Abstract
Knowledge regarding the in vivo performance and periposthetic tissue response of cervical and lumbar total disc replacements (TDRs) continues to expand. This review addresses the following four main questions: 1) What are the latest lessons learned from polyethylene in large joints and how are they relevant to current TDRs? 2) What are the latest lessons learned regarding adverse local tissue reactions from metal-on-metal, CoCr bearings in large joints and how are they relevant to current TDRs? 3) What advancements have been made in understanding the in vivo performance of alternative biomaterials, such as stainless steel and polycarbonate urethane, for TDRs in the past five years? 4) How has retrieval analysis of all these various artificial disc bearing technologies advanced the state of the art in preclinical testing of TDRs? The study of explanted artificial discs and their associated tissues can help inform bearing selection as well as the design of future generations of disc arthroplasty. Analyzing retrieved artificial discs is also essential for validating preclinical test methods.
Keywords: total disc arthroplasty, total disc replacement, TDR, ultra-high molecular weight polyethylene, UHMWPE, wear, oxidation, retrieval analysis, explant analysis, stainless steel, polycarbonate urethane, PCU, polyurethane, cobalt chrome, CoCr, metal-on-metal, adverse local tissue reactions
1. Introduction
Knowledge regarding the in vivo performance and periposthetic tissue response of cervical and lumbar total disc replacements (TDRs) continues to expand. Many different cervical and lumbar artificial discs are currently available and are fabricated from a range of polymers and metals, which may be unfamiliar to a spine surgeon or resident. Some of these biomaterials, such as ultra-high molecular weight polyethylene (hereafter, polyethylene) and cobalt chromium alloy (hereafter, CoCr alloy), have a long-standing history as orthopaedic bearing materials for total joint replacements1. Artificial disc designers have also incorporated novel biomaterials as bearing materials for artificial discs2, including titanium alloys, stainless steel, polycarbonate urethanes (PCU), and polyaryletheretherketone (PEEK). Until recently, it has remained unclear which bearing materials are sufficiently durable, biocompatible, and resistant to mechanical loading for use in cervical or lumbar disc arthroplasty. As noted in previous reviews3, 4, the study of explanted artificial discs and periprosthetic tissues can help inform bearing selection decisions as well as improve the design of future generations of disc arthroplasty.
Bearing selection continues to be an important topic for large total joint replacements used in the hip and the knee5. In response to concerns regarding wear, osteolysis, and instability of polyethylene acetabular liners, major evolutionary changes to the biomaterials and bearing designs used for hip arthroplasty have occurred over the past decade1. In the late 1990s, the vast majority of hip replacements performed in the United States included gamma-sterilized polyethylene liners, whereas today four types of bearing materials are in clinical use (Table 1). Highly cross-linked polyethylene is the most widely used hip bearing material for the acetabulum, however alternative bearings such as metal-on-metal (MOM) and ceramic-on-ceramic (COC) were, for a time, increasingly adopted in the mid 2000s6. These premium “hard-on-hard” bearings are more expensive and sensitive to surgical positioning than highly cross-linked polyethylene6, 7, and their utilization has, consequently, since waned. Of the contemporary bearing material combinations used for large total joint replacement, only metal-on-polyethylene (M-PE) and MOM articulations have been employed for disc replacements. One of the goals of this review is to synthesize the latest developments in bearing technology from orthopaedics with the emerging perspective obtained from analyzed retrieved total disc replacements.
Table 1.
Total Hip Replacement Bearing Materials1
| Type | Femoral Head | Acetabular Liner |
|---|---|---|
| M-PE | CoCr Alloy | Polyethylene |
| C-PE | Ceramic | Polyethylene |
| COC | Ceramic | Ceramic |
| MOM | CoCr Alloy | CoCr Alloy |
In addition to informing bearing selection, analyzing retrieved artificial discs is also essential for validating preclinical test methods8. As we gain experience with the clinical performance of artificial discs, the observations of retrieved implants provide valuable insight for the methodologies used in wear and fatigue testing8. Working with regulatory agencies under the auspices of the American Society for Testing and Materials International (ASTM), biomedical engineers and surgeons have helped to advance the state of the art in wear testing, not only under normal intended use conditions, but also under more demanding, impingement scenarios that are frequently observed in retrieved artificial discs.
None of the advancements described in this review would have been possible without the close and active collaboration between surgeons and biomedical engineers. In recent years, researchers have been studying explanted artificial discs and periprosthetic tissues from retrieved cervical and lumbar artificial discs to better understand the durability and biocompatibility of these implants3, 4. Many of these artificial discs and tissues were collected as part of a public, multi-center, federally funded retrieval research program3, 4, but retrieval studies are also conducted by manufacturers as part of FDA-mandated post-market surveillance programs. This article builds on previous reviews that focused on the motivation, methodologies, and early findings from the first retrieval analyses of artificial discs3, 4. In this article, we concentrate on addressing the following four main questions: 1) What are the latest lessons learned from polyethylene in large joints and how are they relevant to current TDRs? 2) What are the latest lessons learned regarding adverse local tissue reactions from metal-on-metal, CoCr bearings in large joints and how are they relevant to current TDRs? 3) What advancements have been made in understanding the in vivo performance of alternative biomaterials, such as stainless steel and polycarbonate urethane, for TDRs in the past five years? 4) How has retrieval analysis of all these various artificial disc bearing technologies advanced the state-of-the-art in preclinical testing of TDRs?
2. Update on Polyethylene Used in Total Joint Replacements and Disc Replacement
In total hip and knee arthroplasty, the dominant bearing technology for the past 50 years continues to be a CoCr alloy femoral component articulating against a polyethyelene acetabular or tibial component1. Especially within the past two decades, the polyethylene used in hip and knee joint arthroplasty has undergone major changes in terms of formulation5. By contrast, the changes to polyethylene formulation in the spine are comparatively subtle, mirroring the early evolution of sterilization practices for polymer hip and knee components in the early 1990s9. Prior to the mid-1990s, polyethylene was gamma sterilized in air-permeable packaging, which contributed to shelf aging prior to implantation and in vivo oxidation. In the 1990’s, the state-of-the-art for polyethylene shifted to gamma sterilization in a low oxygen environment (often referred to as “gamma inert sterilization”). Gamma sterilization of polyethylene in air was thus gradually abandoned, but variation in industry practices for packaging limited the effectiveness of this transition10. Indeed, the first generation of gamma sterilization in nitrogen by Link, the original producers of the CHARITÉ TDR, were permeable to oxygen, and thus exhibited similar degradation as earlier implants that were gamma sterilized in air9. Oxygen impermeable packaging is the key to successful preservation of polyethylene properties during shelf aging10.
Beginning in 1998, several generations of highly cross-linked polyethylene materials have been introduced and have since become the standard of care in hip arthroplasty1. It is now clear from the orthopaedic literature that highly cross-linked polyethylene reduces wear as well as the risk of osteolysis in total hip replacements (THR)5. However, not all implant designs may be appropriate for highly cross-linked polyethylene. Although elevated crosslinking improves wear resistance, it also reduces the material’s ductility and fracture resistance11. The concomitant decrease in mechanical properties that occurs during the cross-linking process may be deleterious to both knee and spine devices. Thus, gamma inert sterilized polyethylene continues to be used in total knee and disc replacements, at least partly because of the increased stresses that may be encountered in these applications.
Much of the existing retrieval evidence published in journal articles thus far for polyethylene in TDRs is based on the equivalent of historical gamma air-sterilized material that was abandoned almost two decades ago. Why, then, continue to study these early polyethylene TDRs? It turns out that these explants helped to dispel many of the optimistic preconceptions offered by the early promoters of disc arthroplasty. In 2003, Link and Keller12 wrote that “it is reassuring that the anterior column of the lumbar spine appears to be one place in the human body where periprosthetic osteolysis is not a major factor, due to lower ranges of motion and an absence of synovium compared to the hip and knee joint.” During the early 2000s, the issues of wear and osteolysis were poorly understood, and hence, underestimated by the spine community advocating disc arthroplasty as an alternative to fusion.
The first systematic retrieval analyses of a collection of polyethylene TDR components, performed on the CHARITÉ artificial disc, was instrumental in challenging the established paradigm in the spine community that wear was irrelevant for disc arthroplasty. A series of disc retrieval and clinical publications helped to establish osteolysis and polyethylene wear as clinically relevant, albeit rare, complications of disc arthroplasty13–15. Upon examination, the central dome and peripheral rim of the explanted polyethylene components exhibited different wear and damage mechanisms16. The central dome often exhibited burnishing and the microscopic, multidirectional scratching consistent with the adhesive and abrasive wear mechanism characteristic of total hip replacement. On the other hand, the peripheral rim of the retrievals sometimes showed evidence of delamination, cracking, and fracture, damage modes most often associated with gamma-air sterilized total knee replacements. The central dome adhesive/abrasive wear was associated with the expected, dome-to-dome articulation of the disc arthroplasty, whereas the peripheral rim cracking and fractures were associated with impingement. These basic observations, performed on a collection of 21 retrieved CHARITÉ TDRs16, would provide the basis for starting to validate the methods used to test artificial discs8. Subsequent publications focused on quantitatively characterizing the patterns of wear17 and the natural history of oxidative degradation9 of the polyethylene TDRs.
Having demonstrated that clinically relevant wear was rare but indeed possible with historical, gamma-air sterilized polyethylene TDRs, researchers turned to study the characteristics and biological ramifications of wear debris released into the intradiscal periprosthetic tissues18–20. In one of the only long-term studies of retrieved TDR tissue, we observed a chronic inflammatory response within the periprosthetic fibrous tissues from 15 of 16 patients who had undergone revision surgery of historical Charité TDRs20.
Revision for all of these patients was indicated for intractable pain after an average of 9 years implantation (range 3–16 years). The inflammatory cells consisted of lymphocytes, macrophages and giant cells, which were associated with ingested small or uningested large polyethylene particles. Polyethylene particles greater than 2 μm were detected in tissue samples from 15 of 16 patients. In addition, the presence of giant cells and polyethylene wear debris increased with implantation time, and were associated with inflammatory cytokines such as tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) in a subset of CD-68 positive macrophages and giant-cells. The highest number of polyethylene particles was observed in the single patient that showed signs of osteolysis of the sacrum14, 19, 20. Innervation and vascularization were also noted within the retrieved tissue. Inflammation and innervation may contribute to the development of neuroinflammatory-induced pain in TDR patients. These findings point to the complexity of the wear debris interactions in the spine and of the clinically relevant wear debris required to stimulate histiocytes and giant cell formation.
In total hip arthroplasty (THA) tissues, submicron-sized polyethylene particles have been shown to be critical in promoting the phagocytic inflammatory response and stimulating the production of proinflammatory factors. Further evaluation of five TDR patient tissues with wear and inflammation by scanning electron microscopy (SEM), showed over a billion 0.5–2.0 μm polyethylene wear particles per gram of tissue (1.6 × 109/gm)19. Compared to gamma-air sterilized THA revision tissue (2.3 × 109/gm), a significantly lower concentration of particles was found in TDR tissue, however this represents a substantial load within spinal tissue. Moreover, despite differences in loading and kinematics between the lumbar spine and the hip joint, the mean wear particle size and shape were comparable, although the TDR particles tended to be smaller and more round. No correlations were found between visible damage to the UHMWPE core and the concentration or shape of the UHMWPE particles. A positive correlation was found for increasing particle size and increasing rim penetration of the TDR core. The lower concentration of polyethylene particles in TDR tissue might explain why pain rather than osteolysis is the major reason for revision surgery. For the single osteolytic TDR patient and sixth patient evaluated, a high concentration of particles was detected (2.58 × 109/gm).
Based on the data from retrieved, historical TDRs and their periprosthetic tissues, it appears that, overall, the clinical consequences of gamma air-sterilized polyethylene have conformed to expectations from hip and knee arthroplasty, with a few twists or “surprises.” Perhaps the first surprise is that osteolysis is so rare following these historical TDRs, especially because it is clear the periprosthetic particle load and characteristics are at least comparable to hip arthroplasty in some patients. We observed on the order of 1 billion polyethylene particles per gram of the retrieved periprosthetic tissues from these TDR patients, yet—with only one exception in our series—these levels were not associated with osteolysis of the adjacent vertebral bodies19. The second unexpected finding relates to observations of impingement, which were common in the collection of retrieved disc replacements. In addition, we observed new nerve growth in certain samples of the periprosthetic tissues. It remains unclear at present if there is an association between wear particles and the discogenic pain that was the dominant reason for revision of disc arthroplasties incorporating historical polyethylene.
New data is beginning to emerge regarding conventional, gamma inert sterilized polyethylene in cervical and lumbar TDRs. The largest collection of ProDisc retrievals, for example, has been maintained by researchers from the Hospital for Special Surgery (HSS), who have presented their observations over the past five years in a series of conference abstracts21–24. In the most recent analysis from HSS21, 20 lumbar ProDisc retrievals, implanted for an average of one year, showed evidence of impingement in 15/20 (75%) and back side wear in 11/14 (78%) of the components that were disassembled. In a companion HSS study23 of 29 cervical ProDisc retrievals, also implanted for an average of one year, researchers observed impingement in 96% (28/29) of explanted devices. These short-term retrieval findings, coupled with radiographic evidence of impingement in a clinical study25 of the lumbar ProDisc, further highlight the relevance of impingement to contemporary TDR designs in addition to the historical CHARITÉ. In our retrieval collection, we have had the opportunity to characterize 10 TDRs from conventional gamma inert-sterilized polyethylene. These components, implanted for 0.3 to 3.3 years, exhibited an order of magnitude lower oxidation than the historical gamma air sterilized materials analyzed previously. Two of these devices could not be fully examined due to severe iatrogenic damage, however, we observed evidence of impingement on either the polyethylene components or the metallic endplates in all of the remaining devices. Longer term retrievals are necessary to effectively compare wear rates and oxidative properties between TDR designs fabricated from historical gamma air-sterilized and conventional gamma-inert sterilized polyethylene.
From a tissue response perspective, compared with the response to gamma air-sterilized polyethylene, less has been published regarding the response to wear debris or inflammation in periprosthetic spine tissue from conventional gamma inert sterilized polyethylene TDRs. From our own studies, we have observed early tissue responses after a lumbosacral disc replacement with a ProDisc-L implant, which was removed after 14 months for low back pain26. Micro-computed tomography of the tissue showed the presence of third-body debris, which was predominantly heterotopic ossification/bone formation. Histological analysis showed fibrotic tissue with increased vascularization. Other areas of the tissue showed evidence of cell degeneration, and several fields contained fibrocartilage. Similar to previous short-term studies, no phagocytic cells were observed in areas where polyethylene wear debris was found and the amount of wear debris was limited.
Taken together, though limited, the available data provides support for gamma inert-sterilized polyethylene in disc arthroplasty. Research on historical, gamma-air sterilized polyethylene TDRs continues, because it provides an ideal negative control material against which modern polyethylene materials for the spine can be compared and effectively benchmarked. As we shall see in the following sections, the foundation of knowledge gained from polyethylene TDR retrievals forms a strong basis for critically examining, and indeed expanding, the battery of preclinical tests new TDRs must be subjected to prior to their widespread clinical adoption.
3. Adverse Local Tissue Reactions in CoCr alloy, Metal-on-Metal (MOM) TDR
Until recently, CoCr alloy, metal-on-metal (MOM) hip bearings were considered to be a reasonable alternative to reduce long-term wear and short-term dislocation risk in hip arthroplasty, though long-term exposure to metal ions has been a concern with MOM for many decades27. However, there has been a recent increase in the reports in the hip literature of short-term revision due to metal hypersensitivity, osteolysis, and pseudo-tumor formation28, 29. These adverse local tissue reactions to MOM, while infrequent, may result in substantial tissue damage and permanent disability to hip patients. In July 2008, Zimmer issued a voluntary recall for the Durom MOM hip system because its “warnings and instructions for use were inadequate30”. In April 2010, the British MHRA (equivalent to the FDA), issued a Medical Device Alert for all MOM THRs, and called for systems to be put into place to monitor patients implanted with MOM for soft-tissue reactions and unexplained pain31. In August 2010, DePuy Orthopedics issued a voluntary worldwide recall for ASR MOM hip implant systems, due to a higher than expected revision rate in the United Kingdom30. In May 2011, the FDA ordered orthopedic manufacturers to perform post market surveillance studies of MOM hip implants in the US and established a website30 to inform the public and encourage the reporting of adverse events related to MOM bearings. Media articles questioning the appropriateness of MOM hip bearings and mass tort litigation have followed32, 33. Consequently, the short-term revision due to periprosthetic tissue reactions of MOM; heightened scrutiny by the lay press; the prospect of involvement in litigation; and the recent regulatory actions have reduced the level of enthusiasm for this alternative hip bearing in the US. Spine surgeons considering to use MOM TDRs should familiarize themselves with the FDA website for MOM hip implants30, which will be updated regularly as new information becomes available.
The MOM situation for TDR is more complex than for THR. A unique problem for spinal surgeons in understanding the tissue response to particulate debris, metal ions, and corrosion products associated with “Metal-on-Metal” TDR is that—unlike in hip arthroplasty—a variety of alloys are used in artificial discs2. For example, CoCr alloy is used in the Maverick™ Lumbar disc (Medtronic Spinal and Biologics, Memphis, TN), the Flexicore cervical and lumbar discs (Stryker Spine, Allendale, NJ), and in the Kineflex cervical and lumbar discs (Spinal Motion, Mountain View, CA). Stainless steel is used in the Prestige® cervical disc. Titanium alloy is used in the Prestige® LP cervical disc. Finally, commercially pure titanium is used in the titanium shells of the Bryan® artificial disc (although the titanium shells do not articulate in normal circumstances, the shells may produce wear debris if impingement or failure occurs). Thus, the particulate debris, metal ions, and corrosion products, as well as the attendant host response found in peri-prosthetic tissues, varies with the specific alloy used in the device. Therefore, the tissue response seen for one device made of one alloy may not be the same as a tissue response for another alloy.
We have studied seven explanted Maverick TDRs (Medtronic, Memphis, TN). The explanted Maverick devices were made available and funded through Medtronic’s IDE studies or Medtronic’s quality system. The Maverick is machined from cobalt-chromium-molybdenum alloy hot-worked bar stock (ASTM F-1537, Alloy 2). Protruding from the bone-abutting surface is a “keel” which provides for device stability by press-fitting into a prepared channel in the vertebral body. The articulating surface has a male (convex) dome that mates with the female (concave) feature of the superior endplate.
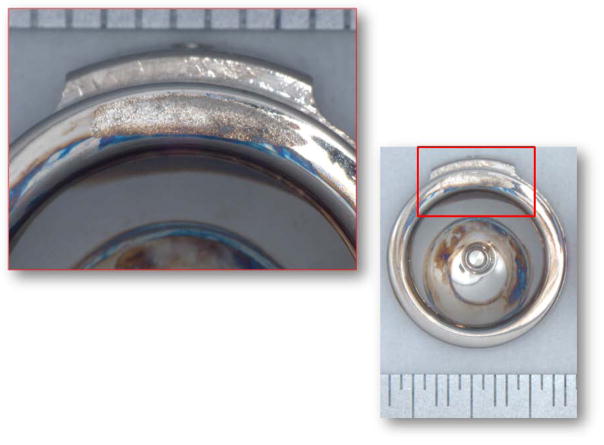
The seven retrieved Maverick discs in our collection were removed from L4/L5 and L5/S1 after 0.6–3.1 years of implantation (ave: 1.3 years). All of the retrieved components appeared polished, but showed varying extent of scratches on the bearing surfaces and rims (Figure 1). There was no macroscopic evidence of plastic deformation, third-body wear, burnishing, pitting or fracture at either the rims or the spherical bearing surfaces of the implants. The primary wear mechanism was microabrasion, which was evident by microscopic scratching of the articulating surfaces in all three sets of components. Two sets of components, implanted for 0.6 and 1.5 years, showed evidence of rim impingement. We found a fan-shaped pattern of microscopic scratches on both the superior and inferior endplates as clear evidence of chronic impingement in this case (Figure 1). Located in the anterior aspect of the device, the impingement zones consisted of microscopic unidirectional, circumferential scratches, suggesting that they were generated by a combination of lateral bending and/or axial rotation. We also consistently found surface deposits, manifested as a smoky or hazy discoloration, on the superior and inferior endplates of all the retrievals. Using low-voltage, EDS analysis, we confirmed that these carbon- and oxygen-rich films were of comparable composition to those tribochemical reaction layers previously observed in well-functioning MOM hip joints34. We are continually collecting retrieved total disc replacements in order to understand long term outcomes and potential complications.
Figure 1.
Micrograph of the superior (left) and inferior (right) endplates of a Maverick retrieved implant (1.5y in vivo) showing scratches and an area of impingement. (Color version of figure is available online.)
The periposthetic tissues associated with one of the retrieved Maverick discs in our collection were also available for analysis. Peri-prosthetic tissues associated with retrieved Maverick devices have showed focal metallosis upon gross examination. A uniform discoloration (metallosis) was not observed in any of the tissue samples at gross. Two minute metallic fragments were observed (as detected by Faxitron high-resolution radiography) remaining in the tissue samples after removal of bone subsequent to decalcification. As seen in Figure 2 and Figure 3, histology showed that necrotic bone, necrotic bone marrow, and necrotic dense fibrous connective tissues were seen in all three peri-prosthetic tissue samples. As seen in Figure 3, focal microscopic metallic debris was only infrequently observed in the histology of some of the tissue samples. Metallic debris was not found in a uniform distribution throughout the tissue samples. Unlike histology from the Prestige devices we previously reported on4, 35, corrosion products were not observed in any of the tissue samples from the current Maverick total disc replacement patient. Cytological (morphologic) changes associated with tissue necrosis were observed in the histology. As seen in Figure 4, karyopyknosis, the irreversible condensation of chromatin in the nucleus of a cell undergoing necrosis or apoptosis was observed in the histology. Karyorrhexis, or fragmentation of the pyknotic nucleus with subsequent accumulation of nuclear dust (heterochromatin nuclei of dead cells), was also observed in the histology. Karyolysis, the complete dissolution of the nucleus was also observed in the histology. The cause of the tissue necrosis was not known. There was no evidence in the histology to suggest that an infection caused the necrosis. However, a focal cell mediated immune response consisting of 25+ lymphocytes and less than 10 macrophages per 400–500x high-powered field was focally observed in peri-prosthetic tissues. The observed host response did not have an acute inflammatory character (no neutrophils). There were no signs of infection in the tissues. Osteolysis was not observed in any of the tissues. Based on the population of lymphocytes observed, there is concern that cobalt TDRs such as the Maverick device may be associated with tissue necrosis and lymphocytic (cell mediated immune) response in some patients.
Figure 2.
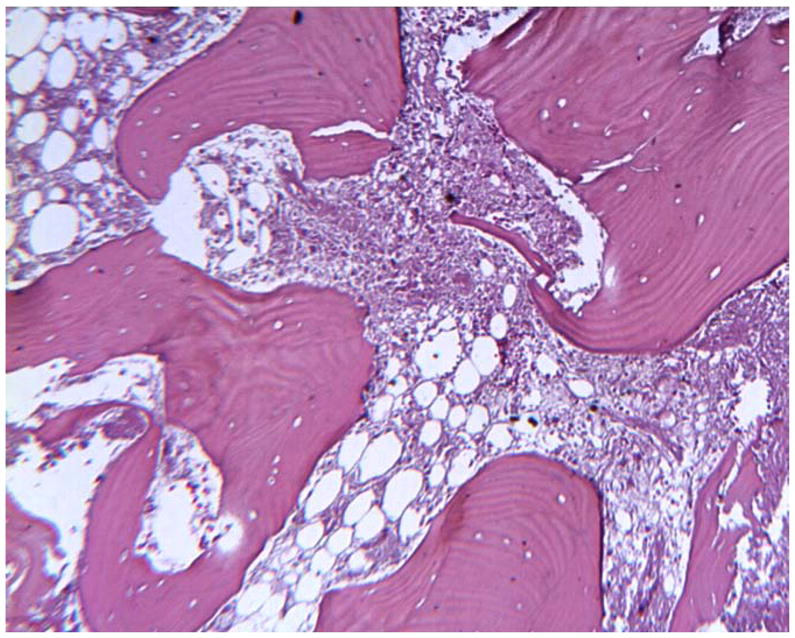
Histology of peri-prosthetic tissues adjacent to a cobalt based TDR showing necrotic bone with empty osteocyte lacunae and necrotic marrow. (H&E, original magnification = 70x). (Color version of figure is available online.)
Figure 3.
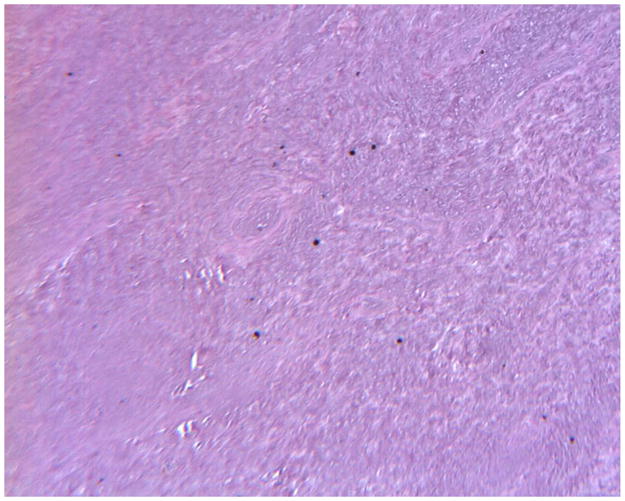
Histology of peri-prosthetic tissues adjacent to a cobalt based TDR showing necrotic tissue and microscopic metallic debris. (H&E, original magnification = 79x). (Color version of figure is available online.)
Figure 4.

Histology of peri-prosthetic tissues adjacent to a cobalt based TDR showing karyopyknosis, karyorrhexis, and nuclear dust in tissues undergoing necrosis. (H&E, original magnification = 500x). (Color version of figure is available online.)
A paucity of information on the host response to CoCr MOM TDRs exists in the literature. In fact, there are only three articles in the recent literature that present histologic findings associated with CoCr MOM TDRs36–38. In addition to the tissue response we describe above, peri-prosthetic tissue reactions to both fixed and mobile bearing CoCr MOM TDRs are known in both the cervical and lumbar spine. The article by Berry et al.38 indicated that “the final pathology report described the tissue as benign and reactive and consistent with a large granuloma. There were uniformly sized, shaped, and colored particles within the granulation tissue, indicative of wear debris.” However, figure 3 in Berry’s article38 shows similar findings to the peri-prosthetic tissues we described above – profound tissue necrosis with either nuclear dust or metallic debris present. Similarly, a recent article by Guyer et al. reported on a single Maverick™ lumbar disc prosthesis case report36. The authors state, “A laparoscopic biopsy found slightly necrotic nonvascular grayish-white tissue. Histology showed predominantly necrotic fibrous and adipose tissue. Bordering the necrosis were some focal, poorly defined, histiocytic palisades, and scattered foreign-body giant cells with surrounding lymphocytic infiltrate36.” Based on the tissue response we describe above as well as the two single case reports in the literature, there is concern that cobalt TDRs such as the Maverick™ device may be associated with tissue necrosis and lymphocytic (cell mediated immune) response in some patients – similar to total hip replacements. Neither the extent of this response nor the cause of this response can be gauged based on these few case reports. In summary, there is evidence of adverse local tissue reactions to CoCr alloy MOM TDRs, but these are thus far limited to suspected39 or histologically confirmed36–38 cases in isolated reports in both cervical and lumbar disc applications.
4. Update on Device and Tissue Response to Alternative Biomaterials used in TDR
Whereas polyethylene and CoCr alloy have a well-established track record as orthopaedic bearing materials that can be traced back over five decades, as noted in the previous section, many different polymers and metal alloys have been incorporated into modern TDRs, especially in the cervical spine. These “alternative biomaterials” have no long-term clinical history as bearing materials, and hence retrieval analysis has the opportunity to provide especially crucial feedback regarding the tribological performance, chemical stability, and tissue response to these new materials in the context of spine arthroplasty. In this section we provide an update on our device and tissue retrieval experience for some of these new biomaterials used in TDRs. Because these alternative biomaterials are isolated to unique TDR designs, the following sections are grouped by device.
4.1. Stainless Steel – Prestige® Cervical Disc System
The use of stainless steel as a bearing material in cervical arthroplasty can be traced to the Bristol/Cummins Disc, which was first used clinically in 1991. Early clinical prototypes of stainless steel cervical discs were evaluated in the late 1990s, leading to development of the Prestige ST (currently referred to as the Prestige® Cervical Disc System) design by Medtronic Spinal and Biologics in 2002. This implant design has 8 components; two endplates that interface via a ball and trough mechanism, two bone screws per endplate (four bone screws total), and one set screw per endplate (two set screws total). A grit-blasted surface on the endplates of the superior and inferior components allows for bone ongrowth. The spherical superior endplate is comprised of a convex (ball) geometry that articulates with the inferior endplate comprised of a concave (trough) geometry. When the device is well fixed and functioning normally, articulation occurs between the ball of the superior endplate and the trough of the inferior endplate. However, if one or both of endplates become loose or subside, then articulation can occur not only between the rims of the endplates, but also between the endplates and screws. In the United States, the FDA approved the Prestige cervical artificial disc in 2007. This TDR is intended to replace a cervical disc from C3–C7 following removal of the disc for intractable radiculopathy and/or myelopathy, and it is implanted using an anterior surgical approach.
We have studied 20 explanted Prestige TDRs from 20 patients after an average implantation time of 2.0 years (0.3–7.0 years). The explanted devices were made available and funded through Medtronic’s IDE studies or Medtronic’s quality system. The explants generally exhibit a slightly discolored, elliptical wear region of varying dimension centered in the bearing center, with the long-axis oriented in the medial-lateral direction (Figure 5). Microabrasive wear is the dominant in vivo wear mechanism, with microscopic scratches generally oriented in the medial-lateral direction.
Figure 5.
Micrograph of the superior (left) and inferior (right) endplates of a Prestige retrieved implant (2.3y in vivo) showing a typical wear scar.
Evidence of anterior impingement has been noted in the Prestige retrieval collection (11/16 explants, 69%) (Figure 6). Evidence of localized screw hole fretting and fretting near the heads of bone screws is also typically observed, but SEM examination has ruled out corrosive material removal at these interfaces. We have seen evidence of locking screw fracture (3 cases) and bone screw fracture (1 case) among our retrievals. We continue to collected Prestige retrievals in order to better understand long term outcomes and potential complications.
Figure 6.
Anterior impingement in a Prestige retrieved implant (2.2y in vivo). (A) Photomicrograph of entire component; (B) detail of impingement region.
From a periprosthetic tissue perspective, we have analyzed samples from approximately fifteen Prestige cervical artificial discs that were explanted between one and seven years. At gross, periprosthetic tissue samples from Prestige explants typically show focal metallosis. At longer post-implantation periods, a uniform discoloration (metallosis) has been observed in periprosthetic tissue samples at gross. High-resolution radiographs of periprosthetic tissues show the presence of infrequent small metallic debris before and after decalcification. Fibrous connective tissues are frequently found in the histology associated with Prestige explants. Focal microscopic metallic debris is observed histologically in periprosthetic tissue samples. Metallic debris is not found in a uniform distribution throughout the tissue samples. As exemplified in Figure 7, in most periprosthetic tissues associated with Prestige explants, a lamellar distribution of metallic debris within the interstices of fibrous tissues is observed, especially at the device interface. In other microscopic fields, focal microscopic metallic debris may also be present within fatty marrow in the intertrabecular spaces of bone. For many periprosthetic tissues sample, nuclear detail is often obscured by intracellular debris, making recognition/differentiation of phagocytes difficult. As seen in Figure 8, metallic debris is found intracellularly within phagocytes (presumably macrophages). Figure 9 shows plate-like corrosion products which are frequently observed in periprosthetic tissue samples from Prestige explants. As we have noted previously, this finding is consistent with histology from other stainless steel surgical devices4, 35. Similarly, as shown in Figure 10, hemosiderin is frequently found in peri-prosthetic tissue samples from Prestige® explants. Hemosiderin (iron storage granules) may be present in areas of old hemorrhage or may be deposited in tissues with a high iron concentration. Histology of one of the explants has shown evidence of an infection.
Figure 7.
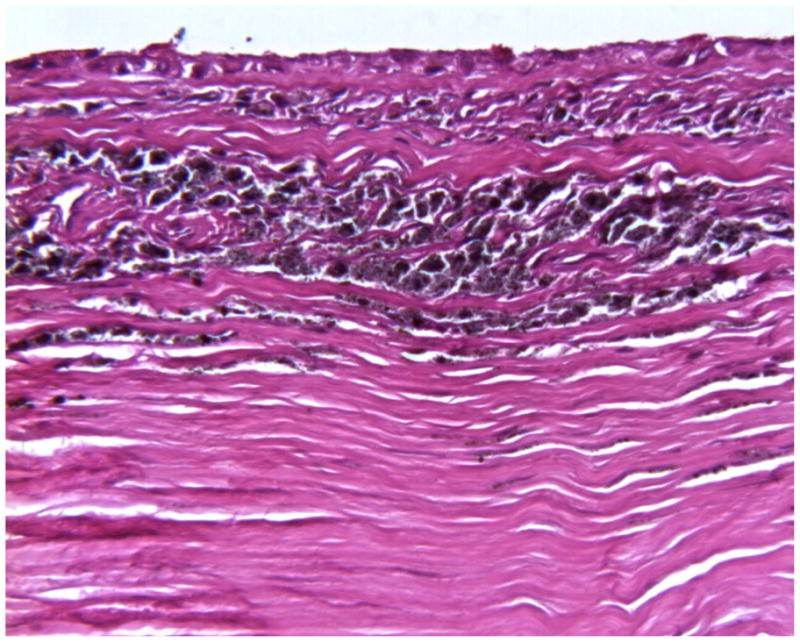
Histology of periprosthetic tissues adjacent to a stainless steel Prestige cervical artificial disc showing intracellular and extracellular metallic debris in the interstices of fibrovascular connective tissues at the device interface. (H&E stain, original magnification = 200x). (Color version of figure is available online.)
Figure 8.
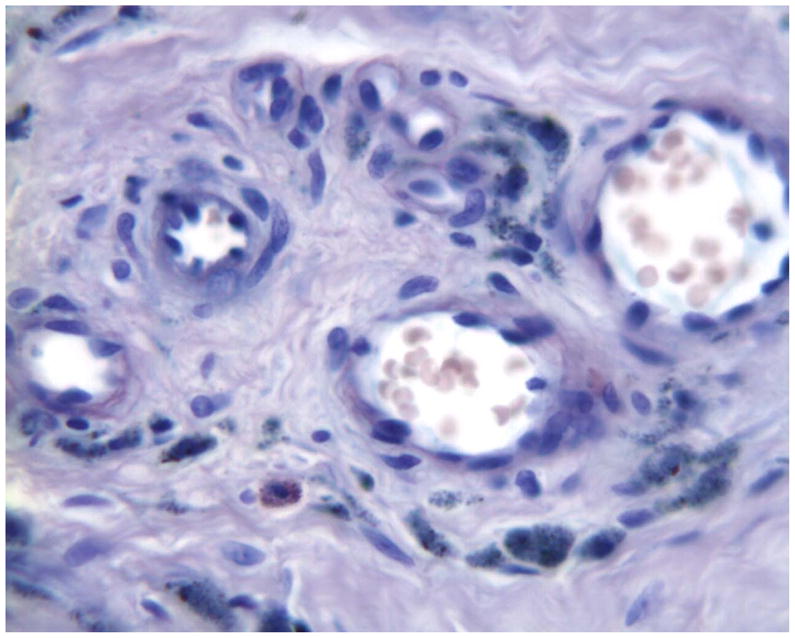
Histology of periprosthetic tissues adjacent to a stainless steel Prestige cervical artificial disc showing intracellular microscopic metallic debris within macrophages in fibrovascular tissues. (Wright-Giemsa stain, original magnification = 500x). (Color version of figure is available online.)
Figure 9.

Histology of periprosthetic tissues adjacent to a stainless steel Prestige cervical artificial disc showing the presence of plate-like corrosion products in fibrovascular connective tissues. (H&E, original magnification = 400x). (Color version of figure is available online.)
Figure 10.
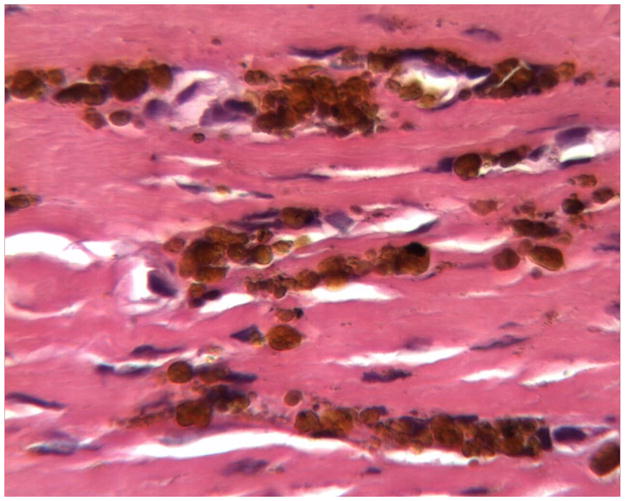
Histology of periprosthetic tissues adjacent to a stainless steel Prestige cervical artificial disc showing hemosiderin (iron storage granules) in fibrovascular connective tissues. (H&E, original magnification = 400x). (Color version of figure is available online.)
The typical host response found in periprosthetic tissue samples adjacent to the Prestige explants is characterized as a mononuclear chronic inflammatory response consisting of less than 15 mononuclear phagocytes (presumably macrophages) per 400–500x high powered field. In addition, less than 5 neutrophils, lymphocytes, eosinophils and foreign body giant cells per 400–500x high powered fields are typically observed. Unlike the host response to Bryan explants (described later in detail), a multinuclear foreign body giant cell response is not commonly observed in periprosthetic tissues adjacent to Prestige devices (although a few foreign body giant cells may be found). ASTM F981-04 interprets less than 15 phagocytes in fields at 400–500x magnification as a score of 1 (mild reaction). The observed chronic inflammatory response represents a typical finding in peri-prosthetic tissues adjacent to the Prestige metal-on-metal spine arthroplasty devices. The observed host responses do not have an acute inflammatory character (few neutrophils). At this time, adverse events associated with degradation products such as necrosis, osteolysis, or tissue degeneration have not been observed in these explants. Neither lymphocyte infiltration nor accumulation of neutrophils has been observed in peri-prosthetic tissues.
Although stainless steel is well understood as a biomaterial, to date only a few intermediate-term artificial disc retrievals and tissues have been studied thus far with greater than five years implantation time. Retrievals show evidence of wear on the explants and wear products in periprosthetic tissues. At present we have not observed the adverse local tissue reactions, previously reported with CoCr MOM bearings, for the stainless steel-on-stainless steel cervical bearings of the Prestige.
4.2. Polyurethanes and Titanium Alloy – Bryan® Disc
The Bryan® cervical artificial disc (Medtronic Spinal and Biologics) is a bi-articular prosthesis fabricated from a pair of identical titanium alloy shells (which are fitted into the endplates of the cervical vertebral body), a polycarbonate urethane (PCU) nucleus (which articulates with the titanium shells), and a flexible polyether urethane (PEU) sheath (meant to prevent tissue ingrowth into the articulating surfaces) which surrounds the polyurethane nucleus. The Bryan cervical artificial disc is indicated for degenerative disc disease (DDD) at one level from C3–C7. Clinical studies of the Bryan started in Europe in 2000 and up to 8 years of clinical follow-up has been performed to date40, 41.
We have analyzed 35 Bryan TDRs retrieved from 30 patients after 3.2y (0.3 to 7.0y). The Bryan retrievals were made available and funded through Medtronic’s IDE studies or Medtronic’s quality system. The nominal height loss of the explanted cores (mean ± SD) was 0.44 ± 0.55 mm (range: 0.04 to 2.6 mm). We generally observed localized, microscopic evidence of adhesive and abrasive wear (confirmed by SEM and interferometry), and attributed the majority of initial height loss to creep because, for many of the retrievals, the initial glossy surface finish of the cores was generally well preserved, even after 7.0y in vivo (Figure 11). We have observed endplate impingement in 9/30 (30%) of retrieved Bryans (Figure 12). In two cases, the rim impingement generated Ti debris and caused third body wear of the PCU core with substantial height loss (1.6 mm and 2.6 mm). In one case, impingement was associated with a full-thickness rim fracture of the PCU core.
Figure 11.
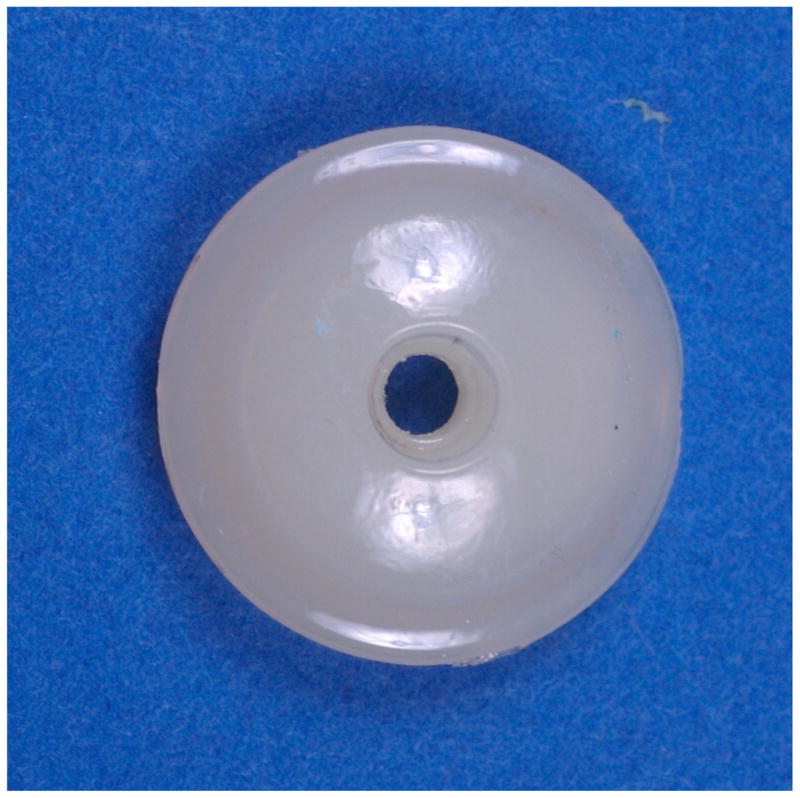
Nucleus of a retrieved Bryan implant (6.1y in vivo) showing its glossy appearance. (Color version of figure is available online.)
Figure 12.
Endplate impingement in a retrieved Bryan implant (6.9y in vivo). (Color version of figure is available online.)
The sheaths typically showed evidence of folding, or permanent deformation in regions where the core made repeated contact. We also found evidence of in vivo degradation of the sheath in a subset of these devices (4/15, 27%), and the manifestation appears to be patient specific rather than associated with implantation time. In the early stages of biodegradation, the sheath gains an opaque or cloudy appearance (Figure 13). Biodegradation of the PEU sheath, in the later stages, leads to surface fissures and, in some cases, full thickness cracks (Figure 13). The mechanism thought to be responsible for biodegradation of the sheath polyurethane is caused by the release of reactive oxygen species by macrophages and foreign body giant cells42. The clinical significance of sheath biodegradation appears to be minimal at the early stages in which the sheath becomes discolored, but if the sheath degrades in vivo over the long term, it remains unclear whether the polymer will generate debris that contributes to an inflammatory reaction around the implant. None of our implants received to date have been revised due to issues related to the sheath. We continue to collect Bryan retrievals to better understand long term outcomes and potential complications.
Figure 13.

Posterior view of the sheath of a retrieved Bryan implant (1.6y in vivo) showing the cloudy appearance along with areas of cracking. (Color version of figure is available online.)
We have analyzed periprosthetic tissue samples from approximately fifteen Bryan cervical artificial discs that have been implanted between one and six years. Unlike MOM TDR’s, few peri-prosthetic tissues show metallosis at gross. As seen in Figure 14 through Figure 16, microscopically, foreign body giant cells and macrophages with intracellular polymeric particulate debris tend to be observed in periprosthetic tissue samples. Polarized light microscopy often reveals weakly birefringent intracellular polymeric debris in the histology of periprosthetic tissue samples (Figure 14). Corrosion products are not frequently found in the histology of periprosthetic tissues. Previous published literature from both preclinical animal studies and human clinical explants have shown that the polyurethane polymers used in the sheath and core of the Bryan cervical disc are birefringent when viewed under polarized light microscopy, and thus are readily recognized in histology sections4, 35, 43, 44. As shown in Figure 14, polarized light microscopy clearly demonstrates birefringent polymeric particulate in periprosthetic tissues. As a complement to polarized light microscopy, Oil Red-O staining, specific for the presence of polymeric wear debris in cleared tissues, can also be used to document the presence of polymeric debris. As shown in Figure 15 and Figure 16, similar to polyethylene particulate in large joint histology, Oil Red-O staining is useful in demonstrating the presence of polyurethane polymeric debris (red) in periprosthetic tissue samples. Histology micrographs document that these complementary techniques reveal red-stained (when Oil Red-O sections were evaluated) birefringent polymeric debris in the tissues, most likely related to wear in the Bryan device. Histology techniques cannot determine whether the polymeric debris is from the sheath or nucleus of the Bryan device. No evidence of osteolysis has been observed in the histology associated with these explants. Histology of one of the explants has shown evidence of an infection.
Figure 14.
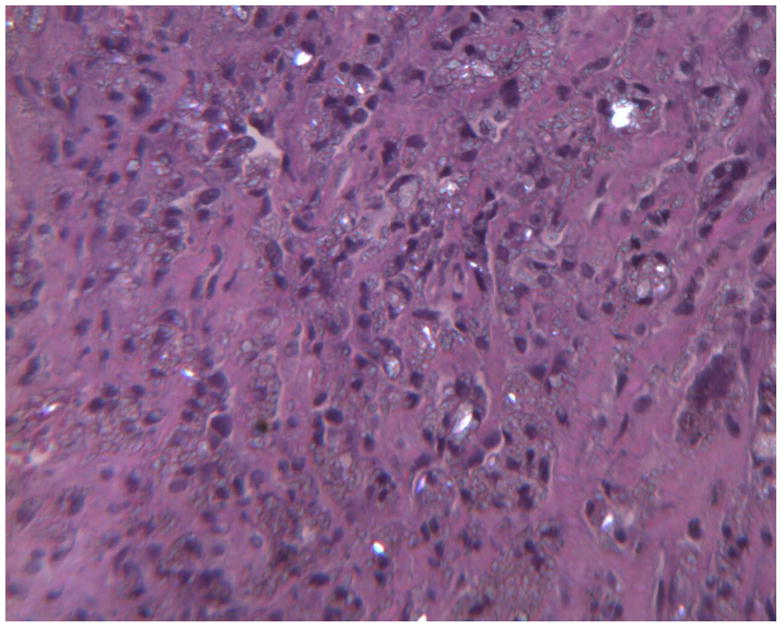
Histology of periprosthetic tissues adjacent to a Bryan polyurethane cervical TDR showing birefringent intracellular polymeric particulate within macrophages. (H&E, original magnification = 200x, partially polarized light). (Color version of figure is available online.)
Figure 16.
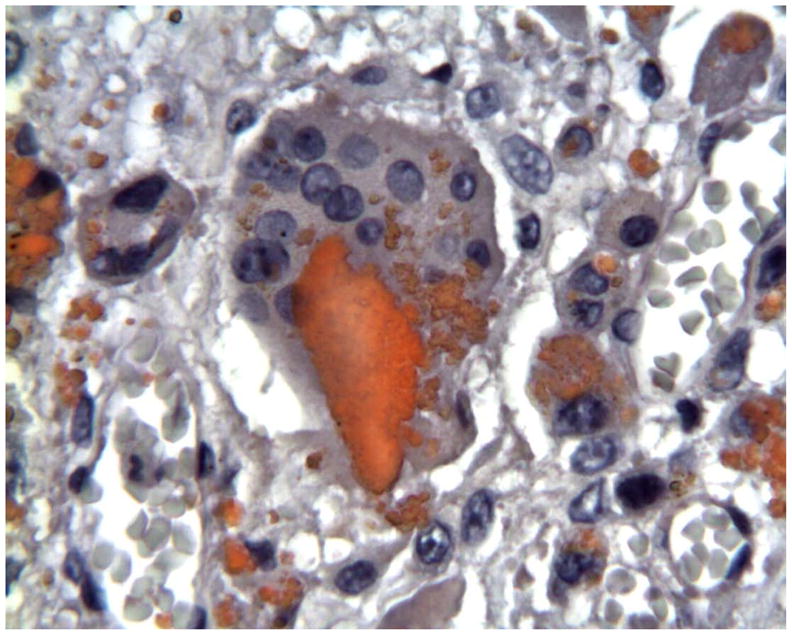
Histology of peri-prosthetic tissues adjacent to a Bryan cervical TDR showing intracellular red stained polymeric debris within macrophages and foreign body giant cells. (Oil Red-O stain with hematoxylin counter stain, original magnification = 500x). (Color version of figure is available online.)
Figure 15.
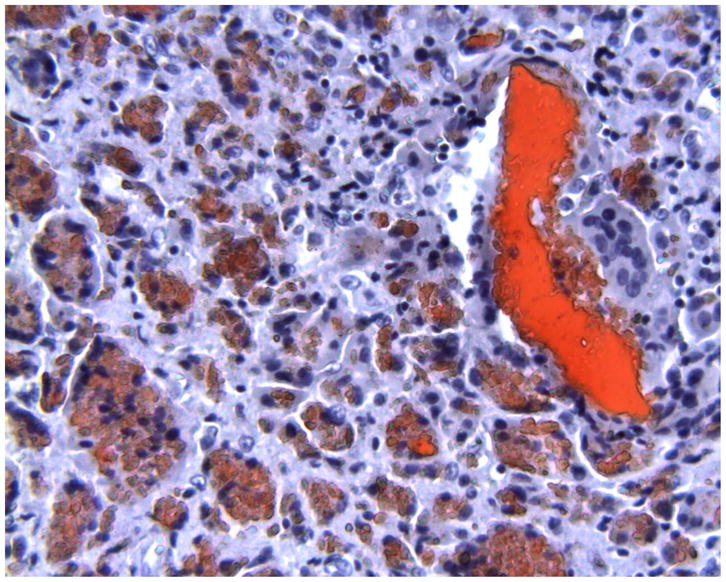
Histology of periprosthetic tissues adjacent to a Bryan polyurethane cervical TDR showing larger red-stained extracellular polymeric particulate debris as well as foreign body giant cells and macrophages with smaller intracellular red-stained polymeric debris. (Oil-Red-O, original magnification = 200x). (Color version of figure is available online.)
Based on the population and type of inflammatory cells observed in the histology of these devices, a moderate to marked chronic inflammatory host response consisting of macrophages and foreign body giant cells is typically observed in the histology. In one of the explants, lobulated epitheliod granulomatous tissues, similar to pseudosynovial tissues found in large joint arthroplasty, are sometimes found in these periprosthetic tissues. In this case, focal lymphocyte infiltration with more than 20 lymphocytes was observed in 500x fields. Less than 5 eosinophils per 500x field were focally observed in these same fields for that explant. A sample histology image is seen in Figure 17. As is the case with Bryan cervical TDR’s, chronic inflammatory host responses with intracellular polymeric debris are frequently observed adjacent to joint arthroplasty devices such as TDR’s.
Figure 17.

Histology of peri-prosthetic tissues adjacent to a Bryan cervical TDR showing an inflammatory response consisting of macrophages, lymphocytes, and eosinophils. (H&E stain, original magnification = 500x). (Color version of figure is available online.)
Case studies of osteolysis and adverse local tissue reactions have been observed with the Bryan artificial disc. In one case, osteolysis was attributed to a periprosthetic infection. The authors are aware of two reported cases of aseptic osteolysis associated with the Bryan; one of these cases has been presented at an international conference in Europe45. It concerned an asymptomatic young lady in whom the osteolysis of the vertebral bodies adjacent to the artificial disc was detected at a routine follow-up examination at 3 years postoperatively. The other case was a middle-aged man who developed at 5 years postoperatively a recurrent radiculopathy due to osteolysis and subsequent reactive hypertrophic bone formation with nerve root compression. In both cases the prosthesis was removed and an interbody fusion with autograft and plate stabilization performed. A full description of these cases is currently being prepared for a future journal publication.
5. The Role of Retrieval Analysis on the Development of New TDR Test Methods
The aforementioned retrieval studies provide standardization bodies with scientific data by which in vitro test methods for TDRs can and should be validated. At present, few standards for TDR characterization exist46–48. The standards which do exist are focused on static and dynamic characterization and intended bearing wear of the devices. Unlike other arthroplasty devices, test methods for the other modes of wear, including impingement and third body wear, remain under development.
Conveniently, retrieval analysis results provide guidance for the characterization techniques that can be used to correlate in vivo and in vitro wear patterns and morphology49. Several studies have succeeded in drawing correlations between the analyses conducted on retrieved and wear tested devices17, 35. These analyses focus on using optical microscopy, scanning electron microscopy, micro computed tomography (MicroCT), and surface profilometry to quantify the characteristic wear mechanisms and damage patterns of the devices. There is now strong consensus that both the worst-case and most clinically relevant wear protocol for M-PE TDR bearing couples is one that employs a simultaneous multi-component motion sequence which results in cross-shear stresses at the bearing interface8. However, for MOM bearing couples the worst-case and clinically relevant wear simulation for TDRs is likely not contained in one test. Rather, a uni-directional test typically presents a worst case for most MOM bearing couples and yet most clinical MOM TDR retrievals demonstrate multidirectional abrasive wear, indicating a simultaneous multi-component motion sequence is more clinically relevant.
Although selecting the correct normal wear scenario for a given bearing couple is laden with trade-offs and the need for justification, when more adverse testing conditions are considered, even less guidance is available. As we have seen in the previous sections of this review, a substantial fraction of retrieved cervical and lumbar TDRs have been documented with evidence of in vivo impingement, regardless of design or bearing material. Using the available retrieval evidence, it is clear that TDR impingement is likely the result of a number of both design and clinical factors, including subsidence or suboptimal positioning.
The first step in developing a clinically relevant impingement wear testing approach begins with understanding the wear mechanisms and contact areas present on the retrieved devices. Next, understanding the ranges of motion and combined loading scenarios which generate the impingement scenario can be achieved virtually using modeling software or on the bench using range of motion testing procedures50, 51. Lastly, the loading scenarios should be replicated using a cyclic profile under lubricated conditions. The in vitro samples should be analyzed for wear rate, morphology and mechanism. The fluid collected during testing should be analyzed for particle size distribution and morphology. All results should be compared to the normal or bearing wear test conducted on the same design and verified mechanistically against the retrieved devices. This approach is founded on the use of retrievals. As with the original TDR wear testing standards, as more artificial discs are evaluated, both material and design performance should become more predictable.
Standardization efforts are underway to increase guidance to the industry in order to assist with more thorough device characterization including testing under adverse conditions. Developing a single set of boundary conditions that can be applied to all designs and material combinations in likely unachievable. Therefore, using the limited retrieval data available is perhaps the only scientifically valid approach to develop a set of justifiable testing conditions that mimic the in vivo performance of TDRs.
6. Summary and Conclusions
Our knowledge of TDR clinical performance based on explanted devices and tissues may no longer be in its infancy, but still remains far from complete. The most retrieval data are currently available for historical TDRs in which polyethylene was effectively gamma irradiated in air. The wear and damage modes associated with these early devices has conformed to expectations of gamma air-irradiated polyethylene from the literature. Contemporary TDRs incorporating conventional gamma inert-sterilized polyethylene, approved by the FDA starting in 2004, exhibit an order of magnitude less oxidation than the historical polyethylene devices based on the short-term retrievals that have been studied to date. Because of the markedly reduced oxidation, it is expected that conventional polyethylene in TDRs will exhibit lower risk of fatigue and fracture than the historical gamma air sterilized polyethylene TDRs. While more advanced, highly cross-linked polyethylene technologies are currently used in the fabrication of hip and knee replacements, it remains unclear whether the improved wear resistance these new materials afford are adequately balanced by the increased fracture risk that accompanies elevated radiation crosslinking.
At present, many of the unanswered questions surrounding polyethylene in TDRs relate to better understanding the inflammatory tissue reactions to wear debris, and how the inflammation can be effectively avoided, either by improved materials science (e.g., radiation cross-linking of the polyethylene) or, potentially, by pharmacological intervention. Furthermore, the relationships between TDR bearing design (e.g., fixed vs. mobile bearing), wear debris release, and impingement damage remain poorly understood. Because of the more complete clinical and retrieval history describing their performance, polyethylene TDRs provide the starting point for validating realistic wear and fatigue test protocols to characterize TDRs during the research and development phase of preclinical discovery. It is especially clear from the retrieval history that it is crucial to characterize TDR response under “normal” or expected use, as well as adverse conditions including impingement, that are frequently observed in explanted devices. Regulatory agencies are considering making adverse testing and impingement characterization essential rather than optional components of a preclinical test program for new TDR designs seeking regulatory approval. The most effective and realistic impingement simulations will incorporate the specific biomechanical analysis and retrieval evidence, if available, to validate the device specific conditions for the impingement testing.
Overall, the clinical data associated with total disc replacement technology, regardless of bearing couple, suggests that device characterization and research efforts should be focused on validating the existing wear testing standards using retrieval data to understand the clinical relevance of test duration (i.e., the number of loading and motion cycles) and profile selection. The same retrieval datasets provide a basis for impingement protocol development and warrant the study of third body debris generation. It remains unclear if the effect of combined adverse conditions such as impingement-related accelerated wear, will lead to third body abrasive conditions at intended bearing surfaces. Further, the effect of all associated particle debris, whether from intended bearing surfaces or from impingement conditions, especially in non-polymeric bearing couples, remains highly contentious and warrants investigation. Regardless of bearing material selection or design, the lessons learned and approaches to retrieval analysis and wear test validation employed in hip and knee arthroplasty should be implemented for TDRs to assure patients, surgeons, and regulators of device safety and effectiveness.
Acknowledgments
The research studies described in this article were supported in part by funding from NIH R01 AR47094, NIH R01 AR56264, and the FDA Critical Path Program. Institutional support for the Maverick, Prestige, and Bryan retrieval programs was received from Medtronic funded IDE studies and Medtronic’s quality system. The authors would like to thank Genevieve Hill and Jonathan Peck, FDA, for their helpful discussions and collaboration on studying impingement in TDR; as well as Frank Chan, Megan Harper, Jacob Shorez, and Mark Dace, Medtronic, for their contributions. The authors gratefully acknowledge Amy Rizzo MT(ASCP) for her extraordinary skill and technical expertise in producing histological sections of periprosthetic tissues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kurtz SM. The UHMWPE Biomaterials Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacement and Medical Devices. 2. Burlington, MA: Academic Press; 2009. [Google Scholar]
- 2.Kurtz SM. Total disc arthroplasty. In: Kurtz SM, Edidin AA, editors. Spine Technology Handbook. New York: Academic Press; 2006. p. 303. [Google Scholar]
- 3.Kurtz SM, Steinbeck MJ, Ianuzzi A, van Ooij A, Punt IM, Isaza J, Ross ERS. Retrieval analysis of motion preserving spinal devices and periprosthetic tissues. SAS Journal. 2009;3(4):161. doi: 10.1016/j.esas.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson PA, Kurtz SM, Toth JM. Explant analysis of total disc replacement. Seminars in Spine Surgery. 2006;18(2):1. doi: 10.1053/j.semss.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurtz SM, Gawel HA, Patel JD. History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene. Clinical orthopaedics and related research. 2011;469(8):2262. doi: 10.1007/s11999-011-1872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. The Journal of bone and joint surgery. 2009;91(7):1614. doi: 10.2106/JBJS.H.01220. [DOI] [PubMed] [Google Scholar]
- 7.Bozic KJ, Ong K, Lau E, Kurtz SM, Vail TP, Rubash HE, Berry DJ. Risk of complication and revision total hip arthroplasty among medicare patients with different bearing surfaces. Clinical orthopaedics and related research. 2010;468(9):2357. doi: 10.1007/s11999-010-1262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurtz S, Siskey R, Ciccarelli L, van Ooij A, Peloza J, Villarraga M. Retrieval analysis of total disc replacements: Implications for standardized wear testing. Journal of ASTM International. 2006;3(6):1. [Google Scholar]
- 9.Kurtz SM, Macdonald D, Ianuzzi A, van Ooij A, Isaza J, Ross ER, Regan J. The natural history of polyethylene oxidation in total disc replacement. Spine. 2009;34(22):2369. doi: 10.1097/BRS.0b013e3181b20230. [DOI] [PubMed] [Google Scholar]
- 10.Costa L, Bracco P, Brach del Prever EM, Kurtz SM, Gallinaro P. Oxidation and oxidation potential in contemporary packaging for polyethylene total joint replacement components. Journal of biomedical materials research. 2006;78(1):20. doi: 10.1002/jbm.b.30454. [DOI] [PubMed] [Google Scholar]
- 11.Baker DA, Bellare A, Pruitt L. The effects of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J Biomed Mater Res A. 2003;66(1):146. doi: 10.1002/jbm.a.10606. [DOI] [PubMed] [Google Scholar]
- 12.Link HD, Keller A. Biomechanics of Total Disc Replacement. In: Buttner-Janz K, Hochschuler SH, McAfee PC, editors. The artificial disc. Berlin: Springer; 2003. p. 33. [Google Scholar]
- 13.van Ooij A, Oner FC, Verbout AJ. Complications of artificial disc replacement: a report of 27 patients with the SB Charite disc. Journal of spinal disorders & techniques. 2003;16(4):369. doi: 10.1097/00024720-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 14.van Ooij A, Kurtz SM, Stessels F, Noten H, van Rhijn L. Polyethylene wear debris and long-term clinical failure of the Charite disc prosthesis: a study of 4 patients. Spine. 2007;32(2):223. doi: 10.1097/01.brs.0000251370.56327.c6. [DOI] [PubMed] [Google Scholar]
- 15.Punt IM, Visser VM, van Rhijn LW, Kurtz SM, Antonis J, Schurink GW, van Ooij A. Complications and reoperations of the SB Charite lumbar disc prosthesis: experience in 75 patients. Eur Spine J. 2008;17(1):36. doi: 10.1007/s00586-007-0506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurtz SM, van Ooij A, Ross ERS, de Waal Malefijt J, Peloza J, Ciccarelli L, Villarraga ML. Polyethylene wear and rim fracture in total disc arthroplasty. Spine J. 2007;7(1):12. doi: 10.1016/j.spinee.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz SM, Patwardhan A, MacDonald D, Ciccarelli L, van Ooij A, Lorenz M, Zindrick M, O’Leary P, Isaza J, Ross R. What is the correlation of in vivo wear and damage patterns with in vitro TDR motion response? Spine. 2008;33(5):481. doi: 10.1097/BRS.0b013e318165e3be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Punt IM, Austen S, Cleutjens JP, Kurtz SM, Ten Broeke RH, van Rhijn LW, Willems PC, Ooij AV. Are periprosthetic tissue reactions observed after revision of total disc replacement comparable to the reactions observed after total hip or knee revision surgery? Spine. 2011 doi: 10.1097/BRS.0b013e3182154c22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Punt I, Baxter R, Ooij AV, Willems P, Rhijn LV, Kurtz S, Steinbeck M. Submicron sized ultra-high molecular weight polyethylene wear particle analysis from revised SB Charite III total disc replacements. Acta Biomater. 2011 doi: 10.1016/j.actbio.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Punt IM, Cleutjens JP, de Bruin T, Willems PC, Kurtz SM, van Rhijn LW, Schurink GW, van Ooij A. Periprosthetic tissue reactions observed at revision of total intervertebral disc arthroplasty. Biomaterials. 2009;30(11):2079. doi: 10.1016/j.biomaterials.2008.12.071. [DOI] [PubMed] [Google Scholar]
- 21.Lebl DR, Cammisa FP, Girardi FP, Lee S, Chen D, Wright TM, Abjornson C. Analysis of wear, surface properties, and fixation of retrieved lumbar total disc replacements. Proceedings of the NASS 26th Annual Meeting; 2011. [Google Scholar]
- 22.Kelly N, Cottrell J, Wright T. Retrieval analysis of ProDisc total disc replacements. Transactions of the 54th Meeting for the Orthopaeidic Research Society; 2008. [Google Scholar]
- 23.Lebl DR, Cammisa FP, Girardi FP, Lee S, Wright TM, Abjornson C. Analysis of wear, surface properties, and fixation of explanted cervical total disc replacements. Proceedings of the NASS 26th Annual Meeting; 2011. [Google Scholar]
- 24.Wright T, Cottrell J. Retrieval analysis of ProDisc total disc replacements. 7th Annual Meeting of the Spine Arthroplasty Society; Berlin, Germany. May 1–4; 2007. p. 124. [Google Scholar]
- 25.Kafer W, Clessienne CB, Daxle M, Kocak T, Reichel H, Cakir B. Posterior component impingement after lumbar total disc replacement: a radiographic analysis of 66 ProDisc-L prostheses in 56 patients. Spine (Phila Pa 1976) 2008;33(22):2444. doi: 10.1097/BRS.0b013e318182c37b. [DOI] [PubMed] [Google Scholar]
- 26.Choma TJ, Miranda J, Siskey R, Baxter R, Steinbeck MJ, Kurtz SM. Retrieval analysis of a ProDisc-L total disc replacement. J Spinal Disord Tech. 2009;22(4):290. doi: 10.1097/BSD.0b013e31816dd2b6. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs JJ, Urban RM, Hallab NJ, Skipor AK, Fischer A, Wimmer MA. Metal-on-metal bearing surfaces. The Journal of the American Academy of Orthopaedic Surgeons. 2009;17(2):69. doi: 10.5435/00124635-200902000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: A consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- 29.Glyn-Jones S, Pandit H, Kwon YM, Doll H, Gill HS, Murray DW. Risk factors for inflammatory pseudotumour formation following hip resurfacing. J Bone Joint Surg Br. 2009;91(12):1566. doi: 10.1302/0301-620X.91B12.22287. [DOI] [PubMed] [Google Scholar]
- 30. [Accessed, August 26, 2011.];Metal-on-Metal Hip Implants, Food and Drug Administration. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/default.htm.
- 31.Medical device alert: All metal-on-metal (MoM) hip replacements. Vol. 1. London: Medicines and Healthcare products Regulatory Agency, Department of Health; 2010. [Google Scholar]
- 32.Meier B. In medicine, new isn’t always improved. The New York Times; Jun 25, 2011. [Google Scholar]
- 33.Meier B. Hip implant complaints surge, even as the dangers are studied. The New York Times; Aug 22, 2011. [Google Scholar]
- 34.Wimmer MA, Fischer A, Buscher R, Pourzal R, Sprecher C, Hauert R, Jacobs JJ. Wear mechanisms in metal-on-metal bearings: the importance of tribochemical reaction layers. J Orthop Res. 2010;28(4):436. doi: 10.1002/jor.21020. [DOI] [PubMed] [Google Scholar]
- 35.Anderson PA, Rouleau JP, Toth JM, Riew KD. A comparison of simulator-tested and -retrieved cervical disc prostheses. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. Journal of neurosurgery. 2004;1(2):202. doi: 10.3171/spi.2004.1.2.0202. [DOI] [PubMed] [Google Scholar]
- 36.Guyer RD, Shellock J, MacLennan B, Hanscom D, Knight RQ, McCombe P, Jacobs JJ, Urban RM, Bradford D, Ohnmeiss DD. Early failure of metal-on-metal artificial disc prostheses associated with lymphocytic reaction: diagnosis and treatment experience in four cases. Spine. 2011;36(7):E492. doi: 10.1097/BRS.0b013e31820ea9a2. [DOI] [PubMed] [Google Scholar]
- 37.Cavanaugh DA, Nunley PD, Kerr EJ, 3rd, Werner DJ, Jawahar A. Delayed hyper-reactivity to metal ions after cervical disc arthroplasty: a case report and literature review. Spine. 2009;34(7):E262. doi: 10.1097/BRS.0b013e318195dd60. [DOI] [PubMed] [Google Scholar]
- 38.Berry MR, Peterson BG, Alander DH. A granulomatous mass surrounding a Maverick total disc replacement causing iliac vein occlusion and spinal stenosis: a case report. The Journal of bone and joint surgery American volume. 2010;92(5):1242. doi: 10.2106/JBJS.H.01625. [DOI] [PubMed] [Google Scholar]
- 39.Tumialan LM, Gluf WM. Progressive vertebral body osteolysis after cervical disc arthroplasty. Spine. 2011;36(14):E973. doi: 10.1097/BRS.0b013e3181fd863b. [DOI] [PubMed] [Google Scholar]
- 40.Walraevens J, Demaerel P, Suetens P, Van Calenbergh F, van Loon J, Vander Sloten J, Goffin J. Longitudinal prospective long-term radiographic follow-up after treatment of single-level cervical disk disease with the Bryan Cervical Disc. Neurosurgery. 2010;67(3):679. doi: 10.1227/01.NEU.0000377039.89725.F3. [DOI] [PubMed] [Google Scholar]
- 41.Goffin J, van Loon J, Van Calenbergh F, Lipscomb B. A clinical analysis of 4- and 6-year follow-up results after cervical disc replacement surgery using the Bryan Cervical Disc Prosthesis. Journal of neurosurgery Spine. 2010;12(3):261. doi: 10.3171/2009.9.SPINE09129. [DOI] [PubMed] [Google Scholar]
- 42.Christenson EM, Dadsetan M, Wiggins M, Anderson JM, Hiltner A. Poly(carbonate urethane) and poly(ether urethane) biodegradation: in vivo studies. J Biomed Mater Res A. 2004;69(3):407. doi: 10.1002/jbm.a.30002. [DOI] [PubMed] [Google Scholar]
- 43.Anderson PA, Rouleau JP, Bryan VE, Carlson CS. Wear analysis of the Bryan Cervical Disc prosthesis. Spine. 2003;28(20):S186. doi: 10.1097/01.BRS.0000092212.42388.79. [DOI] [PubMed] [Google Scholar]
- 44.Anderson PA, Sasso RC, Rouleau JP, Carlson CS, Goffin J. The Bryan Cervical Disc: wear properties and early clinical results. Spine J. 2004;4(6 Suppl):303S. doi: 10.1016/j.spinee.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 45.Goffin J, van Loon J, Van Calenbergh F, Walraevens J, Vander Sloten J. Transactions of the European Association of Neurosurgical Societies (EANS) Groningen (the Netherlands) 2010. Place of cervical artificial disc implantations. [Google Scholar]
- 46.ASTM F2423 Standard Guide for Functional, Kinematic, and Wear Assessment of Total Disc Prostheses, 2011.
- 47.ISO 18192-1: Implants for surgery — Wear of total intervertebral spinal disc prostheses —Part 1:Loading and displacement parameters for wear testing and corresponding environmental conditions for test. 2008.
- 48.ASTM F2346 Standard Test Methods for Static and Dynamic Characterization of Spinal Artificial Discs. 2005.
- 49.ASTM F561 - 05a (2010) Standard Practice for Retrieval and Analysis of Medical Devices, and Associated Tissues and Fluids. 2010.
- 50.Rundell SADJ, Isaza J, Siskey R, MacDonald D, Kurtz SM. Derivation of clinically relevant boundary conditions suitable for evaluation of chronic impingement of lumbar total disc replacement: Application to standard development. Int J ASTM. 2011 [Google Scholar]
- 51.Wilke HJ, Wenger K, Claes L. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. European Spine Journal. 1998;7(2):148. doi: 10.1007/s005860050045. [DOI] [PMC free article] [PubMed] [Google Scholar]