Abstract
The multifunctionality of plant annexins and their importance for coordinating development and responses to biotic and abiotic environment have been largely reviewed. We recently described a tobacco annexin, named Ntann12, which is mainly localized in the nucleus of root cells when the plant is grown under light conditions. We also found that auxin and polar auxin transport are essential for Ntann12 accumulation in root cells. Under dark condition, Ntann12 is no longer detected in the root system. In the present addendum, light, regulating auxin signaling, is evidenced as an essential determinant for the synchronization of growth and development between the shoot and the root during light/dark cycle. A speculative model for Ntann12 is described and discussed with regards to relevant literature data.
Keywords: annexin, polar auxin transport, root, nucleus, tobacco, auxin, light
Introduction
Annexins are soluble proteins capable of Ca2+-dependent or independent association with membrane phospholipids. Annexins are thought to be generally involved in the organization of membrane-associated protein networks and in interaction with components of signaling pathways and therefore they are linked to wide range of cellular and developmental processes (for a review see ref. 1). Plant annexins are phylogenetically distinct and differ structurally from their animal counterparts. Essentially, animal annexins consist of usually four times repeated annexin domains and a variable N-terminal region. The annexin domain, of about 70 amino acids, contains the conserved endonexin fold (K-G-X-G-T-{38}-D/E) and is able to bind Ca2+. For plant annexins, typically only the first and fourth repeated annexin domains have the characteristic endonexin sequence (for a review see ref. 2).
Plant annexins, which comprise a multiple family with several members (e.g., eight in Arabidopsis thaliana),3 are expressed throughout the life cycle and are under developmental and environmental controls.4 The functions of these proteins remain poorly understood, and most of what is described on plant annexin activities comes from in vitro studies, including exocytosis, actin binding, peroxydase activity, callose synthase regulation and ion transport (for a review see ref. 5). Plant annexins have also been found to be stimulated by abiotic stress including salinity, cold, oxidative and mechanic stress (for a review see refs 4 and 5). Besides, light has been reported to affect expression of a number of Arabidopsis and tobacco annexins.3,6 Finally, annexin expression has been shown to be upregulated during pathogen attack or symbiotic interaction.7-9 The majority of plant annexins has been localized in the cytosol and this localization appear to be dependent on the tissue/cell type and on the stimulus. However, they have also been found to be associated, or inserted, to both plasma membranes and endomembranes or to be localized to the plant vacuole, the Golgi and Golgi-derived vesicles and the chloroplast (for a review see ref. 10). Although none of reported plant annexins contain known nuclear targeting sequences, a nuclear localization has been reported for some plant annexins including pea,11 Medicago7,12 and tobacco,6 but the mechanism of their nuclear import has not been elucidated yet. The dynamic property of plant annexins, as Ca2+-dependent proteins that bind to membrane phospholipids, together with their expression patterns, i.e. in various organs and induced by several stress, put forward that these proteins are involved in cell signaling processes and therefore in resulting plant developmental adaptation to environmental changes.
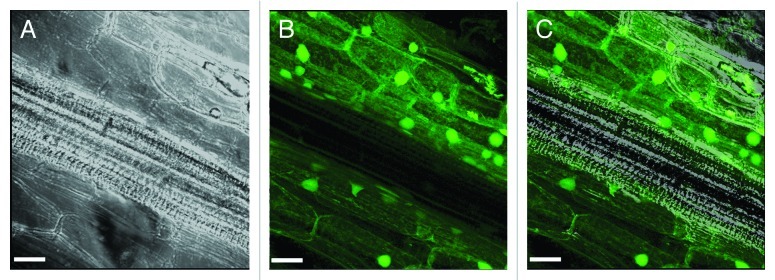
The present addendum focuses on the possible role/function of the tobacco annexin Ntann12 in shoot to root signaling. Ntann12 was identified as a gene induced in tobacco BY-2 cells following infection with the phytopathogenic bacterium Rhodococcus fascians.9 Recently, the biochemical properties of Ntann12 were investigated and the data evidenced the capacity of this protein to bind negatively charged phospholipids in a Ca2+-dependent manner as well as the modulation by Ca2+ concentration of the distribution of the native Ntann12 between cytosol and cell membrane fraction.6 Spatio-temporal analysis revealed that Ntann12 transcript and the corresponding protein are localized within the root system. Besides, pNtann12-GUS transgenic plants indicated that Ntann12 is mainly expressed within the root maturation zone but not in the root cap or in the root elongation zone. At the cellular level, Ntann12 is present in the nucleus (Fig. 1) but also in the cytoplasm of root cells.6
Figure 1. Ntann12 immunolocalization in a tobacco longitudinal root section visualized by fluorescence microscopy. 2D maximum projection of a 32-confocal image stack, corresponding to a root volume of 238.1 µm × 238.1 µm × 57.88 µm. Analysis was done on a Leica SP2 confocal microscope with a 63X objective (n.a. = 1.4); bar = 30 µm. (A) Phase contrast; (B) immunostaining with affinity-purified antiserum against Ntann12; (C) superposition of (A and B) (for Materials and Methods, see ref. 6).
In silico analysis of the 542 bp pNtann12 using PlantPAN13 indicated the occurrence of nine cis-element sequences possibly implicated in light responses, three GT1 consensus sequences and six GATABOX sequences. To clarify the possible role of Ntann12 in light signaling, we designed experiments at the whole plant level and assessed the effect of light and dark conditions on Ntann12 accumulation in tobacco root system. When plants are grown in light conditions, Ntann12 was found to be principally expressed in roots and the corresponding protein was mainly immuno-localized in the nucleus of root cells. In contrast, following plant transfer to darkness for 48 h, Ntann12 expression was inhibited as well as in plants lacking the aerial part and the corresponding protein was no more detectable.6 Altogether, these data suggest that light perceived at the leaves surface is the initial signal activating the expression of Ntann12 in the root system of tobacco plantlets. Since auxin has been associated with several light-regulated processes (for a review see ref. 14), the possibility that auxin could be linked to the signal coming from aerial parts exposed to light was considered and further experiments showed that indole-3-acetic acid (IAA) treatment restored the expression of Ntann12 in the root system in dark condition. Besides, polar auxin transport (PAT) inhibitors such as 1-naphthylphthalamic acid (NPA) or 2,3,5-triiodobenzoic acid (TIBA) inhibited Ntann12 expression in light condition. These results indicate that the expression of Ntann12 and the accumulation of the corresponding polypeptide in the root cells are linked to the perception of light/auxin signaling in the aerial part of the plant that is transmitted to the root via PAT.
Plant Annexin and Light/Auxin Signaling
Plants have evolved light receptors and signaling networks that detect and respond to changes in light intensity and duration and allow plants to adapt and to shape their development according the environmental conditions. Much of this developmental plasticity is achieved by light modulation of auxin levels and auxin signaling systems (for a review see ref. 15). As mentioned above, light has been previously shown to affect the expression of several annexins in Arabidopsis. In hypocotyls, Annat5 expression is increased by red light and this is reversible by application of far red light; in cotyledons, Annat6 has a similar red/far red response.3 These authors suggested that these annexin functions are downstream of phytochrome A. In Mimosa pulvinus, during the nyctinastic movement, the amount of annexin increased at night and the protein was largely cytosolic, while in the morning, when the leaf is held up, it was redistributed to the outermost periphery of the motor cells.16
Auxin plays an important role in controlling root length, lateral root development and root gravitropism (for a review see ref. 17). Auxin is transported through the plant by both passive and active mechanisms and elicits responses in tissues distant from those where it was synthesized. Auxin is actively moved from cell to cell by PAT, a process that is highly regulated by influx and efflux carrier proteins localized on the plasma membrane (for a review see ref. 18). PAT plays crucial roles in the regulation of many aspects of plant growth and development and the inhibition of auxin transport by local application of the PAT inhibitor NPA to the shoot has been shown to inhibit lateral root development.19 The coupling of light and auxin signaling appears to be essential in the regulation of plant development but the precise network in which they are involved has still to be deciphered.
Annexins and Ca2+-Dependent MAPK Signaling
Several phosphorylation sites have been found and experimentally verified for Arabidopsis annexins (for a review see ref. 20) and similarly some putative phosphorylation sites are present in the deduced polypeptide sequence of Ntann12. The occurrence of these phosphorylation sites may regulate binding to other proteins. Accordingly, by using a tandem-affinity purification approach, Rohila et al.21 identified a rice annexin, Os05 g31750, homolog to AnnAt4, that was associated with several complexes involving various protein kinases. The first complex contains a MAPKKKK sterile-20 (Ste20)-like kinase (Os10 g37480), and a putative MAPKKK WNK1 (Os07 g38530). The second complex contains a casein kinase (Os01 g38950), a putative methyltransferase (Os01 g49250) and a MAPK (Os08 g06060). The third complex contains a calcium/calmodulin-dependent protein kinase (Os01 g64970), a RNA recognition motif containing protein (Os02 g12850) and a putative nucleic acid binding protein (Os03 g52490). The fourth complex contains 13 proteins including a receptor-like kinase (Os01 g02580) and two 14-3-3 proteins.21 The association of annexin and MAPKKK indicates that these proteins may be involved in membrane-associated, Ca2+-dependent MAPK signaling.21
Possible Function of Ntann12 in Shoot to Root Signaling
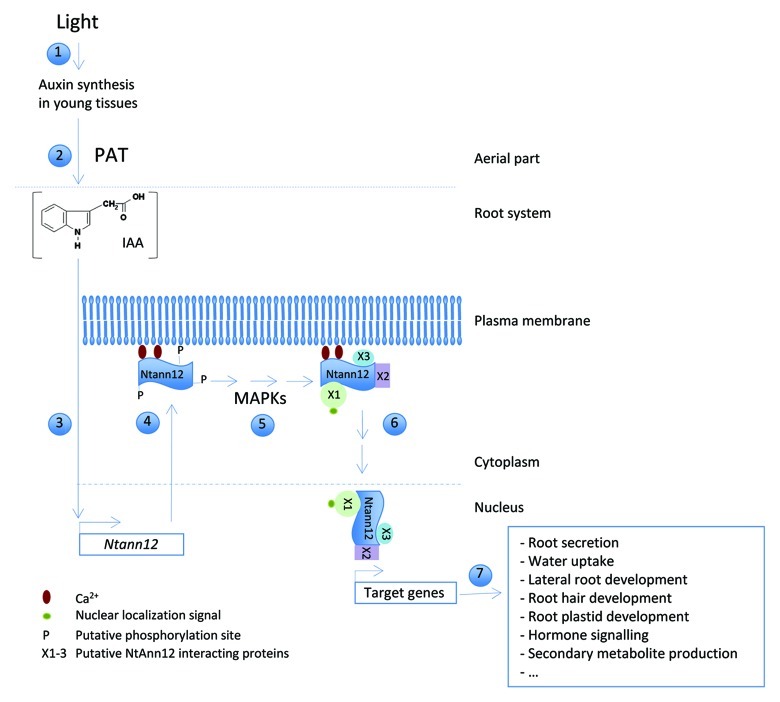
A speculative model for Ntann12 function, modulated by auxin accumulation in root, is suggested in Figure 2. Upon light perception in the plant aerial part, auxin synthesis is induced in young leaves. As a mobile morphogen, auxin can travel from shoot to root system thanks to PAT. This mechanism is essential for synchronization of growth and development between the shoot and the root.22 Auxin accumulation in root cells induces Ntann12 expression; this is supported by the induction of Ntann12 expression in root system following auxin treatment and by the occurrence of an ARF binding motif in the Ntann12 promoter.6 Auxin accumulation was shown to induce an increase in the level of cytosolic Ca2+ in various corn and parsley tissues within minutes after its application.23 Therefore it can be assumed that intracellular Ca2+ mobilization induced by accumulation of auxins in root cells triggers recruitment of Ntann12 to negatively charged phospholipids in a Ca2+-dependent manner.
Figure 2. Ntann12 function in tobacco root: a speculative model. (1) Light induces auxin synthesis in young tissues of the aerial part. (2) Auxin moves from shoot to root system via the PAT. (3) Auxin accumulation in root cells induces Ntann12 expression. (4) The increase of the level of cytosolic Ca2+ induced by auxin accumulation triggers recruitment of Ntann12 to negatively charged phospholipids. (5) Ntann12 interacts with other proteins (X1–X3) possibly via a phosphorylation-dependent regulation. (6) The protein complex reaches the cell nucleus. (7) The nuclear Ntann12 protein-complex may acts as a transcription factor to induce the expression of target genes that are required for the functioning of root system in daylight condition.
Ntann12 contains several putative phosphorylation sites and similarly to what it was described for the rice annexin Os05 g31750, possible interaction of Ntann12 with other proteins, subject to phosphorylation-dependent regulation, might be suspected. Alternatively, Ntann12 may promote other proteins to bind tightly to a membrane, forming a ‘multidomain cooperation’.5 Since in root cells, Ntann12 is mainly localized in the nucleus, it is tempting to speculate that at least one of the Ntann12-protein partner’s possess a nuclear localization signal that help the complex to reach the cell nucleus. Another possibility is that Ntann12 could be modified by ubiquitination and/or sumoylation, which are involved in nucleo-cytoplasmic trafficking (for a review see ref. 24); in accordance, the bovine annexin A1 present in the nuclei was mostly modified with sumo or ubiquitin.25 Finally, the nuclear Ntann12 protein-complex as a whole, or partially, may act as a transcription factor to induce the transcription of target genes necessary for the root system to perform required biochemical, physiological as well as developmental processes during plant exposure to daylight. Such hypothesis is supported by several reports on light-regulated developmental processes in root system. For instance, root secretion of some phytochemicals in Arabidopsis is affected by light/dark cycles.26 Besides, annexins of Arabidopsis seedlings grown in continuous light have been immunolocalized in secretory cells in roots including outer root cap cells and root hairs.27 In addition, in maize roots, the xylem water flux sustained by root pressure occurs during the day and is less important at night.28 More generally, gene profiling of the red light signaling pathways in Arabidopsis roots performed by Molas et al.29 allowed the identification of several factors acting downstream of phytochromes in red-light signaling among which genes involved in lateral root and root hair formation, root plastid development, hormone signaling and phenylpropanoid metabolism.
Concluding Remarks and Prospects
The structural, functional and biochemical properties of annexins and their importance for coordinating development and responses to biotic and abiotic stress have been largely reviewed. To the best of our knowledge, Ntann12 is the first described plant annexin specifically induced in root cells when the plant is exposed to light. Because of the occurrence of light boxes in the Ntann12 promoter, light was initially suspected as the triggering signal for Ntann12 expression. However according to our presented data, it is obvious that light modulation of auxin level via PAT is the effective direct mediator of Ntann12 expression in root cells. This is in agreement with the induction of this gene expression in tobacco leaves incubated in auxin solution6 or infected by R. fascians,9 a bacterium known to produce and secrete auxin upon interaction with plant cells.30 The hypothesis described in this addendum is that Ntann12 would be involved in the sensing in the root of the day/night switch and in the activation of the expression of genes essential for root function. The identification of the proteins or proteins complexes that are possibly associated to Ntann12, and/or the possible modification of this protein by ubiquitination and/or sumoylation will permit to further decipher the suggested role of Ntann12 in the shoot to root signaling.
Acknowledgments
M.B. is Senior Research Associate of the FRS-FNRS (Belgian Fund for Scientific Research). The authors thank Marjorie Vermeersch for technical assistance.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19647
References
- 1.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–61. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 2.Moss SE, Morgan RO. The annexins. Genome Biol. 2004;5:219. doi: 10.1186/gb-2004-5-4-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantero A, Barthakur S, Bushart TJ, Chou S, Morgan RO, Fernandez MP, et al. Expression profiling of the Arabidopsis annexin gene family during germination, de-etiolation and abiotic stress. Plant Physiol Biochem. 2006;44:13–24. doi: 10.1016/j.plaphy.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Mortimer JC, Laohavisit A, Macpherson N, Webb A, Brownlee C, Battey NH, et al. Annexins: multifunctional components of growth and adaptation. J Exp Bot. 2008;59:533–44. doi: 10.1093/jxb/erm344. [DOI] [PubMed] [Google Scholar]
- 5.Laohavisit A, Davies JM. Annexins. New Phytol. 2011;189:40–53. doi: 10.1111/j.1469-8137.2010.03533.x. [DOI] [PubMed] [Google Scholar]
- 6.Baucher M, Oukouomi Lowe Y, Vandeputte OM, Mukoko Bopopi J, Moussawi J, Vermeersch M, et al. Ntann12 annexin expression is induced by auxin in tobacco roots. J Exp Bot. 2011;62:4055–65. doi: 10.1093/jxb/err112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Carvalho Niebel F, Lescure N, Cullimore JV, Gamas P. The Medicago truncatula MtAnn1 gene encoding an annexin is induced by Nod factors and during the symbiotic interaction with Rhizobium meliloti. Mol Plant Microbe Interact. 1998;11:504–13. doi: 10.1094/MPMI.1998.11.6.504. [DOI] [PubMed] [Google Scholar]
- 8.Manthey K, Krajinski F, Hohnjec N, Firnhaber C, Pühler A, Perlick AM, et al. Transcriptome profiling in root nodules and arbuscular mycorrhiza identifies a collection of novel genes induced during Medicago truncatula root endosymbioses. Mol Plant Microbe Interact. 2004;17:1063–77. doi: 10.1094/MPMI.2004.17.10.1063. [DOI] [PubMed] [Google Scholar]
- 9.Vandeputte O, Lowe YO, Burssens S, van Raemdonck D, Hutin D, Boniver D, et al. The tobacco Ntann12 gene, encoding an annexin, is induced upon Rhodoccocus fascians infection and during leafy gall development. Mol Plant Pathol. 2007;8:185–94. doi: 10.1111/j.1364-3703.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- 10.Laohavisit A, Davies JM. Multifunctional annexins. Plant Sci. 2009;177:532–9. doi: 10.1016/j.plantsci.2009.09.008. [DOI] [Google Scholar]
- 11.Clark GB, Dauwalder M, Roux SJ. Immunological and biochemical evidence for nuclear localization of annexin in peas. Plant Physiol Biochem. 1998;36:621–7. doi: 10.1016/S0981-9428(98)80010-7. [DOI] [PubMed] [Google Scholar]
- 12.Kovács I, Ayaydin F, Oberschall A, Ipacs I, Bottka S, Pongor S, et al. Immunolocalization of a novel annexin-like protein encoded by a stress and abscisic acid responsive gene in alfalfa. Plant J. 1998;15:185–97. doi: 10.1046/j.1365-313X.1998.00194.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang W-C, Lee TY, Huang H-D, Huang H-Y, Pan R-L. PlantPAN: Plant promoter analysis navigator, for identifying combinatorial cis -regulatory elements with distance constraint in plant gene groups. BMC Genomics. 2008;9:561. doi: 10.1186/1471-2164-9-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swarup R, Parry G, Graham N, Allen T, Bennett M. Auxin cross-talk: integration of signalling pathways to control plant development. Plant Mol Biol. 2002;49:411–26. doi: 10.1023/A:1015250929138. [DOI] [PubMed] [Google Scholar]
- 15.Halliday KJ, Martínez-García JF, Josse E-M. Integration of light and auxin signaling. Cold Spring Harb Perspect Biol. 2009;1:a001586. doi: 10.1101/cshperspect.a001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshino D, Hayashi A, Temmei Y, Kanzawa N, Tsuchiya T. Biochemical and immunohistochemical characterization of Mimosa annexin. Planta. 2004;219:867–75. doi: 10.1007/s00425-004-1285-7. [DOI] [PubMed] [Google Scholar]
- 17.Overvoorde P, Fukaki H, Beeckman T. Auxin control of root development. Cold Spring Harb Perspect Biol. 2010;2:a001537. doi: 10.1101/cshperspect.a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanneste S, Friml J. Auxin: a trigger for change in plant development. Cell. 2009;136:1005–16. doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Reed RC, Brady SR, Muday GK. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 1998;118:1369–78. doi: 10.1104/pp.118.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konopka-Postupolska D, Clark G, Hofmann A. Structure, function and membrane interactions of plant annexins: an update. Plant Sci. 2011;181:230–41. doi: 10.1016/j.plantsci.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Rohila JS, Chen M, Chen S, Chen J, Cerny R, Dardick C, et al. Protein-protein interactions of tandem affinity purification-tagged protein kinases in rice. Plant J. 2006;46:1–13. doi: 10.1111/j.1365-313X.2006.02671.x. [DOI] [PubMed] [Google Scholar]
- 22.Robert HS, Friml J. Auxin and other signals on the move in plants. Nat Chem Biol. 2009;5:325–32. doi: 10.1038/nchembio.170. [DOI] [PubMed] [Google Scholar]
- 23.Gehring CA, Irving HR, Parish RW. Effects of auxin and abscisic acid on cytosolic calcium and pH in plant cells. Proc Natl Acad Sci U S A. 1990;87:9645–9. doi: 10.1073/pnas.87.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YE, Pernet O, Lee B. Regulation of the nucleocytoplasmic trafficking of viral and cellular proteins by ubiquitin and small ubiquitin-related modifiers. Biol Cell. 2012;104:121–38. doi: 10.1111/boc.201100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirata F, Thibodeau LM, Hirata A. Ubiquitination and SUMOylation of annexin A1 and helicase activity. Biochim Biophys Acta. 2010;1800:899–905. doi: 10.1016/j.bbagen.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Badri DV, Loyola-Vargas VM, Broeckling CD, Vivanco JM. Root secretion of phytochemicals in Arabidopsis is predominantly not influenced by diurnal rhythms. Mol Plant. 2010;3:491–8. doi: 10.1093/mp/ssq004. [DOI] [PubMed] [Google Scholar]
- 27.Clark GB, Lee D, Dauwalder M, Roux SJ. Immunolocalization and histochemical evidence for the association of two different Arabidopsis annexins with secretion during early seedling growth and development. Planta. 2005;220:621–31. doi: 10.1007/s00425-004-1374-7. [DOI] [PubMed] [Google Scholar]
- 28.Lopez F, Bousser A, Sissoëff I, Gaspar M, Lachaise B, Hoarau J, et al. Diurnal regulation of water transport and aquaporin gene expression in maize roots: contribution of PIP2 proteins. Plant Cell Physiol. 2003;44:1384–95. doi: 10.1093/pcp/pcg168. [DOI] [PubMed] [Google Scholar]
- 29.Molas ML, Kiss JZ, Correll MJ. Gene profiling of the red light signalling pathways in roots. J Exp Bot. 2006;57:3217–29. doi: 10.1093/jxb/erl086. [DOI] [PubMed] [Google Scholar]
- 30.Vandeputte O, Öden S, Mol A, Vereecke D, Goethals K, El Jaziri M, et al. Biosynthesis of auxin by the gram-positive phytopathogen Rhodococcus fascians is controlled by compounds specific to infected plant tissues. Appl Environ Microbiol. 2005;71:1169–77. doi: 10.1128/AEM.71.3.1169-1177.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]