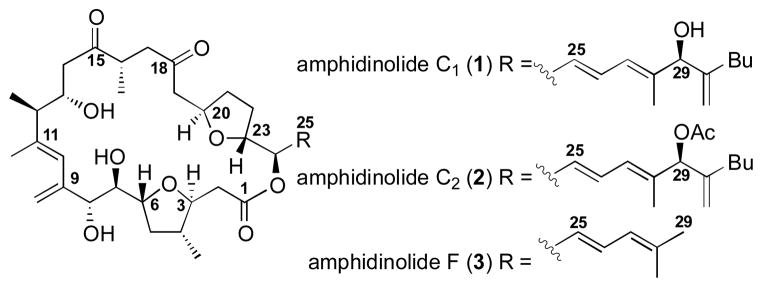
Over 30 members of the diverse amphidinolide family of biologically active macrolides have been isolated from the dinoflagellate Amphidinium sp.[1] From this family, amphidinolides C (1–2)[2] and F(3)[3] stand among the most complex and densely functionalized members (Figure 1).[4] These natural products 1–3 contain eleven stereogenic centers embedded within a 25-membered macrolactone including two trans-disposed tetrahydrofuran ring systems, a 1,4-diketone motif and a highly substituted diene moiety at C9–C11. In addition to the sizable structural challenges present in 1–3, these macrolides have shown significant cytotoxic activity.[2,3] Consequently, compounds 1–3 have attracted considerable synthetic attention from numerous laboratories[5] including our own.[6] Despite these sizable endeavors,[5–6] neither amphidinolide C nor amphidinolide F have been successfully synthesized in the 20+ years since their isolation. It should be noted that the stereochemical assignment of compound 3 is based on analogy to compound 1 and isolation from the same organism. Herein, we disclose the first total synthesis of amphidinolide F (3), which confirms both the absolute and relative stereochemistry of the natural product.
Figure 1.
Structurally Complex Amphidinolide Natural Products.
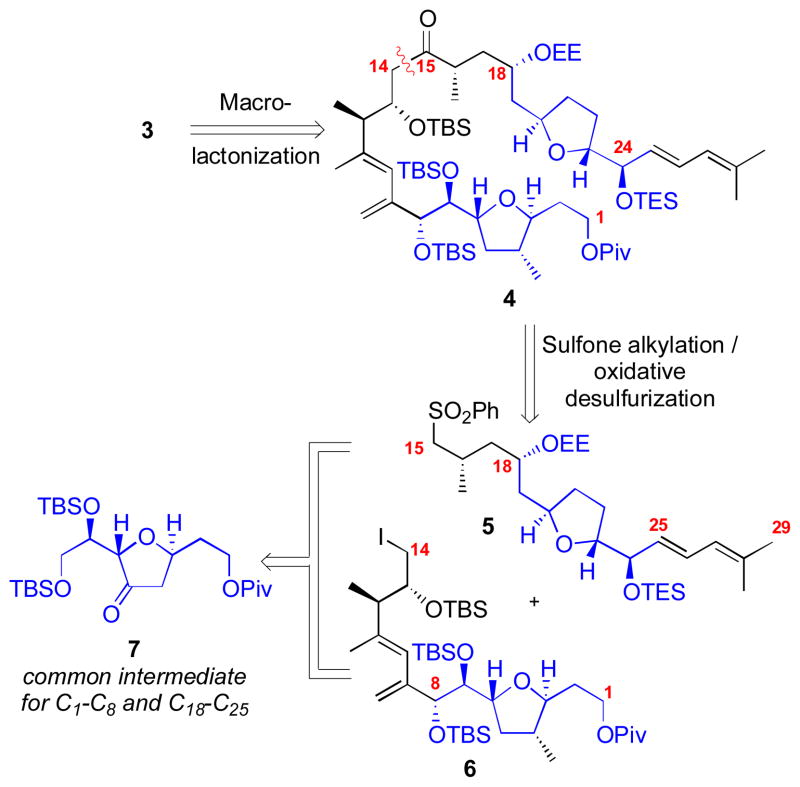
Our initial disconnection in the retrosynthesis involved cleavage of the C1 macrolactone linkage to provide the ketone 4 (Scheme 1). This ketone 4 should be accessible from sulfone 5 and iodide 6 through an umpolung strategy[7] involving a sulfone alkylation/oxidative desulfurization sequence[6a, 8] which would mask the otherwise challenging 1,4-dicarbonyl functionality. We noticed considerable “hidden” symmetry within the tetrahydrofuran (THF) portions of fragments 5 and 6. Specifically, the C1–C8 and the C18–C25 portions contain nearly identical functionalization, oxidation state and stereochemistry. This observation led us to propose that compounds 5 and 6 might be accessible via common intermediate 7. Ketone 7 should provide access to over half the carbon backbone of the macrocycle as well as the majority of the stereochemistry present in amphidinolide F.
Scheme 1.
Retrosynthesis.
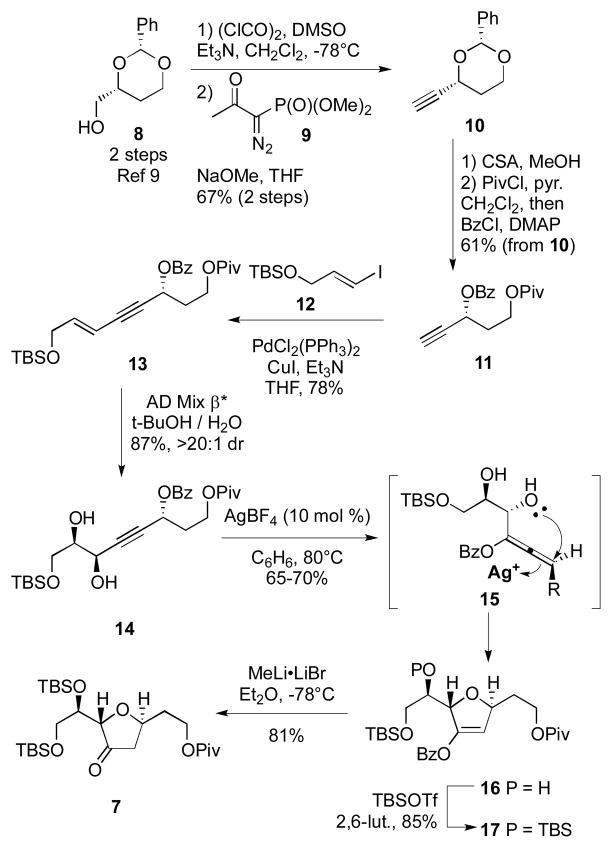
Synthesis of the common intermediate 7 is shown in Scheme 2. Starting from the known alcohol 8,[9] oxidation and Ohira-Bestmann reaction[10] cleanly provided the alkyne 10. Removal of the benzylidine acetal under acidic conditions followed by protection and Shonagashira cross coupling provided enyne 13. Sharpless asymmetric dihydroxylation yielded the diol 14 in excellent yield and diastereoselectivity.[11] Building on the work from Gagosz[12] and Krause,[13] we had hoped to use a gold-catalyzed cyclization to generate the enol ether 16. The presence of the 1,2-diol moiety complicates any cyclization conditions as both furan and pyran formation might be feasible. Unfortunately, all attempts to facilitate this transformation under Au-catalysis failed to generate the desired product. Fortunately, we found that AgBF4[14] nicely provided the desired dihydrofuran 16 in good yield and complete stereoselectivity (>20:1 dr). This transformation was routinely performed on 5-gram scale and provided sufficient quantities of 16 which might serve as a building block for a variety of trans-disposed furan-containing natural products. Subsequent silyl protection and removal of the enol benzoate with MeLi•LiBr[15] produced the common intermediate 7.
Scheme 2.
Synthesis of Common Intermediate.
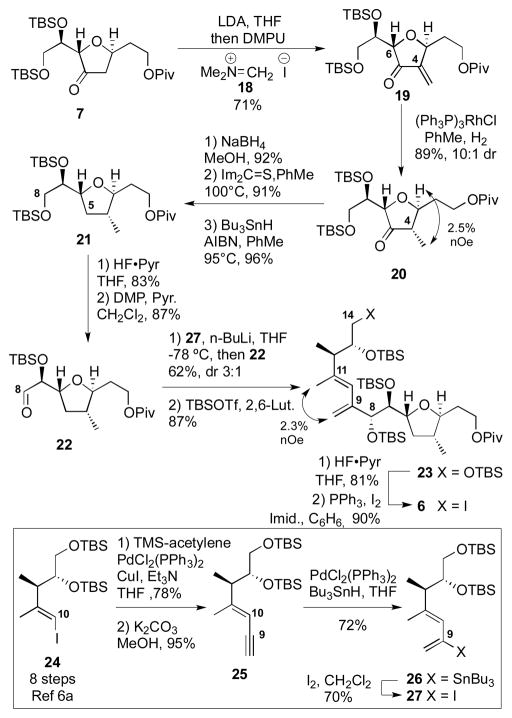
Synthesis of the C1–C14 subunit is shown in Scheme 3. We had hoped to directly trap the enolate derived from ketone 7 with methyl iodide to generate the C4 methyl derivative 20; however, the C6 stereochemistry appeared to be the dominant stereocontrolling element in the alkylation – yielded the undesired C4 methyl stereochemistry. Fortunately, we were able to exploit this directing effect to our advantage through hydrogenation of the exo-methylene compound 19 using Wilkinson’s catalyst to provide the correct stereochemical combination 20. Deoxygenation of the C5 carbonyl followed by deprotection and oxidation at C8 generated the aldehyde 22. Next, we required the stereoselective addition of a 2-metallo-1,3-diene species to the α-silyloxy aldehyde 22. The prerequisite iodide 27 was prepared in 4 steps from the previously prepared iodide 24[6a] through a regioselective hydrostannylation of enyne 25. This regiochemistry is counter to what is typically observed with most Pd-catalyzed hydrostannylations.[16] Treatment of iodide 27 with n-BuLi followed by addition to the aldehyde 22 provided the C8–C9 coupled material in good yield and reasonable diastereoselectivity (3:1 dr).[17] We had been concerned that the organolithium species might undergo 1,3-metallotropic shifts[18] to generate allenyl metallo species as well as scramble the C10–C11 E/Z olefin geometry; however, we did not see evidence of this rearrangement occurring under the reaction conditions. Generation of a related vinyllithium species via a hydrazone using Shapiro conditions led to extensive decomposition. After C8 silylation, incorporation of the C14 iodide via a two-step sequence provided fragment 6.
Scheme 3.
Synthesis of C1–C14 Subunit.
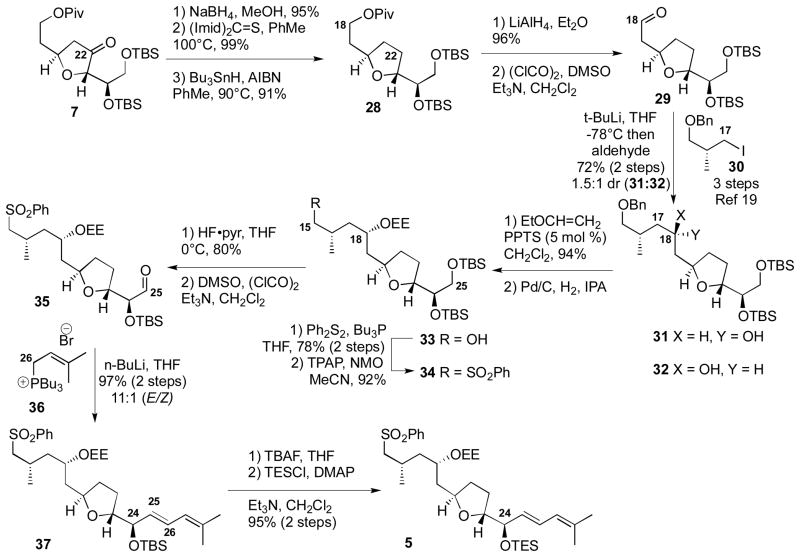
The construction of the second major fragment was also accomplished using common intermediate 7 (Scheme 4). As before, deoxygenation at C22 easily provided tetrahydrofuran 28. Removal of the pivolate at C18 followed by oxidation generated aldehyde 29. Addition of the organolithium species derived from the known iodide 30[19] provided the 2° alcohols 31 and 32 as an inseparable mixture of stereoisomers. We initially had hoped to convert the C18 alcohol into its corresponding dimethyl ketal via oxidation followed by ketalization under Noyori conditions. This approach had proven productive in our prior model system.[6] While the oxidation was effective, we were never able to ketalize the corresponding ketone under a diverse array of conditions. Consequently, we selected protection of alcohol(s) 31 and/or 32 as its ethoxyethyl (EE) ether as a viable alternative. While we believe both C18 alcohols 31 and 32 are viable compounds for the synthetic sequence, we proceeded forward with the 18S isomer 31[17] for practicality reasons including simplification of NMR spectra. Oxidation of the mixture 31 and 32 with TPAP, NMO followed by reduction with L-Selectride gave the 18S isomer 31 in high distereoselectivity [15:1 (31:32), 85% yield over two steps]. After EE protection of alcohol 31, debenzylation and incorporation of the sulfone moiety at C15 provided the compound 34. Selective 1° TBS deprotetion using HF•pyr followed by Swern oxidation revealed the key α-oxy aldehyde 35. We had initially planned to exploit Julia-Kocienski-Blakemore olefination[20] of this aldehyde with the known PT sulfone;[21] however, this reaction showed a preference for the undesired cis alkene. While alternate, multi-step solutions have been developed to circumvent this problem,[5c,5j] we continued to look for a direct solution. Fortunately, use of the Vedejs-type tributyl phosphonium salt 36[22] cleanly generated the desired E alkene 37 in good selectivity (97% yield, 11:1 E:Z). Exchange of the C24 silyl protecting groups provided the C15–C29 fragment 5.
Scheme 4.
Synthesis of C15–C29 Subunit
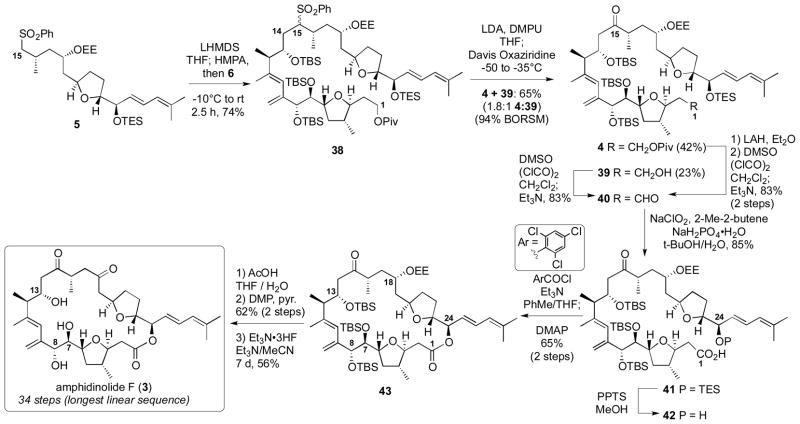
The completion of the total synthesis of amphidinolide F is shown in Scheme 5. The key coupling of the major fragments was accomplished by treatment of sulfone 5 with LHMDS and HMPA followed by the addition of alkyl halide 6 smoothly formed the C14–C15 coupled material 38.[6a] The nucleophilicity of sulfone carbanions was instrumental in the success of this challenging coupling between an α-branched alkyl iodide and an α-branched nucleophile.[23] Next, oxidative desulfurization was accomplished using LDA/DMPU followed by treatment with Davis’ oxaziridine to provide the desired ketone 4 along with the Piv deprotected ketone 39 in a combined 65% yield (94% BORSM). While this type of oxidation has been known for some time,[24] it is only recently starting to gain attention as a viable method for the incorporation of carbonyl moieties in synthesis.[6,8] Interestingly, the Davis oxaziridine proved superior to our previous TMSOOTMS conditions.[6a,8a] Both compounds 4 and 39 were easily converted to the seco acid 42. In contrast to our synthesis of amphidinolide B,[25] macrolactonization proved to be an effective way for construction of the cyclized product 43 with Yamaguchi conditions[26] being optimum. Next, careful deprotection at C18 under aqueous acidic conditions followed by oxidation yielded the sensitive C15, C18-diketone. Finally, global desilylation using Et3N•3HF[27] provided synthetic amphidindolide F (3) which matched with the natural material (1H, 13C, [α]D).[2]
Scheme 5.
Total Synthesis of Amphidinolide F.
In summary, the total synthesis of amphidinolide F has been accomplished in 34 steps (longest linear sequence). Highlights to the synthetic sequence include a silver-catalyzed dihydrofuran formation, use of a common intermediate 7 to access both the C1–C8 and C18–C25 fragments, regioselective hydrostannylation of enyne 25, diasteroselective addition of a 2-lithio-1,3-diene species to aldehyde 22 and the sulfone alkylation/oxidative desulfurization sequence to couple the major subunits and incorporate the C15 carbonyl moiety.
Supplementary Material
Acknowledgments
Financial support was provided by the National Institutes of Health (NIH) (GM63723) and Oregon State University (Harris and Shoemaker Fellowships for SM). Professor Takaaki Kubota and Jun’ichi Kobayashi (Hokkaido University) are acknowledged for providing a copy of the 1H NMR spectra for 3. Professor Max Deinzer and Jeff Morré (OSU) for mass spectra data. Dong Su is acknowledged for assistance in the synthesis of additional amounts of early building block fragment 11 and Somdev Banerjee for synthesis of known iodide 12. Finally, the authors are grateful to Professor James D. White (OSU) and Dr. Roger Hanselmann (Rib-X Pharmaceuticals) for their helpful discussions
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.a) Kobayashi J, Tsuda M. Nat Prod Rep. 2004;21:77–93. doi: 10.1039/b310427n. [DOI] [PubMed] [Google Scholar]; b) Kobayashi J. J Antibiot. 2008;61:271–284. doi: 10.1038/ja.2008.39. [DOI] [PubMed] [Google Scholar]
- 2.a) Kobayashi J, Ishibashi M, Wälchli NR, Nakamura H, Yamasu T, Hirata Y, Sasaki T, Ohizumi Y. J Am Chem Soc. 1988;110:490–94. [Google Scholar]; b) Kubota T, Tsuda M, Kobayashi J. Org Lett. 2001;3:1363–66. doi: 10.1021/ol015741z. [DOI] [PubMed] [Google Scholar]; c) Kubota T, Sakuma Y, Tsuda M, Kobayashi J. Mar Drugs. 2004;2:83–87. [Google Scholar]; d) Kubota T, Suzuki A, Yamada M, Baba S, Kobayashi J. Heterocycles. 2010;82:333–338. [Google Scholar]
- 3.Kobayashi J, Tsuda M, Ishibashi M, Shigemori H, Yamasu T, Hirota H, Sasaki T. J Antibiot. 1991;44:1259–1261. doi: 10.7164/antibiotics.44.1259. [DOI] [PubMed] [Google Scholar]
- 4.Kubota T, Kobayashi J. J Nat Prod. 2007;70:451–460. doi: 10.1021/np0605844. [DOI] [PubMed] [Google Scholar]
- 5.a) Ishiyama H, Ishibashi M, Kobayashi J. Chem Pharm Bull. 1996;44:1819–1822. [Google Scholar]; b) Kubota T, Tsuda M, Kobayashi J. Tetrahedron. 2003;59:1613–1625. [Google Scholar]; c) Shotwell JB, Roush WR. Org Lett. 2004;12:3865–3868. doi: 10.1021/ol048381z. [DOI] [PubMed] [Google Scholar]; d) Mohapatra DK, Rahaman H, Chorghade MS, Gurjar MK. Synlett. 2007:567–570. [Google Scholar]; e) Bates RH, Shotwell JB, Roush WR. Org Lett. 2008;9:4343–4346. doi: 10.1021/ol801852j. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Armstrong A, Pyrkotis C. Tetrahedron Lett. 2009;50:3325–3328. [Google Scholar]; g) Paudyal MP, Rath NP, Spilling CD. Org Lett. 2010;12:2954–2957. doi: 10.1021/ol100959a. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Ferri L, Figadre B. Org Lett. 2010;12:4976–4979. doi: 10.1021/ol1021228. [DOI] [PubMed] [Google Scholar]; (i) Roy S, Spilling CD. Org Lett. 2010;12:5326–5329. doi: 10.1021/ol102345v. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Morra NA, Pagenkopf BL. Org Lett. 2011;13:572–575. doi: 10.1021/ol1030074. [DOI] [PubMed] [Google Scholar]; k) Fischer DA, Williams DR, De R, Fultz RM, Morales-Ramos A, Rodriguez-Reyes D. 243st National American Chemical Society Meeting; San Diego, CA. March 25–29, 2012; p. ORGN-756. [Google Scholar]
- 6.a) Mahapatra S, Carter RG. Org Biomol Chem. 2009;7:4582–4585. doi: 10.1039/b916744g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mahapatra S, Carter RG. 241st National American Chemical Society Meeting; Anaheim, CA. March 27–31, 2011; p. ORGN-295. [Google Scholar]; c) Mahapatra S, Carter RG. Northwest Regional American Chemical Society Meeting; Portland, OR. June 26–30, 2011; 2011. p. NORM-261. [Google Scholar]
- 7.Seebach D. Angew Chem Int Ed. 1979;17:239–258. [Google Scholar]
- 8.a) Zhou XT, Carter RG. Angew Chem Int Ed. 2006;45:1787–90. doi: 10.1002/anie.200503733. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bonaparte AC, Betush MP, Panseri BM, Mastarone DJ, Murphy RK, Murphree SS. Org Lett. 2011;13:1447–1449. doi: 10.1021/ol200135m. [DOI] [PubMed] [Google Scholar]
- 9.a) Flögel O, Amombo MGO, Reißig HU, Zahn G, Brüdgam I, Hartl H. Chem Eur J. 2003;9:1405–1415. doi: 10.1002/chem.200390160. [DOI] [PubMed] [Google Scholar]; b) Herradon B. Tetrahedron: Asymmetry. 1991;2:191–194. [Google Scholar]
- 10.Ohira S. Synth Commun. 1989;19:561–564.Müller S, Liepold B, Roth GJ, Bestmann J. Synlett. 1996:521–522.See also: Goundry WRF, Baldwin JE, Lee V. Tetrehedron. 2003;59:1719–1729.Kitamura M, Tokynaga M, Noyori R. J Am Chem Soc. 1995;117:2931–2932.
- 11.Diastereoselectivity was determined by 1H NMR analysis of compounds 14, 16and 17,which showed a single diastereomer (>20:1 dr).
- 12.Buzas A, Istrate F, Gagosz F. Org Lett. 2006;8:1957–59. doi: 10.1021/ol0606839. [DOI] [PubMed] [Google Scholar]
- 13.Volz F, Krause N. Org Biomol Chem. 2007;5:1519–21. doi: 10.1039/b703995f. [DOI] [PubMed] [Google Scholar]
- 14.Shigemasa Y, Yasui M, Ohrai S, Sakaki M, Sashiwa H, Saimoto H. J Org Chem. 1991;56:910–912. [Google Scholar]
- 15.Turks M, Vogel P. J Org Chem. 2009;74:435–437. doi: 10.1021/jo801981c. [DOI] [PubMed] [Google Scholar]
- 16.This reactivity was first noted by Smith and co-workers as an unwanted side reaction in their synthesis of rapamycin. Smith AB, III, Condon SM, McCauley JA, Leazer JL, Jr, Leahy JW, Maleczka RE., Jr J Am Chem Soc. 1997;119:962–973.For alternative routes to similar stannanes, see: Oehlschager AC, Hutzinger MW, Aksela R, Sharma S, Singh SM. Tetrahedron Lett. 1990;31:165–168.Suzenet F, Blart E, Quintard JP. Synlett. 1998:879–881.Mandal AK, Schneekloth JR, Jr, Kuramochi K, Crews CM. Org Lett. 2006;8:427–430. doi: 10.1021/ol052620g.
- 17.The stereochemistry was established by advanced Mosher ester method. See supporting information for details. Ohtani I, Kusumi T, Kashman Y, Kakisawa H. J Am Chem Soc. 1991;113:4092–4096.
- 18.Hoffmann RW, Polachowski A. Chem Eur J. 1998;4:1724–1730. [Google Scholar]
- 19.a) White JD, Kawaski M. J Org Chem. 1992;57:5292–5300. [Google Scholar]; b) Vong BG, Abraham S, Xiang AX, Theodorakis EA. Org Lett. 2003;5:1617–1620. doi: 10.1021/ol034243i. [DOI] [PubMed] [Google Scholar]; (c) Kopecky DJ, Rychnovsky SD. J Am Chem Soc. 2001;123:8420–8421. doi: 10.1021/ja011377n. [DOI] [PubMed] [Google Scholar]
- 20.a) Kocienski PJ, Bell A, Blakemore PR. Synlett. 2000:365–366. [Google Scholar]; b) Blakemore PR. J Chem Soc, Perkin Trans. 2002;1:2563–2585. [Google Scholar]
- 21.Takano D, Nagamitsu T, Ui H, Shiomi K, Yamaguchi Y, Masuma R, Kuwajima I, Omura S. Org Lett. 2001;3:2289–2291. doi: 10.1021/ol010089t. [DOI] [PubMed] [Google Scholar]
- 22.a) Vedejs E, Marth CF, Ruggeri R. J Am Chem Soc. 1998;110:3940–3948. [Google Scholar]; b) Tamura R, Saegusa K, Kakihana M, Oda D. J Org Chem. 1998;53:2723–2728. [Google Scholar]
- 23.Fuwa H, Okamura Y, Natsugari H. Tetrahedron. 2004;60:5341–52. [Google Scholar]
- 24.Hwu JR. J Org Chem. 1983;48:4432–4433. [Google Scholar]
- 25.Lu L, Zhang W, Carter RG. J Am Chem Soc. 2008;130:7253–7255. doi: 10.1021/ja803012n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull Chem Soc Jpn. 1979;52:1989–1993. [Google Scholar]
- 27.Dunetz JR, Julian LD, Newcom JS, Roush WR. J Am Chem Soc. 2008;130:16407–16416. doi: 10.1021/ja8063205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.