Abstract
Context
A randomized trial demonstrates that adding bevacizumab to carboplatin and paxlitaxel improves survival in advanced non-small-cell lung cancer (NSCLC).
Objective
To examine whether adding bevacizumab to carboplatin and paclitaxel chemotherapy is associated with improved survival in the NSCLC Medicare population.
Design, Setting and Participants
Retrospective cohort study of Medicare beneficiaries aged 65 and older with stage IIIB or IV non-squamous NSCLC diagnosed in 2002–2007 in a Surveillance, Epidemiology and End Results (SEER) region. Patients were categorized into three cohorts based on diagnosis year and the type of initial chemotherapy administered within 4 months of diagnosis: 1) bevacizumab-carboplatin-paclitaxel (BCP) diagnosed 2006–7; 2) carboplatin-paclitaxel diagnosed 2006–7 (CP 2006–7); and, 3) CP diagnosed 2002–5 (CP 2002–5). The effects of BCP and CP on overall survival were compared using Cox proportional hazards models and propensity score analyses including information about patient characteristics recorded in SEER-Medicare.
Main Outcome Measure
Overall survival measured from the first date of chemotherapy treatment until death or the censoring date of December 31, 2009.
Results
4,168 patients had either BCP or CP chemotherapy. The median survival (interquartile range) estimates were 9.7 (4.4–18.6) months, 8.9 (3.5–19.3) months, and 8.0 (3.7–17.2) months for BCP, CP 2006–7, and CP 2002–5 recipients, respectively. One-year survival probabilities (95% confidence interval [CI]) were 39.6% (34.6%–45.4%) for BCP, versus 40.1% (37.4%–43.0%) for CP 2006–7 and 35.6% (33.8%–37.5%) for CP 2002–5. Neither multivariable nor propensity score-adjusted Cox models demonstrated a survival advantage for BCP compared to CP cohorts. In propensity score-stratified models, the hazard ratio (HR) for overall survival for BCP compared with CP 2006–7 was 1.01 (95% CI, 0.89–1.16; P=.85); and compared with CP 2002–5 was 0.93 (95% CI, 0.83–1.06; P=.28). The propensity score-weighted model and propensity score-matching model similarly failed to demonstrate a statistically significant superiority for BCP. Subgroup and sensitivity analyses for key variables did not change these findings.
Conclusions
Adding bevacizumab to carboplatin and paclitaxel was not associated with better survival among Medicare patients with advanced NSCLC.
Non-small-cell lung cancer (NSCLC) is usually diagnosed at advanced (IIIB or IV) stage when cure is rarely attainable.1 Although chemotherapy offers modest quality-of-life and survival advantages over best supportive care,2,3 treatment outcomes remain disappointing with 1-year survival less than 50% and 3-year survival less than 25%.4
Bevacizumab inhibits tumor angiogenesis and subsequent tumor growth and metastases.5 In 2006, a randomized trial conducted by the Eastern Cooperative Oncology Group (ECOG 4599) of 878 patients with advanced NSCLC of non-squamous cell type, demonstrated a significant survival benefit for bevacizumab-carboplatin-paclitaxel (BCP) over carboplatin-paclitaxel (CP), with a hazard ratio (HR) of 0.79 for death (95% confidence interval [CI], 0.67–0.92).6 This trial led to the approval of BCP as treatment for advanced non-squamous NSCLC by the U.S. Food and Drug Administration (FDA) in October 2006.7 However, a recently published meta-analysis of 4 randomized trials did not identify a significant improvement in 1-year overall survival when adding bevacizumab to standard chemotherapy.8
Moreover, the ECOG trial failed to demonstrate a survival advantage for BCP over CP (HR, 0.89; 95% CI, 0.70–1.14) among the subgroup of 366 patients aged 65 years and older.6 An unplanned subset analysis in 224 patients aged 70 years or older at diagnosis from the same trial also suggested no significant differences in overall survival (11.3 vs. 12.1 months) between BCP and CP.9 Notwithstanding the uncertainty about benefits in the over 65 year population, the Center for Medicare and Medicaid Services (CMS) has covered bevacizumab therapy for its enrollees subsequent to FDA approval.10 Little is known about how clinicians have interpreted efficacy studies to formulate treatment recommendations, and given that approximately 2/3 of patients with lung cancer are diagnosed at age 65 years or older,4 establishing the survival advantage of bevacizumab in the Medicare population is a priority for informed decision making.
Using analytic strategies to address confounding and selection bias caused by the lack of treatment randomization in observational studies that may limit ability to make valid inferences about causality, we examined whether adding bevacizumab to first-line CP was associated with improved survival in the Medicare population with advanced non-squamous NSCLC.
METHODS
Data Source
We used population-based data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) program linked to Medicare claims. The SEER database covers 17 cancer registries and captures cancer incidence for approximately 28% of the U.S. population.11 SEER includes information on cancer site, histology, stage, grade, and dates of diagnosis and death, as well as patient demographic characteristics.12,13 Medicare Parts A and B claims contain extensive service level data for hospital inpatient and outpatient care, skilled nursing facility, physician services, durable medical equipment, home health agency, and hospice.12 SEER data for patients diagnosed from January 1, 2002, through December 31, 2007, were matched to Medicare claims data from January 1, 2001, through December 31, 2009. The study was approved by the institutional review board at the Dana-Farber Harvard Cancer Center.
Study Participants
The study cohort included patients aged 65 years or older with pathologically confirmed Stage IIIB or IV non-squamous NSCLC diagnosed between 2002 and 2007 who received first-line chemotherapy with BCP or CP within 4 months of diagnosis. Patients were excluded if they had other primary cancers diagnosed either before or after NSCLC, or died within 30 days of NSCLC diagnosis. To ensure completeness of Medicare claims information, patients who were not continuously eligible for Medicare Parts A and B or who were enrolled in a health maintenance organization at any point from diagnosis to death or 6 months of follow-up were also excluded. Staging information was available in SEER and defined according to the American Joint Committee on Cancer (AJCC) Staging Manual 6th edition.14
The goal of this analysis was to compare survival outcomes for elderly advanced NSCLC patients treated with first-line carboplatin and paclitaxel alone or with bevacizumab. Thus, our primary comparison groups were patients diagnosed in 2006–2007 receiving first-line BCP and those diagnosed in 2006–2007 receiving first-line CP (CP 2006–7). We also constructed a second control group (CP 2002–5) composed of patients diagnosed in 2002–2005 before bevacizumab was commercially available. The CP 2002–5 group was used to mitigate bias caused by selecting patients into either treatment or control group based on patients’ characteristics that might also be associated with outcomes. If, in 2006 and 2007, physicians chose BCP for their healthier patients, then a selection bias might result in better survival for BCP-treated patients. Between 2002–2005, BCP was not available so comparing the CP 2002–5 cohort to the BCP cohort would attenuate this potential bias. At the minimum follow-up observation time of 24 months from diagnosis, 83% of cohort members were deceased.
Identification of First-Line Carboplatin, Paclitaxel, and Bevacizumab
Medicare claims have been shown to have high sensitivity and specificity for identification of chemotherapy agents among elderly patients with lung cancer.15,16 First-line chemotherapy was defined as chemotherapy administered within 4 months after the NSCLC diagnosis. Specific agents were identified from Medicare outpatient, physician, or durable medical equipment claims by using Healthcare Common Procedure Coding System (HCPCS) codes and National Drug Codes (NDCs). The date of the first chemotherapy claim was considered the start date of chemotherapy. Additional agents received within 8 days of the first drug were considered as components of the same regimen. Any patients whose initial chemotherapy was delivered concurrently with radiotherapy, defined as start dates within 8 days of each other, were eliminated.
Survival Outcomes
The primary outcome was all-cause mortality, defined as the number of survival months from the administration of first chemotherapy agent until the date of death or the end of the observation period. Date of death was reported in Medicare enrollment files capturing death through December 31, 2009. Patients alive at the end of follow-up were censored.
Baseline Characteristics
Demographic and clinical data obtained from SEER included age, sex, race/ethnicity, marital status, geographic region, urban residency, ecological surrogates for educational attainment and median income, AJCC stage, and tumor grade according to the categories displayed in Table 1. Race/ethnicity was classified as non-Hispanic white, non-Hispanic black, and other (e.g., Hispanic, Asian/Pacific Islander, American Indian/Alaska Native). Race in SEER is identified from patients’ medical records and registration information, and Hispanic ethnicity in SEER is determined through a Hispanic-surname algorithm that has better sensitivity than that recorded in Medicare data.17 To measure the burden of comorbidities, we applied the Deyo adaptation18 of the Charlson comorbidity index,19 modified to exclude cancer diagnoses, to Medicare inpatient, outpatient, and physician claims during the 12-month period extending from 13 months to 1 month before NSCLC diagnosis using lung cancer-specific weights as described by Klabunde et al.20,21 We then categorized the comorbidity score into 3 groups (0, 1, 2 or more).
Table 1.
Characteristics of Elderly Advanced Non-Squamous NSCLC Patients in the Three Treatment Groups
| Characteristics | BCP No. (%) |
CP 2006–7
|
CP 2002–5
|
||
|---|---|---|---|---|---|
| No. (%) | P value a | No. (%) | P value a | ||
| TOTAL | 318 | 1,182 b | 2,664 b | ||
|
| |||||
| Age at diagnosis, years | |||||
|
| |||||
| 65–69 | 127 (39.9) | 396 (33.5) | 911 (34.2) | ||
|
| |||||
| 70–74 | 84 (26.4) | 356 (30.1) | .07 | 861 (32.3) | .08 |
|
| |||||
| 75–79 | 78 (24.5) | 278 (23.5) | 612 (23.0) | ||
|
| |||||
| 80+ | 29 (9.1) | 152 (12.9) | 280 (10.5) | ||
|
| |||||
| Gender | |||||
|
| |||||
| Female | 156 (49.1) | 529 (44.8) | .18 | 1,255 (47.1) | .51 |
|
| |||||
| Male | 162 (50.9) | 653 (55.2) | 1,409 (52.9) | ||
|
| |||||
| Race/ethnicity | |||||
|
| |||||
| Non-Hispanic White | 279 (87.7) | 981 (83.0) | 2,267 (85.1) | ||
|
| |||||
| Non-Hispanic Black | 15 (4.7) | 98 (8.3) | .07 | 167 (6.3) | .46 |
|
| |||||
| Other | 24 (7.5) | 103 (8.7) | 230 (8.6) | ||
|
| |||||
| Marital status | |||||
|
| |||||
| Not married | 117 (36.8) | 467 (39.5) | .40 | 993 (37.3) | .90 |
|
| |||||
| Married | 201 (63.2) | 715 (60.5) | 1,671 (62.7) | ||
|
| |||||
| Median income (census tract quintile) c | |||||
|
| |||||
| 1 (lowest) | 60 (18.9) | 240 (20.3) | 536 (20.1) | ||
|
| |||||
| 2 | 58 (18.2) | 242 (20.5) | 543 (20.4) | ||
|
| |||||
| 3 | 63 (19.8) | 237 (20.1) | .20 | 533 (20.0) | .23 |
|
| |||||
| 4 | 58 (18.2) | 242 (20.5) | 533 (20.0) | ||
|
| |||||
| 5 (highest) | 79 (24.8) | 221 (18.7) | 519 (19.5) | ||
|
| |||||
| College educated (census tract quintile) d | |||||
|
| |||||
| 1 (lowest) | 66 (20.8) | 234 (19.8) | 533 (20.0) | ||
|
| |||||
| 2 | 58 (18.2) | 244 (20.6) | 537 (20.2) | ||
|
| |||||
| 3 | 62 (19.5) | 238 (20.1) | .38 | 533 (20.0) | .76 |
|
| |||||
| 4 | 57 (17.9) | 241 (20.4) | 537 (20.2) | ||
|
| |||||
| 5 (highest) | 75 (23.6) | 225 (19.0) | 524 (19.7) | ||
|
| |||||
| SEER region | |||||
|
| |||||
| Northeast | 65 (20.4) | 295 (25.0) | 657 (24.7) | ||
|
| |||||
| South | 69 (21.7) | 244 (20.6) | .40 | 548 (20.6) | .42 |
|
| |||||
| Midwest | 51 (16.0) | 173 (14.6) | 409 (15.4) | ||
|
| |||||
| West | 133 (41.8) | 470 (39.8) | 1,050 (39.4) | ||
|
| |||||
| Urban residency e | |||||
|
| |||||
| No | 35 (11.0) | 92 (7.8) | .07 | 211 (7.9) | .07 |
|
| |||||
| Yes | 283 (89.0) | 1,090 (92.2) | 2,453 (92.1) | ||
|
| |||||
| Modified Charlson comorbidity score f | |||||
|
| |||||
| 0 | 210 (66.0) | 665 (56.3) | 1,661 (62.3) | ||
|
| |||||
| 1 | 88 (27.7) | 324 (27.4) | <.001 | 657 (24.7) | <.01 |
|
| |||||
| 2+ | 20 (6.3) | 193 (16.3) | 346 (13.0) | ||
|
| |||||
| Level of differentiation (Tumor grading) | |||||
|
| |||||
| Well/moderately | 50 (15.7) | 128 (10.8) | 277 (10.4) | ||
|
| |||||
| Poorly/no | 85 (26.7) | 345 (29.2) | .06 | 820 (30.8) | .01 |
|
| |||||
| Unknown | 183 (57.5) | 709 (60.0) | 1,567 (58.8) | ||
|
| |||||
| AJCC stage | |||||
|
| |||||
|
| |||||
| IIIB | 56 (17.6) | 344 (29.1) | <.001 | 816 (30.6) | <.001 |
|
| |||||
| IV | 262 (82.4) | 838 (70.9) | 1,848 (69.4) | ||
Abbreviations: NSCLC, non-small-cell lung cancer; BCP, bevacizumab-carboplatin-paclitaxel; CP 2006–7, carboplatin-paclitaxel (diagnoses 2006–7 when bevacizumab was FDA approved for NSCLC); CP 2002–5, carboplatin-paclitaxel (diagnoses 2002–5 when bevacizumab was not available (2002–3) or not approved for NSCLC treatment); SEER, Surveillance, Epidemiology and End Results; AJCC, American Joint Committee on Cancer. Percentages for some categories do not total 100% because of rounding.
The first P value is for the comparison of BCP to CP 2006–7 and the second for the comparison of BCP to CP 2002–5.
2 patients with a missing characteristic variable were excluded from each of the two control groups.
Median income refers to median household or per capita income by 2000 census tract or zip code.
College educated refers to the percentage of persons older than age 25 years with some college education by 2000 census tract.
Urban residency refers to counties of at least 20,000 residents, as opposed to rural residency.
Mordified Charlson comorbidity score was constructed by applying the Deyo adaptation18 of the Charlson comorbidity index,19 modified to exclude cancer diagnoses, to Medicare inpatient, outpatient, and physician claims during the 12-month period extending from 13 months to 1 month before NSCLC diagnosis using lung cancer-specific weights as described by Klabunde et al.20,21
Statistical Analysis
Differences in distribution of baseline characteristics between the BCP group and each of the CP controls were evaluated using the χ2 test. The Kaplan-Meier survival method was used to estimate median survival and we tested for crude differences among the three groups (BCP, CP 2006–7, and CP 2002–5) using a log-rank test. We conducted unadjusted and multivariable Cox proportional hazards models controlling for all demographic and clinical characteristics listed in Table 1 to examine whether the addition of bevacizumab to carboplatin and paclitaxel improved overall survival in patients with advanced non-squamous NSCLC. We compared BCP patients to both CP 2006–7 and 2002–5 cohorts.
We used propensity score analyses22 to balance measurable confounders between the BCP and each of the two CP (2006–7 and 2002–5) groups. A multivariable logistic regression was used to predict treatment (BCP compared with CP) based on confounding covariates, including age, sex, race/ethnicity, marital status, geographic region, urban residency, tumor grading, census tract education, median income, modified Charlson comorbidities, and AJCC stage. Each patient was then assigned an estimated propensity score, which was his/her predicted probability of receiving BCP rather than CP on the basis of his/her observed baseline characteristics.23,24 The cohort was then divided into five strata defined by quintiles of estimated propensity scores.25 Next, we used p-values of the χ2 test to assess whether patients’ baseline characteristics were balanced across the two treatment groups within each stratum. Finally, Cox proportional hazards models were conducted separately within each stratum to compare overall survival of patients treated with BCP vs. CP, and then the five hazard ratios estimated from each stratum were combined into an overall hazard ratio for the whole cohort.26 Cox models were also performed by applying propensity scores to adjust for group differences in three alternative ways: 1) regression adjustment (i.e., inclusion of the propensity score as a linear predictor in the model); 2) propensity score-matching which paired BCP and CP patients that were similar in terms of their measurable characteristics; and, 3) use of the propensity score to create stabilized weights, defined as the inverse probability of treatment weighting (IPTW).27,28 The analyses were first performed on the BCP and CP 2006–7 cohorts, and then repeated on the BCP and CP 2002–5 groups.
We also performed subgroup analyses for two characteristics that were imbalanced between treatment groups, specifically, stage IV disease, more prevalent in the BCP group, and comorbidity, less prevalent in the BCP group. Sensitivity analyses evaluated the potential impact of immortal time bias29 and alternative strategies for treatment assignment on results. Specifically, we measured survival starting from CP treatment day 9, the end of the 8 day interval used to ascertain concurrent bevacizumab therapy, instead of from CP treatment day 1, and we expanded the interval used to identify bevacizumab concurrent administration with CP from 8, to 30 days, while initiating measurement of survival at day 31.
SAS software version 9.2 (SAS Institute, Cary, NC) was used for all analyses. Statistical significance was set at P<.05, and all tests were 2-tailed.
RESULTS
Cohort Description and Baseline Characteristics
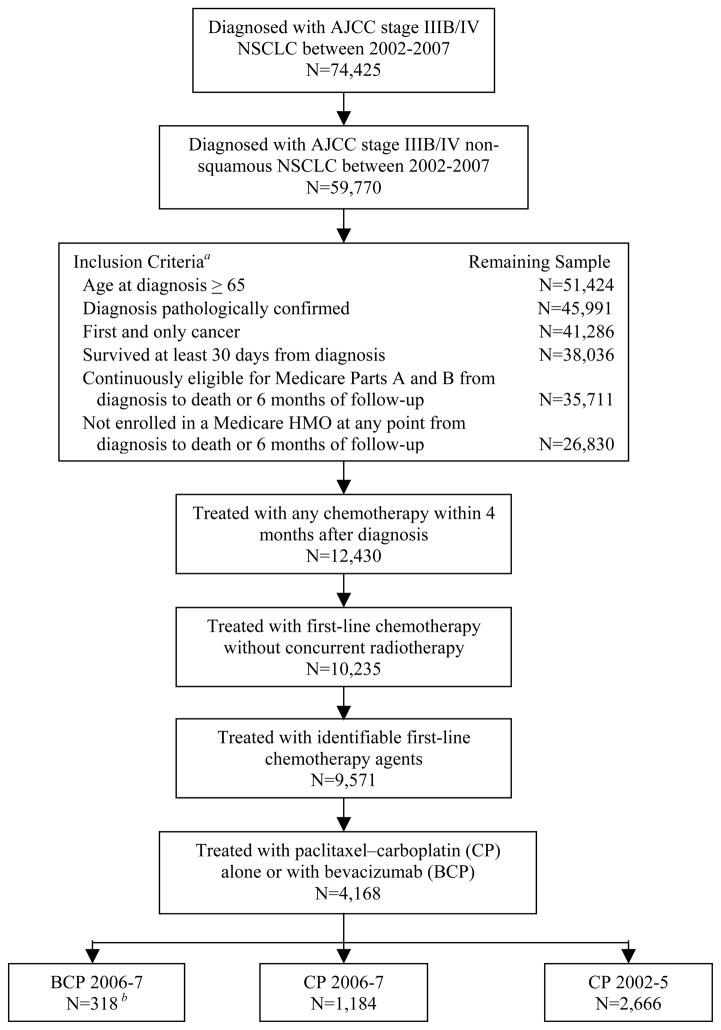
From an initial sample of 59,770 patients diagnosed with advanced non-squamous NSCLC between 2002 and 2007, 26,830 patients met initial study inclusion criteria and 12,430 received chemotherapy within 4 months of diagnosis (Figure 1). Of these, 9,571 patients received identifiable first-line chemotherapy agents, 4,168 of whom received treatment with CP with or without bevacizumab. Within the study cohort, 2,666 (64%) patients were diagnosed between 2002–2005 and made up the CP 2002–5 group. The remaining 1,502 patients were diagnosed in 2006–2007 and of these, 318 (21%) were in the bevacizumab (BCP)-treated cohort and 1,184 (79%) were in the CP 2006–7 cohort. Among patients diagnosed in 2007, 22% were treated with BCP.
Figure 1.
Cohort Assembly: Medicare Enrollees Diagnosed with Stage IIIB/IV Non-Squamous NSCLC in 2002–2007 Treated with Either Paclitaxel–Carboplatin Alone or with Bevacizumab
Abbreviations: NSCLC, non-small-cell lung cancer; AJCC, American Joint Committee on Cancer; HMO, health maintenance organization; BCP, bevacizumab-carboplatin-paclitaxel; CP, carboplatin-paclitaxel.
a The inclusion criteria were applied sequentially as listed.
b 19 patients treated with BCP in 2005 were included in the BCP group.
Characteristics of patients in the BCP, CP 2006–7, and CP 2002–5 groups are shown in Table 1 and were similar in most respects. BCP-treated patients were less likely to have 2 or more comorbidities (6.3% vs. 16.3%, P<.001) and more likely to have stage IV disease (82.4% vs. 70.9%, P<.001) compared with those receiving CP 2006–7 (Table 1). Similarly, compared with those in the CP 2002–5 group, patients receiving BCP were less likely to have 2 or more comorbidities (6.3% vs. 13.0%, P<.01), more likely to have stage IV disease (82.4% vs. 69.4%, P<.001), and more likely to have well/moderately differentiated tumors (15.7% vs. 10.4%, P =.01). There were no significant differences in the distribution of age, sex, race/ethnicity, marital status, income, education, SEER region, and urban residency between BCP and each of the CP controls. After adjusting for stratification of propensity scores, the balance of these observed covariates between BCP and each of the CP controls improved (eTable 1). In addition, propensity score matching resulted in well-balanced BCP (n=318) and each of the CP cohorts (n=318), which were similar in all measurable characteristics except age at diagnosis between BCP and CP 2002–5 (eTable 2), improving covariate balance from the unmatched cohorts.
Survival Outcomes
Kaplan-Meier survival curves are shown in Figure 2. The median overall survival was 9.7 months (interquartile range [IQR], 4.4–18.6 months) for patients receiving BCP, compared with 8.9 months (IQR, 3.5–19.3 months) for those receiving CP 2006–7, and 8.0 months (IQR, 3.7–17.2 months) for those receiving CP 2002–5. The unadjusted 1-year survival probabilities were 39.6% (95% CI, 34.6%–45.4%) for BCP, versus 40.1% (95% CI, 37.4%–43.0%) for CP 2006–7 and 35.6% (95% CI, 33.8%–37.5%) for CP 2002–5.
Figure 2.
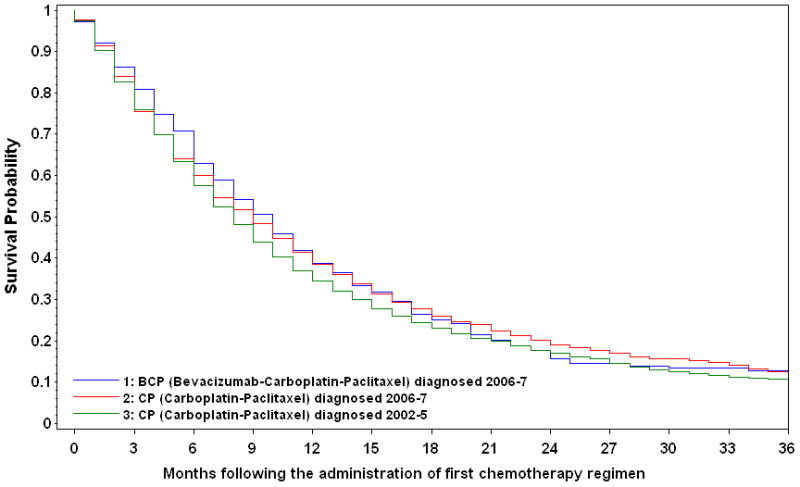
Kaplan-Meier Survival Curves for Medicare Beneficiaries Diagnosed with Advanced Non-Squamous Non-Small-Cell Lung Cancer, by Year of Diagnosis and First-Line Chemotherapy Administration with or without Bevacizumab
| No. at risk Groups | Months following the administration of first chemotherapy regimen | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | 30 | 36 | |
| 1 | 318 | 225 | 133 | 84 | 54 | 31 | 17 |
| 2 | 1182 | 756 | 489 | 328 | 228 | 133 | 68 |
| 3 | 2664 | 1688 | 982 | 652 | 472 | 348 | 283 |
Controlling for demographic and clinical characteristics in adjusted Cox proportional hazards models, we did not find a significant difference in overall survival between patients treated with BCP and those treated with CP 2006–7 (HR, 1.01; 95% CI, 0.88–1.15) or CP 2002–5 (HR, 0.94; 95% CI, 0.83–1.06) (Table 2).
Table 2.
Impact of Bevacizumab in addition to Carboplatin and Paclitaxel on Hazard Ratios for Overall Survival
| Model | Sample Size
|
Hazard Ratio (95% CI)
|
||
|---|---|---|---|---|
| BCP, CP 2006–7 | BCP, CP 2002–5 | BCP vs. CP 2006–7 | BCP vs. CP 2002–5 | |
| Unadjusted model | 318, 1,182 | 318, 2,664 | 1.00 (0.88–1.14) | 0.95 (0.84–1.08) |
|
| ||||
| Multivariable adjusted model a | 318, 1,182 | 318, 2,664 | 1.01 (0.88–1.15) | 0.94 (0.83–1.06) |
|
| ||||
| Propensity score-adjusted model b | ||||
|
| ||||
| Stratification | 318, 1,153 | 311, 2,512 | 1.01 (0.89–1.16) | 0.93 (0.83–1.06) |
|
| ||||
| Within propensity score quintile | ||||
|
| ||||
| 1 (lowest propensity) | 33, 261 | 51, 716 | 0.75 (0.51–1.10) | 0.97 (0.72–1.30) |
|
| ||||
| 2 | 69, 337 | 84, 795 | 1.06 (0.80–1.39) | 0.92 (0.73–1.17) |
|
| ||||
| 3 | 110, 361 | 107, 640 | 1.06 (0.85–1.32) | 0.97 (0.79–1.20) |
|
| ||||
| 4 | 78, 151 | 52, 299 | 1.16 (0.87–1.55) | 0.81 (0.59–1.11) |
|
| ||||
| 5 (highest propensity) | 28, 43 | 17, 62 | 0.84 (0.48–1.47) | 1.07 (0.61–1.91) |
|
| ||||
| Regression adjustment | 318, 1,153 | 311, 2,512 | 1.01 (0.89–1.16) | 0.94 (0.83–1.06) |
|
| ||||
| Weighting (stabilized IPTW) | 318, 1,153 | 311, 2,512 | 0.99 (0.87–1.13) | 0.93 (0.82–1.06) |
|
| ||||
| Matching 1:1 | 318, 318 | 318, 318 | 0.99 (0.79–1.23) | 0.90 (0.72–1.13) |
|
| ||||
| Subgroup analyses | ||||
|
| ||||
|
| ||||
| Stage IV c | 262, 838 | 262, 1,848 | 0.96 (0.83–1.12) | 0.88 (0.77–1.02) |
|
| ||||
| Estimated comorbidity score of 0 d | 210, 665 | 210, 1,661 | 1.04 (0.87–1.23) | 0.92 (0.79–1.08) |
|
| ||||
| Sensitivity analyses a | ||||
|
| ||||
| Patients surviving >8 days from treatment start | 315, 1,173 | 315, 2,637 | 1.00 (0.87–1.15) | 0.91 (0.80–1.03) |
|
| ||||
| Patients first treated with bevacizumab between 1 and 30 days of starting CP and surviving >30 days from treatment start | 342, 1,068 | 342, 2,478 | 0.98 (0.86–1.12) | 0.89 (0.79–1.01) |
Abbreviations: CI, confidence interval; BCP, bevacizumab-carboplatin-paclitaxel; CP 2006–7, carboplatin-paclitaxel (diagnoses 2006–7 when bevacizumab was FDA approved for NSCLC); CP 2002–5, carboplatin-paclitaxel (diagnoses 2002–5 when bevacizumab was not available (2002–3) or not approved for NSCLC treatment); IPTW, inverse probability of treatment weighting.
The model was adjusted for baseline age, sex, race/ethnicity, marital status, geographic region, urban residency, tumor grading, census tract education, median income, modified Charlson comorbidities, and AJCC stage.
The propensity of receiving BCP was estimated using multivariable logistic regression model that included baseline age, sex, race/ethnicity, marital status, geographic region, urban residency, tumor grading, census tract education, median income, modified Charlson comorbidities, and AJCC stage.
The model was adjusted for baseline age, sex, race/ethnicity, marital status, geographic region, urban residency, tumor grading, census tract education, median income, and modified Charlson comorbidities.
The model was adjusted for baseline age, sex, race/ethnicity, marital status, geographic region, urban residency, tumor grading, census tract education, median income, and AJCC stage.
None of the four propensity score-adjusted models demonstrated any evidence to support the superiority of BCP to CP. For example, stratified analyses based on propensity scores showed no significant differences in overall survival between patients receiving BCP and those receiving either CP 2006–7 (HR, 1.01; 95% CI, 0.89–1.16) or CP 2002–5 (HR, 0.93; 95% CI, 0.83–1.06). Similarly, propensity score weighting did not demonstrate a significant improvement in overall survival for the BCP group compared with either the CP 2006–7 (HR, 0.99; 95% CI, 0.87–1.13) or CP 2002–5 groups (HR, 0.93; 95% CI, 0.82–1.06) (Table 2).
Neither subgroup nor sensitivity analyses changed our essential finding that BCP was not associated with a survival advantage. For example, the HR for the stage IV BCP-treated patients compared to stage IV CP 2006–7 patients was 0.96 (95% CI, 0.83–1.12), and compared to stage IV CP 2002–5 patients was 0.88 (95% CI, 0.77–1.02) (Table 2). To contextualize these findings, we also evaluated the association between other measured characteristics and overall survival both for BCP and CP 2006–7 and for BCP and CP 2002–5 cohorts and found that later stage disease (Stage IV vs. IIIB) and higher burden of comorbidity were associated with inferior survival (Table 3).
Table 3.
Crude Median Survival for BCP, CP 2006–7, and CP 2002–5 Treated Patients and Hazard Ratios for Overall Survival for BCP vs. CP 2006–7 or CP 2002–5 Adjusting for Patient Characteristics
| Variable | Crude Median Survival (IQR) in months
|
Hazard Ratio (95% CI)
|
|||
|---|---|---|---|---|---|
| BCP | CP 2006–7 | CP 2002–5 | BCP and CP 2006–7 | BCP and CP 2002–5 | |
| Chemotherapy regimen | |||||
|
| |||||
| CP | 8.9 (3.5–19.3) | 8.0 (3.7–17.2) | Reference | Reference | |
|
| |||||
| BCP | 9.7 (4.4–18.6) | 1.01 (0.88–1.15) | 0.94 (0.83–1.06) | ||
|
| |||||
| Age at diagnosis, years | |||||
|
| |||||
| 65–69 | 9.9 (5.5–20.4) | 9.5 (3.4–19.5) | 8.1 (3.7–17.2) | Reference | Reference |
|
| |||||
| 70–74 | 8.7 (4.9–16.8) | 9.8 (4.4–19.0) | 7.8 (3.7–16.7) | 1.03 (0.90–1.18) | 1.07 (0.98–1.18) |
|
| |||||
| 75–79 | 8.5 (3.6–16.7) | 6.8 (3.2–18.5) | 8.1 (3.2–16.2) | 1.10 (0.95–1.27) | 1.10 (0.99–1.21) |
|
| |||||
| 80+ | 13.1 (2.6–21.8) | 9.3 (3.6–20.1) | 8.4 (3.8–17.2) | 1.07 (0.89–1.29) | 1.08 (0.94–1.23) |
|
| |||||
| Gender | |||||
|
| |||||
| Female | 10.9 (5.2–21.5) | 10.3 (4.1–23.0) | 9.0 (3.8–20.5) | Reference | Reference |
|
| |||||
| Male | 8.3 (3.8–15.4) | 7.7 (3.3–16.1) | 7.4 (3.3–14.6) | 1.37 (1.23–1.54) | 1.28 (1.18–1.38) |
|
| |||||
| Race/Ethnicity | |||||
|
| |||||
| Non-Hispanic White | 8.9 (4.1–16.8) | 8.6 (3.5–19.0) | 7.9 (3.5–16.3) | Reference | Reference |
|
| |||||
| Non-Hispanic Black | 10.9 (4.0–22.1) | 9.5 (3.3–17.9) | 8.0 (3.0–18.5) | 0.93 (0.75–1.15) | 0.91 (0.77–1.06) |
|
| |||||
| Other | 16.9 (7.1–25.5) | 11.2 (4.4–20.8) | 9.8 (4.7–21.9) | 0.80 (0.65–0.98) | 0.79 (0.69–0.91) |
|
| |||||
| Marital status | |||||
|
| |||||
| Not married | 8.8 (3.9–19.4) | 8.3 (3.5–17.1) | 7.8 (3.5–16.3) | Reference | Reference |
|
| |||||
| Married | 9.9 (5.0–17.6) | 9.3 (3.5–20.5) | 8.1 (3.7–17.2) | 0.88 (0.78–0.98) | 0.90 (0.83–0.97) |
|
| |||||
| SEER region | |||||
|
| |||||
| Northeast | 8.6 (5.3–15.9) | 9.7 (4.1–19.0) | 8.6 (3.4–18.2) | Reference | Reference |
|
| |||||
| South | 7.1 (2.7–16.2) | 9.1 (3.4–17.5) | 7.3 (3.5–15.8) | 1.03 (0.86–1.23) | 1.09 (0.96–1.23) |
|
| |||||
| Midwest | 9.7 (4.2–15.5) | 7.6 (3.4–17.1) | 7.3 (3.4–14.9) | 0.97 (0.80–1.18) | 1.04 (0.91–1.19) |
|
| |||||
| West | 11.2 (5.5–20.4) | 9.1 (3.4–20.1) | 8.6 (3.8–17.2) | 0.91 (0.76–1.08) | 1.01 (0.90–1.13) |
|
| |||||
| Urban residency | |||||
|
| |||||
| No | 6.3 (2.6–12.6) | 9.0 (3.5–20.7) | 8.4 (3.2–17.4) | Reference | Reference |
|
| |||||
| Yes | 9.9 (4.9–20.0) | 8.9 (3.5–19.0) | 8.0 (3.6–16.9) | 1.08 (0.87–1.34) | 1.06 (0.91–1.23) |
|
| |||||
| Median income (census tract quintile) a | |||||
|
| |||||
| 1 (lowest) | Reference | Reference | |||
|
| |||||
| 2 | 0.97 (0.81–1.16) | 0.90 (0.80–1.02) | |||
|
| |||||
| 3 | 0.83 (0.69–1.02) | 0.94 (0.83–1.07) | |||
|
| |||||
| 4 | 0.87 (0.71–1.06) | 0.94 (0.82–1.07) | |||
|
| |||||
| 5 (highest) | 0.77 (0.64–0.94) | 0.92 (0.80–1.05) | |||
|
| |||||
| College educated (census tract quintile) a | |||||
|
| |||||
| 1 (lowest) | Reference | Reference | |||
|
| |||||
| 2 | 1.04 (0.87–1.24) | 1.04 (0.92–1.18) | |||
|
| |||||
| 3 | 0.94 (0.78–1.14) | 0.99 (0.87–1.12) | |||
|
| |||||
| 4 | 1.04 (0.86–1.27) | 1.06 (0.93–1.21) | |||
|
| |||||
| 5 (highest) | 1.19 (0.96–1.47) | 1.10 (0.95–1.27) | |||
|
| |||||
| Modified Charlson comorbidity score b | |||||
|
| |||||
| 0 | 9.0 (4.2–20.4) | 9.1 (3.5–20.1) | 8.3 (3.8–17.5) | Reference | Reference |
|
| |||||
| 1 | 9.7 (4.2–16.7) | 9.5 (3.4–20.3) | 7.7 (3.3–15.5) | 0.99 (0.87–1.12) | 1.08 (0.99–1.18) |
|
| |||||
| 2+ | 13.8 (4.9–18.4) | 7.1 (3.6–16.3) | 7.4 (3.0–16.5) | 1.17 (1.00–1.38) | 1.08 (0.96–1.22) |
|
| |||||
| Level of differentiation (Tumor grading) | |||||
|
| |||||
| Well/moderately | 11.6 (6.0–35.7) | 13.9 (5.8–30.4) | 11.0 (4.5–22.1) | Reference | Reference |
|
| |||||
| Poorly/no | 7.1 (3.5–14.6) | 7.5 (3.4–18.5) | 7.3 (3.3–16.2) | 1.57 (1.29–1.92) | 1.38 (1.21–1.57) |
|
| |||||
| Unknown | 9.8 (5.0–20.0) | 8.7 (3.4–17.3) | 8.1 (3.6–16.1) | 1.51 (1.25–1.81) | 1.26 (1.11–1.42) |
|
| |||||
| AJCC Stage | |||||
|
| |||||
| IIIB | 12.6 (7.6–21.8) | 13.8 (5.5–27.9) | 10.9 (5.1–23.1) | Reference | Reference |
|
| |||||
| IV | 8.4 (4.1–17.0) | 7.4 (3.2–15.7) | 7.0 (3.0–14.4) | 1.57 (1.38–1.78) | 1.43 (1.32–1.55) |
Abbreviations: IQR, interquartile range; CI, confidence interval; BCP, bevacizumab-carboplatin-paclitaxel; CP 2006–7, carboplatin-paclitaxel (diagnoses 2006–7 when bevacizumab was FDA approved for NSCLC); CP 2002–5, carboplatin-paclitaxel (diagnoses 2002–2005 when bevacizumab was not available (2002–3) or not approved for NSCLC treatment); SEER, Surveillance, Epidemiology and End Results; AJCC, American Joint Committee on Cancer.
The income and education quintiles were constructed when comparing the BCP group to each of the CP controls, and thus, the cutoff values were not the same across these groups. Hence, there was no crude median survival for subgroups of these two variables.
Mordified Charlson comorbidity score was constructed by applying the Deyo adaptation18 of the Charlson comorbidity index,19 modified to exclude cancer diagnoses, to Medicare inpatient, outpatient, and physician claims during the 12-month period extending from 13 months to 1 month before NSCLC diagnosis using lung cancer-specific weights as described by Klabunde et al.20,21
COMMENT
Using data from SEER-Medicare, we compared survival outcomes for advanced NSCLC patients, non-squamous cell subtype, treated with carboplatin and paclitaxel, the prevailing standard chemotherapy regimen, with or without bevacizumab. We found that in the wake of the 2006 FDA approval decision, adoption of bevacizumab was by no means universal. For patients diagnosed in 2006 only 20% and among those diagnosed in 2007, only 22% received bevacizumab as a component of their first-line chemotherapy regimen. Second, we found no evidence that bevacizumab conferred a survival advantage on recipients in multivariable models that controlled for observable demographic and clinical patient attributes.
Our patterns of care findings in the Medicare population suggest that the medical oncology community requires therapeutic evidence specific to a particular disease prior to adoption. Medical oncologists, particularly those in private practice may have financial incentives to administer new expensive treatment agents if they can purchase them for less money than CMS reimburses. Because bevacizumab is expensive30–32 and was covered by CMS, if indeed, oncologists were subject to powerful treatment incentives as some have suggested,33 we would have expected to observe them in this context. That we did not observe rapid or complete uptake of bevacizumab provides some measure of reassurance that oncologists are circumspect and judicious in their use of new agents with uncertain benefit in the Medicare population.
The magnitude of the survival benefit we describe is lower than that observed for clinical trial participants. In our study, the median survival for BCP-treated patients was 9.7 months versus 12.3 months for participants in the ECOG 4599 trial. The CP patients in our study had median survival of 8.9 (2006–2007 diagnoses) versus 8.1 months (2002–2005 diagnoses) whereas those in ECOG 4599 were 10.3 months. The difference in median survival between BCP and CP 2006–7 was 0.8 and between BCP and CP 2002–5 was 1.6 months in our observational study which is 40–80% of the 2 months survival advantage obtained in ECOG 4599. This is not entirely surprising given that only 44% of CP and 42% of BCP trial participants were at age 65 or older at diagnosis6 whereas in our Medicare cohort, 100% were over age 65 and indeed over 1/3 were over age 75 at diagnosis. The marginally lower median survival rates in our observational cohort as compared to either the complete or elderly subgroup of the efficacy cohort may also be attributed to differences in clinical factors such as performance status and baseline lung function that cannot be ascertained from SEER-Medicare data.
Researchers have called for prospective trials specifically designed for the elderly to better define the role of intensive cancer treatments that are routinely demonstrated to have superiority in clinical trials that recruit patients who are younger and/or healthier than the general population of patients with the index malignancy.34–36 However, elderly-specific trials with bevacizumab face practical barriers. For example, Merza and colleagues studied 106 male elderly patients diagnosed with advanced NSCLC at a Veteran’s Administration medical center, and found that only 10% of patients were candidates for bevacizumab after applying exclusion criteria used in ECOG 4599 and another bevacizumab combination trial.37 Our study helps to determine if the insignificant results from the subset analyses of older patients in ECOG 45996,9 can be extended to broader elderly NSCLC patients treated in real world contexts.
Our study must be interpreted in the context of limitations inherent to all observational studies as well as those that rely on administrative data sources such as Medicare. First, the study was limited to Medicare fee-for-service beneficiaries who were aged 65 years or older and living in a SEER region at diagnosis. This cohort may not be representative of all non-squamous NSCLC patients in the United States12 although it is likely to be more representative than the sample of clinical trial participants. Second, SEER-Medicare lacks essential clinical details, such as the presence of molecular biomarkers, performance status, and baseline pulmonary function which may be associated with the selection of chemotherapy agents, survival, or both. However, the inability to identify patients with poor baseline lung function and limited performance status or other clinical contraindications to bevacizumab such as significant hemoptysis and brain metastases would be expected to widen rather than narrow the apparent gap in survival between BCP and CP patients. In an observational cohort, patients with relative contraindications to bevacizumab are more likely to be included in the CP cohort and this should increase the survival advantage of BCP relative to CP. The fact that we did not observe this lends credence to our finding that there is no sizeable benefit from adding bevacizumab to CP in the Medicare population. Third, because we only have NSCLC diagnoses through 2007, the sample size for BCP-treated patients was small and we cannot exclude the possibility that more recent data and/or larger sample would yield different results. Fourth, differences in second- and third-line chemotherapy between study groups may have contributed to survival outcomes; the overall survival might favor the group which had a higher percentage of patients receiving further lines of chemotherapy. Finally, although we used statistical techniques to mitigate the potential for imbalance between our cohorts based on measured prognostic factors, the potential for selection bias based on unmeasured factors that predisposed patients to be included in a particular treatment group cannot be excluded.
In conclusion, our analyses suggest that the addition of bevacizumab to carboplatin and paclitaxel is not associated with demonstrable improvement in overall survival in the Medicare population. In the future, for malignancies like NSCLC that disproportionately affect the elderly or where CMS covers a large proportion of treatment costs, negotiations with pharmaceutical sponsors of pivotal trials might mandate adequate representation of the elderly and/or preplanned subgroup analyses relevant to the Medicare population. Absent this information, clinicians will need to rely on efficacy data from subgroup analysis or randomized trials, observational data such as this report and their clinical judgment to make treatment recommendations. Given that neither subgroup analyses from efficacy studies nor observational data analyses identify a benefit for adding bevacizumab to standard carboplatin-paclitaxel therapy, bevacizumab should not be considered standard of care in this context. Clinicians should exercise caution in making treatment recommendations and should use bevacizumab judiciously for their older patients.
Supplementary Material
Acknowledgments
The authors acknowledge support from Saul Weingart MD, PhD, Center for Patient Safety, Dana-Farber Cancer Institute, Boston, MA; Sebastian Schneeweiss MD, ScD, Division of Pharmacoepidemiology, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA; and William Lawrence MD, MS, Center for Outcomes and Evidence, Agency for Healthcare Research and Quality, Rockville, MD. No one received financial compensation for his contributions.
Project support was obtained from National Cancer Institute (NCI) RC2CA148185-01 (J.C.W.). This project was also funded under Contract No. HHSA290201000006I (D.S) from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the NCI’s Surveillance, Epidemiology and End Results (SEER) Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute.
SEER-Medicare linked data. This study used the linked SEER-Medicare database. This resource has been made available to the research community through collaborative efforts of the NCI and CMS. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the SEER Program tumor registries in the creation and ongoing creation of the SEER-Medicare database.
Role of the Sponsor: The funding agencies had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Disclosures: None of the authors have potential conflicts of interest.
Disclaimers: The views expressed in this article are those of the authors and do not necessarily represent the views of the AHRQ, the NCI, or the Department of Health and Human Services. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, Department of Public Health, the NCI, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Author Contributions: Dr. Schrag had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Zhu, Sharma, Schrag
Acquisition of data: Schrag
Analysis and interpretation of data: Zhu, Sharma, Gray, Chen, Weeks, Schrag
Drafting of the manuscript: Zhu, Schrag
Critical revision of the manuscript for important intellectual content: Sharma, Gray, Chen, Weeks
Statistical analysis: Zhu, Sharma
Obtained funding: Weeks, Schrag
Administrative, technical, or material support: Zhu, Sharma, Weeks, Schrag
Study supervision: Zhu, Schrag
References
- 1.Iannettoni MD. Staging strategies for lung cancer. JAMA. 2010;304(20):2296–2297. doi: 10.1001/jama.2010.1723. [DOI] [PubMed] [Google Scholar]
- 2.NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26(28):4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2191–2197. doi: 10.1200/JCO.2009.25.4052. [DOI] [PubMed] [Google Scholar]
- 4.Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975–2007. [Accessed on August 20, 2011]. http://seer.cancer.gov/csr/1975_2007/ [Google Scholar]
- 5.Gridelli C, Maione P, Rossi A, De Marinis F. The role of bevacizumab in the treatment of non-small cell lung cancer: current indications and future developments. Oncologist. 2007;12(10):1183–1193. doi: 10.1634/theoncologist.12-10-1183. [DOI] [PubMed] [Google Scholar]
- 6.Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus carboplatin and paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12(6):713–718. doi: 10.1634/theoncologist.12-6-713. [DOI] [PubMed] [Google Scholar]
- 8.Yang K, Wang YJ, Chen XR, Chen HN. Effectiveness and safety of bevacizumab for unresectable non-small-cell lung cancer: a meta-analysis. Clin Drug Investig. 2010;30(4):229–241. doi: 10.2165/11532260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol. 2008;26(1):60–65. doi: 10.1200/JCO.2007.13.1144. [DOI] [PubMed] [Google Scholar]
- 10.The Center for Medicare and Medicaid Services. [Accessed on September 25, 2011];Medicare National Coverage Determinations Manual Chapter 1, Part 2 (Sections 90 – 160.26) Coverage Determinations. https://www.cms.gov/manuals/downloads/ncd103c1_part2.pdf.
- 11.National Cancer Institute. [Accessed on May 2, 2011];Surveillance, Epidemiology and End Results. http://seer.cancer.gov.
- 12.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 13.Engels EA, Pfeiffer RM, Ricker W, Wheeler W, Parsons R, Warren JL. Use of surveillance, epidemiology, and end results-medicare data to conduct case-control studies of cancer among the US elderly. Am J Epidemiol. 2011;174(7):860–870. doi: 10.1093/aje/kwr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene FL, Page DL, Balch CM, Fleming ID, Fritz AG. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. 6. New York: Springer; 2002. [Google Scholar]
- 15.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 Suppl):IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 16.Lamont EB, Herndon JE, 2nd, Weeks JC, et al. Criterion validity of Medicare chemotherapy claims in Cancer and Leukemia Group B breast and lung cancer trial participants. J Natl Cancer Inst. 2005;97(14):1080–1083. doi: 10.1093/jnci/dji189. [DOI] [PubMed] [Google Scholar]
- 17.Bach PB, Guadagnoli E, Schrag D, Schussler N, Warren JL. Patient demographic and socioeconomic characteristics in the SEER-Medicare database: applications and limitations. Med Care. 2002;40(8 Suppl):IV-19–25. doi: 10.1097/00005650-200208001-00003. [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 21.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 23.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79(387):516–524. [Google Scholar]
- 24.D’Agostino RB, Jr, D’Agostino RB., Sr Estimating treatment effects using observational data. JAMA. 2007;297(3):314–316. doi: 10.1001/jama.297.3.314. [DOI] [PubMed] [Google Scholar]
- 25.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 26.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23(19):2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 28.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 30.Mitka M. Targeted therapies take aim against lung cancer and melanoma. JAMA. 2010;304(6):624–626. doi: 10.1001/jama.2010.1055. [DOI] [PubMed] [Google Scholar]
- 31.Grusenmeyer PA, Gralla RJ. Examining the cost and cost-effectiveness of adding bevacizumab to carboplatin and paclitaxel in advanced non-small cell lung cancer. J Clin Oncol. 2006;24(Suppl:18S):abstract. [Google Scholar]
- 32.Goulart B, Ramsey S. A trial-based assessment of the cost-utility of bevacizumab and chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer. Value Health. 2011;14(6):836–845. doi: 10.1016/j.jval.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson M, O’Malley AJ, Earle CC, Pakes J, Gaccione P, Newhouse JP. Does reimbursement influence chemotherapy treatment for cancer patients? Health Aff (Millwood) 2006;25(2):437–443. doi: 10.1377/hlthaff.25.2.437. [DOI] [PubMed] [Google Scholar]
- 34.Jatoi A, Hillman S, Stella P, et al. Should elderly non-small-cell lung cancer patients be offered elderly-specific trials? Results of a pooled analysis from the North Central Cancer Treatment Group. J Clin Oncol. 2005;23(36):9113–9119. doi: 10.1200/JCO.2005.03.7465. [DOI] [PubMed] [Google Scholar]
- 35.Gridelli C, Maione P, Rossi A, et al. Treatment of advanced non-small-cell lung cancer in the elderly. Lung Cancer. 2009;66(3):282–286. doi: 10.1016/j.lungcan.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Gridelli C. Treatment of advanced non-small-cell lung cancer in the elderly: from best supportive care to the combination of platin-based chemotherapy and targeted therapies. J Clin Oncol. 2008;26(1):13–15. doi: 10.1200/JCO.2007.14.1820. [DOI] [PubMed] [Google Scholar]
- 37.Merza T, Howard LM, Junagadhwalla M. Exclusions to use of bevacizumab in elderly veterans with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2007;25(Suppl:687S):abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.