Abstract
Existing 67-channel ERPs, obtained during recognition and working memory paradigms with words or faces, were used to examine early visual processing in schizophrenia patients prone to auditory hallucinations (AH, n = 26) or not (NH, n = 49) and healthy controls (HC, n = 46). Current source density (CSD) transforms revealed distinct, strongly left- (words) or right-lateralized (faces; N170) inferior-temporal N1 sinks (150 ms) in each group. N1 was quantified by temporal PCA of peak-adjusted CSDs. For words and faces in both paradigms, N1 was substantially reduced in AH compared with NH and HC, who did not differ from each other. The difference in N1 between AH and NH was not due to overall symptom severity or performance accuracy, with both groups showing comparable memory deficits. Our findings extend prior reports of reduced auditory N1 in AH, suggesting a broader early perceptual integration deficit that is not limited to the auditory modality.
Keywords: schizophrenia, auditory hallucination, event-related potentials (ERPs), visual N1, current source density (CSD), principal components analysis (PCA)
Impairments of working memory (WM) and recognition memory (RM) are core features in patients with schizophrenia (Barch, 2005). It has been suggested that impairments in early sensory, bottom-up processing contribute to the WM deficit in schizophrenia (Javitt, 2009). Javitt, Shelley, Silipo, & Lieberman (2000) found evidence that schizophrenia is associated with impairments of auditory sensory (echoic) memory, which is evident even in simple tone discrimination tasks. A deficit in precision of encoding physical stimulus properties can in turn produce downstream deficits in cognitive tasks involving utilization of memory traces. Studies have successfully exploited the fine temporal resolution of event-related brain potentials (ERPs) to provide direct measures of the time course of ongoing brain activity during memory processing (e.g., Kayser et al., 2006), which may provide further insight into the stage of processing that underlies memory deficits in schizophrenia. ERP components occurring at different latencies reflect the spatiotemporal sequence of cortical processing, ranging from early sensory perception and attention (e.g., P1 or N1) to later cognitive evaluation (e.g., P3). For instance, Haenschel et al. (2007) found that a deficit in early visual processing, as indexed by a reduction of the P1 component peaking about 100 ms after stimulus onset, contributed to the impaired visual WM performance.
Reductions of early visual (Bruder et al., 1998; Butler et al., 2007; Doniger, Foxe, Murray, Higgins, & Javitt, 2002; Foxe, Doniger, & Javitt, 2001; Neuhaus et al., 2011; Yeap et al., 2006) and auditory (Foxe et al., 2011; Kayser et al., 2001; Rosburg, Boutros, & Ford, 2008; Salisbury, Collins, & McCarley, 2010) ERP components in schizophrenia are often considered as evidence of sensory, bottom-up processing deficits, which may represent an endophenotype for schizophrenia (Foxe et al., 2001, 2011). This has primarily been found in simple target detection tasks, which involve minimal cognitive demand. In fact, task or stimulus processing requirements are of critical importance and may account for conflicting findings concerning early visual processing deficits in schizophrenia (Butler et al., 2007; Foxe et al., 2001). For example, Doniger et al. (2002) observed reduced P1 during the recognition of fragmented images while N1 was preserved, whereas Bruder et al. (1998) found reduced N1 but no P1 reduction during a visuospatial discrimination task. Less is known as to whether electrophysiological indices of early sensory processing are reduced in schizophrenia during more demanding memory tasks. In three ERP studies (Kayser et al., 1999, 2010; Kayser, Tenke, Gil, & Bruder, 2009), in which schizophrenia patients showed poorer RM for words compared to healthy controls, N1 amplitude tended to be smaller in patients, but this group difference did not attain statistical significance.
There is evidence that N1 amplitude may vary with the severity of patients’ symptoms (Rosburg et al., 2008). In particular, reduction of the N1 component to tones has been reported for psychotic patients during periods of auditory hallucinations (Hubl, Koenig, Strik, Garcia, & Dierks, 2007), which could stem from central acoustic interference associated with internal speech (Ford et al., 2001), that is, auditory hallucinations may interfere with processing of tones or spoken words. This raises the possibility that patients having auditory hallucinations may be particularly likely to show reduced N1 amplitude and that an impairment in early auditory processing could contribute to a heightened WM deficit. In a study measuring auditory WM in a word serial position test, which involves storage and rehearsal of phonological and sequential information, patients who reported experiencing auditory hallucinations showed significantly poorer performance than patients who did not report having hallucinations (Bruder et al., 2011). In a multi-site neuroimaging study, patients with auditory hallucinations, when compared to nonhallucinating patients, showed decreased activity in left superior temporal and inferior parietal regions to probe stimuli in a Sternberg WM task (Wible et al., 2009). Although stimuli were visually presented numbers, the auditory verbal language system would be expected to be involved in item rehearsal.
Several lines of evidence suggest that auditory verbal hallucinations involve the left temporal lobe and reduced language lateralization (see Badcock & Hugdahl, 2012, for a review). This hypothesis has received support from fMRI studies showing reduced activation in left superior-temporal and inferior-parietal regions in hallucinators (Ford et al., 2009; Hugdahl et al., 2007; Wible et al., 2009) and dichotic listening studies, which have consistently found hallucinations to be associated with reduced left hemisphere advantage for words or syllables (Bruder et al., 1995; Green, Hugdahl, & Mitchell, 1994; Hugdahl et al., 2008). Moreover, several electrophysiologic studies using rather heterogenous approaches to study auditory hallucinations have also implicated the left superior temporal cortex (see van Lutterveld, Sommer, & Ford, 2011, for a review). For example, using LORETA as an inverse source localization technique (e.g. Pascual-Marqui, Michel, & Lehmann, 1994), Hubl et al. (2007) reported that the N1 reduction to tones during periods of auditory hallucinations was primarily associated with reduced current density sources in the left temporal lobe. It would therefore be of interest to more directly study lateralized neurophysiologic processes in schizophrenia by employing stimuli known to differentially engage the left or right hemisphere in early perceptual processing (cf. Kayser et al., 2001).
This report takes advantage of ERP data recorded from a large sample of schizophrenia patients and healthy controls during working memory and recognition memory tasks using words and faces (Kayser et al., 2010) to examine whether patients' propensity to experience auditory hallucinations affects early visual processing (cf. van Lutterveld et al., 2011). Given the above findings, we predicted that patients who report having auditory hallucinations would show reduced N1 when compared to patients without hallucinations and healthy controls. If patients who are prone to having auditory hallucinations show reduced N1 amplitude during these visual RM and WM paradigms, this would indicate that it is not merely due to direct acoustical interference (i.e., hearing voices), and not limited to the auditory modality, thereby suggesting a broader, and modality-unspecific, cognitive deficit. Alternatively, we may observe reduced early visual ERP components in schizophrenia independent of the tendency to experience auditory hallucinations, which would provide further evidence that disrupted cognition in schizophrenia is caused by a fundamental deficit in bottom-up processes (e.g., Javitt, 2009).
Because ERPs depend on the EEG reference location and the operational definition of an ERP component measure, current source density (CSD) and temporal principal components analysis (PCA) were combined to obtain a reference-free estimate of visual N1 (Kayser & Tenke, 2006a), which is strongly left-lateralized for words (Kayser, Tenke, Gates, & Bruder, 2007; Kayser et al., 2009, 2010). Given the involvement of left temporoparietal cortex in word processing (Price, 2000, 2010) and auditory hallucinations (e.g., Badcock & Hugdahl, 2012; van Lutterveld et al., 2011; Wible et al., 2009), we also predicted that the N1 reduction in hallucinators would be most evident over left temporoparietal sites for words.
Methods
Participants
We analyzed the ERP data of 58 inpatients and 17 outpatients (42 male) at New York State Psychiatric Institute and 46 healthy adults (20 male) who had participated in at least one of two visual memory paradigms. Most participants performed both RM (session 1) and WM (session 2) tasks at two different recording sessions within a week, typically 1–2 days apart. Left-handed individuals (Oldfield, 1971) and those with a history of neurological illness or substance abuse, as well as participants performing at or below chance level, were excluded. Most importantly, only patients for which clinical ratings of auditory hallucinations were available for the time of ERP testing were included. The auditory hallucination item of the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984) was used to form symptom subgroups by separating patients who reported experiencing auditory hallucinations (AH) in the past week (SAPS auditory hallucination item ≥ 1) from those who did not (NH).
Table 1 details the demographic and clinical variables, DSM-IV (28) criteria, and medication status for the two patient subgroups and healthy controls (HC) in the two memory paradigms. Most patients (14 AH, 30 NH) and 42 healthy adults provided data for both memory paradigms. Although the groups did not differ in gender, age (range 18–56 years) or degree of right-handedness (laterality quotient range 20–100), patients had significantly less education than controls. However, the available verbal IQ data (WAIS) suggested that the patients’ verbal skills were well within normal range and did not differ between AH and NH subgroups. Although hallucinators and nonhallucinators did not differ in age of onset (range 9–37 years) or illness duration (range 0–47 years), the total score of the brief psychiatric rating scale (BPRS), which was derived from the Positive and Negative Syndrome Scale (PANSS; Kay, Opler, & Fishbein, 1992), indicated that hallucinators were more disturbed than nonhallucinators. This difference is also reflected in higher positive and negative PANSS subscales in hallucinators than nonhallucinators. Notwithstanding, all electrophysiologic findings reported were not affected by subgroup differences in total BPRS measures of severity (see below).
Table 1.
Means (SD) for demographic and clinical variables, DSM-IV criteria, medication status, and sample sizes for each paradigm.
| Paradigm | Recognition Memory (RM) | Working Memory (WM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group a | AH (n = 19) | NH (n = 32) | HC (n = 44) | AH (n = 21) | NH (n = 47) | HC (n = 44) | ||||||
| Gender (male/female) | 10/9 | 18/14 | 18/26 | 12/9 | 26/21 | 19/25 | ||||||
| Age (years) | 29.8 | (10.2) | 28.6 | (8.0) | 26.2 | (6.2) | 30.6 | (11.0) | 28.8 | (8.0) | 26.4 | (6.6) |
| Education (years) b | 14.2 | (2.6) | 14.3 | (2.4) | 16.3 | (2.1) | 13.6 | (2.8) | 13.8 | (2.6) | 16.3 | (2.1) |
| Handedness (LQ) c | 72.8 | (27.7) | 75.0 | (20.2) | 75.3 | (20.0) | 74.5 | (28.3) | 78.2 | (19.4) | 74.7 | (19.9) |
| Verbal IQ (WAIS) d | 102.7 | (16.0) | 102.9 | (14.9) | 102.9 | (18.0) | 98.0 | (15.4) | ||||
| Onset age (years) | 22.4 | (6.6) | 20.8 | (5.1) | 22.8 | (6.1) | 21.1 | (6.0) | ||||
| Illness duration (years) | 7.5 | (7.6) | 7.8 | (9.0) | 7.9 | (9.0) | 7.7 | (8.4) | ||||
| Total BPRS e | 45.7 | (15.3) | 31.1 | (10.3) | 47.7 | (15.5) | 32.7 | (9.8) | ||||
| PANSS general e | 37.6 | (13.4) | 27.1 | (8.7) | 39.4 | (13.7) | 28.4 | (7.8) | ||||
| PANSS positive e | 19.4 | (7.3) | 12.4 | (5.3) | 20.4 | (7.2) | 12.8 | (5.4) | ||||
| PANSS negative e | 17.1 | (6.6) | 12.5 | (5.4) | 18.2 | (7.4) | 14.1 | (5.7) | ||||
| Schizophrenia, paranoid | 6 | 12 | 8 | 14 | ||||||||
| Schizophrenia, undifferentiated | 4 | 10 | 6 | 16 | ||||||||
| Schizophrenia, catatonic | 1 | |||||||||||
| Schizophrenia, residual | 1 | |||||||||||
| Schizoaffective, depressed | 3 | 5 | 2 | 7 | ||||||||
| Schizoaffective, bipolar | 5 | 2 | 4 | 4 | ||||||||
| Psychosis NOS | 1 | 3 | 1 | 4 | ||||||||
| Unmedicated (> 14 days) | 9 | 11 | 11 | 18 | ||||||||
| Medicated (atypical antipsychotics) | 10 | 21 | 10 | 29 | ||||||||
Note.
AH: Auditory hallucinators; NH: Nonhallucinators; HC: Healthy controls. Group overlap across paradigms: AH, n = 14; NH, n = 30; HC, n = 42.
RM, WM: AH, NH differ significantly from HC (all p ≤ .002).
LQ: Laterality quotient (Oldfield, 1971) can vary between −100.0 (completely left-handed) and +100.0 (completely right-handed).
RM: AH, n = 11; NH, n = 22; WM: AH, n = 14; NH, n = 29.
RM: AH differ significantly from NH (total BPRS, PANSS general, PANSS positive, all p ≤ .001; PANSS negative, p = .01); WM: AH (n = 18) differ significantly from NH (n = 45; total BPRS, PANSS general, PANSS positive, all p ≤ .001; PANSS negative, p = .03).
All patients met DSM-IV (American Psychiatric Association, 1994) criteria for schizophrenia (paranoid, n = 26; undifferentiated, n = 22; catatonic, n = 1; residual, n = 1), schizoaffective disorder (depressed type, n = 11; bipolar type, n = 9), schizophrenoform (n = 1) or psychosis not otherwise specified (n = 5; the clinical profile of these patients consisted of core psychotic symptoms, including thought disorder, disorganized thinking, paranoid thoughts, and hallucinations, placing them clearly in the range of schizophrenia spectrum disorders). Diagnoses were determined by consensus among senior diagnosticians, clinicians and clinical research interviewers (master level or above) on the basis of a semistructured interview (Nurnberger et al., 1994), which included items from commonly-used instruments (SAPS, SANS, SCID-P; Andreasen, 1983, 1984; Spitzer, Williams, Gibbon, & First, 1990). Thirty-two patients (43%) did not receive antipsychotic medications for at least 14 days before testing. The remaining 43 patients were treated with risperidone (n = 11), aripriprazole (n = 10), ziprasidone (n = 9), olanzapine (n = 6), quetiapine (n = 4), or clozapine (n = 3), with chlorpromazine equivalents ranging from 67 to 1067 mg/day (Woods, 2003). The number of medicated and unmedicated patients did not differ between AH and NH subgroups for either paradigm (χ2[1] ≤ 1.18, p ≥ .28).
Patients were compared to 46 healthy volunteers who were recruited from the New York metropolitan area for a payment of US$15/hr, and who were without current or past psychopathology based on a standard screening interview (SCID-NP; First, Spitzer, Gibbon, & Williams, 1996).
All participants had normal or corrected-to-normal vision. The ethnic composition (63 White, 19 Black, 9 Asian, 1 Native-American, 17 more than one race, 12 unknown) was representative for the New York region with similar ratios for each subgroup. The experimental protocol was approved by the institutional review board and was undertaken with the understanding and written consent of each participant.
Stimuli and Procedure
The RM paradigm consisted of continuous recognition memory tasks with common words or unknown faces, as detailed elsewhere (Kayser et al., 2010). Briefly, words were 320 English nouns and faces were 320 black-and-white photographs (160 male) taken from a college yearbook. A series of word (or face) stimuli were foveally presented for 500 ms (constant 2.5-s stimulus onset asynchrony) with 114 stimuli per block and 4 blocks per task (912 trials total). Participants indicated for each item whether it was new (never presented in the series) or old (presented previously) by pressing one of two buttons on a response pad. Participants were instructed to respond to every stimulus as quickly and accurately as possible. There was no overlap between blocks for item repetitions.
Building on our previous implementation of a word serial position task (Kayser et al., 2006), the WM paradigm consisted of two serial position tests (27 trials per block, 4 blocks per task, 216 total trials) with the same word or face stimuli used in the RM paradigm. In each trial, a series of three words (or faces) was presented (500 ms, 1.5-s stimulus onset asynchrony) and, after a 3-s delay, one of the items was repeated (probe). Participants indicated the position of the probe item during the encoding series by pressing one of three buttons after a fixed 1.5-s delay.
Data Acquisition, Recording, and Artifact Procedures
Employing an extended 10–20 system (e.g., Jurcak et al., 2007; Pivik et al., 1993) 67-channel montage, continuous EEG was acquired with a 24-bit Biosemi ActiveTwo system (256 samples/s; DC-128 Hz) using a Lycra stretch electrode cap and external electrodes for nose tip (used as off-line reference) and for monitoring bipolar eye activity (left and right outer canthi; above and below right eye). Volume-conducted blink artifacts were effectively removed from the continuous EEG by means of spatial PCA (NeuroScan, 2003; for further details, see Kayser et al., 2010).
For the RM paradigm, 2-s epochs (300 ms pre-stimulus baseline) were extracted, low-pass filtered at 20 Hz (−24 dB/octave), corrected for horizontal eye movements, and rigorously screened for residual artifacts (Kayser & Tenke, 2006b) or electrode bridging (Tenke & Kayser, 2001). Artifactual or bridged surface potentials were replaced by spherical spline interpolation (Perrin, Pernier, Bertrand, & Echallier, 1989) using the available artifact-free data if possible (i.e., when less than 25% of all EEG channels contained an artifact); otherwise, a trial was rejected. Stimulus-locked ERPs were computed from correct, artifact-free trials separately for each task (i.e., words or faces) but across all other conditions (i.e., old, new, and filler items; cf. Kayser et al., 2010) to maximize the signal-to-noise ratio for the targeted N1 component. The means for the number of trials used to compute these ERP averages (M ± SD, HC vs. NH vs. AH) were 349 ±41 vs. 333 ±41 vs. 317 ±39 for faces, and 372 ±73 vs. 350 ±36 vs. 333 ±42 for words (range 207 to 447).
For the WM paradigm, 9-s epochs (1.5 s pre-stimulus baseline of the first encoding item) were processed in an analogous fashion, although both correct and incorrect trials were included. Shorter 1.5-s subepochs (−102 to 1,398 ms around stimulus onset of each trial item) were extracted from these long, artifact-free ERPs using only the matching stimulus pairs (i.e., probe and corresponding encoding item), which were separately combined for each task. The means for the number of trials underlying these ERPs (M ± SD, HC vs. NH vs. AH) were 149 ±28 vs. 116 ±33 vs. 101 ±39 for faces, and 157 ±32 vs. 156 ±28 vs. 148 ±31 for words (range 62 to 202).
Finally, all ERPs were low-pass filtered (12.5 Hz, −24 dB/octave) and baseline-corrected (100 ms before stimulus onset).
Current Source Density (CSD), N1 Latency Jitter, and Principal Components Analysis (PCA)
The averaged, nose-referenced ERP waveforms were transformed into reference-free current source density (CSD) estimates (µV/cm2 units) using a spherical spline surface Laplacian (Perrin et al., 1989; for details, see Kayser, 2009; Kayser & Tenke, 2006a; Kayser et al., 2007). CSD estimates are based on the second spatial derivative of the recorded surface potentials and represent the magnitude of the radial (transcranial) current flow entering (sinks) and leaving (sources) the scalp, thereby mapping the direction, location and intensity of current generators that underlie an ERP topography (Mitzdorf, 1985; Nicholson, 1973; Nunez & Srinivasan, 2006). Apart from representing a common bridge between scalp-recorded EEG and intracranial local field potential recordings (see Tenke & Kayser, 2012, for a review), a distinct advantage of surface Laplacian methodology is that any EEG reference will yield the same, that is, unique CSD waveforms with reduced volume-conducted contributions, yielding more focused components with unambiguous polarity and sharper topographies compared to ERPs (Kayser et al., 2009).
The visual N1 sink1 to words is strongly left-lateralized in healthy adults, with a maximum over the inferior lateral-parietal scalp locations (e.g., sites P7 and P9 of the extended 10–20 system over visual object processing regions along the occipitotemporal ventral stream; Ungerleider & Haxby, 1994; cf. Kayser et al., 2006, 2007, 2009, 2010), and this distinct left-larger-than-right N1 sink asymmetry is also seen for nose-referenced ERPs (Kayser et al., 1999; Kayser, Fong, Tenke, & Bruder, 2003). In contrast, the visual N1 sink to faces is less asymmetric with a right-parietal maximum (Kayser et al., 2010) and corresponds to the face-sensitive N170 component (e.g., Rossion & Jacques, 2008). These lateralized N1 sinks are likely associated with the structural processing of words (visual word form area; e.g., Cohen et al., 2000) or faces (fusiform face area; e.g., Halgren, Raij, Marinkovic, Jousmaki, & Hari, 2000) involving inferior occipital-temporal regions (e.g., fusiform gyrus).
ERP component latency jitter, either between conditions or between subjects, is a well-known problem for quantifying ERP peaks (e.g., Möcks, 1986). For each paradigm (RM, WM) and task (face, word), individual CSD waveform topographies were temporally adjusted for N1 sink peak latency to create optimized subepochs (−50 to 400 ms) focused on N1 sink activity. After determining the most negative deflection for pooled CSD waveforms using sites P7 and P9 (word; 91–223 ms interval) or P7, P8, P9 and P10 (face; 79–223 ms), all 67 CSD waveforms were jointly aligned with the mean N1 sink peak latency for each task (face, word) and paradigm (RM, WM). For example, if an individual N1 sink peaked at 141 ms in the RM paradigm using words (i.e., using the average of the corresponding CSD waveforms at P7 and P9), all CSDs for this participant, task and paradigm were shifted 2 samples (approximately +8 ms) so that the N1 sink maximum would match the respective mean N1 sink peak latency for words during the RM paradigm (approximately 149 ms). Local N1 sink maxima were unambiguously determined within these intervals in almost all instances (100% for words, 97.2% for faces); if not, the CSD waveforms were not shifted. The N1 sink latency shift did not significantly differ between AH, NH, and HC groups in either task or paradigm (overall range −51 to +31 ms; RM, F[2,92] = 1.48, p > .23; WM, F[2,109] < 1.0, n.s.).
Optimized CSD waveforms were submitted to unrestricted, temporal principal components analysis (PCA) derived from the covariance matrix, followed by Varimax rotation of the covariance loadings (Kayser & Tenke, 2003, 2006a), to determine common sources of variance related to N1 sink activity and to quantify its amplitude. The input matrices consisted of 116 variables (samples between −50 and 400 ms) and 12,730 or 15,008 observations stemming from 95 or 112 participants, 2 tasks (face, word) and 67 electrode sites. This approach provides a concise, efficient simplification of the temporal pattern and spatial distribution of neuronal generators, yielding reference-independent CSD factors with unambiguous component polarity and topography (Kayser & Tenke, 2006a; Tenke & Kayser, 2012).
Statistical Analysis
For each paradigm, N1 sink factor scores were submitted to repeated measures ANOVAs with group (AH, NH, HC) as a between-subjects factor, and task (word, face), hemisphere (left, right), and site (P7/8, P9/10) as within-subjects factors. The selection of two homologous inferior-parietal scalp locations was based on prior research (Kayser et al., 2007). Inasmuch as these sites jointly represented N1 sink maxima, main or interaction effects involving site were not considered.
For analyses of overall performance accuracy, repeated measures ANOVAs with group and task were computed, using the d’-like sensitivity measure dL (Snodgrass & Corwin, 1988) for the RM paradigm (e.g., Kayser et al., 1999, 2010), and percentages of correct responses (chance levels linearly scaled to 50%) for the WM paradigm (Kayser et al., 2006).
As there were no specific hypotheses regarding sex differences, and given the resulting small cell sizes for AH, gender was excluded from all analyses (all reported effects were preserved in secondary analyses including gender). Group differences in accuracy or N1 sink amplitude were examined using planned contrasts (BMDP-4V; Dixon, 1992). All reported correlations coefficients are linear (Pearson). A conventional significance level (p < .05) was applied for all effects. Effect sizes (partial eta squared [η2p]) are reported for group main effects.
Results
Behavioral Data
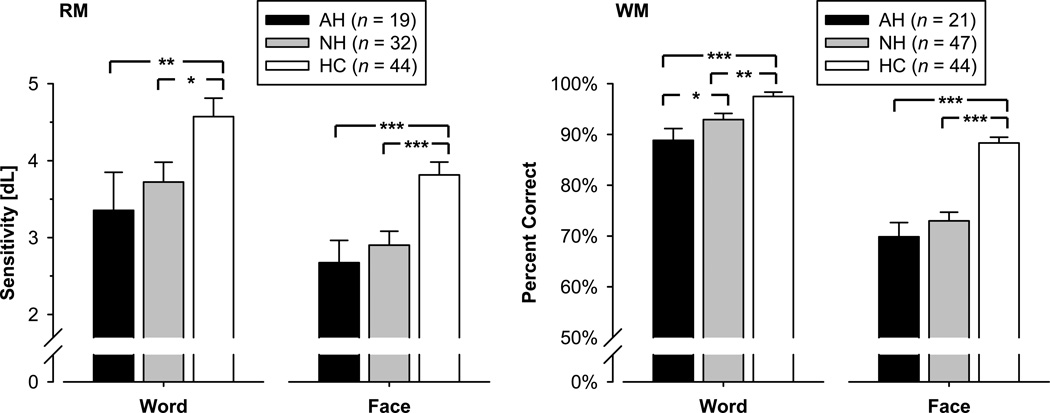
In both paradigms, remembering faces was more difficult than remembering words (task main effect: RM, F[1,92] = 33.2, p < .0001; WM, F[1,109] = 494.7, p < .0001; Figure 1). Patients had poorer performance than controls (group main effect: RM, F[2,92] = 7.56, p = .0009, η2p = .141; WM, F[2,109] = 23.5, p < .0001, η2p = .301; between group contrasts: RM, HC vs. AH, F[1,92] = 11.5, p = .001, HC vs. NH, F[1,92] = 8.93, p = .004; WM, HC vs. AH, F[1,109] = 35.5, p < .0001, HC vs. NH, F[1,109] = 30.5, p < .0001), but hallucinators did not differ significantly from nonhallucinators (RM, F[1,92] < 1.0, n.s.; WM, F[1,109] = 2.57, p > .11). The difference in accuracy between the patient groups and controls was smaller for words than faces during the WM paradigm, which resulted in a significant group × task interaction, F(2,109) = 27.0, p < .0001. A ceiling performance of controls may have contributed to this effect, and no such interaction was observed during the RM paradigm. However, between group contrasts computed in repeated measures ANOVAs separately for each task and paradigm revealed poorer performance of hallucinators compared to nonhallucinators for words during the WM paradigm (see significance brackets in Figure 1).
Figure 1.
Mean (±SEM) accuracy during recognition memory (RM) and working memory (WM) tasks using words or faces. Overall, remembering faces was more difficult than remembering words. Patients performed more poorly than healthy controls (HC), but auditory hallucinators (AH) did not differ significantly from nonhallucinators (NH), except for the word WM task. Significant contrasts between groups for each paradigm and each task are indicated as follows: * p ≤ .05; ** p ≤ .01; *** p ≤ .001.
Electrophysiologic Data
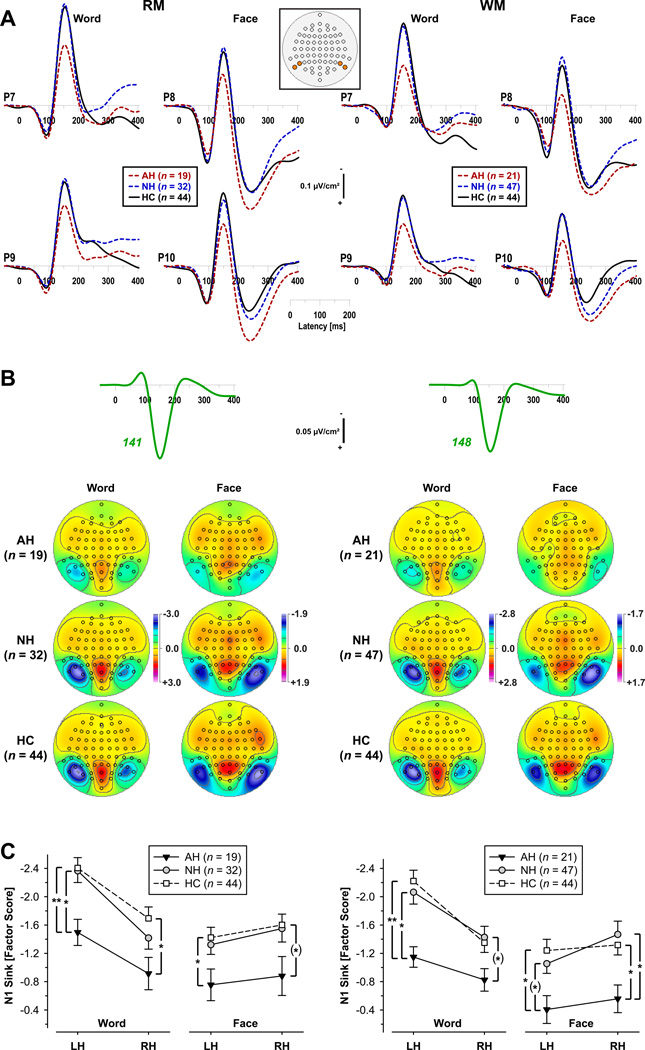
Figure 2A compares the grand mean CSD waveforms of patients and controls at selected left-hemispheric inferior lateral-parietal sites where N1 sink was prominent for words (P7, P9), and at homologous right-hemispheric sites for faces (P8, P10) for both RM and WM paradigms. These corrected (i.e., peak-aligned) grand mean CSDs were highly comparable to those not adjusted for N1 sink peak latency (supplementary Figure S1 depicts these CSDs along with their nose-referenced ERP counterparts). A distinct component structure, consisting of P1 source (approximate peak latency 85 ms), N1 sink (145 ms), and P2 source (240 ms for faces), was observed for the three groups in both paradigms. In all conditions, however, auditory hallucinators revealed marked reductions of N1 sink compared to nonhallucinators and controls who had almost identical N1 sink amplitudes. In contrast, no obvious group differences were seen for P1 source amplitude. Parallel ANOVAs computed for P1 source factors (RM vs. WM, 98 vs. 105 ms peak latency, 6.1% vs. 5.2% explained variance) yielded no significant effects involving group (all F ≤1.88, all p ≥ .16).
Figure 2.
N1 sink amplitude (adjusted for peak latency) for auditory hallucinators (AH), nonhallucinators (NH), and healthy controls (HC) during the recognition memory (RM) and working memory (WM) paradigms using words or faces. A. Grand mean current source density (CSD) [µV/cm2] waveforms at selected inferior lateral-parietal sites for words (left hemisphere sites P7, P9) and faces (right hemisphere sites P8, P10). These locations are highlighted in inset showing the 67-channel EEG montage (top view, nose up). B. Time courses of Varimax-rotated covariance loadings for PCA factors corresponding to N1 sink (RM: 141 ms peak latency; WM: 148 ms) and associated factor score topographies for each task and group. C. Mean (±SEM) N1 sink amplitude at inferior lateral-parietal sites (P7/8, P9/10) comparing AH, NH, and HC participants. Significant contrasts between groups for each paradigm, task, and hemisphere (LH: left; RH: right) are indicated as follows: (*) p ≤ .10; * p ≤ .05; ** p ≤ .01.
As intended, the unrestricted PCA for these CSD waveforms yielded optimized factors representing N1 sink activity, with highly comparable time courses of factor loadings (RM vs. WM, peak latencies 141 vs. 148 ms, explained variance 16.4% vs. 14.8%; Figure 2B). The corresponding factor score topographies of N1 sink were, for each paradigm, restricted to posterior, inferior-lateral regions, revealing a robust left-larger-than-right hemisphere asymmetry for words, and the opposite, less robust asymmetry for faces (Figure 2B). The overall N1 sink amplitude was substantially reduced for auditory hallucinators, although the task-dependent asymmetry was nevertheless preserved.
These observations were confirmed by the statistical analyses. Significant group main effects (RM, F[2,92] = 3.13, p < .05, η2p = .064; WM, F[2,109] = 3.64, p = .03, η2p = .063) stemmed from smaller N1 sink amplitudes for auditory hallucinators compared to nonhallucinators and healthy controls, who did not differ from each other (between group contrasts: RM, AH vs. NH, F[1,92] = 3.90, p = .05, AH vs. HC, F[1,92] = 6.05, p = .02, NH vs. HC, F[1,92] < 1.0, n.s.; WM, AH vs. NH, F[1,109] = 5.96, p = .02, AH vs. HC, F[1,109] = 6.30, p = .01, NH vs. HC, F[1,109] < 1.0, n.s.). There were also highly significant main effects of task (RM, F[1,92] = 36.4, p < .0001; WM, F[1,109] = 49.3, p < .0001) and hemisphere (RM, F[1,92] = 11.7, p < .001; WM, F[1,109] = 8.75, p = .004), as well as highly significant task × hemisphere interactions (RM, F[1,92] = 54.0, p < .0001; WM, F[1,109] = 59.6, p < .0001), but none of these effects interacted with group (all F ≤ 1.88, all p > .15). Larger N1 sink amplitudes to words than faces and over left than right hemisphere sites were seen for all groups in both paradigms (Figure 2C). These task and hemisphere effects were moderated by a robust left-greater-than-right asymmetry for words, and a smaller right-greater-than-left asymmetry for faces. Notwithstanding the absence of significant interactions of group with task or hemisphere, contrasts between groups separately computed for each paradigm, task, and hemisphere indicated that differences in N1 sink amplitude between auditory hallucinators and nonhallucinators, as well as auditory hallucinators and healthy controls, were most robust over the left hemisphere for words.
To address the potential confound of differences in severity between hallucinators and nonhallucinators (cf. Table 1, BPRS and PANSS), analogous repeated measures ANCOVAs were computed for patients with PANSS ratings, using the total BPRS score as a between-subjects covariate. All critical effects reported above were maintained in these analyses, which is not surprising given that the total BPRS score and all PANSS subscales did not substantially co-vary with N1 sink amplitude (correlation range: RM, n = 51, −.08 ≤ r ≤ .21, all p ≥ .14; WM, n = 63, −.05 ≤ r ≤ .20, all p ≥ .20). Furthermore, additional repeated measures ANOVAs with patients grouped into those with a low (< 35) or high (≥ 35) total BPRS score (median split) did not reveal any group differences in N1 sink amplitude (RM, F[1,49] < 1.0, n.s.; WM, F[1,61] < 1.0, n.s.).
PCA-based estimates of N1 sink amplitude were highly comparable across paradigms, as revealed by correlations computed for participants who provided data from both paradigms (AH, n = 14, .81 ≤ r ≤ .98; NH, n = 30, .87 ≤ r ≤ .92; HC, n = 42, .89 ≤ r ≤ .92; all p < .001). To increase statistical power, data stemming from the two paradigms were therefore combined into a single N1 sink estimate by using either the available N1 sink estimate or the pooled mean across paradigms, exploiting that PCA factor scores represent a standardized measure (the overall mean across participants, tasks, and sites is zero with a standard deviation of 1). As expected, the repeated measures ANOVA for the combined sample (AH, n = 26; NH, n = 49; HC, n = 46) confirmed the findings for each paradigm, however, at a more robust significance level (group main effect, F[2,118] = 4.26, p = .02, η2p = .067; between group contrasts: AH vs. NH, F[1,118] = 6.55, p = .01, AH vs. HC, F[1,118] = 7.44, p = .007, NH vs. HC, F[1,118] < 1.0, n.s.).
Finally, we addressed the question whether these findings were specific to auditory hallucinations by also taking into account if patients were experiencing other (i.e., visual, olfactory, somatic/tactile, persecutory) but not auditory hallucinations. Because of the small number of patients exhibiting other forms of hallucinations (as indicated by SAPS) prevented forming specific subgroups of comparable size, patients not reporting auditory hallucinatons (NH) were regrouped into those reporting any other hallucinations (OH) and those without reporting any hallucinations (WH). The repeated measures ANOVA for the pooled N1 sink estimates of the combined sample (AH, n = 26; WH, n = 23; OH, n = 26; HC, n = 46) indicated that auditory hallucinators had significantly reduced N1 sinks (AH: −.85 ±1.22) compared to all other groups (WH: −1.52 ±1.16; OH: −1.66 ±1.95; HC: −1.66 ±1.40), who did not differ from each other (between group contrasts: AH vs. WH, F[1,117] = 3.78, p = .05, AH vs. OH, F[1,117] = 5.90, p = .02, AH vs. HC, F[1,117] = 7.38, p = .008; all other, F[1,117] < 1.0, n.s.).
Discussion
Using visually-presented common words or unknown faces, schizophrenia patients showed significantly poorer RM and WM performance than healthy controls, which agrees with evidence of impaired memory processing in schizophrenia (e.g., Barch, 2005; Pelletier, Achim, Montoya, Lal, & Lepage, 2005). Despite poorer performance, the associated sequence of cortical activation was highly comparable in patients and controls, with matching N1 sink peak latencies (approximately 150 ms) and topographies (inferior lateral-parietal maximum), replicating previous findings (Kayser et al., 1999, 2006, 2009). Patients and controls revealed distinct, stimulus-dependent N1 sink asymmetries (left-larger-than-right for words and vice versa for faces). However, auditory hallucinators had markedly reduced N1 sink for words and faces compared to healthy controls and nonhallucinators, independent of memory paradigm. Avoiding known limitations of reference-dependent ERP measures (Kayser et al., 2006; Kayser & Tenke, 2010; Tenke & Kayser, 2012), the neuronal generators underlying N1 amplitude were concisely summarized by inferior-parietal sinks and quantified by temporal PCA after aligning the individual CSD waveforms for N1 sink peak latency.
This neurophysiological deficit in early visual processing can not be explained by differences in overall symptom severity, which was unrelated to N1 sink amplitude; instead, higher ratings of severity in auditory hallucinators are likely, at least in part, related to the subgroup classification itself. Furthermore, typical concerns of random effects attributable to small, heterogenous samples are untenable given the large sample and the lack of subgroup differences in demographic (gender, age, education, handedness, verbal IQ) or clinical variables (illness duration and onset).
Auditory hallucinations, considered a hallmark symptom of schizophrenia (Wible et al., 2009), involve activation in auditory association cortex as well as non-sensory cortical and subcortical regions and interfere with cognitive processing (reviewed by Allen, Laroi, McGuire, & Aleman, 2008). Although the mechanism responsible for cognitive interference remains unknown, the reduction of N1 sink amplitude to words and faces during WM and RM paradigms has implications for understanding its basis.
First, while N1 reduction to tones has been found in psychotic patients during periods of auditory hallucinations compared to symptom-free intervals (Hubl et al., 2007), it is not limited to the auditory modality but occurs for visual processing as well, and appears to be largely independent of specific stimulus features (i.e., words or faces). Patients who are prone to auditory hallucinations also showed decreased activation in temporoparietal regions compared to nonhallucinators during a visually-presented verbal WM task (Wible et al., 2009), and showed poorer performance than nonhallucinators in both an auditory and visual word serial position test (Bruder et al., 2011). Thus, any interference caused by experiencing auditory hallucinations is not likely due to direct acoustic interference (i.e., hearing voices). However, visually-presented stimuli may be rehearsed using the verbal language system and interference from the “inner voice” could occur in hallucinators (Ford et al., 2001; Wible et al., 2009). Interestingly, restricted perceptual processing may itself cause hallucinatory symptoms, as seen after depriving healthy individuals from visual experience for several days (Merabet et al., 2004).
Second, N1 sink reduction in hallucinators was present despite an adequate, well-above chance memory performance across tasks and paradigms, which was comparable to nonhallucinators. Notwithstanding a slightly better performance of nonhallucinators than hallucinators for words during the WM paradigm (i.e., the easiest condition with ceiling effects), the comparable overall performance level across tasks and paradigms indicates that the reduced N1 sink was not merely due to poorer attention or distractability in hallucinators.
Third, the intact P1 source in hallucinators for unambiguous, high-contrast and meaningful visual stimuli (i.e., words and faces) used in the recognition memory and working memory paradigms, which differ in these regards from more ambiguous stimuli used for visuospatial discrimination tasks (e.g., Butler et al., 2007; Doniger et al., 2002; Foxe et al., 2001), suggests that the N1 sink reduction did not result from a deficit in earlier visual processing. Rather, it involves interference during a subsequent stage of cognitive processing beginning about 150 ms after word or face onset, likely coinciding with stimulus categorization (Rossion & Jacques, 2008) as opposed to low-level perceptual analysis (Butler et al., 2007). This implies that auditory hallucinations, or the impaired functional processes that cause auditory hallucinations, interfere with the integration of incoming perceptual information as reflected by N1, that is, perceptual driven or bottom-up processing involving the ventral occipito-temporal stream is impaired by top-down modulation or cognitive interference (cf. Twomey, Kawabata Duncan, Price, & Devlin, 2011).
Fourth, the fact that visual N1 to words and faces was fully preserved in those schizophrenia patients without auditory hallucinations does not support the bottom-up processing deficit hypothesis in schizophrenia (e.g., Javitt, 2009), at least not without additional qualification. Instead, this finding may indicate that reductions of early perceptual ERP components for any given sample of schizophrenia patients may be primarily due to top-down cognitive interference in patients prone to auditory hallucinations. In this sense, the present findings may offer a cogent explanation for some of the inconsistency in demonstrating a neurophysiological deficit of early visual processing in schizophrenia using electrophysiologic methods. It should also be noted that our experimental procedure and stimuli mainly probed the ventral (parvocellular) visual processing stream or the ‘what' pathway (Ungerleider & Haxby, 1994), whereas Javitt (2009) reviewed evidence that reflects visual processing deficits in schizophrenia involving the dorsal (magnocellular) processing stream or the ‘where' pathway. In any case, this hypothesis regarding early processing deficits in schizophrenia and its relation to specific symptom features is in need of further study.
Lastly, our findings have implications for the hypothesis that auditory hallucinations involve abnormalities of language-related, left-hemisphere function (e.g., Badcock & Hugdahl, 2012). Although N1 sink reduction was greatest for words and over left inferior-parietal sites where N1 sink was largest, the lack of a significant interaction involving group and hemisphere and/or task does not provide statistical support for this hypothesis. Rather, a bilateral inferior-parietal N1 sink reduction in hallucinators was present to both words and faces.
Neurophysiologic abnormalities are more common or more pronounced in the auditory than visual modality (Egan et al., 1994; Ford et al., 1994; Kayser et al., 2009; Pfefferbaum, Ford, White, & Roth, 1989), leading Ford wondering whether this may be related “to whatever dysfunction leads to more frequent auditory than visual hallucinations” (Ford, 1999, p. 668). In fact, our sample did not reveal similar N1 sink reductions for patients experiencing other (e.g., visual, olfactory, somatic/tactile) but not auditory hallucinations, which bolsters the notion that impairments in auditory function play a central role in the etiology of schizophrenia. On the other hand, electrophysiological recording conventions, such as using a linked-ears or -mastoids reference that drastically reduces ERP amplitudes at inferior-parietal sites (cf. Kayser et al., 2003, 2009), may have contributed to the notion of modality-specific ERP reductions in schizophrenia. If the underlying generator of the studied ERP phenomena is in close proximity to the EEG reference, as is the case for secondary visual cortex along the ventral processing stream, the surface potentials as measured at nearby sites (e.g., P7/8, P9/10) will be close to zero. In contrast, neuronal generators within auditory cortex will only marginally be affected by this particular EEG reference choice. For these reasons, it may be easier to demonstrate deficits in auditory rather than visual processing in schizophrenia, even if both modalities were equally affected, because existing group or condition differences will be substantially masked for the visual modality by this EEG reference choice. However, this methodological limitation was averted here by employing a reference-free CSD transform.
Among the shortcomings of previous ERP studies examining the neurophysiology of auditory hallucinations are small sample sizes (e.g., n = 7 in Hubl et al., 2007). Although using ERP data of 75 right-handed patients allowed studying a reasonably-sized subgroup of 26 patients experiencing auditory hallucinations, the sample was heterogenous with regard to diagnostic criteria and medication status. However, it included 32 unmedicated participants, hallucinators and nonhallucinators did not differ in medication status, and previous research has not linked early neurophysiological deficits to antipsychotic medication (cf. Butler et al., 2007; Rosburg et al., 2008).
These findings support the value of a symptom-based approach for dealing with the heterogeneity of schizophrenia, and suggest that visual N1 reductions may not represent a biological marker for schizophrenia per se. Although reduced visual N1 may be dependent on presence of auditory hallucinations during testing (i.e., a marker of current clinical state), it may also represent a stable trait in patients prone to auditory hallucinations. Additional research is required to address this state vs. trait issue, and whether the observed N1 sink reductions for auditory hallucinators are specific to the visual modality, or are also present, and perhaps even more robust, for auditory stimuli (Foxe et al., 2011; Salisbury et al., 2010).
Supplementary Material
Acknowledgments
This research was supported by grant MH066597 from the National Institute of Mental Health (NIMH). We thank Charles L. Brown, III, for developing, providing and improving excellent waveform plotting software.
Footnotes
Preliminary analyses of these data were presented at the 50th Annual Meeting of the Society for Psychophysiological Research (SPR), Portland, OR, September 29 – October 3, 2010.
The chosen nomenclature for ERP (“N1”) and CSD (N1 sink”) components is intended to reflect the crucial difference between reference-dependent surface potentials (ERPs) and their reference-independent surface Laplacian transformations (CSDs), as well as their association. Because the polarity of surface potentials depends on the choice of the EEG reference, which is arbitrary, a negative ERP deflection observed with any given reference may easily revert to a different polarity and/or topography (i.e., become a ‘different ERP component’) after re-referencing the data (i.e., applying a different EEG reference), thereby rendering ERPs non-unique (e.g., Kayser & Tenke, 2010). In contrast, CSDs are unique (i.e., reference-free) and therefore have an unambiguous polarity and topography. Here, the “N1 sink” term associates the radial current flow entering the scalp (i.e., the “sink”) with the label “N1” typically used for a corresponding ERP component (i.e., the negativity peaking at about 150 ms at site P7 when using a nose reference). Thus, the term “N1” is generally used to refer to reference-dependent ERP data, whereas the term “N1 sink” is used to refer to its reference-free CSD counterpart.
References
- Allen P, Laroi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neuroscience and Biobehavioral Reviews. 2008;32:175–191. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, editor. Diagnostic and Statistical Manual of the Mental Disorders. Fourth Edition (DSM-IV) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: The University of Iowa; 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- Badcock JC, Hugdahl K. Cognitive mechanisms of auditory verbal hallucinations in psychotic and non-psychotic groups. Neuroscience and Biobehavioral Reviews. 2012;36:431–438. doi: 10.1016/j.neubiorev.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annual Review of Clinical Psychology. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Alschuler DM, Kroppmann CJ, Fekri S, Gil RB, Jarskog LF, Wexler BE. Heterogeneity of auditory verbal working memory in schizophrenia. Journal of Abnormal Psychology. 2011;120:88–97. doi: 10.1037/a0021661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder G, Kayser J, Tenke C, Rabinowicz E, Friedman M, Amador X, Gorman J. The time course of visuospatial processing deficits in schizophrenia: an event-related brain potential study. Journal of Abnormal Psychology. 1998;107:399–411. doi: 10.1037//0021-843x.107.3.399. [DOI] [PubMed] [Google Scholar]
- Bruder G, Rabinowicz E, Towey J, Brown A, Kaufmann CA, Amador X, Gorman JM. Smaller right ear (left hemisphere) advantage for dichotic fused words in patients with schizophrenia. American Journal of Psychiatry. 1995;152:932–935. doi: 10.1176/ajp.152.6.932. [DOI] [PubMed] [Google Scholar]
- Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G, Javitt DC. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130:417–430. doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Dixon WJ, editor. BMDP Statistical Software Manual: To Accompany the 7.0 Software Release. Berkeley, CA: University of California Press; 1992. [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Archives of General Psychiatry. 2002;59:1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- Egan MF, Duncan CC, Suddath RL, Kirch DG, Mirsky AF, Wyatt RJ. Event-related potential abnormalities correlate with structural brain alterations and clinical features in patients with chronic schizophrenia. Schizophrenia Research. 1994;11:259–271. doi: 10.1016/0920-9964(94)90020-5. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis-I Disorders - Non-patient Edition (SCID-NP) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT. Cortical responsiveness during inner speech in schizophrenia: an event-related potential study. American Journal of Psychiatry. 2001;158:1914–1916. doi: 10.1176/appi.ajp.158.11.1914. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Jorgensen KW, Turner JA, Brown GG, Notestine R, Mathalon DH. Tuning in to the voices: a multisite FMRI study of auditory hallucinations. Schizophrenia Bulletin. 2009;35:58–66. doi: 10.1093/schbul/sbn140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, White PM, Csernansky JG, Faustman WO, Roth WT, Pfefferbaum A. ERPs in schizophrenia: effects of antipsychotic medication. Biological Psychiatry. 1994;36:153–170. doi: 10.1016/0006-3223(94)91221-1. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. Neuroreport. 2001;12:3815–3820. doi: 10.1097/00001756-200112040-00043. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Yeap S, Snyder AC, Kelly SP, Thakore JH, Molholm S. The N1 auditory evoked potential component as an endophenotype for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives, first-episode, and chronic schizophrenia patients. European Archives of Psychiatry & Clinical Neuroscience. 2011;261:331–339. doi: 10.1007/s00406-010-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Hugdahl K, Mitchell S. Dichotic listening during auditory hallucinations in patients with schizophrenia. American Journal of Psychiatry. 1994;151:357–362. doi: 10.1176/ajp.151.3.357. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Haertling F, Rotarska-Jagiela A, Maurer K, Singer W, Linden DE. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia: a study with event-related potentials and functional magnetic resonance imaging. Archives of General Psychiatry. 2007;64:1229–1240. doi: 10.1001/archpsyc.64.11.1229. [DOI] [PubMed] [Google Scholar]
- Halgren E, Raij T, Marinkovic K, Jousmaki V, Hari R. Cognitive response profile of the human fusiform face area as determined by MEG. Cerebral Cortex. 2000;10:69–81. doi: 10.1093/cercor/10.1.69. [DOI] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik WK, Garcia LM, Dierks T. Competition for neuronal resources: how hallucinations make themselves heard. British Journal of Psychiatry. 2007;190:57–62. doi: 10.1192/bjp.bp.106.022954. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Loberg EM, Jorgensen HA, Lundervold A, Lund A, Green MF, Rund B. Left hemisphere lateralisation of auditory hallucinations in schizophrenia: a dichotic listening study. Cognitive Neuropsychiatry. 2008;13:166–179. doi: 10.1080/13546800801906808. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Loberg EM, Specht K, Steen VM, van Wageningen H, Jorgensen HA. Auditory hallucinations in schizophrenia: the role of cognitive, brain structural and genetic disturbances in the left temporal lobe. Frontiers in Human Neuroscience. 2007;1:6. doi: 10.3389/neuro.09.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annual Review of Clinical Psychology. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Archives of General Psychiatry. 2000;57:1131–1137. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. NeuroImage. 2007;34:1600–1611. doi: 10.1016/j.neuroimage.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Fishbein A. Positive and negative syndrome scale (PANSS) rating manual. Toronto, Canada: Multihealth System Inc.; 1992. [Google Scholar]
- Kayser J. Current Source Density (CSD) Interpolation using Spherical Splines: CSD Toolbox (Version 1.1) 2009 [Available at: http://psychophysiology.cpmc.columbia.edu/Software/CSDtoolbox]
- Kayser J, Bruder GE, Friedman D, Tenke CE, Amador XF, Clark SC, Gorman JM. Brain event-related potentials (ERPs) in schizophrenia during a word recognition memory task. International Journal of Psychophysiology. 1999;34:249–265. doi: 10.1016/s0167-8760(99)00082-3. [DOI] [PubMed] [Google Scholar]
- Kayser J, Bruder GE, Tenke CE, Stuart BK, Amador XF, Gorman JM. Event-related brain potentials (ERPs) in schizophrenia for tonal and phonetic oddball tasks. Biological Psychiatry. 2001;49:832–847. doi: 10.1016/s0006-3223(00)01090-8. [DOI] [PubMed] [Google Scholar]
- Kayser J, Fong R, Tenke CE, Bruder GE. Event-related brain potentials during auditory and visual word recognition memory tasks. Cognitive Brain Research. 2003;16:11–25. doi: 10.1016/s0926-6410(02)00205-7. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Optimizing PCA methodology for ERP component identification and measurement: theoretical rationale and empirical evaluation. Clinical Neurophysiology. 2003;114:2307–2325. doi: 10.1016/s1388-2457(03)00241-4. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clinical Neurophysiology. 2006a;117:348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Electrical distance as a reference-free measure for identifying artifacts in multichannel electroencephalogram (EEG) recordings. Psychophysiology. 2006b;43:S51. [Google Scholar]
- Kayser J, Tenke CE. In search of the Rosetta Stone for scalp EEG: converging on reference-free techniques. Clinical Neurophysiology. 2010;121:1973–1975. doi: 10.1016/j.clinph.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Gates NA, Bruder GE. Reference-independent ERP old/new effects of auditory and visual word recognition memory: joint extraction of stimulus- and response-locked neuronal generator patterns. Psychophysiology. 2007;44:949–967. doi: 10.1111/j.1469-8986.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Gates NA, Kroppmann CJ, Gil RB, Bruder GE. ERP/CSD indices of impaired verbal working memory subprocesses in schizophrenia. Psychophysiology. 2006;43:237–252. doi: 10.1111/j.1469-8986.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Gil RB, Bruder GE. Stimulus- and response-locked neuronal generator patterns of auditory and visual word recognition memory in schizophrenia. International Journal of Psychophysiology. 2009;73:186–206. doi: 10.1016/j.ijpsycho.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Kroppmann CJ, Fekri S, Alschuler DM, Gates NA, Bruder GE. Current source density (CSD) old/new effects during recognition memory for words and faces in schizophrenia and in healthy adults. International Journal of Psychophysiology. 2010;75:194–210. doi: 10.1016/j.ijpsycho.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Maguire D, Warde A, Alterescu K, Stickgold R, Pascual-Leone A. Visual hallucinations during prolonged blindfolding in sighted subjects. Journal of Neuro-Ophthalmology. 2004;24:109–113. doi: 10.1097/00041327-200406000-00003. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiological Review. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Möcks J. The influence of latency jitter in principal component analysis of event-related potentials. Psychophysiology. 1986;23:480–484. doi: 10.1111/j.1469-8986.1986.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Neuhaus AH, Karl C, Hahn E, Trempler NR, Opgen Rhein C, Urbanek C, Dettling M. Dissection of early bottom-up and top-down deficits during visual attention in schizophrenia. Clinical Neurophysiology. 2011;122:90–98. doi: 10.1016/j.clinph.2010.06.011. [DOI] [PubMed] [Google Scholar]
- NeuroScan, Inc. SCAN 4.3 - Vol. II. EDIT 4.3 - Offline analysis of acquired data (Document number 2203, Revision D) El Paso, TX: Compumedics Neuroscan; 2003. [Google Scholar]
- Nicholson C. Theoretical analysis of field potentials in anisotropic ensembles of neuronal elements. IEEE Transactions on Biomedical Engineering. 1973;20:278–288. doi: 10.1109/TBME.1973.324192. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. New York: Oxford University Press; 2006. [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. International Journal of Psychophysiology. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Pelletier M, Achim AM, Montoya A, Lal S, Lepage M. Cognitive and clinical moderators of recognition memory in schizophrenia: a meta-analysis. Schizophrenia Research. 2005;74:233–252. doi: 10.1016/j.schres.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [Corrigenda EEG 02274, 1990, EEG Clin. Neurophysiol., 76, 565]. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Roth WT. P3 in schizophrenia is affected by stimulus modality, response requirements, medication status, and negative symptoms. Archives of General Psychiatry. 1989;46:1035–1044. doi: 10.1001/archpsyc.1989.01810110077011. [DOI] [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. Journal of Anatomy. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Boutros NN, Ford JM. Reduced auditory evoked potential component N100 in schizophrenia--a critical review. Psychiatry Research. 2008;161:259–274. doi: 10.1016/j.psychres.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Rossion B, Jacques C. Does physical interstimulus variance account for early electrophysiological face sensitive responses in the human brain? Ten lessons on the N170. NeuroImage. 2008;39:1959–1979. doi: 10.1016/j.neuroimage.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Collins KC, McCarley RW. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophrenia Bulletin. 2010;36:991–1000. doi: 10.1093/schbul/sbp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. Journal of Experimental Psychology: General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R: Patient Edition. Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- Tenke CE, Kayser J. A convenient method for detecting electrolyte bridges in multichannel electroencephalogram and event-related potential recordings. Clinical Neurophysiology. 2001;112:545–550. doi: 10.1016/s1388-2457(00)00553-8. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Generator localization by current source density (CSD): implications of volume conduction and field closure at intracranial and scalp resolutions. Clinical Neurophysiology. 2012 doi: 10.1016/j.clinph.2012.06.005. in press. doi: 10.1016/j.clinph.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey T, Kawabata Duncan KJ, Price CJ, Devlin JT. Top-down modulation of ventral occipito-temporal responses during visual word recognition. NeuroImage. 2011;55:1242–1251. doi: 10.1016/j.neuroimage.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Current Opinion in Neurobiology. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- van Lutterveld R, Sommer IE, Ford JM. The neurophysiology of auditory hallucinations - a historical and contemporary review. Frontiers in Psychiatry. 2011;2:28. doi: 10.3389/fpsyt.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible CG, Lee K, Molina I, Hashimoto R, Preus AP, Roach BJ, Lauriello J. fMRI activity correlated with auditory hallucinations during performance of a working memory task: data from the FBIRN consortium study. Schizophrenia Bulletin. 2009;35:47–57. doi: 10.1093/schbul/sbn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Yeap S, Kelly SP, Sehatpour P, Magno E, Javitt DC, Garavan H, Foxe JJ. Early visual sensory deficits as endophenotypes for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives. Archives of General Psychiatry. 2006;63:1180–1188. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.