Abstract
The last decade has seen great progress in the understanding of the molecular pharmacology, physiological function and therapeutic potential of the G protein-coupled receptors. Free Fatty acids (FFAs) have been demonstrated to act as ligands of several GPCRs including GPR40, GPR43, GPR84, GPR119 and GPR120. We have recently shown that GPR120 acts as a physiological receptor of ω3 fatty acids in macrophages and adipocytes, which mediate potent anti-inflammatory and insulin sensitizing effects. The important role GPR120 plays in the control of inflammation raises the possibility that targeting this receptor could have therapeutic potential in many inflammatory diseases including obesity and type 2 diabetes. In this review, we discuss lipid-sensing GPCRs and highlight potential outcomes of targeting such receptors in ameliorating disease.
Lipid sensing GPCRs as therapeutic targets
G-protein-coupled receptors (GPCRs) comprise a family of cell-surface receptors that respond to various extracellular stimuli such as light, odorants, neurotransmitters and hormones, and trigger a cascade of intracellular signaling. There are approximately 850 predicted human GPCRs 1 that have specific cell type or tissue-specific expression and are involved in various physiological and clinical processes 2. The importance of identification and characterization of GPCRs is underscored by the fact that ~ 30% of all prescription drugs target GPCRs 3. Because these drugs modulate approximately half of the well characterized GPCRs, it is likely that many more GPCRs remain to be explored as potential drug targets. GPCRs in humans and mice show striking orthology, and the fact that highly related receptors are evolutionarily conserved, suggests that these receptors are not functionally redundant2. Because human diseases involving GPCR mutations are extremely rare, occurring in less than 1 per 1000 people, they also provide a stable therapeutic target 4.
Free fatty acids (FFAs) can act as ligands of several GPCRs, including GPR119, GPR84, GPR120, GPR40 (FFAR1), GPR43 (FFAR2) and GPR41 (FFAR3) (Table 2). Fatty acids are categorized by the length of their aliphatic tails; short-chain fatty acids have less than 6 carbons, medium-chain fatty acids have 6-12 carbons and long-chain fatty acids have 12 or more carbons. Fatty acids can act as signaling molecules that modulate receptor signaling and gene expression 5. GPR120 and GPR40 are activated by medium- and long-chain FFAs, and GPR119 is activated by long-chain fatty acids. GPR84 is activated by medium-chain fatty acids, whereas GPR43 and GPR41 are activated by short-chain FFAs 6.
Table 2.
FFA sensing GPCRs and their cognate ligands
| GPCR | Ligands | Synthetic agonists |
|---|---|---|
| GPR40 | Long chain fatty acids (C12-C16) | TAK-875 (ACS Med. Chem. Lett., 2010, 1 (6), pp 290–294), GW9508 [51], MEDICA16 (relatively selective for GPR40), Troglitazone and Rosiglitazone89. |
| GPR41 | Short chain fatty acids (C3-C5) formate, acetate, propionate, butyrate and pentanonate89 | |
| GPR43 | Short chain fatty acids (C2-C3) formate, acetate, preferentially propionate, butyrate and pentanonate89 | ESN-280 and ESN-28270. |
| GPR120 | Long chain fatty acids (C14-1C8) Omega 3 fatty acids, EPA, DHA, palmitoleic acid, α-linolenic acid (ALA) | NCG compound, grifolic acid (partial synthetic agonist) and MEDICA1646. |
| GPR119 | Oleoylethanolamine and N-oleoyldopamine | Several, such as AR231453, WO07120702, JP04269468, JP04269469, WO0604349065 |
| GPR84 | Medium chain fatty acids, Capric acid (C10:0), undecanoic acid (C11:0), and lauric acid (C12:0)62 |
In obesity and type 2 diabetes, elevated levels of plasma FFAs are observed, resulting in lipid accumulation and insulin resistance in target tissues 7. FFAs exert divergent effects on insulin secretion from beta cells. Acute exposure to FFAs stimulates insulin secretion, whereas chronic exposure impairs insulin secretion 7. The dual and opposing effects of FFAs on insulin secretion raise the possibility that FFAs contribute to both hyper- and hypo-insulinemia during the development of type 2 diabetes. Thus, GPCRs that recognize fatty acids are of particular interest in the treatment of type 2 diabetes 8.
Chronic low grade metabolic inflammation, referred to as ‘metaflammation’ 9, has been established in the pathogenesis for obesity, insulin resistance and type 2 diabetes. The contribution of inflammation to insulin resistance has been extensively studied, and immunological changes occurring in adipose tissue, liver, brain, islets, the vasculature and circulating leukocytes, with concomitant changes in cytokines and chemokines are important components of the etiology of insulin resistance and type 2 diabetes 9, 10. Although recent studies have identified the presence of different cells of the adaptive immune system, such as T cells and mast cells in adipose tissue, macrophages are the most abundant leukocyte population and are generally considered as the effector cell contributing to inflammation-mediated insulin resistance 10, 11. In addition, macrophage-mediated inflammation has been associated with many other diseases such as rheumatoid arthritis, cancer, inflammatory bowel disease, cardiovascular disease 12,13, psoriasis 14, multiple sclerosis 15 and periodontitis 16. A very interesting recent study showed that macrophages might mediate metastasis in breast cancer 17. Macrophages can express a wide variety of GPCRs that modulate physiological processes such as inflammation and immunity. Furthermore, the expression of GPCRs differ in pro- and anti-inflammatory macrophages 18. The identification of endogenous and synthetic ligands to target inflammatory disease is therefore of great importance, and FFA-sensing GPCRs expressed in macrophages and insulin target tissues have emerged as therapeutic targets.
Medium- and long-chain fatty acid receptors
GPR120
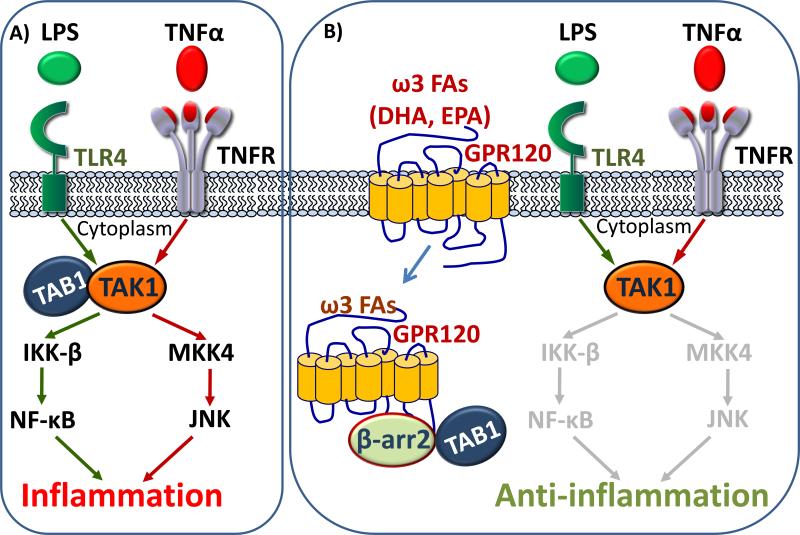
We have shown that GPR120 is a physiological receptor for ω3 fatty acids in macrophages and adipocytes, mediating potent anti-inflammatory and insulin sensitizing effects19. ω3 fatty acids are part of a series of essential polyunsaturated fatty acid family that cannot be synthesized de novo within the human body, but are vital for normal metabolism 20. GPR120 is a member of the rhodopsin-like family of GPCRs and is highly conserved across many species 21. Long-chain fatty acids, in particular palmitoleic acid (PA), and the ω3 fatty acids, α-linolenic acid (ALA), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are established activators of GPR120 19, 22. Some studies have shown that stimulation of GPR120 by these fatty acids increases glucagon like peptide-1 (GLP-1) secretion both in vitro and in vivo, and consequently increases circulating insulin levels. Stimulation of GPR120 by ALA and PA increases cholecystokinin secretion in vivo and in mouse intestinal enteroendocrine cells 23. Upon extensive tissue expression analyses, we found that GPR120 is expressed in macrophages and mature adipocytes and that the expression increases in pro-inflammatory macrophages. We demonstrated that DHA abolishes LPS-mediated phosphorylation and activation of IκB kinase (IKK) and c-Jun N-terminal kinase (JNK) in macrophages; upon GPR120 knockdown, DHA has no effect. Activation of GPR120 by DHA recruits β-arrestin 2 (β-arr 2) to the cytosolic putative binding sites on GPR120, and the GPR120- β-arr 2 complex internalizes. Pro-inflammatory cascades are broadly mediated by the tumor necrosis factor-alpha (TNF-α) and toll-like receptor 4 (TLR4) receptors. Both of these pathways converge at the step of TGF-beta activated kinase 1 (TAK1) interacting with TGF-beta activated kinase 1 binding protein 1 (TAB1) and mediate downstream inflammatory effects via activation of NF-κB and JNK. The internalized GPR120-β-arr 2 complex interacts with TAB1, thereby inhibiting the TAB1 interaction with TAK1 and in effect, inhibiting the downstream pro-inflammatory pathways.
GPR120 activation in primary adipose tissue and 3T3-L1 adipocytes led to an increase in glucose transport and translocation of GLUT4 to the plasma membrane, and this effect was abolished upon GPR120- and Gαq/11- KO. Knockdown of GLUT4 using siRNA abolished the stimulatory effects of GPR120 agonists, whereas knockdown of β-arrestin 1 and 2 was without effect. GPR120 activation in primary adipose tissue and 3T3-L1 adipocytes led to an increase in glucose transport and translocation of GLUT4 to the plasma membrane, and this effect was abolished upon GPR120- and Gαq/11- KO. Knockdown of GLUT4 using siRNA abolished the stimulatory effects of GPR120 agonists, whereas knockdown of β-arrestin 1 and 2 was without effect.
Interestingly, we demonstrated that the anti-inflammatory effects of ω3 fatty acids mediated by GPR120 are exclusively dependent on β-arr2, but independent of Gαq/11, regardless of the fact that GPR120 can be a Gαq/11-coupled receptor in other contexts 19. These mechanistic differences in macrophage and adipocytes is consistent with the concept of ‘functionally selective’, or ‘biased agonism’, which postulates that some agonists can cause unique agonist-specific active state conformations of receptors, which can result in a differential effector response in various cell types. In such scenarios, the efficacy of a drug is affected by the cell type, because cells vary in the relative stoichiometry of intracellular signaling components 24. The mechanism by which activated GPR120 inhibits inflammation in the macrophage is summarized in Figure 1. Because activated GPR120 inhibits TLR2, TLR4 and TNFR-mediated inflammation, it is possible that a wide range of diseases mediated by one, or any of these pathways, can be targeted via GPR120 agonists.
Figure 1. Mechanism of anti-inflammation upon GPR120 activation by omega 3 fatty acids in macrophage.
A) Activated TLR4 and TNFR by LPS and TNFα respectively, converge on cytoplasmic association of TAK1 with TAB1, mediating pro-inflammatory cascades by activating NF-B and JNK1. B) Activation of GPR120 by ω3 FAs internalizes GPR120 which binds to β-arrestin 2 and sequesters TAB1, inhibiting inflammation.
We performed in vivo studies with GPR120 knockout (KO) mice and wild-type (WT) littermates on a standard chow diet, a 60% high-fat diet (HFD) and a 60% high-fat diet containing ω3 FAs (ω3 diet). On standard chow, the GPR120 KO animals were more insulin resistant compared to WT controls. Both WT and GPR120 KO animals were equally insulin resistant upon HFD feeding. However, upon ω3 supplementation, the WT animals showed significantly improved glucose tolerance compared to both WT and KO animals on HFD alone (Table 1). ω3 supplementation was without effect in the GPR120 KO animals. Because the HFD typically contains negligible amount of ω3 fatty acids, and standard chow contains more ω3 compared to HFD, we hypothesized that the phenotype of these animals was directly correlated to the amount of ω3 fatty acids obtained from diet, and validated this using lipidomic analyses. We determined that circulating concentrations of ω3 FAs were much lower in mice on a HFD compared to chow diets, and supplementing the HFD with ω3 FAs led to an increase in plasma ω3 FA levels. To confirm the contribution of macrophages to the in vivo phenotype, we performed bone marrow transplants to obtain functional macrophage-specific GPR120 knockout mice, and found that ω3 supplementation was only effective in the WT, but not macrophage-specific GPR120 knockout mice 19.
Table 1.
Metabolic phenotype of free fatty acid receptor knock out mice.
| GPCR | HF diet phenotype | reference |
|---|---|---|
| GPR120KO | Normal chow: GPR120 KO animals were more insulin resistant compared with WT controls. High fat diet: Both WT and GPR120 KO animals were equally insulin resistant. However, upon ω3 supplementation, the WT animals had significantly improved glucose tolerance compared to both WT and KO animals on HFD. ω3 supplementation was without effect in the GPR120 KO animals. |
19 |
| GPR119KO | Normal chow: GPR119 KO mice show normal plasma glucose and lipids, but have lower body weight and lower post-prandial levels of active glucagon-like peptide 1 (GLP-1)67. Nutrient-stimulated GLP-1 release is attenuated in GPR119–/– mice. High fat diet: GPR119KO mice gain weight similarly to wild type littermates and show similar results during glucose and insulin tolerance tests. |
67 |
| GPR40KO | High fat diet: for 8 wks. Loss of GPR40 protects mice from obesity-induced hyperinsulinemia, hepatic steatosis, hypertriglyceridemia, increased hepatic glucose output, hyperglycemia, and glucose intolerance. | 52 |
| High fat diet: for 11 wks: GPR40KO mice develop fasting hyperglycemia, become obese and glucose intolerant and insulin resistant with similar liver steatosis as WT littermates. | 55 | |
| GPR84KO | Normal chow: Normal T- and B-cell proliferation in KO mice, increased Th2 production in KO mice, regulates early IL-4 gene expression in activated T cells. | 64 |
| GPR43KO | Normal chow: GPR43KO mice are phenotypically normal. High fat diet: GPR43KO show decreased body mass, increased lean mass, Improved glucose tolerance, increased energy expenditure, increased core body temperature, increased food intake, decreased triglycerides in liver, decreased plasma cholesterol, decreased lipid in brown adipose tissue and decreased crown-like structures in fat. |
73 |
| GPR41KO | GPR41KO mice have reduced PYY expression, increased intestinal transit rate and reduced extraction of energy from short chain FFAs that are produced by the microbial fermentation of indigestible dietary polysaccharides. These results suggest that GPR41 regulates host energy balance through mechanisms that are dependent upon the gut microbiota. | 85 |
Our study demonstrates that GPR120 plays an important role in the control of inflammation, and raises the possibility that many inflammatory disease states (including obesity, diabetes, cancer and cardiovascular disease) could potentially be attenuated by ω3 FA supplementation. The current rise in inflammatory diseases underscores the importance of exploring effective and safe therapeutic strategies. The anti-inflammatory potential of ω3 FAs has been well investigated and shown to have therapeutic potential in numerous diseases 12. There have been multiple clinical trials assessing the benefits of dietary supplementation with fish oils in the prevention and/or treatment of several inflammatory and autoimmune diseases including, rheumatoid arthritis13, periodontitis 16, bronchial asthma 25, cardiovascular disease 26, cancer 27 and neuroprotection 28. In general the effects observed in these clinical trials vary widely from subtle to moderate, and the most beneficial dose in humans is still under investigation 29. However, in some studies ω3 supplementation did not improve the inflammatory status, for example in patients treated with 3.4 g/d of EPA+DHA the serum triglycerides were significantly lowered but no improvement in inflammatory status was observed after 8 weeks of treatment 30. The concept of achieving effective functional dosing in vivo is discussed in a recent review in the context of inflammatory disease 31 . Schall et al., discuss that time, target and dose are the three crucial factors that act synergistically to determining effective therapy. Before ruling out the potentially therapeutic beneficial effects of ω3 supplementation in studies where no effect on inflammatory status is observed, further attention must be given to the dose used, the duration of treatment while also controlling for omega 6 and saturated fatty acid intake while administering omega 3 supplements.
Beneficial effects of ω3 diets in inflammatory disease might involve improvements in macrophage function related to reversal of defective macrophage phagocytosis of apoptotic cells (efferocytosis) 32. In obesity, type 2 diabetes and atherosclerosis, elevated levels of saturated FAs and/or decreased levels of ω3 FAs contribute to decreased efferoctyosis. There is an increased content of saturated FAs and decreased DHA and eicosapentaenoic acid (EPA) compared to controls, in membrane lipid composition of ob/ob and ob/ob;Ldlr(-/-) macrophages. Defective macrophage efferocytosis in ob/ob macrophages can be reversed by treatment with EPA or by feeding ob/ob mice a ω3-rich diet 32, demonstrating the beneficial effects of ω3 supplements in genetic models of obesity.
The importance, and need for ω3 supplementation is highlighted by the fact, that the dietary intake ratio of ω6:ω3 fatty acids in humans has changed dramatically from 1:1 in early humans, to 10:1 in the United States as of 2002. This is due to a combined effect of decreased intake of ω3, and increased intake of ω6 26. The American Heart Association (AHA) recommends ω3 FA intake as prevention agents against cardiovascular disease 26, and ω3 fatty acids are also prescribed for patients with hypertriglyceridemia 33. Interestingly, GPR120 is expressed in the taste buds, and GPR120 KO mice show a diminished preference for linoleic acid and oleic acid, and diminished taste nerve responses to several fatty acids 34. These results show that GPR120 mediates the taste of fatty acids34. Further studies are required to elucidate whether GPR120 expression in taste buds can cause preferential disposition towards a healthy and beneficial ω3 diet.
Type 2 diabetic subjects often require a combination of dietary, pharmacological and lifestyle interventions. One study in a diet-induced obese mouse model demonstrated that low-dose thiazolidinedione treatment in combination with dietary ω3 supplementation is a very viable and attractive option, because it can help offset the side effects of thiazolidinedione (TZD) treatment alone, such as edema, weight gain, bone loss and possible risk of heart failure 35. Clinically, ω3 FAs have been successfully used in combination with simvastatin in the treatment of various lipid disorders such as increased Low-density lipoprotein, triglycerides, non-high-density lipoprotein cholesterol and lipoprotein particle size. In subjects with persistent hypertriglyceridemia, prescription ω3-acid ethyl ester (P-OM3; Lovaza) 4g/day plus simvastatin 40mg/day and dietary counseling improved non-HDL-C and other lipid and lipoprotein parameters to a greater extent than simvastatin alone 36.
Clinical studies of ω3 fatty acids
Clinical studies have established that dietary intake of ω3 fatty acids should be around 4g/day for treating very high triglyceride levels (≥ 500 mg/dL) 37. Low-dose supplementation with EPADHA (400mg/day) or ALA (2 g/day) did not significantly reduce the rate of major cardiovascular events among patients who had a myocardial infarction, and who were receiving state-of-theart antihypertensive, antithrombotic and lipid-modifying therapy 38. The dose of ω3 fatty acids used in clinical trials varies widely from approximately 400 mg - 16.2 g per day, and the most beneficial dose in humans is still under investigation 27, 29, 30, 39. The most commonly reported adverse effect of ω3 fish oil supplements is a fishy aftertaste and eructations, but several others have been reported, including nausea and gastric bleeding 12, 26, 40, 41. Among the more serious effects, one study suggested that omega 3 supplements may result in a slightly higher risk of hemorrhagic stroke 42. Consumption of high levels of fish oil can also result in hypervitaminosis, a condition associated with high levels of Vitamins -A and –D, that are usually added to the fish oils 43.
Clinically, low doses of ω3 FAs are largely ineffective, even in the context of combination therapy. Should fish oils prove impractical as a therapeutic agent, the identification of the GPR120 receptor suggests that synthetic DHA/EPA mimetics could be developed that might provide the same and potentially greater anti-inflammatory effects. Synthetic agonists have certainly been more potent than endogenous ligands, as demonstrated by synthetic agonists of liver X receptors (LXRs) 44 and farnesoid X receptor (FXRs) 45. A recent report suggested potential synthetic agonists of GPR120 based on molecular modeling 46, that could be further explored to obtain clinical benefits.
GPR40 (FFAR1)
Free fatty acid receptor 1 (FFAR1) or GPR40, plays an important part in the mechanisms that link obesity and type 2 diabetes. GPR40 mediates both acute and chronic effects of FFAs on insulin secretion, and GPR40 signaling is linked to impaired glucose homeostasis. GPR40 is preferentially expressed in pancreatic beta cells, where it plays a role in the FFA enhancement of glucose-stimulated insulin secretion 47-50. GPR40 is also expressed in endocrine cells of the gastrointestinal tract, including cells that express incretin hormones such as GLP-1 and glucose-dependent insulinotropic polypeptide (GIP). In addition, GPR40 mediates FFA-stimulated incretin secretion 51.
Loss of GPR40 protects mice from obesity-induced hyperinsulinemia, hepatic steatosis, hypertriglyceridemia, increased hepatic glucose output, hyperglycemia, and glucose intolerance, and overexpression in mice leads to beta cell dysfunction, hyperinsulinemia and diabetes 52. Loss of GPR40 function through small interfering RNA (siRNA) 47, 53 or pharmacological inhibition 54 suppresses FFA-stimulated potentiation of glucose-stimulated insulin secretion. However, these findings are controversial, as more recently, Kebede et. al. have shown that GPR40 KO mice develop fasting hyperglycemia and they became as obese, glucose intolerant, and insulin resistant as their WT littermates given 8 weeks of HFD feeding. 55, Table 1. Furthermore, some studies report that deletion of GPR40 could not improve dysfunction of pancreatic islets and insulin resistance caused by a high fat diet in vivo 56, 57 and over-expression of GPR40 in islets improves glucose tolerance 58.
Due to its biological activity and tissue distribution, GPR40 is an attractive drug target for type 2 diabetes, but a debate still exists as to whether an agonist or antagonist may be therapeutic. A GPR40 agonist, GW9508, activates both GPR40 and GPR120 and stimulates glucose-stimulated insulin secretion in insulin-secreting MIN6 cells 54. TZDs including rosiglitazone and MEDICA16 also activate GPR40 59, 60. A recent study showed a small molecule antagonist of GPR40, DC260126, decreased serum insulin levels and improved insulin tolerance, but not glucose tolerance in Zucker rats 61.
GPR84
GPR84 functions as a receptor for medium-chain FFAs and is highly expressed in leukocytes 62. Activation of GPR84 in monocytes/macrophages amplifies LPS-stimulated IL-12 p40 production 62 and expression of GPR84 is markedly induced in macrophages (including glial cells) under inflammatory conditions 63. GPR84 KO mice have normal T cell and B cell proliferation and increased Th2 cytokine production 64, (Table 1). The leukocyte-specific expression of GPR84 suggests it may be involved in linking fatty acid metabolism to immunological regulation 62. Further studies of KO animals upon high fat diet feeding are required to better elucidate the mechanisms by which this receptor modulates glucose homeostasis.
GPR119
GPR119 is a Gαs-coupled receptor that is expressed in the pancreas, ileum and colon and binds long-chain fatty acids including oleoylethanolamide (OEA), lysophosphatidylcholine (LPC), lipid amides, and retinoic acid, with OEA being the most potent, and efficacious. Some controversy exists about retinoic acid and LPC being bona fide ligands 65. Activation of GPR119 by synthetic agonists results in an increase the release of insulin and GLP1, and significantly improves glucose tolerance 66. This highly desirable combination of stimulating both insulin and incretin release has attracted the interest of numerous pharmaceutical companies, and clinical trials are ongoing to determine the efficacy of GPR119 agonists in human patients.
GPR119 KO mice on normal chow show normal plasma glucose and lipids, but have lower body weight and lower post-prandial levels of active glucagon-like peptide 1 (GLP-1) 67, (Table 1). Furthermore, nutrient-stimulated GLP-1 release is attenuated in GPR119–/– mice, suggesting that GPR119 plays a role in physiological regulation of GLP-1 secretion. On a high fat diet, GPR119 KO mice gain weight similarly to WT littermates, and glucose and insulin tolerance tests did not reveal a genotypic difference, suggesting that GPR119 is not essential for the maintenance of glucose homeostasis.
Short-chain fatty acid receptors
GPR43 and GPR41 are both activated by short-chain FFAs such as formate, acetate, propionate, butyrate, and pentanoate 60, 68, 69. GPR43 and GPR41 differ in their specificity for ligands with different length carbon chains (Table 2). GPR41 is activated equally by propionate, butyrate, and pentanoate, whereas GPR43 prefers propionate to other short-chain FFAs 68, 69.
GPR43 (FFAR2)
Free Fatty Acid Receptor 2 or GPR43 is activated by short-chain fatty acids and is involved in the regulation of fatty-acid and glucose homeostasis in adipose tissue and the intestines; thus it has potential therapeutic relevance in the treatment of type 2 diabetes, insulin resistance and obesity 70. GPR43 expression is induced during adipocyte differentiation, and increased by high fat feeding in rodents, suggesting it may also affect adipocyte function 71-73. Adipocytes treated with natural ligands, acetate and propionate, exhibit a reduction in lipolytic activity that is not observed in GPR43 KO mice 71, 73. Furthermore, in humans, acetate reduces plasma FFA levels 74, 75. Therefore, GPR43 plays a role in regulating plasma lipid profiles and aspects of the metabolic syndrome, a term that refers to a cluster of related abnormalities including insulin resistance, dyslipidemia and hypertension. 71.
GPR43 is highly enriched in immune cells, particularly in the polymorphonuclear cells 60, 68, 69, and to a lesser extent in bone marrow, spleen and fetal liver 68. Expression is induced by leukocyte differentiation into monocytes and neutrophils, suggesting GPR43 has a role in the activation process of leukocytes 76. GPR43 is expressed in enteroendocrine cells expressing peptide Y (PYY) 71, 77 and in mucosa and in mucosal mast cells that contain 5-hydroxytryptamine (5-HT) 78. Short chain FFAs stimulate release of PYY 79 and 5-HT 80 from the ileum and colon.
GPR43 is also observed in rat and human colon wall and functions as a tumor suppressor by mediating SCFA-induced cell proliferation inhibition and apoptotic cell death in colon cancer 81.
GPR43 KO mice on high-fat diet results in decreased body mass and increased lean mass compared to WT littermates, (Table 1). The KO animals have improved glucose tolerance, increased energy expenditure, increased core body temperature, increased food intake, decreased triglyceride levels in the liver, decreased plasma cholesterol, decrease in lipid in brown adipose tissue, decreased crown like structures in the fat 82. Targeting GPR43 by dietary manipulation seems attractive at first glance, however studies with the GPR43 agonist, butyrate have raised concerns as a possible associated link with the development of colon cancer. The contribution of butyrate resulting from highly fermentable dietary fibers, to colon cancer is currently controversial with conflict between in vitro and in vivo studies, referred to as the “butyrate paradox” 83.
FFAR3 (GPR41)
Free fatty acid receptor 3 (FFAR3) or GPR41 is expressed abundantly in adipose tissue 68 and has been implicated in leptin production by stimulation of short chain FFAs in adipocytes 84. Leptin production is increased by the over-expression of exogenous GPR41 and abolished by the knock down of GPR41 expression with siRNA 84. Acute oral administration of propionate increases circulating leptin levels in mice, which suggests that the effects of propionate are mediated partially via GPR41 in vivo but other short chain FFARs activated by propionate, (i.e. FFAR2) may also be involved in this response 68, 84. GPR41 KO mice have reduced PYY expression, increased intestinal transit rate and reduced extraction of energy from short chain FFAs that are produced by the microbial fermentation of indigestible dietary polysaccharides (Table 1). These results suggest that GPR41 regulates host energy balance through mechanisms that are dependent upon the gut microbiota 85.
The fact that endogenous agonists of both GPR41 and GPR43 are by-products generated by fermentation of dietary fiber by the gut microbes suggests the importance and relevance of the biome in inflammatory diseases. The gut hosts ~1 × 1014 bacteria from 500–1,000 different species, that are ten times greater than the total number of cells in humans. Research in the last few years has revealed that gut microbes shape and influence the intestinal immune system. The current view that the impact of the intestinal microflora on host immunity stops at the gut 86 is being challenged with new studies identifying an intrinsic role of these microbes contributing to metabolic disease 87. Tables 1 and 2 summarize the expression, ligands and physiological roles of the GPCRs discussed in this review.
Concluding remarks
Fatty acids are not only essential nutrients, but play important roles as endocrine regulators of lipid and carbohydrate metabolism through the activation of their cognate GPCRs. FFARs play significant roles in nutritional regulation by sensing both - and short-chain fatty acids. The deorphanization of more GPCRs is likely to identify additional receptors of endogenous signaling molecules, including FFAs. Our recent study established GPR120 as a physiological receptor of ω3 fatty acids in macrophage and adipocytes that mediates potent anti-inflammatory and insulin sensitizing effects. Targeting fatty acid sensing GPCRs to ameliorate inflammatory disease is an exciting and growing area, and many clinical trials are currently underway to explore the beneficial anti-inflammatory therapeutic effects of ω3 fatty acids in a broad spectrum of disease. Hippocrates in his wisdom clearly nailed it when he said almost 2500 years ago, “Let food be your medicine and let medicine be your food. Only nature heals, provided it is given the opportunity”88. In the context of the global diabetes and inflammatory disease epidemic, we should have heeded his words sooner.
Acknowledgements
We thank Dr Min Lu and Dr William S Lagakos for critically reading this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ellis C, Smith A. Highlighting the pitfalls and possibilities of drug research. Nat Rev Drug Discov. 2004;3:238–278. doi: 10.1038/nrd1332. [DOI] [PubMed] [Google Scholar]
- 2.Vassilatis DK, et al. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci U S A. 2003;100:4903–4908. doi: 10.1073/pnas.0230374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kebede MA, et al. Lipid receptors and islet function: therapeutic implications? Diabetes Obes Metab 11 Suppl. 2009;4:10–20. doi: 10.1111/j.1463-1326.2009.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Insel PA, et al. Impact of GPCRs in clinical medicine: monogenic diseases, genetic variants and drug targets. Biochim Biophys Acta. 2007;1768:994–1005. doi: 10.1016/j.bbamem.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Miyauchi S, et al. New frontiers in gut nutrient sensor research: free fatty acid sensing in the gastrointestinal tract. J Pharmacol Sci. 2010;112:19–24. doi: 10.1254/jphs.09r09fm. [DOI] [PubMed] [Google Scholar]
- 7.Haber EP, et al. Pleiotropic effects of fatty acids on pancreatic beta-cells. J Cell Physiol. 2003;194:1–12. doi: 10.1002/jcp.10187. [DOI] [PubMed] [Google Scholar]
- 8.Gouell S, Hsaio K. Monitoring the activity of G protein-coupled receptors (GPCRs) modulated by lipid or free fatty acid agonists. http://www.promega.com/cnotes/cn023/cn023_13.pdf.
- 9.Gregor MF, Hotamisligil GS. Inflammatory Mechanisms in Obesity. Annu Rev Immunol. 2010 doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 10.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 11.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011 doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 12.Fetterman JW, Jr., Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am J Health Syst Pharm. 2009;66:1169–1179. doi: 10.2146/ajhp080411. [DOI] [PubMed] [Google Scholar]
- 13.James M, et al. Fish oil and rheumatoid arthritis: past, present and future. Proc Nutr Soc. 2010;69:316–323. doi: 10.1017/S0029665110001564. [DOI] [PubMed] [Google Scholar]
- 14.Ricketts JR, et al. Nutrition and psoriasis. Clin Dermatol. 2010;28:615–626. doi: 10.1016/j.clindermatol.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Shinto L, et al. Omega-3 fatty acid supplementation decreases matrix metalloproteinase-9 production in relapsing-remitting multiple sclerosis. Prostaglandins Leukot Essent Fatty Acids. 2009;80:131–136. doi: 10.1016/j.plefa.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naqvi AZ, et al. n-3 fatty acids and periodontitis in US adults. J Am Diet Assoc. 2010;110:1669–1675. doi: 10.1016/j.jada.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qian B, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lattin JE, et al. Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res. 2008;4:5. doi: 10.1186/1745-7580-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh DY, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol. 2008;19:242–247. doi: 10.1097/MOL.0b013e3282ffaf6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fredriksson R, et al. Seven evolutionarily conserved human rhodopsin G protein-coupled receptors lacking close relatives. FEBS Lett. 2003;554:381–388. doi: 10.1016/s0014-5793(03)01196-7. [DOI] [PubMed] [Google Scholar]
- 22.Hirasawa A, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka T, et al. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:523–527. doi: 10.1007/s00210-007-0200-8. [DOI] [PubMed] [Google Scholar]
- 24.Kenakin T. Differences between natural and recombinant G protein-coupled receptor systems with varying receptor/G protein stoichiometry. Trends Pharmacol Sci. 1997;18:456–464. doi: 10.1016/s0165-6147(97)01136-x. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz J. Role of polyunsaturated fatty acids in lung disease. Am J Clin Nutr. 2000;71:393S–396S. doi: 10.1093/ajcn/71.1.393s. [DOI] [PubMed] [Google Scholar]
- 26.Kris-Etherton PM, et al. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 27.Yee LD, et al. Omega-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am J Clin Nutr. 2010;91:1185–1194. doi: 10.3945/ajcn.2009.29036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, et al. Omega-3 polyunsaturated fatty acid supplementation confers long-term neuroprotection against neonatal hypoxic-ischemic brain injury through anti-inflammatory actions. Stroke. 2010;41:2341–2347. doi: 10.1161/STROKEAHA.110.586081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim W, et al. n-3 polyunsaturated fatty acids--physiological relevance of dose. Prostaglandins Leukot Essent Fatty Acids. 2010;82:155–158. doi: 10.1016/j.plefa.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skulas-Ray AC, et al. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr. 2011;93:243–252. doi: 10.3945/ajcn.110.003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schall TJ, Proudfoot AE. Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat Rev Immunol. 2011;11:355–363. doi: 10.1038/nri2972. [DOI] [PubMed] [Google Scholar]
- 32.Li S, et al. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ Res. 2009;105:1072–1082. doi: 10.1161/CIRCRESAHA.109.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoy SM, Keating GM. Omega-3 ethylester concentrate: a review of its use in secondary prevention post-myocardial infarction and the treatment of hypertriglyceridaemia. Drugs. 2009;69:1077–1105. doi: 10.2165/00003495-200969080-00008. [DOI] [PubMed] [Google Scholar]
- 34.Cartoni C, et al. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30:8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuda O, et al. n-3 fatty acids and rosiglitazone improve insulin sensitivity through additive stimulatory effects on muscle glycogen synthesis in mice fed a high-fat diet. Diabetologia. 2009;52:941–951. doi: 10.1007/s00125-009-1305-z. [DOI] [PubMed] [Google Scholar]
- 36.Davidson MH, et al. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29:1354–1367. doi: 10.1016/j.clinthera.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 37.Bays HE, et al. The effect of prescription omega-3 fatty acids on body weight after 8 to 16 weeks of treatment for very high triglyceride levels. Postgrad Med. 2009;121:145–150. doi: 10.3810/pgm.2009.09.2061. [DOI] [PubMed] [Google Scholar]
- 38.Kromhout D, et al. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 39.Sorgi PJ, et al. Effects of an open-label pilot study with high-dose EPA/DHA concentrates on plasma phospholipids and behavior in children with attention deficit hyperactivity disorder. Nutr J. 2007;6:16. doi: 10.1186/1475-2891-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonilla DL, et al. Incorporation of a dietary omega 3 fatty acid impairs murine macrophage responses to Mycobacterium tuberculosis. PLoS One. 2010;5:e10878. doi: 10.1371/journal.pone.0010878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iso H, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285:304–312. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- 42.Ellekjaer EF, et al. Lifestyle factors and risk of cerebral infarction. Stroke. 1992;23:829–834. doi: 10.1161/01.str.23.6.829. [DOI] [PubMed] [Google Scholar]
- 43.Bays HE. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol. 2007;99:35C–43C. doi: 10.1016/j.amjcard.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 44.Sparrow CP, et al. A potent synthetic LXR agonist is more effective than cholesterol loading at inducing ABCA1 mRNA and stimulating cholesterol efflux. J Biol Chem. 2002;277:10021–10027. doi: 10.1074/jbc.M108225200. [DOI] [PubMed] [Google Scholar]
- 45.Soisson SM, et al. Identification of a potent synthetic FXR agonist with an unexpected mode of binding and activation. Proc Natl Acad Sci U S A. 2008;105:5337–5342. doi: 10.1073/pnas.0710981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Q, et al. Structure-activity relationships of GPR120 agonists based on a docking simulation. Mol Pharmacol. 2010;78:804–810. doi: 10.1124/mol.110.066324. [DOI] [PubMed] [Google Scholar]
- 47.Itoh Y, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 48.Tomita T, et al. Expression of the gene for a membrane-bound fatty acid receptor in the pancreas and islet cell tumours in humans: evidence for GPR40 expression in pancreatic beta cells and implications for insulin secretion. Diabetologia. 2006;49:962–968. doi: 10.1007/s00125-006-0193-8. [DOI] [PubMed] [Google Scholar]
- 49.Tomita T, et al. GPR40 gene expression in human pancreas and insulinoma. Biochem Biophys Res Commun. 2005;338:1788–1790. doi: 10.1016/j.bbrc.2005.10.161. [DOI] [PubMed] [Google Scholar]
- 50.Itoh Y, Hinuma S. GPR40, a free fatty acid receptor on pancreatic beta cells, regulates insulin secretion. Hepatol Res. 2005;33:171–173. doi: 10.1016/j.hepres.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 51.Edfalk S, et al. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steneberg P, et al. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 2005;1:245–258. doi: 10.1016/j.cmet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Shapiro H, et al. Role of GPR40 in fatty acid action on the beta cell line INS-1E. Biochem Biophys Res Commun. 2005;335:97–104. doi: 10.1016/j.bbrc.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 54.Briscoe CP, et al. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol. 2006;148:619–628. doi: 10.1038/sj.bjp.0706770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kebede M, et al. The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes. 2008;57:2432–2437. doi: 10.2337/db08-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lan H, et al. Lack of FFAR1/GPR40 does not protect mice from high-fat diet-induced metabolic disease. Diabetes. 2008;57:2999–3006. doi: 10.2337/db08-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Latour MG, et al. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes. 2007;56:1087–1094. doi: 10.2337/db06-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagasumi K, et al. Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes. 2009;58:1067–1076. doi: 10.2337/db08-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hara T, et al. Flow cytometry-based binding assay for GPR40 (FFAR1; free fatty acid receptor 1). Mol Pharmacol. 2009;75:85–91. doi: 10.1124/mol.108.052225. [DOI] [PubMed] [Google Scholar]
- 60.Nilsson NE, et al. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047–1052. doi: 10.1016/s0006-291x(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, et al. DC260126, a small-molecule antagonist of GPR40, improves insulin tolerance but not glucose tolerance in obese Zucker rats. Biomed Pharmacother. 2010;64:647–651. doi: 10.1016/j.biopha.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 62.Wang J, et al. Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J Biol Chem. 2006;281:34457–34464. doi: 10.1074/jbc.M608019200. [DOI] [PubMed] [Google Scholar]
- 63.Bouchard C, et al. G protein-coupled receptor 84, a microglia-associated protein expressed in neuroinflammatory conditions. Glia. 2007;55:790–800. doi: 10.1002/glia.20506. [DOI] [PubMed] [Google Scholar]
- 64.Venkataraman C, Kuo F. The G-protein coupled receptor, GPR84 regulates IL-4 production by T lymphocytes in response to CD3 crosslinking. Immunol Lett. 2005;101:144–153. doi: 10.1016/j.imlet.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 65.Jones RM, et al. GPR119 agonists for the treatment of type 2 diabetes. Expert Opin Ther Pat. 2009;19:1339–1359. doi: 10.1517/13543770903153878. [DOI] [PubMed] [Google Scholar]
- 66.Chu ZL, et al. N-oleoyldopamine enhances glucose homeostasis through the activation of GPR119. Mol Endocrinol. 2010;24:161–170. doi: 10.1210/me.2009-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lan H, et al. GPR119 is required for physiological regulation of glucagon-like peptide-1 secretion but not for metabolic homeostasis. J Endocrinol. 2009;201:219–230. doi: 10.1677/JOE-08-0453. [DOI] [PubMed] [Google Scholar]
- 68.Brown AJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 69.Le Poul E, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 70.Tiwari A. GPR43: an emerging target for the potential treatment of type 2 diabetes, obesity and insulin resistance. Curr Opin Investig Drugs. 2010;11:385–393. [PubMed] [Google Scholar]
- 71.Hong YH, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–5099. doi: 10.1210/en.2005-0545. [DOI] [PubMed] [Google Scholar]
- 72.Tontonoz P, et al. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 73.Ge H, et al. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. 2008;149:4519–4526. doi: 10.1210/en.2008-0059. [DOI] [PubMed] [Google Scholar]
- 74.Laurent C, et al. Effect of acetate and propionate on fasting hepatic glucose production in humans. Eur J Clin Nutr. 1995;49:484–491. [PubMed] [Google Scholar]
- 75.Suokas A, et al. Acute cardiovascular and metabolic effects of acetate in men. Alcohol Clin Exp Res. 1988;12:52–58. doi: 10.1111/j.1530-0277.1988.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 76.Senga T, et al. LSSIG is a novel murine leukocyte-specific GPCR that is induced by the activation of STAT3. Blood. 2003;101:1185–1187. doi: 10.1182/blood-2002-06-1881. [DOI] [PubMed] [Google Scholar]
- 77.Tazoe H, et al. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol 59 Suppl. 2008;2:251–262. [PubMed] [Google Scholar]
- 78.Karaki S, et al. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–360. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- 79.Cherbut C, et al. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol. 1998;275:G1415–1422. doi: 10.1152/ajpgi.1998.275.6.G1415. [DOI] [PubMed] [Google Scholar]
- 80.Fukumoto S, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 81.Tang Y, et al. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int J Cancer. 2010 doi: 10.1002/ijc.25638. [DOI] [PubMed] [Google Scholar]
- 82.Bjursell M, et al. Improved glucose control and reduced body fat mass in free fatty acid receptor 2 (Ffar2) deficient mice fed a high fat diet. Am J Physiol Endocrinol Metab. 2010 doi: 10.1152/ajpendo.00229.2010. [DOI] [PubMed] [Google Scholar]
- 83.Lupton JR. Microbial degradation products influence colon cancer risk: the butyrate controversy. J Nutr. 2004;134:479–482. doi: 10.1093/jn/134.2.479. [DOI] [PubMed] [Google Scholar]
- 84.Xiong Y, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Samuel BS, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Philpott DJ, Girardin SE. Gut microbes extend reach to systemic innate immunity. Nat Med. 2010;16:160–161. doi: 10.1038/nm0210-160. [DOI] [PubMed] [Google Scholar]
- 87.Vijay-Kumar M, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Georgiou NA, et al. Pharma-nutrition interface: the gap is narrowing. Eur J Pharmacol. 2011;651:1–8. doi: 10.1016/j.ejphar.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 89.Ichimura A, et al. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat. 2009;89:82–88. doi: 10.1016/j.prostaglandins.2009.05.003. [DOI] [PubMed] [Google Scholar]