Abstract
Escherichia coli polymerase V (pol V/UmuD'2C) is a low-fidelity DNA polymerase that has recently been shown to avidly incorporate ribonucleotides (rNTPs) into undamaged DNA. The fidelity and sugar selectivity of pol V can be modified by missense mutations around the “steric gate” of UmuC. Here, we analyze the ability of three steric gate mutants of UmuC to facilitate translesion DNA synthesis (TLS) of a cyclobutane pyrimidine dimer (CPD) in vitro, and to promote UV-induced mutagenesis and cell survival in vivo. The pol V (UmuC_F10L) mutant discriminates against rNTP and incorrect dNTP incorporation much better than wild-type pol V and although exhibiting a reduced ability to bypass a CPD in vitro, does so with high-fidelity and consequently produces minimal UV-induced mutagenesis in vivo. In contrast, pol V (UmuC_Y11A) readily misincorporates both rNTPs and dNTPs during efficient TLS of the CPD in vitro. However, cells expressing umuD'C (Y11A) were considerably more UV-sensitive and exhibited lower levels of UV-induced mutagenesis than cells expressing wild-type umuD'C or umuD'C (Y11F). We propose that the increased UV-sensitivity and reduced UV-mutability of umuD'C (Y11A) is due to excessive incorporation of rNTPs during TLS that are subsequently targeted for repair, rather than an inability to traverse UV-induced lesions.
Keywords: SOS mutagenesis, pol V, UV-mutagenesis, ribonucleotide incorporation
1. Introduction
Escherichia coli possesses five DNA polymerases (pols), three of which, pol II, pol IV and pol V, are induced as part of the cell’s SOS response to DNA damage [1]. Pol II is encoded by polB [2]; pol IV by dinB [3]; and pol V by the umuDC genes [4]. All are believed to participate in “translesion DNA Synthesis” (TLS), a process that allows replication to proceed past DNA lesions that normally block the cell’s replicase, pol III, and endanger cell survival. In some instances, TLS is error-free, as in the case of pol IV-dependent TLS of certain adducts formed at the N2 position of guanine [5, 6]. However, in many cases, it is error-prone and leads to a >100-fold increase in cellular mutagenesis [1]. Most damage-induced mutagenesis in E.coli is dependent upon the main TLS polymerase, pol V [7, 8], a complex consisting of a UmuD'2C heterotrimer [4].
It is, therefore, not too surprising that E.coli goes to great lengths to minimize the level of pol V in the cell, so as to restrict its activity to the precise time and place where it is required. In addition to tight transcriptional regulation by the LexA protein [9–11], the UmuDC proteins are subject to multiple mechanisms of posttranslational control. Like the LexA repressor, UmuD undergoes RecA-mediated autodigestion [12, 13]. However, unlike LexA, which is inactivated by autocleavage, conversion of UmuD to UmuD' activates the protein for its mutagenic functions [14]. Both UmuD and UmuD' proteins form homodimers in vitro, yet when mixed together they preferentially form heterodimers [15]. As a consequence, mutagenically active homodimeric UmuD' is only produced when it is present in excess of UmuD. In vivo, this occurs ~45–60 minutes post-DNA damage [16, 17] and presumably only under conditions where there is a persistent activating signal within the damaged cell. Both UmuD and UmuC are rapidly degraded by the Lon protease [18, 19], whilst UmuD' (in a UmuD/D' heterodimer) is degraded by the ClpXP protease [18, 20]. As a consequence, the normal intracellular levels of UmuD'2C are only estimated to reach a maximum of somewhere between 60–200 molecules per cell [16, 21, 22]. Indeed, E.coli does not tolerate high intracellular levels of the Umu proteins particularly well, since in addition to the increased risk of uncontrolled mutagenesis, deregulation of umu gene expression can cause other complex phenotypic changes. For example, when overproduced in E. coli, the UmuDC proteins confer a cold-sensitive growth phenotype [23–25]. In addition, when overexpressed in a recA+ lexA+ background, the UmuD'C proteins also inhibit RecA-dependent recombination [26] and this phenotype is exacerbated in certain umuD' and umuC mutants [27]. UmuD'2C-dependent inhibition of recombination has also been observed in vitro [28], and is due to the fact that the Umu proteins bind to the deep helical groove of a RecA nucleoprotein filament [29] and prevent heteroduplex formation [28].
Besides exhibiting low-fidelity DNA synthesis on undamaged and damaged DNA, pol V, as we recently discovered, can also misincorporate ribonucleotides (rNTPs) during replication [30]. The relatively low sugar discrimination of pol V is explained by the increased flexibility of the “steric gate” residue of UmuC that controls deoxyribonucleotide vs. ribonucleotide selection [31–34]. We investigated this observation further by generating certain missense mutations at, or near, the steric gate. In particular, we generated variants of pol V containing F10L, Y11A or Y11F substitutions in UmuC. We found that when replicating undamaged DNA, the potentially neutral Y11F substitution was indeed similar to wild-type pol V in its ability to discriminate between ribo- and deoxyribonucleotides in vitro. In contrast, F10L significantly increases discrimination between ribo- and deoxyribonucleotides, and the Y11A UmuC substitution obviated any sugar discrimination and effectively converted the mutant into a bona fide RNA polymerase [30].
The effect of steric gate mutants on the ability of Y-family TLS polymerases to bypass DNA lesions varies. For example, the F13V and F12A steric gate mutations E.coli DinB (pol IV) and S.acidocaldarius Dbh respectively, significantly diminish the efficiency of TLS past an N2-furfuryl-dG adduct [5]. A corresponding F34L substitution in human pol η also leads to a reduced ability to bypass T-T CPDs [35]. In contrast, the effect of a Y112A substitution in the steric gate of human pol κ, depended on the type of the lesion encountered [36]. With regard to E.coli pol V, bacteria expressing the UmuC F10L mutant are unable to bypass a site-specifically placed (6-4) photoproduct [37] and it was previously hypothesized that the hypersensitivity to UV-light conferred by umuC Y11A might be largely due to an inability to bypass UV-induced lesions in vivo [38].
In the present manuscript, we describe the ability of E.coli umuC F10L, Y11A and Y11F steric gate mutants to influence pol V-dependent bypass of a site-specific CPD in vitro and the role these variants play in UV-survival and UV-induced mutagenesis in vivo when they are expressed under physiological conditions.
2. Materials and methods
2.1. Bacterial strains and plasmids
The E. coli K-12 strain used for UV-survival and mutagenesis assays was RW584, (full genotype: ΔumuDC596 ::ermGT lexA51 (Def) recA730 thr-1 araD139 Δ(gpt-proA) 62 lacY1 tsx-33 supE44 galK2 hisG4 rpsL31 xyl-5 mtl-1 argE3 thi-1 sulA211). The low-copy-number plasmids used in this study are derived from pGB2 [39]. The three umuC substitutions were all synthesized and sequences confirmed by Genscript (Piscataway, NJ). Plasmid pRW134 encodes wild-type E.coli umuD'C expressed from its natural promoter [40]. Similarly, plasmids pJM964, pJM963 and pJM952 are all derivatives of pRW134 and express UmuC F10L, Y11A and Y11F respectively, from the native umu promoter [30].
Where noted, bacteria were grown in LB media containing 50 µg/ml spectinomycin, 50 µg/ml kanamycin or 100 µg/ml ampicillin.
2.2. Purification of wild-type pol V and steric gate mutants
The E. coli B strain, RW644 (BL21(λDE3) ΔpolB ::Spec ΔdinB61 ::ble ΔumuDC596 ::ermGT), [41] was used for the expression and purification of pol V variants. RW644 was transformed with the UmuD expression plasmid, pARAD2 along with either pHUC25 (wild-type UmuC), pJM948 (UmuC_F10L), pJM949 (UmuC_Y11A), or pJM950 (UmuC_Y11F) and the resulting pol V complex purified by Ni-NTA agarose affinity chromatography, followed by Superdex 200 size exclusion and Hydroxyapatite chromatography as previously described [30, 41].
2.3. In vitro replication assays
Undamaged pSO and pSOcpd, containing a unique CPD adduct, were constructed as previously described [42]. 5'-32P labeled M13-TT (5’ – GAT-CGA-TGG-TAC-GGA-CG) and SSP1 (5’-TGG-TAC-GGA-CGT-GCT-T) primers were annealed to pSO and pSOcpd ssDNA templates at a 1.5:1 molar ratio by heating in an annealing buffer (50 mM Tris-HCl (pH 8), 5 mM MgCl2, 50 µg/ml BSA, 1.42 mM 2-mercaptoethanol) for 10 min at 100°C followed by slow cooling to room temperature. 4 µM RecA (New England Biolabs, Ipswich, MA) was incubated with 1 mM ATPγS and 0.25 µM 48-mer single-stranded oligonucleotide in the 1× reaction buffer [20 mM Tris-HCl pH 7.5, 8 mM MgCl2, 8 mM DTT, 80 µg/ml BSA, 4% glycerol] at 37 °C for 5 min to produce RecA*. Purified pol V variants (80 nM) were first combined with RecA*(0.25 µM) to form pol V Mut and then added to the main reaction mixture that had been preincubated for 3 min at 37°C. This mixture contained 1 mM ATP, 50 µM dNTPs (all four or each one individually) or rNTPs, 2 nM DNA templates, 100 nM SSB (Epicentre Biotechnologies, Madison WI), 50 nM β clamp, and 5 nM γ complex in the 1× reaction buffer. Reactions were incubated at 37°C for 20 mins and terminated by adding 10 µl of 2× loading buffer [97% formamide, 10 mM EDTA, 0.1% xylene cyanol, 0.1% bromophenol blue]. The products were heat-denatured and resolved by denaturing PAGE (8 M urea, 15% acrylamide), followed by visualization on a Fuji image analyzer FLA-5100.
2.4. UV survival assays
Cells were grown overnight at 37°C in LB media plus spectinomycin. The overnight culture was diluted 1:100 in fresh LB media containing spectinomycin and grown until an OD600 ~0.4. Cells were harvested by centrifugation and resuspended in an equal volume of SM buffer [43]. Cells were irradiated with UV-light (254nm) in a petri dish at a UV-fluence of 2 J/m2. Aliquots were removed at each UV dose and serial dilutions of the culture spread in triplicate on LB agar plates containing spectinomycin. Plates were incubated overnight at 37°C before counting the number of viable colonies. All experiments were repeated in triplicate and were performed under yellow light to avoid unwanted photoreactivation. Error bars represent the standard error of the mean (SEM).
2.5. UV mutagenesis assays
Cells were grown and irradiated as described above for the UV survival assays. However, after irradiation, strains were seeded on Davis and Mingioli salts [44] plus glucose (0.4% wt/vol); agar (1.0% wt/vol); proline, threonine, valine, leucine, and isoleucine (all at 100 µg/ml); thiamine (0.25 µg/ml); and histidine (1 µg/ml). On these plates, less than 1000 viable bacteria grew on the low level of histidine to form small detectable colonies after 2 days. When ~ 4 × 107 bacteria were seeded, they grew to form a lawn, concomitantly exhausting the low level of histidine. His+ mutants grew up through the lawn and were counted after 4 days. The number of pre-existing spontaneous mutants was determined by seeding on minimal plates lacking histidine. The UV-induced mutation frequencies were calculated as previously described [45] and takes into account spontaneously arising mutants, as well as any cell killing that reduces the number of pre-existing His+ mutants in the culture. Experiments were carried out at least three times and were performed under yellow light to avoid unwanted photoreactivation.
3. Results
3.1. TLS of a CPD by UmuC variants in vitro
Using a recently developed novel method [41], we have purified three E.coli pol V variants with substitutions in the vicinity of the steric gate in UmuC in order to characterize their sugar selectivity and base substitution fidelity [30]. To extend these studies, we determined the effect of the steric gate mutations on the ability of pol V to facilitate TLS of a single site-specific thymine-thymine CPD in vitro. To do so, we performed “standing-start” primer extension assays using a circular single-stranded DNA template containing a unique T-T CPD lesion [42]. The reactions were catalyzed by wild-type pol V or F10L, Y11A, or Y11F UmuC variants [30] and carried out in the presence of transactivating RecA*, which is required for pol V activity [46, 47], along with accessory cofactors (β-clamp and SSB protein) that significantly enhance the catalytic activity of pol V in vitro [41]. As seen in Fig. 1, all pol V variants were able to facilitate TLS of the CPD. However, while TLS catalyzed by wild-type pol V, Y11A, and Y11F were similarly efficient (Fig.1E, G, H), F10L exhibited reduced bypass of the CPD, and in contrast to the other enzymes, had a strong pause site opposite the 5'T of the CPD (Fig.1F).
Fig. 1.
TLS of a T-T CPD by wild-type and mutant pol V. Time-course studies of TLS by wild-type pol V (A, E), F10L (B, F), Y11A (C, G), or Y11F (D, H) were conducted for 2, 4, 8, and 16 min (lanes 1–4 respectively) using primers with 3' ends juxtaposed to the undamaged (A–D) or CPD-containing T-T (E–H) and 100 µM dNTPs. The numbers next to the polymerase designations at the bottom of the image represent the extent of total primer elongation on the damaged template (first number) and primer extension past the 5' T of TT-CPD (second number, which corresponds to the complete lesion bypass), and is expressed as a percentage of the total primer termini. The numbers reported are from the 16 min time point (lane 4). The position of the primer and well are indicated by arrows at the bottom and top of the gel respectively. The position of undamaged Ts, or the T-T CPD in the template are also indicated on the left of the gel.
As noted above, we have recently found that pol V is characterized by unusually low sugar selectivity that could be modified by mutating the steric gate residue, Y11, or the neighboring residue, F10 [30]. To determine whether rNTP incorporation might influence TLS of the CPD, we compared in vitro assays in the presence of rNTPs, dNTPs, or a mixture of both at a physiologically relevant ratio of 9:1 (rNTPs:dNTPs) [48]. The reaction products were then subjected to alkali cleavage, which hydrolyzes the DNA backbone at incorporated rNMPs (Fig. 2). In the absence of dNTPs, TLS was negligible when catalyzed by wild-type pol V and Y11F and completely abolished in the case of F10L (Fig. 2A, B and D, lanes 1, 2), suggesting that these polymerases are unable to insert ATP opposite the covalently linked T-T bases of the CPD. There is also no evidence that rNTPs were incorporated at any significant levels when a nucleotide mix was used, despite the fact that all four rNTPs were present in 9-fold excess. This conclusion is based on the absence of any noticeable change in product distribution profiles after alkali treatment (compare lanes 3 and 4). In contrast, in reactions containing Y11A, most of the elongation products were cleaved by alkali (Fig. 2C). It should be noted that all reactions, including those indicated as performed in the absence of any rNTPs (Fig. 2C, lanes 5, 6), actually contain ATP, as it is an integral part of the reaction mixture required for proper assembly of the replication complex. Therefore, Y11A appears to favor ATP incorporation opposite both undamaged and damaged Ts. When the fidelity of TLS was assayed in single nucleotide reactions, ~60% of primers were extended by the incorporation of ATP opposite the 3' and 5'T and 80% of the primers were further elongated, resulting in formation of rA:C mispair (Fig. 3C, lane 1). The same products formed when either dATP or dCTP were added to the reactions (Fig. 3C, lanes 2 and 4) suggesting that Y11A prefers ATP over these deoxynucleotides while replicating UV-damaged DNA. In contrast, dGTP and, to a larger extent, dTTP outcompeted ATP on the CPD template (Fig. 3C, lanes 3, 5). As seen with ATP (lane 1), dTTP incorporation generally terminated after misincorporation opposite C (5' to the TT-CPD), although significantly longer (by up to ~ 18 nucleotides, 6 of which would be wrong) replication products were also formed (Fig. 4C, lane 3). In reactions where dGTP and ATP were in competition, the major stop sites for dGTP were opposite the 5'T of CPD and opposite G at the +3 position after the TT-CPD (Fig. 3C, lane 4). Therefore, it appears that on the damaged DNA, Y11A is much more error-prone for dNTP misincorporations (wrong base/correct sugar) than when copying an undamaged template [30], where it preferentially incorporates the Watson-Crick rNTP (wrong sugar /correct base).
Fig. 2.
Alkaline digestion of TLS products obtained in reactions catalyzed by wild-type and mutant pol V in the presence of dNTPs and/or rNTPs. Wild-type pol V (A), F10L (B), Y11A (C), or Y11F (D) catalyzed primer extensions were conducted using primers with 3' ends juxtaposed to the T-T CPD and 100 µM rNTPs (r, lanes 1, 2), 90 µM rNTPs + 10 µM dNTPs (r/d, lanes 3, 4), or 100 µM dNTPs (d, lanes 5, 6) for 5 min. Half of each reaction was subjected to alkaline hydrolysis (OH−) (lanes 2, 4, 6).
Fig. 3.
Specificity of nucleotide incorporation opposite a TT-CPD by wild-type and mutant pol V. Reactions catalyzed by wild-type pol V (A), F10L (B), Y11A (C), or Y11F (D) were performed in the presence 100 µM individual dNTP for 5 min. The identity of the nucleotide incorporated and percentage of primer extensions is indicated below each track. All reactions contained 1 mM ATP. Reactions with no additional nucleotide are indicated by a dash (-). The extended sequence of the template, the position of the primer, as well as the 3'T and 5'T of the CPD are shown on the right.
Fig. 4.
UV survival and mutagenesis of a recA730 lexA51 (Def) ΔumuDC strain expressing wild-type UmuD'C or steric gate variants on a low-copy plasmid from the native umu promoter. (A) UV survival: Exponentially growing cells were exposed to various doses of UV-light and the number of viable cells determined after plating serial dilutions on LB plates containing spectinomycin. The number of viable colonies was determined after overnight incubation at 37°C. Error bars indicate the standard error of the mean (B) UV mutagenesis: Exponentially growing cells were exposed to 20 J/m2 UV light and plated on Davis and Mingioli minimal plates containing a trace amount of Histidine. The number of viable cells and UV-induced His+ revertants was determined after four days incubation at 37 °C. Cell viability after exposure to 20 J/m2 was in the range of 85–90% survival for wild-type, F10L and Y11F mutants and ~60–70% for vector control, pGB2, and the Y11A mutant.
In contrast, but similar to the increased fidelity seen during replication of an undamaged DNA [30], F10L was quite accurate on the CPD-containing template, predominantly incorporating dATP opposite the 3'T of the CPD (Fig. 3B, lane 2). Contrary to the low sugar selectivity observed during nucleotide insertion opposite the undamaged Ts by wild-type pol V and Y11F [30], these polymerases showed almost no incorporation of ATP opposite the TT-CPD (Fig. 3A & D, lanes 1), which is consistent with the lack of primer extension in the presence of all four rNTPs (Fig. 2A & D, lanes 1& 2). As shown in Fig. 3A and 3D, wild-type pol V and Y11F exhibited a preference for dATP even though other dNTPs were also incorporated at different levels in the order of dGTP ≥dTTP >dCTP.
3.2. UV-sensitivity of E.coli expressing umuC steric gate mutants
Since all three UmuC steric gate mutants were able to bypass a single CPD in vitro (to varying degrees), we wanted to determine how this ability might contribute to UV-survival in vivo. To do so, we cloned the steric gate umuC mutants into pRW134, a low-copy-number plasmid expressing wild-type umuD'C from its natural promoter [40]. The resulting plasmids,pJM964 (umuD'C_F10L); pJM963 (umuD'C_Y11A); and pJM952 (umuD'C_Y11F), along with pRW134 (wild-type umuD'C) and pGB2 (vector control) [39] were introduced into the ΔumuDC, recA730 lexA51 (Def) E.coli K-12 strain, RW584 [49] and their effects on UV-survival was analyzed (Fig. 4A). We chose this strain background, because the lexA51 (Def) allele encodes a mutant LexA protein that is unable to act as a transcriptional repressor and as a consequence all SOS-regulated genes are fully derepressed. In addition, the recA730 allele encodes for a mutant RecA protein that spontaneously forms RecA nucleoprotein filaments in vivo (RecA*) and as a consequence, pol V’s activity is enhanced [50]. Under these conditions and as expected, both wild-type umuD'C and umuD'C_Y11F had a significant protective effect on UV-survival. The umuD'C_F10L expressing plasmid also promoted considerable UV-resistance that was virtually indistinguishable from wild-type pol V (Fig. 4A), despite exhibiting a somewhat diminished capacity of the F10L mutant to bypass a T-T CPD in vitro. Surprisingly, despite catalyzing efficient replication of the DNA template with a CPD lesion in vitro, the umuD'C_Y11A mutant conferred the least UV-resistance of all the pol V variants analyzed and the umuD'C_Y11A strain was only slightly more UV-resistant than the strain bearing the control vector, pGB2 (Fig 4A).
Our observations differ significantly from those of Shurtleff et al, who reported that umuD'C_Y11A conferred UV-hypersensitivity on wild-type (recA+ lexA+) E.coli [38]. However, we believe that the difference in the phenotypes can be simply explained by the expression conditions of UmuD'C employed in the two studies. In the Shurtleff et al., study UmuD'C_Y11A was expressed on a low copy plasmid from the Oc1 umu promoter. The Oc1 promoter mutation not only leads to the selective derepression of the umu operon whilst all other lexA -regulated genes remain repressed, but also increases the strength of the −10 promoter element [51]. As a consequence, UmuD'C is expressed at significantly higher cellular levels from the Oc1 umu promoter than from its native promoter in both, recA+ lexA+ (Fig. S1A & B) and recA730 lexA51 (Def) (Fig. S1C & D) backgrounds. Overexpression of wild-type UmuD'C from the Oc1 promoter in a recA+ lexA+ strain is known to inhibit RecA-dependent recombination [[26], Fig. S2A], and we deduce that the UV-hypersensitivity observed by Shurtleff et al., [[38], Fig. S3B], is largely caused by enhanced inhibition of RecA-dependent recombination by UmuD'C_Y11A when it is overexpressed from the Oc1 promoter in a recA+ lexA+ strain (Fig. S2A). Indeed, no inhibition of recombination (Fig. S2), or UV-hypersensitivity (Fig. S3A & C and Fig. 4) is observed when UmuD'C_Y11A is expressed on a low-copy plasmid from its natural promoter either in the recA+ lexA+ strain or in the recA730 lexA (Def) strain employed in the current study.
3.3. UV-mutability of E.coli expressing umuC steric gate mutants
We were interested in determining the effects that the various umuC steric gate alleles have on the level of pol V-dependent UV-induced mutagenesis in vivo in the absence of any additional effects of recombination-inhibition. UV-induced mutagenesis was therefore monitored in the recA730 lexA (Def) ΔumuDC strain, RW584, harboring low-copy number plasmids expressing the Umu proteins from their native promoter. In this strain background, wild-type pol V promoted high levels of UV-induced mutagenesis (Fig. 4B). Similar to spontaneous mutagenesis [30], UV-induced mutagenesis in the umuD'C_Y11F strain was higher than the wild-type strain (Fig. 4B). In contrast, umuD'C_F10L was virtually UV non-mutable (Fig. 4B). Such observations are, however, entirely consistent with our in vitro studies showing that UmuC_F10L bypasses a T-T CPD with high fidelity in vitro (Fig. 3). Despite exhibiting low fidelity DNA and RNA synthesis during bypass of a T-T- CPD in vitro (Fig 1), expression of umuD'C_Y11A also resulted in minimal UV-induced mutagenesis. Rather than invoking a change in polymerase properties between the in vitro and in vivo assays, we suggest that the low levels of UV-induced mutagenesis do not reflect an inability of the mutant pol V enzyme to traverse UV-induced lesions in vivo but more likely reflect the action of additional DNA repair pathways that are induced by excessive ribonucleotide incorporation during TLS in vivo, but which are absent from our in vitro assays.
4. Discussion
We have recently characterized three steric gate mutants of UmuC (F10L, Y11A and Y11F) for their ability to incorporate ribonucleotides into undamaged DNA in vitro. Given their proximity to the active site of pol V, steric gate mutants not only alter sugar discrimination, but are likely to affect the ability of the polymerase to perform TLS [34]. The goal of our study was, therefore, aimed at determining the effect of steric gate mutants on the ability of pol V to bypass the most common UV-induced DNA lesion (a thymine-thymine CPD) in vitro, as well as to analyze their effects on UV-induced mutagenesis and cell survival in vivo.
We have recently discovered that wild-type pol V can efficiently incorporate ribonucleotides into DNA when replicating an undamaged template [30]. Our molecular modeling studies of the UmuC active site suggest that its ability to incorporate rNTPs results from a non-conserved loop structure in the vicinity of the polymerase steric gate causing increased flexibility that allows for accommodation of the 2'-OH of the incoming ribonucleotide [30]. In contrast to the situation observed on an undamaged template, we found no evidence of ribonucleotide incorporation during pol V-dependent TLS of the T-T CPD in vitro (Fig. 2). It seems reasonable to suggest that accommodation of the covalently linked thymines of the CPD in the polymerase active site diminishes its overall flexibility, thus preventing binding of an incoming ribonucleotide (Fig. 5A & B). However, we cannot exclude the possibility of ribonucleotide incorporation during TLS of other DNA lesions that do not constrain the movement of Y11.
Fig. 5.
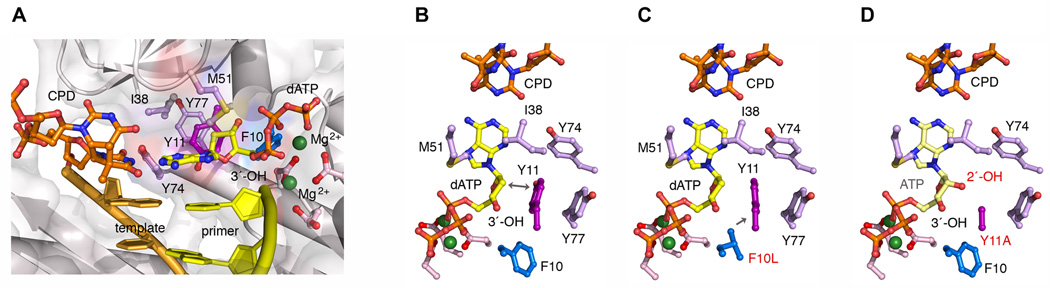
Structure models of UmuC and variants. (A) A model of wild-type UmuC inserting dATP opposite a thymine-thymine CPD. The UmuC model is based on human DNA pol η structure (PDB 3MR3) with amino acid substitutions made following the sequence alignment by HHpred (http://hhpred.tuebingen.mpg.de/hhpred). Residues surrounding the incoming nucleotide are modeled and labeled according to the UmuC sequence. The protein structure is shown in ribbon diagram covered with semi-transparent surface. Y11, the steric gate, is shown in purple sticks, F10 in blue, and other residues (mostly hydrophobic) are shown in light purple. M51 replaces the Arg conserved among polη orthologs. Close –up view of a thymine-thymine CPD and dATP in the active site of wild-type UmuC (B), and F10L_UmuC (C), and ATP in the active site of Y11A_UmuC (D). The view is approximately looking down the DNA helical axis. The protein and DNA polymer chains are omitted for clarity. In the native UmuC (A & B), the deoxyribose of the primer terminus is juxtaposed to Y11 and the side-chain of Y11 cannot rotate away to accommodate a ribose. In the case of F10L (C), the branched side-chain presses on the benzene ring of Y11 (indicated by the arrow), which requires Y11 to rotate slightly. As a result, the side-chain of Y11 is closer to the C2´ than in the native protein and cannot accommodate misinsertions opposite the CPD. Y11A (D) leaves a void around the incoming nucleotide, which not only relaxes selection of the sugar as a modeled ribose can be readily accommodated, but also relaxes the minor groove alignment for correct Watson-Crick base pair. O2´ is modeled with Y11A based on the ATPγS structure from PDB-3EQC.
In vitro, the F10L mutant exhibited a slightly reduced ability to traverse the major lesion that is induced by UV-light in vivo, (a T-T CPD) compared to wild-type pol V, or Y11A and Y11F variants, but it was nevertheless sufficient to confer wild-type levels of UV-resistance in vivo. Our observations therefore suggest that the ability of the UmuC_F10L mutant to bypass CPDs in vivo is much more important to overall cell survival than its inability to bypass the much less prevalent 6-4 T-T dimer [37]. The F10L variant bypassed the T-T CPD with higher fidelity than wild-type pol V and this equated to much lower levels of UV induced mutagenesis in vivo. In this regard, the UmuC_F10L mutant is akin to human polη, which is also unable to bypass a 6-4 photoproduct, yet is characterized by its ability to bypass T-T CPDs accurately and efficiently and in doing so, protects humans from the deleterious consequences of UV-irradiation [52, 53]. Molecular modeling of the F10L mutant onto the structure of human polη [54], suggests that the higher base substitution fidelity is achieved by the branched side-chain of L10 impinging on the benzene ring of Y11, which restricts nucleotide misincorporation opposite the T-T CPD (Fig. 5C). Presumably, the rigidity would also preclude pol V from accommodating the more distorting UV-induced 6-4 photoproduct and explain why the F10L mutant is unable to facilitate bypass of the 6-4PP lesion in vivo [37].
The UmuC Y11A substitution resulted in several unforeseen phenotypes. In vitro, the mutant enzyme is characterized by low fidelity and proficiency for incorporating ribonucleotides into undamaged DNA [30]. Here, we show that it bypasses a T-T CPD with equal or greater efficiency than wild-type pol V (Fig. 1). In contrast to wild-type pol V, it also incorporates ribonucleotides during TLS (Fig. 2).
Molecular modeling indicates that substitution of Y11 with the much smaller alanine residue leaves a void in the active site pol V, and this apparently allows it to accommodate both the T-T CPD and a ribonucleotide simultaneously (Fig. 5D). Based on these properties, we expected that the Y11A mutant would give rise to considerable UV-resistance and a very high level of cellular mutagenesis in vivo. However, neither phenotype was observed. In the recA730 lexA (Def) ΔumuDC strain, umuD'C_Y11A conferred minimal UV-resistance (Fig. 4A). Most strikingly, umuD'C_Y11A also promoted low levels of UV-induced mutagenesis that were similar to those observed for the much higher fidelity umuD'C_F10L mutant (Fig. 4B).
We suggest that both the lower than expected UV-resistance and lower UV-mutability can be explained by excessive error-prone incorporation of rNTPs into the E.coli genome that occurs during pol V_Y11A-dependent TLS of UV-induced DNA lesions. We recognize that the presence of multiple ribonucleotides in the TLS “patch” by itself can decrease a cell’s viability because a reactive 2' hydroxyl on the ribose ring makes a DNA strand with embedded rNMPs more susceptible to spontaneous or enzymatic cleavage. However, we believe that a more plausible scenario involves rNMPs processing. The incorporation of errant ribonucleotides in DNA can trigger a repair pathway specifically targeted to rNMP removal and in the process of TLS “patch” repair, misincorporated deoxyribonucleotides are also removed from the genome. Presumably, the RNA targeted repair pathway involves higher fidelity enzyme(s) that are unable to bypass UV-photoproducts as efficiently as pol V. This would not only result in the observed decrease in mutagenesis, but also in reduced UV-resistance.
Supplementary Material
Acknowledgements
This work was supported in part by the National Institute of Child Health and Human Development/National Institutes of Health Intramural Research Program to RW; the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health Intramural Research Program to WY; the Special Coordination Funds for Promoting Science and Technology of the Japanese Ministry of Education, Culture, Sports, Science and Technology to KK; and National Institutes of Health [GM21422, ESO12259] to MFG.
Abbreviations
- pol
DNA polymerase
- rNTPs
ribonucleotides
- dNTPs
deoxyribonucleotides
- ATPγS
adenosine 5’[γ-thio]triphosphate
- CPD
cyclobutane pyrimidine dimer
- ssDNA
single-stranded DNA
- pol
DNA polymerase
- SSB
single-stranded DNA binding protein
- TLS
translesion DNA synthesis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflict of interest
References
- 1.Friedberg EC, Walker GC, Siede W, Wood R, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. 2nd ed. Washington, DC: ASM Press; 2006. [Google Scholar]
- 2.Bonner CA, Hays S, McEntee K, Goodman MF. DNA polymerase II is encoded by the DNA damage-inducible dinA gene of Escherichia coli. Proc. Natl. Acad. Sci. USA. 1990;87:7663–7667. doi: 10.1073/pnas.87.19.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner J, Gruz P, Kim SR, Yamada M, Matsui K, Fuchs RPP, Nohmi T. The dinB gene encodes an novel Escherichia coli DNA polymerase (DNA pol IV) involved in mutagenesis. Mol Cell. 1999;4:281–286. doi: 10.1016/s1097-2765(00)80376-7. [DOI] [PubMed] [Google Scholar]
- 4.Tang M, Shen X, Frank EG, O'Donnell M, Woodgate R, Goodman MF. UmuD'2C is an error-prone DNA polymerase, Escherichia coli DNA pol V. Proc Natl Acad Sci USA. 1999;96:8919–8924. doi: 10.1073/pnas.96.16.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarosz DF, Godoy VG, Delaney JC, Essigmann JM, Walker GC. A single amino acid governs enhanced activity of DinB DNA polymerases on damaged templates. Nature. 2006;439:225–228. doi: 10.1038/nature04318. [DOI] [PubMed] [Google Scholar]
- 6.Yuan B, Cao H, Jiang Y, Hong H, Wang Y. Efficient and accurate bypass of N2-(1-carboxyethyl)-2'-deoxyguanosine by DinB DNA polymerase in vitro and in vivo. Proc Natl Acad Sci U S A. 2008;105:8679–8684. doi: 10.1073/pnas.0711546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato T, Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977;156:121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- 8.Steinborn G. Uvm mutants of Escherichia coli K12 deficient in UV mutagenesis. I. Isolation of uvm mutants and their phenotypical characterization in DNA repair and mutagenesis. Mol Gen Genet. 1978;165:87–93. doi: 10.1007/BF00270380. [DOI] [PubMed] [Google Scholar]
- 9.Bagg A, Kenyon CJ, Walker GC. Inducibility of a gene product required for UV and chemical mutagenesis in Escherichia coli. Proc Natl Acad Sci USA. 1981;78:5749–5753. doi: 10.1073/pnas.78.9.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández de Henestrosa AR, Ogi T, Aoyagi S, Chafin D, Hayes JJ, Ohmori H, Woodgate R. Identification of additional genes belonging to the LexA-regulon in Escherichia coli. Mol. Microbiol. 2000;35:1560–1572. doi: 10.1046/j.1365-2958.2000.01826.x. [DOI] [PubMed] [Google Scholar]
- 11.Courcelle J, Khodursky A, Peter B, Brown PO, Hanawalt PC. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics. 2001;158:41–64. doi: 10.1093/genetics/158.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burckhardt SE, Woodgate R, Scheuermann RH, Echols H. UmuD mutagenesis protein of Escherichia coli: overproduction, purification and cleavage by RecA. Proc Natl Acad Sci USA. 1988;85:1811–1815. doi: 10.1073/pnas.85.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinagawa H, Iwasaki H, Kato T, Nakata A. RecA protein-dependent cleavage of UmuD protein and SOS mutagenesis. Proc Natl Acad Sci USA. 1988;85:1806–1810. doi: 10.1073/pnas.85.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nohmi T, Battista JR, Dodson LA, Walker GC. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc Natl Acad Sci USA. 1988;85:1816–1820. doi: 10.1073/pnas.85.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Battista JR, Ohta T, Nohmi T, Sun W, Walker GC. Dominant negative umuD mutations decreasing RecA-mediated cleavage suggest roles for intact UmuD in modulation of SOS mutagenesis. Proc Natl Acad Sci USA. 1990;87:7190–7194. doi: 10.1073/pnas.87.18.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sommer S, Boudsocq F, Devoret R, Bailone A. Specific RecA amino acid changes affect RecA-UmuD'C, interaction. Mol.Microbiol. 1998;28:281–291. doi: 10.1046/j.1365-2958.1998.00803.x. [DOI] [PubMed] [Google Scholar]
- 17.Opperman T, Murli S, Smith BT, Walker GC. A model for a umuDC-dependent prokaryotic DNA damage checkpoint. Proc Natl Acad Sci USA. 1999;96:9218–9223. doi: 10.1073/pnas.96.16.9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank EG, Ennis DG, Gonzalez M, Levine AS, Woodgate R. Regulation of SOS mutagenesis by proteolysis. Proc Natl Acad Sci USA. 1996;93:10291–10296. doi: 10.1073/pnas.93.19.10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez M, Frank EG, Levine AS, Woodgate R. Lon-mediated proteolysis of the Escherichia coli UmuD mutagenesis protein: in vitro degradation and identification of residues required for proteolysis. Genes & Dev. 1998;12:3889–3899. doi: 10.1101/gad.12.24.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez M, Rasulova F, Maurizi MR, Woodgate R. Subunit-specific degradation of the UmuD/D', heterodimer by the ClpXP protease: The role of trans recognition in UmuD' stability. EMBO J. 2000;19:5251–5258. doi: 10.1093/emboj/19.19.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodgate R, Ennis DG. Levels of chromosomally encoded Umu proteins and requirements for in vivo UmuD cleavage. Mol.Gen.Genet. 1991;229:10–16. doi: 10.1007/BF00264207. [DOI] [PubMed] [Google Scholar]
- 22.Boudsocq F, Campbell M, Devoret R, Bailone A. Quantitation of the inhibition of Hfr x F− recombination by the mutagenesis complex UmuD'C. J. Mol. Biol. 1997;270:201–211. doi: 10.1006/jmbi.1997.1098. [DOI] [PubMed] [Google Scholar]
- 23.Opperman T, Murli S, Walker GC. The genetic requirements for UmuDC-mediated cold sensitivity are distinct from those for SOS mutagenesis. J.Bacteriol. 1996;178:4400–4411. doi: 10.1128/jb.178.15.4400-4411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murli S, Opperman T, Smith BT, Walker GC. A role for the umuDC gene products of Escherichia coli in increasing resistance to DNA damage in stationary phase by inhibiting the transition to exponential growth. J.Bacteriol. 2000;182:1127–1135. doi: 10.1128/jb.182.4.1127-1135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton MD, Walker GC. umuDC-mediated cold sensitivity is a manifestation of functions of the UmuD(2)C complex involved in a DNA damage checkpoint control. J.Bacteriol. 2001;183:1215–1224. doi: 10.1128/JB.183.4.1215-1224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommer S, Bailone A, Devoret R. The appearance of the UmuD'C, protein complex in Escherichia coli switches repair from homologous recombination to SOS mutagenesis. Mol. Microbiol. 1993;10:963–971. doi: 10.1111/j.1365-2958.1993.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 27.Sommer S, Coste G, Bailone A. Specific amino acid changes enhance the antirecombination activity of the UmuD'C, complex. Mol.Microbiol. 2000;35:1443–1453. doi: 10.1046/j.1365-2958.2000.01809.x. [DOI] [PubMed] [Google Scholar]
- 28.Rehrauer WM, Bruck I, Woodgate R, Goodman MF, Kowalczykowski SC. Modulation of recombination function by the mutagenic UmuD'C, protein complex. J.Biol.Chem. 1998;273:32384–32387. doi: 10.1074/jbc.273.49.32384. [DOI] [PubMed] [Google Scholar]
- 29.Frank EG, Cheng N, Do CC, Cerritelli ME, Bruck I, Goodman MF, Egelman EH, Woodgate R, Steven AC. Visualization of two binding sites for the Escherichia coli UmuD'2C complex (DNA pol V) on RecA-ssDNA filaments. J.Mol.Biol. 2000;297:585–597. doi: 10.1006/jmbi.2000.3591. [DOI] [PubMed] [Google Scholar]
- 30.Vaisman A, Kuban W, McDonald JP, Karata K, Yang W, Goodman MF, Woodgate R. Critical amino acids in Escherichis coli responsible for sugar discrimination and base-substitution fidelity. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks233. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Astatke M, Ng K, Grindley ND, Joyce CM. A single side chain prevents Escherichia coli DNA polymerase I (Klenow fragment) from incorporating ribonucleotides. Proc Natl Acad Sci U S A. 1998;95:3402–3407. doi: 10.1073/pnas.95.7.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLucia AM, Chaudhuri S, Potapova O, Grindley ND, Joyce CM. The properties of steric gate mutants reveal different constraints within the active sites of Y-family and A-family DNA polymerases. J Biol Chem. 2006;281:27286–27291. doi: 10.1074/jbc.M604393200. [DOI] [PubMed] [Google Scholar]
- 33.DeLucia AM, Grindley ND, Joyce CM. An error-prone family Y DNA polymerase (DinB homolog from Sulfolobus solfataricus) uses a 'steric gate' residue for discrimination against ribonucleotides. Nucleic Acids Res. 2003;31:4129–4137. doi: 10.1093/nar/gkg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown JA, Suo Z. Unlocking the sugar "steric gate" of DNA polymerases. Biochemistry. 2011;50:1135–1142. doi: 10.1021/bi101915z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niimi A, Limsirichaikul S, Yoshida S, Iwai S, Masutani C, Hanaoka F, Kool ET, Nishiyama Y, Suzuki M. Palm mutants in DNA polymerases α and η alter DNA replication fidelity and translesion activity. Mol.Cell.Biol. 2004;24:2734–2746. doi: 10.1128/MCB.24.7.2734-2746.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niimi N, Sassa A, Katafuchi A, Gruz P, Fujimoto H, Bonala RR, Johnson F, Ohta T, Nohmi T. The steric gate amino acid tyrosine 112 is required for efficient mismatched-primer extension by human DNA polymerase κ. Biochemistry. 2009;48:4239–4246. doi: 10.1021/bi900153t. [DOI] [PubMed] [Google Scholar]
- 37.Sommer S, Becherel OJ, Coste G, Bailone A, Fuchs RP. Altered translesion synthesis in E. coli Pol V mutants selected for increased recombination inhibition. DNA repair. 2003;2:1361–1369. doi: 10.1016/j.dnarep.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Shurtleff BW, Ollivierre JN, Tehrani M, Walker GC, Beuning PJ. Steric gate variants of UmuC confer UV hypersensitivity on Escherichia coli. J Bacteriol. 2009;191:4815–4823. doi: 10.1128/JB.01742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Churchward G, Belin D, Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984;31:165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- 40.Szekeres ESJ, Woodgate R, Lawrence CW. Substitution of mucAB or rumAB for umuDC alters the relative frequencies of the two classes of mutations induced by a site-specific T-T cyclobutane dimer and the efficiency of translesion DNA synthesis. J.Bacteriol. 1996;178:2559–2563. doi: 10.1128/jb.178.9.2559-2563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karata K, Vaisman A, Goodman MF, Woodgate R. Simple and efficient purification of E.coli DNA polymerase V: cofactor requirements for optimal activity and processivity in vitro. DNA Repair. 2012;11:431–440. doi: 10.1016/j.dnarep.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karata K, Vidal AE, Woodgate R. Construction of a circular single-stranded DNA template containing a defined lesion. DNA Repair. 2009;8:852–856. doi: 10.1016/j.dnarep.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller JH. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 44.Davis BD, Mingioli ES. Mutants of Escherichia coli requiring methionine or vitamin B12. J.Bacteriol. 1950;60:17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sedgwick SG, Bridges BA. Survival, mutation and capacity to repair single strand DNA breaks after gamma irradiation in different exr− strains of Escherichia coli. Mol.Gen.Genet. 1972;119:93–102. doi: 10.1007/BF00269129. [DOI] [PubMed] [Google Scholar]
- 46.Schlacher K, Cox MM, Woodgate R, Goodman MF. RecA acts in trans to allow replication of damaged DNA by DNA polymerase V. Nature. 2006;442:883–887. doi: 10.1038/nature05042. [DOI] [PubMed] [Google Scholar]
- 47.Jiang Q, Karata K, Woodgate R, Cox MM, Goodman MF. The active form of DNA polymerase V is UmuD'2C-RecA-ATP. Nature. 2009;460:359–363. doi: 10.1038/nature08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neuhard J, Nygaard P. In: Purines and pyrimidines, Escherichia coli and Salmonella typhimurium: Cellular and molecular biology. Ingraham JL, Low KB, Neidhardt FC, Magasanik B, Schaechter M, Umbarger HE, editors. Washington D.C.: American Society for Microbiology; 1987. pp. 445–473. [Google Scholar]
- 49.Vandewiele D, Fernández de Henestrosa AR, Timms AR, Bridges BA, Woodgate R. Sequence analysis and phenotypes of five temperature sensitive mutator alleles of dnaE encoding modified a-catalytic subunits of Escherichia coli DNA polymerase III holoenzyme. Mutat.Res. 2002;499:85–95. doi: 10.1016/s0027-5107(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 50.Sweasy JB, Witkin EM, Sinha N, Roegner-Maniscalco V. RecA protein of Escherichia coli has a third essential role in SOS mutator activity. J.Bacteriol. 1990;172:3030–3036. doi: 10.1128/jb.172.6.3030-3036.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sommer S, Knezevic J, Bailone A, Devoret R. Induction of only one SOS operon, umuDC is required for SOS mutagenesis in Escherichia coli. Mol.Gen.Genet. 1993;239:137–144. doi: 10.1007/BF00281612. [DOI] [PubMed] [Google Scholar]
- 52.Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, Araki M, Iwai S, Takio K, Hanaoka F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase |. Nature. 1999;399:700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- 53.Masutani C, Araki M, Yamada A, Kusumoto R, Nogimori T, Maekawa T, Iwai S, Hanaoka F. Xeroderma pigmentosum variant (XP-V) correcting protein from HeLa cells has a thymine dimer bypass DNA polymerase activity. EMBO J. 1999;18:3491–3501. doi: 10.1093/emboj/18.12.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biertümpfel C, Zhao Y, Kondo Y, Ramon-Maiques S, Gregory M, Lee JY, Masutani C, Lehmann AR, Hanaoka F, Yang W. Structure and mechanism of human DNA polymerase η. Nature. 2010;465:1044–1048. doi: 10.1038/nature09196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.