B. A., Bermingham, N. A., He, Y., Sun, Y., Jan, Y. N., Zoghbi, H. Y. & Bellen, H. J. (2000) Neuron 25, 549-561. [DOI] [PubMed] [Google Scholar]
- 10.Giniger, E., Jan, L. Y. & Jan, Y. N. (1993) Development (Cambridge, U.K.) 117, 431-440. [DOI] [PubMed] [Google Scholar]
- 11.Fambrough, D., Pan, D., Rubin, G. M. & Goodman, C. S. (1996) Proc. Natl. Acad. Sci. USA 93, 13233-13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wittenburg, N., Eimer, S., Lakowski, B., Rohrig, S., Rudolph, C. & Baumeister, R. (2000) Nature 406, 306-309. [DOI] [PubMed] [Google Scholar]
- 13.Bailey, C. H., Bartsch, D. & Kandel, E. R. (1996) Proc. Natl. Acad. Sci. USA 93, 13445-13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubnau, J., Chiang, A. S. & Tully, T. (2003) J. Neurobiol. 54, 238-253. [DOI] [PubMed] [Google Scholar]
- 15.Shellenbarger, D. L. & Mohler, J. D. (1978) Dev. Biol. 62, 432-446. [DOI] [PubMed] [Google Scholar]
- 16.Presente, A., Shaw, S., Nye, J. S. & Andres, A. J. (2002) Genesis 34, 165-169. [DOI] [PubMed] [Google Scholar]
- 17.Linsey, D. M. & Zimm, G. G. (1992) The Genome of Drosophila Melanogaster (Academic, San Diego).
- 18.Lieber, T., Kidd, S., Alcamo, E., Corbin, V. & Young, M. W. (1993) Genes Dev. 7, 1949-1965. [DOI] [PubMed] [Google Scholar]
- 19.Rebay, I., Fehon, R. G. & Artavanis-Tsakonas, S. (1993) Cell 74, 319-329. [DOI] [PubMed] [Google Scholar]
- 20.Yang, M. Y., Armstrong, J. D., Vilinsky, I., Strausfeld, N. J. & Kaiser, K. (1995) Neuron 15, 45-54. [DOI] [PubMed] [Google Scholar]
- 21.Kennerdell, J. R. & Carthew, R. W. (2000) Nat. Biotechnol. 18, 896-898. [DOI] [PubMed] [Google Scholar]
- 22.Hall, J. C. (1994) Science 264, 1702-1714. [DOI] [PubMed] [Google Scholar]
- 23.McBride, S. M., Giuliani, G., Choi, C., Krause, P., Correale, D., Watson, K., Baker, G. & Siwicki, K. K. (1999) Neuron 24, 967-977. [DOI] [PubMed] [Google Scholar]
- 24.Tully, T. & Quinn, W. G. (1985) J. Comp. Physiol. A 157, 263-277. [DOI] [PubMed] [Google Scholar]
- 25.Tully, T., Preat, T., Boynton, S. C. & Del Vecchio, M. (1994) Cell 79, 35-47. [DOI] [PubMed] [Google Scholar]
- 26.Pascual, A. & Preat, T. (2001) Science 294, 1115-1117. [DOI] [PubMed] [Google Scholar]
- 27.Fehon, R. G., Kooh, P. J., Rebay, I., Regan, C. L., Xu, T., Muskavitch, M. A. & Artavanis-Tsakonas, S. (1990) Cell 61, 523-534. [DOI] [PubMed] [Google Scholar]
- 28.Brand, A. H. & Perrimon, N. (1993) Development (Cambridge, U.K.) 118, 401-415. [DOI] [PubMed] [Google Scholar]
- 29.de Belle, J. S. & Heisenberg, M. (1994) Science 263, 692-695. [DOI] [PubMed] [Google Scholar]
- 30.Zars, T., Fischer, M., Schulz, R. & Heisenberg, M. (2000) Science 288, 672-675. [DOI] [PubMed] [Google Scholar]
- 31.Yu, H., Saura, C. A., Choi, S. Y., Sun, L. D., Yang, X., Handler, M., Kawarabayashi, T., Younkin, L., Fedeles, B., Wilson, M. A., et al. (2001) Neuron 31, 713-726. [DOI] [PubMed] [Google Scholar]
- 32.Feng, R., Rampon, C., Tang, Y. P., Shrom, D., Jin, J., Kyin, M., Sopher, B., Miller, M. W., Ware, C. B., Martin, G. M., et al. (2001) Neuron 32, 911-926. [DOI] [PubMed] [Google Scholar]
- 33.Costa, R. M., Honjo, T. & Silva, A. J. (2003) Curr. Biol. 13, 1348-1354. [DOI] [PubMed] [Google Scholar]
- 34.Davis, G. W., Schuster, C. M. & Goodman, C. S. (1996) Neuron 17, 669-679. [DOI] [PubMed] [Google Scholar]
- 35.Dubnau, J., Chiang, A. S., Grady, L., Barditch, J., Gossweiler, S., McNeil, J., Smith, P., Buldoc, F., Scott, R., Certa, U., et al. (2003) Curr. Biol. 13, 286-296. [DOI] [PubMed] [Google Scholar]
- 36.Selkoe, D. J. (2002) Science 298, 789-791. [DOI] [PubMed] [Google Scholar]
- 37.Haass, C. (1996) Curr. Opin. Neurol. 9, 254-259. [PubMed] [Google Scholar]
- 38.Ye, Y., Lukinova, N. & Fortini, M. E. (1999) Nature 398, 525-529. [DOI] [PubMed] [Google Scholar]
- 39.Levitan, D., Doyle, T. G., Brousseau, D., Lee, M. K., Thinakaran, G., Slunt, H. H., Sisodia, S. S. & Greenwald, I. (1996) Proc. Natl. Acad. Sci. USA 93, 14940-14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Strooper, B., Annaert, W., Cupers, P., Saftig, P., Craessaerts, K., Mumm, J. S., Schroeter, E. H., Schrijvers, V., Wolfe, M. S., Ray, W. J., et al. (1999) Nature 398, 518-522. [DOI] [PubMed] [Google Scholar]