Abstract
BACKGROUND
Prostate-Specific Antigen (PSA) is a serine protease whose expression is maintained in all stages of prostate cancer. A role for PSA in the pathobiology for prostate cancer has not been firmly established. Experimental studies to date support a role for PSA through mechanisms such as release or processing of growth factors and degradation of the extracellular matrix. Exposure of prostate cancer cells to exogenous PSA also results in gene expression changes. These in vitro and biochemical assays rely on the use of commercially available PSA. Contamination of these commercial preparations can significantly impact the results of these in vitro studies.
METHODS
We characterized PSA and trypsin-like activity of PSA preparations obtained from three commercial sources: Calbiochem, Fitzgerald, and AbD Serotec. Silver stained gels were used to compare the purity of each preparation and mass spectrometry was performed to characterize contaminating proteases.
RESULTS
PSA activity varied between PSA preparations with AbD Serotec PSA having highest degree of activity. Significant trypsin-like activity, which was inhibited by aprotinin, was observed in PSA preparations from Calbiochem and Fitzgerald, but not AbD Serotec. These former two PSA preparations also contained the greatest degree of non-PSA contaminants by silver stain and mass spectrometry.
CONCLUSIONS
Commercially available preparations of PSA contain contaminating proteins, including trypsin-like protease activity, that could potentially complicate the interpretation of results obtained from in vitro studies assessing PSA proteolysis of potential protein substrates and effects of PSA on gene expression.
Keywords: PSA, trypsin, contamination, commercially
INTRODUCTION
Prostate-Specific Antigen (PSA) is a 33 kDa glycosylated serine protease belonging to the human kallikrein gene family (reviewed in [1]). PSA is aptly named, in that it is exclusively produced in large amounts by both normal and malignant prostate epithelial cells but is not produced in any significant amounts by other normal tissue in the human male. On this basis, PSA is used extensively as a biomarker to screen for prostate cancer, to detect recurrence following local therapies, and to follow response to systemic therapies for metastatic disease [2,3]. While important in these contexts as a biomarker, the functional significance of PSA in the development and progression of prostate cancer is not known. However, previous studies have demonstrated that PSA can cleave a number of proteins that are important in cancer cell biology that include insulin-like growth factor binding proteins [4], the small latent for of TGFß2 [5], parathyroid-hormone-related protein (PTHrP) [6,7] and the extracellular matrix components fibronectin and laminin [8]. Additional studies have demonstrated that exogenous PSA could affect the growth of osteoblastic and endothelial cells and could also significantly alter gene expression in a number of model systems [9–15].
For the most part, these in vitro biochemical and cellular studies have been performed using PSA that has been purified from human semen. PSA is the most abundant (i.e., mg/ml concentration) of several kallikreins that are present in the seminal fluid that include KLK2, [4,11]. These kallikreins share considerable sequence homology with PSA and are of similar molecular weight [16,17]. In the freshly ejaculated semen PSA’s major function is to maintain the semen in a semi-liquid state through its ability to cleave the major gel-forming proteins semenogelin I (SgI) and semenogelin II (SgII), which are synthesized and secreted by the seminal vesicles [18–20]. PSA was first purified from seminal fluid by Wang et al. [21] and was later demonstrated to be a serine protease [18,22,23]. In early studies, PSA was reported to possess trypsin-like activity, which is a feature common to the majority of proteins in the kallikrein family [18,22]. However, once the structure and sequence of PSA became known, it was apparent that PSA was a chymotrypsin-like protease due to the presence of serine at the base of the S1 specificity pocket [22,24,25]. With this information, the Lilja laboratory developed an additional purification step for PSA in which the seminal fluid is run through a column containing immobilized aprotinin which removes the arginine-restricted trypsin-like proteases present in the seminal fluid [26].
Unlike these earlier studies in which investigators purified their own PSA, it is now common for researchers to use commercially available PSA for their experiments. Enzymatically active PSA is available for purchase from a number of commercial vendors who purify PSA from human seminal plasma by precipitation and/or various chromatographic techniques. In our laboratory we have used such commercial PSA in the development of a PSA-activated form of proaerolysin, a potent protein toxin [27]. Over the course of these studies, we observed that preparations of commercially available PSA could activate the wild type proaerolysin which contained an arginine-rich furin activation motif. These results suggested that trypsin-like protease may be contaminating the purified PSA used in these experiments. Given the remarkable proteolytic activity of trypsinlike kallikreins as KLK2 [28,29], even small amounts of contamination could confound the results. In addition, as these commercial PSA preparations are used with increasing frequency to probe the role of PSA in the pathobiology of prostate cancer, it is critical for the interpretation of the results of these experiments that contaminants are not present that would confound the results. Thus, in this study we set out to determine the degree of contamination in commercially available PSA preparations purchased from three vendors and systematically compared their PSA-like and trypsin-like activities under identical conditions. Finally, we employed a mass spectrometric approach to identify the contaminating proteins.
MATERIALS AND METHODS
Materials
Enzymatically active PSA purified from human seminal plasma was acquired from Calbiochem (Catalog #539834, La Jolla, CA), AbD Serotec (Catalog #7820-0504, Oxford, UK), and Fitzgerald Industries International (Catalog #30C-CP1095, Acton, MA) and stored as recommended by the manufacturer. Information supplied with PSA from each of these vendors indicated that in each case PSA is purified from human seminal fluid supplied in a liquid form in sterile filtered PBS, pH 7.4–7.6 with <0.1% azide as a preservative. In each case protein was described as ≥95% pure by SDS–PAGE. For the PSA obtained from Calbiochem and AbD Serotec, activity was confirmed by vendor using peptide substrates. No details on the purification methodology was provided in the description of PSA on the product website or with the purchased product.
A previously characterized PSA substrate Mu-SRKSQQY-AMC was purchased from California Peptide Research (Napa, CA) [30]. Proaerolysin was obtained from Protox Therapeutics (Vancouver, BC Canada). Aprotinin was from Sigma (Catalog A1153, St. Louis, MO). Red blood cells (RBCs) were obtained from discarded material obtained from the Clinical Chemistry laboratory at Johns Hopkins. Unless otherwise specified, all other materials were from Sigma.
Measurementof PSA Activity
PSA activity was determined using the Mu-SRKSQQY-AMC substrate as previously described [30]. The PSA concentration was 5 μg/ml with a PSA substrate concentration of 300 μM. The trypsin inhibitor aprotinin was used at 10 μM where appropriate. All assays were performed in PSA buffer (50 mM Tris, 100 mM sodium chloride, pH 7.5) at 37°C. Assays were performed in triplicate in a black half-area 96-well plate and read every 3 min for 42 min by a Beckman Coulter DTX-880 plate reader (excitation 370 nm, emission 465 nm).
Measurementof Trypsin Activity
Trypsin-like proteolysis of peptide substrates was measured using a trypsin substrate (Sigma #B7260) or a previously described substrate for KLK2 (Gly-Lys-Ala-Phe-Arg-AMC) [29]. Trypsin-like proteolysis of protein substrates was determined by measuring the degree of RBCS hemolysis mediated by activation of proaerolysin as previously described. Proaerolysin is a trypsin-activated pore forming protoxin that contains a furin peptide activation domain with the sequence Lys-Val-Arg-Ala-Arg-Arg (KVRARR) [27]. The PSA concentration was 10 μg/ml and the proaerolysin concentration was 500 nM. The trypsin inhibitor aprotinin was used at 10 μM where appropriate. After washing three times in PBS, RBCs were added to 2% final volume and incubated at 37°C for 1 hr. After a brief centrifugation the absorbance of the supernatant at 415 nm was determined by a Thermo Scientific Nanodrop. Experiments were performed in triplicate.
Mass Spectrometry
PSA preparations from each vendor were reduced with DTT then alkylated with iodoacetamide prior to an overnight trypsin digestion as previously described [31]. Digested peptides were analyzed by liquid chromatography/tandem mass spectrometry (LCMS/MS) using a LTQ Orbitrap Velos mass spectrometer. All MS/MS spectra were analyzed with Mascot v.2.2 using the NCBI 167nr database and with Scaffold v.3 as previously described [32].
RESULTS
To assay PSA enzymatic activity a previously characterized fluorescent substrate was used that contained the amino acid tyrosine in the P1 position of the cleavage site. Hydrolysis data demonstrated a considerable difference in PSA activity amongst the three sources of PSA. PSA purchased from AbD Serotec had the highest PSA activity (Fig. 1). The addition of the trypsin-like kallikrein inhibitor aprotinin to the incubation mixture did not significantly affect hydrolysis of the PSA substrate for any of the PSA preparations.
Fig. 1.
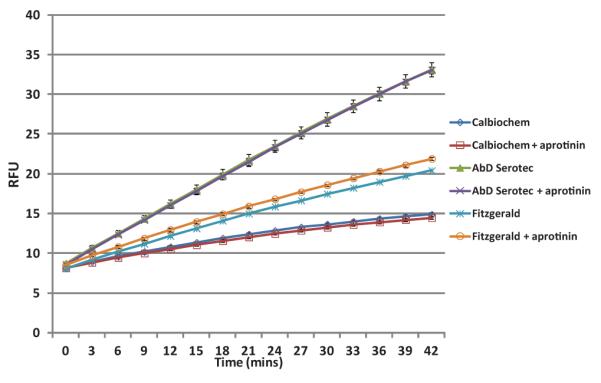
Comparison of the enzymatic activity of three commercial PSA preparations.Data show PSA (5 μg/ml) hydrolysis of the fluorophore AMC from the substrate Mu-SRKSQQY-AMC (300 μM) over 40 min incubationin the absence or presence of 10 μM aprotinin. Assay was performed in triplicate and standard error bars are shown.[Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/pros]
To assess trypsin-like activity, two fluorescent peptide substrates were used. For each substrate there was insufficient trypsin-like activity present to hydrolyze either substrate to a detectable degree after a 1 hr incubation period (data not shown).
In contrast to the small molecule peptide substrates, sufficient protease activity was present in PSA preparations from two of the three vendors to produce activation of the proaerolysin protein toxin and lysis of RBCs (Fig. 2). This activation was completely abolished by the addition of aprotinin consistent with the presence of a trypsin-like protease or kallikrein within the preparation.
Fig. 2.
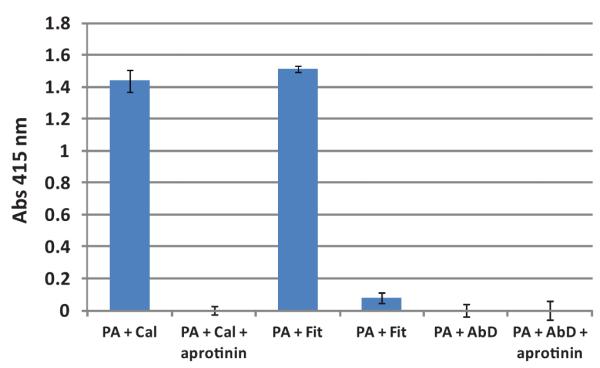
Hydrolysis of RBCs by proaerolysin activated by protease activity within PSA preprations. Proaerolysin (500 nM) was preincubated with PSA (10 μg/ml) followed by the addition of RBCs (2% final volume). Release of hemoglobin into the supernatant from lysed RBCs was measured spectrophotometrically at absorbance of 415 nm. (Cal, Calbiochem; Fit, Fitzgerald; AbD, AbD Serotec PSA preparation). [Color figure canbe seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/pros]
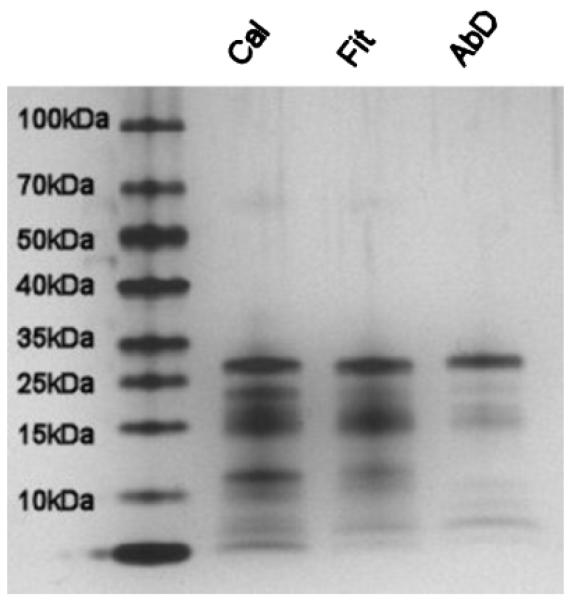
Mass spectrometry was subsequently performed on each PSA preparation to identify the contaminating proteins (Fig. 3). The ProteinProphet algorithm within the Scaffold software was used to calculate the protein identification probabilities [32]. This methodology uses the peptide fragmentation to determine probability that a given protein is in the mixture. Proteins are grouped as those with 0–19, 20–49, 50–79, 80–94%, and greater than 95% probability. Enumeration of the proteins identified in each preparation by this method with probability index above 50% revealed 12 proteins in the AbDSerotec PSA, 27 proteins in the Fitzgerald PSA and 84 proteins in the Calbiochem PSA preparation. Table I lists the proteins with a protein identification probability of >95% for at least one sample. Keratins 1, 2, 9, 10, 14, 16 were observed in some or all of the samples and were considered as contamination and were not included in this table.
Fig. 3.
Purity of PSA preparations by silver stained gel analysis. PSA from eachvendor was assayed for purity by polyacrylamide gel electrophoresis. Briefly, 400 ng of PSA was separated on a 4–15% minigel (Bio-Rad) then fixed and silver stained according to the manufacturers instructions(Pierce).
TABLE I.
Proteins Identifiedby In-solution Digestion and MS Analysis
| Protein identification probability |
|||
|---|---|---|---|
| Protein | AbD Serotec | Fitzgerald | Calbiochem |
| Prostate specific antigen isoform 1 preproprotein | >95% | >95% | >95% |
| Prolactin-induced protein | 0 | >95% | >95% |
| Heat shock 90 kDa protein 1, beta | >95% | >95% | 0 |
| Epididymal secretory protein E1 precursor | >95% | >95% | >95% |
| Lactoferrin | 0 | 0 | >95% |
| Prosaposin isoform b preproprotein | 0 | >95% | >95% |
| Fibronectin 1 isoform 3 preproprotein | 0 | 76% | >95% |
| Acidic epididymal glycoprotein-like 1 isoform 1 precursor | 0 | >95% | >95% |
| Alpha-2-glycoprotein 1, zinc | 0 | 76% | >95% |
| G antigen, family C, 1 | 0 | >95% | 0 |
| Heat shock 70 kDa protein 1B | >95% | >95% | 0 |
| Keratin 14 | 0 | >95% | 0 |
| Prostaglandin H2 D-isomerase | 60% | 76% | >95% |
| Tissue inhibitor of metalloproteinase 1 precursor | 0 | >95% | >95% |
| Quiescin Q6 sulfhydryl oxidase 1 isoform a | 0 | 76% | >95% |
| Preproacrosin | 0 | 0 | >95% |
| WAP four-disulfide core domain 2 precursor | 0 | 0 | >95% |
| CD59 antigen preproprotein | 0 | 0 | >95% |
| Triosephosphate isomerase 1 | >95% | 0 | 0 |
| Phosphatidylethanolamine binding protein | 0 | 0 | >95% |
| Total proteins (excluding PSA) | 5 | 13 | 14 |
Protein identification probabilities were generated using Scaffold’s version of the ProteinProphet algorithm[32].
All of the proteins listed in Table I, with the exception of the G-antigen, have previously been shown to be present in human semen [33–35]. Besides PSA, epididymal secretory protein E1 and prostaglandin H2 D-isomerase were the only proteins observed in all three samples. Peptides from acrosin, a wellcharacterized trypsin-like protease with a role in fertilization [36], were identified only in the sample from Calbiochem. No other proteases, including any other kallikreins were detected in any of the samples.
DISCUSSION
A steadily increasing number of studies have pointed to a role for PSA in the pathobiology of prostate cancer, although an exact mechanism has yet to be clearly defined [1,37]. To perform such studies our own laboratories and others have relied on the use of commercially available preparations of PSA. The purpose of this study was to evaluate a series of commercial PSA preparations for the presence of contaminating proteolytic activity that could confound the interpretation of mechanistic and biochemical studies with PSA. In this study, we observed significant differences in the PSA activity between the three preparations. In addition, we found sufficient contaminating trypsin-like proteolytic activity in two of three preparation to activate the cytolytic protein toxin proaerolysin. Finally, mass spectrometry revealed a large number of contaminating seminal proteins in these “purified” PSA preparations. Of the three preparations tested, the PSA purchased from AbD Serotec had the highest level of PSA activity, demonstrated minimal trypsin-like activity, and had the least number of contaminating proteins detectable by mass spectrometry.
The findings of a trypsin-like activity contaminating the PSA preparations is not a new discovery as such activity was described when PSA was first discovered and characterized in the 1980s [18,22]. The realization that PSA had to be a chymotrypsin-like protease based on its structure led to the inclusion of purification steps such as the use of aprotinin columns to remove the trypsin-like activity [26]. These additional purification steps appear to have been omitted in some of the commercial processes currently used to purify PSA from human seminal plasma. These results emphasize that investigators must use caution in interpreting results when purified exogenous PSA is used in biochemical or gene expression assays.
We were unable to ascertain in this study the exact nature of the contaminating trypsin-like protease. A primary candidate would be KLK2, which is present in the seminal fluid at μg/ml quantities. KLK2 is highly homolgous and of approximately the same molecular weight as PSA (aka KLK3) [16]. KLK4 and KLK11 are also present in the seminal fluid and possess trypsin-like activity [16,17]. None of these kallikreins were detected in our mass spectrometry analysis. However, these kallikreins were also not detected in two other published proteomic analyses of seminal fluid [33,34] while a third, by Pilch et al. [35], reported the presence of both KLK 2 and 11. We have performed proteomic analyses of prostatic fluid isolated from radical prostatectomy specimens. Although we observe a large number of proteins in these samples, we have been unable to detect KLK 2, 4, or 11 in these samples (unpublished data).
Methods to solve this problem could be to either add additional steps to the PSA purification process such as aprotinin columns to remove specific activities or immunoaffinity chromatography [38] with anti-PSA antibodies. Limitations of this latter method are the cost and the concern that the purifying antibody does not cross react with highly homologous kallikreins such as KLK2. Recombinant production could also be employed. However, prior studies have demonstrated that PSA produced in these recombinant systems is enzymatically inactive due to lack of or incomplete removal of the pro-PSA peptide [39–41]. To circumvent this problem, our laboratory has generated a modified form of PSA in which the native pro-PSA peptide has been replaced by a peptide recognized by furin [37]. This method results in production of increased amounts of enzymatically active PSA. Such methods could conceivably be optimized to produce sufficient amounts of purified PSA for commercial purposes.
REFERENCES
- 1.Williams SA, Singh P, Isaacs JT, Denmeade SR. Does PSA play a role as a promoting agent during the initiation and/or progression of prostate cancer? Prostate. 2007;67:312–329. doi: 10.1002/pros.20531. [DOI] [PubMed] [Google Scholar]
- 2.Lilja H, Christensson A, Dahlen U, Matikainen MT, Nilsson O, Pettersson K, Lövgren T. Prostate-specific antigen in serum occurs predominantly in complex with α1-antichymotrypsin. Clin Chem. 1991;37:1618–1625. [PubMed] [Google Scholar]
- 3.Stenman UH, Leinonen J, Alfthan H, et al. A complex between prostate specific antigen and α1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: Assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991;51:222–226. [PubMed] [Google Scholar]
- 4.Cohen P, Graves HC, Peehl DM, Kamarei M, Giudice LC, Rosenfeld RG. Prostate-specific antigen (PSA) is an insulin-like growth factor binding protein-3 protease found in seminal plasma. J Clin Endocrinol Metab. 1992;75:1046–1053. doi: 10.1210/jcem.75.4.1383255. [DOI] [PubMed] [Google Scholar]
- 5.Dallas SL, Zhao S, Cramer SD, Chen Z, Peehl DM, Bonewald LF. Preferential production of latent transforming growth factor beta-2 by primary prostatic epithelial cells and its activation by prostate-specific antigen. J Cell Physiol. 2005;202(2):361–370. doi: 10.1002/jcp.20147. [DOI] [PubMed] [Google Scholar]
- 6.Iwamura M, Hellman J, Cockett AT, Lilja H, Gershagen S. Alteration of the hormonal bioactivity of parathyroid hormone-related protein (PTHrP) as a result of limited proteolysis by prostate-specific antigen. Urology. 1996;48:317–325. doi: 10.1016/S0090-4295(96)00182-3. [DOI] [PubMed] [Google Scholar]
- 7.Cramer SD, Chen Z, Peehl DM. Prostate specific antigen cleaves parathyroid hormone-related protein in the PTH-like domain: Inactivation of PTHrP-stimulated cAMP accumulation in mouse osteoblasts. J Urol. 1996;156(2 Pt 1):526–531. doi: 10.1097/00005392-199608000-00076. [DOI] [PubMed] [Google Scholar]
- 8.Lilja H, Piironen TP, Rittenhouse HG, Mikolajczyk SD, Slawin KM. Prostate-specific antigen. In: Vogelzang NJ, Shipley WU, Scardino PT, Coffey DS, editors. Comprehensive textbook of genitourinary oncology. Lippincott Williams and Wilkins Publishers; Philadelphia: 2000. pp. 638–650. [Google Scholar]
- 9.Romanov VI, Whyard T, Adler HL, Waltzer WC, Zucker S. Prostate cancer cell adhesion to bone marrow endothelium: The role of prostate-specific antigen. Cancer Res. 2004;64:2083–2089. doi: 10.1158/0008-5472.can-03-3487. [DOI] [PubMed] [Google Scholar]
- 10.Gygi CM, Leibovitch IY, Adlington R, Baldwin JE, Chen B, Mccoull W, Pritchard GJ, Becker GW, Dixon EP, Little SP, Sutkowski DM, Teater C, Neubauer BL. Prostate-specific antigen(PSA)-mediated proliferation, androgenic regulation and inhibitory effects of LY312340 in HOS-TE85 (TE85) human osteosarcoma cells. Anticancer Res. 2002;22(5):2725–2732. [PubMed] [Google Scholar]
- 11.Killian CS, Corral DA, Kawinski E, Constantine RI. Mitogenic response of osteoblast cells to prostate-specific antigen suggests an activation of latent TGF-beta and a proteolytic modulation of cell adhesion receptors. Biochem Biophys Res Commun. 1993;192:940–947. doi: 10.1006/bbrc.1993.1506. [DOI] [PubMed] [Google Scholar]
- 12.Nadiminty N, Lou W, Lee SO, Mehraein-Ghomi F, Kirk JS, Conroy JM, Zhang H, Gao AC. Prostate-specific antigen modulates genes involved in bone remodeling and induces osteoblast differentiation of human osteosarcoma cell line SaOS-2. Clin Cancer Res. 2006;12:1420–1430. doi: 10.1158/1078-0432.CCR-05-1849. [DOI] [PubMed] [Google Scholar]
- 13.Goya M, Ishii G, Miyamoto S, Hasebe T, Nagai K, Yonou H, Hatano T, Ogawa Y, Ochiai A. Prostate-specific antigen induces apoptosis of osteoclast precursors: Potential role in osteoblastic bone metastases of prostate cancer. Prostate. 2006;66:1573–1584. doi: 10.1002/pros.20375. [DOI] [PubMed] [Google Scholar]
- 14.Fortier AH, Nelson BJ, Grella DK, Holaday JW. Antiangiogenic activity of prostate-specific antigen. J Natl Cancer Inst. 1999;91:1635–1640. doi: 10.1093/jnci/91.19.1635. [DOI] [PubMed] [Google Scholar]
- 15.Fortier AH, Holaday JW, Liang H, Dey C, Grella DK, Holland-Linn J, Vu H, Plum SM, Nelson BJ. Recombinant prostate specific antigen inhibits angiogenesis in vitro and in vivo. Prostate. 2003;56(3):212–219. doi: 10.1002/pros.10256. [DOI] [PubMed] [Google Scholar]
- 16.Yousef GM, Diamandis EP. An overview of the kallikrein gene families in humans and other species: Emerging candidate tumour markers. Clin Biochem. 2003;36:443–452. doi: 10.1016/s0009-9120(03)00055-9. [DOI] [PubMed] [Google Scholar]
- 17.Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: Structure, function, and association to disease. Endocrine Rev. 2001;22:184–204. doi: 10.1210/edrv.22.2.0424. [DOI] [PubMed] [Google Scholar]
- 18.Lilja H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J Clin Invest. 1985;76:1899–1903. doi: 10.1172/JCI112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lilja H, Abrahamsson P-A, Lundwall A. Semenogelin, the predominant protein in human semen. J Biol Chem. 1989;264:1894–1900. [PubMed] [Google Scholar]
- 20.Malm J, Hellman J, Hogg P, Lilja H. Enzymatic action of prostate-specific antigen (PSA or hK3): Substrate specificity and regulation by Zn2+, a tight-binding inhibitor. Prostate. 2000;45(2):132–139. doi: 10.1002/1097-0045(20001001)45:2<132::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. Invest Urol. 1979;17(2):159–163. [PubMed] [Google Scholar]
- 22.Watt KW, Lee PJ, M’Timkulu T, Chan WP, Loor R. Human prostate-specific antigen: Structural and functional similarity with serine proteases. Proc Natl Acad Sci. 1986;83(10):3166–3170. doi: 10.1073/pnas.83.10.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akiyama K, Nakamura T, Iwanaga S, Hara M. The chymotrypsin-like activity of human prostate-specific antigen, [gamma]-seminoprotein. FEBS Lett. 1987;225(1–2):168–172. doi: 10.1016/0014-5793(87)81151-1. [DOI] [PubMed] [Google Scholar]
- 24.Lundwall A, Lilja H. Molecular cloning of human prostate specific antigen cDNA. FEBS Lett. 1987;214(2):317–322. doi: 10.1016/0014-5793(87)80078-9. [DOI] [PubMed] [Google Scholar]
- 25.Schaller J, Akiyama K, Tsuda R, Hara M, Marti T, Rickli EE. Isolation, characterization and amino-acid sequence of gamma-seminoprotein, a glycoprotein from human seminal plasma. Eur J Biochem. 1987;170(1–2):111–120. doi: 10.1111/j.1432-1033.1987.tb13674.x. [DOI] [PubMed] [Google Scholar]
- 26.Christensson A, Laurell C-B, Lilja H. Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine protease inhibitors. Eur J Biochem. 1990;194:755–765. doi: 10.1111/j.1432-1033.1990.tb19466.x. [DOI] [PubMed] [Google Scholar]
- 27.Williams SA, Merchant RF, Garrett-Mayer E, Isaacs JT, Buckley JT, Denmeade SR. A prostate-specific antigen–activated channel-forming toxin as therapy for prostatic disease. J Natl Cancer Inst. 2007;99(5):376–385. doi: 10.1093/jnci/djk065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikolajczyk SD, Millar LS, Kumar A, Saedi MS. Human glandular kallikrein, hK2, shows arginine-restricted specificity and forms complexes with plasma protease inhibitors. Prostate. 1998;34(1):44–50. doi: 10.1002/(sici)1097-0045(19980101)34:1<44::aid-pros6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 29.Janssen S, Jakobsen CM, Rosen DM, Ricklis RM, Reineke U, Christensen SB, Lilja H, Denmeade SR. Screening a combinatorial peptide library to develop a human glandular kallikrein 2-activated prodrug as targeted therapy for prostate cancer. Mol Cancer Ther. 2004;3(11):1439–1450. [PubMed] [Google Scholar]
- 30.Denmeade SR, Lou W, Malm J, Lovgren J, Lilja H, Isaacs JT. Specific and efficient peptide substrates for assaying the proteolytic activity of prostate specific antigen. Cancer Res. 1997;57:4924–4930. [PMC free article] [PubMed] [Google Scholar]
- 31.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem. 1996;68(5):850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 32.Keller A, Nesvizhiskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74(20):5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 33.Fung KY, Glode LM, Green S, Duncan MW. A comprehensive characterization of the peptide and protein constituents of human seminal fluid. Prostate. 2004;61:171–181. doi: 10.1002/pros.20089. [DOI] [PubMed] [Google Scholar]
- 34.Utleg AG, Yi EC, Xie T, Shannon P, White JT, Goodlett DR, Hood L, Lin B. Proteomic analysis of human prostasomes. Prostate. 2003;56:150–161. doi: 10.1002/pros.10255. [DOI] [PubMed] [Google Scholar]
- 35.Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7:R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klemm U, Muller-Esterl W, Engel W. Acrosin, the peculiar sperm-specific serine protease. Hum Genet. 1991;87:635–641. doi: 10.1007/BF00201716. [DOI] [PubMed] [Google Scholar]
- 37.Williams SA, Jelinek CA, Litvinov I, Cotter RJ, Isaacs JT, Denmeade SR. Enzymatically active prostate-specific antigen promotes growth of human prostate cancers. Prostate. 2011;71:1595–1607. doi: 10.1002/pros.21375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pérez L, Zulueta O, Melchor A, Hernández L, López R, Cazanave J, Béquer D. Purification of human Prostatic-Specific Antigen (hPSA) from seminal plasma by immunoaffinity chromatography using a monoclonal antibody anti total PSA. Hybridoma. 2011;30:247–251. doi: 10.1089/hyb.2010.0111. [DOI] [PubMed] [Google Scholar]
- 39.Takayama TK, Fujikawa K, Davie EW. Characterization of the precursor of prostate-specific antigen. J BiolChem. 1997;272:21582–21588. doi: 10.1074/jbc.272.34.21582. [DOI] [PubMed] [Google Scholar]
- 40.Lovgren J, Rajakoski K, Karp M, Lundwall A, Lilja H. Activation of the zymogen form of prostate-specific antigen by human glandular kallikrein 2. Biochem Biophys Res Comm. 1997;238:549–555. doi: 10.1006/bbrc.1997.7333. [DOI] [PubMed] [Google Scholar]
- 41.Kumar A, Mikolajczyk SD, Goel AS, Millar LS, Saedi MS. Expression of pro form of prostate-specific antigen by mammalian cells and its conversion to mature, active form by human kallikrein 2. Cancer Res. 1997;57:3111–3114. [PubMed] [Google Scholar]