Abstract
BRCA1, a product of a familial breast and ovarian cancer susceptibility gene, localizes to centrosomes and physically interacts with γ-tubulin, a key centrosomal protein for microtubule nucleation and anchoring at centrosomes. Here, we performed a rigorous analysis of centrosome localization of BRCA1, and found that BRCA1 is specifically associated with mother centrioles in unduplicated centrosomes, and daughter centrioles acquire BRCA1 prior to initiation of duplication, and thus duplicated centrosomes are both bound by BRCA1. We further found that BRCA1 suppresses centrosomal aster formation. In addition, we identified a new domain of BRCA1 critical for γ-tubulin binding, which confers not only its localization to centrosomes, but also its activity to suppress centrosomal aster formation.
Keywords: BRCA1, centrosome, centriole, microtubules, aster formation
Introduction
BRCA1 is a product of a familial breast and ovarian cancer susceptibility gene1 and is mutated in 5–10% of the total breast cancer incidence.2 BRCA1 participates in various cellular functions, including transcription,3,4 DNA damage repair5-8 and protein ubiquitination.9,10 BRCA1 has also been implicated in the numeral homeostasis of centrosomes and the centrosome-based microtubule (MT) organization.11-13 The centrosome is a small non-membranous organelle composed of a pair of centrioles and surrounding proteins referred to as pericentriolar material (PCM). Some PCM proteins localize to centrosomes transiently during the cell cycle, while some continuously localize to centrosomes. The centrioles in the pair are structurally different from each other, one with a set of appendages at the distal ends (mother centriole) and another without them (daughter centriole). The centrosome, like DNA, duplicates once in each cell cycle: it initiates duplication at G1-S transition by physical separation of mother and daughter centrioles, followed by synthesis of procentrioles near the preexisting centrioles. During S and G2, duplicated centrosomes continue to mature and by late G2, two mature centrosomes are generated.14,15 Disruption of the regulatory mechanisms of the cell cycle-dependent duplication of the centrosome can lead re-duplication of centrosomes, resulting in generation of more than two centrosomes (centrosome amplification). Centrosome amplification increases the frequency of defective mitoses and chromosome segregation errors and is one of the major causes of chromosome instability in cancers.16,17 BRCA1 has been shown to suppress centrosome duplication partly through its ubiquitin ligase activity in association with BARD1,18,19 and thus loss or functional inactivation of BRCA1 leads to a high frequency of centrosome amplification.11
The primary function of the centrosome is to organize MTs, including interphase cytoplasmic MT network and formation of mitotic spindles. The centrosome-based organization of MTs involves several distinct processes, including nucleation, anchoring and elongation/release of MTs. MT nucleation is initiated by the γ-tubulin ring complex (γTuRC), a multi-protein complex containing γ-tubulin.20-22 The MT anchoring complex at the sub-distal appendages captures the MT nucleated γTuRC to form and maintain the radial MT arrays (asters).23 Thus, although MT nucleation occurs near mother and daughter centrioles, the MT aster is formed primarily on mother centrioles.24 BRCA1 has been shown to be involved in the control of the centrosome-based MT dynamics and mitotic spindle assembly via physical interaction with γ-tubulin and spindle-pole proteins such as TPX2, NuMA and XRHAMM.12,13
Mutational loss or inactivation of BRCA1 is expected to promote tumor progression not only through induction of centrosome amplification, but also through disruption of MT dynamics. In high-grade breast tumors, the tissue architecture is severely disrupted, which lays a favorable ground for tumor cells to expand. Because the tissue architecture is established and maintained by the proper polarity of the cells within the tissue, and the centrosome plays an essential role for properly polarizing cells, numeral integrity and controlled activity of the centrosome are critical for establishment and maintenance of the tissue architecture. Indeed, in those breast tumors, centrosome amplification as well as abnormal arrays of MTs are frequently observed.25 Consistently, the recent network modeling to identify genes potentially associated with higher risk of breast cancer has revealed the significant association between breast cancer susceptibility and centrosome dysfunction.26
Several studies have shown that BRCA1 localizes to centrosomes,27-29 suggesting that BRCA1 may control MT dynamics and/or centrosome number at the centrosome. However, centrosomal localization of BRCA1 was later challenged by one group: through the examination of exogenously introduced fluorescent protein-tagged BRCA1, they have concluded that BRCA1 does not localize to centrosomes.30 The question of whether BRCA1 localizes to centrosomes had not been resolved. Here, we undertook a rigorous analysis of centrosome localization of BRCA1. We found that BRCA1 localized specifically to mother centrioles of unduplicated centrosomes and with daughter centrioles prior to initiation of centrosome duplication. It has been shown that BRCA1 binds to γ-tubulin through a.a. 500–800.31 We identified the second γ-tubulin-interacting domain (a.a. 802–1002), and this region confers centrosome localization of BRCA1 during interphase. Moreover, BRCA1 possesses an activity to suppress aster formation at interphase centrosomes, and the newly identified γ-tubulin-interacting domain is required and sufficient for this activity.
Results
BRCA1 binds to both mitotic and interphase centrosomes
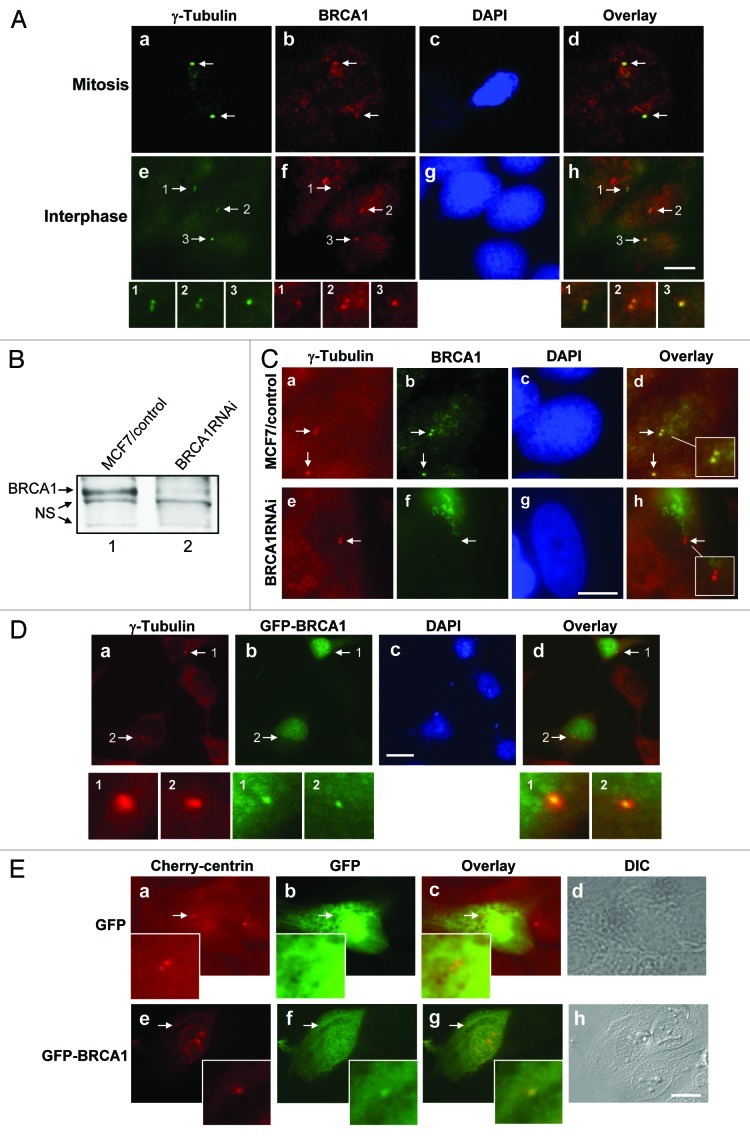
Because centrosomal localization of BRCA1 had been continuously debated due to the conflicting data published by different laboratories,27-30 we undertook a careful examination of whether BRCA1 localized to centrosomes. We used MCF7 cells that display several characteristics of differentiated mammary epithelium retaining both BRCA1 and p53 in their wild type (wt) forms.5,32 We first examined the centrosome localization of BRCA1 in MCF7 cells by immunostaining with anti-γ-tubulin and several commercially available anti-BRCA1 antibodies. As previously reported, the anti-BRCA1 antibody Ab-2, which was raised against a.a. 768–793, detected BRCA1 at mitotic (Fig. 1A, a–d) as well as interphase centrosomes (e–h). The anti-BRCA1 antibody that was raised against a.a.1–300 (Ab-1) failed to detect BRCA1 at centrosomes, suggesting that the epitope (a.a. 1–300) may be not accessible by the antibody (data not shown). The anti-BRCA1 antibody C-20, which was raised against a.a. 1844–1863, detected BRCA1 at centrosomes in both interphase and mitotic cells (data not shown).
Figure 1. Centrosome localization BRCA1. (A) MCF7 cells were pre-extracted, fixed and co-immunostained for centrosomes (anti-γ-tubulin antibody) and BRCA1 [anti-BRCA1 antibody (Ab-2)], and stained for DNA with DAPI. Mitotic cell (a‒d) and interphase cell with duplicated centrosomes (e-h) are shown. Arrows point to the centrosomes. Magnified images of the indicated areas are shown. Scale bar, 10 μm. (B) MCF7 cells were co-transfected with siRNA targeting BRCA1 or a randomized sequence control together with a drug selection marker plasmid. The cells sub-cloned after drug selection were examined for BRCA1 expression. NS; non-specific protein bands. (C) BRCA1RNAi and MCF7/control cells were co-immunostained with anti-γ-tubulin and anti-BRCA1 antibodies. Arrows point to the centrosomes. The insets show the magnified images of the indicated areas. Scale bar, 10 μm. (D) HeLa cells were transiently transfected with GFP-wt BRCA1, and immunostained with anti-γ-tubulin and anti-GFP antibodies. Arrows point to the centrosomes. Panels 1 and 2 show the magnified images of the indicated areas. Scale bar, 20 μm. (E) HeLa cells expressing Cherry centrin were transfected with either GFP or GFP-wt BRCA1. At 24 h post-transfection, cells were examined by fluorescent microscopy. Arrows point to the positions of centrosomes, and insets show the magnified images of the indicated areas. Scale bar, 10 μm.
To test the specificity of the Ab-2 anti-BRCA1 antibody, we generated MCF7 cells silenced for endogenous expression of BRCA1 by transfecting a small interfering RNA (siRNA) sequence targeting BRCA1 along with a drug selection marker plasmid. A randomized sequence was used as a control. The drug-resistant colonies were sub-cloned and examined for BRCA1 expression (Fig. 1B). BRCA1 expression was successfully silenced to < 10% of the normal levels. We co-immunostained MCF7/control and BRCA1RNAi cells with anti-γ-tubulin and anti-BRCA1 (Ab-2) antibodies (Fig. 1C). The anti-BRCA1 antibody-reactive signal was readily detected at centrosomes in MCF7/control cells (a–d). In contrast, the anti-BRCA1 antibody-reactive signal was no longer detected at centrosomes in BRCA1RNAi cells (e–h), demonstrating the specificity of the anti-BRCA1 antibody.
To further exclude the possibility that the centrosomal localization of BRCA1 is the artifact due to the non-specific association of the anti-BRCA1 antibody to centrosomes, we transiently transfected green fluorescent protein (GFP)-tagged BRCA1 into cells. The transfected cells were pre-extracted and co-immunostained with anti-γ-tubulin and anti-GFP antibodies (Fig. 1D). GFP-BRCA1 was clearly detected at centrosomes, further demonstrating the centrosomal localization of BRCA1.
We next transiently transfected cells expressing the red (cherry) fluorescent protein (CFP)-tagged centrin, a known centriole marker,33 with GFP-BRCA1, and the transfected cells were directly examined by fluorescent microscopy. Although centrosomal localization of GFP-BRCA1 was less clear by live cell imaging than immunostaining with a pre-extraction procedure due to the autofluorescent activity of non-centrosomal GFP-BRCA1, GFP-BRCA1 was readily detected at centrosomes in interphase cells (Fig. 1E, e–h). Centrosomal localization of GFP-BRCA1 in live cells during mitosis could not be determined, because no GFP-BRCA1 positive cells proceeded into mitosis due to the toxicity associated with overexpression of wt BRCA1.
BRCA1 binds specifically to mother centrioles
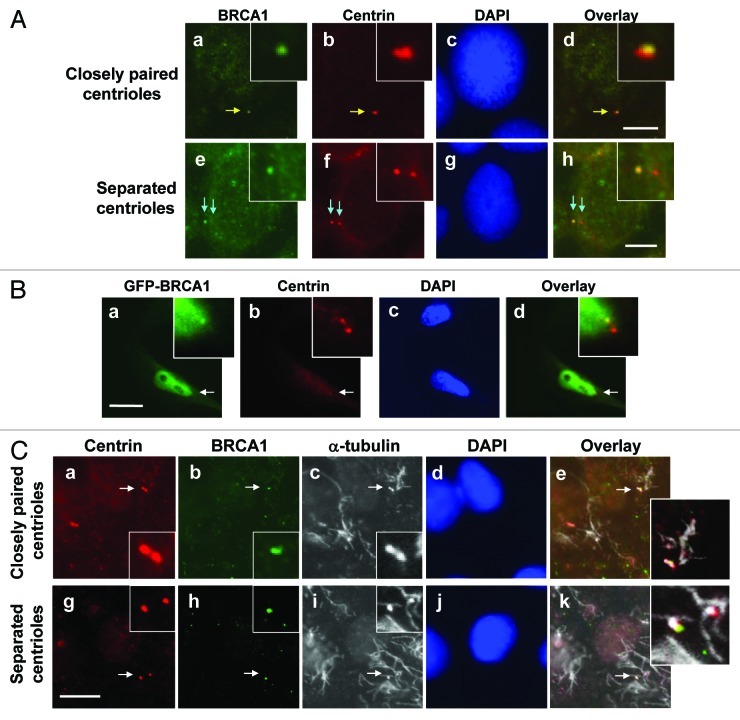
To further characterize the centrosomal localization of BRCA1 in interphase cells, we co-immunostained serum-starved MCF7 cells with anti-centrin (for centrioles) and anti-BRCA1 (Ab-2) antibodies. Under a serum-deprived condition, the majority (80–90%) of cells contained unduplicated centrosomes, among which ~50% of centrioles were closely paired, while ~50% were physically separated by a readily discernible distance. In the cells with closely paired centrioles, dot signals detected by anti-BRCA1 antibody were, in most cases, found more closely associated with one of the paired centrioles rather than uniformly associated with both centrioles (Fig. 2A, a–d). In the cells with physically separated centrioles, BRCA1 was found at only one of the two centrioles (e–h). We also examined the serum-starved cells transiently transfected with GFP-BRCA1, and found that GFP-BRCA1 also localized exclusively to one of the paired centrioles (Fig. 2B).
Figure 2. Characterization of centrosome localization of BRCA1. MCF7 cells were co-immunostained for BRCA1 [anti-BRCA1 antibody (Ab-2)] and centrioles (anti-centrin antibody) (A). Yellow arrows point to the closely paired centrioles (a‒d), and blue arrows point to the separated centrioles (e‒h). The insets show the magnified images of the indicated areas. Scale bar, 8 μm. (B) HeLa cells were transfected with GFP-wt BRCA1, and immunostained with anti-GFP and anti-γ-tubulin antibodies. Arrows point to the centrosomes, and insets show the magnified images of the indicated areas. Scale bar, 10 μm. (C) G1 phase MCF7 cells enriched by serum-starvation followed by serum-stimulation for 8 h were treated with nocodazole for 4 h. Cells were briefly pre-extracted, fixed, and co-immunostained with mouse anti-centrin and rabbit anti-BRCA1 antibodies. Antibody-antigen complexes were detected with Alexa fluor 488-conjugated anti-rabbit IgG (green) and Alexa fluor 594-conjugated anti-mouse IgG (red) antibodies. MTs (and centrioles) were then immunostained using mouse anti-α-tubulin antibody, followed by Alexa fluor 680-conjugated anti-mouse IgG antibody. Although the Alexa fluor 680-anti-mouse IgG antibody detects both mouse anti-centrin and mouse anti-α-tubulin antibodies, it does not interfere with the data analysis. Arrows point to the position of the centrioles, and insets show the magnified image of the indicated areas. Scale bar, 10 μm.
Proteins that localize to centrosomes in a MT-dependent manner usually reside at the periphery of centrosomes, while those localizing to centrosomes in a MT-independent manner are usually found at close proximity to centrioles.34 We thus tested the MT dependency of centrosomal association of BRCA1. MCF7 cells were treated with nocodazole, to depolymerize MTs, and immunostained for centrioles (Fig. 2C, a and g), BRCA1 (b and h) and MTs (c and i). Under the MT-depolymerized condition, BRCA1 remained associated with the centrosomes when centrioles are closely paired (Fig. 2C, a–e) and with one of the centrioles when centrioles were separated (g–k). Thus, BRCA1 localizes to interphase centrosomes in a MT-independent manner, suggesting that BRCA1 localizes in the proximity of the centrioles.
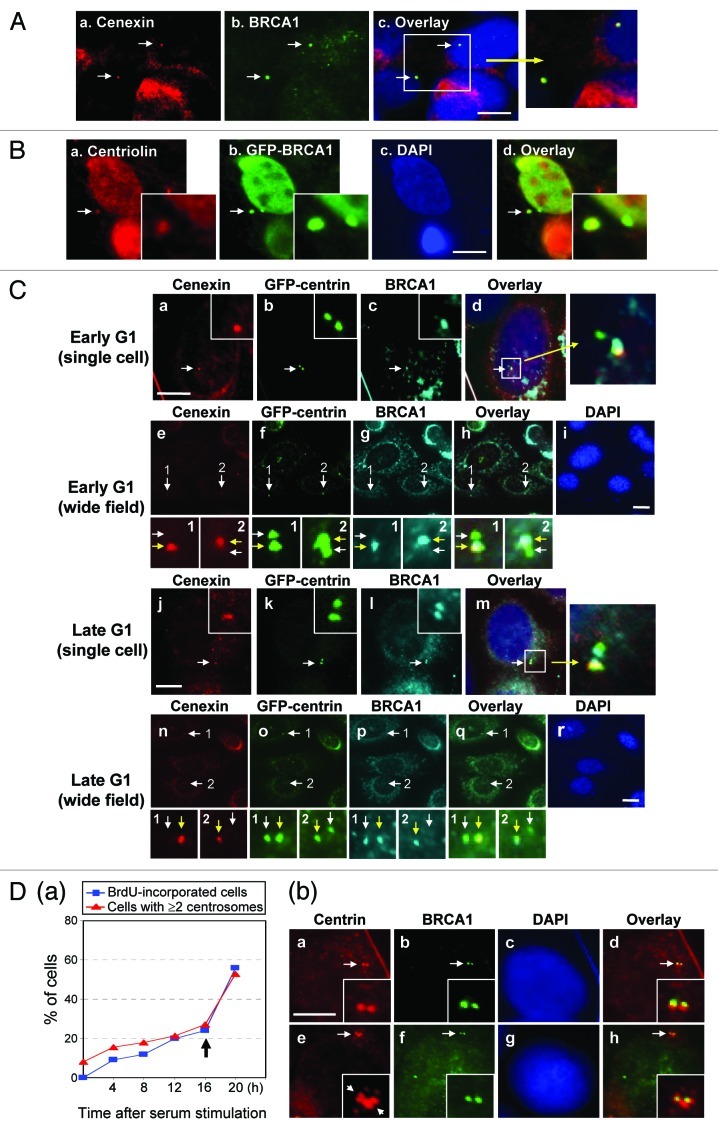
To test whether BRCA1 specifically localizes to either a mother or daughter centriole, we co-immunostained MCF7 cells for BRCA1 and cenexin, a protein specifically present at the mature mother centriole that is acquired by the daughter centriole at G2/M.35 The signals detected by anti-BRCA1 antibody overlapped with those detected by anti-cenexin antibody, indicating that BRCA1 specifically associates with mother centrioles (Fig. 3A). We also tested the centrosomal localization of GFP-BRCA1 by co-immunostaining GFP and centriolin, another protein known to specifically associate with mother centrioles.36 We found that GFP-BRCA1 co-localized with centriolin (Fig. 3B), further indicating that BRCA1 specifically localizes to mother centrioles.
Figure 3. BRCA1 specifically associates with mother centrioles in unduplicated centrosomes. (A) MCF7 cells in G1 phase were co-immunostained for cenexin and BRCA1. Arrows point to the position of the cenexin-positive mature centriole. The panel on the right shows the magnified image of the indicated area. Scale bar, 5 μm. (B) HeLa cells transfected with GFP-wt BRCA1 were immunostained with anti-centriolin and anti-GFP antibodies. Arrows point to the position of the centriolin-positive mature centriole. Insets show the magnified images of the indicated areas. Scale bar, 5 μm. (C) MCF7/GFP-centrin cells in early and late G1 were co-immunostained for cenexin and BRCA1. Panels e-i and n-r show the wide field immunostaining images. Panels 1 and 2 show the magnified images of the indicated areas, in which white arrows point to the daughter centrioles, and yellow arrows point to the mother centrioles. Scale bar in panels (a–d) and (j‒m), 8 μm, and in panels (e‒i) and (n‒r), 10 μm. (D) MCF7 cells were serum-starved for 36 h, and serum-stimulated in the presence of BrdU. At indicated time points, cells were immunostained for incorporated BrdU and centrioles, and the rates of BrdU-incorporation and centrosome duplication were determined from > 100 cells (a). MCF7 cells after 16 h serum-stimulation were co-immunostained for centrin and BRCA1 (b). Arrows in the inset of panel e point to duplicated centrioles. Scale bar, 8 μm.
We next examined the centrosome localization kinetics of BRCA1 during the centrosome duplication cycle. MCF7 cells stably expressing GFP-centrin (MCF7/GFPcentrin cells) were synchronized for cell cycling by serum starvation followed by serum stimulation. At early and late G1, cells were co-immunostained for cenexin and BRCA1 (Fig. 3C). When a centriole pair were physically separated, yet had not initiated centriole duplication as seen in early G1 cells, we found that BRCA1 co-localized with cenexin at mother centrioles (Fig. 3C, a–i). As it is known that the mother centriole normally initiates procentriole formation before the daughter centriole,36 we found that the cenexin-positive centriole always initiates procentriole formation before the cenexin-negative centriole (Fig. 3C, j–r, a yellow arrow points to the centriole that has initiated procentriole formation). However, BRCA1 was found on both mother and daughter centrioles (l and p), suggesting that the daughter centriole acquired BRCA1 prior to the initiation of procentriole formation.
At 16 h after serum stimulation, ~20% of synchronized MCF7 cells underwent centrosome duplication, and ~60% of cells duplicated centrosomes by 20 h (Fig. 3D, left graph). Thus, serum stimulation for 16 h should enrich cells with centrosomes that were immediately before or after initiation of duplication. Co-immunostaining of centrin and BRCA1 in the serum-stimulated cells for 16 h showed that both centrioles of a pair that had physically separated from each other, but not initiated procentriole formation, were bound by BRCA1 (Fig. 3D, a–d). Moreover, BRCA1 was found at all duplicated centrosomes examined (e–h). Thus, BRCA1 localizes to daughter centrioles prior to procentriole formation, hence duplicated centrosomes are both bound by BRCA1.
BRCA1 suppresses centrosomal aster formation
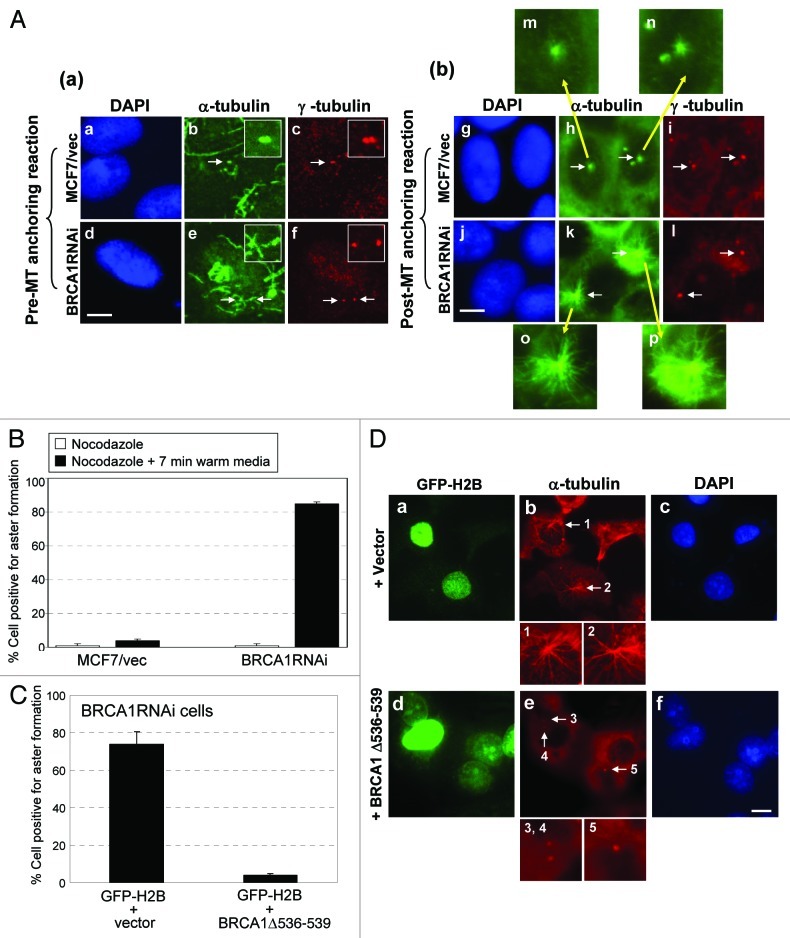
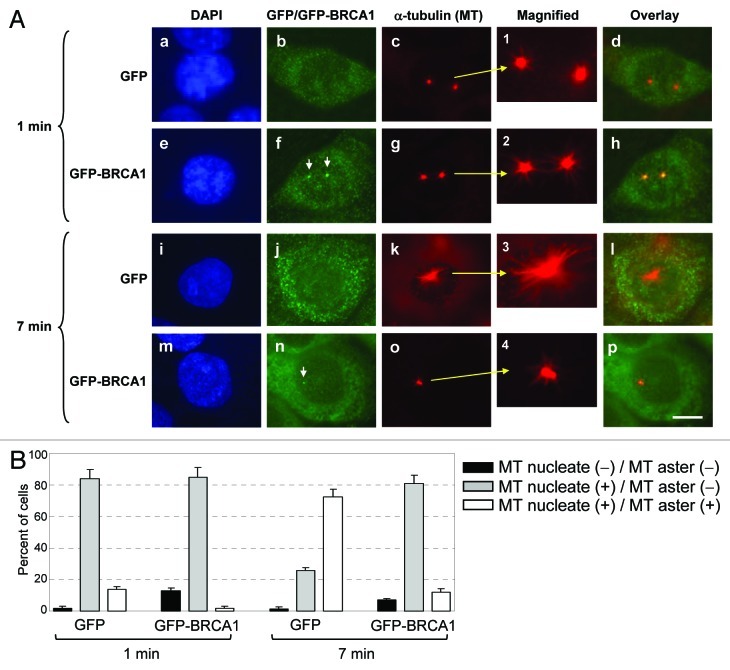
Involvement of BRCA1 in the MT organizing activity of centrosomes has previously been shown.12,13 The centrosome-based MT organization proceeds through several steps: MT nucleation is initiated by the γTuRC, and then the MT anchoring complex at the sub-distal appendages, which is present only at mother centrioles, anchors the MT-nucleated γTuRCs. The nucleated MTs then elongate to form radial MT arrays (asters).23 Thus, although MT nucleation occurs at both mother and daughter centrioles, the MT aster is formed primarily on mother centrioles.24 Because BRCA1 specifically associates with mother centrioles, we tested whether BRCA1 is involved in the regulation of centrosomal aster formation. The BRCA1RNAi and control cells (see Fig. 1B) were subjected to the aster formation assay; cells were first treated with nocodazole on ice to completely depolymerize interphase MTs (Fig. 4A, a–f). After removal of nocodazole, cells were incubated in fresh warm media for 7 min, and the ability of the centrosome to nucleate, anchor and elongate MTs was determined by co-immunostaining for either centrosomes (anti-γ-tubulin or anti-centrin antibody) and MTs (anti-α-tubulin antibody). The aster forming activity of centrosomes was assessed according to the previously established protocol,37 in which the centrosomes with > 30 MTs (> 4 μm long) were scored as positive for aster formation. MCF7/control cells showed a low frequency of aster formation (< 5%) (Fig. 4A, g–i and 4B), while the majority (> 85%) of BRCA1RNAi cells were positive for aster formation (Fig. 4A, j–l and 4B). Wide-field immunostaining images are shown in Figure S1. To exclude the possibility of the non-specific activity of the siRNA used for silencing BRCA1, we transiently re-introduced BRCA1 that lacks the sequence targeted by the siRNA (BRCA1Δ547–551). Re-introduction of BRCA1 restored the aster formation suppressing activity in BRCA1RNAi cells (Fig. 4C and D), demonstrating that the observed effect on centrosomal aster formation in BRCA1RNAi cells is caused by silencing of BRCA1 expression. We thus concluded that BRCA1 possesses the activity to suppress centrosomal aster formation.
Figure 4. BRCA1 suppresses centrosomal aster formation. (A) MCF7/control and BRCA1RNAi cells were treated with nocodazole on ice, and co-immunostained for MTs with anti-α-tubulin antibody and centrosomes with anti-γ-tubulin antibody (a). Arrows point to the positions of centrosomes. Insets show magnified images of the indicated areas. MCF7/control and BRCA1RNAi cells in parallel cultures were incubated in fresh warm media for 7 min to allow for MT re-growth. Cells were then co-immunostained for MTs and centrosomes (b). Arrows point to the positions of centrosomes. Panels m-p show magnified images of the indicated areas. Scale bar, 10 μm. The centrosomal aster formation in MCF7/control and BRCA1RNAi cells were assessed as positive if centrosomes had a MT aster with greater than 30 MTs (> 4 μm long). The results are shown in (B) as the average ± standard error (S.E). from three experiments. For each experiment, > 200 cells were examined. (C) BRCA1RNAi cells were transfected with BRCA1 resistant to the siRNA used for silencing BRCA1 expression (BRCA1Δ547–551) along with GFP-H2B as a transfection marker. As a control, a vector was transfected. The GFP-positive cells were examined for MT anchoring (aster formation). The results were shown as the average ± SE from three experiments. For each experiment, > 200 cells were examined. The representative immunostaining images are shown in (D). Scale bar, 10 μm.
We next tested whether BRCA1 suppressed centrosomal aster formation via targeting MT nucleation or anchoring/elongation. The assay to distinguish these processes has been previously described,23 where positive MT nucleation is assessed by the presence of short MT arrays radiating from centrosomes after 1 min of MT re-growth period (instead of 7 min in the MT anchoring assay). To test whether BRCA1 suppresses MT nucleation or anchoring/elongation, HCC1937 breast cancer cells that express low levels of truncated BRCA15,38 were transiently transfected with either GFP or GFP-BRCA1. It should be noted that centrosomes of HCC1937 cells behave similar to MCF7 cells silenced for BRCA1 expression in the MT nucleation/anchoring assay (Fig. S2). The transfected cells were subjected to the assay with either 1 or 7 min of MT re-growth period after nocodazole washout. Cells were then co-immunostained with anti-GFP and anti-α-tubulin antibodies (Fig. 5A and B). With 1 min of MT re-growth period, centrosomes in both GFP- and GFP-BRCA1-transfected cells nucleated numerous short MTs at similar levels (Fig. 5A, a–d and e–h) and frequencies (Fig. 5B), demonstrating that BRCA1 does not suppress MT nucleation at centrosomes. However, with 7 min of MT re-growth period, the majority of centrosomes (~75%) in the control GFP-transfected cells formed large asters (Fig. 5A, i–l and Fig. 5B), while the large aster formation was observed only in ~10% of the GFP-BRCA1-transfected cells (Fig. 5A, m–p and Fig. 5B). Wide field immunostaining images are shown in Figure S3. Thus, BRCA1 suppresses MT anchoring and/or elongation at centrosomes, but not MT nucleation.
Figure 5. BRCA1 suppresses centrosomal MT anchoring/elongation, but not MT nucleation. (A) HCC1937 cells were transfected with either GFP-vector or -wt BRCA1, and subjected to the assay as described in the legend to Figure 4, with either 1 min or 7 min of MT re-growth period. Cells were briefly extracted prior to fixation, and co-immunostained with anti-GFP and anti-α-tubulin antibodies. The GFP-BRCA1 signals at centrosomes are indicated by arrows in panel f and n. Scale bar, 10 μm. The frequencies of the GFP-positive cells with centrosomes that either nucleated or formed asters were determined, and the results are shown in (B) as average ± SE from three experiments. For each experiment, > 200 cells were examined.
Characterization of γ-tubulin interaction and centrosome localization activities of BRCA1
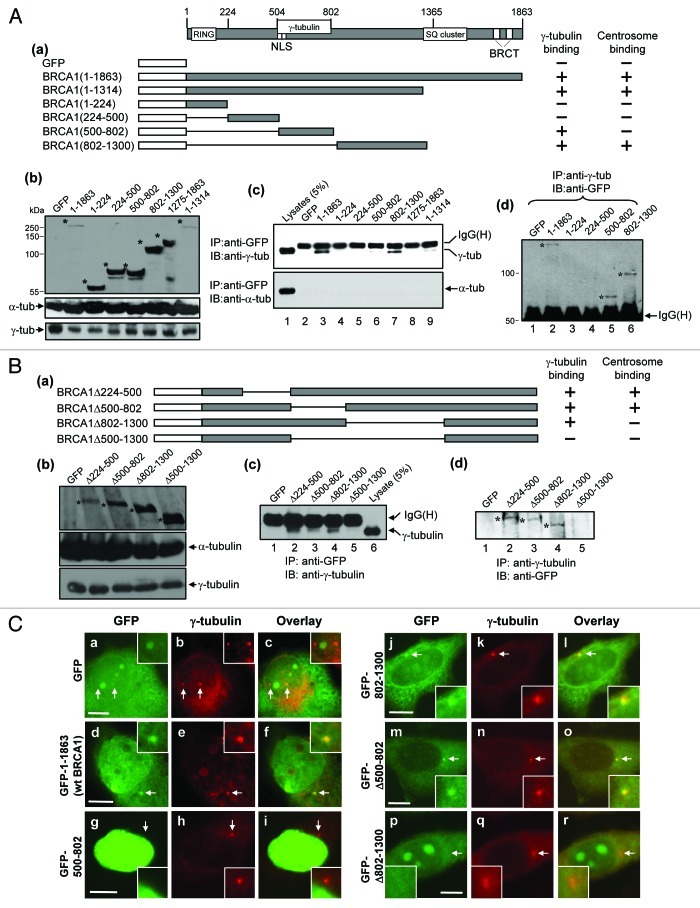
BRCA1 has been shown to physically interact with γ-tubulin through the region spanning a.a. 502–802.27 Since γ-tubulin is a key component of γTuRC, the γ-tubulin-binding may be critical for BRCA1 to suppress centrosomal aster formation. We thus revisited the interaction between BRCA1 and γ-tubulin. A series of GFP-tagged BRCA1 mutants were constructed (Fig. 6A, a) and transiently transfected into HeLa cells. Expression of the transfected proteins was confirmed by immunoblot analysis (Fig. 6A, b, top panel). It should be noted that expression of wt as well as mutant BRCA1 proteins did not alter the endogenous levels of γ-tubulin (Fig. 6A, b, bottom panel). The lysates prepared from the transfected cells were immunoprecipitated with anti-GFP-antibody, and the immunoprecipitates were immunoblotted with anti-γ-tubulin and anti-α-tubulin (negative control) antibodies (Fig. 6A, c). Wt BRCA1 (a.a. 1–1863, lane 3, top panel) and the a.a. 1–1314 mutant (lane 9) co-immunoprecipitated γ-tubulin. The a.a. 500–802 fragment harboring the previously identified γ-tubulin association domain also co-immunoprecipitated γ-tubulin (lane 6). Importantly, the a.a. 802–1300 fragment also co-immunoprecipitated γ-tubulin (lane 7). The reciprocal experiment also showed that γ-tubulin co-immunoprecipitated a.a. 500–802 fragment as well as a.a. 802–1300 fragment (Fig. 6A, d). Thus, there are two distinct domains in BRCA1 that can physically interact with γ-tubulin.
Figure 6. Mapping of the regions of BRCA1 critical for interaction with γ-tubulin and centrosome localization. (A) A series of GFP-BRCA1 fragments were generated (a), and transiently transfected into HeLa cells. As a control, GFP-vector and -wt BRCA1 were transfected. The lysates from the transfected cells were immunoblotted with anti-GFP (top panel), anti-γ-tubulin (bottom panel) and anti-α-tubulin antibody (middle panel) (b). The corresponding bands of the transfected wt and mutant BRCA1 proteins are indicated by asterisks. The lysates were also subjected to immunoprecipitation using anti-GFP antibody, and the immunoprecipitates were immunoblotted with either anti-γ-tubulin (c, top panel) or anti-α-tubulin antibody (c, bottom panel). The lysates (5% of the amounts used for immunoprecipitation) was included as a reference. The reciprocal co-immunoprecipitation assay was performed with anti-γ-tubulin antibody, and immunoblotted with anti-GFP antibody (d). The corresponding bands of GFP-wt and -mutant BRCA1 proteins co-immunoprecipitated with γ-tubulin are indicated by asterisks. (B) A series of GFP-BRCA1 deletion mutants were constructed (a), which were transfected into HeLa cells. The lysates from the transfected cells were immunoblotted with anti-GFP (top panel), anti-γ-tubulin (bottom panel) and anti-α-tubulin antibody (middle panel) (b). The corresponding bands of the transfected wt and mutant BRCA1 are indicated by asterisks. The lysates were immunoprecipitated with either anti-GFP antibody or anti-γ-tubulin antibodies, and immunoblotted with anti-γ-tubulin antibody (c) and anti-GFP antibody (d), respectively. The corresponding bands of GFP-wt and -mutant BRCA1 proteins co-immunoprecipitated with γ-tubulin are indicated by asterisks. (C) The transfected cells described above in (A and B) were also immunostained with anti-γ-tubulin and anti-GFP antibodies. Arrows point to the position of the centrosomes, and insets show the magnified images of the indicated areas. Scale bar, 8 μm. > 50 GFP-positive cells were examined for each experiment. The GFP-tagged BRCA1 mutants/fragments were counted as positive for centrosome localization if > 80% cells had the GFP signal at centrosomes.
To corroborate the above findings, we constructed additional BRCA1 deletion mutants (Fig. 6B, a). These mutants tagged with GFP were transfected into cells, and the lysates were immunoblotted with anti-GFP-antibody, which showed that all transfectants expressed the respective proteins (Fig. 6B, b, top panel). There was no significant change in the levels of γ-tubulin among the transfected cells, indicating that expression of those BRCA1 mutants did not alter the endogenous levels of γ-tubulin (Fig. 6B, b, bottom panel). The lysates were immunoprecipitated with anti-GFP-antibody, and the immunoprecipitates were immunoblotted with anti-γ-tubulin antibody (Fig. 6B, c). The mutants deleted for a.a. 224–500 (BRCA1Δ224–500), a.a. 500–802 (BRCA1Δ500–802) and a.a. 802–1300 (BRCA1Δ802–1300) all co-immunoprecipitated γ-tubulin (lanes 2–4), but the mutant deleted for a.a. 500–1300 (BRCA1 Δ500–1300) failed to do so (lane 5). The reciprocal co-immunoprecipitation assay also showed that the BRCA1 Δ500–1300 mutant failed to be co-immunoprecipitated with γ-tubulin (Fig. 6B, d, lane 5). Thus, BRCA1 contains two separate γ-tubulin association domains: γ-tubulin binding region (γBR)1 (a.a. 500–802) and γBR2 (a.a.802–1300).
We next examined the BRCA1 mutants described above for their abilities to localize to centrosomes. HeLa cells were transiently transfected with GFP-tagged wt and mutant BRCA1 proteins. The control GFP protein was not found at centrosomes (Fig. 6C, a‒c), while GFP-wt BRCA1 localized to centrosomes (d‒f). The GFP-BRCA1(500–802), a fragment encompassing γBR1, failed to localize at centrosomes (g‒i), while the GFP-BRCA1(Δ500–802), which lacks γBR1, localized at centrosomes (m‒o), demonstrating that association with γ-tubulin through γBR1 is not essential for centrosomal localization of BRCA1. In contrast, the GFP-BRCA1(802–1300), a fragment encompassing γBR2, localized to centrosomes (j‒l), while the GFP-BRCA1(Δ802–1300), which lack γBR2, failed to do so (p‒r). These results indicate that the region encompassing a.a. 802–1300 is essential for centrosomal localization of BRCA1.
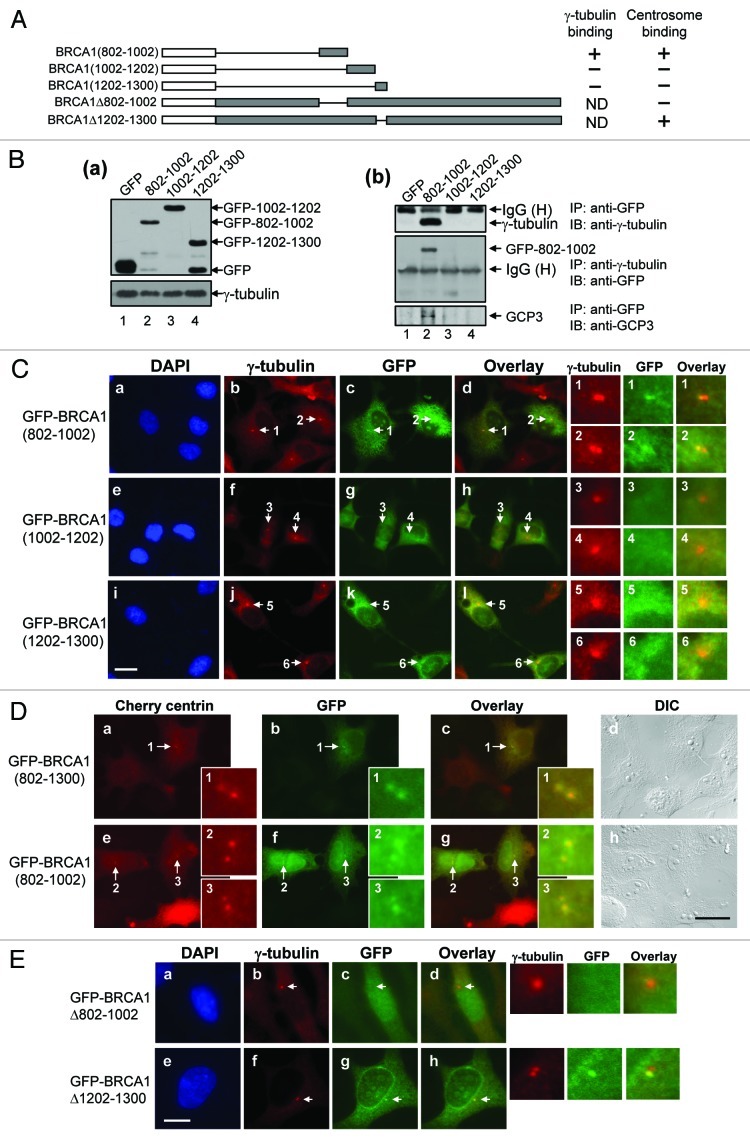
To further dissect the γBR2 for association with γ-tubulin and centrosome localization, we generated a series of small fragments within the γBR2 (a.a. 802–1002, a.a. 1002–1202, a.a. 1202–1300) tagged with GFP (Fig. 7A), which were transfected into HeLa cells. The lysates from the transfectants were immunoprecipitated with anti-GFP antibody, and the immunoprecipitates were immunoblotted with anti-γ-tubulin antibody (Fig. 7B, a and b). All transfectants expressed the transfected GFP-BRCA1 fragments at similar levels. Among the BRCA1 fragments, only the a.a. 802–1002 fragment co-immunoprecipitated γ-tubulin (Fig. 7B, b, top panel, lane 2). The reciprocal co-immunoprecipitation assay showed that only the a.a. 802–1002 fragment was co-immunoprecipitated with γ-tubulin (Fig. 7B, b, second panel, lane 2). Thus, the γBR2 resides within a.a. 802–1002. We also tested whether other γTuRC proteins (GCP) also associate with BRCA1. To this end, the anti-GFP antibody immunoprecipitates were immunoblotted with anti-GCP-3 antibody (Fig. 7B, b, third panel, lane 2). Like γ-tubulin, GCP-3 was co-immunoprecipitated with a.a. 802–1002 fragment, but not with other fragments, suggesting that BRCA1 possesses the ability to associate with γTuRC.
Figure 7. Dissection of the region (a.a. 802–1300) of BRCA1 for γ-tubulin binding and centrosome localization. A series of small BRCA1 fragments within a.a. 802–1300 tagged with GFP were generated (A), and transfected into HeLa cells. As a control, GFP-vector was transfected. The lysates from the transfected cells were immunoblotted with anti-GFP and anti-γ-tubulin antibodies (B-a). The lysates were immunoprecipitated with anti-GFP antibody and immunoblotted with anti-γ-tubulin (B-b, top panel) and anti-GCP-3 antibody (B-b, bottom panel). The lysates were also immunoprecipitated with anti-γ-tubulin antibody, and immunoblotted with anti-GFP antibody (B-b, middle panel). (C) The transfected cells described above were immunostained with anti-γ-tubulin and anti-GFP antibodies. Arrows point to the centrosomes. Panels 1–6 on the right show the magnified image of the indicated areas. Scale bar, 20 μm. (D) The GFP-BRCA1 fragments were transfected into HeLa cells expressing Cherry centrin, and examined by fluorescent microscopy. The images of the cells transfected with GFP-BRCA1(802–1300) and BRCA1(802–1002) are shown. GFP-BRCA1(1002–1202) and BRCA1(1202–1300) were not found at centrosomes (data not shown). Arrows point to the areas of centrosomes, and insets 1–3 show the magnified images of the indicated areas. Scale bar, 20 μm. (E) GFP-BRCA1 mutants deleted for either a.a. 802–1002 or a.a. 1202–1300 were also generated (A), transfected into HeLa cells, and immunostained with anti-γ-tubulin and anti-GFP antibodies. Arrows point to the centrosomes, and magnified images of the indicated areas are shown on the right. Scale bar, 10 μm.
We next examined these BRCA1 fragments tagged with GFP for centrosomal localization, and found that only the a.a. 802–1002 fragment was able to localize to centrosomes (Fig. 7C). Live cell imaging of GFP-BRCA1 mutants in cells expressing Cherry-centrin also showed that only the a.a. 802–1002 fragment was found at centrosomes [representative images of GFP-802–1002 and GFP-802–1300 (positive control) are shown in Fig. 7D]. We next tested the BRCA1 mutant deleted for a.a. 802–1002 (BRCA1Δ802–1002) for its ability to localize to centrosomes. As a control, the BRCA1 mutant deleted for a.a. 1202–1300 (BRCA1Δ1202–1300) was used. Although these deletion mutants could not be tested for their γ-tubulin binding activities because they retained γBR1, the BRCA1Δ1202–1300 mutant was found at centrosomes (Fig. 7E, e–h), while the BRCA1Δ802–1002 mutant failed to localize at centrosomes (Fig. 7E, a–d). Taken together, we concluded that the region spanning a.a. 802–1002 confers both γ-tubulin association and centrosomal localization of BRCA1.
Centrosomal localization and/or γ-tubulin association at γBR2 is required for BRCA1 to suppress centrosomal aster formation
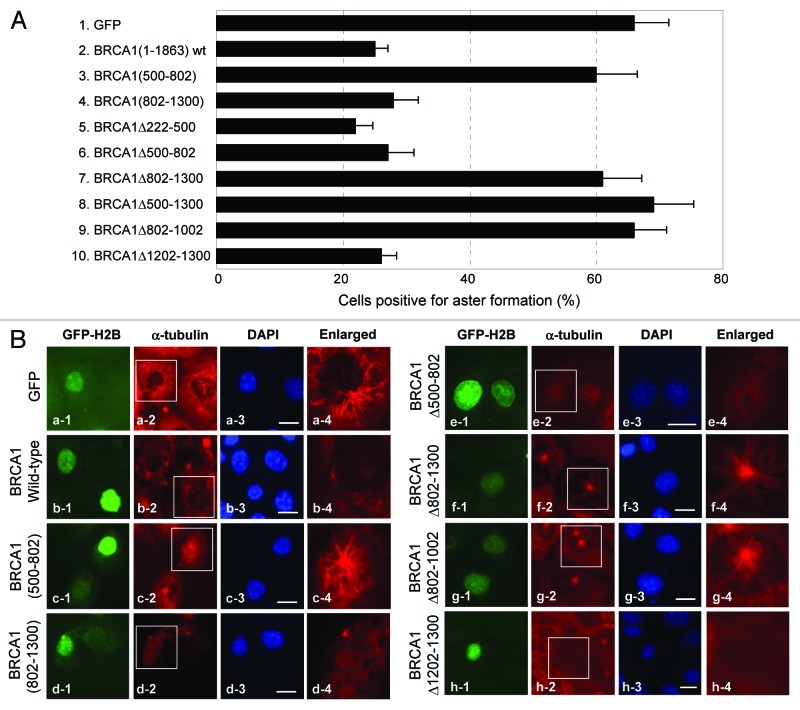
To further explore the BRCA1-mediated regulation of aster formation at centrosomes, HCC1937 cells were transiently transfected with GFP-wt and mutant BRCA1 described in Figures 6A and B and 7A, and the transfected cells were subjected to the aster formation assay (Fig. 8A and B). It should be noted here that, although the ectopic (non-centrosomal) MT nucleation has been known to occur (e.g., by overexpression of γ-tubulin),39 which might interfere with the data analysis, we found there was no noticeable occurrence of non-centrosomal aster formation during the 7 min recovery period, even when γ-tubulin was overexpressed (data not shown). Expression of GFP-wt BRCA1 suppressed centrosomal aster formation (A, column 2; B, b1–4) as expected. The BRCA1 a.a. 500–802 fragment, which comprises γBR1, failed to suppress aster formation (A, column 3; B, c1–4), and BRCA1 mutant deleted for this region (BRCA1Δ500–802) retained the aster formation suppressing activity (A, column 6; B, e1–4), suggesting that the interaction with γ-tubulin through γBR1 does not play a role in the suppression of centrosomal aster formation. In contrast, the a.a. 802–1300 fragment where γBR2 resides suppressed aster formation as effectively as wt BRCA1 (A, column 4; B, d1–4), while the mutant deleted for this region (BRCA1Δ802–1300) lost the aster formation suppressing activity (A, column 7; B, f1–4). Thus, a.a. 802–1300 comprises the region critical for BRCA1 to suppress centrosomal aster formation. Deletion of a.a. 802–1002, a part of the γBR2 domain that also confers the centrosomal localization of BRCA1, failed to suppress aster formation (A, column 9; B, g1-g4), indicating that the interaction with γ-tubulin at γBR2 and/or centrosome localization may be critical for BRCA1 to control centrosomal aster formation. Noteworthily, the 802–1300 fragment lacking the N-terminal RING domain (a.a. 1–78, see Fig. 6A), which is known to be essential for the ubiquitin ligase activity of BRCA1,10,40 retains the activity to suppress aster formation, indicating that the ubiquitination activity is not essential for BRCA1 to suppress aster formation.
Figure 8. Evaluation of wt and mutant BRCA1 for suppressing centrosomal aster formation. (A) The GFP-wt and mutant BRCA1 described in Figures 6 and 7 were transiently transfected into HCC1937 cells along with GFP-H2B as a transfection marker, and the transfected cells were subjected to the aster formation assay. The results are shown as average ± SE from three experiments. For each experiment, > 200 cells were examined. The representative immunostaining images are shown in (B). The panel on the right (a-4 to h-4) shows the magnified image of the indicated area (a-2 to h-2). Scale bar, 20 μm.
Discussion
The centrosome localization of BRCA1 had been debated for last several years. Although several studies had shown that BRCA1 localized to centrosomes in interphase and mitotic cells,27-29 it was later challenged by one study, which failed to detect the exogenously introduced GFP-tagged BRCA1 to localize to centrosomes.30 In that study, the cells stably overexpressing GFP-wt BRCA1 were used, and these cells proceeded through the cell cycle and enter mitosis without any cell cycle perturbation, which is difficult to reconcile with the known toxicity associated with wt BRCA1. This fact raised the possibility that those cells stably overexpressing GFP-wt BRCA1 might have acquired mutation(s) of either the transfected BRCA1 or other genes that counteract the toxicity associated with wt BRCA1. Indeed, when we transiently transfected GFP-wt BRCA1 into cells, cells become arrested in interphase, and no mitotic cells were detected. Moreover, when the rare viable cycling GFP-positive MCF7 cells arising from the transfection of GFP-wt BRCA1 were sub-cloned and examined for GFP-BRCA1 in those cloned cells, we found that virtually all of them expressed either the GFP-moiety alone or GFP-BRCA1 proteins that were largely truncated (P. Tarapore, unpublished observation). Thus, it is possible that GFP-BRCA1 expressed in the cells used in the above study may have undergone truncation/deletion mutations that abrogate its ability to localize to centrosomes. Here, we rigorously examined the centrosome localization of BRCA1 with various experimental approaches. The commercially available anti-BRCA1 antibody (e.g., Ab-2, Neomarkers) detected BRCA1 at centrosomes. Moreover, this antibody could no longer detect reactive signals at centrosomes in cells silenced for BRCA1 expression, demonstrating the immunocytochemical specificity of this antibody. We further showed that transiently transfected GFP-BRCA1 localizes to centrosomes in interphase cells by live cell imaging. We then extended these analyses to uncover the unique centrosomal localization pattern of BRCA1: BRCA1 specifically associates with the mother centriole of the unduplicated centrosome, and the daughter centriole acquires BRCA1 immediately before initiation of duplication, and thus duplicated centrosomes are both bound by BRCA1. At molecular levels, BRCA1 has been shown to physically interact with γ-tubulin, and the γ-tubulin binding region of BRCA1 was found to be located in a.a. 500–802 (γBR1).27 Here, we identified a second γ-tubulin binding region within a.a 802–1002 (γBR2), and found that this region confers the centrosomal localization of BRCA1. However, since we tested the BRCA1 and γ-tubulin interaction by the co-immunoprecipitation assay, it remains to be determined whether other protein(s) are involved in their interaction. Considering that γ-tubulin is present at centrosomes throughout the centrosome cycle, the unique centrosome localization pattern of BRCA1 argues against the possibility of centrosome localization of BRCA1 to be solely mediated by direct interaction with γ-tubulin, and it is more likely that other centrosomal protein(s) are involved in BRCA1-γ-tubulin interaction.
We found that BRCA1 possesses the activity to suppress centrosomal aster formation, and the newly identified γ-tubulin binding domain (a.a. 800–1002, denoted as γBR2) is critical for this activity. BRCA1 is known to act as an E3 ubiquitin ligase in association with BARD1,9,10 and BRCA1-BARD1 has been shown to mono-ubiquitinate γ-tubulin on Lysine (K) 48 and K344 as a post-translational modification.18 It has previously been shown that the ubiquitination of γ-tubulin by BRCA1 plays a role in suppression of aster formation.12 Indeed, we found that BARD1 also localizes to interphase centrosomes (data not shown). However, the BRCA1 fragment (a.a. 802–1300) encompassing γBR2, which lacks the ubiquitination activity, suppressed aster formation as efficiently as wt BRCA1, indicating that the ubiquitination activity is not essential for BRCA1 to suppress aster formation. Although it is not essential, it remains as a possibility that BRCA1-dependent ubiquitination of γ-tubulin may play an additional role in the suppression of centrosomal aster formation. We tested this possibility using non-ubiquitinatable γ-tubulin mutant (K48R/K344R). The K48R/K344R γ-tubulin mutant was able to associate with centrosomes as similar efficiency with wt γ-tubulin (Fig. S4A). The aster formation assays failed to detect any significant difference in the efficiency of centrosomal aster formation among the HCC1937 cells transfected with the vector control, wt γ-tubulin or K48R/K344R mutant (Fig. S4B and C). However, when wt BRCA1 was co-expressed, the K48R/K344R mutant noticeably alleviated the BRCA1-dependent suppression of centrosomal aster formation compared with wt γ-tubulin (Fig. S4B and C). Thus, non-ubiquitinatable γ-tubulin appears to act dominant negatively for aster formation in a manner dependent on the presence of BRCA1, which indirectly suggests that γ-tubulin ubiquitination may play an additional role in the BRCA1-mediated suppression of centrosomal aster formation. However, it is known that ubiquitination of γ-tubulin occurs at a minimal level in cells,18 and the study described above were performed by overexpression of mutated γ-tubulins, which may critically alter the protein structure and, consequentially, the function, of γ-tubulin. The role of γ-tubulin ubiquitination in the BRCA1-associated suppression of centrosomal aster formation clearly needs further investigation.
The centrosomal aster formation follows several distinct steps, including MT nucleation, MT anchoring and elongation/release of MTs. MT nucleation is initiated by γTuRC, and the MT anchoring machinery at the subdistal appendage, a structure unique to the mother centriole, anchors the MT-nucleated γTuRCs. Thus, the mother centriole serves as the primary site of MT anchoring to form asters.41 We found that BRCA1 suppresses aster formation not by targeting MT nucleation, but by targeting either MT anchoring or elongation. At present, the molecular mechanisms underlying the MT anchoring and elongation at mother centrioles are not well understood, and it is difficult to determine whether BRCA1 targets MT anchoring or elongation to suppress aster formation. We found that BRCA1 has the ability to physically associate with not only γ-tubulin, but also GCP3, one of the components of γTuRC. Thus, it is possible that BRCA1 may directly block the anchoring of the MT-nucleated γTuRC at mother centrioles. Alternatively, the MT-nucleated γTuRC can be anchored at mother centrioles, but BRCA1 may block the elongation of MTs nucleated by γTuRC. Further understanding of the aster forming process at centrosomes will clarify this issue.
Disruption of the tissue architecture is one of the major characteristics of carcinomas, which can provide a favorable ground for cancer cells to expand. The tissue architecture is established and maintained by the proper polarity of cells that compose the tissues, and the proper cell polarity is primarily determined and maintained by the position of centrosomes within a cell and the MTs organized by them. Thus, the MT organizing activity of the centrosome of each cell must be finely and continuously tuned in response to the dynamic environment in vivo, and BRCA1 is likely participating in such a fine-tuning of the MT organizing activity of the centrosome.
Materials and Methods
Cells and transfection
MCF7 and HeLa cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS), and HCC1937 cells in RPMI1640 supplemented with 10% FBS. Transfection was performed using Fugene 6 reagent (Roche).
Plasmids and antibodies
pGFP-BRCA1 mutants were constructed using the peGFP-C1 vector (Clontech). The RSET-CFP (cherry fluorescent protein) was a gift of Dr. R. Tsien. The CFP-BRCA1 was constructed using the PCR-based protocol. A 19 bp oligonucleotide sequence targeted within exon 11 (a.a. 534–540) of BRCA1 and a randomized 19 bp oligonucleotide sequence (control) were cloned into the pSUPER siRNA vector (OligoEngine). The plasmids encoding FLAG-HA-tagged wt γ-tubulin and K48R/K344R mutant are gifts from Dr. J. Parvin. Anti-BRCA1 antibodies used in this study are Ab-1 (Calbiochem), Ab-2 (Neomarkers) and C-20 (Santa Cruz). Rabbit anti-γ-tubulin antibody was generated in our laboratory. Mouse anti-γ-tubulin (clone GTU88), mouse anti-α-tubulin (DM1A) and mouse anti-FLAG (M2) antibodies were purchased from Sigma Immunochemicals. Mouse anti-cenexin, mouse anti-centrin, rabbit anti centriolin and rabbit anti-GCP-3 antibodies are gifts from Drs. K. Gull, J. Salisbury, S. Doxsey and Y. Zheng, respectively.
Indirect immunofluorescence
For immunostaining of BRCA1, cells were briefly extracted in 0.75% Triton X-100, 2 mM EGTA, 5 mM PIPES (pH 7.0) for 15 sec, followed by a brief wash with PBS, and fixed with either methanol for 5 min at -20°C or 10% formalin/methanol for 20 min at 25°C. Cells were probed with primary antibodies, and the antibody-antigen complexes were detected with Alexa fluor 488-, 594-, 647 or 680-conjugated antibodies (Molecular Probes). Cells were also stained for DNA with 4', 6-diamidino-2-phenylindole (DAPI). For live cell imaging, Cherry-centrin was used as a marker for centrioles. The pGFP-BRCA1, pGFP-BRCA1(802–1300) and pGFP-BRCA1(802–1002) were transfected into HeLa cells expressing Cherry-centrin, and examined at 24 h post-transfection. The same GFP-tagged proteins were also transfected into MCF7 cells expressing Cherry-centrin and examined at 36 h post-transfection.
Aster formation assay
The assay was performed as described previously.23,25 Briefly, cells were treated with nocodazole (1.5 μg/ml) for 40 min on ice to depolymerize interphase MTs, washed with PBS to remove the nocodazole, and incubated in fresh warm media for 1 or 7 min at 37°C to allow MT re-growth.
Immunoblot and immunoprecipitation
For immunoblot analysis, cells were lysed in SDS/NP-40 lysis buffer [1% SDS, 1% NP-40, 50 mM Tris (pH 8.0), 150 mM NaCl, 4 mM Pefabloc SC, 2 µg/ml leupeptin, 2 µg/ml aprotinin]. The lysates were briefly sonicated, boiled for 5 min and cleared by a 10 min centrifugation (20,000 × g) at 4°C. The supernatant was further denatured at 95°C for 5 min in sample buffer [2% SDS, 10% glycerol, 60 mM Tris (pH 6.8), 5% β-mercaptoethanol, 0.01% bromophenol blue], resolved by SDS-PAGE, and transferred to Immobilon sheets (Millipore). The blots were incubated in blocking buffer [5% (wt/vol) nonfat dry milk in TBS + 0.2% Tween 20 (TBS-T)] for 1 h at 25°C and then primary antibody overnight at 4°C. The blots were then rinsed in TBS-T and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at 25°C, and the antibody-antigen complex was visualized by ECL chemiluminescence (Amersham). For immunoprecipitation, cells were lysed in Triton-X lysis buffer [1% Triton-X-100, 50 mM Hepes (pH 7.4), 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 4 mM Pefabloc SC, 2 µg/ml leupeptin, 2 µg/ml aprotinin, 25 mM NaF, 80 mM β-glycerophosphate]. The lysates were cleared by centrifugation for 15 min (20,000 × g) at 4°C. The lysates containing 200 µg of total proteins were incubated with antibodies, and the antibody-antigen complexes were collected with protein G-agarose.
Supplementary Material
Acknowledgments
We thank all the members of the Fukasawa laboratory for helpful comments. This work was supported by National Institute of Health (GM087328).
Glossary
Abbreviations:
- MT
microtubule(s)
- PCM
pericentriolar materials
- γTuRC
γ-tubulin ring complex
- wt
wild-type
- siRNA
small interfering RNA
- GFP
green fluorescent protein
- CFP
cherry fluorescent protein
- γBR
γ-tubulin binding region
- DAPI
4’,6-diamidino-2-phenylindole
- BrdU
bromodeoxyuridine
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21396
References
- 1.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 2.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–9. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 3.Chapman MS, Verma IM. Transcriptional activation by BRCA1. Nature. 1996;382:678–9. doi: 10.1038/382678a0. [DOI] [PubMed] [Google Scholar]
- 4.Monteiro AN, August A, Hanafusa H. Evidence for a transcriptional activation function of BRCA1 C-terminal region. Proc Natl Acad Sci USA. 1996;93:13595–9. doi: 10.1073/pnas.93.24.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scully R, Chen J, Ochs RL, Keegan K, Hoekstra M, Feunteun J, et al. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90:425–35. doi: 10.1016/S0092-8674(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 6.Gowen LC, Avrutskaya AV, Latour AM, Koller BH, Leadon SA. BRCA1 required for transcription-coupled repair of oxidative DNA damage. Science. 1998;281:1009–12. doi: 10.1126/science.281.5379.1009. [DOI] [PubMed] [Google Scholar]
- 7.Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–8. doi: 10.1016/S1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 8.Zhong Q, Chen CF, Li S, Chen Y, Wang CC, Xiao J, et al. Association of BRCA1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science. 1999;285:747–50. doi: 10.1126/science.285.5428.747. [DOI] [PubMed] [Google Scholar]
- 9.Hashizume R, Fukuda M, Maeda I, Nishikawa H, Oyake D, Yabuki Y, et al. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J Biol Chem. 2001;276:14537–40. doi: 10.1074/jbc.C000881200. [DOI] [PubMed] [Google Scholar]
- 10.Xia Y, Pao GM, Chen HW, Verma IM, Hunter T. Enhancement of BRCA1 E3 ubiquitin ligase activity through direct interaction with the BARD1 protein. J Biol Chem. 2003;278:5255–63. doi: 10.1074/jbc.M204591200. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Weaver Z, Linke SP, Li C, Gotay J, Wang XW, et al. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell. 1999;3:389–95. doi: 10.1016/S1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- 12.Sankaran S, Starita LM, Groen AC, Ko MJ, Parvin JD. Centrosomal microtubule nucleation activity is inhibited by BRCA1-dependent ubiquitination. Mol Cell Biol. 2005;25:8656–68. doi: 10.1128/MCB.25.19.8656-8668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joukov V, Groen AC, Prokhorova T, Gerson R, White E, Rodriguez A, et al. The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell. 2006;127:539–52. doi: 10.1016/j.cell.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 14.Doxsey S. Re-evaluating centrosome function. Nat Rev Mol Cell Biol. 2001;2:688–98. doi: 10.1038/35089575. [DOI] [PubMed] [Google Scholar]
- 15.Fukasawa K. Oncogenes and tumour suppressors take on centrosomes. Nat Rev Cancer. 2007;7:911–24. doi: 10.1038/nrc2249. [DOI] [PubMed] [Google Scholar]
- 16.D’Assoro AB, Lingle WL, Salisbury JL. Centrosome amplification and the development of cancer. Oncogene. 2002;21:6146–53. doi: 10.1038/sj.onc.1205772. [DOI] [PubMed] [Google Scholar]
- 17.Fukasawa K. Centrosome amplification, chromosome instability and cancer development. Cancer Lett. 2005;230:6–19. doi: 10.1016/j.canlet.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Starita LM, Machida Y, Sankaran S, Elias JE, Griffin K, Schlegel BP, et al. BRCA1-dependent ubiquitination of gamma-tubulin regulates centrosome number. Mol Cell Biol. 2004;24:8457–66. doi: 10.1128/MCB.24.19.8457-8466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato K, Hayami R, Wu W, Nishikawa T, Nishikawa H, Okuda Y, et al. Nucleophosmin/B23 is a candidate substrate for the BRCA1-BARD1 ubiquitin ligase. J Biol Chem. 2004;279:30919–22. doi: 10.1074/jbc.C400169200. [DOI] [PubMed] [Google Scholar]
- 20.Job D, Valiron O, Oakley B. Microtubule nucleation. Curr Opin Cell Biol. 2003;15:111–7. doi: 10.1016/S0955-0674(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 21.Moritz M, Braunfeld MB, Sedat JW, Alberts B, Agard DA. Microtubule nucleation by gamma-tubulin-containing rings in the centrosome. Nature. 1995;378:638–40. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- 22.Wiese C, Zheng Y. Gamma-tubulin complexes and their interaction with microtubule-organizing centers. Curr Opin Struct Biol. 1999;9:250–9. doi: 10.1016/S0959-440X(99)80035-9. [DOI] [PubMed] [Google Scholar]
- 23.Delgehyr N, Sillibourne J, Bornens M. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J Cell Sci. 2005;118:1565–75. doi: 10.1242/jcs.02302. [DOI] [PubMed] [Google Scholar]
- 24.Piel M, Meyer P, Khodjakov A, Rieder CL, Bornens M. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J Cell Biol. 2000;149:317–30. doi: 10.1083/jcb.149.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lingle WL, Lutz WH, Ingle JN, Maihle NJ, Salisbury JL. Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc Natl Acad Sci USA. 1998;95:2950–5. doi: 10.1073/pnas.95.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pujana MA, Han JD, Starita LM, Stevens KN, Tewari M, Ahn JS, et al. Network modeling links breast cancer susceptibility and centrosome dysfunction. Nat Genet. 2007;39:1338–49. doi: 10.1038/ng.2007.2. [DOI] [PubMed] [Google Scholar]
- 27.Hsu LC, White RL. BRCA1 is associated with the centrosome during mitosis. Proc Natl Acad Sci USA. 1998;95:12983–8. doi: 10.1073/pnas.95.22.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lotti LV, Ottini L, D’Amico C, Gradini R, Cama A, Belleudi F, et al. Subcellular localization of the BRCA1 gene product in mitotic cells. Genes Chromosomes Cancer. 2002;35:193–203. doi: 10.1002/gcc.10105. [DOI] [PubMed] [Google Scholar]
- 29.Maul GG, Jensen DE, Ishov AM, Herlyn M, Rauscher FJ., 3rd Nuclear redistribution of BRCA1 during viral infection. Cell Growth Differ. 1998;9:743–55. [PubMed] [Google Scholar]
- 30.Hut HM, Rembacz KP, van Waarde MA, Lemstra W, van Cappellen WA, Kampinga HH, et al. Dysfunctional BRCA1 is only indirectly linked to multiple centrosomes. Oncogene. 2005;24:7619–23. doi: 10.1038/sj.onc.1208859. [DOI] [PubMed] [Google Scholar]
- 31.Hsu LC, Doan TP, White RL. Identification of a gamma-tubulin-binding domain in BRCA1. Cancer Res. 2001;61:7713–8. [PubMed] [Google Scholar]
- 32.Thomas JE, Smith M, Tonkinson JL, Rubinfeld B, Polakis P. Induction of phosphorylation on BRCA1 during the cell cycle and after DNA damage. Cell Growth Differ. 1997;8:801–9. [PubMed] [Google Scholar]
- 33.Salisbury JL. Centrin, centrosomes, and mitotic spindle poles. Curr Opin Cell Biol. 1995;7:39–45. doi: 10.1016/0955-0674(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 34.Fry AM, Mayor T, Meraldi P, Stierhof YD, Tanaka K, Nigg EA. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J Cell Biol. 1998;141:1563–74. doi: 10.1083/jcb.141.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lange BM, Gull K. A molecular marker for centriole maturation in the mammalian cell cycle. J Cell Biol. 1995;130:919–27. doi: 10.1083/jcb.130.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gromley A, Jurczyk A, Sillibourne J, Halilovic E, Mogensen M, Groisman I, et al. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J Cell Biol. 2003;161:535–45. doi: 10.1083/jcb.200301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Job D, Valiron O, Oakley B. Microtubule nucleation. Curr Opin Cell Biol. 2003;15:111–7. doi: 10.1016/S0955-0674(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 38.Tomlinson GE, Chen TT, Stastny VA, Virmani AK, Spillman MA, Tonk V, et al. Characterization of a breast cancer cell line derived from a germ-line BRCA1 mutation carrier. Cancer Res. 1998;58:3237–42. [PubMed] [Google Scholar]
- 39.Shu HB, Joshi HC. Gamma-tubulin can both nucleate microtubule assembly and self-assemble into novel tubular structures in mammalian cells. J Cell Biol. 1995;130:1137–47. doi: 10.1083/jcb.130.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu LC, Wang ZW, Tsan JT, Spillman MA, Phung A, Xu XL, et al. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat Genet. 1996;14:430–40. doi: 10.1038/ng1296-430. [DOI] [PubMed] [Google Scholar]
- 41.Bornens M. Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol. 2002;14:25–34. doi: 10.1016/S0955-0674(01)00290-3. [DOI] [PubMed] [Google Scholar]
- 42.White RA, Pan Z, Salisbury JL. GFP-centrin as a marker for centriole dynamics in living cells. Microsc Res Tech. 2000;49:451–7. doi: 10.1002/(SICI)1097-0029(20000601)49:5<451::AID-JEMT7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.