Abstract
Photosynthetic organisms synthesize carotenoids for harvesting light energy, photoprotection, and maintaining the structure and function of photosynthetic membranes. A light-sensitive, phytoene-accumulating mutant, pds1-1, was isolated in Chlamydomonas reinhardtii and found to be genetically linked to the phytoene desaturase (PDS) gene. PDS catalyzes the second step in carotenoid biosynthesis—the conversion of phytoene to ζ-carotene. Decreased accumulation of downstream colored carotenoids suggested that the pds1-1 mutant is leaky for PDS activity. A screen for enhancers of the pds1-1 mutation yielded the pds1-2 allele, which completely lacks PDS activity. A second independent null mutant (pds1-3) was identified using DNA insertional mutagenesis. Both null mutants accumulate only phytoene and no other carotenoids. All three phytoene-accumulating mutants exhibited slower growth rates and reduced plating efficiency compared to wild-type cells and white phytoene synthase mutants. Insight into amino acid residues important for PDS activity was obtained through the characterization of intragenic suppressors of pds1-2. The suppressor mutants fell into three classes: revertants of the pds1-1 point mutation, mutations that changed PDS amino acid residue Pro64 to Phe, and mutations that converted PDS residue Lys90 to Met. Characterization of pds1-2 intragenic suppressors coupled with computational structure prediction of PDS suggest that amino acids at positions 90 and 143 are in close contact in the active PDS enzyme and have important roles in its structural stability and/or activity.
Introduction
Carotenoids are a diverse class of isoprenoid pigments with important functions in nature. In plants and green algae they are C40 molecules with a long chain of conjugated double bonds that can absorb light energy and quench harmful molecules such as triplet chlorophylls and singlet oxygen [1], [2]. Plants synthesize carotenoids in chloroplasts for harvesting light energy, photoprotection, and maintaining the structure and function of photosynthetic membranes [1], [3], [4]. In photosynthetic tissues most carotenoids are bound to proteins localized in thylakoid membranes [5], [6], [7]. Besides their role in photosynthesis, carotenoids act as attractants for pollination and seed dispersal. In seeds, carotenoids help prevent seed aging and increase seed viability [8], [9]. Carotenoids can also be converted to the plant hormone, abscisic acid (ABA) [10], [11], [12], which promotes seed dormancy. Dietary carotenoids in animals have many functions as antioxidants, pigments, and precursors to vitamin A. A diet rich in carotenoids helps prevent eye diseases and can reduce the risk of cancers and UV damage to skin in humans [13], [14], [15].
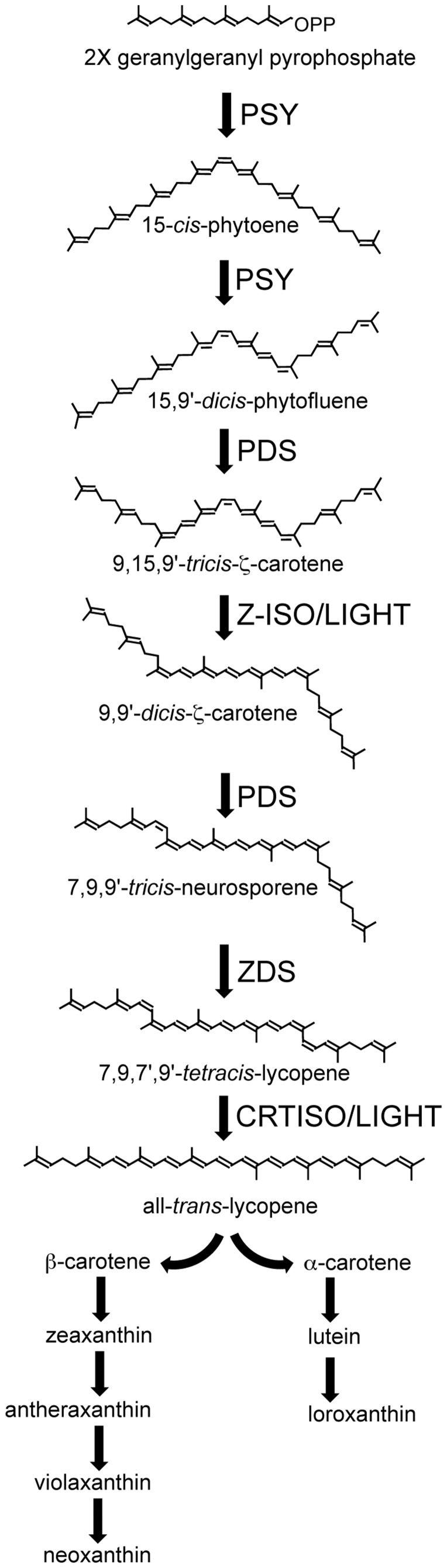
Carotenoid biosynthesis (Figure 1) involves four types of reactions: 1) condensation of two colorless geranylgeranylpyrophosphates (GGPP) molecules to form the colorless phytoene molecule, 2) desaturation and isomerization of phytoene to form red colored lycopene, 3) cyclization of lycopene to form beta-carotene and alpha-carotene and 4) addition of oxygen groups to form xanthophylls [16].
Figure 1. Carotenoid biosynthesis in plants and green algae.
Geranylgeranyl pyrophosphate, phytoene, and phytofluene are all colorless compounds. Colored carotenoids include ζ-carotene and all carotenoids downstream. Xanthophylls include zeaxanthin, antheraxanthin, violaxanthin, neoxanthin, lutein, and loroxanthin (found in C. reinhardtii).
In plants and green algae, the first committed step of carotenoid biosynthesis is catalyzed by phytoene synthase (PSY), which joins two molecules of the colorless C20 compound geranylgeranyl diphosphate (GGPP) to form the colorless C40 carotene, 15-cis-phytoene. Two conjugated double bonds are then added to 15-cis-phytoene by phytoene desaturase (PDS), giving ζ-carotenes their characteristic light-yellow color. PDS catalyzes two successive dehydrogenation reactions, converting 15-cis-phytoene via the intermediate 15,9′-dicis-phytofluene to 9,15,9′-tricis-ζ-carotene. Plant and algal mutants affecting PSY and PDS activity accumulate GGPP and phytoene, respectively, resulting in albino seedlings for plants [17], [18], [19], [20], [21] and white-colored cells in algae [22].
C. reinhardtii lts1 mutants impaired in PSY were previously characterized by McCarthy et al. [22], who isolated eleven “white” or carotenoid-less mutants, all of which were found to be affected in PSY activity and did not accumulate phytoene as would be expected for mutants with defects in PDS activity [22]. Vila et al. attempted to generate phytoene-accumulating mutants by post-transcriptional silencing of PDS expression through small interfering RNA (siRNA) and antisense RNA targeted to PDS [23]. Although they showed that PDS mRNA levels were reduced, carotenoid levels were unaffected, and phytoene did not accumulate [23]. Here we describe the successful isolation and characterization of C. reinhardtii mutants affecting PDS and offer an explanation as to why previous screens were unsuccessful.
Materials and Methods
Strains and growth conditions
The wild-type C. reinhardtii strains used in this work, 4A+ (mt+) and 4Ax5.2- (mt−), are in the 137c genetic background [24]. The polymorphic wild-type strain, S1D2 (mt−), was used in genetic linkage tests [25]. The lts1-210 mutant has a null mutation in the PSY gene [22]. Cells were maintained on Tris-acetate-phosphate (TAP) agar medium [26] at 25°C in complete darkness. Unless otherwise specified, experiments were performed on cells grown in 50 ml of liquid TAP to a density of ∼5×106 cells ml−1 in complete darkness with shaking at 120 rpm.
For norflurazon experiments, cells were spotted onto 35 ml of TAP-agar with norflurazon concentrations of 0.5 µM, 1 µM, 5 µM, 10 µM, 50 µM and 100 µM. Norflurazon was dissolved in methanol and diluted so that 100 µl were added per 35 ml of TAP-agar. TAP-only plates contained 100 µl of methanol. For pigment analysis, 4A+ cells were grown in 50 ml TAP plus 0 µM, 5 µM, or 10 µM norflurazon to a density of ∼5×106 cells ml−1, and 4×107 cells were harvested for high performance liquid chromatography (HPLC) analysis.
For light sensitivity assays, cells were inoculated into 150 µl of TAP in 96-well plates and grown for 2 days in the dark at 25°C. 5 µl of cells were then spotted onto TAP-agar and grown for 5 days in the dark. Cells were then shifted to 10 µMol photons m−2 sec−1 (vLL), 100 µMol photons m−2 sec−1 (LL), or 500 µMol photons m−2 sec−1 (HL) for 7 days. Dark-only cells were grown completely in the dark for 12 days. Cells were grown either in the dark or in LL for 2 weeks at 25°C prior to HPLC.
To determine plating efficiency, cells were grown to 2×106 cells ml−1 and then counted using a hemacytometer. Since pds1-3 cells tend to clump, all strains were incubated in 30 ml of water for 2 hours prior to cell counting allowing them to become single cells. The cells were then centrifuged at 3000× g for 5 min, and the resulting pellet was gently suspended in liquid TAP and plated onto TAP-agar plates using glass beads. The plates were incubated in the dark at 25°C for 2 weeks before colony forming units (CFU) were counted. Growth of white mutants compared to dark green wild-type cells was tested by mixing lts1 or pds1 cells in equal ratio to wild-type cells and plating onto TAP-agar. Plates were inoculated with 2500 cells for pds1 strains and 1650 cells for wild-type and lts1-210 strains and grown for 2 weeks in the dark.
To determine growth rates of 4A+, lts1, pds1-3, and pds1-1, 1×106 cells were used to inoculate each of three 100 ml TAP cultures. The cultures were allowed to grow in the dark at 25°C with shaking at 120 rpm, and cells were counted every 12 hours for 1 week. Cell densities were measured with a Multisizer3 Coulter Counter (Beckman Coulter, Fullerton, CA).
Mutagenesis
The pds1-1, pds1-2, P3-84, and pds1-2 suppressor mutants were all generated using UV mutagenesis [22]. 4A+ cells were mutagenized to create pds1-1 mutants, and pds1-1 in turn, was mutagenized to generate the P3-84 strain. The pds1-2 suppressor strains were generated by mutagenizing P3-84 cells. For each mutagenesis, 20 ml of cells (∼5×106 cells ml−1) in an open 150 mm glass Petri dish were exposed to 90,000 µJ UV light cm−2. Cells were incubated overnight in the dark then plated onto TAP-agar with glass beads and further grown in the dark at 25°C until colonies became visible. For pds1 enhancer mutants, light green, green brown, and white mutants were picked and further screened via HPLC for phytoene accumulation. To isolate suppressor mutants, P3-84 cells were UV mutagenized at 55,000 µJ UV light cm−2. A total of 35 TAP-agar plates were grown with 1.25×107 mutagenized cells/plate. After plating onto TAP-agar, mutagenized cells were allowed to grow in the dark for 5 days followed by 2 weeks at a light intensity of 1 µMol photons m−2 sec−1. Green colonies were picked for further analysis.
The mutant pds1-3 was generated by DNA insertional mutagenesis [24] using the pBC1 plasmid conferring paromomycin resistance. pBC1 was linearized with XbaI and 0.5 µg of the plasmid was used per transformation. Following transformation cells were resuspended in liquid TAP and placed in the dark with shaking at 120 rpm at 25°C to recover overnight. After recovery, mutagenized cells were centrifuged and resuspended in 300 µl TAP before being plated onto TAP-agar containing 10 µg ml−1 paromomycin. The cells were then kept in the dark at 25°C for 4 weeks to select for paromomycin-resistant colonies.
HPLC analysis
Pigments were extracted and analyzed by HPLC from dark-grown liquid TAP cultures or from cells grown on TAP-agar as described previously [22]. Pigments were extracted from 1×108 cells by vortexing in 200 µl of acetone for 30 seconds. After centrifugation at 20,000× g for 1 min, the supernatant was filtered through a 0.45-µM nylon filter and stored in the dark until HPLC analysis, when 25 µl of the pigment extract was separated on a reverse-phase C18 Spherisorb S5 ODS1 4.6-×250-mm cartridge column (Waters, Milford, MA) at 30°C. The carotenoids and chlorophylls were identified by their absorbance at 445 and 296 nm using a diode array detector. A standard curve of known concentrations of each purified compound was used for calculating chlorophyll and carotenoid concentrations. Since no commercially purified phytoene was available to create a standard curve, phytoene levels were compared using peak areas derived from HPLC analysis.
Genetic analysis
All crosses were carried out according to Harris [26]. Because pds1 mutants were extremely light sensitive, zygospores derived from pds1 mutants were only exposed to 5 hours of vLL to induce germination. Germinated zygospores were dissected, and the resulting progeny were grown in complete darkness at 25°C on TAP-agar plates until colonies could be detected. The pds1-1 (mt+), pds1-3 (mt+), and P3-84 (mt+) strains were crossed to 4Ax5.2 (mt−). Progeny produced from crosses between 4Ax5.2 and pds1-3 were tested for paromomycin resistance by growing the cells on TAP-agar plus 10 µg/ml paromomycin for 2 weeks in the dark.
For genetic linkage analysis, the pds1-1 mutant was crossed to the S1D2 (mt−) strain. Genomic DNA was extracted from progeny resulting from this cross and used to amplify a 268-bp DNA fragment of the PDS gene with primers PDS4 (5′–ACCTTTCTGTTACACAAACCATGC-3′) and PDS7 (5′-TACACTGGTTTGGCACTCGTAGA-3′). The 268-bp PCR product was digested with ScrFI overnight before being run on a 3% Metaphor agarose gel (Cambrex, East Rutherford, NJ).
Vegetative diploids were generated by crossing pds1-1 to an arginine-deficient strain with the arg7-8 mutation [26], [27]. Progeny from this cross were maintained on TAP-agar supplemented with 50 µg/ml of L-arginine. The pds1-1 arg7-8 (mt−) double mutants were selected by their light green color and their inability to grow on TAP-agar without arginine and then crossed to an arginine-deficient strain with the arg7-1 allelic mutation. The mating mix was plated directly on TAP-agar without arginine plates and grown in LL at 25°C. After 10 days in the light, surviving colonies were picked and tested for their ploidy using mating-type PCR [27].
DNA analysis
DNA and RNA were extracted from cells grown in liquid. For restriction enzyme site-directed amplification (RESDA)-PCR analysis, DNA was extracted from cells grown on TAP-agar plates for 14 days in the dark. DNA was extracted from cells as described previously [28], but without CsCl purification.
The PDS and PSY genes were sequenced from genomic DNA isolated from 4A+, pds1-1, P3-84, and pds1-2 suppressor mutants. Sequencing primers were designed using Primer3 software [29] against the annotated PDS and PSY genes in the C. reinhardtii nuclear genome sequence from the Department of Energy Joint Genome Institute (JGI, http://genome.jgi-psf.org/Chlre4/Chlre4.home.html). PCR fragments were sequenced using the DYEnamic ET Terminator Cycle Sequencing kit (Amersham Biosciences, Piscataway, NJ) and then analyzed using an ABI 3100 automated DNA sequencer (Applied Biosystems, Foster City, CA). Primer pairs used to amplify and sequence regions carrying mutations in the PDS locus were: 1) C490019_17A (5′-GGACACCACCCAATCGTTCT-3′) and C490019_17B (5′-CTACAGCCGCCCTTACTGAC-3′) and 2) C490019_4A (5′-ATACGAACATATATACGTGGCACACT-3′) and C490019_4B (5′-ATGTTTAGCTCCTTGAAGACATTCAT-3′). Primers T-PSYF1 and PSYR2 [22] were used to amplify and sequence mutations in the PSY locus.
RNA analysis
Total RNA for quantitative PCR (qPCR) and reverse-transcriptase (RT) PCR was prepared by first centrifuging cultures for 5 min at 3000 rpm followed by RNA extraction using 2 ml of Trizol reagent (Invitrogen, Carlsbad, CA) per 50 ml culture. Total RNA was resuspended in 30 µl DEPC-H20 and treated with 1.5 µl RQ Rnase-free DNAse (Promega, Madison, WI) for 1 hour at 37°C, and RNA was purified from the reaction using RNAeasy columns (Qiagen, Valencia, CA).
First-strand cDNA was synthesized using Superscript Reverse Transcriptase III (Invitrogen, Carlsbad, CA). The first-strand synthesis reaction was set up with 1 µl of 50 µM oligo-dT(20) primer, 1 µl 10 mM dNTPs, and 500 ng total RNA in a total volume of 13 µl. The cDNA synthesis reaction was incubated at 65°C for 5 minutes and quenched on ice for 1 minute before adding 4 µl 5× FS buffer, 1 µl 0.1 M DTT, 1 µl RnaseOUT, and 200 U enzyme. This was followed by a 50°C incubation for 1 hour, and finally 70°C for 15 min. 1 µl RNaseH was added to the reaction and incubated at 37°C for 20 min. 2 µl of the first-strand cDNA reaction was used as template for PCR amplification of specific transcripts with specific primers. Primers used for the amplification of tubulin as a positive control were tub-3 (5′-CGCCAAAGTACATCTCCATCC-3′) and tub-4 (5′-TAGGGGCTCTTCTTGGACA-3′) which produced a 285 bp fragment from genomic DNA and a 107 bp fragment from cDNA. Primers used to amplify the PDS transcript were PDSF_4 (5′-CTGCATGGAAGGATGAGGAT-3′) and MS069 (5′- TTGATCTCGGTGGGAAACA-3′).
For quantitative RT-PCR (qPCR), first-strand cDNA was synthesized from 1 µg total RNA with random primers (5′NNNNNNNNN) using Omniscript reverse transcriptase (Qiagen, Valencia, CA) according to the manufacturer's protocol. qPCR reactions were set up using 1 µl cDNA synthesis reaction diluted to 5 µl with sterile water as template, 2 µl of each primer at 2.5 µM concentration, and 10 µl 2× Sybr-green master mix (Qiagen, Valencia, CA) in a final volume of 20 µl. qPCR reactions were run on an ABI-7300 qPCR machine, with standard cycling. Transcript levels were quantified using the delta-delta Ct method. CBLP was used as the endogenous control gene, amplified with primers SWQ43 (5′-CAAGACCATCAAGCTGTGGA-3′) and SWQ44 (5′-ACACGATGATGGGGTTGGT-3′) which targeted the third exon. Primer pairs in the second exon of PDS, PDSF_4 (5′-CTGCATGGAAGGATGAGGAT-3′) and PDSR_4 (5′GAGTCGGGCATAGCAAAGAT-3′), and in the 3′ UTR, PDSF_3 (5′-ATCCGGAGGATTCAGGAGAC-3′) and PDSR_3 (5′-CAGAAGTCCGCACACTCAAA-3′), with approximately 150 bp amplicons were used for PDS expression analysis. Transcript levels were quantified using the delta-delta Ct method.
Isolation and analysis of flanking genomic sequences
The insertion site of pBC1 in the DNA insertional mutant, pds1-3, was identified using RESDA-PCR [30]. A set of primary and secondary specific nested primers was designed to amplify genomic DNA flanking the vector insert. Flanking sequence was isolated with primary primer MS010 (5′-AATGCGGGCGTTGCAAGTCAAATC-3′) and secondary primer MS011A (5′-AATCTGCAAGCACGCTGCCTGATC-3′). Degenerate primers and the Q0 specific primer were those described in González-Ballester et al. [30], with the addition of a fifth degenerate primer constructed identically to the original four, replacing the original restriction enzyme cutting sites with the StyI site.
Two sequential PCR reactions were required to amplify the flanking sequence. The primary RESDA-PCR reaction was set up in a volume of 25 µl as follows: 5 pmol specific primary primer, 15 pmol degenerate primer, 2.5 µl Eppendorf 10× PCR Buffer Advanced, 2.5 µl 200 µM dNTPs, 0.3 µl Eppendorf Taq polymerase, and ∼80 ng genomic DNA template suspended in TE buffer. Primary reactions were diluted 1∶25 and used as template in secondary RESDA-PCR reactions which were set up in a volume of 25 µl as follows: 5 pmol specific secondary primer, 5 pmol Q0 specific primer, 2.5 µl Eppendorf 10× PCR Buffer Advanced, 2.5 µl 200, µM dNTPs, 0.3 µl Eppendorf Taq polymerase, and 1.5 µl diluted primary reaction. RESDA-PCR primary cycling parameters were as described in Dent et al. [24], whereas secondary cycling parameters were as described in González-Ballester et al. [30]. Secondary RESDA-PCR reactions were separated on 1% agarose gels, and reactions with amplification product(s) were purified for sequencing using either the Qiagen MinElute PCR purification kit (Qiagen, Valencia, CA), or the QIAquick gel extraction kit (Qiagen, Valencia, CA) for reactions that amplified multiple bands. 40–50 ng of the DNA obtained was sequenced with the plasmid specific primer RMD225 (5′-ATAAGCTTGATATCGAATTC-3′).
RESDA-PCR of pds1-3 yielded C. reinhardtii genomic DNA flanking sequence that was used to design the primer MS039 (5′-GCCACGCCCTTGTAGTTGTA-3′) for further analysis of the insertion site. PCR with primer MS039 and RESDA-PCR secondary vector specific primers RMD225, RMD 271 (5′-CGAGCTCCCCGCTCGAGGTCGACG-3′), and MS011A (5′-AATCTGCAAGCACGCTGCCTGATC-3′) was performed. Primers were also designed within the PDS gene model at two locations upstream of the recovered flanking sequence: MS041A (5′-CTCCCTAACTCCCGCTCTTC-3′) and MS041B (5′-GTCCACGGTGGTCAGCTT-3′) were designed 500 bp upstream while MS031A (5′-GGTGGGTCATTTAGCACCTC-3′) and MS031B (5′-ATCCTCATCCTTCCATGCAG-3′) were designed 2.5 kb upstream.
Bioinformatics and structural modeling
ChloroP 1.1 (http://www.cbs.dtu.dk/services/ChloroP/) was used to predict the presence and length of potential chloroplast transit peptides from translated protein sequences [31].
PDS protein sequences from C. reinhardtii (GenBank accession XP_001690859.1), Synechocystis sp. PCC 6803 (GenBank accession CAA44452.1) and Arabidopsis thaliana (GenBank accession AAA20109.1) were retrieved from NCBI protein database at http://www.ncbi.nlm.nih.gov/sites/entrez?db=protein [32]. Ostreococcus tauri PDS protein sequence was from the Joint Genome Institue (JGI, http://genome.jgi-psf.org/Ostta4/Ostta4.home.html, protein ID 21852). The protein sequences were aligned using ClustalW version 1.83 [33] at http://www.ch.embnet.org/software/ClustalW.html and shaded according to Blosum 62 matrix.
The C. reinhardtii PDS protein (GenBank accession XP_001690859.1) was submitted to 3DLigandSite web server (Ligand binding site prediction Server) at http://www.sbg.bio.ic.ac.uk/3dligandsite/ [34].
Results
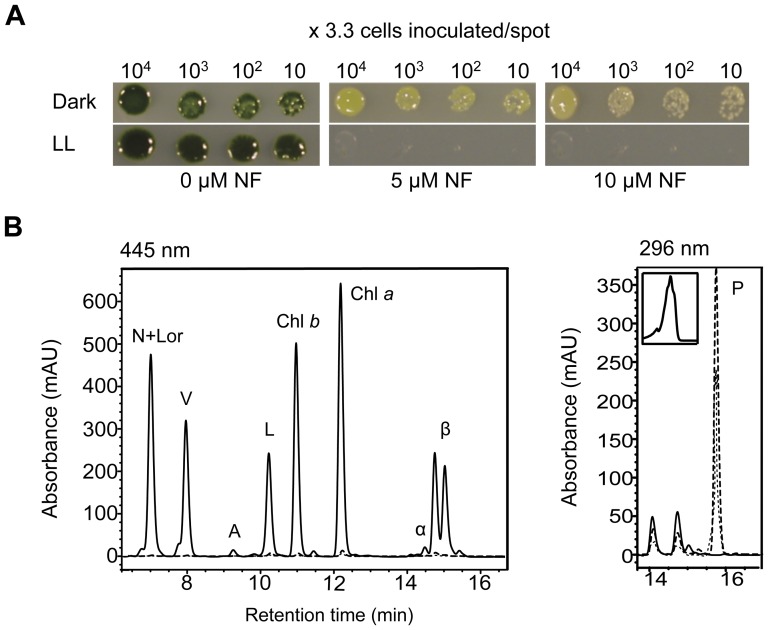
C. reinhardtii wild-type cells (4A+) were grown on norflurazon to determine the expected phenotype of pds1 mutants. Norflurazon is a bleaching herbicide that specifically inhibits PDS activity and therefore carotenoid biosynthesis [35], [36], [37]. When wild-type cells were grown on norflurazon, dark green cells became light green to almost white with increasing concentrations of norflurazon (Figure 2A). Cell growth was inhibited by norflurazon concentrations above 10 µM in the dark, whereas in low light cell growth was completely inhibited at 5 µM and higher. HPLC analysis of dark-grown cells showed that norflurazon-treated cells accumulated phytoene and had severe reductions in chlorophyll and carotenoids levels (Figure 2B). Phytoene was identified by its absorbance spectrum at 296 nm and its retention time (Figure 2B). Chlorophylls and other carotenoids were detected at 445 nm and also identified by their absorbance spectra and retention times (Figure 2B).
Figure 2. Phenotype of wild-type C. reinhardtii cells grown norflurazon.
A). Growth of wild-type C. reinhardtii cells on 0, 5, and 10 µM norflurazon (NF) in LL (100 µMol photons m−2 sec−1) or in the dark. B). Overlay of HPLC results of carotenoid and chlorophyll pigments detected in dark-grown wild-type cells treated with 0 µM (solid lines), 5 µM (dashed lines), and 10 µM (dotted lines) NF. N+Lor (neoxanthin+loroxanthin); V (violaxanthin); A (antheraxanthin); L (Lutein); Chl a and Chl b (chlorophyll a and b); α-, ß- (α- and ß-carotenes); P (phytoene). Inset shows absorbance spectrum of phytoene peak at 296 nm.
A phytoene accumulating mutant: pds1-1
Based on the results of norflurazon inhibition, C. reinhardtii mutants that are defective in PDS activity were predicted to have a light to very pale green color. From a UV mutagenesis screen, 135 light green, pale green, white, and green/brown color mutants were picked and analyzed by HPLC for pigment abnormalities. The pds1-1 mutant was identified from this screen—it was light green and accumulated phytoene (Figure 3 and 4). However, pds1-1 still produced carotenoids downstream of phytoene including ß-carotene, lutein, antheraxanthin, violaxanthin, and neoxanthin (Figure 4) at ∼5% the levels found in wild-type cells (Table 1). The pds1-1 mutant accumulated 5-fold more chlorophyll than lts1 mutants but only ∼12% the level detected in wild-type cells. The chlorophyll to colored carotenoid ratio for wild-type cells was 3.2∶1, whereas in pds1-1 the ratio was 8.7∶1. Wild-type and lts1 mutants did not accumulate any phytoene, whereas pds1-1 mutants accumulated significant levels of phytoene (Figure 4).
Figure 3. Light sensitivity of wild-type, lts1 and pds1 mutants.
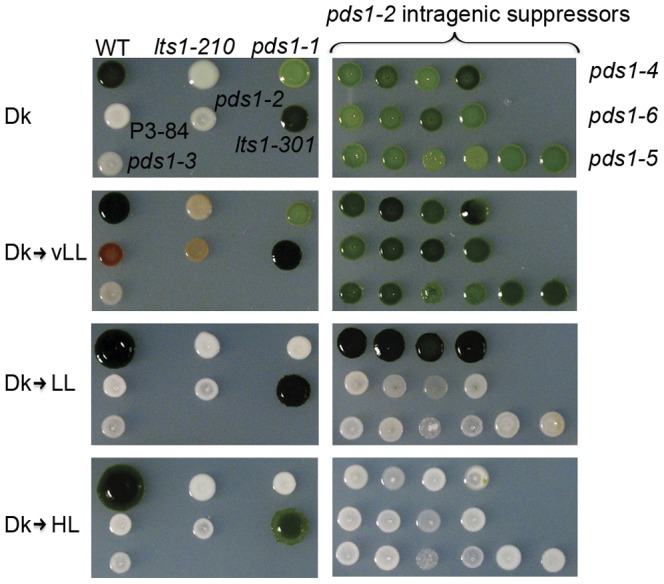
Cells were spotted onto TAP-agar and grown for 5 days in the dark before being exposed to light. All cells were grown for a total of 19 days. WT (wild-type), lts1-210 (null psy), pds1-1 (leaky pds1), P3-84 (lts1-301 pds1-2), pds1-2, lts1-301 (leaky psy), and pds1-3 (null pds1) are in the left column. In the right column are intragenic suppressors of pds1-2 mutants (pds1-4, pds1-5, pds1-6), all in the lts1-301 genetic background. Light intensities: Dk (dark), vLL (10 µMol photons m−2 sec−1), LL (100 µMol photons m−2 sec−1), HL (500 µMol photons m−2 sec−1).
Figure 4. Chlorophyll and carotenoid profiles of PDS-activity deficient mutants.
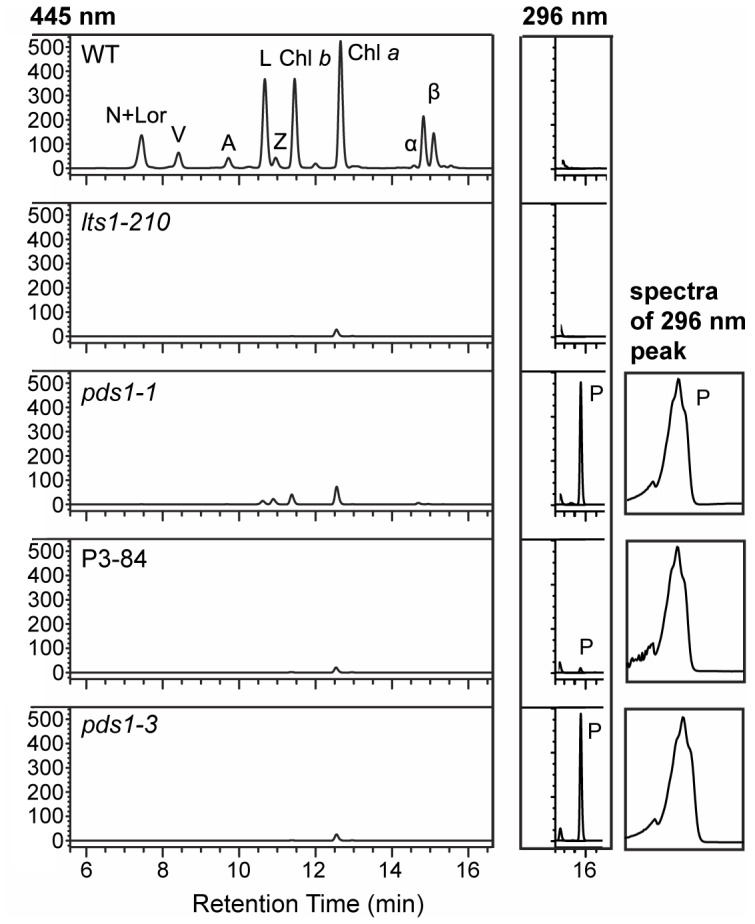
Chlorophylls and carotenoids were detected at 445 nm and phytoene was detected at 296 nm. Absorbance spectra are shown for the 296 nm phytoene peak present in both pds1-1 and pds1-3 mutants and small peak detected in P3-84. Pigments were extracted from a total of 1×108 cells for each sample and analyzed via HPLC coupled with a diode array detector. N+Lor (neoxanthin+loroxanthin); V (violaxanthin); A (antheraxanthin); L (lutein); Z (zeaxanthin); Chl a and Chl b (chlorophyll a and b); α-, ß- (α- and ß-carotenes); P (phytoene).
Table 1. Quantification of chlorophyll and carotenoid content of dark-grown lts1 and pds1 mutants.
| total chl (fmol/cell) | chl a/b ratio | total colored carotenoids (fmol/cell) | total xanthophylls (fmol/cell) | lutein (fmol/cell) | zeaxanthin (fmol/cell) | phytoene (total peak area) | |
| Wild-type | 0.5201±0.0404 | 2.17±0.23 | 0.1616±0.0307 | 0.1079±0.0208 | 0.0377±0.0291 | 0.0069±0.0030 | 0 |
| lts1-301 (leaky psy ) | 0.2297±0.0243 | 2.24±0.51 | 0.0391±0.0056 | 0.0306±0.0051 | 0.0111±0.0032 | 0.0099±0.0016 | 0 |
| lts1-210 (null psy ) | 0.0128±0.0041 | 39.24±1.09 | 0 | 0 | 0 | 0 | 0 |
| pds1-1 (leaky pds ) | 0.0655±0.0076 | 2.67±0.15 | 0.0075±0.0006 | 0.0061±0.0006 | 0.0025±0.0006 | 0.0031±0.0 | 3,605±177 |
| pds1-2 (null pds ) | 0.0113±0.0020 | 13.84±0.27 | 0 | 0 | 0 | 0 | 2,642±232 |
| pds1-3 (null pds ) | 0.0177±0.0084 | 13.84±0.27 | 0 | 0 | 0 | 0 | 4,048±907 |
| lts1-301 pds1-2 | 0.0096±0.0031 | 14.44±0.42 | 0 | 0 | 0 | 0 | 167±34 |
| suppressor pds1-4 | 0.1726±0.0643 | 2.08±0.12 | 0.0313±0.0107 | 0.0247±0.0076 | 0.0088±0.0023 | 0.0059±0.0019 | 0 |
| suppressor pds1-5 | 0.2464±0.0917 | 2.12±0.32 | 0.0363±0.0075 | 0.0283±0.0055 | 0.0102±0.0022 | 0.0070±0.0029 | 24±11 |
| suppressor pds1-6 | 0.2297±0.0744 | 2.32±0.27 | 0.0298±0.0072 | 0.0236±0.0074 | 0.0088±0.0022 | 0.0066±0.0029 | 8±0.68 |
Chlorophyll (Chl) and carotenoid quantities (represented as fmol/cell) were extracted from a total of 1×108 cells for each sample with 200 µl of acetone. Colored carotenoids detected in C. reinhardtii include α-carotene, β-carotene, lutein, violaxanthin, antheraxanthin, neoxanthin, loroxanthin, zeaxanthin. Total xanthophylls include lutein, loroxanthin, violaxanthin, antheraxanthin, neoxanthin, and zeaxanthin. Averages and standard deviations are from three independent cultures.
Similar to lts1 mutants, the pds1-1 mutant was found to be very light sensitive. After growth in the dark for four days, pds1-1 died after being exposed to more than 24 hours of vLL (Figure 3). In vLL cells died and turned brown, whereas at higher light intensities (LL and HL) cells bleached completely and turned white (Figure 3). In contrast, wild-type cells grew well at all light intensities including HL.
Genetic analysis of pds1-1 revealed that the pds1 phenotype is caused by a single, recessive nuclear mutation. Crosses between pds1-1 and wild-type cells produced tetrads that segregated 2∶2 for the pds1-1 mutant phenotype (light colored, phytoene accumulation, and reduced levels of colored carotenoids) and the wild-type phenotype (dark green, no phytoene, and normal levels of carotenoids) (Table 2). Dominance testing using heterozygous pds1-1/PDS1 vegetative diploids showed the pds1-1 mutation is recessive.
Table 2. Tetrad analysis of pds1-1 and pds1-3 crossed to wild-type.
| PD∶NPD (complete tetrads) | Total progeny | WT progeny | Mutant progeny | Mutant progeny recombinant for paromomycin marker | |
| pds1-1 ( mt+ )×WT ( mt− ) | 10∶0 | 106 | 54 | 52 | N/A |
| pds1-3 ( mt+ )×WT ( mt− ) | 7∶0 | 145 | 88 | 57 | 0 |
WT = wild type, PD = parental ditype, NPD = non-parental ditype.
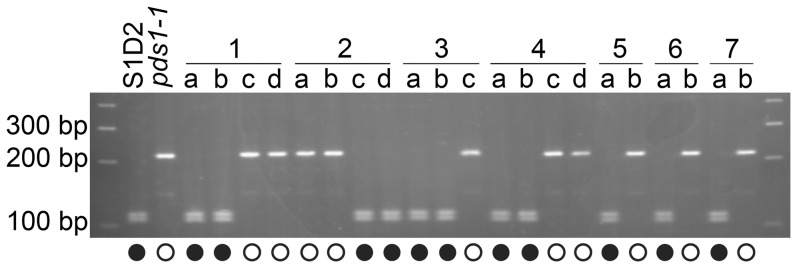
The pds1-1 mutant was crossed to the polymorphic wild-type strain S1D2 in order to map the mutation relative to the annotated PDS gene. A total of 21 progeny were isolated from this cross: 12 from complete tetrads and 9 from incomplete tetrads. A marker for the PDS locus on chromosome 12 amplified a 268 bp PCR product from both pds1-1 and S1D2. Digestion of the 268 bp PCR product with ScrFI yielded 215 and 52 bp fragments from pds1-1, whereas 111, 104, 26, and 25 bp fragments were produced from S1D2. DNA fragments smaller than 100 bp could not be visualized. When the PDS marker was tested on DNA isolated from the progeny, the light green phenotype cosegregated with the polymorphism found in pds1-1 (215 and 52 bp), while dark green progeny yielded fragments similar to S1D2 (111, 104, 26, and 25 bp) (Figure 5). This result shows that the light green phenotype is linked to the PDS locus and that a mutation in PDS is likely to be responsible for the light green color and phytoene accumulation in pds1-1.
Figure 5. The PDS gene is genetically linked to the pds1-1 mutant phenotype.
A marker located within the PDS locus cosegregated with the light green, phytoene-accumulating mutant phenotype of pds1-1. Amplification and ScrFI digestion of a 268 bp fragment of the PDS gene containing a single nucleotide polymorphism in exon 2 was used to score progeny from crosses between pds1-1 and a polymorphic wild-type strain (S1D2). Seven full and partial tetrads were scored: individual progeny within tetrads are labeled “a, b, c, d”. Solid circles indicate dark green progeny with wild-type carotenoid composition while open circles indicate light green progeny with phytoene accumulation.
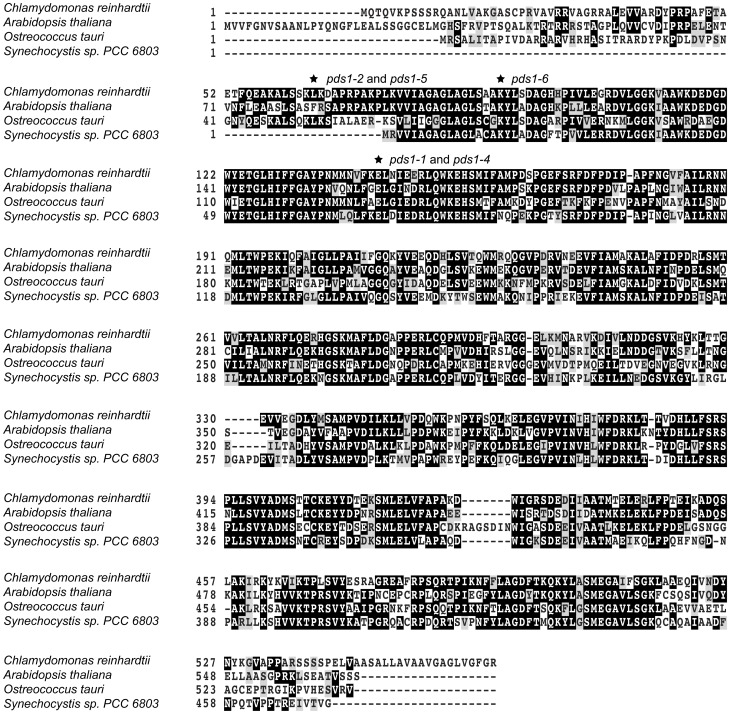
The PDS locus was sequenced from pds1-1 to discover if a mutation in this locus was responsible for the phytoene-accumulating, light green phenotype. The Chlamydomonas nuclear genome sequence of PDS is 4030 bp and the predicted protein is 564 amino acids long. Amplification and sequencing of the PDS locus identified a single base pair change in exon two of pds1-1. The point mutation consisted of a G/C to A/T transition, resulting in an E143K missense change in deduced PDS protein sequence (Figure 6). A multiple sequence alignment of predicted PDS protein sequences from wild-type C. reinhardtii, O. tauri, Synechocystis sp PCC6803, and A.thaliana and revealed that the amino acid change occurred in the conserved dinucleotide (FAD)-dependent oxidoreductase/amine oxidase domain of the PDS protein (Figure 6).
Figure 6. Multiple sequence alignment of PDS protein sequences.
Alignment of PDS amino acid sequences from eukaryotic green algae, Chlamydomonas and Ostreococcus; a plant, Arabidopsis; and a cyanobacterium, Synechocystis sp. PCC6803. Conserved residues were scored using Blosum 62 matrix, with darker shading indicating higher conservation and no shading low conservation. Asterisks mark positions of mutations in pds1 alleles.
Isolation of pds1-2 as an intragenic enhancer of pds1-1
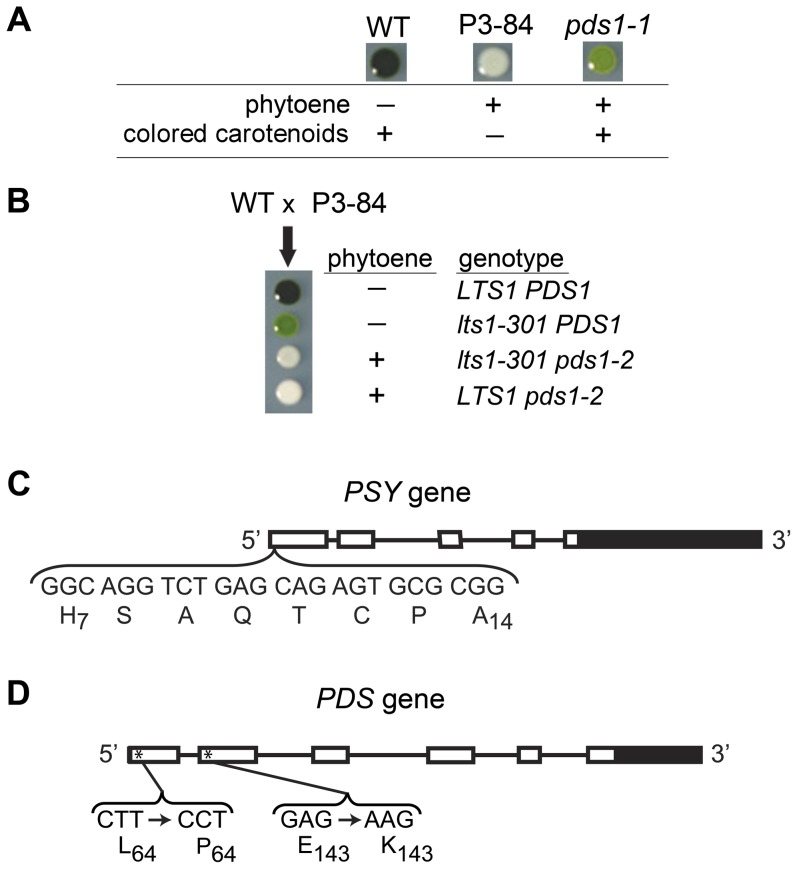
Because pds1-1 still synthesizes colored carotenoids, a second round of UV mutagenesis was conducted to find enhancer mutants that eliminated PDS activity. Light green pds1-1 cells were mutagenized, and a white mutant, P3-84, was isolated (Figure 3 and Figure 7A) that had a similar pigment profile as null psy (lts1-210) mutants, except that P3-84 accumulated a low level of phytoene (Figure 4). When P3-84 was crossed to wild-type cells, the tetratype tetrad progeny gave unexpected pigment phenotypes: two white progeny with phytoene accumulation and no other carotenoids, one dark green mutant with wild-type carotenoid composition and levels, and one light green mutant with lower carotenoid levels (Figure 7B). The original light green, phytoene-accumulating pds1-1 pigment phenotype was not recovered. To determine if the P3-84 mutant phenotype was due to mutations in either the PSY or PDS gene, both genes were sequenced. Sequencing results revealed new mutations in both PSY and PDS genes in P3-84. An in-frame deletion of 24 bp removed eight amino acid residues from positions 7 to 14 (H7SAQTCPA14) in the putative chloroplast transit peptide of PSY (Figure 7C). This new allele of PSY was named lts1-301. The PDS locus was found to carry two point mutations: the original pds1-1 mutation (E143K) and an additional T to C transition resulting in the conversion of a leucine residue at position 64 to a proline residue (L64P) (Figure 7D, Table 3). This double mutant allele of PDS was named pds1-2.
Figure 7. Analysis of enhancer strain P3-84 (lts1-301 pds1-2) and intragenic suppressors of pds1-2 mutants.
A). Color and pigment phenotype of dark-grown wild type (WT), P3-84, and pds1-1 mutants. (−) indicates no accumulation while (+) indicates presence of phytoene or colored carotenoids. Colored carotenoids include all carotenoids downstream of phytofluene. B). Tetratype tetrad phenotype from crosses between wild-type and P3-84 cells. The presence (+) or absence (−) of phytoene is indicated for each progeny along with their corresponding genotypes. C). Structure of the PSY gene in C. reinhardtii. UTRs are indicated by solid boxes, exons by open boxes and four introns by lines. The bracket highlights the eight amino acids and their corresponding nucleotides deleted from the putative chloroplast transit peptide in lts1-301, P3-84, and in pds1-2 suppressor mutants. Subscript numbers note position of the amino acid residue in the wild-type PSY protein. D). Structure of the PDS gene in C. reinhardtii. UTRs are indicated by solid boxes, exons by open boxes and five introns by lines. The brackets highlight the two missense mutations found in P3-84. Subscript numbers note position of the amino acid residue in the wild-type PDS protein. Asterisks mark approximate location of mutations.
Table 3. Summary of mutants described in this work.
| Strain(s) | Genotype | PSY mutation | PDS mutation |
| lts1-210 | lts1-210 | W60stop | n/a |
| lts1-301 | lts1-301 | in-frame deletion of PSY residues 7–14 (HSAQTCPA) | n/a |
| SP60.90 | pds1-1 | n/a | E143K |
| pds1-2 | pds1-2 | n/a | L64P E143K |
| T29-3 | pds1-3 | n/a | pBC1 insertion |
| P3-84 | lts1-301 pds1-2 | in-frame deletion of PSY residues 7–14 (HSAQTCPA | L64P E143K |
| csp6,10,14,15 | lts1-301 pds1-4 | in-frame deletion of PSY residues 7–14 (HSAQTCPA | L64P |
| csp3,4,5,9,11,16,17 | lts1-301 pds1-5 | in-frame deletion of PSY residues 7–14 (HSAQTCPA | L64F E143K |
| csp1,7,8,12,13 | lts1-301 pds1-6 | in-frame deletion of PSY residues 7–14 (HSAQTCPA | L64P K90M E143K |
The PDS and PSY sequencing results from P3-84 explained the unexpected tetratype phenotypes recovered in the cross between P3-84 and wild-type. The two parental phenotypes were represented: dark green wild-type and white P3-84 (Figure 7A and 7B). For the two unexpected phenotypes, the light-green, no phytoene accumulating phenotype belonged to progeny with reduced PSY activity (Figure 7B). Sequencing of PSY and PDS genes from this progeny (lts1-301) revealed that it has the eight amino acid chloroplast transit peptide deletion in PSY and no mutations in PDS (Figure 7C). The lts1-301 strain synthesizes wild-type carotenoids, but at reduced levels, indicating that PSY function is reduced or “leaky” (Table 1) possibly because of inefficient transport of the PSY protein into the chloroplast. The second unexpected phenotype, white plus phytoene accumulation, belonged to progeny with wild-type PSY and the two mutations in PDS (L64P and E143K) (Figure 7B). This second white progeny, pds1-2, is an intragenic enhancer mutant for pds1-1 since the only carotenoid detected was phytoene (Table 1). Both P3-84 (lts1-301 pds1-2) and pds1-2 survive only in the dark. Similar to lts1-210 and pds1-1 mutants, they die when cultured under very low light. In contrast, lts1-301 is very light tolerant, growing almost as well as wild-type cells in HL (Figure 3).
pds1-3 is a null allele derived from DNA insertional mutagenesis
An additional white, phytoene-accumulating mutant, pds1-3, was isolated from a DNA insertional mutagenesis screen based on its sensitivity to light and white color. Similar to pds1-1 mutants, pds1-3 bleached and died at vLL intensities (Figure 3), and it also accumulated phytoene (Figure 4). Unlike pds1-1, however, it does not synthesize any colored carotenoids (Table 1).
Tetrads from crosses with wild-type segregated 2∶2 for the pds1-3 and wild-type phenotypes (Table 2), indicating that the pds1-3 mutant phenotype is controlled by a single gene. Co-segregation of the mutant phenotype with paromomycin resistance also indicated that the mutation is tagged by the transforming plasmid (Table 2).
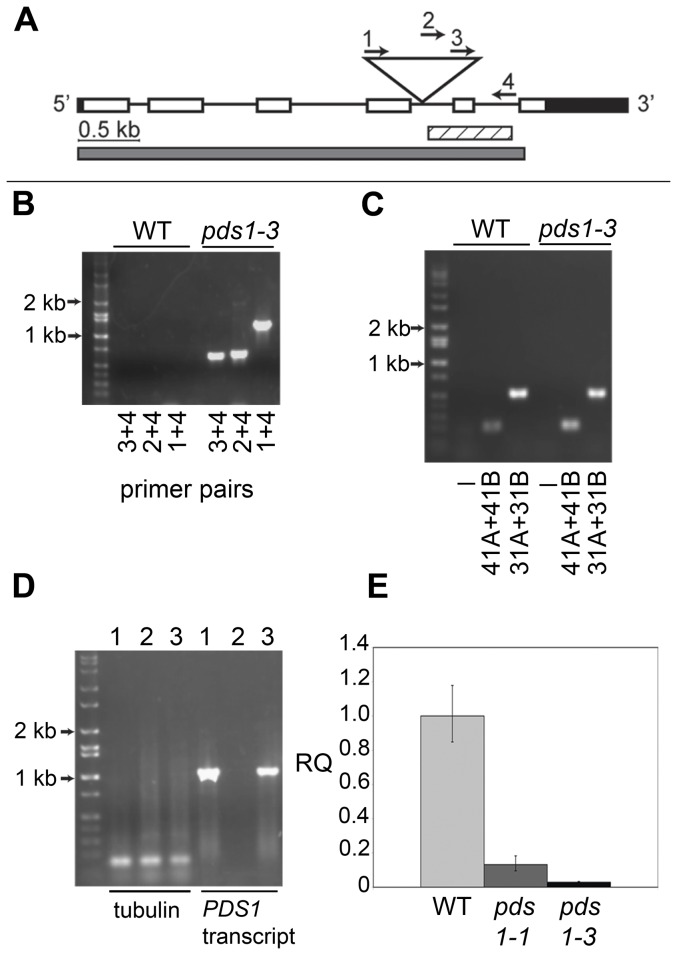
To identify the mutation responsible for the white, phytoene-accumulating phenotype of the pds1-3 mutant, RESDA-PCR was used to recover a flanking sequence tag for one end of the vector insert in pds1-3. The flanking sequence was used as a query in a BLAST search for homologous sequences [38] against the Department of Energy (DOE) Joint Genome Institute (JGI) Chlamydomonas reinhardtii v4 genome (www.jgi.doe.gov/chlamy) and found to have significant identity to a 331 bp sequence on Chromosome 12. Flanking sequence analysis indicated that the insertion interrupts an intron in PDS (Figure 8A). A primer was designed within the Chlamydomonas genomic DNA flanking the putative insert location obtained from RESDA-PCR for pds1-3, and PCR with this primer and three nested primers within the vector was performed (Figure 8A). Successful amplification confirmed the location of the plasmid vector in pds1-3 genomic DNA (Figure 8B). Recovery of flanking sequence at the other end of the insert was unsuccessful, however. One reason may be because the insertion of foreign DNA into Chlamydomonas genomic DNA is often accompanied by a deletion [24]. To determine whether a significant deletion was present in pds1-3, PCR primers were designed within the PDS genomic DNA on the side of the insertion for which no flanking sequence could be recovered. MS031A and MS031B primers amplified a 498 bp product from pds1-3 genomic DNA, 500 bp downstream from the insertion point, and primers MS041A and MS041B amplified a 200 bp fragment 2.5 kb distant from the site of insertion. Successful amplification and DNA sequencing of these PCR products indicated that a large deletion did not accompany the plasmid insertion (Figure 8C).
Figure 8. Analysis of pds1-3 DNA insertional mutant.
A). Schematic of the C. reinhardtii PDS gene showing DNA insertion location (triangle), region amplified in flanking sequence tag (striped bar), and region of transcript not detectable by RT-PCR in pds1-3 (gray bar). UTRs are indicated by black bars and exons by open bars. Genomic DNA spanning the 5′ UTR to the 4th exon could be amplified by PCR in pds1-3. B). No amplification in wild-type (WT) and amplification in pds1-3 with three vector-specific primers (1, 2, 3) and one primer in PDS genomic DNA (4), indicated by arrows in panel A, confirming insert location in pds1-3. C). Successful amplification of genomic DNA and DNA sequencing of PDS on the opposite side of insertion from flanking sequence tag in wild-type and pds1-3. Amplification products were obtained from genomic DNA 2.5 kb (primers MS031A and MS031B, in exon 1) and 500 bp (primers MS041A and MS041B, in exon 4) distant from insertion site. D). Amplification of PDS transcript (gray bar in panel A) from total RNA in wild-type (1), pds1-3 (2), and pds1-1 (3), with the amplification of tubulin as a positive control. E). Relative PDS transcript levels in wild-type cells (light gray bar), pds1-1 (dark gray bar ), and pds1-3 (black bar). Relative quantification (RQ) fold-change values to the calibrator (WT PDS transcript levels) are shown.
The PDS transcript is present in pds1-1 (Figure 8D), although at a reduced level: ∼13% of the level found in wild-type (Figure 8E). In contrast, no PDS transcript was detectable by RT-PCR or qPCR in pds1-3 (Figures 8D and 8E).
Growth defects of pds1 mutants
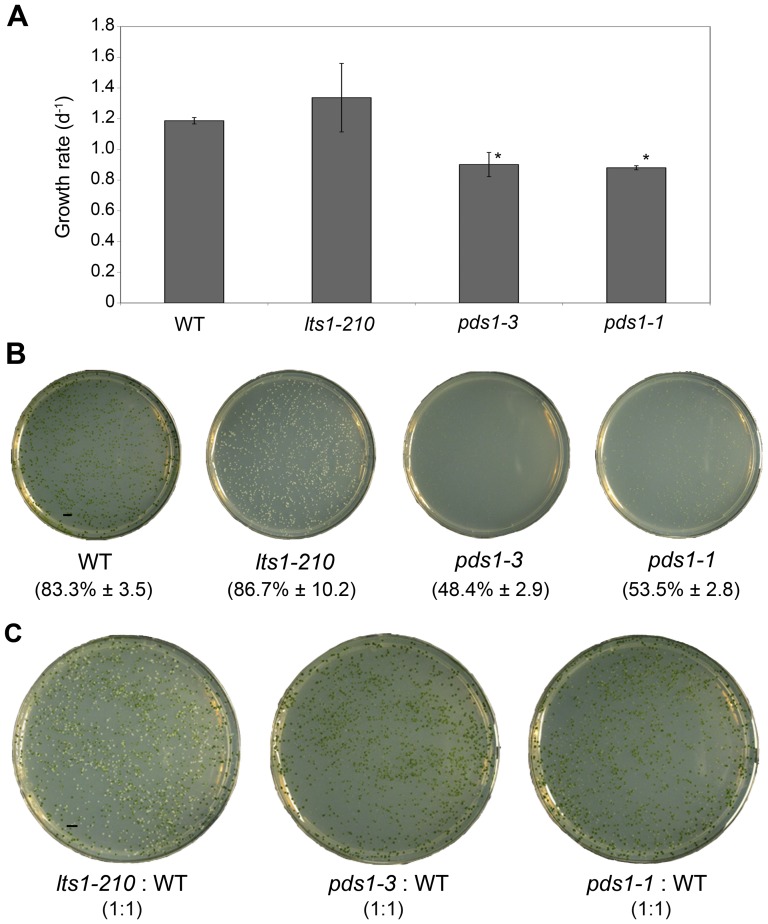
Multiple independent alleles of lts1 but no pds1 mutants were isolated in a previous screen for white mutants of C. reinhardtii [22]. To understand why pds1 mutants were not found, the growth rates of pds1 mutants were compared to lts1-210 and wild-type cells. Comparison of growth rates in liquid TAP medium in the dark showed that pds1-1 and pds1-3 mutants grew more slowly than either lts1-210 or wild-type cells (Figure 9A).
Figure 9. Results of plating assays of wild-type, lts1-210, pds1-3, and pds1-1.
A). Growth rate per day for wild-type, lts1-210 (null psy), pds1-3, and pds1-1. Biological triplicates of each strain were grown in the dark in liquid TAP on a shaker. *Significantly differently from lts1-210 and wild-type values under the same conditions using a two-tailed t test (P<0.05). B). Percent of cells that survived plating with glass beads (% survival ± standard deviation) after 12 days of growth in the dark. Scale bar represents 5 mm. C). ∼1∶1 ratio of carotenoid mutant to wild-type cells after 12 days of growth in the dark. After accounting for plating efficiencies, the expected CFU/plate was 1250 CFU/plate for pds1 and 1320 CFU/plate for wild-type and lts1-210 strains. Scale bar represents 5 mm.
Differences in growth between pds1 mutants and white lts1-210 or wild-type cells were more pronounced in plating assays. First, the plating efficiency of pds1, lts1, and wild-type strains was measured as colony-forming units (CFU). After 3 weeks growth in the dark, the plating efficiency of pds1-1 was calculated as 53.5%±2.8; pds1-3 was 48.4%±2.9; lts1-210 was 86.7%±10.2; and wild type was 83.3%±3.5 (Figure 9B).
A second plating experiment was performed to assess how visible pds1 mutants are in a background of wild-type cells when grown in a ∼1∶1 ratio (Figure 9C). After accounting for the ∼50% and ∼80% observed plating efficiencies for pds1 strains and wild-type/lts1-210 strains, respectively, the expected CFU was 1250 CFU/plate for pds1 mutants and 1320 CFU/plate for wild-type and lts1-210 strains. On TAP-agar plates with a 1∶1 ratio of lts1-210 to wild-type cells, white lts1-210 colonies were easily identified; they were as densely populated and equal in diameter to wild-type colonies (Figure 9C). In contrast, it was difficult to identify light green pds1-1 and white pds1-3 mutants among wild-type colonies because their colonies were frequently half the diameter or smaller than wild-type colonies and fewer in number (Figure 9C). Of the carotenoid mutants tested, pds1-3 colonies were the smallest and the least dense. Because of their small size, it was difficult to determine the color of some pds1 colonies and as a result, they could have been mistaken for extremely small wild-type colonies or not been detected at all in a screen for white mutants [22].
Intragenic pds1-2 suppressor mutants
To gain further insight into amino acid residues important for PDS structure and function, mutations that suppressed the white phenotype of pds1-2 were isolated. Sixteen light green pds1-2 suppressor mutants falling into three allelic classes were isolated from UV mutagenesis of the white P3-84 strain (lts1-301 pds1-2).
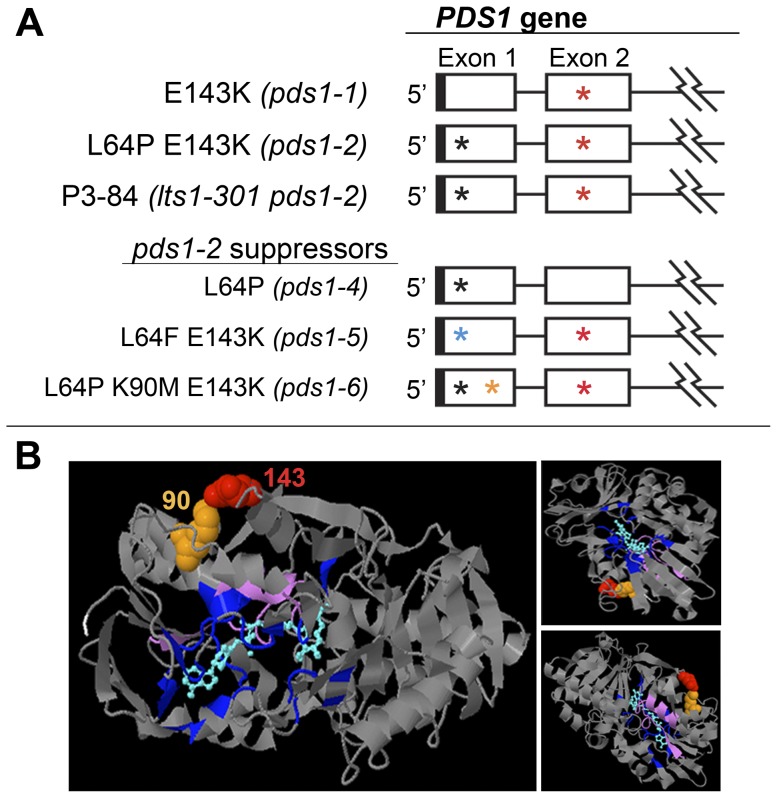
PSY and PDS genes were both sequenced from the pds1-2 suppressor mutants to identify any revertants and/or additional mutations. All 16 suppressor mutants retained the chloroplast transit peptide mutation in the PSY gene (lts1-301) from strain P3-84 (Figure 7C). Four of these strains, csp6, csp10, csp14, and csp15, had a reversion of the pds1-1 mutation: a transition from “A/T” (Lys143) back to wild-type “G/C” (Glu143) (Figures 6 and 10A; Table 3). These suppressors retained the L64P mutation, which was named pds1-4. The second class of intragenic pds1-2 suppressor mutants, pds1-5, had the original pds1-1 mutation (E143K) plus a new pds1 mutation, which converted the pds1-2 mutation (L64P) in exon one to L64F (Figure 7, 10A). Seven pds1-5 strains were isolated: csp3, csp4, csp5, csp9, csp11, csp16 and csp17 (Figure 3, csp17 not shown; Table 3). The third class of intragenic pds1-2 suppressor mutants had three mutations in PDS: E143K from pds1-1, L64P from pds1-2 and a new mutation, K90M. The methionine at position 90 resulted from a transversion mutation that changed the wild-type “A” to a “T (Figures 6 and 10A). In this third allelic class, pds1-6, five strains were isolated: csp1, csp7, csp8, csp12, and csp13 (Figure 3, csp8 not shown; Table 3).
Figure 10. Analysis of intragenic suppressors of pds1-2 mutants.
A). Schematic depiction of pds1-1, P3-84, pds1-2, and pds1-2 suppressors (pds1-4, pds1-5, and pds1-6). Cartoon of the C. reinhardtii PDS gene showing only exons one and two. Jagged lines indicate a partial depiction of the PDS gene. UTRs are indicated by solid boxes, exons by open boxes and introns by lines. Asterisks mark positions of mutations in pds1-1, pds1-2, P3-84 and pds1-2 suppressor mutants. Black and blue asterisks represent mutations L64P and L64F, respectively. Orange asterisk in exon 1 signifies location of the K90M mutation, and red asterisks in exon 2 signify the E143K mutation. B). 3DLigandSite structural prediction of the C. reinhardtii PDS protein showing positions of amino acid residues mutated in pds1-1 and pds1-6 mutants, E143K and K90M, respectively. L64P and L64F were not mapped because the first 71 amino acids of the N-terminus had no structural prediction. Ligand and wild-type amino acids corresponding to mutated residues were colored as follows: position 90 (spacefilling, orange); position 143 (spacefilling, red); ligand [NAD(P)/FAD] (cyan); predicted ligand binding sites (indigo); start of predicted N-terminus (amino acid residue 72, white); and carotenoid binding site proposed by Armstrong et al. (amino acid residues 492–517, lavender). Three different perspectives of the predicted structure are shown.
Light green intragenic suppressor mutants of pds1-2 were more light tolerant than light green pds1-1 and white pds1 mutants but less light tolerant than medium green lts1-301 single mutants (Figure 3). All suppressor mutants grew well under vLL, but only pds1-4 mutants could survive in LL. The pds1-5 and pds1-6 mutants bleached and died in LL (Figure 3). No suppressor mutant survived in HL. Although the suppressor mutants were less light tolerant than lts1-301 single mutants, pigment analysis of pds1-2 suppressor mutants showed that all three classes synthesize the full spectrum of wild-type colored carotenoids, but at ∼20% of the levels found in wild-type cells, similar to lts1-301 (Table 1). Comparison of total xanthophylls, known for their photoprotective properties [39], [40], did not reveal any significant differences between suppressor mutants and lts1-301. Zeaxanthin, a xanthophyll particularly important for photoprotection [40], [41], [42], was elevated 1.6 fold in lts1-301 compared to suppressor mutants and 1.4 fold higher than in wild-type cells. The levels of lutein were significantly lower than in wild-type cells but not significantly different among suppressor mutants or lts1-301 mutants. Two of the three pds1-2 suppressor mutant classes still accumulated phytoene. The pds1-5 and pds1-6 strains accumulated phytoene at ∼17% and ∼6%, respectively, of the levels present in the starting strain P3-84 (Table 1). Like wild-type cells, pds1-4 mutants did not accumulate any phytoene (Table 1).
PDS structural prediction
The 3DLigandSite web server predicted that the C. reinhardtii PDS protein has a structure most similar to a human monoamine oxidase, C2c70B. Both C2c70B and the C. reinhardtii PDS proteins were classified as oxidoreductases and had 12% identity to each other. 3DLigandSite predicted the presence of a dinucleotide-binding motif [NAD(P) or FAD] in the center of the PDS protein (cyan blue, Figure 10B) and potential ligand binding sites (indigo, Figure 10B) [43], [44]. The C-terminus of bacterial carotenoid dehydrogenases was proposed to contain a hydrophobic carotenoid-binding pocket [46], which was also found conserved among cyanobacteria, algae, and plants [45], [46]. In C. reinhardtii this region spans amino acid residues 492–517 (Figure 10B, lavender).
In the predicted PDS protein structure, amino acid residue 143 (mutated in both pds1-1 and in pds1-2 suppressor mutants) and amino acid residue 90 (mutated in pds1-6), were adjacent to one another, and in spacefilling mode, in physical contact (Figure 10B, Glu143, red and Lys90, orange). In the wild-type PDS protein these amino acids are Glu143 and Lys90. Amino acid residue 64, which is affected in pds1-2 and the suppressor mutants could not be visualized, because no structure was predicted for the first 71 amino acid residues of the N-terminus (or the last 22 residues of the C-terminus) of C. reinhardtii PDS.
Discussion
Metabolic pathways are commonly regulated at their early steps in order to conserve resources and control accumulation of possibly harmful intermediates. Studies in other organisms indicate that the first two steps of carotenoid biosynthesis, catalyzed by PSY and PDS, are likely points of regulation for the whole pathway. PSY was reported to be regulated in tomato [47], pepper [48], mustard [49], [50], corn [51], [52], [53], sunflower [54] and algae [55], [56] by light and/or carotenoid content. PDS was found to be regulated or rate-limiting in algae [56], [57], [58], [59], potato [60], pepper [48], and tomato [46], [47].
Previous studies have identified many C. reinhardtii lts1 mutants affecting PSY, but no C. reinhardtii pds1 mutants had been isolated until this study. A phytoene-accumulating C. reinhardtii mutant was previously reported in a study by Stolbova [61], who observed that the light-sensitive lts4 mutant accumulated phytoene, but without any significant change in pigment composition [61]. The lts4 mutation was mapped to chromosome 11, indicating that it was not a pds1 mutant since the only copy of the C. reinhardtii PDS gene is on chromosome 12 [62]. The lts4 mutation might instead be linked to a plastid terminal oxidase (PTOX) or to plastoquinone biosynthesis, both of which are necessary for phytoene desaturation [19], [63], [64].
Similar to lts1 mutants, pds1 mutants are extremely light sensitive and pale in color. Mutants lacking PSY (lts1-210) or PDS activity (pds1-2 and pds1-3) accumulate no colored carotenoids (Figure 4) and die when exposed to even very low light intensities (Figure 3). Surprisingly, the leaky pds1-1 mutant was as light sensitive as lts1 and pds1 null mutants even though it was able to accumulate colored carotenoids. The light sensitive phenotype of pds1-1 indicates that the amount of colored carotenoids present in pds1-1 provides insufficient protection from even very low light.
The deleterious effect of phytoene accumulation in C. reinhardtii
The major difference between the white lts1 and pds1 mutants is the accumulation of phytoene in pds1. Phytoene-accumulating pds1-1 and pds1-3 mutants grew more slowly in complete darkness and had a lower plating efficiency than wild-type or lts1-210 cells (Figure 9). Together, these phenotypes could explain the difficulty in isolating pds1 mutants in previous screens [22].
Light sensitivity assays of pds1-2 suppressor mutants also suggest that phytoene accumulation has a deleterious effect on the fitness of C. reinhardtii cells. All three classes of suppressor mutants accumulated significant amounts of colored carotenoids, but the pds1-5 and pds1-6 mutants that accumulated low levels of phytoene were more light sensitive than pds1-4 mutants that did not (Figure 3). The pds1-4 mutants did not have more photoprotective carotenoids than either pds1-5 and pds1-6 mutants (Table 1), so their ability to survive in higher light intensities cannot be attributed to the presence of higher levels of total colored carotenoids or to a specific photoprotective carotenoid such as lutein or zeaxanthin.
A harmful effect of phytoene accumulation might explain the occurrence of the lts1-301 mutation in the pds1-1 enhancer strain, P3-84 (lts1-301 pds1-2). The mutation in PSY might have arisen secondarily, enhancing the growth and survival of cells with loss of PDS function by mitigating the accumulation of phytoene. The lts1-301 mutation decreases the flux of metabolites entering carotenoid biosynthesis and therefore reduces the amount of phytoene that accumulates in the cell (Figure 4).
In pds mutants of plants, the possible effects of phytoene accumulation are difficult to assess. Generally, studies have shown that impairment of PDS activity result in phytoene accumulation and pleiotropic defects in plants. Arabidopsis [18], [19], [65], maize [20], [66], rice [17], [66], [67], and tobacco plants [21] with impaired PDS activity accumulate phytoene, are lethal at the seedling stage, have stunted growth, exhibit albinism, and in the case of maize and rice, seeds experience vivipary. Light-exposed, norflurazon-treated plants also accumulate phytoene and produce albino seedlings or white leaf sectors [36], [47], [68], [69]. However, these morphological defects are not exclusive to plants with reduced PDS activity and phytoene accumulation. Mutants in other steps of the carotenoid pathway and in metabolic pathways that feed substrates directly into carotenoid biosynthesis also produce mutants with albinism, vivipary, and stunted growth. These mutants include psy mutants [21], zds mutants [17], [70], GGPP synthase mutants [71] and mutants of the plastidic methylerythritol 4-phosphate (MEP) pathway [72]. Carotenoid and abscisic acid deficiency is probably the primary cause of the adverse phenotypes in plant pds mutants.
Insight into the structure of the C. reinhardtii PDS protein
Amino acid residues affected in pds1-1, pds1-2, and intragenic suppressors of pds1-2 mutants must play an important role in PDS structure and/or function. Phyre prediction of the PDS protein structure placed amino acid residues affected in pds1-1 and pds1-6 mutants in close proximity with one another. In wild-type PDS, these amino acids are negatively charged Glu143 and positively charged Lys90, respectively. 3DLigandSite did not identify them as residues required for FAD/NAD(P) binding. Because of their predicted physical proximity to each other, it is possible that these residues form an ion pair that is important for proper folding of PDS. In pds1-1, Glu143(−) is converted to Lys143(+). This would result in two positively charged residues, Lys143(+) and Lys90(+), in direct contact, which would presumably introduce electrostatic repulsion and possibly promote protein destabilization. Electrostatic repulsion might be alleviated in pds1-6, which substitutes Lys90(+) with an uncharged methionine. The pds1-6 mutants carrying this amino acid change were light green in color, not dark green like wild-type cells, indicating that full PDS activity was not recovered. Changes to amino acid residues 90 and 143 result in less PDS activity, but further investigation is required to determine if this is due to decreases in PDS enzyme specific activity or PDS protein accumulation. The reason for lower PDS activity in the six mutant alleles of pds1 could be addressed by immunoblot analysis with a specific anti-PDS antibody.
P3-84, pds1-2, and all intragenic suppressor mutants have mutations that affect amino acid residue 64, but no structure was predicted for the first 71 amino acid residues of the PDS N-terminus. In wild-type cells, this residue is hydrophobic leucine, whereas in P3-84 and pds1-2 this residue was converted to cyclic proline, which in conjunction with the pds1-1 mutation severely impaired PDS activity. The replacement of proline with a different hydrophobic amino acid, phenylalanine, allowed pds1-5 suppressor mutants to partially recover PDS activity. The first 70–80 residues of the N-terminus of PDS is not present in the cyanobacterium Synechocystis indicating that this region is not critical for PDS activity (Figure 6). Instead, it may be involved in chloroplast targeting or perhaps insertion into the thylakoid membrane. Several studies using protein blotting and immunogold labeling have localized PDS proteins in the thylakoid membranes [20], [73].
In summary, we have isolated and characterized six alleles of pds1 in C. reinhardtii. Comparisons of lts1 and pds1 mutants suggest that phytoene accumulation is deleterious and that PDS may be an important control point in understanding and engineering carotenoid biosynthesis. Homology modeling and structural analysis of the pds1 mutations have also provided insight into the PDS protein structure and function.
Acknowledgments
We thank Marilyn Kobayashi for assistance with UV mutagenesis, Sarah McCarthy for design of the PDS marker, Brian Chin for the pBC1 vector, and Setsuko Wakao for critical reading of the manuscript.
Funding Statement
This work was supported by award number R01GM058799 from the National Institute of General Medical Sciences (to KKN). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Frank HA, Cogdell RJ (1996) Carotenoids in photosynthesis. Photochem Photobiol 63: 257–264. [DOI] [PubMed] [Google Scholar]
- 2. Demmig-Adams B, Adams WW (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43: 599–626. [Google Scholar]
- 3. Havaux M (1998) Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci 3: 147–151. [Google Scholar]
- 4.Yamamoto HY, Bassi R (2004) Carotenoids: Localization and Function. In: Ort DR, Yocum CF, Heichel IF, editors. Oxygenic Photosynthesis: The Light Reactions: Springer Netherlands. pp. 539–563. [Google Scholar]
- 5. Bassi R, Pineau B, Dainese P, Marquardt J (1993) Carotenoid-binding proteins of photosystem II. Eur J Biochem 212: 297–303. [DOI] [PubMed] [Google Scholar]
- 6. Croce R, Weiss S, Bassi R (1999) Carotenoid-binding sites of the major light-harvesting complex II of higher plants. J Biol Chem 274: 29613–29623. [DOI] [PubMed] [Google Scholar]
- 7. Herrin DL, Battey JF, Greer K, Schmidt GW (1992) Regulation of chlorophyll apoprotein expression and accumulation. J Biol Chem 267: 8260–8269. [PubMed] [Google Scholar]
- 8. Calucci L, Capocchi A, Galleschi L, Ghiringhelli S, Pinzino C, et al. (2004) Antioxidants, free radicals, storage proteins, puroindolines, and proteolytic activities in bread wheat (Triticum aestivum) seeds during accelerated aging. J Agric Food Chem 52: 4274–4281. [DOI] [PubMed] [Google Scholar]
- 9. Howitt CA, Pogson BJ (2006) Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ 29: 435–445. [DOI] [PubMed] [Google Scholar]
- 10. Rock CD, Zeevaart JAD (1991) The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosyntheis. Proc Natl Acad Sci USA 88: 7496–7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276: 1872–1874. [DOI] [PubMed] [Google Scholar]
- 12. Taylor HF, Smith TA (1967) Production of plant growth inhibitors from xanthophylls: a possible source of dormin. Nature 215: 1513–1514. [DOI] [PubMed] [Google Scholar]
- 13. Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43: 228–265. [DOI] [PubMed] [Google Scholar]
- 14. Farré G, Sanahuja G, Naqvi S, Bai C, Capell T, et al. (2010) Travel advice on the road to carotenoids in plants. Plant Sci 179: 28–48. [Google Scholar]
- 15. Giovannucci E (1999) Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst 91: 317–331. [DOI] [PubMed] [Google Scholar]
- 16. Cunningham FX, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 49: 557–583. [DOI] [PubMed] [Google Scholar]
- 17. Fang J, Chai C, Qian Q, Li C, Tang J, et al. (2008) Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo-oxidation in rice. Plant J 54: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qin G, Gu H, Ma L, Peng Y, Deng XW, et al. (2007) Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid, and gibberellin biosynthesis. Cell Res 17: 471–482. [DOI] [PubMed] [Google Scholar]
- 19. Norris SR, Barrette TR, DellaPenna D (1995) Genetic dissection of carotenoid synthesis in Arabidopsis defines plastoquinone as an essential component of phytoene desaturation. Plant Cell 7: 2139–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hable WE, Oishi KK, Schumaker KS (1998) Viviparous -5 encodes phytoene desaturase, an enzyme essential for abscisic acid (ABA) accumulation and seed development in maize. Mol Gen Genet 257: 167–176. [DOI] [PubMed] [Google Scholar]
- 21. Busch M, Seuter A, Hain R (2002) Functional analysis of the early steps of carotenoid biosynthesis in tobacco. Plant Physiol 128: 439–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCarthy SS, Kobayashi MC, Niyogi KK (2004) White mutants of Chlamydomonas reinhardtii are defective in phytoene synthase. Genetics 168: 1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vila M, Couso I, León R (2008) Carotenoid content in mutants of the chlorophyte Chlamydomonas reinhardtii with low expression levels of phytoene desaturase. Metab Eng 43: 1147–1152. [Google Scholar]
- 24. Dent RM, Haglund CM, Chin BL, Kobayashi MC, Niyogi KK (2005) Functional Genomics of Eukaryotic Photosynthesis Using Insertional Mutagenesis of Chlamydomonas reinhardtii. Plant Physiol 137: 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gross CH, Ranum LPW, Lefebvre PA (1988) Extensive restriction fragment length polymorphisms in a new isolate of Chlamydomonas reinhardtii. Curr Genet 13: 503–508. [DOI] [PubMed] [Google Scholar]
- 26.Harris EH (1989) The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Labortory Use: Academic Press, San Diego. [DOI] [PubMed] [Google Scholar]
- 27. Werner R, Mergenhagen D (1998) Mating type determination of Chlamydomonas reinhardtii by PCR. Plant Mol Biol Rep 16: 295–299. [Google Scholar]
- 28. Davies JP, Weeks DP, Grossman AR (1992) Expression of the arylsulfatase gene from the β2-tubulin promoter in Chlamydomonas reinhardtii . Nucleic Acids Res 20: 2959–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozen S, Skaletsky H (1999) Primer3 on the WWW for General Users and for Biologist Programmers. In: Misener S, Krawetz SA, editors. Bioinformatics Methods and Protocols: Humana Press. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 30. González-Ballester D, de Montaigu A, Galván A, Fernández E (2005) Restriction enzyme site-directed amplification PCR: A tool to identify regions flanking a marker DNA. Anal Biochem 340: 330–335. [DOI] [PubMed] [Google Scholar]
- 31. Emanuelsson O, Nielsen H, Heijne Gv (1999) ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Science 8: 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pruitt KD, Tatusova T, Maglott DR (2007) NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res 35: D61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thompson JD, Higgins DG, Gibson T (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignmet through sequence weighting position-specific gap penalties and weight matric choice. Nucleic Acids Res 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wass MN, Sternberg MJE (2009) Prediction of ligand binding sites using homologous structures and conservation at CASP8. Proteins: Structure, Function, and Bioinformatics 77: 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Breitenbach J, Zhu C, Sandmann G (2001) Bleaching herbicide norflurazon Inhibits phytoene desaturase by competition with the cofactors. J Agric Food Chem 49: 5270–5272. [DOI] [PubMed] [Google Scholar]
- 36. Simkin AJ, Breitenbach J, Kuntz M, Sandmann G (2000) In vitro and in situ inhibition of carotenoid biosynthesis in Capsicum annuum by bleaching herbicides. J Agric Food Chem 48: 4676–4680. [DOI] [PubMed] [Google Scholar]
- 37. Mayer M, Barlet D, Beyer P, Kleinig H (1989) The in vitro mode of action of bleaching herbicides on the desaturation of 15-cis-phytoene and cis-ζ-carotene in isolated daffodil chromoplasts. Pesticide Biochem Physiol 34: 111–117. [Google Scholar]
- 38. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50: 333–359. [DOI] [PubMed] [Google Scholar]
- 40. Niyogi KK, Björkman O, Grossman AR (1997) The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci USA 94: 14162–14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baroli I, Do AD, Yamane T, Niyogi KK (2003) Zeaxanthin accumulation in the absence of a functional xanthophyll cycle protects Chlamydomonas reinhardtii from photooxidative stress. Plant Cell 15: 992–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Holt NE, Zigmantas D, Valkunas L, Li X-P, Niyogi KK, et al. (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307: 433–436. [DOI] [PubMed] [Google Scholar]
- 43. Fraser PD, Linden H, Sandmann G (1993) Purification and reactivation of recombinant Synechococcus phytoene desaturase from an overexpressing strain of Escherichia coli . Biochem J 291: 687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Al-Babili S, von Lintig J, Haubruck H, Beyer P (1996) A novel, soluble form of phytoene desaturase from Narcissus pseudonarcissus chromoplasts is Hsp70-complexed and competent for flavinylation, membrane association and enzymatic activation. Plant J 9: 601–612. [DOI] [PubMed] [Google Scholar]
- 45. Armstrong GA, Alberti M, Leach F, Hearst JE (1989) Nucleotide sequence, organization, and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter capsulatus . Mol Gen Genet 216: 254–268. [DOI] [PubMed] [Google Scholar]
- 46. Pecker I, Chamovitz D, Linden H, Sandmann G, Hirschberg J (1992) A single polypeptide catalyzing the conversion of phytoene to zeta-carotene is transcriptionally regulated during tomato fruit ripening. Proc Natl Acad Sci USA 89: 4962–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Giuliano G, Bartley GE, Scolnik PA (1993) Regulation of carotenoid biosynthesis during tomato development. Plant Cell 5: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Simkin AJ, Zhu C, Kuntz M, Sandmann G (2003) Light-dark regulation of carotenoid biosynthesis in pepper (Capsicum annuum) leaves. J Plant Physiol 160: 439–443. [DOI] [PubMed] [Google Scholar]
- 49. Welsch R, Beyer P, Hugueney P, Kleinig H, von Lintig J (2000) Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta 211: 846–854. [DOI] [PubMed] [Google Scholar]
- 50. von Lintig J, Welsch R, Bonk M, Giuliano G, Batschauer A, et al. (1997) Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in Sinapis alba and Arabidopsis thaliana seedlings. Plant J 12: 625–634. [DOI] [PubMed] [Google Scholar]
- 51. Li F, Vallabhaneni R, Wurtzel ET (2008) PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress-induced root carotenogenesis. Plant Physiol 146: 1333–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li F, Vallabhaneni R, Yu J, Rocheford T, Wurtzel ET (2008) The maize phytoene synthase gene family: overlapping roles for carotenogenesis in endosperm, photomorphogenesis, and thermal stress tolerance. Plant Physiol 147: 1334–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li F, Tsfadia O, Wurtzel ET (2009) The phytoene synthase gene family in the grasses. Plant Signal Behav 4: 208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Campisi L, Fambrini M, Michelotti V, Salvini M, Giuntini D, et al. (2006) Phytoene accumulation in sunflower decreases the transcript levels of the phytoene synthase gene. Plant Growth Reg 48: 79–87. [Google Scholar]
- 55. Schäfer L, Sandmann M, Woitsch S, Sandmann G (2006) Coordinate up-regulation of carotenoid biosynthesis as a response to light stress in Synechococcus PCC7942. Plant Cell Environ 29: 1349–1356. [DOI] [PubMed] [Google Scholar]
- 56. Bohne F, Linden H (2002) Regulation of carotenoid biosynthesis genes in response to light in Chlamydomonas reinhardtii . Biochim Biophys Acta 1579: 26–34. [DOI] [PubMed] [Google Scholar]
- 57. Chamovitz D, Sandmann G, Hirschberg J (1993) Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacterial reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J Biol Chem 268: 17348–17353. [PubMed] [Google Scholar]
- 58. Grünewald K, Eckert M, Hirschberg J, Hagen C (2000) Phytoene desaturase is localized exclusively in the chloroplast and up-regulated at the mRNA level during accumulation of secondary carotenoids in Haematococcus pluvialis (Volvocales, Chlorophyceae). Plant Physiol 122: 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Steinbrenner J, Sandmann G (2006) Transformation of the green alga Haematococcus pluvialis with a phytoene desaturase for accelerated astaxanthin biosynthesis. Appl Env Microbiol 72: 7477–7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Diretto G, Al-Babili S, Tavazza R, Papacchioli V, Beyer P, et al. (2007) Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway. PLoS ONE 2: e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stolbova AV (1999) The lts4 photosensitive mutation maps to the short arm of Chlamydomonas reinhardtii Dangeard chromosome XI. Genetika 35: 111–113. [Google Scholar]
- 62. Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, et al. (2007) The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, et al. (1999) Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell 11: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Carol P, Kuntz M (2001) A plastid terminal oxidase comes to light: implications for carotenoid biosynthesis and chlororespiration. Trends Plant Sci 6: 31–36. [DOI] [PubMed] [Google Scholar]
- 65. Wang T, Iyer LM, Pancholy R, Shi X, Hall TC (2005) Assessment of penetrance and expressivity of RNAi-mediated silencing of the Arabidopsis phytoene desaturase gene. New Phytol 167: 751–760. [DOI] [PubMed] [Google Scholar]
- 66. Wurtzel ET, Luo R, Yatou O (2001) A simple approach to identify the first rice mutants blocked in carotenoid biosynthesis. J Exp Bot 52: 161–166. [PubMed] [Google Scholar]
- 67. Miki D, Shimamoto K (2004) Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol 45: 490–495. [DOI] [PubMed] [Google Scholar]
- 68. Wetzel CM, Rodermel SR (1998) Regulation of phytoene desaturase expression is independent of leaf pigment content in Arabidopsis thaliana . Plant Mol Biol 37: 1045–1053. [DOI] [PubMed] [Google Scholar]
- 69. Aluru MR, Zola J, Foudree A, Rodermel SR (2009) Chloroplast photooxidation-induced transcriptome reprogramming in Arabidopsis immutans white leaf sectors. Plant Physiol 150: 904–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Conti A, Pancaldi S, Fambrini M, Michelotti V, Bonora A, et al. (2004) A deficiency at the gene coding for ζ-carotene desaturase characterizes the sunflower non dormant-1 mutant. Plant Cell Physiol 45: 445–455. [DOI] [PubMed] [Google Scholar]
- 71. Maluf MP, Saab IN, Wurtzel ET, Sachs MM (1997) The viviparous12 maize mutant is deficient in abscisic acid, carotenoids, and chlorophyll synthesis. J Exp Bot 48: 1259–1268. [Google Scholar]
- 72. Page JE, Hause G, Raschke M, Gao W, Schmidt J, et al. (2004) Functional analysis of the final steps of the 1-deoxy-D-xylulose 5-phosphate (DXP) pathway to isoprenoids in plants using virus-induced gene silencing. Plant Physiol 134: 1401–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Linden H, Lucas M, de Felipe MR, Sandmann G (1993) Immunogold localization of phytoene desaturase in higher plant chloroplasts. Physiol Plant 88: 229–236. [Google Scholar]