Abstract
Clinical reports suggest a positive association between fat consumption and the incidence of hyperactivity, impulsivity and cognitive abnormalities. To investigate possible mechanisms underlying these disturbances under short-term conditions, we examined in Sprague-Dawley rats the influence of 7-day consumption of a high-fat diet (HFD) compared to chow on anxiety, novelty-seeking and exploratory behaviors and also on acetylcholine (ACh) neurotransmission that may mediate these behaviors. The HFD consumption, which elevated circulating fatty acids but produced no change in caloric intake or body weight, stimulated novelty-seeking and exploration in an open field, while reducing anxiety in an elevated plus maze. Using the Ellman assay to measure ACh esterase (AChE) activity that breaks down ACh, the second experiment showed HFD consumption to significantly reduce AChE activity in the frontal cortex, hypothalamus and midbrain. With measurements of [125I]-epibatidine or [125I]-bungarotoxin binding to nicotinic ACh receptors (nAChRs) containing β2 or α7 subunits, respectively, the results also showed HFD consumption to increase both β2-nAChR binding in the medial prefrontal cortex and substantia nigra and α7-nAChR binding in the lateral and ventromedial hypothalamus. When treated with an acute dose of the nicotinic antagonist, mecamylamine (0.5 mg/kg, sc), the HFD animals responded with significantly reduced exploratory and novelty-seeking behaviors, whereas the chow-consuming rats exhibited no response. These findings suggest that the exploratory and novelty-seeking behaviors induced by dietary fat may be mediated by enhanced nicotinic cholinergic activity, which is accompanied by increased density of β2-nAChRs in cortical and midbrain regions associated with impulsivity and locomotor activity and of α7-nAChRs in hypothalamic regions associated with arousal and energy balance.
Keywords: Acetylcholine, nicotinic receptors, rat, novelty-seeking, high-fat diet
1. Introduction
The consumption of palatable, fat-rich diets has increased significantly over the past several decades (WHO, 2003). In addition to contributing to the worldwide epidemic of obesity (Harrington and Elliott, 2009), this rise in high-fat diet (HFD) consumption has been linked to hyperactivity in children, impulsivity in adolescents, and cognitive deficits in adults (Crichton et al., 2010; Pauli-Pott et al., 2010; Wiles et al., 2009). There are only a few reports in animals examining the behavioral effects of dietary fat intake. Recent studies show chronic consumption of a HFD to cause deficits in memory and learning in adult mice (Greenwood and Winocur, 2005; Valladolid-Acebes et al., 2010; Winocur et al., 2005) and reduce anxiety in adult rats (Maniam and Morris, 2010). Additionally, in very young animals or animals exposed to an acute stressor, HFD consumption stimulates exploration in an open field, a behavior related to novelty-seeking (Boukouvalas et al., 2008; Soulis et al., 2007). Binging on fat is also found to predispose animals to increased cocaine-seeking and -taking behavior (Puhl et al., 2011), suggesting that this diet may generally enhance risk-taking behaviors. Whereas this ability of dietary fat to produce behaviors related to emotion, impulse control and cognition is becoming more generally recognized, the neurobiological mechanisms underlying these effects, especially during the initial pre-obesity stages of unhealthy eating, remain to be determined.
The neurotransmitter, acetylcholine (ACh), which is known to influence behaviors related to novelty-seeking and exploration through its actions in various cortical and subcortical brain areas, is likely to have a role in mediating the changes in emotional behaviors induced by HFD consumption. Pharmacological studies with specific agonists and antagonists suggest that ACh via nicotinic ACh receptors (nAChRs) acts in the medial prefrontal cortex (mPFC) to increase impulsive behaviors (Raybuck and Gould, 2010) and the central nucleus of the amygdala (CeA) to stimulate anxiety (Zarrindast et al., 2008). Additionally, in the substantia nigra (SN) and ventral tegmental area (VTA), the activation of nAChRs stimulates locomotor- and reward-related behaviors (Alburges et al., 2007; Wise, 2009). Within regions of the hypothalamus such as the lateral hypothalamus (LH), ventromedial hypothalamus (VMH) and paraventricular hypothalamic nucleus (PVN), nAChR’s have been shown to control arousal, stress-responding and energy balance (Imaki et al., 1995; Pasumarthi and Fadel, 2010; Ribeiro et al., 2007; Takahashi et al., 1995; Yoburn and Glusman, 1984). In the paraventricular nucleus of the thalamus (PVT), cholinergic stimulation regulates states of arousal and cognition (Pasumarthi and Fadel, 2008). These behavioral effects may be receptor- and site-specific, with the high affinity β2 subunit-containing nAChRs (β2-nAChRs) predominating in extra-hypothalamic regions and the α7 subunit-containing nAChRs (α7-nAChRs) predominating in hypothalamic regions (Marks et al., 1996; Tribollet et al., 2004; Zaninetti et al., 2002). While this evidence is consistent with the idea that nicotinic cholinergic signaling may mediate the behavioral changes induced by dietary fat, the effects that HFD consumption has on this system have yet to be characterized. There is only one animal study directly investigating this possibility, showing long-term (6 months) fat consumption that increased weight gain to reduce the activity in the cortex and hypothalamus of the enzyme, acetylcholinesterase (AChE), which breaks down ACh (Kaizer et al., 2004). Additional in vitro studies have also suggested that free fatty acids (FFA), which are elevated both peripherally and centrally by fat intake (Ahren et al., 1997; Buettner et al., 2000), can inhibit AChE activity and enhance the activity of nAChRs (Nishizaki et al., 1997; Nishizaki et al., 1999; Vajreswari et al., 2002).
To investigate the relationship of dietary fat to cholinergic function in the brain, this study in Sprague-Dawley rats, which are similar in their caloric intake and body weight but differ in their serum FFA levels, was designed to: 1) characterize the disturbances in anxiety, novelty-seeking, and exploration produced by short-term HFD consumption; 2) determine whether these HFD-induced behaviors are associated with changes in cholinergic neurotransmission and nicotinic receptor binding in particular brain regions; and 3) test directly whether blocking the nicotinic cholinergic system attenuates the behaviors induced by fat consumption. The overall hypothesis being tested was that fat consumption stimulates ACh and nAChRs in specific brain regions and that this neurochemical change contributes to the HFD-induced disturbances in anxiety, novelty-seeking and exploratory behaviors.
2. Results
2.1 Experiment 1: Behavioral and metabolic changes induced by a high-fat diet
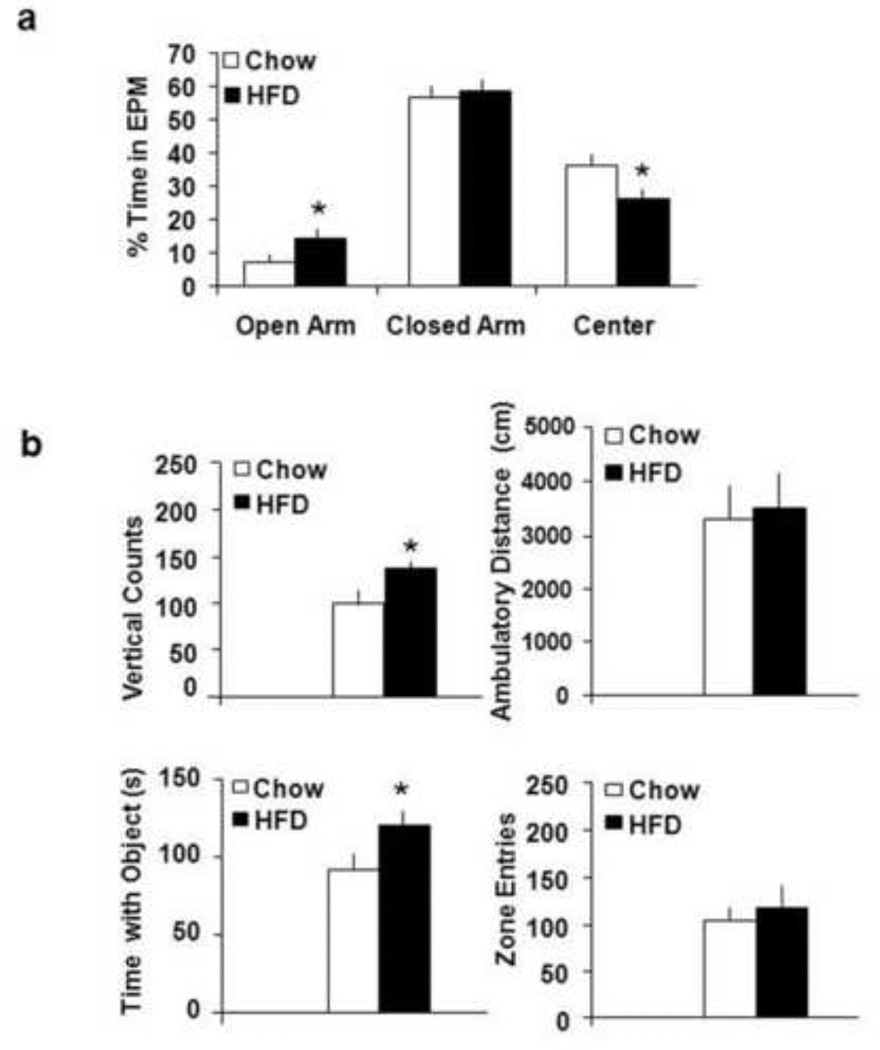
The fat- and chow-consuming rats were tested for anxiety in an elevated plus maze (EPM) or for novelty-seeking and exploration in an open field activity chamber with a novel object, and also their serum was measured for levels of circulating FFA. The results showed that the rats consuming a HFD for 7 days (n=8), compared to the chow rats (n=8), ate a similar number of daily calories (115±6 vs 99±5 kcal, p=0.36, not significant, ns) and were similar in body weight (312±7 vs 308±6 g, p=0.58, ns) but had markedly elevated FFA levels (0.31±0.03 vs 0.12±0.01, p<0.05). Using a RM-ANOVA, we identified a significant interaction between diet and multiple behaviors in the EMP [F(2,24)=4.91, p<0.05], with the HFD rats compared to the chow rats being less anxious. This was indicated by a 50% increase in percent of time spent in the open arm of the EPM (p<0.05) and a significant reduction in percent of time spent in the center square (−27%, p<0.05), with no difference in percent of time spent in the closed arm (ns) (Fig. 1a). In addition, the measures of open field activities revealed a significant interaction [F(3,32)=4.21, p<0.05] between diet and behaviors such as the rats’ interaction with a novel object (time with object and zone entries) and their rearing behavior (vertical counts). The HFD-consuming rats spent more time interacting with the novel object (+38%, p<0.05) and displayed enhanced rearing behavior (+40%, p<0.05), a sign of exploration, and they showed no differences in entries made into the quadrant containing the novel object (ns) or in ambulatory distance (ns) (Fig. 1b), suggesting a lack of effect on general motor-related behaviors. These findings demonstrate that short-term consumption of fat, while significantly elevating FFA levels without affecting caloric intake and body weight, reduces anxiety and promotes certain behaviors related to novelty-seeking and exploration.
Figure 1. Consequences of dietary fat consumption on behavioral patterns.
a) Effects of HFD consumption on anxiety, as measured by percent time spent in the open arm, closed arm, or center of an EPM. The data showed a significant increase in time spent within the open arm (* p<0.05) and a decrease in the time spent in the center of the maze (* p<0.05) in animals consuming HFD compared to chow diet. b) Effects of HFD consumption on novelty-seeking and exploratory behavior. The data showed that fat compared to chow consumption increased both the time spent interacting with this object (* p<0.05) and rearing behavior in the open field (* p<0.05), while having no effect on ambulatory distance or zone entries. Abbreviations: HFD - high-fat diet
2.2 Experiment 2: Changes in AChE activity induced by a high-fat diet
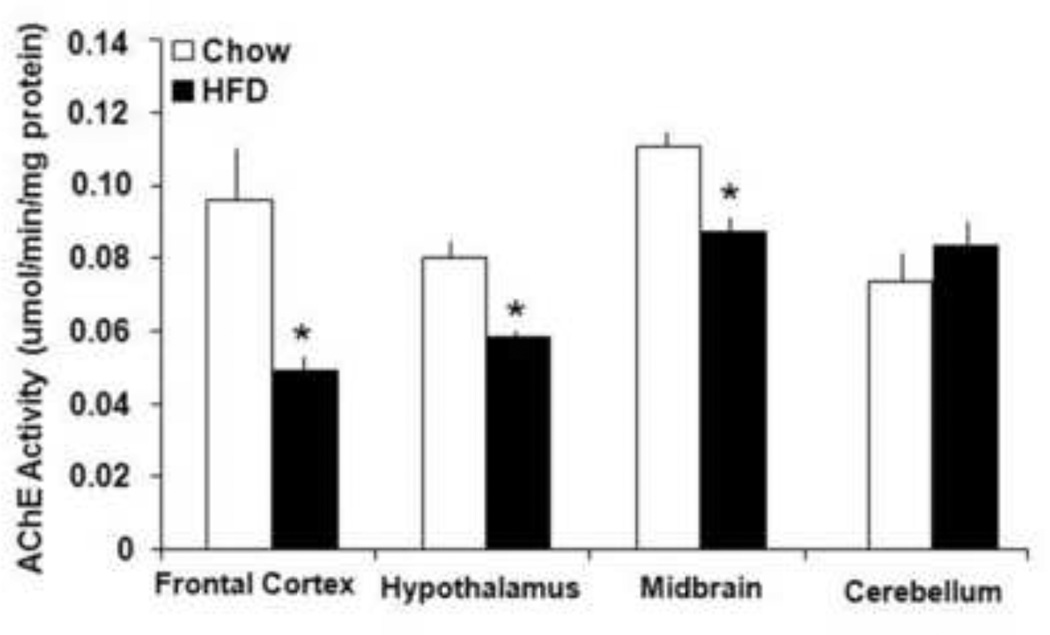
This experiment was designed to examine cholinergic neurotransmission by measuring the activity of AChE, an enzyme responsible for the breakdown of ACh, in the frontal cortex, hypothalamus, midbrain region and cerebellum. The animals eating HFD (n=8) compared to chow diet (n=8) for 7 days showed similar values of caloric intake (121±5 vs 129±6 kcal, p=0.75, ns) and body weight (347±10 vs 335±8 g, p=0.54, ns). The data demonstrated that the two diets differed in their effects on AChE [F(3,32)=4.0, p<0.05]. Specifically, the HFD, which significantly elevated FFA (0.36±0.02 vs 0.16±0.009 mEq/L, p<0.05), markedly reduced the activity of AChE in the four brain areas (Fig. 2). This change was somewhat larger in the frontal cortex (−48%, p<0.05) than the hypothalamus (−28%, p<0.05) and midbrain area (−22%, p<0.05), with no change observed in the cerebellum (ns). With reduced AChE activity normally associated with increased ACh content in the synaptic cleft (Hartmann et al., 2007), these results suggest that ACh levels may be enhanced in these brain regions in rats ingesting a fat-rich diet.
Figure 2. Changes in AChE induced by HFD consumption.
Reduced AChE activities in homogenized frontal cortex, hypothalamus and midbrain region, but not cerebellum of rats exposed a HFD or chow diet. * Represents significant differences between HFD and chow diet animals (p<0.05). Abbreviations: HFD - high-fat diet; AChE – acetylcholinesterase
2.3 Experiment 3: Changes in nAChR binding induced by a high-fat diet
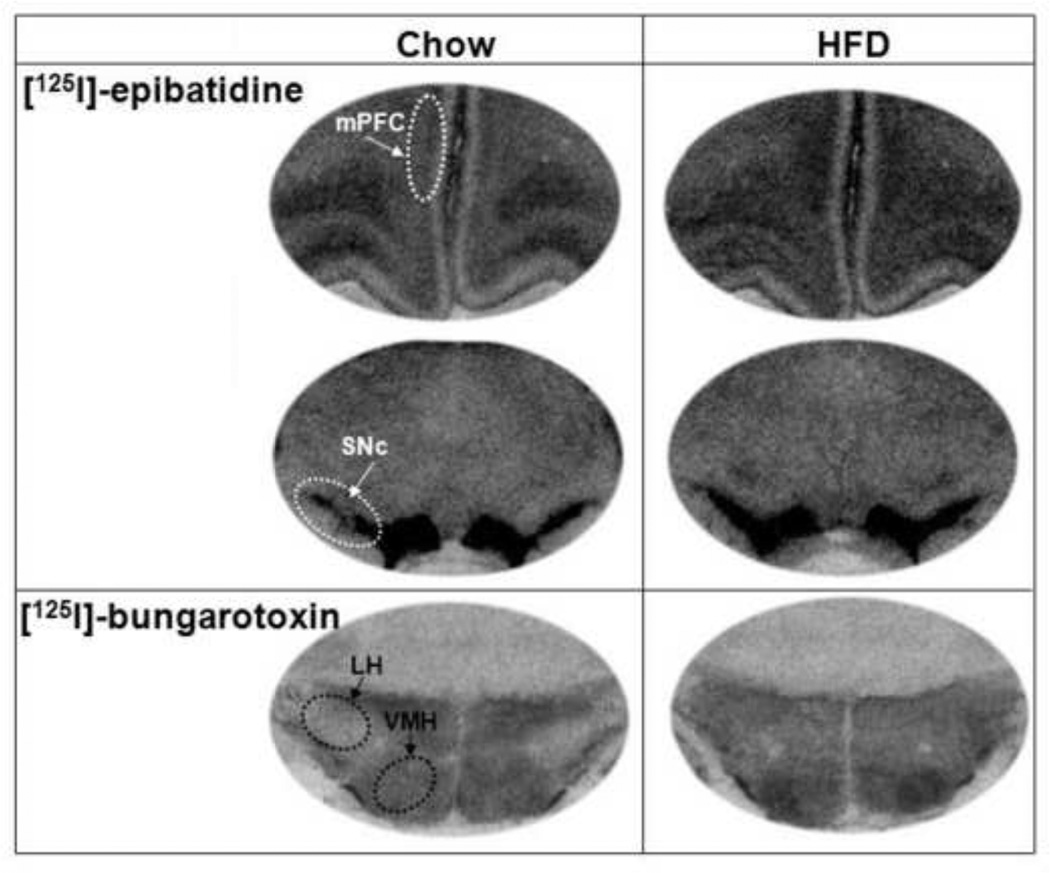
Quantitative autoradiography was used to measure the binding of [125I]-epibatidine to β2-nAChRs and of [125I]- bungarotoxin (BTX) to α7-nAChRs in several brain nuclei of fat- and chow-consuming rats (n=8/group). Once again, these two groups had similar caloric intake (118±8 vs 112±5 kcal, p=0.68, ns) and body weights (393±12 vs 379±10 g, p=0.41, ns) during their 7-day feeding regime. In both groups, the pattern of specific binding of these two ligands rarely overlapped, with intense [125I]-BTX binding found in the hypothalamus and amygdala and [125I]-epibatidine binding found in the thalamus and midbrain, with only the cortex showing binding of both ligands, as previously described (Pauly et al., 1991; Tribollet et al., 2004). The specific binding of [125I]-epibatidine to β2-nAChRs was dense in the mPFC region, SNc, PVT and VTA, yielding binding values within a range of (229–328 fmol/mg brain protein) but very little binding in the hypothalamus and amygdala, and the binding of [125I]- BTX to α7-nAChRs was clearly visible in the different hypothalamic areas as well as the mPFC and CeA, although highest in the LH and mPFC (260–331 fmol/mg brain protein), lower in the PVN, VMH and CeA (80–165 fmol/mg brain protein), and absent in the PVT.
When comparing the HFD- to chow-consuming rats (n=8/group), quantitative analysis of [125I]-epibatidine binding (fmol/mg brain protein) showed an interaction between brain area and diet [F(3,32)=4.23, p<0.05]. Post hoc comparisons revealed a small but significant increase in β2-nAChRs, specifically in the mPFC (+10%, p<0.05) and SNc (12%, p<0.05) but not the VTA (ns) (Table 1). A significant interaction effect between brain area and diet was also detected with [125I]-BTX binding [F(4,40)=4.08, p<0.05]. In contrast to β2-nAChR density, the binding of [125I]-BTX to α7-nAChR sites in the HFD compared to chow rats, while unaltered in the mPFC (ns) and CeA (ns), was significantly increased in two areas of the hypothalamus, the LH (11%, p<0.05) and VMH (25%, p<0.05), but not in the PVN (ns) (Table 1). This stimulatory effect of HFD consumption on β2-nAChR binding in the mPFC and SNc and α7-nAChR binding in the LH and VMH is clearly depicted in the autoradiographs presented in Fig. 3. Collectively, these binding data indicate that the consumption of a HFD can lead to both a receptor-specific and site-specific stimulation of nAChR binding.
Table 1.
Effects of HFD consumption on the binding of [125I]-epibatidine and [125I]-bungarotoxin in several brain regions
| [125I]-epibatidine | [125I]-bungarotoxin | |||
|---|---|---|---|---|
| Chow | HFD | Chow | HFD | |
| mPFC | 238±6.6 | 261±3.9* | 323±7.3 | 319±8.4 |
| CeA | ----- | ----- | 162±2.4 | 161±3.9 |
| PVT | 294±13.3 | 315±6.2 | ----- | ----- |
| PVN | ----- | ----- | 123±10.8 | 111±8.7 |
| LH | ----- | ----- | 265±16.2 | 296±5.8* |
| VMH | ----- | ----- | 94±8.5 | 118±4.9* |
| VTA | 298±10.3 | 311±8.9 | ----- | ----- |
| SNc | 255±10.9 | 285±8.1* | ----- | ----- |
All values are expressed as fmol/mg brain protein, with an asterisk (*) representing a significant effect of diet on nAChR binding (p<0.05) and dashes representing brain regions where no ligand binding was observed.
Abbreviations: mPFC - medial prefrontal cortex; CeA – central nucleus of the amygdala; PVT - thalamic paraventricular nucleus; PVN - hypothalamic paraventricular nucleus; LH - lateral hypothalamus; VMH - ventromedial hypothalamus; VTA - ventral tegmental area; and SNc - substantia nigra pars compacta.
Figure 3. Autoradiographs illustrating the effects of HFD consumption on nAChR binding.
Images depicting a stimulatory effect of dietary fat on [125I]-epibatidine binding in the mPFC and SNc regions (top 2 panels) and [125I]-bungarotoxin binding in the LH and VMH regions (bottom panel). Abbreviations: HFD - high-fat diet; mPFC - medial prefrontal cortex; LH - lateral hypothalamus; VMH - ventromedial hypothalamus; and SNc - substantia nigra pars compacta
2.4 Experiment 4: Attenuation of HFD-induced behaviors by administration of mecamylamine
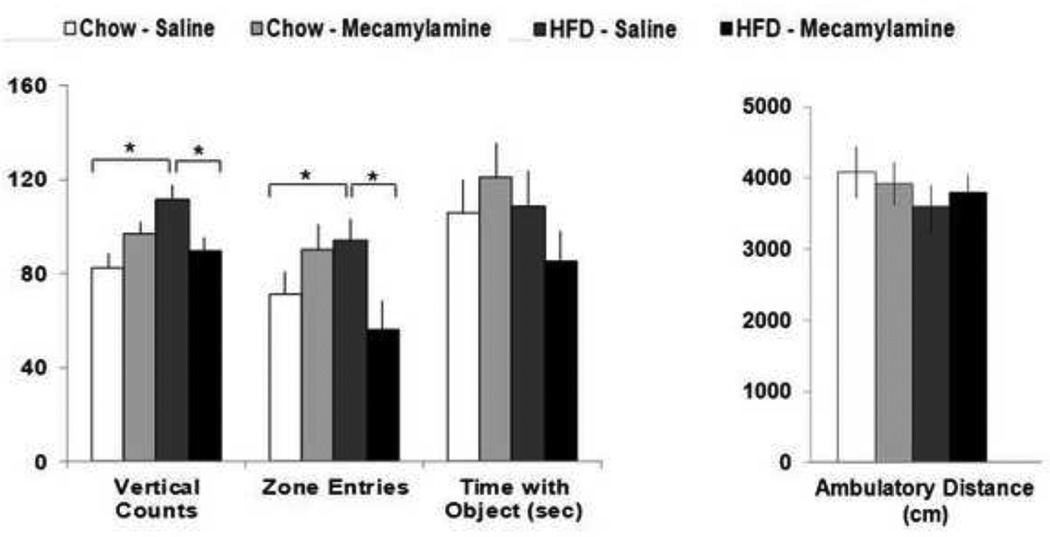
With evidence in Experiments 1 and 3 showing high fat consumption to increase novelty-seeking behavior and nicotinic receptor binding in cortical, midbrain and hypothalamic regions, this last experiment was performed to determine whether peripheral administration of a general nicotinic antagonist can block the effects of one-week HFD consumption on behaviors related to novelty-seeking and exploration. A two-way ANOVA was used to compare four specific behaviors (ambulatory distance, vertical counts, time in zone with novel object, and number of entries into novel object quadrant) of animals (n=8/diet/treatment) fed a HFD or chow diet after a single injection of saline or mecamylamine at a low dose of 0.5 mg/kg. Collectively, the results demonstrated that mecamylamine, while not altering these behaviors in chow-consuming rats, was able to attenuate the novelty-seeking and exploratory behaviors induced by the HFD (Fig. 4). While the four treatment groups showed no differences in ambulatory distance [F(1,30)=1.23, ns], analysis of their rearing behavior (vertical counts) revealed a significant interaction between diet and drug [F(1,30)=5.94, p<0.025], with post-hoc comparisons showing the 35% increase (p<0.05) in rearing of the HFD-saline compared to chow-saline rats to be significantly attenuated by mecamylamine (ns vs. chow-saline) (Fig. 4). This is in contrast to the chow-fed animals, which showed no changes in rearing behavior (ns) after this drug, with only an upward trend (p=0.06) detected between the chow-saline and chow-mecamylamine rats in measures of vertical counts. This significant drug × diet interaction, while not seen with the measure of time spent in the zone containing the novel object [F(1,30)=1.89, ns], was clearly evident with the measure of entries made into this zone [F(1,30)=5.32, p<0.025], which revealed a 32% increase in entries (p<0.05) in the HFD-saline group compared to the chow-saline rats. Similar to the results with rearing behavior, mecamylamine had no effect in the chow animals (ns) but, in the HFD rats, significantly reduced the number of entries into the quadrant containing the novel object by 40% (p<0.05) compared to the HFD-saline group, bringing this measure to similar values as the chow-saline control group (ns). These results provide evidence that acute treatment with this nicotinic cholinergic antagonist can attenuate the novelty-seeking and exploratory behaviors induced by the HFD.
Figure 4. Effects of mecamylamine on HFD-induced novelty-seeking and exploration.
Data showing an attenuation of HFD-induced increases in vertical counts and zone entries in response to an acute mecamylamine injection. * Represents significant differences between designated groups (p<0.05). Abbreviations: HFD - high-fat diet
3. Discussion
The goal of this study was to determine whether short-term HFD consumption, in rats with normal caloric intake and body weight, can lead to abnormal behavioral patterns that may be linked to changes in cholinergic neurotransmission. The findings demonstrate that rats consuming a diet rich in fat, resulting in increased circulating levels of FFA, exhibit reduced anxiety along with greater novelty-seeking and exploratory behaviors. These behavioral changes in fat-consuming rats are accompanied by an increase in cholinergic activity and nAChR binding in select brain regions and are attenuated with peripheral administration of the nicotinic antagonist, mecamylamine. These findings provide evidence suggesting that short-term consumption of fat can contribute to abnormal patterns of behavior, possibly by stimulating nicotinic cholinergic neurotransmission in specific hypothalamic and extra-hypothalamic brain regions.
3.1 Consumption of a high-fat diet leads to changes in behavioral patterns
In rats consuming a HFD compared to a chow diet with lower fat content, the results of the first experiment revealed a significant reduction in anxiety in an EPM and increase in novelty-seeking and exploratory behavior in an open field activity chamber. This finding is consistent with published studies, showing fat consumption to reduce stress sensitivity in rodent models (Maniam and Morris, 2010; Prasad and Prasad, 1996; Teegarden and Bale, 2007). With the EPM often used to measure exploratory behavior (Moreira et al., 2007; Rodgers and Dalvi, 1997), our finding may also signify enhanced exploration of a novel environment, in this case of the open arm, resulting from reduced anxiety. With more direct measures of exploration and novelty-seeking, our findings demonstrate that rats consuming a HFD are more likely to explore an open field and interact with a novel object. Consistent with this are other reports in prepubescent or acutely-stressed fat-consuming animals, which with measures of sniffing and open field activity provide some evidence for increased exploratory and locomotor behavior (Boukouvalas et al., 2008; Soulis et al., 2007). With our HFD and chow rats exhibiting no baseline differences in their locomotor activity, perhaps because of the two acclimation sessions they had prior to novelty testing in the open field, our results additionally indicate that the changes in anxiety, novelty-seeking and exploratory behaviors induced by the HFD are not due to any non-specific effects of this diet on activity level. This is the first direct evidence, in adult animals of normal body weight and caloric intake, that dietary fat is responsible for the observed reduction of anxiety and increase in novelty-seeking and exploration.
3.2 Increased cholinergic neurotransmission with high-fat diet consumption
With evidence indicating that ACh in extra-hypothalamic and hypothalamic regions functions to influence behaviors related to exploration and novelty-seeking (see Section 1), the next goal was to determine whether cholinergic neurotransmission in these areas may be disturbed by dietary fat. In rats consuming a HFD, measurements of the ACh metabolizing enzyme, AChE, revealed reduced enzymatic activity specifically in the frontal cortex, hypothalamus and midbrain area, reflecting a possible increase in ACh levels in these regions. This result agrees with a previous study, which used a long-term feeding paradigm (6 months on a HFD) that increased weight gain and produced metabolic disturbances and revealed a suppressive effect on AChE activity in the cortex and hypothalamus (Kaizer et al., 2004). It also receives indirect support from another study, showing 8 weeks on a HFD to reverse the Neurochemical effects of neonatal parathion exposure that depletes central ACh stores (Slotkin et al., 2009). The possibility that ACh levels are increased by the HFD along with this enzymatic change is supported by studies in mutant mice lacking the AChE gene or in mice injected with an AChE antagonist, both of which lead to elevated levels of extracellular ACh (Hartmann et al., 2007). One possible mechanism mediating the effects of fat consumption on cholinergic function may be related to circulating energy molecules, such as FFA. These metabolites are markedly elevated in the HFD-consuming rats and have been shown to reduce the activity in vitro of AChE (Tel et al., 2010; Vajreswari et al., 2002). Although not measured in the current study, there is published evidence also suggesting that other energy signaling molecules, such as glucose and ghrelin, can function through cholinergic signaling pathways and cause changes in behaviors such as food reward and memory (Dickson et al., 2010; Ragozzino et al., 1994). Together, this evidence supports the idea that short-term (7 day) fat consumption, which increases metabolic factors such as FFA, is effective in stimulating the cholinergic system in cortical, hypothalamic and midbrain regions and perhaps contributing to the observed changes in behavior.
3.3 Increased nAChR binding induced by high-fat diet consumption
Since chronic stimulation of nAChRs with a cholinergic agonist is known to increase nAChR binding in multiple extra-hypothalamic and hypothalamic brain regions (Pauly et al., 1996; Sparks and Pauly, 1999; Trauth et al., 1999), a possible increase in ACh levels resulting from the fat-induced reduction of AChE activity may also be accompanied by changes in receptor binding. Using quantitative autoradiography, we examined the density of [125I]-epibatidine (β2-nAChR) and [125I]-BTX (α7-nAChR) binding sites in different extra-hypothalamic and hypothalamic brain regions. Our findings with autoradiographic measurements of NAChRs showed the consumption of a HFD to increase the density of β2-nAChRs specifically in the mPFC and SNc, while having no effect on these receptors in the VTA and PVT, and also to increase the density of α7-nAChRs specifically in the LH and VMH, but not the mPFC, CeA or PVN. Similar to these results in rats consuming a HFD, other reports measuring nicotine-induced [3H]-ACh or [3H]-nicotine binding have demonstrated an increase in receptor density in the hypothalamus, specifically the VMH, and also in the midbrain, specifically the SN region, but not in the thalamus which shows a resistance to such agonist-induced changes in binding (Kellar et al., 1989; Pauly et al., 1991). Although not directly measured in this study, the cellular mechanisms responsible for the receptor upregulation observed in the mPFC, SN, LH and VMH may involve an enhanced rate of synthesis or decrease in degradation of nAChRs. While an early study suggested that the greater susceptibility of specific brain regions to agonist-induced receptor changes may be related to their higher baseline levels of the nAChRs (Pauly et al., 1991), our findings are not consistent with this, showing changes in nAChR binding to occur specifically in regions exhibiting mid-range values of baseline binding. Overall, our results indicate that the β2-nAChRs specifically in the mPFC and SN and α7-nAChRs specifically in the VMH and LH are particularly sensitive to the stimulatory effects of dietary fat.
3.4 Role of β2-nAChRs in mediating behavioral changes induced by dietary fat
The changes in behavioral patterns induced by consumption of fat may stem from disturbances in cholinergic signaling in specific cortical and midbrain regions. Our findings indicate that animals consuming a HFD, while being less anxious, show increased behaviors related to novelty-seeking and exploration and that these behaviors are associated with enhanced cholinergic neurotransmission within the frontal cortex and midbrain area, along with increased β2-nAChR binding in the mPFC and SNc. The role of β2-nAChR sites in the mPFC in mediating enhanced novelty-seeking and exploration is suggested by pharmacological evidence, showing reduced impulse-related behaviors in animals administered a specific α4β2 nAChR antagonist in this brain region (Tsutsui-Kimura et al., 2010a; Tsutsui-Kimura et al., 2010b). Also, the fat-induced increase in the density of β2-nAChR sites in the SNc, a region where nicotine is known to activate dopaminergic projections to motor regions (Keath et al., 2007), suggests that dietary fat may be promoting novelty-seeking and exploration through direct activation of the midbrain motor circuits. Collectively, our findings in fat- compared to chow-consuming animals suggest that enhanced cholinergic signaling through β2-nAChRs in the mPFC and SNc can stimulate impulse- and motor-regulating circuits, thereby driving animals to seek more interaction with novel objects and explore an open field. With novelty seeking and exploratory behavior playing an important role in animal survival strategy (Kelley et al., 2004; Korpela et al., 2011), our findings can additionally suggest that a HFD, which is most desirable for its high energy content, also prepares rats for future novel and possibly threatening conditions
3.5 Role of α7-nAChRs in mediating behavioral changes induced by dietary fat
Our results in HFD- compared to chow-consuming animals, showing a reduction in AChE activity within the hypothalamus and increase in α7-nAChRs specifically in the LH and VMH, indicate that fat is stimulating hypothalamic pathways related to energy and arousal that, in turn, can promote enhanced exploratory and novelty-seeking behaviors. In addition to affecting feeding behavior (Miyata et al., 1999; Yang et al., 1999), recent literature has focused on the important role of ACh in the LH in promoting behavioral arousal by interacting with local peptide and neurotransmitter systems (Henny and Jones, 2006; Kane et al., 2000; Pasumarthi and Fadel, 2010). This stimulatory effect is most likely mediated by local nicotinic receptors, since treatment with a nicotinic agonist is shown to stimulate orexin expression, a peptide in the LH which is known to promote arousal (Estabrooke et al., 2001; Kane et al., 2000; Li et al., 2000). Similarly, the VMH, a region rich in energy-sensing neurons (Kang et al., 2004) and originally suggested to function as a satiety center (Takahashi et al., 1995), is now believed to have a role in promoting arousal, based on evidence showing this region to be activated during periods of food anticipation and heightened arousal (Ribeiro et al., 2007; Ribeiro et al., 2009). Unlike the stimulatory effect of dietary fat on α7-nAChRs in the LH and VMH, no changes in receptor binding occurred in the CeA or PVN, where ACh is associated with the activation of stress responses (Ohmori et al., 1995; Zarrindast et al., 2008; Zhang and Zheng, 1997). Since our current findings reveal reduced anxiety in animals consuming a HFD, this behavior is likely to be mediated by other neurotransmitter systems, such as γ-aminobutyric acid, which is stimulated by feeding (Rada et al., 2003) and known to reduce anxiety and stress responding specifically in the CeA (Sanders and Shekhar, 1995) and PVN (Imaki et al., 1995) regions. Together with these studies, our evidence suggests that dietary fat, by stimulating cholinergic signaling via α7-nAChRs, can activate hypothalamic energy-sensing and arousal circuits in the LH and VMH, rather than anxiety circuits, to promote novelty-seeking and exploratory behaviors.
3.6 Nicotinic cholinergic mechanisms mediate the HFD-induced behaviors
The current findings suggest that nicotinic cholinergic mechanisms mediate the novelty-seeking and exploratory behaviors induced by a fat-rich diet. In addition to showing an increase in the density of α7 and β2 nicotinic receptor sites, our results with pharmacological manipulations demonstrate that the nicotinic receptor antagonist, mecamylamine, can attenuate certain HFD-induced behaviors characteristic of novelty-seeking and exploration and that this occurs at a low dose that has little behavioral effect in animals eating a chow diet, as shown here and in previously published studies (Kolokotroni et al., 2011). These results with mecamylamine are consistent with recent reports showing a similar low dose to attenuate nicotine-induced behaviors related to novelty-seeking, such as impulsive choice (Kirshenbaum et al., 2011; Kolokotroni et al., 2011). Whereas there was no difference observed in Experiment 4 with novel object interaction time, this may be due to the marked changes in the number of entries made into the novel object quadrant, which precluded a significant change in quadrant time. In a secondary analysis of zone entries and time spent in zone for individual animals, we found these two measures to be inversely related (−0.62, p <0.05), further supporting the possibility that the increase in number of entries into the novel object quadrant most likely masked a change in time spent interacting with the object. Although not directly measured in the current study, it is important to note that mecamylamine has previously been shown to have anxiolytic properties even at low doses (Newman et al., 2001; Newman et al., 2002) and thus may be having an additive effect to the dietary fat in reducing anxiety. While mecamylamine as a general nicotinic antagonist makes it difficult to identify the receptor-specificity of these observed behavioral changes, the available evidence suggests that both α7 and β2 nAChRs are involved in impulse and novelty-related behaviors. This is indicated by results with a specific antagonist of the α4β2 nAChR, which can block the effects of nicotine on premature responding in various tests of impulsivity (Blondel et al., 2000; Tsutsui-Kimura et al., 2010a), and with α7 nAChR null mice, which demonstrate impaired performance in similar impulse-related tasks (Hoyle et al., 2006). Together with our evidence showing increased density of both α7 and β2 receptor subtypes after HFD consumption, an effect similar to that occurring with prolonged nicotine treatment (Flores et al., 1997; Rasmussen and Perry, 2006; Schwartz and Kellar, 1983), it is likely that mecamylamine by blocking these receptor sites can attenuate the behavioral abnormalities induced by a diet rich in fat.
3.7 Conclusion
We conclude that the behavioral changes induced by HFD consumption, increased novelty-seeking and exploration, may be related to enhanced nicotinic cholinergic signaling in specific cortical, hypothalamic and midbrain regions associated with such behaviors. Evolutionarily, it is possible that dietary fat consumption, by activating cholinergic circuits, stimulates central survival mechanisms that in turn promote these active-coping behaviors such as exploration of novel environments. With our findings suggesting that a brief, 7-day period of HFD consumption leads to such distinct patterns of behavior along with increased cholinergic neurotransmission, it is possible that such changes can occur with even shorter periods of consumption. They may also be a direct consequence of the elevated FFA acting on central cholinergic circuits, rather than the long-term effects of dietary fat on physiological systems related to body weight.
4. Experimental Procedures
4.1 Animals
Adult, male Sprague-Dawley rats (approximately 2 months of age), weighing in between 250 – 300 g at the start of all experiments (Charles River Breeding Labs, Kingston, NY), were individually housed (22 °C, with lights off at 1:00 p.m. for 12 hr) in a fully accredited American Association for the Accreditation of Laboratory Animal Care facility, according to institutionally approved protocols as specified in the National Institutes of Health Guide to the Use and Care of Animals and also with the approval of the Rockefeller University Animal Care and Use Committee. All animals were given 1 week to acclimate to lab conditions, during which time they were maintained ad libitum on laboratory chow (LabDiet Rodent Chow 5001, St. Louis, MO; 12% fat, 60% carbohydrate, and 28% protein) and water. All protocols fully conformed to the Guiding Principles for Research Involving Animals and Human Beings as set forth by the American Physiological Society. All efforts were made to minimize the use and suffering of animals
4.2 Diets
For the experimental period, rats were either maintained ad libitum on standard rodent chow (12% fat, 3.3 kcal/g) or a HFD (50% fat, 5.2 kcal/g), which was the same as that described in prior publications (Leibowitz et al., 2004; Dourmashkin et al., 2006). It consisted of fat from 75% lard (Armour, Omaha, NE) and 25% vegetable oil (Wesson vegetable oil, Omaha, NE), of carbohydrate from 30% dextrin, 30% cornstarch (ICN Pharmaceuticals, Costa Mesa, CA), and 40% sucrose (Domino, Yonkers, NY), and of protein from casein (Bioserv, Frenchtown, NJ) and 0.03% L-cysteine hydrochloride (ICN Pharmaceuticals). The diet was supplemented with minerals (USP XIV Salt Mixture Briggs; ICN Pharmaceuticals) and vitamins (Vitamin Diet Fortification Mixture; ICN Pharmaceuticals). The macronutrient composition was calculated as percentage of total kcal, with the HFD containing 50% fat, 25% carbohydrate, and 25% protein. This semi-solid diet was stored at 4°C until use. On a daily basis, fresh diet was weighed out in metal bowls and placed into the appropriate animal cages.
4.3 Test procedures
Animals were first adapted for one week to standard housing conditions and then split into two groups matched for body weight. One group was trained to consume a HFD, first by receiving a small 15-kcal meal of this diet each day for 3 days with chow also available and then by being placed on the HFD ad libitum for an additional 7 days. The other group was maintained on the chow diet for the entire experimental period. Caloric intake and body weight was recorded daily, and to avoid any non-specific effects of calories or weight on the behavioral and neurochemical measures, rats in both groups were specifically chosen to have similar caloric intake and equal body weights. This swas done first by rank ordering the rats by caloric intake, which positively correlated with body weight (r=+0.68 – +0.75) and then eliminating the top tertile or bottom tertile for the HFD and chow groups, respectively. Thus, with this subgrouping, the number of subjects in each experiment, generally starting at 12 per group, was reduced to approximately 8 per group.
In Experiment 1, the effects of HFD versus chow consumption on anxiety and novelty-seeking behaviors were examined. Shortly after dark onset on day 7, rats consuming a HFD or chow (n=8/group) were placed on EPM for 5 min, and then, at the same time on day 8, they were each tested for novelty-seeking and exploration in an open field activity chamber for 20 min. After all behavioral testing was completed; tail vein blood was collected and analyzed for serum levels of circulating FFA (see below).
In Experiment 2, rats (n=8/group) were allowed to consume either a HFD or chow as described above and then, shortly after dark onset on day 7 of diet consumption, were sacrificed by rapid decapitation. Brain regions of interest were dissected and analyzed for AChE activity, as described below and tail vein blood was collected for measures of circulating FFA (see below)
To examine the effects of dietary fat on nAChR binding in specific brain nuclei, Experiment 3, in an additional set of animals (n=8/group) exposed to chow or a HFD for 7 days, used quantitative autoradiography to measure the binding of [125I]-epibatidine to β2-nAChRs and [125I]-bungarotoxin (BTX) to α7-nAChRs. On day 7 of diet exposure shortly after dark onset, animals were sacrificed using rapid decapitation, and their brains were removed, dipped into cold isopentane (−25°C) for 30 s, and then kept at −80°C until further use. Additionally, tail vein blood was collected on day 7 for measurements of circulating FFA.
Experiment 4 examined the ability of a nicotinic receptor antagonist to reduce the novelty-seeking behavior induced by a HFD in Experiment 1. Mecamylamine hydrochloride (Sigma Aldrich, St. Louis, MO) was diluted in saline and injected subcutaneously at a dose of 0.5 mg/kg in a 0.5 ml volume. This low dose of mecamylamine was specifically chosen based on literature suggesting that it can block impulsive behaviors produced by low dose nicotine while having limited behavioral effects on its own (Kolokotroni et al., 2011). Animals were initially separated into chow and HFD groups and allowed to consume their respective diets for a total of 7 days. After this, they were matched for caloric intake and body weight, yielding 16 animals/diet group. On day 8, animals from the chow and HFD groups were injected (s.c.) with either saline vehicle or mecamylamine in a between-subject design and, 20 minutes later, were tested for 20 minutes in the open field for locomotor, exploratory and novelty-seeking behavior, as described below.
4.4 Elevated plus maze
The EPM apparatus consisted of two open arms (10 × 50 cm), which lay perpendicular to two closed arms (10 × 50 cm) with 30 cm high wooden walls, and a common central platform as previously described (Avena et al., 2008; Walf and Frye, 2007). The whole apparatus was placed 55 cm above the floor. The test was carried out by an experimenter blind to the manipulation. Each rat was placed at the central platform of the apparatus and allowed to explore the maze for 5 min under dim red light. A video camera recorded movements of the rats, which were later analyzed for the percent of time spent in the open or closed arms as well as the central platform out of the total 300 seconds of the test. The criterion used to determine a full arm entry was both forepaws into an arm. Anxiety was determined by more time spent in the closed arms of the EPM, while the time spent in the open arms represented a reduction in anxiety behavior. The apparatus was thoroughly cleaned with soap and water and dried between each run.
4.5 Open Field Measures
The open field activity chamber consisted of a computerized Plexiglas box (43.2 × 43.2 cm) with a white floor, black walls, and infrared photocells (MED Associates, Georgia, VT, USA). Novelty-seeking testing occurred in a test room separate from the vivarium under dim red light. All animals were acclimated, for 20 min each day, to the testing chambers on 2 consecutive days (day 6 and day 7 of diet consumption). They were then tested for novelty-seeking (20 min) in these same chambers, with a novel square object placed in the far right quadrant of the chamber and measurements taken of entries made into the quadrant containing the novel object and time spent in the quadrant with the object. During this same 20 minute session, locomotor behavior (ambulatory distance), a measure of motor function, and rearing behavior (vertical counts), a measure of enhanced exploration (Prut and Belzung, 2003), were also recorded.
4.6 Fatty acid determinations
In Experiments 1–3, serum from tail vein blood was assayed for non-esterified fatty acid levels, with an E-Max Microplate Reader using a colorimetric assay kit from WAKO Pure Chemical Industries, Ltd (Osaka, Japan).
4.7 Acetylcholinesterase activity
Animals were sacrificed using rapid decapitation, and their brains were quickly dissected on ice to remove the entire frontal cortex, hypothalamus, midbrain area and cerebellum for determinations of AChE activity. Tissue from the brain regions of interest was then individually homogenized at 4°C in filtered 50 mM TrisHCl buffer, pH 7.4 (1:10 w/v) and centrifuged at 1,000 × g for 10 min to remove nuclei and debris. The protein content of each sample’s supernatant was determined using the Bradford Assay (Bradford, 1976). AChE activity was then measured using acetylthiocholine iodide and 5,5'-dithiobis-2-nitrobenzoic acid (DTNB), according to the methods of Ellman and colleagues (Ellman et al., 1961). Briefly, 0.32 mM DTNB (color regent) and 1.0 mM acetylthiocholine iodide (substrate) were added to the homogenates, and the absorbance at 412 nm was measured using an E-max microplate reader. Enzyme activity of AChE is expressed as µmol/min/mg protein at 25°C.
4.8 Quantitative autoradiography
Brains were sectioned (16 µm) at −20°C on a cryostat microtome, thaw mounted on gelatin-coated microscope slides, and desiccated overnight at 4°C using an air vacuum. Multiple sets of adjacent sections were collected for measurements of β2- and α7-nAChRs on sections that had consistent anatomical representation. Brain sections were collected from Bregma 3.2 to 2.7 mm for the mPFC; Bregma −1.8 to −1.88 mm for the PVN and the PVT, Bregma −2.8 to −3.14 mm for the LH and CeA; and from Bregma −5.6 to −5.8 mm for the VTA and SN pars compacta (Paxinos and Watson, 1997). All sections, clearly depicting the region of interest, were chosen at the same level for each animal within each group. For each animal, 3 adjacent brain sections were used for the binding assay and averaged for statistical analysis.
The binding of [125I]-epibatidine to β2-nAChRs was measured using a slightly modified method from Tizabi and colleagues (Tizabi and Perry, 2000). Briefly, brain sections were pre-incubated at room temperature for 15 min in a 50 mM TrisHCl buffer containing (pH 7.4) 120 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2 and were then incubated for 60 min at room temperature in the same buffer solution containing 0.165 nM [125I]-epibatidine (2200 Ci/mmol, Perkin-Elmer, Boston, MA,). Nonspecific binding was determined in adjacent sections with the addition of 300 mM nicotine hydrogen tartrate to the incubation buffer. All sections were then rinsed twice (5 min each) in ice-cold buffer, dipped briefly into ice-cold distilled water, and air-dried at room temperature.
The binding of [125I]-BTX to α7-nAChR sites was measured as previously described (Sparks and Pauly, 1999) with slight modification. Briefly, slides containing the brain regions of interest were pre-incubated at room temperature for 15 min in a 50 mM TrisHCl (pH 7.4) buffer containing 0.1% BSA and were then transferred to the incubation solution which contained the same buffer solution in addition to 2 nM [125I]-BTX (120 Ci/mmol, Perkin-Elmer, Boston, MA,). In order to determine non-specific binding, adjacent sections were incubated in this same incubation buffer, but with 300 uM nicotine hydrogen tartrate (Sigma Aldrich, St. Louis, MO) added. Sections were allowed to incubate in these two solutions for 2 h at room temperature and then quickly dipped in ice-cold Tris buffer, rinsed 3 times (10 min each) in ice-cold buffer, dipped in distilled water, and finally air-dried at room temperature.
For both binding assays, the air-dried sections were exposed to Kodak BioMax MR (Sigma Aldrich, St. Louis, MO) film along with [125I] standards (American Radiolabeled Chemicals, St. Louis, MO) for 4 h (β2-nAChR) or 5 days (α7-nAChR). After appropriate exposure times, the films were developed using an automatic developer and images scanned and saved for further analysis. Quantitative densitometric analysis of binding was performed using NIH software, Image J v1.43 for Windows. In order to determine specific binding, non-specific binding, which represented less than 5% of the total binding, was subtracted from total binding in adjacent sections. The values of binding were determined using a standard curve constructed from [125I] standards with a known amount of activity and expressed as (fmol/mg brain protein).
4.9 Statistical analysis
The values in the figures are expressed as mean ± SEM. Statistical analyses to compare the effects of dietary fat to chow on caloric intake, body weight and serum fatty acid levels were performed using an unpaired Student’s t-test, with significant differences accepted at p<0.05. Data in the first three experiments, involving behavior, receptor binding or AChE activity, were first analyzed using a repeated measure analysis of variance (RM-ANOVA). In Experiment 4, the effects of mecamylamine on diet-induced behavioral changes were analyzed using a two-way ANOVA, with diet (two levels) and drug (two levels) as independent variables. Where significant main effects were obtained, follow-up pairwise comparisons were made between groups of interest using either two-tailed t-tests (RM-ANOVA) or a post-hoc Holm-Sidak test (two-way ANOVA), where p<0.05 was considered significant. The probability values given in the text or legends to the figures and tables reflect the results of these tests.
Research Highlights.
Consumption of a high-fat diet reduces anxiety and enhances novelty-seeking behavior in rats.
Dietary fat intake stimulates central cholinergic activity and nicotinic receptor binding.
Pharmacological blockade of nicotinic receptors attenuates fat-induced behavioral changes.
Nicotinic cholinergic mechanisms are involved in behavioral effects of dietary fat consumption.
Acknowledgements
The authors would like to thank Dr. Jessica Barson for her assistance with the statistical analysis of the current data and Ms. Karen Chen for her help with the animal behavior measures. This research was supported by USPHS Grant DA-21518 and USPHS Grant AA-12882.
Glossary
- ACh
Acetylcholine
- AChE
acetylcholinesterase
- ANOVA
analysis of variance
- BTX
bungarotoxin
- CeA
central nucleus of the amygdala
- DA
dopamine
- EPM
elevated plus maze
- HFD
High-fat diet
- LH
lateral hypothalmus
- mPFC
medial prefrontal cortex
- nAChRs
nicotinic acetylcholine receptors
- PVN
paraventricular hypothalamic nucleus
- PVT
paraventricular thalamic nucleus
- SNc
substantia nigra pars compacta
- VMH
ventromedial hypothalamus
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahren B, Simonsson E, Scheurink AJ, Mulder H, Myrsen U, Sundler F. Dissociated insulinotropic sensitivity to glucose and carbachol in high-fat diet-induced insulin resistance in C57BL/6J mice. Metabolism. 1997;46:97–106. doi: 10.1016/s0026-0495(97)90175-x. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Hoonakker AJ, Hanson GR. Nicotinic and dopamine D2 receptors mediate nicotine-induced changes in ventral tegmental area neurotensin system. Eur J Pharmacol. 2007;573:124–132. doi: 10.1016/j.ejphar.2007.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav. 2008;94:309–315. doi: 10.1016/j.physbeh.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel A, Sanger DJ, Moser PC. Characterisation of the effects of nicotine in the five-choice serial reaction time task in rats: antagonist studies. Psychopharmacology (Berl) 2000;149:293–305. doi: 10.1007/s002130000378. [DOI] [PubMed] [Google Scholar]
- Boukouvalas G, Antoniou K, Papalexi E, Kitraki E. Post weaning high fat feeding affects rats' behavior and hypothalamic pituitary adrenal axis at the onset of puberty in a sexually dimorphic manner. Neuroscience. 2008;153:373–382. doi: 10.1016/j.neuroscience.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buettner R, Newgard CB, Rhodes CJ, O'Doherty RM. Correction of diet-induced hyperglycemia, hyperinsulinemia, and skeletal muscle insulin resistance by moderate hyperleptinemia. Am J Physiol Endocrinol Metab. 2000;278:E563–E569. doi: 10.1152/ajpendo.2000.278.3.E563. [DOI] [PubMed] [Google Scholar]
- Crichton GE, Murphy KJ, Bryan J. Dairy intake and cognitive health in middle-aged South Australians. Asia Pac J Clin Nutr. 2010;19:161–171. [PubMed] [Google Scholar]
- Dickson SL, Hrabovszky E, Hansson C, Jerlhag E, Alvarez-Crespo M, Skibicka KP, Molnar CS, Liposits Z, Engel JA, Egecioglu E. Blockade of central nicotine acetylcholine receptor signaling attenuate ghrelin-induced food intake in rodents. Neuroscience. 2010;171:1180–1186. doi: 10.1016/j.neuroscience.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CM, Davila-Garcia MI, Ulrich YM, Kellar KJ. Differential regulation of neuronal nicotinic receptor binding sites following chronic nicotine administration. J Neurochem. 1997;69:2216–2219. doi: 10.1046/j.1471-4159.1997.69052216.x. [DOI] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G. High-fat diets, insulin resistance and declining cognitive function. Neurobiol Aging. 2005;26(Suppl 1):42–45. doi: 10.1016/j.neurobiolaging.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Harrington DW, Elliott SJ. Weighing the importance of neighbourhood: a multilevel exploration of the determinants of overweight and obesity. Soc Sci Med. 2009;68:593–600. doi: 10.1016/j.socscimed.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Kiewert C, Duysen EG, Lockridge O, Greig NH, Klein J. Excessive hippocampal acetylcholine levels in acetylcholinesterase-deficient mice are moderated by butyrylcholinesterase activity. J Neurochem. 2007;100:1421–1429. doi: 10.1111/j.1471-4159.2006.04347.x. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE. Vesicular glutamate (VGlut), GABA (VGAT), and acetylcholine (VACht) transporters in basal forebrain axon terminals innervating the lateral hypothalamus. J Comp Neurol. 2006;496:453–467. doi: 10.1002/cne.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle E, Genn RF, Fernandes C, Stolerman IP. Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology (Berl) 2006;189:211–223. doi: 10.1007/s00213-006-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaki T, Xiao-Quan W, Shibasaki T, Yamada K, Harada S, Chikada N, Naruse M, Demura H. Stress-induced activation of neuronal activity and corticotropin-releasing factor gene expression in the paraventricular nucleus is modulated by glucocorticoids in rats. J Clin Invest. 1995;96:231–238. doi: 10.1172/JCI118026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaizer RR, da Silva AC, Morsch VM, Correa MC, Schetinger MR. Diet-induced changes in AChE activity after long-term exposure. Neurochem Res. 2004;29:2251–2255. doi: 10.1007/s11064-004-7033-3. [DOI] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD. Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology. 2000;141:3623–3629. doi: 10.1210/endo.141.10.7707. [DOI] [PubMed] [Google Scholar]
- Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes. 2004;53:549–559. doi: 10.2337/diabetes.53.3.549. [DOI] [PubMed] [Google Scholar]
- Keath JR, Iacoviello MP, Barrett LE, Mansvelder HD, McGehee DS. Differential modulation by nicotine of substantia nigra versus ventral tegmental area dopamine neurons. J Neurophysiol. 2007;98:3388–3396. doi: 10.1152/jn.00760.2007. [DOI] [PubMed] [Google Scholar]
- Kellar KJ, Giblin BA, Lumpkin MD. Regulation of brain nicotinic cholinergic recognition sites and prolactin release by nicotine. Prog Brain Res. 1989;79:209–216. doi: 10.1016/s0079-6123(08)62480-2. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Ann N Y Acad Sci. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AP, Jackson ER, Brown SJ, Fuchs JR, Miltner BC, Doughty AH. Nicotine-induced impulsive action: sensitization and attenuation by mecamylamine. Behav Pharmacol. 2011;22:207–221. doi: 10.1097/FBP.0b013e328345ca1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolokotroni KZ, Rodgers RJ, Harrison AA. Acute nicotine increases both impulsive choice and behavioural disinhibition in rats. Psychopharmacology (Berl) 2011;217:455–473. doi: 10.1007/s00213-011-2296-2. [DOI] [PubMed] [Google Scholar]
- Korpela K, Sundell J, Ylonen H. Does personality in small rodents vary depending on population density? Oecologia. 2011;165:67–77. doi: 10.1007/s00442-010-1810-2. [DOI] [PubMed] [Google Scholar]
- Li MD, Parker SL, Kane JK. Regulation of feeding-associated peptides and receptors by nicotine. Mol Neurobiol. 2000;22:143–165. doi: 10.1385/MN:22:1-3:143. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. Palatable cafeteria diet ameliorates anxiety and depression-like symptoms following an adverse early environment. Psychoneuroendocrinology. 2010;35:717–728. doi: 10.1016/j.psyneuen.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Grun EU, Collins AC. ST/b and DBA/2 mice differ in brain alpha-bungarotoxin binding and alpha 7 nicotinic receptor subunit mRNA levels: a quantitative autoradiographic analysis. Brain Res Mol Brain Res. 1996;39:207–222. doi: 10.1016/0169-328x(96)00027-7. [DOI] [PubMed] [Google Scholar]
- Miyata G, Meguid MM, Fetissov SO, Torelli GF, Kim HJ. Nicotine's effect on hypothalamic neurotransmitters and appetite regulation. Surgery. 1999;126:255–263. [PubMed] [Google Scholar]
- Moreira CM, Masson S, Carvalho MC, Brandao ML. Exploratory behaviour of rats in the elevated plus-maze is differentially sensitive to inactivation of the basolateral and central amygdaloid nuclei. Brain Res Bull. 2007;71:466–474. doi: 10.1016/j.brainresbull.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Newman MB, Nazian SJ, Sanberg PR, Diamond DM, Shytle RD. Corticosterone-attenuating and anxiolytic properties of mecamylamine in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:609–620. doi: 10.1016/s0278-5846(00)00178-0. [DOI] [PubMed] [Google Scholar]
- Newman MB, Manresa JJ, Sanberg PR, Shytle RD. Anxiolytic effects of mecamylamine in two animal models of anxiety. Exp Clin Psychopharmacol. 2002;10:18–25. doi: 10.1037//1064-1297.10.1.18. [DOI] [PubMed] [Google Scholar]
- Nishizaki T, Ikeuchi Y, Matsuoka T, Sumikawa K. Short-term depression and long-term enhancement of ACh-gated channel currents induced by linoleic and linolenic acid. Brain Res. 1997;751:253–258. doi: 10.1016/s0006-8993(96)01405-9. [DOI] [PubMed] [Google Scholar]
- Nishizaki T, Nomura T, Matsuoka T, Enikolopov G, Sumikawa K. Arachidonic acid induces a long-lasting facilitation of hippocampal synaptic transmission by modulating PKC activity and nicotinic ACh receptors. Brain Res Mol Brain Res. 1999;69:263–272. doi: 10.1016/s0169-328x(99)00117-5. [DOI] [PubMed] [Google Scholar]
- Ohmori N, Itoi K, Tozawa F, Sakai Y, Sakai K, Horiba N, Demura H, Suda T. Effect of acetylcholine on corticotropin-releasing factor gene expression in the hypothalamic paraventricular nucleus of conscious rats. Endocrinology. 1995;136:4858–4863. doi: 10.1210/endo.136.11.7588217. [DOI] [PubMed] [Google Scholar]
- Pasumarthi RK, Fadel J. Activation of orexin/hypocretin projections to basal forebrain and paraventricular thalamus by acute nicotine. Brain Res Bull. 2008;77:367–373. doi: 10.1016/j.brainresbull.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasumarthi RK, Fadel J. Stimulation of lateral hypothalamic glutamate and acetylcholine efflux by nicotine: implications for mechanisms of nicotine-induced activation of orexin neurons. J Neurochem. 2010;113:1023–1035. doi: 10.1111/j.1471-4159.2010.06666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli-Pott U, Albayrak O, Hebebrand J, Pott W. Association between inhibitory control capacity and body weight in overweight and obese children and adolescents: dependence on age and inhibitory control component. Child Neuropsychol. 2010;16:592–603. doi: 10.1080/09297049.2010.485980. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J Pharmacol Exp Ther. 1991;258:1127–1136. [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Robinson SF, van de Kamp JL, Collins AC. Chronic nicotine and mecamylamine treatment increase brain nicotinic receptor binding without changing alpha 4 or beta 2 mRNA levels. J Pharmacol Exp Ther. 1996;278:361–369. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain, in Stereotaxic Coordinates. Vol. San Diego, C.A.: Academic Press, Inc.; 1997. [Google Scholar]
- Prasad A, Prasad C. Short-term consumption of a diet rich in fat decreases anxiety response in adult male rats. Physiol Behav. 1996;60:1039–1042. doi: 10.1016/0031-9384(96)00135-7. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Puhl MD, Cason AM, Wojnicki FH, Corwin RL, Grigson PS. A history of bingeing on fat enhances cocaine seeking and taking. Behav Neurosci. 2011;125:930–942. doi: 10.1037/a0025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Mendialdua A, Hernandez L, Hoebel BG. Extracellular glutamate increases in the lateral hypothalamus during meal initiation, and GABA peaks during satiation: microdialysis measurements every 30 s. Behav Neurosci. 2003;117:222–227. doi: 10.1037/0735-7044.117.2.222. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Arankowsky-Sandoval G, Gold PE. Glucose attenuates the effect of combined muscarinic-nicotinic receptor blockade on spontaneous alternation. Eur J Pharmacol. 1994;256:31–36. doi: 10.1016/0014-2999(94)90612-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen BA, Perry DC. An autoradiographic analysis of [125I]alpha-bungarotoxin binding in rat brain after chronic nicotine exposure. Neurosci Lett. 2006;404:9–14. doi: 10.1016/j.neulet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiol Learn Mem. 2010;94:353–363. doi: 10.1016/j.nlm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AC, Sawa E, Carren-LeSauter I, LeSauter J, Silver R, Pfaff DW. Two forces for arousal: Pitting hunger versus circadian influences and identifying neurons responsible for changes in behavioral arousal. Proc Natl Acad Sci U S A. 2007;104:20078–20083. doi: 10.1073/pnas.0710096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AC, LeSauter J, Dupre C, Pfaff DW. Relationship of arousal to circadian anticipatory behavior: ventromedial hypothalamus: one node in a hunger-arousal network. Eur J Neurosci. 2009;30:1730–1738. doi: 10.1111/j.1460-9568.2009.06969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220:214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Wrench N, Ryde IT, Lassiter TL, Levin ED, Seidler FJ. Neonatal parathion exposure disrupts serotonin and dopamine synaptic function in rat brain regions: modulation by a high-fat diet in adulthood. Neurotoxicol Teratol. 2009;31:390–399. doi: 10.1016/j.ntt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulis G, Papalexi E, Kittas C, Kitraki E. Early impact of a fat-enriched diet on behavioral responses of male and female rats. Behav Neurosci. 2007;121:483–490. doi: 10.1037/0735-7044.121.3.483. [DOI] [PubMed] [Google Scholar]
- Sparks JA, Pauly JR. Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57Bl/6 mice. Psychopharmacology (Berl) 1999;141:145–153. doi: 10.1007/s002130050818. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Ishimaru H, Ikarashi Y, Maruyama Y. Decrease of norepinephrine and preservation of acetylcholine in the hypothalamus of VMH obese rats. Brain Res Bull. 1995;36:97–99. doi: 10.1016/0361-9230(94)00173-x. [DOI] [PubMed] [Google Scholar]
- Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61:1021–1029. doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Tel G, Ozturk M, Duru ME, Harmandar M, Topcu G. Chemical composition of the essential oil and hexane extract of Salvia chionantha and their antioxidant and anticholinesterase activities. Food Chem Toxicol. 2010;48:3189–3193. doi: 10.1016/j.fct.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Perry DC. Prenatal nicotine exposure is associated with an increase in [125I]epibatidine binding in discrete cortical regions in rats. Pharmacol Biochem Behav. 2000;67:319–323. doi: 10.1016/s0091-3057(00)00379-8. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Tsutsui-Kimura I, Ohmura Y, Izumi T, Yamaguchi T, Yoshida T, Yoshioka M. Nicotine provokes impulsive-like action by stimulating alpha4beta2 nicotinic acetylcholine receptors in the infralimbic, but not in the prelimbic cortex. Psychopharmacology (Berl) 2010a;209:351–359. doi: 10.1007/s00213-010-1804-0. [DOI] [PubMed] [Google Scholar]
- Tsutsui-Kimura I, Ohmura Y, Izumi T, Yamaguchi T, Yoshida T, Yoshioka M. Endogenous acetylcholine modulates impulsive action via alpha4beta2 nicotinic acetylcholine receptors in rats. Eur J Pharmacol. 2010b;641:148–153. doi: 10.1016/j.ejphar.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Vajreswari A, Rupalatha M, Rao PS. Effect of altered dietary n-6-to-n-3 fatty acid ratio on erythrocyte lipid composition and membrane-bound enzymes. J Nutr Sci Vitaminol (Tokyo) 2002;48:365–370. doi: 10.3177/jnsv.48.365. [DOI] [PubMed] [Google Scholar]
- Valladolid-Acebes I, Stucchi P, Cano V, Fernandez-Alfonso MS, Merino B, Gil-Ortega M, Fole A, Morales L, Ruiz-Gayo M, Olmo ND. High-fat diets impair spatial learning in the radial-arm maze in mice. Neurobiol Learn Mem. 2010 doi: 10.1016/j.nlm.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Diet, nutrition and the prevention of chronic diseases. WHO Technical Report Series. 2003 [PubMed]
- Wiles NJ, Northstone K, Emmett P, Lewis G. 'Junk food' diet and childhood behavioural problems: results from the ALSPAC cohort. Eur J Clin Nutr. 2009;63:491–498. doi: 10.1038/sj.ejcn.1602967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, Blaha V, Meguid MM, Oler A, Miyata G. Infusion of nicotine into the LHA enhances dopamine and 5-HT release and suppresses food intake. Pharmacol Biochem Behav. 1999;64:155–159. doi: 10.1016/s0091-3057(99)00111-2. [DOI] [PubMed] [Google Scholar]
- Yoburn BC, Glusman M. The effects of intrahypothalamic hemicholinium-3 on muricide, irritability and feeding. Pharmacol Biochem Behav. 1984;20:829–833. doi: 10.1016/0091-3057(84)90001-7. [DOI] [PubMed] [Google Scholar]
- Zaninetti M, Tribollet E, Bertrand D, Raggenbass M. Nicotinic cholinergic activation of magnocellular neurons of the hypothalamic paraventricular nucleus. Neuroscience. 2002;110:287–299. doi: 10.1016/s0306-4522(01)00536-x. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Solati J, Oryan S, Parivar K. Effect of intra-amygdala injection of nicotine and GABA receptor agents on anxiety-like behaviour in rats. Pharmacology. 2008;82:276–284. doi: 10.1159/000161129. [DOI] [PubMed] [Google Scholar]
- Zhang JF, Zheng F. The role of paraventricular nucleus of hypothalamus in stress-ulcer formation in rats. Brain Res. 1997;761:203–209. doi: 10.1016/s0006-8993(97)00257-6. [DOI] [PubMed] [Google Scholar]