Abstract
Background and purpose
By 2010 there had been 14 published trials of surgery for intracerebral haemorrhage reported in systematic reviews or to the authors, but the role and timing of operative intervention remains controversial and the practice continues to be haphazard. This study attempted to obtain individual patient data from each of the 13 studies published since 1985 in order to better define groups of patients that might benefit from surgery.
Methods
Authors of identified published papers were approached by mail, email and at conferences and invited to take part in the study. Data were obtained from 8 studies (2186 cases). Individual patient data included patient's age, GCS at presentation, volume and site of haematoma, presence of IVH, method of evacuation, time to randomisation and outcome.
Results
Meta-analysis indicated that there was improved outcome with surgery if it was undertaken within 8 hours of ictus (p = 0.003), or the volume of the haematoma was 20-50ml. (p = 0.004), or the GCS was between 9 and 12 (p = 0.0009), or the patient was aged between 50 and 69 (p = 0.01). In addition there was some evidence that more superficial haematomas with no IVH might also benefit (p = 0.09).
Conclusions
There is evidence that surgery is of benefit if undertaken early before the patient deteriorates. This work identifies areas for further research. Ongoing studies in subgroups of patients, such as STICH II, will confirm whether these interpretations can be replicated.
Keywords: surgery, meta-analysis, intracerebral haemorrhage
Introduction
Spontaneous supratentorial Intracerebral Haemorrhage (ICH) has a high morbidity and mortality and places a significant burden on health and social services. The role and timing of operative neurosurgical intervention remains controversial and the practice and timing of surgery continues to be haphazard. Operative intervention is thought to be beneficial in stopping bleeding, preventing rebleeding and removing the mass effect to prevent secondary brain damage. To date fourteen trials have been undertaken to investigate the role of surgery for spontaneous ICH with varying conclusions (See Table S1). The first randomised trial was published in 1961 (McKissock(1)) and suggested that there was no significant advantage for surgery. As the use of the CT scan in stroke increased and operative techniques and care facilities improved, more trials were undertaken with four small single centre trials reported between 1989 and 1992(2-5). Further small studies reported between 1998 and 2006(6-10), and four large studies were published in 2001(11), 2004(12), 2005(13) and 2009(14). Few studies have shown statistically significant differences and some have favoured surgery while others have favoured conservative treatment. The meta-analysis of the published outcomes from these studies shows a significant benefit from surgery both for the outcome of mortality (15) and for the combined outcome of death or disability(16) (See Figures S1 and S2). However there are many differences between the studies in the types of population included and in the outcomes measured.
The aim of this study was to pool all available original data from all trials of surgery versus conservative treatment in spontaneous ICH in order to carry out an individual patient data meta-analysis. Such pooled raw data makes it possible to test subpopulations of ICH patients and their response to surgery. There were no raw data available from studies prior to 1985.
Methods
Study selection and data items
The randomised controlled trials of surgery for ICH since CTs became available were identified from the ongoing Cochrane Review of Prasad et al.(17). Presentation of the study design at conferences and reviewing of relevant literature led to the identification of two further papers: those of Pantazis et al.(10) and Wang et al.(14). The authors of the published papers were contacted by mail, email and at stroke and neurosurgery conferences and were invited to take part in the study. They were asked to describe the format of their datasets and indicate which variables were available. Data requested included patient's age and sex, Glasgow Coma Score (GCS) at presentation, volume and site of haematoma and presence of intraventricular haemorrhage (IVH), method of evacuation of haematoma, time to randomisation and outcome at three to six months.
Definition of outcome
Different outcome measures were used by different studies. Glasgow Outcome Scale (GOS) had been used by three studies (3, 7, 13) while extended GOS (GOSE) had only been used by Mendelow(13). Barthel was used by three studies (6, 7, 13), and Rankin was the measure most commonly available in the data provided by six studies(2, 6-8, 12, 13). Batjer (4)had used ‘independent at home’ and Hosseini(9) had used Karnofsky. Chen(11) had used a five category variable based on Barthel: death, vegetative state, Barthel< 60, Barthel > 60 and excellent with no neurological deficit.
The primary outcome for this analysis was defined as unfavourable outcome. Unfavourable outcome was defined as death plus the vegetative state or severe disability on the 5 point GOS. If this was not available then a Rankin of 3 or more was considered as unfavourable; and if this was not available an outcome of Barthel of 90 or less was considered unfavourable. An outcome of moderate disability on the GOS implies that an individual is able to carry out shopping tasks and use public transport. Thus favourable outcome was regarded as being independent outside the home. This differs from the prognosis based outcome derived from the GOSE that was used in the STICH trial (13). The outcome classification from the Chen(11) trial did not provide sufficient information to code directly in this way so the classification of “excellent” was the only category defined as a favourable outcome. These decisions were made prior to the analysis of the data
Pre-specified subgroup analysis
For the analysis, each of the continuous baseline variables was grouped. Categories were chosen to reflect the admission criteria in the trials, previous publications and criteria generally used in making treatment decisions. Age was grouped into three categories: less than 50, 50 to 69 and 70 or more. GCS was grouped into three groups: 3-8, 9-12 and 13-15; volume was classified into four groups: less than 20 ml, 20 – 50 ml, 50 – 80 ml and 80mls or more. These decisions were made prior to the analysis of the data.
The data were analysed using SPSS to cross tabulate the outcome by treatment group for each baseline variable group and the values entered into the Revman program to calculate the odds ratios and to demonstrate the Forest plots.
Results
Study selection
Numerous attempts were made to contact each first author by mail and email. Where this method was unsuccessful further attempts were made using third party contacts made at conferences or via other researchers and by holding meetings with the trialists during conferences. This was a long process. Contact was established with the authors of all studies. Hattori(12) was unable to take part. Batjer's and Chen's(5) data were not retrievable. The main authors of each of the other contacted studies agreed to be involved in this study and to provide data for it. Full data sets have been supplied by Juvela, Morgenstern, Zuccarello, Teernstra, Chen, Mendelow and Wang (3, 6-8, 11, 13, 14). After an exhaustive search in various universities taking more than a year the original data of Auer(2) was not located but comprehensive tables from a contemporary thesis analysis of the original data were obtained(18). Hosseini(9) supplied some aggregate data but of insufficient detail to include in an individual patient data analysis. In addition the study has still not been published and therefore not yet subjected to peer review. Pantazis(10) supplied full data for only 92 of his 108 cases. He was unable to locate the data for the other 16 cases and therefore the decision was made not to include this data in case it was a biased selection of the cases.
Full data sets therefore have been supplied by seven authors (2086 patients) and grouped data by an eighth author (100 patients).
Study characteristics
Table 1 summarises the characteristics of the patients in each of the trials. Only one data set contained details of the depth of the haematoma from the cortical surface(13). This would give an indication of the amount of healthy tissue that has to be passed through to reach the clot. Seven studies had recorded presence or absence of IVH but only one of these had any measure of the severity the IVH(13).
Table 1. Characteristics of data sets.
Median, (quartiles) and minimum and maximum values of continuous variables. Percentage of categorical variables.
| Author | Year | No. of cases | Male % | Age | GCS | Volume of haematoma | Time to randomisation | Lobar haematoma % | IVH present % | Favourable outcome % (responders) |
|---|---|---|---|---|---|---|---|---|---|---|
| Auer | 1989 | 100 | NR* | 49% <50yrs | 48% 8 or less | 49% <50ml | NR | 45 | 32 | 25% |
| Juvela | 1989 | 52 | 58 | 51 (42-58) 24-65 | 12 (7-14) 4-15 | 58 (36-77) 17-152 | NR | 15 | 62 | 12% |
| Morgenstern | 1998 | 34 | 65 | 51 (43-63) 22-77 | 11 (10-14) 5-15 | 50 (30-76) 11-170 | 4 (1-6) 0 -11 | 24 | NR | 19% (31) |
| Zuccarello | 1999 | 20 | 55 | 64 (59-70) 27-80 | 12 (9-14) 4-15 | 33 (19-61) 16-105 | 7 (4-10) 2-19 | 50 | 50 | 45% |
| Chen | 2001 | 500 | 72 | 58 (49-65) 12-74 | 11 (9-13) 7-15 | 30 (22-45) 12-130 | 35% <8hr 22%>24hr | 5 | 4 | 23% |
| Teernstra | 2003 | 70 | 57 | 70 (61-74) 46-87 | 9 (7-11) 4-15 | 59 (33-81) 10-132 | 4 (2-9) 0-62 | 54 | 31 | 11% |
| Mendelow | 2005 | 1033 | 57 | 62 (52-70) 19-93 | 12 (9-14) 5-15 | 38 (24-62) 4-210 | 20 (10-36) 2-72 | 35 | 39 | 18% (965) |
| Wang | 2009 | 377 | 63 | 56 (50-65) 40-75 | 12 (10-14) 9-15 | 32 (28-37) 25-44 | 3 (2-8) 1-67 | 0 | 19 | 45% |
• NR – Not recorded
Since only dichotomised data were obtained from Auer it is not always possible to include his data in the tables. He categorised GCS as 3 to 8 and 9 to 15, age as 60 and below or above 60, and volume as below 50 ml or 50 ml and above.
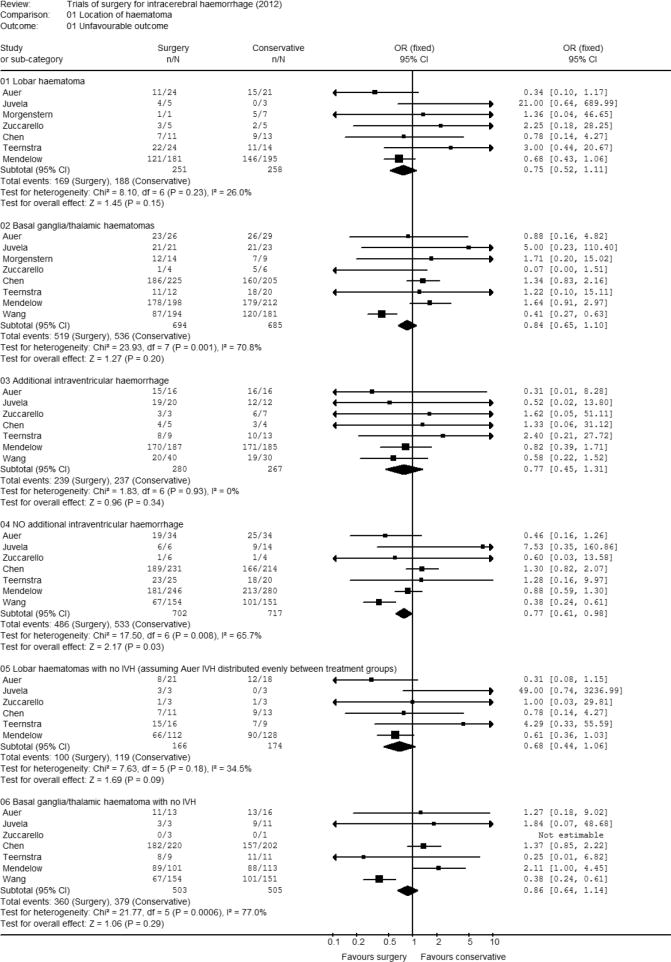
Location of haematoma – Lobar or deep and presence of IVH
All studies recorded location of haematoma. Outcome was recorded for 509 patients with lobar haematoma (Figure 1). The majority of the patients (376, 74%) were from the Mendelow et al. trial and the overall odds ratio was 0.75 (95% CI 0.52, 1.11; p= 0.15) showing a non significant trend towards surgery giving a favourable outcome. Outcome was recorded for 1379 patients with basal ganglia or thalamic haematomas. The studies of Chen, Mendelow and Wang contribute most patients to this analysis: 430, 410 and 375 respectively. The odds ratio was 0.84 (95% CI 0.65, 1.10) demonstrating no significant difference (p = 0.20) in outcome according to whether the patients were allocated to surgery or conservative treatment. However the test for heterogeneity demonstrates a significant difference amongst these studies suggesting that the results seen for the Wang study differ from those seen in the other studies.
Figure 1. Meta-analysis by location of haematoma.
Seven studies recorded presence or absence of IVH. In total there were 1419 patients with no intraventricular haemorrhage 526 from Mendelow, 445 from Chen and 305 from Wang. The odds ratio was 0.77 (95% CI 0.61, 0.98; p=0.03) indicating a significantly more favourable outcome with surgery than with conservative treatment when there was no intraventricular haemorrhage. There were 547 patients with IVH, 373 being from the Mendelow trial. The odds ratio was similar at 0.77 (95% CI 0.45, 1.31) but there was no evidence for an improvement in outcome from surgery for the ICH when there was IVH due to the reduced power because of a smaller sample size.
Further analysis was undertaken studying only those patients with lobar haematomas who did not have IVH. Full data were available from 5 studies (301 cases) with incomplete data from the fourth (Auer) (39 cases). From the reported analyses we know that there were six patients who had lobar haematomas and IVH in his data set and that 31 out of 32 patients with IVH had an unfavourable outcome. Thus we can investigate a range of differences assuming all six patients were in the conservative group, or all six were in the surgical group or there were three in each group. All these analyses give an odds ratio favouring surgery with significance levels varying between p = 0.04 and p = 0.15. Assuming that the patients with IVH in Auer's data were equally distributed between the two groups gives an odds ratio 0.68 (95% CI 0.44, 1.06; p=0.09).
Analysis of patients with a basal ganglia or thalamic haematoma and no IVH had an odds ratio of 0.86 (95%CI 0.64, 1.14; p=0.29). There was a great deal of heterogeneity in this data (p = 0.0006) with only the Wang and Teernstra data demonstrating any tendency towards favourable outcome with surgery.
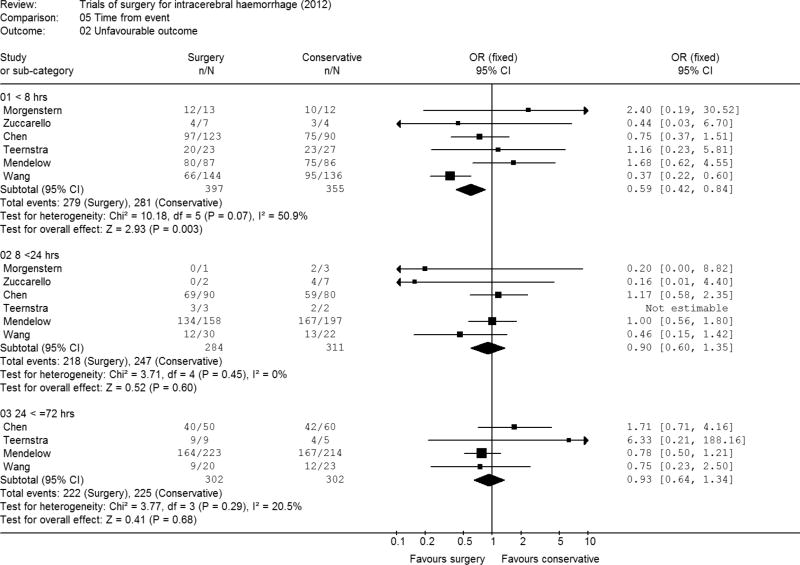
Time to randomisation
Two studies recruited patients in a 48 hour time window but the data was not recorded for individual patients (2, 3). Time was, therefore, only available for six studies and for one of the studies this had been recorded in a limited way as less than 8 hours, 8 to 24 hours and 24 to 72 hours. Because of this lack of full data this categorisation was therefore used for the analysis. About a third of the patients fell into each categorisation (752 patients were randomised with 8 hours, another 595 by 24 hrs, and the other 604 by 72 hours) Only four studies included patients randomised more than 24 hours after ictus (Figure 2). These analyses suggested that early operation (within 8 hours of ictus was beneficial) with an odds ratio of 0.59 (95% CI 0.42, 0.84; p=0.003).
Figure 2. Meta-analysis by time of randomisation.
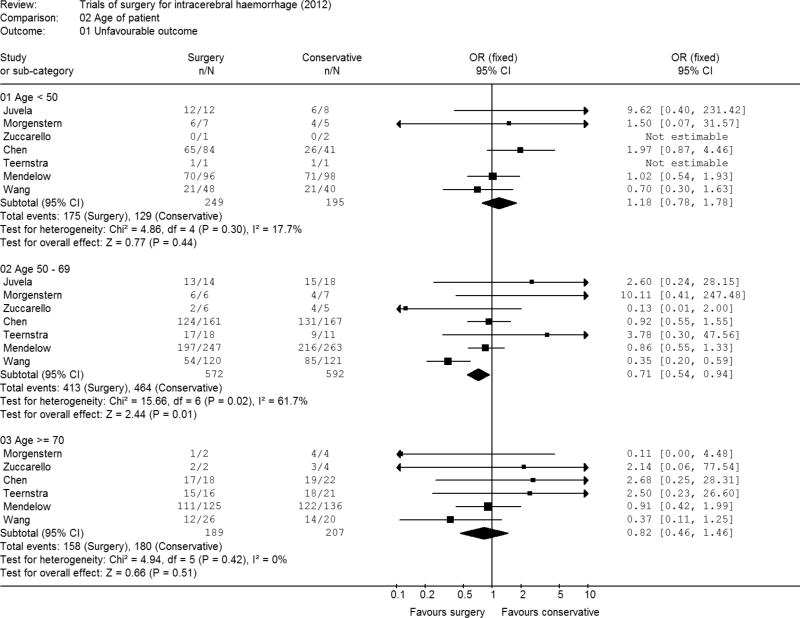
Age of patient
In total 444 patients were aged under 50 and the Chen study with a third of these patients had double the number in the surgery group compared to the conservative group. The studies tended to favour conservative rather than surgical therapy (Figure 3) with the overall odds ratio for patients under 50 being a non significant 1.18 (95% CI 0.78, 1.78; p = 0.44). Amongst the 1164 patients in the intermediate age group of 50 to 69 the odds ratio was significantly in favour of surgery 0.71 (95% CI 0.54, 0.94; p=0.01) but again there was evidence of heterogeneity. For the 396 patients aged 70 or over it was 0.82 (95% CI 0.46, 1.46; p=0.51). In general older patients fared worse than younger patients 85% of patients aged 70 or above had an unfavourable outcome compared with 75% of those aged 50 to 69 and 68% of patients aged under 50.
Figure 3. Meta-analysis by age of patient.
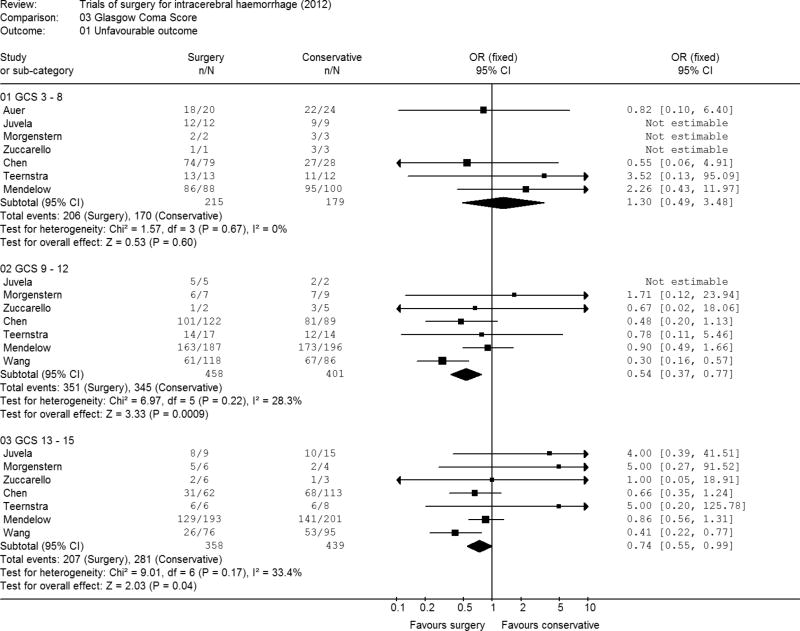
GCS at randomisation
There were 394 patients with a GCS of 3 to 8 (Figure 4). The odds ratio in favour of conservative treatment was 1.30 (95% CI 0.49, 3.48; p=0.60). All studies showed no significant difference in outcome between surgery and conservative treatment though tending to favour conservative treatment. Chen showed imbalances in the allocation of patients in this GCS range: 79 allocated to surgery and 28 to conservative treatment.
Figure 4. Meta-analysis by Glasgow Coma Score.
There were 859 patients with a GCS of between 9 and 12. This group of patients demonstrated a significantly improved outcome with surgery (odds ratio 0.54; 95% CI 0.37, 0.77) (p = 0.0009). All individual studies showed a tendency towards improved outcome with surgery for this group of patients and the test for heterogeneity was non-significant (p=0.22).
There were 797 patients with a GCS between 13 and 15. These patients also showed a significant difference in outcome between surgery and initial conservative treatment (odds ratio 0.74; 95%CI 0.55, 0.99) (p = 0.04).
In general, patients in coma had a poorer outcome than conscious patients: 95% of patients with a GCS 8 or less had an unfavourable outcome, compared with 81% of patients with a GCS of 9-12, and 60% of patients with a GCS of 13-15.
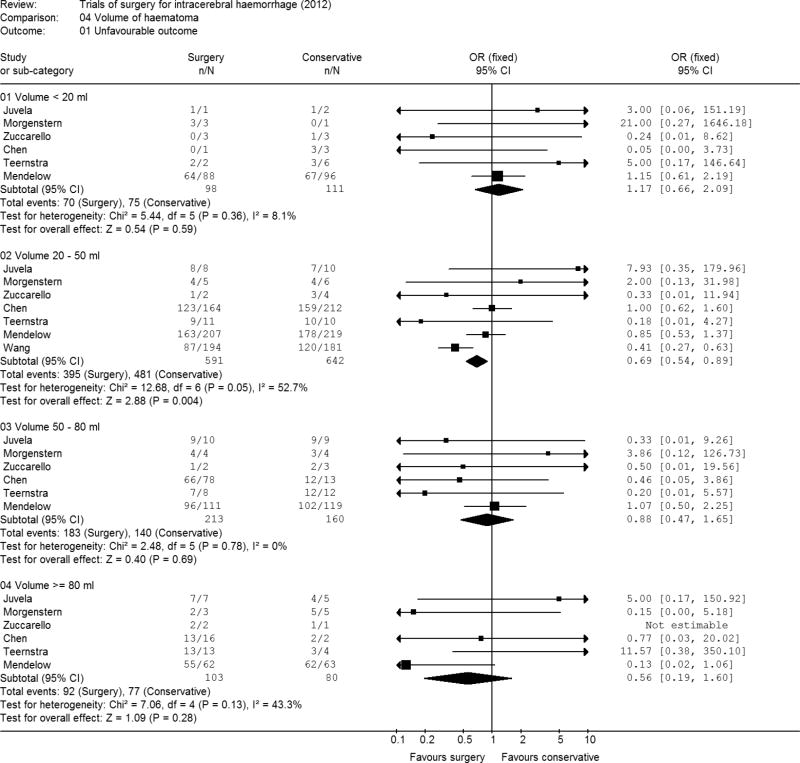
Volume of haematoma
There were 209 patients with a haematoma of less than 20 ml, 1233 with a haematoma of 20 to 49 ml, 373 with a haematoma of 50 to 79 ml and 183 patients with a haematoma of 80 ml or more. The only category showing a significant treatment effect was the group with the volume of between 20 and 49 ml. (Figure 5). The odds ratio for this group was 0.69 (95% CI 0.54, 0.89; p=0.004). The smallest volume of haematomas (<20mls.) was the only category to show a tendency to favour conservative treatment. There is no evidence of a significant benefit for surgery at other volumes. In general, larger volumes were associated with worse outcomes: 93% of patients with a haematoma of 80 mls. or more had an unfavourable outcome compared with 69% of patients with a haematoma of less than 20 mls.
Figure 5. Meta-analysis by volume of haematoma.
Discussion
Summary of evidence
This study set out to obtain the raw data from as many of the prospective randomised controlled trials of surgical treatment for spontaneous supratentorial ICH as possible. This has allowed exploration of clinically plausible hypotheses and pre-specified subgroup analysis ideas in larger data sets than was available in the individual trials.
This study considered one intervention: removal of the clot by physical means. This clot removal by surgery includes craniotomy, aspiration, endoscopic aspiration, suction, and catheter aspiration. The intervention has been explored in terms of site, additional IVH, age of patient, volume of haematoma, GCS and timing of the intervention.
The overall data has permitted us to re-evaluate the prognostic features associated with ICH but it should be remembered that the interventions of surgery may have influenced these. Nevertheless, the well known prognostic factors are clearly evident in this data. A separate analysis of the control arm of clinical trials of interventions (pharmacological and surgical) is underway and will be reported separately. Advancing age, volume of clot and level of consciousness remain powerful predictors of outcome in this data set.
The treatment of spontaneous supratentorial ICH has remained and will remain controversial until a definitive prospective randomised controlled trial comes up with firm evidence in favour of a particular treatment. To date, both medical and surgical trials have failed to do this. Traditional treatment of ICH has been to observe the patient's clinical condition and to consider and offer surgery once the patient deteriorates. This means that secondary brain damage has taken place before the intervention of surgery. It would be more logical to intervene before the onset of secondary brain damage and this has been the theory behind many of the surgical trials over the last half century. Other aspects of intervention include prevention of expansion of the clot and treatment of the source of bleeding. Medical therapies have been aimed at these last two pathophysiological events but surgery would deal with all three. Analysis of trials to date has suggested that there is a treatment effect but none has been conclusive. Meta analysis of these trials gives some indication as to effectiveness but the different methods, treatment intervals, outcome assessments and methods of treatment make comparison difficult. Future studies should aim to standardise the collection of data. Actual measurements should be recorded rather than grouped data such as the grouped time to randomisation recorded in the Chen study(11). All recognised predictive variables should be recorded and outcome should be measured at six months as well as any additional outcome points. Outcome should be measured using the extended version of the GOS as well as modified Rankin and other recognised assessments of outcome.
Overall there is no evidence that haematomas located in the deeper regions, basal ganglia or thalamus, may benefit from surgery although more recent studies using minimally invasive techniques combined with clot lysis using urokinase suggest that this may prove to be a fruitful area for further research(14, 19, 20).
There is, however, a suggestion that patients with lobar haematomas and no IVH might benefit from surgery. Our meta-analysis supports their selection as an appropriate population for the ongoing STICH II study(21).
Our analysis confirms the benefits seen from surgery in the Cochrane (second edition) review (17). From this it has emerged that the factors that are most likely to show benefit with physical removal of the clot (that is different types of surgery) are earlier intervention (timing), the treatment of lobar and superficial haematomas and the exclusion of patients with ventricular haemorrhage and/or hydrocephalus. The question of early intervention has been the central theme of the STICH trials and this is the first indication that early surgery is beneficial. It would in fact be logical to hypothesise that the earlier the clot is removed, the better. However there have been studies that have suggested that ultra early surgery might lead to worse outcomes(22) so further work is needed. This will be a pre-specified subgroup in STICH II (21).
Limitations
Meta-analysis can be useful to identify trends and to set up hypotheses for further trials. The results have to be interpreted with caution because of the different methods used in each of the individual trials. Methods to evaluate studies that use meta-analysis have been proposed, the most recent being the PRISMA recommendations(23), which updates the Quorum (Quality of reporting of Meta-analyses) statement(24). The data that we have analysed here are difficult to report using these standards.. In addition Brand (25)has pointed out the difficulty of conducting trials in a double blind way in the surgical arena.
Conclusions
Our data suggest that improved outcomes can be achieved with early surgery within 8 hours of ictus, with haematomas of 20 – 50 ml, for patients with a GCS of 9 or more or for patients aged 50 to 69. In particular our analyses suggest that when the GCS is 8 or lower early surgery does not significantly improve outcome. Taking this argument further suggests that once the GCS has dropped to 8 or below, then irretrievable damage has already occurred and surgery will not be successful in rescuing the patient.
This work has shown that the information available to date does favour earlier surgical intervention in patients with ICH. The ongoing studies STICH II, MISTIE and CLEAR III are focused on areas that this analysis shows may offer promising results(19, 21, 26). Our work also endorses the AHA guidelines(27) calling for more research to establish the groups that might benefit from surgery. At present the recommendations for or against surgery are based on conflicting evidence. These trials should provide robust evidence on which to base future recommendations.
Supplementary Material
Table S1. Table of potential data sources
Figure S1. Meta-analysis of published data for all 14 trials: Mortality
Figure S2. Meta-analysis of published data for death and disability
Acknowledgments
Sources of funding: Funding for this work was provided initially by NIH (P50 NS044283-03 subaward P021-040-N633-1105), and UK Stroke Association (TSA 2004/19). The funding sources provided salary support for Dr Gregson. They had no role in writing the manuscript or in the decision to submit.
Footnotes
Author's contributions: Barbara A Gregson designed the study, analysed the data and drafted the paper.
Joseph Broderick, Ludwig M Auer, Lewis B Morgenstern, A David Mendelow designed the study, provided the datasets, aided the interpretation and critically reviewed the paper.
Xian-Cheng Chen, Seppo Juvela, George Pantazis, Onno P M Teernstra, Wen-Zhi Wang, Mario Zuccarello provided datasets, aided the interpretation and critically reviewed the paper.
Hunt Batjer aided the interpretation and critically reviewed the paper.
Disclosures: Professor Broderick has received honoraria from Genentech, PhotoThera and Oakstone Medical Publishing and trial supplies from EKOS Corporation Genentech, and Schering Plough for other studies. Professor Mendelow has received honoraria from Stryker, Codman and NovoNordisc. Both place the honoraria in an educational/research fund in their departments.
References
- 1.McKissock W, Richardson A, Taylor J. Primary intracerebral haemorrhage: a controlled trial of surgical and conservative treatment in 180 unselected cases. Lancet. 1961;2:221–6. [Google Scholar]
- 2.Auer LM, Deinsberger W, Niederkorn K, Gell G, Kleinert R, Schneider G, et al. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. Journal of Neurosurgery. 1989;70:530–5. doi: 10.3171/jns.1989.70.4.0530. [DOI] [PubMed] [Google Scholar]
- 3.Juvela S, Heiskanen O, Poranen A, Valtonen S, Kuurne T, Kaste M, et al. The treatment of spontaneous intracerebral hemorrhage. A prospective randomised trial of surgical and conservative treatment. J Neurosurg. 1989;70:755–8. doi: 10.3171/jns.1989.70.5.0755. [DOI] [PubMed] [Google Scholar]
- 4.Batjer H, Reisch J, Allen B, Plaizier L, Jen Su C. Failure of surgery to improve outcome in hypertensive putaminal hemorrhage. A prospective randomised trial. Arch Neurol. 1990;47:1103–6. doi: 10.1001/archneur.1990.00530100071015. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Yang H, Cheng Z. A prospective randomised trial of surgical and conservative treatment of hypertensive intracerebral haemorrhage. Acta Acad Shanghai Med. 1992;19:237–40. [Google Scholar]
- 6.Morgenstern LB, Frankowski RF, Shedden P, Pasteur W, Grotta JC. Surgical treatment for intracerebral hemorrhage (STICH): a single-center, randomized clinical trial. Neurology. 1998;51:1359–63. doi: 10.1212/wnl.51.5.1359. [DOI] [PubMed] [Google Scholar]
- 7.Zuccarello M, Brott T, Derex L, Kothari R, Sauerbeck L, Tew J, et al. Early surgical treatment for intracerebral hemorrage. A randomized feasibility study. Stroke. 1999;30:1833–9. doi: 10.1161/01.str.30.9.1833. [DOI] [PubMed] [Google Scholar]
- 8.Teernstra OPM, Evers SMAA, Lodder J, Leffers P, Franke CL, Blaauw G. Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA) Stroke. 2003;34:968–74. doi: 10.1161/01.STR.0000063367.52044.40. [DOI] [PubMed] [Google Scholar]
- 9.Hosseini H, Leguerinel C, Hariz M, Melon E, Palfi S, Deck P, et al. Stereotactic aspiration of deep intracerebral hematomas under computed tomographic control: a multicentric prospective randomised trial. Cerebrovasular Diseases. 2003;16S:57. [Google Scholar]
- 10.Pantazis G, Tsitsopoulos P, Mihas C, Katsiva V, Stavrianos V, Zymaris S, et al. Early surgical treatment vs conservative management for spontaneous supratentorial intracerebral hematomas: A prospective randomized study. Surgical Neurology. 2006;66:492–501. doi: 10.1016/j.surneu.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Wu J, Zhou X, YZhang Y, Wang Z, Qin Z, et al. The randomized multicentric prospective controlled trial in the standardized treatment of hypertensive intracerebral hematomas: The comparison of surgical therapeutic outcomes with conservative therapy. Chinese Journal of clinical Neuroscience. 2001;4:365–8. [Google Scholar]
- 12.Hattori N, Katayama Y, Maya Y, Gatherer A. Impact of stereotactic hematoma evacuation on activities of daily living during the chronic period following spontaneous putaminal hemorrhage: a randomised study. Journal of Neurosurgery. 2004;101:417–20. doi: 10.3171/jns.2004.101.3.0417. [DOI] [PubMed] [Google Scholar]
- 13.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–97. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 14.Wang WZ, Jiang B, Liu HM, Li D, Lu CZ, Zhao YD, et al. Minimally invasive craniopuncture therapy vs. conservative treatment for spontaneous intracerebral hemorrhage: results from a randomized clinical trial in China. Int J Stroke. 2009;4:11–6. doi: 10.1111/j.1747-4949.2009.00239.x. [DOI] [PubMed] [Google Scholar]
- 15.Mendelow A. Surgical management of intracerebral hemorrhage. In: Carhuapoma J, Mayer S, Hanley D, editors. Intracerebral Hemorrhage. Cambridge: Cambridge University Press; 2009. pp. 165–75. [Google Scholar]
- 16.Mendelow A, Gregson B. Surgery for Intracerebral Hemorrhage. In: Mohr JP, Wolf P, Grotta J, Moskowitz M, Mayberg M, von Kummer R, editors. Stroke: Pathophysiology, Diagnosis and Management. 5th. Saunders; 2011. [Google Scholar]
- 17.Prasad K, Mendelow AD, Gregson BA. Surgery for primary supratentorial intracerebral haemorrhage. Cochrane Database of Systematic Reviews. 2008 doi: 10.1002/14651858.CD000200. Art. No.: CD000200. [DOI] [PubMed] [Google Scholar]
- 18.Deinsberger W. Spontane Nichttraumatische Intracerebrale Haematome: Verbesserung der Prognose durch die endoskopische Haematomentleerung [MD] Graz: Karl Franzens Universitat; 1987. [Google Scholar]
- 19.Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D. Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. Acta Neurochirurgica - Supplement. 2008;105:147–51. doi: 10.1007/978-3-211-09469-3_30. [DOI] [PubMed] [Google Scholar]
- 20.Zhou H, Zhang Y, Liu L, Huang Y, Tang Y, Su J, et al. Minimally invasive stereotactic puncture and thrombolysis therapy improves long-term outcome after acute intracerebral hemorrhage. Journal of Neurology. 2011;258:661–9. doi: 10.1007/s00415-011-5902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendelow AD, Gregson B, Mitchell P, Murray G, Rowan E, Gholkar A, et al. Surgical Trial in Lobar Intracerebral Haemorrhage (STICH II) Protocol. Trials. 2011;12:124. doi: 10.1186/1745-6215-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgenstern LB, Demchuk AM, Kim DH, Frankowski RF, Grotta JC. Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage. Neurology. 2001;56:1294–9. doi: 10.1212/wnl.56.10.1294. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 25.Brand RA, Brand RA. Standards of reporting: the CONSORT, QUORUM, and STROBE guidelines. Clinical Orthopaedics & Related Research. 2009;467:1393–4. doi: 10.1007/s11999-009-0786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan T, Awad I, Keyl P, Lane K, Hanley D. Preliminary report of the clot lysis evaluating accelerated resolution of intraventricular hemorrhage (CLEAR-IVH) clinical trial. Acta Neurochirurgica - Supplement. 2008;105:217–20. doi: 10.1007/978-3-211-09469-3_41. [DOI] [PubMed] [Google Scholar]
- 27.Morgenstern LB, Hemphill JC, 3rd, Anderson C, Becker K, Broderick JP, Connolly ES, Jr, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–29. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Table of potential data sources
Figure S1. Meta-analysis of published data for all 14 trials: Mortality
Figure S2. Meta-analysis of published data for death and disability