Abstract
Lipid A is a key component of the outer membrane of Gram-negative bacteria and stimulates proinflammatory responses via the Toll-like receptor 4 (TLR4)-MD2-CD14 pathway. Its endotoxic activity depends on the number and length of acyl chains and its phosphorylation state. In Salmonella enterica serovar Typhimurium, removal of the secondary laurate or myristate chain in lipid A results in bacterial attenuation and growth defects in vitro. However, the roles of the two lipid A phosphate groups in bacterial virulence and immunogenicity remain unknown. Here, we used an S. Typhimurium msbB pagL pagP lpxR mutant, carrying penta-acylated lipid A, as the parent strain to construct a series of mutants synthesizing 1-dephosphorylated, 4′-dephosphorylated, or nonphosphorylated penta-acylated lipid A. Dephosphorylated mutants exhibited increased sensitivity to deoxycholate and showed increased resistance to polymyxin B. Removal of both phosphate groups severely attenuated the mutants when administered orally to BALB/c mice, but the mutants colonized the lymphatic tissues and were sufficiently immunogenic to protect the host from challenge with wild-type S. Typhimurium. Mice receiving S. Typhimurium with 1-dephosphorylated or nonphosphorylated penta-acylated lipid A exhibited reduced levels of cytokines. Attenuated and dephosphorylated Salmonella vaccines were able to induce adaptive immunity against heterologous (PspA of Streptococcus pneumoniae) and homologous antigens (lipopolysaccharide [LPS] and outer membrane proteins [OMPs]).
INTRODUCTION
Salmonella enterica serovar Typhimurium is a facultative intracellular enteric pathogen that causes a typhoid-like systemic infection in mice and a self-limiting gastroenteritis in humans (22). One of its major virulence factors is lipopolysaccharide (LPS), which is localized to the outer leaflet of the Gram-negative bacterial outer membrane. LPS is composed of three domains: lipid A, core oligosaccharide, and O-antigen. LPS confers resistance to bile salts and hydrophobic antibiotics, and it protects the pathogen from the complement system and from killing by macrophages (39). Lipid A is a hydrophobic moiety that anchors LPS into the asymmetric outer membrane. It is required for growth and is an important barrier that provides resistance against antimicrobial peptides and environmental stresses that affect cell viability. For pathogens, lipid A functions as an immunomodulatory molecule that stimulates a strong innate immune response via the Toll-like receptor 4 (TLR4)-MD2-CD14 pathway resulting in the activation of nuclear factor κB (NF-κB) and upregulation of costimulatory molecules and inflammatory cytokines. A strong inflammatory response, however, is deleterious to the host, as it can lead to endotoxic shock or death (9).
Salmonella lipid A is similar to that of Escherichia coli (34). It is a glucosamine disaccharide that carries phosphate groups at positions 1 and 4′ and contains four primary (glucosamine-linked) hydroxyacyl chains and two secondary acyl chains (Fig. 1A) (34). Several enzymes are involved in the modification of the number of acyl chains or the phosphate groups of lipid A, and a majority of the genes encoding these enzymes are regulated by two global regulatory systems, PhoP/PhoQ and PmrA/PmrB. These genes include pagL, pagP, lpxR, arnT, eptA, and lpxT (10, 11, 35, 44, 45). The gene products of pagL and lpxR catalyze 3-O-deacylation and removal of the 3′-acyloxyacyl moiety from the Salmonella lipid A, respectively, and the product of pagP catalyzes transfer of a palmitate chain from a phospholipid to lipid A to form the hepta-acylated lipid A, which affects either the organism's immunogenicity or its resistance to antimicrobial peptides (11, 35, 45). On the other hand, ArnT, EptA, and LpxT are responsible for decoration of the phosphate groups by 4-amino-4-deoxy-l-arabinose (l-Ara4N), phosphoethanolamine (pEtN), and phosphate, respectively, which also modulate the bacterial sensitivity to antimicrobial peptides (10, 34).
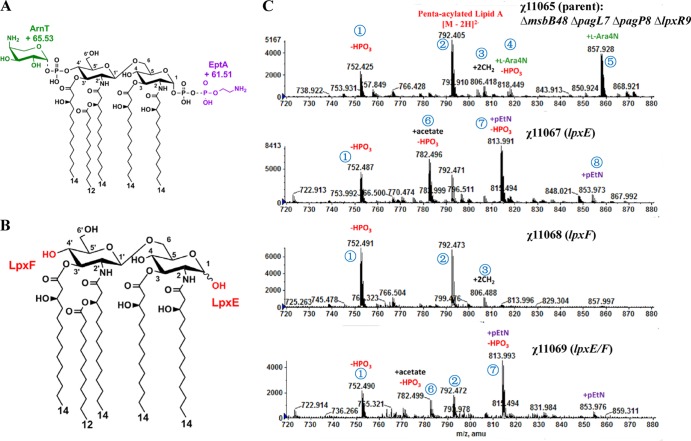
Fig 1.
Lipid A structure of the S. Typhimurium msbB mutant and its derivatives. (A) Covalent modifications of lipid A in the msbB pagL pagP lpxR mutant. The known covalent modifications of lipid A are indicated. (B) Covalent modifications of lipid A in the msbB pagL pagP lpxR mutant in the presence of LpxE or LpxF. (C) Lipid A profiles from ESI-MS analysis of χ11065 (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9), χ11067 (ΔmsbB48 ΔpagL7 ΔpagP81::Plpp lpxE ΔlpxR9), χ11068 (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR93::Plpp lpxF), and χ11069 (ΔmsbB48 ΔpagL7 ΔpagP81::Plpp lpxE ΔlpxR93::Plpp lpxF) grown in LB medium at 37°C. The msbB pagL pagP lpxR mutant makes a penta-acylated lipid A as indicated by the [M − 2H]2− peak at m/z 792.55. Removal of one phosphate group shifts the lipid A [M − 2H]2− peak by m/z −39.98. The addition of l-Ara4N to the 4′ phosphate, catalyzed by ArnT, shifts the lipid A [M − 2H]2− peak by m/z +65.529. The addition of pEtN to the 1-phosphate, catalyzed by EptA, shifts the MS peak by m/z +61.505. See Table S3 in the supplemental material for detailed information on lipid A derivatives.
The major factors contributing to lipid A-mediated endotoxicity are the number and length of acyl chains present and the phosphorylation state of the disaccharide backbone (7, 36). The negative charge carried by phosphate moieties at the 1 and 4′ positions serve as sites for adjacent LPS molecules to be cross-linked by cations (Mg2+ and Ca2+) or modified by l-Ara4N and pEtN moieties (29). In addition, the pattern of lipid A phosphorylation significantly affects its endotoxicity, as lipid A lacking one or both phosphate groups in Helicobacter pylori (24), Leptospira interrogans (33), Porphyromonas gingivalis (6), or Francisella novicida (32) is less toxic and has reduced affinity for antimicrobial peptides compared to the bisphosphorylated lipid A precursors. This phenotype, at least in Francisella tularensis, is due to the activity of two phosphatases (LpxE and LpxF). LpxE selectively removes the 1-phosphate group from either hexa-acylated (46) or penta-acylated lipid A (15), whereas LpxF can remove the 4′-phosphate moiety only from penta-acylated lipid A or tetra-acylated lipid A (47). Both LpxE and LpxF are integral inner membrane enzymes that dephosphorylate LPS and lipid A in the periplasmic leaflet of the inner membrane (46). No homologs of these genes are known in S. Typhimurium strain UK-1, and this study was carried out to understand the role of these lipid A phosphate moieties in the virulence and immunogenicity of Salmonella.
In order to determine the significance of lipid A phosphorylation in Salmonella, we used a msbB pagL pagP lpxR mutant strain of S. Typhimurium as the parent strain (19) and generated a series of variants, each expressing F. novicida lpxE and/or lpxF from the chromosome. We systematically investigated the in vitro phenotypes as well as the virulence and immunogenicity of these recombinant strains in BALB/c mice. We observed that removal of either or both phosphate groups rendered the strains more sensitive to detergents in vitro. Dephosphorylation of the lipid A of these strains also resulted in attenuation in mice following oral administration, yet the strains retained their abilities to induce protective adaptive immune responses against both homologous and heterologous antigens.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Strains and plasmids used in this work are listed in Table S1 in the supplemental material. S. Typhimurium was cultured at 37°C in LB medium (2) or in N minimal medium (41), pH 5.8, supplemented with 0.1% Casamino Acids, 38 mM glycerol, and 10 μM MgCl2, or on LB agar. A 350 mM EGTA solution at pH 8.0 (adjusted with NaOH) was used as a stock solution. LB-0 denotes LB without NaCl. EGTA (350 mM; Sigma, St. Louis, MO) was added to LB-0 to a final concentration of 6.5 mM to obtain LB-EGTA. MacConkey agar base (Difco) was used to prepare 0.2% galactose MacConkey agar. Diaminopimelic acid (DAP) was added (50 μg/ml) to media to facilitate growth of strains carrying the Δasd mutation (26). LB agar containing 5% sucrose was used for SacB-based counterselection during the mutagenesis process by allelic exchange. MOPS minimal medium (28) with or without 10 μg/ml p-aminobenzoic acid (pABA) was used to confirm the phenotype of ΔpabA ΔpabB double mutants of S. Typhimurium. S. pneumoniae WU2 was cultured on brain heart infusion agar containing 5% sheep blood or in Todd-Hewitt broth with 0.5% yeast extract.
Phenotypic characterization of strains.
Construction of suicide plasmids and the corresponding mutant strains are described in the supplemental material. LPS profiles of all Salmonella strains were examined by silver staining methods described previously (13). The full spectrum of the lipid A species isolated from each strain was determined by negative-ion electrospray ionization-mass spectrometry (ESI-MS) (20) after the samples were isolated by the Bligh/Dyer method (4). In general, the doubly charged regions will contain peaks corresponding to bisphosphorylated lipid A species due to ionization of each phosphate as well as peaks corresponding to monophosphorylated lipid A species due to the ability of the single phosphate to have two negative charges. Nonphosphorylated lipid A species were detected only in the singly charged region, presumably due to ionization of a hydroxyl moiety.
Data acquisition and analysis were performed using Analyst QS software. The MICs of different antimicrobial substances were determined by using 96-well plates following our protocol described elsewhere (18).
Cytokine assays of the cell line supernatant and in serum from mice.
Crude LPS was purified from a 20-ml culture using Tri-reagent (Sigma) as described previously (49); then the deoxycholate-phenol method (12) was used to remove the trace amounts of lipoprotein. LPS was quantified with a stand curve by the 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) method described by Osborn (30). The levels of interleukin 6 (IL-6), IL-1β, and tumor necrosis factor alpha (TNF-α) in the human monocytic leukemia cell line Mono Mac 6 (MM6) (Lonza, Braunschweig, Germany) and TNF-α and granulocyte-macrophage colony-stimulating factor (GM-CSF) in the murine macrophage cell line RAW264.7 were determined to check the LPS reactogenicity (20). Cytokine concentrations from the cell line supernatant and from the mouse serum were determined by using the Bio-plex protein array system (Bio-Rad) according to the manufacturer's recommendations (20).
Animal experiments.
Six-week-old, female BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA). All animal procedures were approved by the Arizona State University Animal Care and Use Committees. Mice were acclimated for 7 days after arrival before experiments were started.
The virulence of the Salmonella strains in mice was determined by the median lethal dose (LD50) according to our standard procedure (20). To evaluate colonization, mice were inoculated via the oral route with 1 × 109 CFU of each strain in 20 μl of buffered saline with gelatin (BSG). At days 3 and 6, blood samples were collected from the mice before the animals were euthanized for harvesting of spleens and livers. Each tissue was homogenized in BSG (1 ml), and dilutions (10−1 to 10−6 depending on the tissue) were plated onto LB agar plates to determine the number of viable bacteria. These experiments were done twice, and data were combined to calculate the LD50 and colonization.
To evaluate immunogenicity of vaccine strains in mice, the plasmid pYA4088, carrying the gene pspA from S. pneumoniae, or empty vector pYA3493 was transformed into the vaccine strains (see Table S1 in the supplemental material), and the arabinose-regulated synthesis of PspA and LacI was confirmed by immunoblotting (17, 18). The cultured vaccine strains were orally administered to the mice following our standard procedures (20, 21). Briefly, vaccine strains were grown statically overnight in the appropriate media. The next day, 1 ml of this culture was inoculated into 50 ml of the appropriate media and grown with aeration at 37°C to an optical density at 600 nm (OD600) of 0.8 to 0.9. Bacteria were harvested by centrifugation at 24°C and resuspended in 0.5 ml BSG. A volume of 20 μl of bacterial suspension contains around 109 CFU. Food and water were removed from the mouse cage for 6 h, and then mice were orally inoculated with 20 μl of BSG containing 1 × 109 CFU of each strain on day 0. Mice were given a booster at week 5 of the same dose of the same strain. Blood was obtained by mandibular vein puncture at biweekly intervals, and serum was collected after centrifugation. The vaginal tract of each mouse was washed with 50 μl BSG, and the wash fluids were pooled to analyze the secretory IgA. The immunogenicity of vaccine strains was evaluated by determining the titers of serum antibodies against S. Typhimurium LPS, recombinant PspA (rPspA), and Salmonella outer membrane protein (SOMP) by enzyme-linked immunosorbent assay (ELISA) as described previously (18).
Protection against virulent pneumococcal challenge.
The protection rates of the attenuated Salmonella delivering rPspA were assessed at week 9 postimmunization by intraperitoneal challenge of mice with 2 × 104 CFU of S. pneumoniae WU2 in BSG (200 μl) (27), which is equivalent to 100 times the LD50 for BALB/c mice. Challenged mice were monitored daily for 30 days.
Statistical analysis.
Data are shown as means ± standard errors of the means (SEM). Bonferroni's multiple comparison test in a two-way analysis of variance (ANOVA) was employed to evaluate differences in antibody titers in the mouse serum or vaginal wash fluids. Dunnett's multiple comparison test in one-way ANOVA was used to evaluate differences in cytokine levels and colonization levels by various strains. The LD50 was estimated using a probit analysis based on XLSTAT. All analyses were performed using GraphPad PRISM 5.0. A P value of <0.05 was considered statistically significant.
RESULTS
Recombinant LpxE and LpxF exhibit phosphatase activity on penta-acylated lipid A.
Negative electrospray ionization-mass spectrometry (ESI-MS) of the total lipid A species isolated from the mutants grown in LB medium was performed to attribute alterations to specific mutations (Fig. 1). In the spectrum of χ11065 (parent strain; ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9), the peaks include m/z 792.5 ([M − 2H]2−) for penta-acylated lipid A, m/z 857.9 ([M − 2H]2−) for penta-acylated lipid A with l-Ara4N at the 4′ position, and m/z 752.5([M − 2H]2−) for 1-dephospho-penta-acylated lipid A, which is a minor product of mild acid hydrolysis of LPS. No evidence of palmitate addition was noted, consistent with deletion of the pagP gene. The spectrum of lipid A derived from strain χ11067 (ΔmsbB48 ΔpagL7 ΔpagP81::Plpp lpxE ΔlpxR9) showed the following peaks: m/z 792.5 ([M − 2H]2−) for penta-acylated lipid A, m/z 752.5 ([M − 2H]2−) for 1-dephosphorylated penta-acylated lipid A (due to the action of LpxE), and m/z 813.99([M − 2H]2−) for 1-dephosphorylated penta-acylated lipid A with pEtN at the 4′ position. Lipid A peaks identified in the spectrum of strain χ11068 (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR93::Plpp lpxF), included m/z 792.5 ([M − 2H]2−) for penta-acylated lipid A and m/z 752.5([M − 2H]2−) for 4′-dephosphorylated penta-acylated lipid A (due to LpxF). These data showed that a portion of the lipid A was still bisphosphorylated, indicating that LpxF did not fully dephosphorylate the 4′ position of lipid A in this construct. For strain χ11069 (ΔmsbB48 ΔpagL7 ΔpagP81::Plpp lpxE ΔlpxR93::Plpp lpxF), the lipid A spectrum was similar to that of strain χ11067 (ΔmsbB48 ΔpagL7 ΔpagP81::Plpp lpxE ΔlpxR), demonstrating that the lipid A was not completely di-dephosphorylated by LpxE and LpxF (Fig. 1C). Nevertheless, in the singly charged region of the lipid A spectrum for strain χ11069, nonphosphorylated lipid A was observed (see Fig. S1 in the supplemental material).
Dephosphorylation of lipid A alters several phenotypes of the mutants.
To investigate if changes in the phosphorylation state of lipid A caused any alteration to the overall LPS profile, LPS from various mutant strains was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by silver staining. We observed no significant differences in terms of the ladder pattern of the O antigen or the core region of LPS (see Fig. S2A in the supplemental material). The effect of each mutation on susceptibility to P22 transduction was also determined. The sensitivity of χ11065 (parent) to infection with P22 phage was similar to that of the wild type. However, the efficiency of P22 infection was at least 5-fold lower in the dephosphorylated strains, as determined by the number of bacterial colonies obtained after P22 infection (data not shown).
Strains lacking the myristate chain or phosphate groups contain an altered LPS structure that impacts membrane permeability (29). To investigate this correlation, an isolated colony of each mutant strain was patched on LB, LB-0 (no salt, low ionic strength), LB-0+EGTA (OM disruptor), or MacConkey (contains bile salts, natural detergent) agar plates. All mutants grew normally on LB agar or on LB-0 (LB without salt) agar (data not shown). Differences in growth were observed on MacConkey agar plus 1% galactose (see Fig. S2B in the supplemental material). χ11065 (parent), which has both lipid A phosphate groups intact, showed growth similar to that of the wild type. The mutant strain lacking in part both 1- and 4′-phosphate groups (χ11069 [lpxE lpxF]) exhibited clear sensitivity to detergent (see Fig. S2B).We also determined the relative sensitivities of the S. Typhimurium mutants to the detergent deoxycholate salt (DOC) and a representative antimicrobial peptide polymyxin B as measured by MICs (see Table S4 in the supplemental material). Wild-type χ3761 and χ11065 each withstood up to 6.25 mg/ml of DOC and >10 mg/ml of ox bile (see Table S4), which is in agreement with their growth on different media (see Fig. S2B). As expected, the MIC of DOC was slightly lower (3.1 mg/ml) for mutants containing either the 1-dephosphorylated (χ11067) or 4′-dephosphorylated (χ11068) lipid A and significantly lower (0.8 mg/ml) when both phosphates were removed (χ11069). χ11065 (msbB mutant with penta-acylated lipid A) was more sensitive to polymyxin B than χ3761 (wild-type UK-1), which is consistent with other reports (25). The susceptibilities of mutants carrying the 1-dephosphorylated or 4′-dephosphorylated lipid A to polymyxin B were the same as that of the wild-type UK-1 and, therefore, lower than that of the parent strain χ11065 (see Table S4). Removal of the 1-phosphate (χ11067 [lpxE] or χ11069 [lpxE lpxF]) significantly reduced the motility of S. Typhimurium, but partial removal of the 4′-phosphate group had a minimal effect when tested on 0.3% soft agar (Fig. 1C; also, see Table S4).
Dephosphorylation of lipid A attenuates S. Typhimurium while retaining immunogenicity.
To evaluate the significance of lipid A phosphorylation for the virulence of S. Typhimurium, we compared the LD50 of individual mutants in BALB/c mice inoculated orally. The LD50 of wild-type strain χ3761 was 2.8 × 104 CFU (Table 1), which is consistent with our previous observations (17, 18). The LD50 was a log higher (3.9 × 105 CFU) for the χ11065 (parent) strain, which contains the penta-acylated lipid A. However, dephosphorylation of lipid A at either the 1 or 4′ position significantly increased the LD50 of strains χ11067 (lpxE), χ11068 (lpxF), and χ11069 (lpxE lpxF) to >108 CFU, suggesting attenuation of S. Typhimurium. We observed some clinical manifestations of disease (scruffy coat and lethargy) and some deaths in mice receiving the highest doses (108 or 109 CFU) of χ11067 (lpxE) and χ11068 (lpxF). These symptoms were not observed in mice that received χ11069 (lpxE lpxF). All mice that survived immunization with χ11067, χ11068, or χ11069 were protected from UK-1 challenge (Table 1), indicating that the mutants, although attenuated, remained sufficiently immunogenic to induce protection against the wild-type UK-1 challenge. Because not all of the mice inoculated with 103 or 104 CFU doses of χ11065 and χ3761 survived, any dependence of protection rate on the dosage of immunization could not be fully evaluated.
Table 1.
Mouse survival after oral inoculation with S. Typhimurium strainsa
| Strain | No. of surviving mice/total receiving dose (CFU) ofb: |
LD50 (CFU)c | ||||||
|---|---|---|---|---|---|---|---|---|
| 103 | 104 | 105 | 106 | 107 | 108 | 109 | ||
| c11065 (parent)d | 5/5 | 9/10 | 6/12 | 3/10 | 0/7 | 0/2 | — | 3.9 × 105 |
| χ11067 (lpxE) | — | — | — | 7/7 | 7/7 | 7/10 | 7/9 | >109 |
| χ11068 (lpxF) | — | — | — | 7/7 | 9/10 | 5/9 | 4/9 | 6.9 × 108 |
| χ11069 (lpxE lpxF) | — | — | — | 7/7 | 7/7 | 10/10 | 9/9 | >109 |
| χ3761 (wild type) | 6/7 | 5/9 | 2/10 | 0/9 | — | — | — | 2.8 × 104 |
Surviving mice were challenged with 1 × 109 CFU of χ3761 30 days after the initial inoculation. All mice originally receiving doses of 105 CFU or more survived challenge, while only a portion of those receiving a dose of 103 or 104 CFU survived.
—, dose not tested.
Data are based on probit analysis.
ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9.
Dephosphorylation of lipid A reduces the colonization of bacteria in mice.
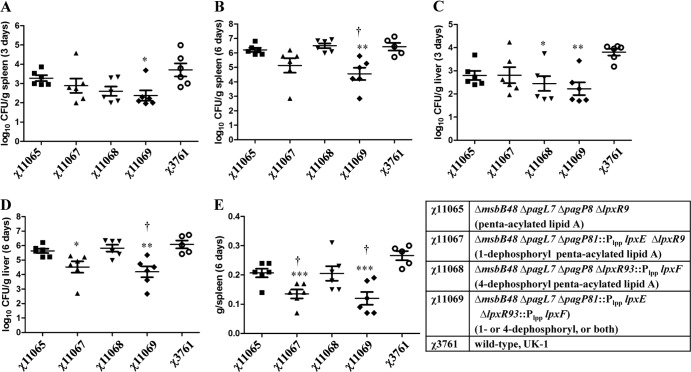
To determine tissue colonization by each mutant, the bacterial burden in spleen or liver was determined 3 and 6 days after oral inoculation (Fig. 2). The median log10 CFU of wild-type χ3761 recovered from one gram of spleen or liver was ∼3.5 on day 3 and increased to ∼6.0 on day 6, which is in agreement with our previous reports (17, 18). Among the four mutants, only strain χ11069 (lpxE lpxF) had a lower median log10 CFU recovered from the spleen on day 3 (relative to the value for the wild type, P < 0.05), but it did reach a median log10 CFU of 4 by day 6. No differences in colonization of mice were observed in the other mutant strains at the two tested times (Fig. 2). Similar results were also observed in liver colonization of mice (Fig. 2). In addition, only mutants lacking the 1-phosphate (χ11067) or both 1- and 4′-phosphates (χ11069) induced smaller spleen sizes than the wild-type induced, but they still induced larger spleen sizes than were seen in the BSG control mice (about 0.1 g per spleen) (Fig. 2E), indicating that the mutants still induced inflammatory responses in vivo.
Fig 2.
Colonization of mice by χ3761 and each of its lipid A mutants. Viable bacterial counts (log10 CFU per gram) recovered from spleen (A and B) and liver (C and D) of BALB/c mice (n = 6) at 3 and 6 days after oral inoculation by the indicated S. Typhimurium mutant strains are shown. Strain χ3761 is the wild-type strain that was used as a control. (E) Weights of spleens (in grams) (E) from six BALB/c mice 6 days after oral inoculation by the indicated strains of S. Typhimurium. Horizontal bars show the means; error bars indicate SEM. ***, P < 0.001; **, P < 0.01; *, P < 0.05 (relative to the value for wild-type UK-1). ††, P < 0.01; †, P < 0.05 (relative to the value for χ11065 [ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9]).
Dephosphorylation of lipid A alters innate immunity in cell lines and mice. (i) In vitro assay using MM6 cells.
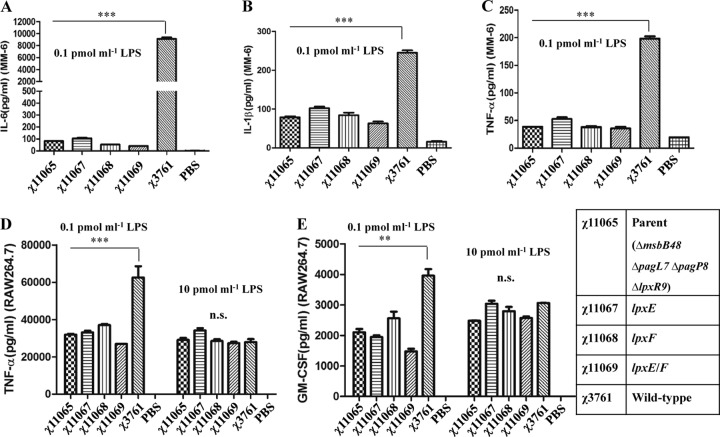
To determine whether purified LPS from the msbB mutant and the isogenic dephosphorylated lipid A strains differed in their abilities to induce inflammatory responses, the levels of IL-6, IL-1β, and TNF-α secreted by MM6 cells upon stimulation with purified LPS were assayed by the Bioplex method. LPS (0.1 pmol/ml) derived from wild-type UK-1 induced 10,000 pg/ml IL-6, 280 pg/ml IL-1β, and 200 pg/ml TNF-α, but the LPS from the mutants induced significantly (P < 0.001) lower levels of the three cytokines tested (Fig. 3A to C). Similar results were achieved upon stimulation with 10 pmol/ml LPS (see Fig. S3 in the supplemental material).
Fig 3.
LPS-induced cytokine production in cultured Mono Mac 6 (MM6) and RAW264.7 cells. IL-6 (A), IL-1β (B), and TNF-α (C) in the supernatant of the MM6 cell culture stimulated with 0.1 pmol/ml LPS for 24 h were quantified by Bioplex assay. TNF-α (D) and GM-CSF (E) released into the culture supernatant of RAW264.7 cells stimulated for 24 h with 0.1 pmol/ml or 10 pmol/ml of LPS, respectively, were quantified by Bioplex assay. Means and SEM of three independent experiments, each performed in triplicate, are given. ***, P < 0.001; **, P < 0.01; *, P < 0.05 (relative to the levels obtained by stimulation with LPS from wild-type χ3761). †††, P < 0.001; ††, P < 0.01; †, P < 0.05 (relative to levels obtained by stimulation with LPS from the parent strain, χ11065 [ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9]).
(ii) In vitro assay using RAW264.7 cells.
Levels of the proinflammatory cytokines TNF-α and GM-CSF produced by cultured RAW264.7 cells upon incubating with purified LPS from each strain was also determined. At a low concentration (0.1 pmol/ml), LPS derived from the wild-type strain (χ3761) induced approximately 6,300 pg/ml of TNF-α (Fig. 3D) and 4,000 pg/ml of GM-CSF (Fig. 3E). The levels were over 50% lower when the cells were stimulated by LPS derived from any of the four mutants (Fig. 3D and E) and did not differ significantly between the mutant strains. The only exception was the amount of GM-CSF induced when LPS purified from strain χ11069 (lpxE and lpxF) was used (1,500 pg/ml versus 2,100 pg/ml for other strains). However, no significant differences were observed between cytokine profiles when a higher concentration (10 pmol/ml) of LPS was used (Fig. 3D and E). We observed lower induction of TNF-α and GM-CSF when it was stimulated by 10 pmol/ml compared to 0.1 pmol/ml of χ3761 LPS, which may be due to inhibition of the TLR4 pathway by the high dose of LPS in RAW264.7 cells.
(iii) Innate immune response in mice.
S. Typhimurium has a complex network to regulate its gene expression in different microenvironment niches; thus, in vitro models do not truly represent the situation in vivo. To better understand the inflammatory and immunomodulatory potential of each mutant, the levels of cytokines in pooled mouse serum were determined 6 days after oral inoculation of mice with 109 CFU of each strain. The cytokines evaluated included hallmarks of inflammation (IL-1α, IL-1β, IL-6, MIP-1β, TNF-α, G-CSF, and GM-CSF), cytokines involved in chemotaxis (KC, MCP-1, and IL-17), and immunomodulatory cytokines [IL-2, IL-9, IL-10, IL-12(p70), IL-13, and IFN-γ]. The levels of all the assayed cytokines were significantly elevated in mice receiving any strain compared to those receiving saline (BSG) as a control (see Table S5 in the supplemental material).
Strains that had undergone modifications of lipid A, either by removal of one acyl chain (χ11065), by removal of the 1-phosphate by LpxE (χ11067), or by removal of the 4′-phosphate by LpxF (χ11068), all induced significantly (P < 0.01 to P < 0.001) lower levels of induced proinflammatory or immunomodulatory cytokines in mice than the wild-type strain (χ3761). For the majority of these cytokines, the levels were even lower in mice receiving strain χ11069, in which the 1- and 4′-phosphates were both removed.
Delivery of a heterologous antigen and induction of protective adaptive immunity.
To evaluate the effect of lipid A modifications on the ability of recombinant attenuated Salmonella vaccines (RASV) to induce adaptive immune responses in mice, each of the deletion or insertion-deletion mutations resulting in various alterations of the lipid A structure was moved into the χ9241 background. This strain contains the pabA and pabB attenuating mutations (along with ΔasdA) and is routinely used in our laboratory to evaluate the significance of various mutations in developing attenuated Salmonella vaccines (18).
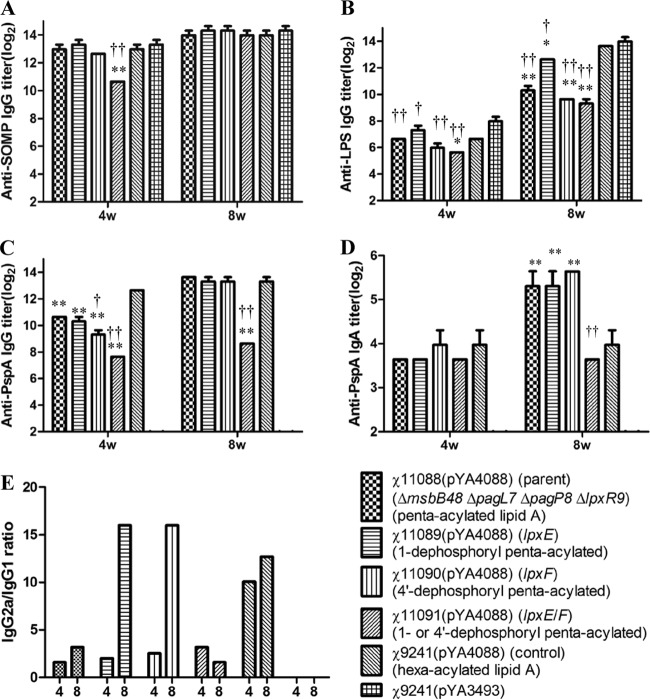
Figure 4 summarizes titers of antibody against rPspA or against self-antigens (SOMP and LPS) of S. Typhimurium in pooled mouse sera. χ11091 (dephosphorylated at both lipid A phosphate groups) induced a lower level of IgG against Salmonella OMP than other mutant strains at 4 weeks; however, at 8 weeks, no differences in titers of IgG against OMP were observed among all mutants (Fig. 4A). In contrast, titers of IgG against LPS from mice immunized with lipid A variants were significantly (P < 0.05 or P < 0.01) lower than those in sera from mice immunized with the parent strain χ9241 (Fig. 4B), especially at the later time point (8 weeks).
Fig 4.
Serum IgG and vaginal IgA immune responses in immunized and control mice. The total serum IgG against SOMP (A), Salmonella LPS (B), and rPspA (C), the total vaginal wash fluid IgA against rPspA (D), and the IgG2a/IgG1 ratio (E) were measured by ELISA. Mice (n = 10 or 13) received the first immunization on day 0 and a booster dose at week 5. Data represent reciprocal anti-IgG titers in pooled sera from mice orally immunized with attenuated Salmonella carrying either pYA4088 (pspA) or pYA3493 (control). No immune responses to PspA were detected in mice immunized with the S. Typhimurium vaccine strain carrying the control plasmid pYA3493 (reciprocal titers, <1:50 for IgG and <1:25 for IgA). Error bars represent variations between triplicate wells. ***, P < 0.001; **, P < 0.01; *, P < 0.05 [compared with the value for χ9241(pYA4088)]. †††, P < 0.001; ††, P < 0.01; †, P < 0.05 [compared with the value for χ11088(pYA4088)].
The relative abilities of the vaccine strains to induce antibody responses against the heterologous pneumococcal antigen PspA were distinct. At 4 weeks, anti-PspA IgG titers induced by each mutant strain were significantly (P < 0.01) lower than the titer induced by the parent strain χ9241(pYA4088). In addition, removing the 4′-phosphate (χ11090 [lpxF]) further affected its immunogenicity. The titers were, however, indistinguishable at 8 weeks (measured 3 weeks after the booster); the only exception was strain χ11091, which contains 1- and 4′-dephosphorylated lipid A. χ11088, χ11089 (lpxE), and χ11090 (lpxF) induced significantly higher levels of secretory IgA in the vaginal tract than χ9241 (P < 0.01) (Fig. 4D). Anti-PspA titers in mice receiving the control strain χ9241(pYA3493) were not detectable.
To determine if alteration in the lipid A phosphorylation state induces a Th1/Th2 switch response, the ratios of titers of IgG2a and IgG1 against PspA were determined (Fig. 4E). All vaccine strains induced Th1 responses typical of Salmonella during the immunization period. χ11089 (lpxE) and χ11090 (lpxF) induced similar Th1/Th2 responses; however, both strains induced higher Th1 responses than χ11088 (parent) and χ11091 (lpxE lpxF) at 8 weeks.
To examine the ability of RASV-rPspA vaccines to protect against pneumococcal infection, mice were challenged intraperitoneally (i.p.) with 100 LD50s of S. pneumoniae WU2 4 weeks after a booster vaccination. Immunization with any of the pspA-expressing strains provided significant protection against challenge (P < 0.001) compared with control χ9241 containing pYA3493 (empty vector). Importantly, there was no significant difference in the levels of protection conferred by any of the vaccine strains tested (see Table S6 in the supplemental material).
DISCUSSION
LPS is a major molecular component of the outer membrane of Gram-negative bacteria. LPS not only provides stability to the bacterial cell wall but is also a potent virulence factor that is believed to contribute to bacterial-induced pathologies, including general bacterial sepsis, inflammatory bowel disorders, etc. (1). The lipid A component of LPS in particular, with its 1- and 4′-phosphates, plays an immunostimulatory role by inducing proinflammatory responses. S. Typhimurium employs modifications of lipid A as one of its several means to modulate the host immune response. For example, lipid A 3-O-deacylation by PagL and palmitoylation by PagP reduce the ability of lipid A to activate the host TLR4 pathway and help the pathogen to evade the immune system (16, 23). Similarly, the l-Ara4N and/or pEtN moieties together neutralize the negative charges of the 1- and 4′-phosphate groups on lipid A to reduce its affinity for cationic peptides and thus confer resistance to antimicrobial peptides (11, 35, 45). In this work, we determined that phosphate groups at both the 1 and 4′ positions of Salmonella lipid A affected Salmonella virulence and immunogenicity.
Nonphosphoryl or monophosphoryl lipid A structures are a natural phenomenon in several pathogenic bacteria, such as Helicobacter pylori (24), Leptospira interrogans (33), Porphyromonas gingivalis (6), Francisella novicida (32), and the plant endosymbiont Rhizobium etli (3), as a result of specific phosphatases present in these bacteria. Dephosphorylation of lipid A confers certain advantages on the pathogens' interactions with the host. For example, in F. novicida, the modified lipid A structure aids the pathogen in immune evasion and ultimately protects it from immune clearance. In fact, a shift to a phosphorylated form of lipid A results in decreased virulence or in total attenuation (48). Salmonella does not contain genes that encode a lipid A 1 (LpxE)- or 4′ (LpxF)-phosphatase. It was proposed that UgtL in Salmonella could remove the 1-phosphate group and contribute to resistance to cationic antimicrobial peptides (40); however, studies in our laboratory suggest that UgtL is not a lipid A 1-phosphatase but rather mediates the addition of l-Ara4N to lipid A and therefore provides resistance to polymyxin B (Q. Kong, D. A. Six, Q. Liu, C. R. H. Raetz, and R. Curtiss III, unpublished data).
Two inner membrane phosphatases from Francisella novicida, designated LpxE and LpxF, can selectively remove the 1- and 4′-phosphate groups of lipid A, respectively (46, 47). In this study, we generated various mutant strains of S. Typhimurium that synthesize LpxE or LpxF or both in a msbB lpxR pagL pagP mutant background (χ11065) (19). Use of the msbB pagL pagP lpxR mutant as a parent strain serves two purposes. First, this strain produces a penta-acylated lipid A (3 + 2 type) both in vitro and in vivo, which is required for optimal phosphatase activity of LpxF (47). Second, elimination of PagL, PagP, and LpxR enables us to study the precise effects of various lipid A phosphorylation states on virulence and immunogenicity, independent of lipid A acyl chain variability, which could interfere with the interaction between LPS and TLR4/MD2 complex in vivo. Introducing lpxE (χ11067) or lpxF (χ11068) resulted in the removal of the 1- or 4′-phosphate group from S. Typhimurium lipid A (Fig. 1). Complete dephosphorylation of Salmonella lipid A upon expression of both lpxE and lpxF (χ11069) (Fig. 1) was not achieved, primarily due to incomplete dephosphorylation by LpxF. We were able to detect dephosphorylation at both phosphates to produce nonphosphorylated lipid A (see Fig. S1 in the supplemental material) (6). The increased polymyxin B resistance exhibited by strain χ11069 (lpxE lpxF) is consistent with the strain's containing nonphosphorylated lipid A. The latter observation was also made in E. coli, where expressing F. novicida lpxE and lpxF from a plasmid resulted in complete removal of both phosphate groups from LPS (14). Removal of the 1- or 4′-phosphate group from lipid A by LpxE or LpxF did not dramatically affect the O-antigen pattern of S. Typhimurium (see Fig. S2A in the supplemental material). However, expressing lpxE and lpxF (χ11069) led to increased sensitivity to bile (see Table S4 in the supplemental material). This defect may be due to the impaired membrane integrity caused by disruption of the ionic interactions between neighboring lipid A phosphate groups (29).
When the ability of purified LPS from each of the lipid A variants was tested on human MM6 cells or RAW264.7 cells (Fig. 3) for the ability to induce proinflammatory cytokines, the LPSs from each strain containing penta-acylated lipid A (by virtue of the ΔmsbB and ΔpagP mutations) were less potent than that obtained from the wild-type strain χ3761. The three mutants tested did not differ significantly from their parent strain. We speculate that the less reactogenic phenotype derives predominantly from the presence of penta-acylated lipid A, as it is known that hexa-acylated lipid A has optimal inflammatory activity and the activity decreases approximately 100-fold when one acyl chain is removed (7, 42). While removal of the 1-phosphate from hexa-acylated LPS significantly reduced stimulation of the TLR4-MD2-CD14 pathway (28), the phosphate groups of penta-acylated LPS did not play a vital role in stimulating the innate immune response in this study (Fig. 3), which is not consistent with the notion that removal of either of these phosphate groups reduces endotoxic activity 100-fold (7, 36). We speculate that the incomplete removal of phosphate groups (Fig. 1C), the presence of 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo), and O-antigen or core heterogeneity in the structure of LPS of our mutant strains may account for this unexpected result (31, 50). In addition, the observations made from in vitro assays performed using cell lines to determine the induced cytokine profiles were not consistent with those made using mouse sera (see Table S5 in the supplemental material). The wild-type UK-1 strain (χ3761) induced levels of chemokines and cytokines similar to those induced by strain χ11065 (parent) harboring penta-acylated LPS in vivo. Dephosphorylation of lipid A significantly diminished proinflammatory responses and other cytokines produced in mice. The mutant strains containing either monophosphorylated lipid A (χ11067 [lpxE]) or nonphosphorylated lipid A (χ11069 [lpxE lpxF]) induced lower levels of proinflammatory cytokines than χ11065 and wild-type UK-1 strain (χ3761) (see Table S5). The proinflammatory cytokines induced by the purified LPS in the in vitro assays differ from the cytokines induced by a live Salmonella mutant in vivo (Fig. 2; also, see Table S3 in the supplemental material). The different outcomes likely reflect the different systems (cell lines versus whole animal) as well as the different stimuli used to trigger innate immune responses. For the in vitro assay, only a single pathogen-associated molecular pattern (PAMP), purified LPS, was involved in stimulating a TLR4-dependent innate immune response. For the in vivo assay, the delivery of live Salmonella introduced multiple PAMPs (LPS, flagella, lipoprotein, etc.) (43) and could have various effects depending on the bacterial load and whether the strains are effectively phagocytosed (8, 43).
Nevertheless, χ11068 (lpxF) seemed to have unexpectedly high endotoxic activity in vivo (see Table 5 in the supplemental material). The following reasons may account for this anomaly. (i) Consistent with incomplete 4′-dephosphorylation seen in vitro, LpxF may not completely remove the 4′-phosphate group from LPS in vivo. (ii) Murine TLR4 may efficiently bind to 4′-dephosphorylated LPS, as suggested by the finding that penta-acylated 4′-dephosphorylated lipid A is more TLR4 stimulatory than LPS containing 1-dephosphorylated lipid A in HEK293 with hTLR4-MD2 (5). (iii) Chemokine or cytokine secretion in the murine system is a dynamic process that has a different time course for different strains, such that the 6-day time point may not be optimal for comparing cytokine production induced by χ11068 (lpxF) to that induced by the other strains tested.
S. Typhimurium mutant strains synthesizing the monophosphorylated or nonphosphorylated penta-acylated lipid A were dramatically attenuated in mice when administered orally. We attribute this phenotype to the loss of the phosphate group(s) on lipid A leading to milder induction of innate immunity. The fact that strain χ11065, with five acyl chains and with both phosphate groups intact, exhibited an LD50 (105 CFU) similar to that of the wild-type UK1 strain further supports our hypothesis. Nevertheless, despite the attenuation of virulence, inoculation with these mutants provides complete protection against a challenge with a lethal dose of wild-type Salmonella UK-1 (Table 1). These results suggest that nonphosphorylated or monophosphorylated mutants, though attenuated, retain the ability to induce acquired immunity in mice, which is in agreement with our previous report, in which the Salmonella mutant synthesizing 1-dephosphorylated hexa-acylated lipid A was completely attenuated but still retained its immunogenicity (20). Overall, these findings demonstrate that removal of a phosphate group(s) from Salmonella LPS results in reduced endotoxic responses, attenuation of the pathogen, and reduction in LPS-dependent sepsis and shock (37).
Reducing lipid A-mediated endotoxicity is an important safety feature while developing live bacterial vaccines. The reduction of endotoxicity must be balanced with maintaining sufficient immunogenicity to provide a robust adaptive immune response. To assess the immunogenicity of the S. Typhimurium vaccine strains containing either mono-phosphorylated lipid A (χ11089 or χ11090) or nonphosphorylated lipid A (χ11091) compared to the parent strain (χ11088), we determined the serum antibody titers generated by individual strains against both self-antigens (SOMP and LPS) and a heterologous antigen (PspA). As expected, anti-LPS serum IgG responses differed based on the nature of the lipid A species present on the vaccine strain. Interestingly, titers of serum IgG against outer membrane proteins were essentially indistinguishable, suggesting that alteration in LPS did not affect the immunogenicity of SOMPs. This perhaps also contributed to the protection conferred by each of these strains against virulent Salmonella challenge. However, removal of both phosphate groups (χ11091 [lpxE lpxF]), but not one, significantly diminished the titers of IgG against PspA (Fig. 4C). These results together suggest the significance of lipid A phosphate in serving as an adjuvant to induce a systemic immune response against a protective antigen (PspA in this case). Our data relating to the anti-PspA IgA titer also support the notion that mucosal immune responses to heterologous antigens may be mediated via TLR4, and MyD88-dependent signaling pathway (19, 38).
In conclusion, we found that the removal of either one or both phosphate groups on lipid A renders S. Typhimurium significantly sensitive to detergent and more resistant to antimicrobial peptides than their isogenic parent. Although attenuated, the strains with mono- or nonphosphorylated lipid A are immunogenic and induce a protective innate and adaptive immune response. Thus, we propose that incorporation of monophosphorylated, but not nonphosphorylated, LPS into live Salmonella vaccine strains may be beneficial in reducing endotoxic activity to enhance safety at high doses and retain immunogenicity, and Salmonella vaccines synthesizing the nonphosphorylated lipid A may be useful for developing subunit vaccines, such as those based on outer membrane proteins, outer membrane vesicles and heterologous polysaccharides, without requirement to remove the endotoxin.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ziqiang Guan for assistance in preparing MS figures and Kenneth Roland for critically reading the manuscript.
This work was supported by NIH R01 grants AI056289 and AI060557 and by grant 37863 from the Bill and Melinda Gates Foundation to R.C. and by NIH grant GM-51796 to C.R.H.R. The mass spectrometry facility in the Department of Biochemistry of the Duke University Medical Center is supported by the LIPID MAPS Large Scale Collaborative Grant, number GM-069338, from the NIH.
Footnotes
Published ahead of print 2 July 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Bainbridge BW, Darveau RP. 2001. Porphyromonas gingivalis lipopolysaccharide: an unusual pattern recognition receptor ligand for the innate host defense system. Acta Odontol. Scand. 59:131–138 [DOI] [PubMed] [Google Scholar]
- 2. Bertani G. 1951. Studies on lysogenesis I: the mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhat UR, Forsberg LS, Carlson RW. 1994. Structure of lipid A component of Rhizobium Leguminosarum bv Phaseoli lipopolysaccharide—unique nonphosphorylated lipid A containing 2-amino-8-deoxygluconate, galacturonate, and glucosamine. J. Biol. Chem. 269:14402–14410 [PubMed] [Google Scholar]
- 4. Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 37:911–917 [DOI] [PubMed] [Google Scholar]
- 5. Coats SR, et al. 2011. The lipid A phosphate position determines differential host Toll-like receptor 4 responses to phylogenetically related symbiotic and pathogenic bacteria. Infect. Immun. 79:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coats SR, et al. 2009. Human Toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1-and 4′-phosphatase activities. Cell Microbiol. 11:1587–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erridge C, Bennett-Guerrero E, Poxton IR. 2002. Structure and function of lipopolysaccharides. Microbes Infect. 4:837–851 [DOI] [PubMed] [Google Scholar]
- 8. Figueira R, Holden DW. 2012. Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology 158:1147–1161 [DOI] [PubMed] [Google Scholar]
- 9. Ghosh S, May MJ, Kopp EB. 1998. NF-kappa B and rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225–260 [DOI] [PubMed] [Google Scholar]
- 10. Gunn JS, et al. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27:1171–1182 [DOI] [PubMed] [Google Scholar]
- 11. Guo L, et al. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95:189–198 [DOI] [PubMed] [Google Scholar]
- 12. Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618–622 [DOI] [PubMed] [Google Scholar]
- 13. Hitchcock PJ, Brown TM. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver stained polyacrylamide gels. J. Bacteriol. 154:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ingram BO, Masoudi A, Raetz CRH. 2010. Escherichia coli mutants that synthesize dephosphorylated lipid A molecules. Biochemistry 49:8325–8337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karbarz MJ, Six DA, Raetz CRH. 2009. Purification and characterization of the lipid A 1-phosphatase LpxE of Rhizobium leguminosarum. J. Biol. Chem. 284:414–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawasaki K, China K, Nishijima M. 2007. Release of the lipopolysaccharide deacylase PagL from latency compensates for a lack of lipopolysaccharide aminoarabinose modification-dependent resistance to the antimicrobial peptide polymyxin B in Salmonella enterica. J. Bacteriol. 189:4911–4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kong Q, Liu Q, Jansen AM, Curtiss R., III 2010. Regulated delayed expression of rfc enhances the immunogenicity and protective efficacy of a heterologous antigen delivered by live attenuated Salmonella enterica vaccines. Vaccine 28:6094–6103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kong Q, Liu Q, Roland KL, Curtiss R., III 2009. Regulated delayed expression of rfaH in an attenuated Salmonella enterica serovar Typhimurium vaccine enhances immunogenicity of outer membrane proteins and a heterologous antigen. Infect. Immun. 77:5572–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kong Q, et al. 2011. Palmitoylation state impacts induction of innate and acquired immunity by the Salmonella enterica serovar Typhimurium msbB mutant. Infect. Immun. 79:5027–5038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kong Q, et al. 2011. Salmonella synthesizing 1-dephosphorylated lipopolysaccharide exhibits low endotoxic activity while retaining its immunogenicity. J. Immunol. 187:412–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kong Q, et al. 2011. Effect of deletion of genes Involved in lipopolysaccharide core and O-antigen synthesis on virulence and immunogenicity of Salmonella enterica serovar Typhimurium. Infect. Immun. 79:4227–4239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Layton AN, Galyov EE. 2007. Salmonella-induced enteritis: molecular pathogenesis and therapeutic implications. Expert Rev. Mol. Med. 9:1–17 [DOI] [PubMed] [Google Scholar]
- 23. Miller SI, Ernst RK, Bader MW. 2005. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 3:36–46 [DOI] [PubMed] [Google Scholar]
- 24. Moran AP, Lindner B, Walsh EJ. 1997. Structural characterization of the lipid a component of Helicobacter pylori rough and smooth form lipopolysaccharides. J. Bacteriol. 179:6453–6463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murray SR, Ernst RK, Bermudes D, Miller SI, Low KB. 2007. PmrA(Con) confers pmrHFIJKL-dependent EGTA and polymyxin resistance on msbB Salmonella by decorating lipid A with phosphoethanolamine. J. Bacteriol. 189:5161–5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakayama K, Kelly SM, Curtiss R., III 1988. Construction of an Asd+ expression-cloning vector: stable maintenance and high level expression of cloned genes in a Salmonella vaccine strain. Nat. Biotechnol. 6:693–697 [Google Scholar]
- 27. Nayak AR, et al. 1998. A live recombinant avirulent oral Salmonella vaccine expressing pneumococcal surface protein A induces protective responses against Streptococcus pneumoniae. Infect. Immun. 66:3744–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neidhardt FC, Bloch PL, Smith DF. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nikaido H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Osborn MJ. 1963. Studies on gram-negative cell wall. 1. Evidence for role of 2-keto-3-deoxyoctonate in lipopolysaccharide of Salmonella typhimurium. Proc. Natl. Acad. Sci. U. S. A. 50:499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park BS, et al. 2009. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191–1195 [DOI] [PubMed] [Google Scholar]
- 32. Phillips NJ, Schilling B, McLendon MK, Apicella MA, Gibson BW. 2004. Novel modification of lipid A of Francisella tularensis. Infect. Immun. 72:5340–5348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Que-Gewirth NLS, et al. 2004. A methylated phosphate group and four amide-linked acyl chains in Leptospira interrogans lipid A—the membrane anchor of an unusual lipopolysaccharide that activates TLR2. J. Biol. Chem. 279:25420–25429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raetz CRH, Reynolds CM, Trent MS, Bishop RE. 2007. Lipid A modification systems in gram-negative bacteria. Annu. Rev. Biochem. 76:295–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reynolds CM, et al. 2006. An outer membrane enzyme encoded by Salmonella typhimurium IpxR that removes the 3′-acyloxyacyl moiety of lipid A. J. Biol. Chem. 281:21974–21987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rietschel ET. 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8:217–225 [DOI] [PubMed] [Google Scholar]
- 37. Roger T, et al. 2009. Protection from lethal Gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc. Natl. Acad. Sci. U. S. A. 106:2348–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salam MA, Katz J, Michalek SM. 2010. Role of Toll-like receptors in host responses to a virulence antigen of Streptococcus mutans expressed by a recombinant, attenuated Salmonella vector vaccine. Vaccine 28:4928–4936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shaio MF, Rowland H. 1985. Bactericidal and opsonizing effects of normal serum on mutant strains of Salmonella typhimurium. Infect. Immun. 49:647–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shi YX, Cromie MJ, Hsu FF, Turk J, Groisman EA. 2004. PhoP-regulated Salmonella resistance to the antimicrobial peptides magainin 2 and polymyxin B. Mol. Microbiol. 53:229–241 [DOI] [PubMed] [Google Scholar]
- 41. Snavely MD, Miller CG, Maguire ME. 1991. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J. Biol. Chem. 266:815–823 [PubMed] [Google Scholar]
- 42. Teghanemt A, Zhang DS, Levis EN, Weiss JP, Gioannini TL. 2005. Molecular basis of reduced potency of underacylated endotoxins. J. Immunol. 175:4669–4676 [DOI] [PubMed] [Google Scholar]
- 43. Thiennimitr P, Winter SE, Baumler AJ. 2012. Salmonella, the host and its microbiota. Curr. Opin. Microbiol. 15:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Touze T, Tran AX, Hankins JV, Mengin-Lecreulx D, Trent MS. 2008. Periplasmic phosphorylation of lipid A is linked to the synthesis of undecaprenyl phosphate. Mol. Microbiol. 67:264–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trent MS, Pabich W, Raetz CRH, Miller SI. 2001. A PhoP/PhoQ-induced lipase (PagL) that catalyzes 3-O-deacylation of lipid a precursors in membranes of Salmonella typhimurium. J. Biol. Chem. 276:9083–9092 [DOI] [PubMed] [Google Scholar]
- 46. Wang XY, Karbarz MJ, McGrath SC, Cotter RJ, Raetz CRH. 2004. MsbA transporter-dependent lipid A 1-dephosphorylation on the periplasmic surface of the inner membrane—topography of Francisella novicida LpxE expressed in Escherichia coli. J. Biol. Chem. 279:49470–49478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang XY, McGrath SC, Cotter RJ, Raetz CRH. 2006. Expression cloning and periplasmic orientation of the Francisella novicida lipid A 4′-phosphatase LpxF. J. Biol. Chem. 281:9321–9330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang XY, Ribeiro AA, Guan ZQ, Abraham SN, Raetz CRH. 2007. Attenuated virulence of a Francisella mutant lacking the lipid A 4′-phosphatase. Proc. Natl. Acad. Sci. U. S. A. 104:4136–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yi EC, Hackett M. 2000. Rapid isolation method for lipopolysaccharide and lipid A from Gram-negative bacteria. Analyst 125:651–656 [DOI] [PubMed] [Google Scholar]
- 50. Yoshizaki H, et al. 2001. First total synthesis of the Re-type lipopolysaccharide. Angew. Chem. Int. Edit. 40:1475–1480 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.