Abstract
Objectives
Counseling and medication adherence can affect opioid agonist treatment outcomes. We investigated the impact of two counseling intensities and two medication dispensing methods in patients receiving buprenorphine (BUP) in primary care.
Methods
In a 12-week trial, patients were assigned to Physician Management (PM) with weekly BUP dispensing (n = 28) vs. PM and directly observed, thrice-weekly BUP and cognitive behavioral therapy (PM+DOT/CBT; n = 27) based on therapist availability. Fifteen minute PM visits were provided at entry, after induction and then monthly. CBT was weekly 45-minute sessions provided by trained therapists.
Results
Treatment groups differed on baseline characteristics of years of opioid use, history of detoxification from opioids, and opioid negative urines during induction. Analyses adjusting for baseline characteristics showed no significant differences between groups on retention or drug use based on self-report or urines. Patient satisfaction was high across conditions, indicating acceptability of CBT counseling with observed medication. The number of CBT sessions attended was significantly associated with improved outcome, and session attendance was associated with a greater abstinence the following week.
Conclusions
Although the current findings were non-significant, DOT plus individual CBT sessions was feasible and acceptable to patients. Additional research evaluating the independent effect of directly observed medication and CBT counseling is needed.
Keywords: Buprenorphine/Therapeutic Use, Primary Health Care, Opioid Related Disorders
Physician office-based prescription of buprenorphine for opioid agonist maintenance treatment greatly expands treatment options and availability of services for opioid dependent individuals. Early studies established that buprenorphine in a primary care setting improved treatment outcomes and thus led to legislation authorizing its use (H. R. 4365, 2000). Of note, the majority of those early studies included drug counseling and directly observed medication consistent with methadone regulations of the time (Fiellin et al., 2002; Fudala et al., 2003; Johnson et al., 2000; Kosten et al., 1993; O’Connor et al., 1998; Schottenfeld et al., 1997). Despite this, few studies have directly evaluated level of counseling and observed medication in buprenorphine maintenance in primary care and current legislation does not require directly observed medication or specify the type or amount of counseling (but only provides the ability to refer patients to appropriate care).
Although psychosocial counseling has been shown to improve outcomes among patients receiving methadone (Abbott et al., 2003; Hayes et al., 2004; McLellan et al., 1993; Woody et al., 1995), findings in patients receiving buprenorphine have been less clear. Several studies have shown that counseling (Community Reinforcement Approach or Behavioral Drug Counseling) with contingent vouchers improves treatment outcome compared to standard treatment (Bickel et al., 2008; Chawarski et al., 2008). One study directly compared level of nurse provided counseling in office-based primary-care buprenorphine, but was unable to detect a differences between brief and extended counseling (Fiellin et al., 2006). However this study, as well as those above, included individual weekly counseling in the comparison condition. Despite these findings no studies have compared counseling without vouchers to a no counseling or physician brief counseling condition (Arfken et al., 2010).
An important consideration in evaluating counseling for buprenorphine maintenance is the feasibility of coordinating psychosocial services in primary care offices in a time and cost-efficient manner. The previously referenced study (Fiellin et al., 2006) used nurses to provide counseling since they are readily available in primary care, less expensive than physicians, and familiar with medical settings. An alternative to this approach is onsite counseling provided by a trained professional. Cognitive Behavioral Therapy (CBT) is an empirically supported psychosocial intervention for a variety of psychiatric disorders (McGinn, 2000; Olatunji et al., 2010), has a strong evidence base supporting its efficacy for treating addictive disorders, and has demonstrated durability of treatment effects (Budney et al., 2007; Carroll et al., 2008; Carroll, Rounsaville, Nich et al., 1994; Lee & Rawson, 2008; Longabaugh & Morgenstern, 1999). CBT for addictive disorders is often provided in 12 weekly, one hour, individual sessions by specialized therapists, although group CBT is also common. One pilot study evaluated CBT in the context of opioid agonist maintenance (Abrahms, 1979), showing improvement in secondary clinical outcomes such as anxiety and depression, but to our knowledge no study has evaluated the feasibility and acceptability of providing CBT for opioid agonist maintenance in a primary care context.
Buprenorphine dosing research has established that withdrawal effects, intoxication and adverse medical effects are similar across daily, alternate day, or every 3-day observed dosing (Amass et al., 1998; Bickel et al., 1999). Clinical outcomes of retention and drug use have also shown similar rates between daily and thrice-weekly observed dosing (Schottenfeld et al., 2000). Similarly, provision of weekly dispensing of daily medication has shown similar clinical outcomes to more frequent dispensing (Bell et al., 2007; Fiellin et al., 2006). Notably, in one study, patients with higher levels of documented medication adherence had improved buprenorphine treatment outcomes (Fiellin et al., 2006). Given potential problems associated with buprenorphine diversion, additional evaluation of observed versus dispensed dosing is needed (Dasgupta et al., 2010; Vicknasingam et al., 2010). The current study was designed to evaluate feasibility, acceptability, and initial efficacy of therapist provided CBT counseling with directly observed medication administration compared to physician management only with weekly provision of medication.
Methods
Participants
82 patients who met DSM-IV criteria for current opioid dependence (American Psychiatric Association, 1994) and qualified for opioid agonist maintenance treatment signed initial informed consents. Enrollment began on 5/12/2004 and ended on 10/3/2005. Exclusion criteria were 1) current dependence on alcohol, benzodiazepine or sedatives; 2) current suicide or homicide risk; 3) current psychotic disorder or major depression; 4) inability to read or understand English, and 4) life-threatening or unstable medical problems. Women of childbearing age agreed to adequate contraception and monthly pregnancy monitoring. One patient was excluded for current psychotic disorder and 1 for current major depression. Seven patients chose to enter alternative treatment and 3 were lost to follow-up prior to treatment initiation. Of the 70 who initiated buprenorphine induction, 12 patients did not complete the 14 day buprenorphine induction period, and 58 patients were assigned to a condition (see Table 1). The study was approved by the Human Investigation Committee of Yale University School of Medicine.
Table 1.
Demographic and clinical characteristics of opioid dependent patients receiving buprenorphine/naloxone in primary care assigned to weekly Physician Management (PM) plus directly observed medication (DOT) plus weekly Cognitive Behavioral Therapy (PM+DOT+CBT) or PM with take home medication
| Characteristic | PM+DOT+CBT n = 28 | PM n = 30 | p |
|---|---|---|---|
| Age, years, mean, (SD) | 39.8 (12.1) | 38.1 (11.4) | .60 |
| % Male, (n) | 79% (22) | 70% (21) | .46 |
| %White, (n) | 61% (17) | 83% (25) | .05 |
| % Full-time employment, (n) | 46% (13) | 40% (12) | .62 |
| % High School or greater, (n) | 86% (25) | 90% (27) | .65 |
| Monthly income, $, mean (SD) | 1818 (1828) | 1635 (1635) | .71 |
| % Never married, (n) | 39% (11) | 43% (13) | .99 |
| Opioid dependence, years, mean, (SD) | 12.2 (10.1) | 6.2 (5.6) | .01 |
| % Prescription Drug Use, (n) | 39% (11) | 50% (15) | .41 |
| % History intravenous drug use, (n) | 25% (7) | 7% (2) | .05 |
| % Prior attempted detoxification, (n) | 65% (17) | 27% (7) | .005 |
Design
The study took place in the adult primary care center of an urban teaching hospital. Participants were assigned to 14 weeks of either physician management (PM) and weekly medication dispensing or PM and thrice weekly directly observed buprenorphine therapy (DOT) plus weekly CBT based on therapist availability to provide CBT.
Buprenorphine Induction and Maintenance
Prior to treatment arm assignment, patients were inducted onto buprenorphine/naloxone using a 2-day protocol (8 mg on day one, 12 mg on day 2) by trained nursing staff. All patients received two-weeks of buprenorphine/naloxone stabilization using the sublingual tablet formulation. For patients assigned to PM and weekly dispensing, nursing staff provided take home bottles with 7 doses. The initial maintenance dose was 12 mg SL daily. Take-home medication was provided for the days patients did not have their medication dispensed in the office. To provide a similar daily dose of buprenorphine/naloxone to be dispensed thrice weekly for DOT the initial maintenance dose for the PM+DOT+CBT arm was buprenorphine 24 mg SL on Mondays and Wednesdays and 36 mg SL on Fridays. The dosing protocol for both treatment arms allowed for two dose upgrades equivalent to 14 and 16 mg/day based upon patient discomfort or evidence of ongoing illicit opioid or cocaine use via urine toxicology testing. In the final two weeks of treatment patients were transferred to either a different buprenorphine provider or methadone maintenance or were tapered off of buprenorphine based on physician and patient agreement.
Treatments
Physician Management (PM)
PM is a brief (15 minutes per session), manual-guided, medically focused treatment adapted from a Standard Medical Management manual (Fiellin et al., 2006). PM was provided by primary care Internal Medicine physicians with experience in office-based buprenorphine treatment. Each visit followed a manualized protocol with fidelity guided by the use of structured encounter notes that included the pre-specified components. At each session the physician 1) reviewed the patient’s drug use, urine toxicology results, symptoms, and progress; 2) assessed the impact of drug use on medical, psychiatric, social, employment, and legal functioning; 3) educated the patient about opioid dependence and agonist maintenance treatment; 4) encouraged the patient to become or remain abstinent and adhere to treatment recommendations; 5) encouraged lifestyle changes and attendance at self-help groups; 6) identified and addressed medical complications of opioid use or buprenorphine treatment; and 7) referred the patient as appropriate to other services in the community. Patients were scheduled for PM visits prior to induction, after induction and then at the end of each month, for a total of 5 visits for patients who completed treatment. Additional visits were provided only for urgent medical or psychiatric concerns. Weekly urine toxicology tests results were conducted and included in the medical record for physician review.
Cognitive Behavioral Therapy (CBT)
Manualized CBT was provided in 12 weekly 45 minute sessions following induction. We chose to use individual CBT since most prior studies used CBT in this format and to provide greater fidelity to the manualized format. The CBT manual was a slight modification of Carroll’s manual for cocaine addiction (Carroll, 1998) adapted for use in buprenorphine maintained patients. Our adapted CBT addressed the same critical tasks outlined by Rounsaville and Carroll (Rounsaville & Carroll, 1992): fostering motivation for abstinence, functional analysis of behavior, coping skills training, changing reinforcement contingencies, enhancing management of negative affect, and improving interpersonal functioning and social supports.
Two master’s-level and three doctoral-level therapists provided CBT in the trial. Each of the therapists had at least 3 years’ experience providing substance abuse treatment. Each of the doctoral-level therapists had provided manualized CBT on research protocols involving substance use or associated psychiatric disorders. Training of therapists consisted of a two-day CBT workshop followed by at least two training cases and individual supervision. Therapists were considered “certified in CBT” when at least one half of the CBT-consistent adherence and competence items for each session were rated at midpoint or higher for two separate training patients.
Therapists typically allocated the first third of the session for introduction and review, the second third to train on specific coping skills, and the final third to review high-risk situations and assign homework. Following each session, the therapist completed a brief, structured progress note, including session length and use of core CBT session components. Weekly group supervision involved review of therapists’ taped sessions for competence and adherence, addressing patient issues, and review of progress notes.
Measures
Sociodemographic, medical, psychosocial, drug use and treatment information was collected prior to treatment. Treatment completion was defined as continued participation in treatment through the 14th week. Retention was evaluated as the number of weeks participants remained in treatment. Patients who missed three consecutive instances of medication (n = 11) or three consecutive counseling sessions (n = 2) were considered drop outs. Urine samples were collected during weekly research assessments. Urine toxicology analyses were performed using a semi-quantitative homogenous enzyme immunoassay for opiates, oxycodone, benzodiazepines, cocaine, and methadone. Rates of benzodiazepine positive tests were too low (4%) to evaluate. Missing urines were coded as positive for opioids. The maximum consecutive weeks of abstinence from illicit opioids (opiates, oxycodone, or methadone) were computed for each patient. In addition, the proportion of urines negative for all opioids (opiates, oxycodone, or methadone) and for cocaine were computed for the induction period and the first and second halves of treatment to evaluate potential emergent effects of CBT (Carroll, Rounsaville, Gordon et al., 1994).
Self-reported opioid abstinence for 30 day segments prior to treatment and at each month of treatment was assessed using Time Line Follow Back methodology (Sobel & Sobel, 1992). Patient satisfaction was evaluated using the Primary Care Buprenorphine Satisfaction Scale (Barry et al., 2007). Process measures included the number of PM and CBT sessions attended, missed, rescheduled or canceled and session lengths. A protective transfer criteria was set for patients with unremitting illicit drug use (3 consecutive weeks of positive opioid and/or cocaine urine specimens after dose escalations) or development of significant psychiatric symptoms (Johnson et al., 2000). No patients met these criteria.
Data analysis
Primary outcome measures to evaluate feasibility and initial efficacy were retention, urine toxicologies and patient satisfaction. Secondary outcomes were self-reported drug use, and session attendance. Univariate comparisons of patients were conducted using chi-square tests for categorical variables and T-tests for interval and continuous variables. Retention was examined using Kaplan-Meier survival analysis on weeks in treatment. For outcomes with repeated measures, we used repeated-measures ANOVA for data with no-missing values (urine toxicology tests for induction, and first and second half of treatment), and mixed-model analysis of variance for data with missing values (self-reported opioid use). Since there were group differences in baseline characteristics, we evaluated differences in assigned condition both unadjusted and adjusted for baseline differences using analysis of covariance (ANCOVA) for continuous measures and logistic regression for categorical measures.
Results
Demographic and baseline characteristics are presented in Table 1. Patients assigned to PM had significantly fewer years of opioid use and were less likely to have had previously received detoxification treatment for opioids. Patients assigned to PM only were marginally more likely to be white (p = .05) and less likely to have a history of IV drug use (p = .05). These factors were included in the adjusted outcome tests (ANCOVA’s).
Treatment outcomes
Table 2 presents retention, urinalysis, and self-reported outcomes across the two conditions. Mean values are presented for unadjusted values, although P values for both unadjusted and adjusted for baseline differences are presented. None of the adjusted tests showed significant differences by assigned condition. For each variable the direction of differences remained the same for the adjusted tests (in favor of the PM condition), but the magnitudes were smaller than unadjusted tests.
Table 2.
Treatment outcome and process characteristics of opioid dependent patients receiving buprenorphine/naloxone in primary care assigned to weekly Physician Management (PM) plus directly observed medication (DOT) plus weekly Cognitive Behavioral Therapy (PM+DOT+CBT) or PM with take home medication
| Characteristic | PM+DOT+CBT n = 28 | PM n = 30 | P | P controlling for baseline variables* |
|---|---|---|---|---|
| Retention | ||||
| Treatment Completion, % (n) | 68% (19) | 87% (26) | .09 | .32 |
| Weeks in treatment, M (SD) | 11.9 (3.7) | 13.0 (2.7) | .10 | .35 |
| Urine Toxicology | ||||
| Maximum weeks of continuous opioid abstinence, M (SD) | 5.2 (4.9) | 7.0 (4.7) | .15 | .73 |
| Self-reported days of opioid use, M (SD) | .04 | .17 | ||
| Month prior to treatment | 27.7 (8.7) | 26.8 (8.6) | ||
| Month 1 | 2.8 (6.1) | 0.5 (0.9) | ||
| Month 2 | 1.6 (1.9) | 0.4 (1.3) | ||
| Month 3 | 1.9 (2.5) | 0.4 (1.3) | ||
| Self-reported days of cocaine use, M (SD) | .13 | .49 | ||
| Month prior to treatment | 2.4 (0.7) | 1.1 (0.7) | ||
| Month 1 | 2.0 (0.7) | 0.7 (0.7) | ||
| Month 2 | 1.9 (0.7) | 0.8 (0.7) | ||
| Month 3 | 1.9 (0.8) | 0.6 (0.7) | ||
| Self-reported days of alcohol use, M (SD) | .60 | .67 | ||
| Month prior to treatment | 3.9 (0.9) | 2.7 (0.9) | ||
| Month 1 | 3.3 (0.9) | 2.4 (0.9) | ||
| Month 2 | 2.4 (1.0) | 2.2 (0.9) | ||
| Month 3 | 2.6 (1.0) | 2.5 (0.9) | ||
Analyses controlled for prior detoxification, IV use, years of opioids, race, and percent of opioid abstinent during induction.
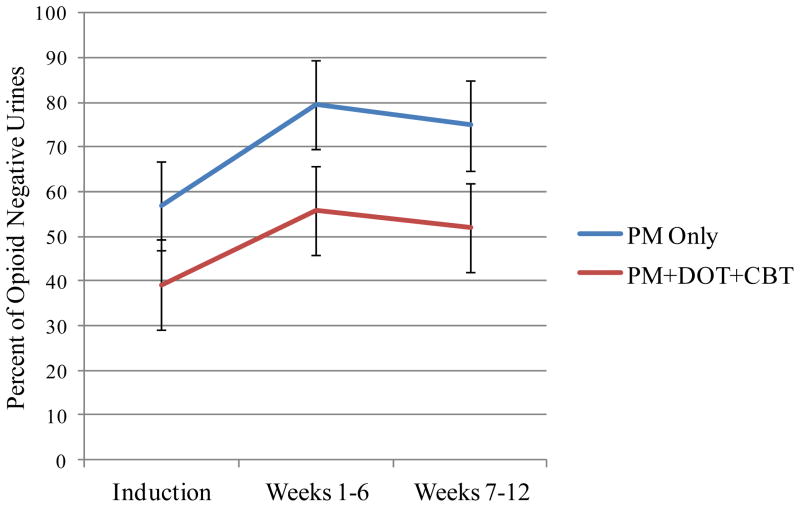
There were no significant differences between conditions on treatment retention although the direction of the effect favored the PM condition (see Table 2). Figure 1 presents the percentage of urines negative for opioid from the induction period, through the first and second half of the study. As can been seen in the figure, the percent of negative urine toxicology analyses improved from the induction period to the first and second half of the study (p = .001), although the first and second half of the study did not differ from each other. Compared to participants assigned to PM+DOT+CBT, participants assigned to PM provided a higher percentage of negative urines across the entire study (70.4 vs. 49.0, p = .003). However, condition did not interact with time (p = .70) indicating that differences between groups evident during induction (before treatment assignment) were maintained throughout the study. Similarly, patients in PM reported less opioid use, and all patients reported a reduction in opioid use from intake (p = <.001), though condition did not interact with time (p = .89, unadjusted, p = .90, adjusted).
Figure 1.
Percent of opioid negative urine toxicology analyses over time for patients assigned to weekly Physician Management (PM) plus directly observed medication (DOT) plus weekly Cognitive Behavioral Therapy (PM+DOT+CBT) or PM with take home medication.
There was a marginally significant difference in percent of urine toxicology analyses negative for cocaine (p = .09). Patients in the PM condition had marginally higher percent negative (M = 88.1), than patients in PM+DOT+CBT (M = 73.1). However, the groups did not differ over the whole study (p = .63), nor was there an effect of time (p = .91), and group did not significantly interact with time (p = .21). There were no differences between conditions on self-reported cocaine use (Table 2), nor was there change over time (p = 32). There were also no differences on the overall measure of patient satisfaction (Table 2), nor on any of the individual satisfaction items (data not shown).
Process outcomes
The mean (SD) daily dose of buprenorphine during the maintenance phase for the PM+DOT+CBT condition was 12.3 (0.9) milligrams and 12.6 (1.7) for the PM condition and did not differ (p=0.39).
Patients attended a mean of 4.2 (SD = 1.7) PM visits and did not differ by assigned condition (Table 2). In addition, the number of sessions missed by patients did not differ by condition (p = .22, unadjusted, p = .08, adjusted, M = 1.0 for PM, M = 0.6 for PM+DOT+CBT). Sessions were approximately 19 minutes in length and did not differ by group.
Patients assigned to PM+DOT+CBT attended a mean of 7.5 (out of 12 possible) CBT sessions (Median = 9.0, SD = 3.5), with 57% attending 8 or more sessions. Mean session length was 42.3 minutes (SD = 7.2). Of the 336 possible sessions (28×12), 58% (195) were attended. Of the 268 sessions that were explicitly scheduled either by the research staff or by the patient and therapist at the end of a session, 195 (73%) were attended, 40 (15%) were considered a no-show or attended too late to complete a partial session, and 33 (12%) were cancelled or rescheduled. Higher proportion of CBT sessions attended was positively associated with a higher percentage of urines negative for opioids (r = .37, p = .05) and maximum continuous weeks of opioid abstinence (r = .50, p = .007). To evaluate the relationship between attendance at any specific session and the subsequent week’s opioid urine test results, we conducted a General Estimating Equation (GEE) including patient as a random variable and controlling for session. Findings indicated that urine toxicology analyses for prior session attendance were marginally (p = .08) more likely to be opioid negative (M = 67%), than those without prior attendance (M = 55%).
Discussion
In this pilot trial of CBT plus directly observed medication we were unable to detect a difference in patient retention, the maximum number of consecutive weeks of opioid abstinence, or patient satisfaction compared to physician management only with weekly dispensing when baseline group differences were statistically controlled. Although inconsistent with our hypothesis (Kraft et al., 1997; McLellan et al., 1993; Woody et al., 1995), these null findings are generally consistent with a recent meta-analysis evaluating the addition of behavioral counseling to opioid agonist maintenance (methadone or buprenorphine; Amato et al., 2008). Amato and colleagues found no significant advantage of added counseling on retention, urines during treatment, and self-reported use of the primary substance, but counseling did improve the number of participants abstinent at end of follow-up. The current results are also consistent with null findings from our previous evaluation of nurse provided counseling and medication dispensing in primary care-based buprenorphine/naloxone (Fiellin et al., 2006).
In our previous trial we found patients were less satisfied with more frequent clinic visits (three times per week, vs. once per week; Barry et al., 2007). However, in the current trial, patient satisfaction was high and did not differ between conditions, indicating acceptability of CBT counseling with observed medication. In addition, patients in the CBT condition attended a mean 7.5 sessions of a possible 12, indicating a reasonable dose of CBT. However, over a quarter of scheduled CBT sessions were not conducted due to no-shows and cancellations. Interestingly, the number of CBT sessions attended was significantly associated with improved outcome, and session attendance was associated with a greater abstinence the following week, thus CBT attendance could be a marker for improved prognosis overall. However, these findings are correlational. Session attendance may increase the probability of drug abstinence, patients who continue to use drugs may avoid attending sessions, or other factors may influence these variables.
We chose to use a relatively brief, 12-session dose of CBT since prior studies with substance abuse have demonstrated effects with this duration of treatment, and because we believed that a shorter time frame would be easier to implement in a primary care context. Since the current findings suggest that more sessions may improve outcome, a longer therapeutic window may be needed to optimize the dose of CBT. However, a number of implementation issues limit the practicality of providing extended individual CBT. We found a number of logistic difficulties of providing CBT, including office space availability, transportation and parking difficulties, and coordination of physician, nurse, therapist and patient schedules. While these difficulties can be addressed, they may require added resources with attendant costs. One alternative may be an adaptive or stepped-care model of treatment in which only patients with poor initial treatment response receive CBT. Alternative CBT delivery systems should also be evaluated. These include remote counseling via phone or web-based systems, automated computer-and web-based CBT programs, and brief, mobile-delivered interventions. Remote and electronic systems may provide more frequent, more flexible, and greater therapeutic contact, be preferable by some patients, and be more feasible in a primary care context (Moore et al., 2011). Despite their potential promise, to date no study has compared such a system on its own to a control in the context of agonist maintenance.
Several limitations of the study should be noted. The sample size was relatively small; the study had reduced power to detect anything but very large effects. However, the results in the current trial were not in the expected direction. This may be due to the manipulation of both medication dosing and counseling in the same condition. Secondary analysis of our previous trial indicated that patients preferred longer weekly medication dispensing rather than thrice weekly dispensing (Barry et al., 2007), thus the thrice weekly observed medication in the current trial may have canceled out a potential benefit of counseling. An additional trial evaluating the independent effect of each of these variables is needed. A third limitation of the trial was the lack of random assignment. Patients were assigned to condition based on therapist availability. This led to a number of differences on baseline variables, including years of opioid dependence and prior detoxification. We attempted to control for these differences statistically, which provided similar null findings to the unadjusted results, but controlling via randomization would be preferred. A final limitation was the lack of assessment following the intervention. Since CBT effects are often noted following the end of counseling (Carroll, Rounsaville, Nich et al., 1994), future studies should include a reasonable follow-up period to evaluate possible emergent effects.
Conclusions
Despite these limitations, the current study demonstrated that weekly, professionally-delivered CBT sessions with observed medication is feasible and acceptable to opioid dependent patients receiving buprenorphine/naloxone maintenance. Additional research on observed medication and counseling for buprenorphine patients in primary care is warranted.
Acknowledgments
Role of the Funding Source
The research was supported by the National Institute on Drug Abuse Grants K01 DA022398 (B.M.), RO1 DA019511 (B.M., D.B., C.C., L.S., M.C., & D.F.), K12 DA00167 (D.F.), K24DA000445 (R.S.), K23 DA024050 (D.B.), a Robert Wood Johnson Generalist Physician Faculty Scholar Award (D.F.), and through the State of Connecticut, Department of Mental Health and Addiction Services support of the Connecticut Mental Health Center (B.M.). These funding sources had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest.
Disclosures
This study was presented in part at the annual scientific meeting of the College on Problems of Drug Dependence (June 2006), Scottsdale, AZ.
References
- Abbott PJ, Moore BA, Delaney HD. Community reinforcement approach and relapse prevention: 12 and 18-month Follow-up. J Maint Addict. 2003;2:35–50. [Google Scholar]
- Abrahms JL. A cognitive-behavioral versus nondirective group treatment program for opioid-addicted persons: An adjunct to methadone maintenance. Int J Addict. 1979;14:503–511. doi: 10.3109/10826087909054598. [DOI] [PubMed] [Google Scholar]
- Amass L, Bickel WK, Crean JP, Blake J, Higgins ST. Alternate-day buprenorphine dosing is preferred to daily dosing by opioid-dependent humans. Psychopharmacology. 1998;136:217–225. doi: 10.1007/s002130050559. [DOI] [PubMed] [Google Scholar]
- Amato L, Minozzi S, Davoli M, Vecchi S, Ferri MMF, Mayet S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database of Systematic Reviews. 2008 doi: 10.1002/14651858.CD004147.pub2. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Arfken CL, Johanson C-E, di Menza S, Schuster CR. Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: National surveys of physicians. J Subst Abuse Treat. 2010;39:96–104. doi: 10.1016/j.jsat.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Barry DT, Moore BA, Pantalon MV, et al. Patient satisfaction with primary care office-based buprenorphine/naloxone treatment. J Gen Intern Med. 2007;22:242–245. doi: 10.1007/s11606-006-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J, Shanahan M, Mutch C, et al. A randomized trial of effectiveness and cost-effectiveness of observed versus unobserved administration of buprenorphine-naloxone for heroin dependence. Addiction. 2007;102:1899–1907. doi: 10.1111/j.1360-0443.2007.01979.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Amass L, Crean JP, Badger GJ. Buprenorphine dosing every 1, 2, or 3 days in opioid-dependent patients. Psychopharmacology. 1999;146:111–118. doi: 10.1007/s002130051096. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, Buchhalter AR, Badger GJ. Computerized behavior therapy for opioid-dependent outpatients: a randomized controlled trial. Exp Clin Psychopharmacol. 2008;16:132–143. doi: 10.1037/1064-1297.16.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha H, Higgins ST. Voucher-based incentives and behavior therapy for adult marijuana dependence. J Cons Clin Psychol. 2006;74:307–316. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM. A cognitive-behavioral approach: Treating cocaine addiction. Rockville, MD: NIDA; 1998. [Google Scholar]
- Carroll KM, Ball SA, Martino S, et al. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. Am J Psychiat. 2008;165:881–888. doi: 10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Rounsaville BJ. Enduring effects of a computer-assisted training program for cognitive behavioral therapy: A 6-month follow-up of CBT4CBT. Drug Alcohol Depend. 2009;100:178–181. doi: 10.1016/j.drugalcdep.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Gordon LT, et al. Psychotherapy and pharmacotherapy for ambulatory cocaine abusers. Arch Gen Psychiatry. 1994;51:177–187. doi: 10.1001/archpsyc.1994.03950030013002. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One year follow-up of psychotherapy and pharmacotherapy for cocaine dependence: Delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Chawarski MC, Mazlan M, Schottenfeld RS. Behavioral drug and HIV risk reduction counseling (BDRC) with abstinence-contingent take-home buprenorphine: a pilot randomized clinical trial. Drug Alcohol Depend. 2008;94:281–284. doi: 10.1016/j.drugalcdep.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Dasgupta N, Bailey EJ, Cicero T, et al. Post-marketing surveillance of methadone and buprenorphine in the United States. Pain Med. 2010;11:1078–1091. doi: 10.1111/j.1526-4637.2010.00877.x. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, O’Connor PG, Chawarski M, Pantalon MV, Schottenfeld RS. Office versus narcotic treatment program-based buprenorphine for opioid dependence. Drug Alcohol Depend. 2002;66:S55–56. [Google Scholar]
- Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. New Eng J Med. 2006;355:365–374. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. New Eng J Med. 2003;349:949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Wilson KG, Gifford EV, et al. A Preliminary Trial of Twelve-Step Facilitation and Acceptance and Commitment Therapy With Polysubstance-Abusing Methadone-Maintained Opiate Addicts. Behav Ther. 2004;35:667–688. [Google Scholar]
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. New Eng J Med. 2000;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Schottenfeld R, Ziedonis D, Falcioni J. Buprenorphine versus methadone maintenance for opioid dependence. J Nerv Ment Dis. 1993;181:358–364. doi: 10.1097/00005053-199306000-00004. [DOI] [PubMed] [Google Scholar]
- Kraft MK, Rothbard AB, Hadley TR, McLellan AT, Asch DA. Are supplementary services provided during methadone maintenance really cost-effective? Am J Psychiat. 1997;154:1214–1219. doi: 10.1176/ajp.154.9.1214. [DOI] [PubMed] [Google Scholar]
- Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug & Alcohol Review. 2008;27:309–317. doi: 10.1080/09595230801919494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longabaugh R, Morgenstern J. Cognitive-behavioral coping-skills therapy for alcohol dependence. Current status and future directions. Alcohol Research & Health: the Journal of the National Institute on Alcohol Abuse & Alcoholism. 1999;23:78–85. [PMC free article] [PubMed] [Google Scholar]
- McGinn LK. Cognitive behavioral therapy of depression: theory, treatment and empirical status. Am J Psychotherapy. 2000;54:257–262. doi: 10.1176/appi.psychotherapy.2000.54.2.257. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Arndt IO, Metzger DS, Woody GE, O’Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA. 1993;269:1953–1959. [PubMed] [Google Scholar]
- Moore BA, Fazzino T, Garnet B, Cutter CJ, Barry DT. Computer-based interventions for drug use disorders: A systematic review. J Subst Abuse Treat. 2011;40:215–223. doi: 10.1016/j.jsat.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor PG, Oliveto AH, Shi JM, et al. A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinc. Amer J Med. 1998;105:100–105. doi: 10.1016/s0002-9343(98)00194-6. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Cisler JM, Deacon BJ. Efficacy of cognitive behavioral therapy for anxiety disorders: a review of meta-analytic findings. Psychiatr Clin North Am. 2010;33:557–577. doi: 10.1016/j.psc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Carroll KM. Comprehensive Textbook of Substance Abuse. 2. New York: Williams and Wilkins; 1992. Individual psychotherapy for drug abusers; pp. 496–508. [Google Scholar]
- Schottenfeld RS, Pakes J, O’Connor P, Chawarski M, Oliveto A, Kosten TR. Thrice-weekly versus daily buprenorphine maintenance. Biol Psychiatry. 2000;47:1072–1079. doi: 10.1016/s0006-3223(99)00270-x. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54:713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- Sobel LC, Sobel MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Allen JP, Litten RZ, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: Human Press; 1992. pp. 41–72. [Google Scholar]
- Vicknasingam B, Mazlan M, Schottenfeld RS, Chawarski MC. Injection of buprenorphine and buprenorphine/naloxone tablets in Malaysia. Drug Alcohol Depend. 2010;111:44–49. doi: 10.1016/j.drugalcdep.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Waiver authority for physicians who dispense or prescribe certain narcotic drugs for maintenance treatment of detoxification treatment, H. R. 4365 § 3501, 3502 (2000).
- Woody GE, McLellan AT, Luborsky L, O’Brien CP. Psychotherapy in community methadone programs: a validation study. Am J Psychiat. 1995;152:1302–1308. doi: 10.1176/ajp.152.9.1302. [DOI] [PubMed] [Google Scholar]