Abstract
Interest in pharmacological treatments for obesity that act in the brain to reduce appetite has increased exponentially over recent years, but failures of clinical trials and withdrawals due to adverse effects have so far precluded any success. Treatments that do not act within the brain are, in contrast, a neglected area of research and development. This is despite the fact that a vast wealth of molecular mechanisms exists within the gut epithelium and vagal afferent system that could be manipulated to increase satiety. Here we discuss mechano- and chemosensory pathways from the gut involved in appetite suppression, and distinguish between gastric and intestinal vagal afferent pathways in terms of their basic physiology and activation by enteroendocrine factors. Gastric bypass surgery makes use of this system by exposing areas of the intestine to greater nutrient loads resulting in greater satiety hormone release and reduced food intake. A non-surgical approach to this system is preferable for many reasons. This review details where the opportunities may lie for such approaches by describing nutrient-sensing mechanisms throughout the gastrointestinal tract.
Keywords: enteroendocrine cell, nutrient sensing, vagus nerve, obesity
Introduction
Obesity is a major health issue in the modern era, with its increasing prevalence focussing attention on a problem not of famine or infection, but the outcome of surplus. Most developed societies are experiencing an epidemic of obesity and its closely related co-morbidity type 2 diabetes (Chan et al., 1994; Colditz et al., 1995). The prevalence of obesity worldwide has more than doubled since 1980, with 2.6 million deaths directly related to the disease each year and predictions that by 2015, over 1.5 billion adults will be overweight [body mass index (BMI) >25] or obese [BMI >30, World Health Organization (WHO) 2011]. Obesity further contributes to increased incidence of diseases such as ischaemic heart disease, stroke, hypertension, obstructive sleep apnoea, non-alcoholic steatohepatitis, polycystic ovary syndrome and numerous forms of cancer (Calle et al., 2003; Ning et al., 2010). Although being overweight or obese is a serious risk to both physical and mental health, it is extremely resistant to behavioural intervention (Wadden et al., 2004). Very recent evidence suggests that increased drive to eat upon dieting is a result of decreased gut hormone release, rather than being entirely a psychological phenomenon (Sumithran et al., 2011). To date, therapeutic approaches to obesity management have been largely aimed at appetite control via actions within the CNS, and have had limited efficacy and/or unacceptable adverse effects. Probably, the most effective current interventions in obesity are firstly, gastric bypass surgery, whereby nutrient is shunted to distal regions of the intestine, where it provokes greater release of satiety hormones via a chemosensory mechanism (Bueter and le Roux, 2011), although intestinal bypass procedures improve glucose homeostasis disproportionately to weight loss (Rubino et al., 2010), indicating there are additional consequences of this surgery besides satiety. The second major intervention is gastric banding, which restricts the volume that can be contained within the stomach before it becomes overstretched and excessively activates mechanosensory nerves. These treatments have their own drawbacks that are beyond the scope of this review, but they exemplify the potential of peripherally directed approaches to obesity. Not surprisingly, there is growing interest in developing non-surgical, pharmacological therapies that lead to a reduction in energy intake by altering satiety and/or hunger and, thereby, weight loss. Targeting the initiation of the satiety signal arising from the gastrointestinal (GI) tract is, therefore, an attractive therapeutic treatment for obesity.
Pathways from the gut involved in appetite suppression
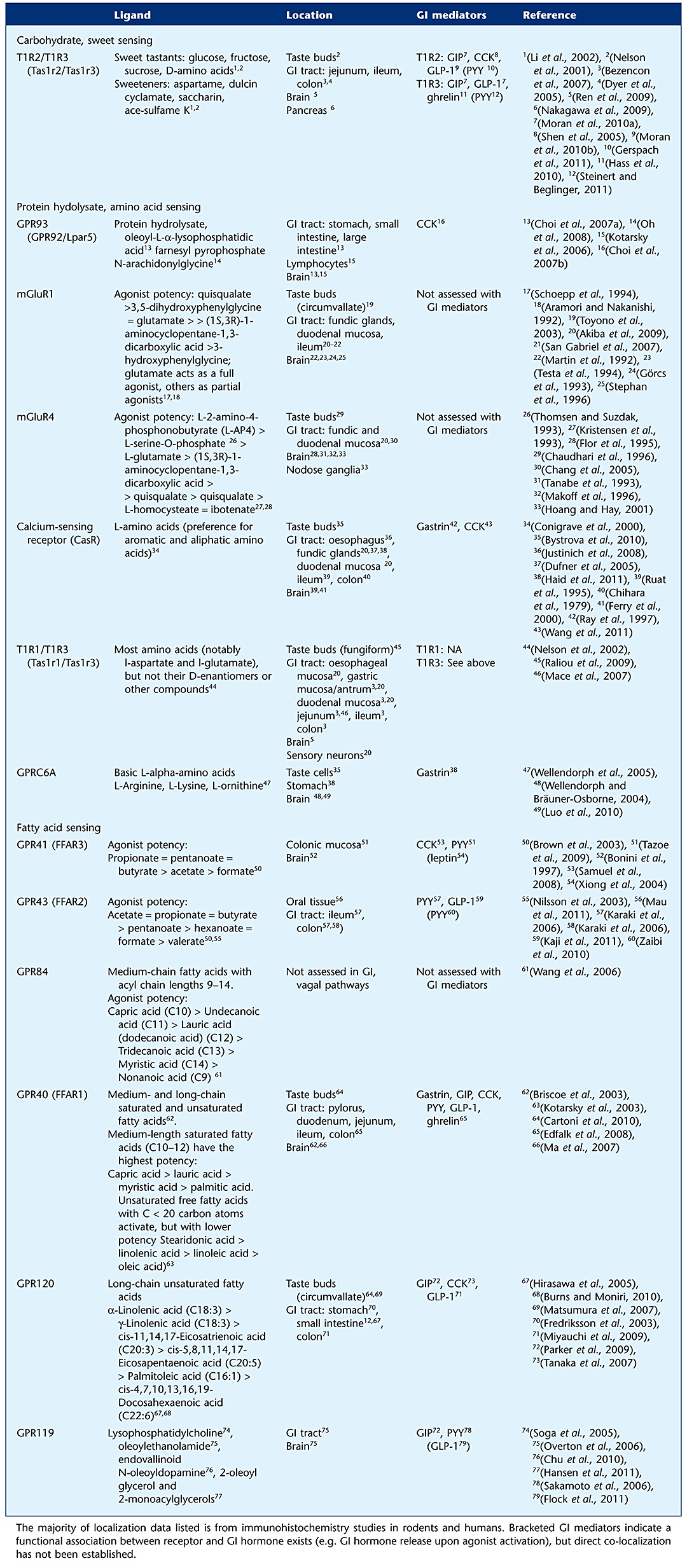
Satiation following food intake occurs via two principal routes within the GI tract – distension of the stomach, and the release of GI peptides in response to the presence of luminal nutrients. Table 1 shows the range of nutrient receptors that are thought to be expressed on gut epithelial cells and elsewhere. From this, it is clear that there is the capacity for detection of all major classes of nutrients, and discrimination according to specific molecular variants of amino acids and fatty acids. Many of these nutrient receptors are GPCR, and mirror a role they may have elsewhere in the body. For example, the calcium-sensing receptor functions to detect both amino acids and extracellular calcium concentrations, which is crucial in parathyroid function, but also in gastric hormone release (see later Buchan et al., 2001). In some cases, specific GPCR are co-localized with specific hormones (Hirasawa et al., 2005), but on the whole, the organization of this system is poorly understood, and the relative role of each type of receptor is unclear. There are some parallels with the taste system that operates in the lingual epithelium, and many components of this pathway are found in the gut. In particular, sweet taste receptors are coupled via the G-protein α-gustducin, and via PLCβ, lead to opening of the transient receptor potential channel transient receptor potential channel-melastatin subtype (TRPM)5, which in turn allows calcium influx and excitation and secretion of mediators such as glucagon-like peptide-1 (GLP-1, see later).
Table 1.
Ligand, location and GI mediators associated with nutrient-sensing receptors
 |
While hormones released from GI tract can signal satiety directly to the CNS, strong functional evidence exists to show that vagal afferent neurons are a primary route to convey mechanical and chemical cues from the GI tract to the brainstem and higher brain centres. Within these brain regions, gustatory, olfactory and textural inputs are then integrated with past experience to control feeding behaviour (Broberger and Hokfelt, 2001; French and Cecil, 2001).
While outside the focus of this review, the role of spinal sensory afferents in food intake regulation is less well known. Spinal sensory innervation of the GI tract is thought to be mainly involved in signalling pain in response to potentially injurious stimuli such as acid-pepsin attack, over-distension or spasm. The cell bodies of gastric and intestinal spinal afferents reside mainly in the thoracic and lumbar dorsal root ganglia adjacent to the spinal cord. Recordings from these afferent pathways show that responses to nutrients do occur (Ranieri et al., 1973; Perrin et al., 1981; Mei et al., 1984); however, evidence so far suggests their role in food intake regulation is minor. For example, bilateral abdominal splanchnectomy in rats does not lead to significant weight loss, in comparison to vagotomy (Furness et al., 2001). Celiac-superior mesenteric ganglionectomy, however, has been shown to reduce acute intake during intraduodenal carbohydrate or fatty acid infusion in rats, while a role of splanchnic afferents in conveying gastric mechanical signals has been suggested (Ozaki and Gebhart, 2001; Sclafani et al., 2003). Further experiments are required to determine the exact contribution of spinal afferents to the GI satiety signal, and whether these afferents signal this directly, and/or indirectly (for example, via mediating changes in intestinal blood supply).
Vagal sensory innervation of the GI tract arises from afferent neurones with cell bodies in the nodose and jugular ganglia, and with endings concentrated in the upper GI tract. Nerve tracing studies have shown that vagal afferent nerves terminate within the muscular layers of the GI tract, within the mucosa, or at both sites. Mechanically sensitive vagal afferent endings form specialized endings within the muscularis externae associated with myenteric ganglia as intraganglionic laminar endings (tension receptors) or as in series intramuscular arrays (putative stretch receptors) running in parallel to circular or longitudinal muscle fibres (Neuhuber, 1987; Berthoud et al., 1992; Zagorodnyuk et al., 2001; Powley and Phillips, 2011). Mucosal vagal afferents (mucosal receptors), in contrast, terminate within the parenchyma of mucosal villi in close contact with the basal lamina, but not with the epithelial surface (Berthoud et al., 1995) and are both mechanosensitive and chemosensitive. This anatomy largely precludes a direct chemosensory action by luminal stimuli, and indicates that specialized epithelial cells serve as primary GI chemosensory cells, and signal to adjacent vagal afferent endings, or enteric neurons, in a paracrine manner. These specialized cells represent less than 1% of the mucosal cell population but together form the largest endocrine organ of the body. They are regionally distributed throughout the GI tract and possess an array of transduction machinery to sense luminal stimuli, and in turn, release over 20 different gut peptides or bioactive molecules (for review, see Cummings and Overduin, 2007; Rindi et al., 2004). Many of these gut peptides undergo rapid proteolytic breakdown in circulation or have high liver clearance, indicating that physiological effects beyond their site of local release within the lamina propria may be limited. Accordingly, paracrine activation of vagal afferent endings is likely to represent an important mode of satiety signalling.
Gastric vagal afferent satiety signals
Investigations of the role in satiety of vagal afferents innervating the stomach have generally been conducted by disruption of the vagal innervation to all abdominal viscera, but this unfortunately provides no discrete evidence of the contribution of gastric vagal afferents (for review, see Ritter, 2004). However, disruption of vagal pathways in rodents with closed pyloric cuffs (to prevent post-gastric-mediated satiation) led to increased consumption and attenuated the reduction of food intake following gastric preloads (Lorenz, 1983). These data highlight a distinct role of gastric vagal afferents in satiety signalling. In humans, there are two main surgical approaches to obesity, both of which involve reduction in the size of the stomach. One is gastric reduction alone, whereby the volume of the stomach is reduced by resection or using an extraluminal band; the other is by gastric resection combined with bypass of a large part of the small intestine. Both types of surgery are effective treatments for obesity, which argues in favour of approaches that target the gastric satiety signal (Elder and Wolfe, 2007). Gastric restriction is accomplished by creating a small gastric pouch and restricting gastric emptying by reducing the size of the gastric outlet, inducing early satiety presumably by increased activation of gastric mechanoreceptors. Other mechanisms of action of bariatric surgery include malabsorption. However, after bariatric surgery, 80% of weight loss is achieved due to diminished energy intake rather than malabsorption (Elder and Wolfe, 2007); therefore, gastric restriction alone is effective in obesity control. The consequence of reducing the size of the stomach is that a smaller meal volume is able to exert sufficient mechanical stimulus to activate vagal mechanoreceptive afferents (Blackshaw et al., 1987), which signal fullness to the CNS. Correspondingly, vagal electrical stimulation has been shown to reduce food intake and body weight in rats (Laskiewicz et al., 2003) and more specifically, electrical modulation of gastric vagal nerves is being used successfully for the treatment of obesity in humans (Toouli et al., 2007). Gastric bypass surgery provides an additional mechanism to gastric restriction, by direct exposure of the distal small intestine to unabsorbed nutrient, which activates intestinal chemosensory mechanisms (see later).
There are two functional classes of mechanosensitive vagal afferents in the stomach, mucosal receptors and tension receptors, according to the location of their mechanoreceptive fields (examples in Figures 1 and 3; Iggo, 1955; 1958; Davison, 1972; Clarke and Davison, 1975; Cottrell and Iggo, 1984a,b; Page and Blackshaw, 1998; Page et al., 2002). Both of these classes give rise to behavioural changes when activated.
Figure 1.
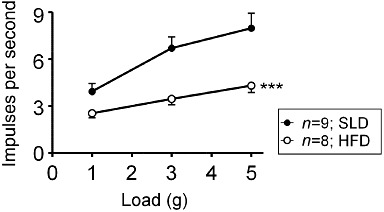
Response of gastric tension receptors to circular stretch in mice fed either a standard laboratory diet (SLD; 7% energy from fat) or a HFD (60% energy from fat). The response to stretch is significantly reduced in mice fed a HFD (P < 0.00; two-way anova). From Kentish et al. (2011).
Figure 3.
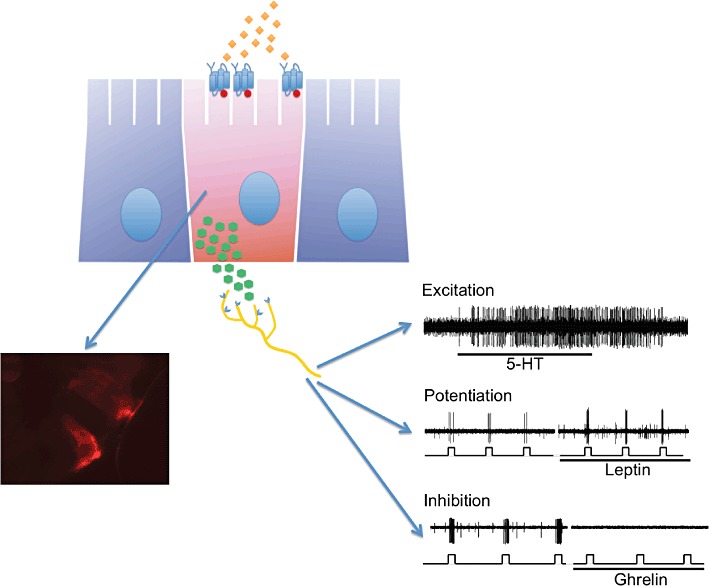
Release mechanism and activation of enteroendocrine cells. Nutrient (diamonds) activate GPCR (blue) on enteroendocrine cell (pink), which via G-proteins (red) activate mediator release on to receptors (blue) on vagal afferents (yellow). Fluorescence micrograph inset shows cell in human colonic epithelium activated in vitro by lauric acid [phosphorylated extracellular-regulated kinase (pERK) immunohistochemistry, unpublished], Nerve recording insets show direct and modulatory effects on vagal afferents of released mediators. Data from Page et al. (2002), Kentish et al. (2011) and unpublished. Recordings are from gastric mucosal afferents in mice. Square waves indicate timing of mechanical stimuli. Note that ghrelin is probably constitutively released rather than induced by nutrient receptors.
We have shown that mucosal receptors are generally silent at rest and respond mechanically to light stroking of the mucosa, generating a burst of action potentials each time the stimulus passes over the receptive field (Page and Blackshaw, 1998; Page et al., 2002). They are insensitive to distension and contraction of the gastric wall. There is evidence that they are important in the initiation of satiety, nausea and vomiting by chemical and osmotic stimuli (Andrews and Sanger, 2002). In the stomach, solid food is triturated into small particles before emptying into the duodenum. Mucosal afferents in the antrum and pylorus are the most likely to mediate this discrimination of particle size and provide negative feedback onto control of gastric motor patterns that promote emptying (Becker and Kelly, 1983; McIntyre et al., 1997; Tuleu et al., 1999). Their high sensitivity to mechanical stimulation of the mucosa also makes them ideal candidates for detecting undigested food to initiate satiety signals.
Tension receptors often have a resting discharge that may be modulated in phase with ongoing contractions. They show slowly adapting responses to normal contractions and distension with a linear relationship to wall tension (Blackshaw et al., 1987; Page and Blackshaw, 1998; Page et al., 2002). Tension receptors signal the level of gastric distension to the CNS, which is important not only in triggering reflexes controlling GI function, but also critical in signalling food intake and generating sensations such as satiety and fullness. Because of their exquisite sensitivity to distension, their signalling of an isovolumetric load is amplified after formation of a gastric pouch (Blackshaw et al., 1987; Blackshaw and Grundy, 1989), as occurs in bariatric surgery, providing a markedly increased satiety signal. We have recently reported that the mechanosensitivity of gastric vagal afferent tension receptors is significantly reduced after chronic consumption of a high-fat diet (HFD) suggesting that the satiety signal would be reduced in these circumstances (see (Kentish et al., 2011; Figure 1). Such a mechanism may have evolved to maximize assimilation of energy from calorie-rich foods in anticipation of famine. A number of studies have shown that obese humans have increased gastric capacity (Geliebter, 1988; Kim et al., 2001), which would result in increased energy intake. This could occur due to a reduction in the gastric distension-stimulated vagal afferent satiety signal. Another recent study has shown that the sensitivity of jejunal afferents to satiety stimuli is also reduced after a HFD (Daly et al., 2011). They revealed that the major mechanism for this decrease in sensitivity is a reduction in the excitability of the neuronal cell membrane (Daly et al., 2011). An alteration in the ion channels involved in mechanotransduction may explain this reduction in excitability of vagal afferents. For example, transient receptor potential vanilloid type-1 (TRPV1) channels have been implicated in obesity. Capsaicin activation of TRPV1 channels has been shown to prevent obesity, while the relative expression of TRPV1 in visceral adipose tissue was significantly reduced in mice fed with a HFD (Zhang et al., 2007). In addition, researchers have shown that consumption of red pepper (a source of capsaicin) along with caffeine significantly reduced energy intake in humans (Yoshioka et al., 2001). Therefore, it is possible that the reduction in mechanosensitivity of tension receptors is due to an alteration in the expression of TRPV1 channels in these particular vagal afferent neurones. However, acid-sensing ion channels (ASIC3s) have also been implicated in gastro-oesophageal vagal afferent mechanosensitivity (Page et al., 2005) and therefore further research in this area is required to find the mechanism responsible for the reduction in mechanosensitivity after chronic consumption of a HFD.
Gastric mechanosensitive vagal afferents can also be modulated by peptides or hormones known to affect appetite. In fact, many of these peptides have been shown to be located in gastric epithelial cells and are therefore in an ideal position to be released and act upon local gastric vagal afferent endings.
Gastric hormones have been shown to have potent effects on satiety, and there is an abundance of evidence that this occurs via vagal mechanisms (Date et al., 2002; Peters et al., 2005). A rich variety of hormones are released from the epithelium of which numerous are implicated in the role of satiety signalling. Their role, source and targets are detailed below and in Figure 2.
Figure 2.
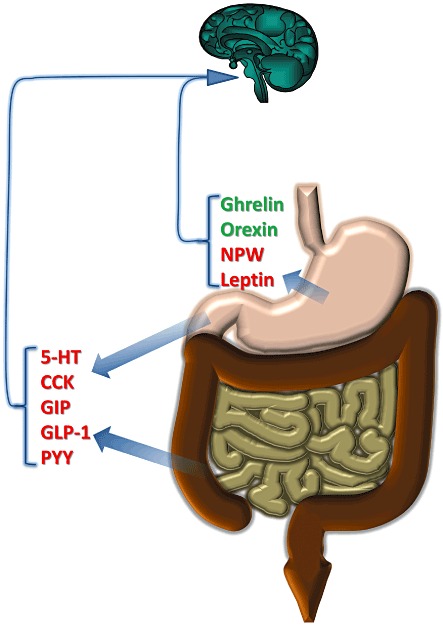
Release of mediators from different regions and effects on food intake. Red indicates inhibition of food intake; green is promotion. Although many mediators act via vagal pathways, some may instead/in addition directly in the brain. See text for details.
Leptin
Traditionally, it was thought that only leptin released from adipocytes was responsible for the long-term regulation of food intake. However, in addition to its role as an adipocytic mediator, leptin is also a gut peptide that interacts with vagal afferents. Epithelial cells in the stomach synthesize and secrete leptin in rodents (Bado et al., 1998) and in humans (Sobhani et al., 2000). Leptin-secreting cells in the epithelium were initially identified as pepsinogen-secreting chief cells (Bado et al., 1998; Sobhani et al., 2000); however, subsequent studies have also detected leptin in the secretory granules of endocrine P cells in the gastric fundus and pylorus (Cinti et al., 2000). Leptin release from the stomach is regulated by feeding (Attoub et al., 1999) (although which nutrients are responsible is unknown), acetylcholine released by the vagus nerve (Sobhani et al., 2002) and small intestinal hormones such as cholecystokinin (CCK) and secretin (Bado et al., 1998; Sobhani et al., 2000). Cell bodies of abdominal vagal afferents in the nodose ganglia synthesize receptors and transmitters, which are subsequently transported to the nerve terminals (Figure 3). Expression of leptin receptor mRNA has been detected in both rodent and human nodose ganglia (Buyse et al., 2001; Burdyga et al., 2002), while vagal afferent neurons possess functionally active leptin receptors (Buyse et al., 2001).
Ghrelin
This orexigenic (appetite-stimulating) signal from the stomach is an endogenous ligand for the growth hormone secretagogue (GHS) receptor (Inui, 2001; Date et al., 2002). Ghrelin has been shown to be located exclusively in endocrine X/A-cells (now designated Gr cells), with the highest concentrations found in the oxyntic glands of the gastric fundus and to a lesser extent the gastric antrum (Date et al., 2000). Ghrelin is secreted from gastric endocrine cells into the circulation, and while the exact stimulus for ghrelin release is not known, there are many stimuli that can inhibit the process. At least part of ghrelin signalling from the stomach is mediated via an ascending neural network through the vagus nerve and brainstem nuclei that ultimately reaches the hypothalamus (Asakawa et al., 2001; Date et al., 2002). We have shown that peripheral vagal afferent endings in the stomach are found in close apposition to ghrelin containing epithelial cells (Kentish et al., 2011) GHS receptors are localized to vagal afferents that project to the rat stomach and we have shown GHS receptor expression in the mouse nodose ganglia (Page et al., 2007). There is some controversy around the role of the vagus nerve in the orexic effects of ghrelin. Date and colleagues (Date et al., 2002) showed that either truncal or selective gastric vagotomy or perivagal capsaicin abolished the action of ghrelin when given intravenously in rats. Findings of Asakawa and colleagues (Asakawa et al., 2001) support this view, showing that ghrelin inhibits the resting discharge in whole vagal nerve recordings and that lesioning vagal afferent fibres inhibits the appetite-stimulating actions of ghrelin. In contrast, Arnold and colleagues (Arnold et al., 2006) showed no effect of vagotomy on the acute effects of ghrelin administered intraperitoneally. We have reported that ghrelin inhibits the mechanosensitivity of gastric tension receptors but not mucosal receptors (Page et al., 2007). After chronic consumption of a HFD or acute food restriction, this selective inhibition of gastric tension receptors is extended to include gastric mucosal receptors (Kentish et al., 2011). Since gastric mucosal receptors are considered to be important in detecting particulate content, which reduces gastric emptying and food intake (McIntyre et al., 1997; Tuleu et al., 1999), ghrelin is likely to reduce this satiety signal during fasting and in obesity. In fasted healthy volunteers, ghrelin administration has been shown to increase the rate of gastric emptying in addition to elevating hunger ratings (Falken et al., 2010); this may be a consequence of the decreased mechanosensitivity of gastric mucosal receptors by ghrelin. Together, these data indicate marked plasticity in the mechanism of action of ghrelin under different feeding states.
Neuropeptide W (NPW)
Neuropeptide W is a recently discovered peptide that activates the orphan G-protein-coupled receptors GPR7 and GPR8 (Tanaka et al., 2003). NPW has been suggested to exert a modulatory role in the control of food intake in the brain since central administration of human NPW in rats stimulated food intake (Shimomura et al., 2002; Levine et al., 2005). However, NPW has also been shown to be present in antral epithelial gastrin (G) cells of rat, mouse and human stomach (Mondal et al., 2006). It is known that these cells respond to dietary amino acids via the G-protein-coupled calcium sensing receptor, which induces excitation via phospholipase C (Buchan et al., 2001). Gastric mucosal expression of NPW mRNA in fasted or food restricted animal models is reduced and normalized upon refeeding (Caminos et al., 2008). In addition, GPR7 knockout mice were shown to be hyperphagic and became obese (Ishii et al., 2003). Together, this suggests an anorectic role for gastric NPW (Mondal et al., 2003). However, preliminary studies within our laboratory indicate that NPW reduces the mechanosensitivity of gastric vagal afferents and thus, like ghrelin, has a peripheral orexigenic effect (Li et al., 2011). This NPW-induced reduction in vagal afferent mechanosensitivity is lost after chronic consumption of a HFD (Li et al., 2011) possibly as a protective mechanism as a result of an energy imbalance.
Orexin
Orexins are neuropeptides first localized in neurons within the lateral hypothalamus (Nambu et al., 1999; Takahashi et al., 1999). They are involved in feeding behaviour by stimulating appetite and food consumption (Wolf, 1998). Two types of orexins have been identified, orexin A and B (Sakurai et al., 1998), derived from a common precursor. Early reports suggested that orexins were restricted to the hypothalamus, however, subsequently, it has been shown that a subset of neurons and endocrine cells of the GI tract also contain orexin A and express functional receptors (Kirchgessner and Liu, 1999). Orexin A immunoreactivity has been found in endocrine cells in gastric pyloric glands of rodents, where a subset co-localizes with gastrin, in addition to endocrine cells in the human stomach (Kirchgessner and Liu, 1999; De Miguel and Burrell, 2002; Nakabayashi et al., 2003). Orexin-1 receptor has also been localized in vagal afferent neurons of humans and rats, while orexin A has been shown to inhibit jejunal vagal afferent responses to CCK (see later Burdyga et al., 2003; 2010). Although not directly investigated, it is possible that orexin A released from gastric endocrine cells could modulate the response of gastric vagal afferents to mechanical stimulation, thereby influencing gastric vagal afferent signalling of satiety.
Non-peptide transmitters such as endocannabinoids and nitric oxide have also been implicated in satiety signalling. There is substantial evidence for the role of nitric oxide in feeding behaviour in the hypothalamus (Czech, 1996; Yamada et al., 1996; Czech, 1998; Czech et al., 1998; Calapai et al., 1999; Morley et al., 1999) but little is known about its role in satiety signalling in the periphery. Studies have shown that nitric oxide is important in the actions of many appetite control peptides including ghrelin, neuropeptide Y, orexin A and leptin (Morley et al., 1999; Gaskin et al., 2003; Farr et al., 2005). For example, the effect of the peptides CCK, neuropeptide Y and ghrelin on food intake is lacking in nitric oxide synthase knockout mice (Morley et al., 2011). Indeed, we have recently reported a role for endogenous nitric oxide as a peripheral modulator of gastro-oesophageal sensory function (Page et al., 2009). Nitric oxide synthase containing cells located within the gastric mucosa are an endogenous source of nitric oxide, which are in close proximity to vagal afferent endings (Page et al., 2009). Therefore, there is a peripheral nitric oxide-vagal afferent pathway that may be involved in appetite regulation. Preliminary data from our laboratory further indicate that there is a switch in the effect of endogenous nitric oxide on vagal afferent mechanosensitivity – from inhibitory in fed animals to excitatory in fasted animals (Elliott et al., 2011). This switch is due to signal transduction via alternate second messenger pathways in response to nitric oxide.
Intestinal vagal afferent satiety signals
The intestine, the major site of macronutrient breakdown and nutrient absorption, is extensively innervated by vagal afferents with peak density in the duodenum and lower density in the ileum and distal intestine (Jagger et al., 1997). Intestinal vagal afferents that terminate within muscle layers are directly responsive to mechanical stimuli (Clarke and Davison, 1978), similar to those innervating muscle layers of the stomach. Mucosal vagal afferents, however, are likely to respond directly to chemical signals from primary sense cells within the intestinal mucosa (Figure 3). In support of a paracrine action of enteroendocrine cells, subsets of intestinal vagal afferents are known to express receptors for a number of key hormones released by gut epithelial cells, including those released in response to luminal nutrients (Kakei et al., 2002; Raybould et al., 2003; Nakagawa et al., 2004; Vahl et al., 2007; Bucinskaite et al., 2009); a number of these important signal mediators are discussed below.
Intestinal vagal afferents are responsive to a wide array of luminal stimuli, including hyperosmotic and hypo-osmotic solutions, acids and bases, and breakdown products of carbohydrates, proteins and fats (Clarke and Davison, 1978; Mei, 1978; Mei and Garnier, 1986; Lal et al., 2001). Intestinal perfusion with water, for example, has been shown to specifically activate subsets of intestinal vagal afferents in cats and rats (Mei and Garnier, 1986; Zhu et al., 2001). Infusions of hyperosmotic solutions exceeding 500 mosM also directly activate nodose ganglion neurons in rats, and significantly inhibit food intake in pigs (Houpt et al., 1983; Zhu et al., 2001). This osmolarity is within the normal postprandial range of ingesta within the intestine and indicates that non-nutritive characteristics of ingesta contribute to the intestinal satiety signal, and signal largely via vagal pathways. Intestinal nutrients exert powerful effects on food intake and satiety via vagal pathways. Subdiaphragmatic vagotomy or abdominal afferent denervation using the neurotoxin capsaicin largely or completely blocks the inhibition of food intake following intestinal infusion of carbohydrates or fatty acids (Yox and Ritter, 1988; Walls et al., 1995). Correspondingly, expression of the immediate early gene product, c-fos, in vagal brainstem nuclei, closely follows intraduodenal infusion of these nutrients (Phifer and Berthoud, 1998). Together, these data highlight the critical role of intestinal vagus signals in satiety, and the rationale of targeting the vagus in managing obesity. There are also many examples of intestinal nutrient infusion slowing gastric emptying via vagal reflex pathways in animals and humans (Wilkinson and Johnston, 1973; Roze et al., 1977; Raybould and Holzer, 1992; Schwartz et al., 1993). In healthy humans, this rate is slowed to 1–3 kcal·min−1, a rate matched to the absorptive capacity of the small intestine (Brener et al., 1983; Raybould, 1998). However, it is important to appreciate that while slowing of gastric emptying may interact with intestinal satiety signalling, the suppression of food intake by intestinal signals per se does not require gastric emptying to be slowed.
Glucagon-like peptide-1
Much research effort has been focussed on intestinal L-cells due to their ability to broadly detect digestion products of carbohydrates, fats and proteins, and in response, secrete the incretin hormone GLP-1. L-cells are distributed throughout the GI tract, with greatest density in the distal intestine (ileum, colon); yet not insignificant numbers are present in the proximal small intestine in most species (Bryant et al., 1983; Eissele et al., 1992). These cells use alternative post-translational processing of proglucagon to produce the bioactive peptides GLP-1, GLP-2 and oxyntomodulin, although most attention has focussed on the actions of GLP-1 (Holst, 2007). GLP-1 is released rapidly in proportion to caloric load and to the length of small intestine exposed to nutrient, and acts to increase satiation, stimulate insulin release, suppress glucagon secretion and slow gastric emptying (Schirra and Goke, 2005; Horowitz and Nauck, 2006; Little et al., 2006). In addition, complex carbohydrates that reach the distal intestine may be fermented to short-chain fatty acids, which can also interact with L-cells via other nutrient sensors (Holst, 2007; Reimann, 2010). While these distal L-cell mechanisms can powerfully influence GI motility and food intake (as the ileal and colonic brake mechanisms), they may not be significantly recruited under normal GI transit due to effective upper gut absorption; they are also less likely to signal via a vagal pathway.
Our own work has shown that a subset of proximal intestine L-cells in both mice and humans express GPCRs for sweet taste (Sutherland et al., 2007; Young et al., 2010). Sweet taste receptors are under dynamic luminal and metabolic control, and link the luminal presence of a broad range of sweet tastants, including sweeteners, to the intestinal release of GLP-1 (for review, see Young, 2011). In L-cell-based systems, and recently in human intestine, it has been shown that blockade of sweet taste receptors reduces glucose-stimulated GLP-1 [and peptide YY (PYY)] release, while mice that are deficient in sweet taste molecule expression show dysregulated glucose-stimulated GLP-1 release (Jang et al., 2007; Margolskee et al., 2007; Gerspach et al., 2011). Sweet taste receptors provide one example, among others, of sensors expressed on L-cells, which may transduce luminal carbohydrate signals (Tolhurst et al., 2009). A similarly diverse list of candidate GPCRs in the intestine respond to breakdown products of amino and fatty acids leading to the release of GLP; these have been reviewed by others [see Table 1 (Little and Feinle-Bisset, 2011b; Reimann, 2010)].
The functional importance of GLP-1 in food intake control is now well established – genetic variation around the GLP-1 allele strongly correlates with total food volume in mice strains (Kumar et al., 2008), while mice deficient in GLP-1 receptors are partially resistant to HFD-induced obesity (Hansotia et al., 2007). Evidence indicates that effects of gut-released GLP-1 on food intake are mediated largely via a vagal pathway arising from the proximal intestine (Hayes et al., 2011). Subdiaphragmatic vagotomy completely blocks the satiating effect of intraperitoneal GLP-1 infusion (targeting intestinal vagal afferents) in rats, indicating that these effects are dependent on vagal afferent activation (Punjabi et al., 2011). Indeed, GLP-1 receptors have been localized on abdominal vagal afferents, on nodose ganglion neurons and in brainstem neurons (notably within the dorsal vagal complex) (Drucker and Asa, 1988; Goke et al., 1995; Imeryuz et al., 1997; Merchenthaler et al., 1999; Kakei et al., 2002; Nakagawa et al., 2004). While GLP-1 has also been shown to excite vagal afferents directly, effects on satiety and gastric emptying were long considered to involve endocrine actions at central sites within brainstem and hypothalamic nuclei (Imeryuz et al., 1997; Kakei et al., 2002; Nagell et al., 2006; Nakade et al., 2006; Bucinskaite et al., 2009; Holmes et al., 2009). However, the relative importance of central site of action for gut-released GLP-1 is uncertain as GLP-1 undergoes rapid degradation within minutes at the site of peripheral release and within the circulation and liver by the ubiquitous enzyme dipeptidyl peptidase-IV (DPP-IV), with less than 10% reaching systemic circulation in intact form (Deacon et al., 1996; Holst, 2007). Moreover, circulating levels of active GLP-1 do not significantly rise after regular chow meals in non-anaesthetized rats (Punjabi et al., 2011), suggesting that the normal signalling mode of gut-derived GLP-1 is predominantly paracrine (vagal), rather than endocrine. It should be noted, however, that GLP-1 is also produced centrally within autonomic neurons of the ventrolateral and caudal nuclei of the solitary tract, which project to various hypothalamic nuclei, and direct activation of GLP-1 receptors within the caudal brainstem is capable of reducing food intake (Merchenthaler et al., 1999; Vrang et al., 2007; Hayes et al., 2008; Llewellyn-Smith et al., 2011). However, blockade of central GLP-1 receptors does not block the anorexia induced by intraperitoneal GLP-1 in rats, whereas peripheral GLP-1 receptor blockade led to increased light phase food intake in rats (Williams et al., 2009), arguing against a prominent role of central GLP-1 in intestinal vagal afferent-mediated satiety. In summary, it appears that gut-derived GLP-1 is the primary satiety signal triggered upon food intake, and that vagal processing is sufficient to exert this effect.
It is also well established that the peripheral administration of GLP-1 or GLP-1 analogues (e.g. exenatide, liraglutide) dose dependently reduce food intake leading to weight loss in animal models of obesity and in lean and obese humans (for review, see Hayes et al., 2010). In rats, this reduced food intake with liraglutide is due to combined actions at GLP-1 receptors on peripheral vagal afferents of the intestine (or hepatic portal region) and centrally (Kanoski et al., 2011). Peripheral GLP-1 also directs energy balance on both a short- and long-term basis via interaction with leptin, which is hypothesized to increase intestinal vagal afferent sensitivity to GLP-1, as occurs for another anorexigenic peptide, CCK. Thus, a satiating effect of GLP-1 in fasted mice is revealed by co-administration with leptin, and GLP-1 is ineffective in reducing food intake in leptin receptor-deficient mice. Both CNS and peripheral sites of action appear plausible for this action (for review, see Barrera et al., 2011).
Glucose-dependent insulinotropic peptide (GIP)
GIP-secreting K-cells are located within the proximal small intestine, and are responsive to digestion products of carbohydrates and fat, as well as certain amino acids (Baggio and Drucker, 2007). GIP is rapidly released postprandially in proportion to calorific load (Pilichiewicz et al., 2007a), and acts to stimulate insulin release and promote lipid storage in adipocytes, the latter an emerging role that may link overnutrition to obesity (Yip et al., 1998; Baggio and Drucker, 2007; Kim et al., 2007). There is recent interest in GIP in the obesity setting, given that GIP knockout mice are resistant to diet-induced obesity (Miyawaki et al., 2002). Indeed, it has been proposed that part of the benefits of Roux-en-Y gastric bypass in obesity management may be due to surgical removal of intestinal K-cells (for review, see Paschetta et al., 2011). However, in contrast to GLP-1, exogenous GIP does not alter the rate of gastric emptying in response to intestinal carbohydrates, and correspondingly, vagal afferents in rodents lack receptors and functional responses to GIP (Nishizawa et al., 1996; Meier et al., 2004; Nakagawa et al., 2004). Moreover, while the density of intestinal K-cells may increase in obesity, GIP has no direct effects on hunger, desire to eat, satiety or prospective consumption in humans (Cho and Kieffer, 2011; Edholm et al., 2011). It should be noted that K-cells co-secrete the peptide xenin, which exerts satiating effects at a peripheral and/or central site of action, the latter which may occur independent of known hypothalamic satiety centres (Leckstrom et al., 2009; Taylor et al., 2011). It remains to be established whether a peripheral vagal pathway is involved in this action of xenin.
Peptide YY
PYY is produced within L-cells that co-secrete GLP-1 in both the proximal and distal intestine, and is released in response to fatty acids, carbohydrates and to a lesser extent, amino acids (for review, see Holst, 2007). PYY release is in proportion to caloric load and acts to both increase satiation and slow gastric emptying (Batterham et al., 2006; Pilichiewicz et al., 2007b; Helou et al., 2008). Two endogenous forms mediate these effects, with PYY3–36– the predominant circulating form – produced following cleavage of PYY1–36 in circulation by the ubiquitous enzyme DPP-IV (Karra et al., 2009). Along with other members of this peptide family (neuropeptide Y, pancreatic polypeptide), PYY peptides interact with specific GPCRs Y1, 2, 4, 5 and 6 with differential specificity; PYY1–36 binds to all Y-class receptors, while PYY3–36 shows high affinity for Y2 receptors, and lower affinity for Y1 and Y5 receptors. Intraperitoneal administration of PYY3–36 in rodents was shown to exert a dose-dependent anorectic effect on food intake (Batterham et al., 2002), which could be completely blocked by subdiaphragmatic vagotomy (Abbott et al., 2005). Indeed, Y2 receptors have been identified on both intestinal vagal afferents and within the hypothalamus (arcuate nucleus) indicating that anorectic effects of Y2 receptor activation may be achieved via paracrine activation of intestinal vagal afferents, via direct central activation by circulating PYY3–36, or by both pathways (Zhang et al., 1997; Fetissov et al., 2004; Koda et al., 2005). Like GLP-1, PYY has complex effects on food intake upon central administration in rodents. Direct administration to the hypothalamic arcuate nucleus inhibits food intake via a Y2 receptor-dependent manner, while i.c.v. administration exerts orexigenic effects in the hypothalamic paraventricular nucleus via a Y1 and Y5 receptor-dependent manner (for review, see Cummings and Overduin, 2007). Together, these findings highlight the complex role of Y2 receptors in both vagal and central pathways regulating satiety.
Vagal expression of Y2 receptors is known to be modified by feeding status in rats, and in association with prevailing levels of the gut hormone CCK. Thus, Y2 receptor levels are low in fasted mice and high in fed mice, or in fasted mice infused with CCK (Burdyga et al., 2008). This action of CCK is mediated via release of the vagal afferent neuronal peptide derived from cocaine- and amphetamine-regulated transcript (CART), which acts in an autocrine manner to increase Y2 receptor levels (De Lartigue et al., 2011; reviewed in Dockray and Burdyga, 2011). Pharmacological blockade of Y2 receptors in rats has been shown to abolish feeding inhibition by PYY3–36, while germline knockout mice deficient in Y2 receptors are hyperphagic and develop obesity (Batterham et al., 2002; Scott et al., 2005); similar findings have been revealed in a PYY-specific knockout mouse (Naveilhan et al., 1999). Thus, there is ample evidence to suggest that vagal Y2 receptors are an important satiety-signalling mechanism.
Cholecystokinin
CCK-secreting I-cells are located largely within the proximal small intestine and are more responsive to digestion products of fatty and amino acids than to carbohydrates (Rehfeld, 1978; Cummings and Overduin, 2007). It is well established that CCK release occurs in proportion to caloric load, but independent of the length of small intestine exposed (Liddle et al., 1985; Little et al., 2006). Release of CCK by fatty acids is critically dependent on acyl chain length, with only fatty acids ≥12 carbon atoms able to trigger release, and subsequent suppression of food intake (Hunt and Knox, 1968; McLaughlin et al., 1999; Feltrin et al., 2004). Short-chain fatty acids, in contrast, engage specific nutrient detectors in the distal intestine, which may influence satiety via non-vagal pathways as part of the ileal or colonic brake mechanisms (for review, see Kaemmerer et al., 2010). Activation of intestinal vagal afferents by fat involves the packaging of fatty acids within enterocytes into chylomicrons, followed by the production and basolateral release of apolipoprotein A-IV; apolipoprotein A-IV then triggers CCK release, activating CCK1 receptors on adjacent vagal afferent endings (Glatzle et al., 2003; Whited et al., 2006; Lo et al., 2007). An alternate signal pathway for CCK-dependent activation of vagal afferents has also been proposed for oleoylethanolamine, a lipid amide that also triggers intestinal release of apolipoprotein A-IV in a manner dependent on the activation of peroxisome proliferator-activated receptor alpha (Fu et al., 2003). However, much less is known of how CCK is released by proteins to activate intestinal vagal afferents. Evidence so far has revealed that the proton-coupled oligopeptide transporter PepT1 (peptide transporter 1) is indirectly involved via a CCK-dependent pathway (Darcel et al., 2005; Matsumura et al., 2005; Liou et al., 2011).
There is abundant evidence from our laboratory, and others, that intestinal vagal afferents express the CCK1 receptor, and are responsive to CCK (Cottrell and Iggo, 1984a; Moran et al., 1987; Blackshaw and Grundy, 1990; Broberger et al., 2001; Lal et al., 2001; Partosoedarso et al., 2001). Exogenous administration of CCK potently suppresses food intake and slows gastric emptying in rats and humans – effects blocked by vagotomy in rats (Smith et al., 1985; Schwartz et al., 1993; Sullivan et al., 2007; Brennan et al., 2008). In a similar manner, pharmacological blockade of CCK1 receptors dose dependently inhibits food intake and gastric emptying effects of exogenous and endogenous CCK in rats and humans (Fried et al., 1991; Moran et al., 1992; 1994; Yox et al., 1992; Beglinger et al., 2001). Genetic polymorphisms around the CCK (and leptin) allele in humans have also been shown to associate with specific eating patterns and meal size (de Krom et al., 2007), supporting a specific role for CCK in human satiety signalling. Despite strong evidence of a peripheral site of action, a central site for CCK satiety has been shown, based on expression and function of CCK receptors in hypothalamic satiety centres, and evidence that blood–brain barrier permeant CCK1 receptor antagonists modify food intake in vagotomized rats (Schick et al., 1990; Honda et al., 1993; Reidelberger et al., 2004). A recent and elegant magnetic resonance study in humans, however, has revealed that activation of brain regions upon intestinal exposure to intralipid was consistent with activation via a vagal pathway, and could be abolished by pharmacological blockade of CCK1 receptors (Lassman et al., 2010). Satiation in humans in response to long-chain fatty acids, and gastric emptying of hexose sugars are also reported to be blocked by the CCK1 receptor antagonist loxiglumide (Matzinger et al., 2000; Little et al., 2010), highlighting the importance of CCK satiety signals in response to a range of dietary macronutrients. Levels of CCK1 receptors in vagal afferents do not show significant nutritional plasticity in health, but serve as triggers to modulate levels of other anorexic and orexigenic receptors with feed status (for review, see (Dockray and Burdyga, 2011). CCK activation of vagal afferents and satiety signalling, though, is inhibited by orexigenic hormones including ghrelin, orexin A and the endogenous cannabinoid receptor agonist, anandamide (Burdyga et al., 2006a; 2010; 2006b; De Lartigue et al., 2011). In contrast, CCK activation of a subset of CCK1 receptor expressing vagal afferents is likely to be potentiated by anorectic actions of melaninocortin-4 receptor agonists (such as α-melanocyte-stimulating hormone) acting via a central presynaptic mechanism (Wan et al., 2008; Gautron et al., 2010), as well as by leptin.
CCK acts predominantly as a short-term satiety signal, as long-term administration in animals and humans does not alter body weight, as reduced meal size is effectively offset by increased meal frequency. In fact, the satiating actions of intraperitoneal CCK administration are lost as early as 24 h in rodents (Crawley and Beinfeld, 1983; West et al., 1984). However, anorectic effects of CCK are augmented by the long-term satiety hormone leptin, when CCK responses are potentiated at the level of vagal afferents, the brainstem and hypothalamus (for review, see Strader and Woods, 2005; Dockray and Burdyga, 2011). Accordingly, the adiposity of an individual, which is tightly linked to circulating leptin levels, is likely to sculpt responsiveness to short-term CCK signals. For example, anorectic effects of CCK would likely be decreased in both lean individuals and in leptin-resistant states, such as obesity. It is also important to note that rats deficient in CCK1 receptor expression are obese and hyperphagic, while mice lacking CCK1 receptors are lean, but become obese following a HFD (Schwartz et al., 1999; Bi et al., 2007); variable effects of a HFD have been reported in CCK knockout mice (Lacourse et al., 1999; Lo et al., 2011). Together, these data indicate that CCK exerts acute anorectic effects on meal size and timing, but exerts these actions predominantly via peripheral vagal actions to control food intake.
Serotonin (5-HT)
Serotonin-secreting enterochromaffin (EC) cells are a prominent enteroendocrine cell type distributed throughout the GI tract. EC cells release 5-HT in response to a wide array of chemical and mechanical cues, including acids, bases, carbohydrates, long- and short-chain fatty acids, as well as following distension or exposure to high-osmolarity stimuli (for reviews, see Grundy, 2006; 2008; Blackshaw et al., 2007; Bertrand, 2009; Raybould, 2009; Bertrand and Bertrand, 2010). Vagal afferents in rats and humans are known to express the 5-HT3 receptor, while in rats, both 5-HT3A and 5-HT3B subtypes are expressed and may form homomeric or heteromeric receptors each with different agonist sensitivity (Glatzle et al., 2002; Morales and Wang, 2002; Lang and Grafe, 2007). Following GI release, 5-HT exerts paracrine effects via activation of 5-HT receptors present on enteric nerves and vagal nerve endings within the mucosa; circulating 5-HT levels are kept low due to specific uptake into platelets. A number of other bioactive compounds have been identified in EC cell populations, including chromogranin A, melatonin, ATP, GABA, uroguanylin and dynorphin although less is known of their actions in the GI tract (see Bertrand and Bertrand, 2010).
While parenteral 5-HT administration has been shown to reduce meal size and duration in rats, vagotomy appears to increase this effect (Fletcher and Burton, 1985; 1986). Additionally, outcomes of studies using 5-HT3 receptor antagonists to influence satiety in animals and humans have generally been inconsistent, raising questions for a peripheral role of 5-HT in food intake regulation (for review, see Aja, 2006). Indeed, most attention has been focussed on central sites of satiety actions of 5-HT acting on 5-HT1 and 5-HT2 receptor subtypes (for review, see Atkinson, 2008). Despite this, evidence in rodents has indicated a role of peripheral 5-HT3 receptors in vagal pathways activated by carbohydrate, fatty acids and hyperosmolar stimuli leading to suppression of food intake, slowing of gastric emptying and pancreatic secretion (Li et al., 2000; Zhu et al., 2001; Raybould et al., 2003; Savastano et al., 2005; 2007; Wu et al., 2005). It has also been suggested that similar populations of intestinal vagal afferents activated by CCK in rats are responsive to 5-HT (Li et al., 2004); this could potentiate satiety signal arising in this common pool of vagal afferents, particularly in the presence of mixed meal components. A similar mechanism has been shown for gastric vagal afferent mechanosensitivity, which is augmented by both CCK and 5-HT and has been suggested is the primary peripheral satiety action of peripheral 5-HT (Bozkurt et al., 1999; Daughters et al., 2001; Hayes et al., 2006). Additional experiments are required to ascertain the precise role of peripheral GI release of 5-HT and vagal 5-HT3 receptors in food intake regulation, although this contribution is likely to be less than other direct vagal mediators of satiety.
Intestinal vagal afferent signals in obesity
Studies in rodents following a HFD, or in diet-induced obesity consistently show reduced suppressive effects of intestinal nutrients on food intake compared to control animals (Covasa et al., 2001; Little and Feinle-Bisset, 2011b). Moreover, the GI release of GLP-1 and PYY is reported to be attenuated in diet-induced obesity in rodents, while CCK and 5-HT release is increased (Spannagel et al., 1996; Anini and Brubaker, 2003; Batterham et al., 2006; Bertrand et al., 2011). Satiety effects of exogenous (intraperitoneal) CCK, however, are attenuated in rodents fed with a HFD (Covasa and Ritter, 1998; Nefti et al., 2009) as are nodose ganglion levels of CCK1 receptor expression, changes that mirror those reported in vagal afferents following injury or nerve damage (Zhang et al., 1996; Broberger et al., 2001). In contrast, anorectic effects of exogenous PYY and GLP-1 appear to be preserved in rodent models of diet-induced or genetic obesity (Neary et al., 2005; Renshaw and Batterham, 2005; Vrang et al., 2006; Madsen et al., 2011; Tomas et al., 2011). A recent study in mice directly tested intestinal afferent function in diet-induced obesity and showed reduced sensitivity of jejunal afferents (primarily vagal) to low-level distension and reduced excitability of identified jejunal vagal afferents within the nodose ganglion to CCK and 5-HT exposure (Daly et al., 2011). Corresponding reductions in vagal afferent expression of receptors for CCK, 5-HT and other anorexic GI peptides have been reported in the nodose ganglion of mice subjected to short- and long-term HFDs, along with reduced brainstem expression of c-fos following a meal stimulus (Donovan et al., 2009; Nefti et al., 2009).
In humans, reports indicate that both fasting and postprandial levels of circulating PYY and GLP-1 are also lower in obese, compared to lean individuals (Batterham et al., 2003; le Roux et al., 2006; Little and Feinle-Bisset, 2011a). CCK levels, in contrast, are either increased (Baranowska et al., 2000) or normal in obese individuals (Lieverse et al., 1994). There are, however, a number of caveats in comparing many of these human studies. One example is that the reduced slowing of gastric emptying that occurs on a HFD (Cunningham et al., 1991) would itself act to increase intestinal exposure and increase GI peptide release. Despite this generalized attenuation in GI peptide release, the anorectic effects of exogenously administered CCK, PYY and GLP-1 appear to be preserved in obese individuals (Flint et al., 2001; Batterham et al., 2003). In summary, it appears that attenuated GI peptide release and lower sensitivity of intestinal vagal pathways can both contribute to reduced signalling of satiety and reduced suppression of food intake in obesity.
Conclusions
Together, these data indicate that sensing of mechanical and nutrient cues within the wall of the GI tract, and signalling of satiety by vagal pathways, represent a major regulatory interface in the control of food intake and satiation. As such, therapies that target such vagal mechanisms are likely to yield effective new management strategies, and a viable target to curb obesity and metabolic disease. Although a number of mechanisms have already been implicated in these actions, their precise mechanisms in the gut and mode of vagal signalling of satiety remain to be fully revealed. This is a promising new arena for clinical and translational research in obesity management.
Acknowledgments
This work was supported by the National Health & Medical Research Council of Australia (AJP, LAB, RLY), Wellcome Trust (UK) (LAB, MP) and AstraZeneca AB (Sweden) (AJP, ES, LAB, RLY).
Glossary
- ASIC
acid-sensing ion channel
- BMI
body mass index
- CART
cocaine- and amphetamine-regulated transcript
- CCK
cholecystokinin
- DPP-IV
dipeptidyl peptidase 4
- EC
enterochromaffin
- FFAR
free fatty acid receptor
- GHS
growth hormone secretagogue
- GI
gastrointestinal
- GIP
glucose-dependent insulinotropic peptide
- GLP
glucagon-like peptide
- GPRC6A
G-protein-coupled receptor family C group 6 member A
- HFD
high-fat diet
- mGluR
metabotropic glutamate receptor
- NPW
neuropeptide W
- PepT1
peptide transporter 1
- PYY
peptide tyrosine-tyrosine
- T1R
taste receptor subtype 1
- TRPM
transient receptor potential channel-melastatin subtype
- TRPV
transient receptor potential channel-vanilloid subtype
- WHO
World Health Organization
Conflict of interest
None of the authors have any conflict of interest, financial, academic or otherwise.
References
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Aja S. Serotonin-3 receptors in gastric mechanisms of cholecystokinin-induced satiety. Am J Physiol Regul Integr Comp Physiol. 2006;291:R112–R114. doi: 10.1152/ajpregu.00159.2006. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Watanabe C, Mizumori M, Kaunitz J. Luminal L-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats. Am J Physiol Gastrointest Liver Physiol. 2009;297:G781–G791. doi: 10.1152/ajpgi.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol. 2002;2:650–656. doi: 10.1016/s1471-4892(02)00227-8. [DOI] [PubMed] [Google Scholar]
- Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252–259. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- Aramori I, Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992;8:757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- Atkinson TJ. Central and peripheral neuroendocrine peptides and signalling in appetite regulation: considerations for obesity pharmacotherapy. Obes Rev. 2008;9:108–120. doi: 10.1111/j.1467-789X.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- Attoub S, Levasseur S, Buyse M, Goiot H, Laigneau JP, Moizo L, et al. Physiological role of cholecystokinin B/gastrin receptor in leptin secretion. Endocrinology. 1999;140:4406–4410. doi: 10.1210/endo.140.10.7079. [DOI] [PubMed] [Google Scholar]
- Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Baranowska B, Radzikowska M, Wasilewska-Dziubinska E, Roguski K, Borowiec M. Disturbed release of gastrointestinal peptides in anorexia nervosa and in obesity. Diabetes Obes Metab. 2000;2:99–103. doi: 10.1046/j.1463-1326.2000.00070.x. [DOI] [PubMed] [Google Scholar]
- Barrera JG, Sandoval DA, D'Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev. 2011;7:507–516. doi: 10.1038/nrendo.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Becker JM, Kelly KA. Antral control of canine gastric emptying of solids. Am J Physiol. 1983;245:G334–G338. doi: 10.1152/ajpgi.1983.245.3.G334. [DOI] [PubMed] [Google Scholar]
- Beglinger C, Degen L, Matzinger D, D'Amato M, Drewe J. Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1149–R1154. doi: 10.1152/ajpregu.2001.280.4.R1149. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Kressel M, Neuhuber WL. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat Embryol (Berl) 1992;186:431–442. doi: 10.1007/BF00185458. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995;191:203–212. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- Bertrand PP. The cornucopia of intestinal chemosensory transduction. Front Neurosci. 2009;3:1–9. doi: 10.3389/neuro.21.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP, Bertrand RL. Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci. 2010;153:47–57. doi: 10.1016/j.autneu.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Bertrand RL, Senadheera S, Markus I, Liu L, Howitt L, Chen H, et al. A Western diet increases serotonin availability in rat small intestine. Endocrinology. 2011;152:36–47. doi: 10.1210/en.2010-0377. [DOI] [PubMed] [Google Scholar]
- Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- Bi S, Chen J, Behles RR, Hyun J, Kopin AS, Moran TH. Differential body weight and feeding responses to high-fat diets in rats and mice lacking cholecystokinin 1 receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293:R55–R63. doi: 10.1152/ajpregu.00002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Responses of vagal efferent fibres to stimulation of gastric mechano- and chemoreceptors in the anaesthetized ferret. J Auton Nerv Syst. 1989;27:39–45. doi: 10.1016/0165-1838(89)90127-6. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst. 1990;31:191–201. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D, Scratcherd T. Involvement of gastrointestinal mechano- and intestinal chemoreceptors in vagal reflexes: an electrophysiological study. J Auton Nerv Syst. 1987;18:225–234. doi: 10.1016/0165-1838(87)90121-4. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Bonini J, Anderson S, Steiner D. Molecular cloning and tissue expression of a novel orphan G protein-coupled receptor from rat lung. Biochem Biophys Res Commun. 1997;234:190–193. doi: 10.1006/bbrc.1997.6591. [DOI] [PubMed] [Google Scholar]
- Bozkurt A, Oktar BK, Kurtel H, Alican I, Coskun T, Yegen BC. Capsaicin-sensitive vagal fibres and 5-HT3-, gastrin releasing peptide- and cholecystokinin A-receptors are involved in distension-induced inhibition of gastric emptying in the rat. Regul Pept. 1999;83:81–86. doi: 10.1016/s0167-0115(99)00050-6. [DOI] [PubMed] [Google Scholar]
- Brener W, Hendrix TR, McHugh PR. Regulation of the gastric emptying of glucose. Gastroenterology. 1983;85:76–82. [PubMed] [Google Scholar]
- Brennan IM, Little TJ, Feltrin KL, Smout AJ, Wishart JM, Horowitz M, et al. Dose-dependent effects of cholecystokinin-8 on antropyloroduodenal motility, gastrointestinal hormones, appetite, and energy intake in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E1487–E1494. doi: 10.1152/ajpendo.90791.2008. [DOI] [PubMed] [Google Scholar]
- Briscoe C, Tadayyon M, Andrews J, Benson W, Chambers J, Eilert M, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- Broberger C, Hokfelt T. Hypothalamic and vagal neuropeptide circuitries regulating food intake. Physiol Behav. 2001;74:669–682. doi: 10.1016/s0031-9384(01)00611-4. [DOI] [PubMed] [Google Scholar]
- Broberger C, Holmberg K, Shi TJ, Dockray G, Hokfelt T. Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res. 2001;903:128–140. doi: 10.1016/s0006-8993(01)02468-4. [DOI] [PubMed] [Google Scholar]
- Brown A, Goldsworthy S, Barnes A, Eilert M, Tcheang L, Daniels D, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Bryant MG, Bloom SR, Polak JM, Hobbs S, Domschke W, Domschke S, et al. Measurement of gut hormonal peptides in biopsies from human stomach and proximal small intestine. Gut. 1983;24:114–119. doi: 10.1136/gut.24.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan AM, Squires PE, Ring M, Meloche RM. Mechanism of action of the calcium-sensing receptor in human antral gastrin cells. Gastroenterology. 2001;120:1128–1139. doi: 10.1053/gast.2001.23246. [DOI] [PubMed] [Google Scholar]
- Bucinskaite V, Tolessa T, Pedersen J, Rydqvist B, Zerihun L, Holst JJ, et al. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol Motil. 2009;21:e978–e978. doi: 10.1111/j.1365-2982.2009.01317.x. [DOI] [PubMed] [Google Scholar]
- Bueter M, le Roux CW. Gastrointestinal hormones, energy balance and bariatric surgery. Int J Obes (Lond) 2011;35:S35–S39. doi: 10.1038/ijo.2011.146. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Spiller D, Morris R, Lal S, Thompson DG, Saeed S, et al. Expression of the leptin receptor in rat and human nodose ganglion neurones. Neuroscience. 2002;109:339–347. doi: 10.1016/s0306-4522(01)00474-2. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Spiller D, Jiang W, Thompson D, Attwood S, et al. Localization of orexin-1 receptors to vagal afferent neurons in the rat and humans. Gastroenterology. 2003;124:129–139. doi: 10.1053/gast.2003.50020. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Feeding-dependent depression of melanin-concentrating hormone and melanin-concentrating hormone receptor-1 expression in vagal afferent neurones. Neuroscience. 2006a;137:1405–1415. doi: 10.1016/j.neuroscience.2005.10.057. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol. 2006b;290:G1289–G1297. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- Burdyga G, de Lartigue G, Raybould HE, Morris R, Dimaline R, Varro A, et al. Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J Neurosci. 2008;28:11583–11592. doi: 10.1523/JNEUROSCI.2493-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons: kinetics, and role in influencing neurochemical phenotype. Am J Physiol Gastrointest Liver Physiol. 2010;299:G63–G69. doi: 10.1152/ajpgi.00059.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R, Moniri N. Agonism with the omega-3 fatty acids alpha-linolenic acid and docosahexaenoic acid mediates phosphorylation of both the short and long isoforms of the human GPR120 receptor. Biochem Biophys Res Commun. 2010;396:1030–1035. doi: 10.1016/j.bbrc.2010.05.057. [DOI] [PubMed] [Google Scholar]
- Buyse M, Ovesjo ML, Goiot H, Guilmeau S, Peranzi G, Moizo L, et al. Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. Eur J Neurosci. 2001;14:64–72. doi: 10.1046/j.0953-816x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- Bystrova M, Romanov R, Rogachevskaja O, Churbanov G, Kolesnikov S. Functional expression of the extracellular-Ca2+-sensing receptor in mouse taste cells. J Cell Sci. 2010;123:972–982. doi: 10.1242/jcs.061879. [DOI] [PubMed] [Google Scholar]
- Calapai G, Corica F, Corsonello A, Sautebin L, Di Rosa M, Campo GM, et al. Leptin increases serotonin turnover by inhibition of brain nitric oxide synthesis. J Clin Invest. 1999;104:975–982. doi: 10.1172/JCI5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Caminos JE, Bravo SB, Garcia-Rendueles ME, Ruth Gonzalez C, Garces MF, Cepeda LA, et al. Expression of neuropeptide W in rat stomach mucosa: regulation by nutritional status, glucocorticoids and thyroid hormones. Regul Pept. 2008;146:106–111. doi: 10.1016/j.regpep.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, et al. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30:8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- Chang H, Yoo B, Lim S, Jeong S, Kim W, Park J. Metabotropic glutamate receptor 4 expression in colorectal carcinoma and its prognostic significance. Clin Cancer Res. 2005;11:3288–3295. doi: 10.1158/1078-0432.CCR-04-1912. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Yang H, Lamp C, Delay E, Cartford C, Than T, et al. The taste of monosodium glutamate: membrane receptors in taste buds. J Neurosci. 1996;16:3817–3826. doi: 10.1523/JNEUROSCI.16-12-03817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara K, Arimura A, Schally AV. Immunoreactive somatostatin in rat hypophyseal portal blood: effects of anesthetics. Endocrinology. 1979;104:1434–1441. doi: 10.1210/endo-104-5-1434. [DOI] [PubMed] [Google Scholar]
- Cho YM, Kieffer TJ. K-cells and glucose-dependent insulinotropic polypeptide in health and disease. Vitam Horm. 2011;84:111–150. doi: 10.1016/B978-0-12-381517-0.00004-7. [DOI] [PubMed] [Google Scholar]
- Choi S, Lee M, Shiu AL, Yo SJ, Aponte GW. Identification of a protein hydrolysate responsive G protein-coupled receptor in enterocytes. Am J Physiol Gastrointest Liver Physiol. 2007a;292:G98–112. doi: 10.1152/ajpgi.00295.2006. [DOI] [PubMed] [Google Scholar]
- Choi S, Lee M, Shiu AL, Yo SJ, Hallden G, Aponte GW. GPR93 activation by protein hydrolysate induces CCK transcription and secretion in STC-1 cells. Am J Physiol Gastrointest Liver Physiol. 2007b;292:G1366–G1375. doi: 10.1152/ajpgi.00516.2006. [DOI] [PubMed] [Google Scholar]
- Chu Z, Carroll C, Chen R, Alfonso J, Gutierrez V, He H, et al. N-oleoyldopamine enhances glucose homeostasis through the activation of GPR119. Mol Endocrinol. 2010;24:161–170. doi: 10.1210/me.2009-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S, Matteis RD, Pico C, Ceresi E, Obrador A, Maffeis C, et al. Secretory granules of endocrine and chief cells of human stomach mucosa contain leptin. Int J Obes Relat Metab Disord. 2000;24:789–793. doi: 10.1038/sj.ijo.0801228. [DOI] [PubMed] [Google Scholar]
- Clarke GD, Davison JS. Tension receptors in the oesophagus and stomach of the rat. J Physiol. 1975;244:41P–42P. [PubMed] [Google Scholar]
- Clarke GD, Davison JS. Mucosal receptors in the gastric antrum and small intestine of the rat with afferent fibres in the cervical vagus. J Physiol. 1978;284:55–67. doi: 10.1113/jphysiol.1978.sp012527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Quinn SJ, Brown EM. L-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc Natl Acad Sci U S A. 2000;97:4814–4819. doi: 10.1073/pnas.97.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell DF, Iggo A. The responses of duodenal tension receptors in sheep to pentagastrin, cholecystokinin and some other drugs. J Physiol. 1984a;354:477–495. doi: 10.1113/jphysiol.1984.sp015389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell DF, Iggo A. Tension receptors with vagal afferent fibres in the proximal duodenum and pyloric sphincter of sheep. J Physiol. 1984b;354:457–475. doi: 10.1113/jphysiol.1984.sp015388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covasa M, Ritter RC. Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin. Peptides. 1998;19:1407–1415. doi: 10.1016/s0196-9781(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Covasa M, Marcuson JK, Ritter RC. Diminished satiation in rats exposed to elevated levels of endogenous or exogenous cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 2001;280:R331–R337. doi: 10.1152/ajpregu.2001.280.2.R331. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Beinfeld MC. Rapid development of tolerance to the behavioural actions of cholecystokinin. Nature. 1983;302:703–706. doi: 10.1038/302703a0. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KM, Daly J, Horowitz M, Read NW. Gastrointestinal adaptation to diets of differing fat composition in human volunteers. Gut. 1991;32:483–486. doi: 10.1136/gut.32.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech DA. Possible involvement of nitric oxide in chlordiazepoxide-induced feeding in the mouse. Pharmacol Biochem Behav. 1996;55:327–331. doi: 10.1016/s0091-3057(96)00101-3. [DOI] [PubMed] [Google Scholar]
- Czech DA. A nitric oxide synthase inhibitor NG-nitro-L-arginine, attenuates glucoprivic feeding and deprivation-induced drinking in the mouse. Pharmacol Biochem Behav. 1998;60:601–607. doi: 10.1016/s0091-3057(98)00016-1. [DOI] [PubMed] [Google Scholar]
- Czech DA, Klosterman AE, Le Sueur KT. N(G)-nitro-L-arginine methyl ester reduces stress-related feeding in the rat tail-pinch model. Pharmacol Biochem Behav. 1998;60:91–96. doi: 10.1016/s0091-3057(97)00551-0. [DOI] [PubMed] [Google Scholar]
- Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol. 2011;589:2857–2870. doi: 10.1113/jphysiol.2010.204594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcel NP, Liou AP, Tome D, Raybould HE. Activation of vagal afferents in the rat duodenum by protein digests requires PepT1. J Nutr. 2005;135:1491–1495. doi: 10.1093/jn/135.6.1491. [DOI] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- Daughters RS, Hofbauer RD, Grossman AW, Marshall AM, Brown EM, Hartman BK, et al. Ondansetron attenuates CCK induced satiety and c-fos labeling in the dorsal medulla. Peptides. 2001;22:1331–1338. doi: 10.1016/s0196-9781(01)00460-0. [DOI] [PubMed] [Google Scholar]
- Davison JS. Response of single vagal afferent fibres to mechanical and chemical stimulation of the gastric and duodenal mucosa in cats. Q J Exp Physiol Cogn Med Sci. 1972;57:405–416. doi: 10.1113/expphysiol.1972.sp002176. [DOI] [PubMed] [Google Scholar]
- De Lartigue G, Dimaline R, Varro A, Raybould H, De la Serre CB, Dockray GJ. Cocaine- and amphetamine-regulated transcript mediates the actions of cholecystokinin on rat vagal afferent neurons. Gastroenterology. 2011;138:1479–1490. doi: 10.1053/j.gastro.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel MJ, Burrell MA. Immunocytochemical detection of orexin A in endocrine cells of the developing mouse gut. J Histochem Cytochem. 2002;50:63–69. doi: 10.1177/002215540205000107. [DOI] [PubMed] [Google Scholar]
- Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol. 1996;271:E458–E464. doi: 10.1152/ajpendo.1996.271.3.E458. [DOI] [PubMed] [Google Scholar]
- Dockray GJ, Burdyga G. Plasticity in vagal afferent neurones during feeding and fasting: mechanisms and significance. Acta Physiol (Oxf) 2011;201:313–321. doi: 10.1111/j.1748-1716.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- Donovan MJ, Paulino G, Raybould HE. Activation of hindbrain neurons in response to gastrointestinal lipid is attenuated by high fat, high energy diets in mice prone to diet-induced obesity. Brain Res. 2009;1248:136–140. doi: 10.1016/j.brainres.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ, Asa S. Glucagon gene expression in vertebrate brain. J Biol Chem. 1988;263:13475–13478. [PubMed] [Google Scholar]
- Dufner M, Kirchhoff P, Remy C, Hafner P, Müller M, Cheng S, et al. The calcium-sensing receptor acts as a modulator of gastric acid secretion in freshly isolated human gastric glands. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1084–G1090. doi: 10.1152/ajpgi.00571.2004. [DOI] [PubMed] [Google Scholar]
- Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates FFA stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edholm T, Degerblad M, Gryback P, Hilsted L, Holst JJ, Jacobsson H, et al. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterol Motil. 2011;22:1191–1200. doi: 10.1111/j.1365-2982.2010.01554.x. [DOI] [PubMed] [Google Scholar]
- Eissele R, Goke R, Willemer S, Harthus HP, Vermeer H, Arnold R, et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest. 1992;22:283–291. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology. 2007;132:2253–2271. doi: 10.1053/j.gastro.2007.03.057. [DOI] [PubMed] [Google Scholar]
- Elliott LA, O'Donnell TA, Isaacs NJ, Blackshaw LA, Page AJ. Opposite effects of nitric oxide on peripheral vagal afferents in feeding and fasting. Gastroenterology. 2011;140:S833–S834. [Google Scholar]
- Falken Y, Hellstrom PM, Sanger GJ, Dewit O, Dukes G, Gryback P, et al. Actions of prolonged ghrelin infusion on gastrointestinal transit and glucose homeostasis in humans. Neurogastroenterol Motil. 2010;22:e192–e200. doi: 10.1111/j.1365-2982.2009.01463.x. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Kumar VB, Morley JE. Orexin-A-induced feeding is dependent on nitric oxide. Peptides. 2005;26:759–765. doi: 10.1016/j.peptides.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Feltrin KL, Little TJ, Meyer JH, Horowitz M, Smout AJ, Wishart J, et al. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol. 2004;287:R524–R533. doi: 10.1152/ajpregu.00039.2004. [DOI] [PubMed] [Google Scholar]
- Ferry S, Traiffort E, Stinnakre J, Ruat M. Developmental and adult expression of rat calcium-sensing receptor transcripts in neurons and oligodendrocytes. Eur J Neurosci. 2000;12:872–884. doi: 10.1046/j.1460-9568.2000.00980.x. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Byrne LC, Hassani H, Ernfors P, Hokfelt T. Characterization of neuropeptide Y Y2 and Y5 receptor expression in the mouse hypothalamus. J Comp Neurol. 2004;470:256–265. doi: 10.1002/cne.11047. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Burton MJ. The anorectic action of peripherally administered 5-HT is enhanced by vagotomy. Physiol Behav. 1985;34:861–866. doi: 10.1016/0031-9384(85)90004-6. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Burton MJ. Microstructural analysis of the anorectic action of peripherally administered 5-HT. Pharmacol Biochem Behav. 1986;24:1133–1136. doi: 10.1016/0091-3057(86)90467-3. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord. 2001;25:781–792. doi: 10.1038/sj.ijo.0801627. [DOI] [PubMed] [Google Scholar]
- Flock G, Holland D, Seino Y, Drucker D. GPR119 regulates murine glucose homeostasis through incretin receptor-dependent and independent mechanisms. Endocrinology. 2011;152:374–383. doi: 10.1210/en.2010-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor P, Lukic S, Rüegg D, Leonhardt T, Knöpfel T, Kuhn R. Molecular cloning, functional expression and pharmacological characterization of the human metabotropic glutamate receptor type 4. Neuropharmacology. 1995;34:149–155. doi: 10.1016/0028-3908(94)00149-m. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Höglund P, Gloriam D, Lagerström M, Schiöth H. Seven evolutionarily conserved human rhodopsin G protein-coupled receptors lacking close relatives. FEBS Lett. 2003;554:381–388. doi: 10.1016/s0014-5793(03)01196-7. [DOI] [PubMed] [Google Scholar]
- French SJ, Cecil JE. Oral, gastric and intestinal influences on human feeding. Physiol Behav. 2001;74:729–734. doi: 10.1016/s0031-9384(01)00617-5. [DOI] [PubMed] [Google Scholar]
- Fried M, Erlacher U, Schwizer W, Lochner C, Koerfer J, Beglinger C, et al. Role of cholecystokinin in the regulation of gastric emptying and pancreatic enzyme secretion in humans. Studies with the cholecystokinin-receptor antagonist loxiglumide. Gastroenterology. 1991;101:503–511. doi: 10.1016/0016-5085(91)90031-f. [DOI] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- Furness JB, Koopmans HS, Robbins HL, Clerc N, Tobin JM, Morris MJ. Effects of vagal and splanchnic section on food intake, weight, serum leptin and hypothalamic neuropeptide Y in rat. Auton Neurosci. 2001;92:28–36. doi: 10.1016/S1566-0702(01)00311-3. [DOI] [PubMed] [Google Scholar]
- Gaskin FS, Farr SA, Banks WA, Kumar VB, Morley JE. Ghrelin-induced feeding is dependent on nitric oxide. Peptides. 2003;24:913–918. doi: 10.1016/s0196-9781(03)00160-8. [DOI] [PubMed] [Google Scholar]
- Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J. Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions. J Comp Neurol. 2010;518:6–24. doi: 10.1002/cne.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter A. Gastric distension and gastric capacity in relation to food intake in humans. Physiol Behav. 1988;44:665–668. doi: 10.1016/0031-9384(88)90333-2. [DOI] [PubMed] [Google Scholar]
- Gerspach AC, Steinert RE, Schonenberger L, Graber-Maier A, Beglinger C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metab. 2011;301:E317–E325. doi: 10.1152/ajpendo.00077.2011. [DOI] [PubMed] [Google Scholar]
- Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR, et al. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology. 2002;123:217–226. doi: 10.1053/gast.2002.34245. [DOI] [PubMed] [Google Scholar]
- Glatzle J, Wang Y, Adelson DW, Kalogeris TJ, Zittel TT, Tso P, et al. Chylomicron components activate duodenal vagal afferents via a cholecystokinin A receptor-mediated pathway to inhibit gastric motor function in the rat. J Physiol. 2003;550:657–664. doi: 10.1113/jphysiol.2003.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Görcs T, Penke B, Böti Z, Katarova Z, Hámori J. Immunohistochemical visualization of a metabotropic glutamate receptor. Neuroreport. 1993;4:283–286. doi: 10.1097/00001756-199303000-00014. [DOI] [PubMed] [Google Scholar]
- Grundy D. Serotonin and sensory signalling from the gastrointestinal lumen. J Physiol (Lond) 2006;575:1–2. doi: 10.1113/jphysiol.2006.113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D. 5-HT system in the gut: roles in the regulation of visceral sensitivity and motor functions. Eur Rev Med Pharmacol Sci. 2008;12:63–67. [PubMed] [Google Scholar]
- Haid D, Widmayer P, Breer H. Nutrient sensing receptors in gastric endocrine cells. J Mol Histol. 2011;42:355–364. doi: 10.1007/s10735-011-9339-1. [DOI] [PubMed] [Google Scholar]
- Hansen K, Rosenkilde M, Knop F, Wellner N, Diep T, Rehfeld J, et al. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96:E1409–E1417. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]
- Hansotia T, Maida A, Flock G, Yamada Y, Tsukiyama K, Seino Y, et al. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest. 2007;117:143–152. doi: 10.1172/JCI25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass N, Schwarzenbacher K, Breer H. T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res. 2010;339:493–504. doi: 10.1007/s00441-009-0907-6. [DOI] [PubMed] [Google Scholar]
- Hayes MR, Chory FM, Gallagher CA, Covasa M. Serotonin type-3 receptors mediate cholecystokinin-induced satiation through gastric distension. Am J Physiol Regul Integr Comp Physiol. 2006;291:R115–R123. doi: 10.1152/ajpregu.00002.2006. [DOI] [PubMed] [Google Scholar]
- Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149:4059–4068. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav. 2010;100:503–510. doi: 10.1016/j.physbeh.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Kanoski SE, De Jonghe BC, Leichner TM, Alhadeff AL, Fortin SM, et al. The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1479–R1485. doi: 10.1152/ajpregu.00356.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helou N, Obeid O, Azar ST, Hwalla N. Variation of postprandial PYY 3-36 response following ingestion of differing macronutrient meals in obese females. Ann Nutr Metab. 2008;52:188–195. doi: 10.1159/000138122. [DOI] [PubMed] [Google Scholar]
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- Hoang CJ, Hay M. Expression of metabotropic glutamate receptors in nodose ganglia and the nucleus of the solitary tract. Am J Physiol Heart Circ Physiol. 2001;281:H457–H462. doi: 10.1152/ajpheart.2001.281.1.H457. [DOI] [PubMed] [Google Scholar]
- Holmes GM, Browning KN, Tong M, Qualls-Creekmore E, Travagli RA. Vagally mediated effects of glucagon-like peptide 1: in vitro and in vivo gastric actions. J Physiol. 2009;587:4749–4759. doi: 10.1113/jphysiol.2009.175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Honda T, Wada E, Battey JF, Wank SA. Differential gene expression of CCK(A) and CCK(B) receptors in the rat brain. Mol Cell Neurosci. 1993;4:143–154. doi: 10.1006/mcne.1993.1018. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Nauck MA. To be or not to be – an incretin or enterogastrone? Gut. 2006;55:148–150. doi: 10.1136/gut.2005.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houpt TR, Houpt KA, Swan AA. Duodenal osmoconcentration and food intake in pigs after ingestion of hypertonic nutrients. Am J Physiol. 1983;245:R181–R189. doi: 10.1152/ajpregu.1983.245.2.R181. [DOI] [PubMed] [Google Scholar]
- Hunt JN, Knox MT. A relation between the chain length of fatty acids and the slowing of gastric emptying. J Physiol. 1968;194:327–336. doi: 10.1113/jphysiol.1968.sp008411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955;128:593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A. The electrophysiological identification of single nerve fibres, with particular reference to the slowest-conducting vagal afferent fibres in the cat. J Physiol. 1958;142:110–126. doi: 10.1113/jphysiol.1958.sp006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol. 1997;273:G920–G927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci. 2001;2:551–560. doi: 10.1038/35086018. [DOI] [PubMed] [Google Scholar]
- Ishii M, Fei H, Friedman JM. Targeted disruption of GPR7, the endogenous receptor for neuropeptides B and W, leads to metabolic defects and adult-onset obesity. Proc Natl Acad Sci U S A. 2003;100:10540–10545. doi: 10.1073/pnas.1334189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger A, Grahn J, Ritter RC. Reduced vagal sensory innervation of the small intestinal myenteric plexus following capsaicin treatment of adult rats. Neurosci Lett. 1997;236:103–106. doi: 10.1016/s0304-3940(97)00767-2. [DOI] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinich C, Mak N, Pacheco I, Mulder D, Wells R, Blennerhassett M, et al. The extracellular calcium-sensing receptor (CaSR) on human esophagus and evidence of expression of the CaSR on the esophageal epithelial cell line (HET-1A) Am J Physiol Gastrointest Liver Physiol. 2008;294:G120–G129. doi: 10.1152/ajpgi.00226.2006. [DOI] [PubMed] [Google Scholar]
- Kaemmerer E, Plum P, Klaus C, Weiskirchen R, Liedtke C, Adolf M, et al. Fatty acid binding receptors in intestinal physiology and pathophysiology. World J Gastrointest Pathophysiol. 2010;1:147–153. doi: 10.4291/wjgp.v1.i5.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji I, Karaki S, Tanaka R, Kuwahara A. Density distribution of free fatty acid receptor 2 (FFA2)-expressing and GLP-1-producing enteroendocrine L cells in human and rat lower intestine, and increased cell numbers after ingestion of fructo-oligosaccharide. J Mol Histol. 2011;42:27–38. doi: 10.1007/s10735-010-9304-4. [DOI] [PubMed] [Google Scholar]
- Kakei M, Yada T, Nakagawa A, Nakabayashi H. Glucagon-like peptide-1 evokes action potentials and increases cytosolic Ca2+ in rat nodose ganglion neurons. Auton Neurosci. 2002;102:39–44. doi: 10.1016/s1566-0702(02)00182-0. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152:3103–3112. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, et al. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–360. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- Karra E, Chandarana K, Batterham RL. The role of peptide YY in appetite regulation and obesity. J Physiol. 2009;587:19–25. doi: 10.1113/jphysiol.2008.164269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish S, Li H, Philp LK, O'Donnell TA, Isaacs NJ, Young RL, et al. Diet induced adaptation of vagal afferent function. J Physiol. 2011;590((Pt 1)):209–221. doi: 10.1113/jphysiol.2011.222158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Camilleri M, Murray JA, Stephens DA, Levine JA, Burton DD. Is there a role for gastric accommodation and satiety in asymptomatic obese people? Obes Res. 2001;9:655–661. doi: 10.1038/oby.2001.89. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Nian C, McIntosh CH. Activation of lipoprotein lipase by glucose-dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP-activated protein kinase cascade. J Biol Chem. 2007;282:8557–8567. doi: 10.1074/jbc.M609088200. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Liu M. Orexin synthesis and response in the gut. Neuron. 1999;24:941–951. doi: 10.1016/s0896-6273(00)81041-7. [DOI] [PubMed] [Google Scholar]
- Koda S, Date Y, Murakami N, Shimbara T, Hanada T, Toshinai K, et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- Kotarsky K, Nilsson N, Flodgren E, Owman C, Olde B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem Biophys Res Commun. 2003;301:406–410. doi: 10.1016/s0006-291x(02)03064-4. [DOI] [PubMed] [Google Scholar]
- Kotarsky K, Boketoft A, Bristulf J, Nilsson N, Norberg A, Hansson S, et al. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- Kristensen P, Suzdak P, Thomsen C. Expression pattern and pharmacology of the rat type IV metabotropic glutamate receptor. Neurosci Lett. 1993;155:159–162. doi: 10.1016/0304-3940(93)90697-j. [DOI] [PubMed] [Google Scholar]
- de Krom M, van der Schouw YT, Hendriks J, Ophoff RA, van Gils CH, Stolk RP, et al. Common genetic variations in CCK, leptin, and leptin receptor genes are associated with specific human eating patterns. Diabetes. 2007;56:276–280. doi: 10.2337/db06-0473. [DOI] [PubMed] [Google Scholar]
- Kumar KG, Byerley LO, Volaufova J, Drucker DJ, Churchill GA, Li R, et al. Genetic variation in Glp1r expression influences the rate of gastric emptying in mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R362–R371. doi: 10.1152/ajpregu.00640.2007. [DOI] [PubMed] [Google Scholar]
- Lacourse KA, Swanberg LJ, Gillespie PJ, Rehfeld JF, Saunders TL, Samuelson LC. Pancreatic function in CCK-deficient mice: adaptation to dietary protein does not require CCK. Am J Physiol. 1999;276:G1302–G1309. doi: 10.1152/ajpgi.1999.276.5.G1302. [DOI] [PubMed] [Google Scholar]
- Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;281:G907–G915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- Lang PM, Grafe P. Chemosensitivity of unmyelinated axons in isolated human gastric vagus nerve. Auton Neurosci. 2007;136:100–104. doi: 10.1016/j.autneu.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Laskiewicz J, Krolczyk G, Zurowski G, Sobocki J, Matyja A, Thor PJ. Effects of vagal neuromodulation and vagotomy on control of food intake and body weight in rats. J Physiol Pharmacol. 2003;54:603–610. [PubMed] [Google Scholar]
- Lassman DJ, McKie S, Gregory LJ, Lal S, D'Amato M, Steele I, et al. Defining the role of cholecystokinin in the lipid-induced human brain activation matrix. Gastroenterology. 2010;138:1514–1524. doi: 10.1053/j.gastro.2009.12.060. [DOI] [PubMed] [Google Scholar]
- Leckstrom A, Kim ER, Wong D, Mizuno TM. Xenin, a gastrointestinal peptide, regulates feeding independent of the melanocortin signaling pathway. Diabetes. 2009;58:87–94. doi: 10.2337/db08-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AS, Winsky-Sommerer R, Huitron-Resendiz S, Grace MK, de Lecea L. Injection of neuropeptide W into paraventricular nucleus of hypothalamus increases food intake. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1727–R1732. doi: 10.1152/ajpregu.00638.2003. [DOI] [PubMed] [Google Scholar]
- Li H, Kentish S, O'Donnell TA, Witten G, Blackshaw LA, Page AJ. Modulatory effect of NPW on mechanosensitivity of vagal afferents in obesity. Gastroenterology. 2011;140:S34–S34. [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hao Y, Zhu J, Owyang C. Serotonin released from intestinal enterochromaffin cells mediates luminal non-cholecystokinin-stimulated pancreatic secretion in rats. Gastroenterology. 2000;118:1197–1207. doi: 10.1016/s0016-5085(00)70373-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu XY, Owyang C. Serotonin and cholecystokinin synergistically stimulate rat vagal primary afferent neurones. J Physiol (Lond) 2004;559:651–662. doi: 10.1113/jphysiol.2004.064816. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985;75:1144–1152. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieverse RJ, Jansen JB, Masclee AA, Rovati LC, Lamers CB. Effect of a low dose of intraduodenal fat on satiety in humans: studies using the type A cholecystokinin receptor antagonist loxiglumide. Gut. 1994;35:501–505. doi: 10.1136/gut.35.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou AP, Chavez DI, Espero E, Hao S, Wank SA, Raybould HE. Protein hydrolysate-induced cholecystokinin secretion from enteroendocrine cells is indirectly mediated by the intestinal oligopeptide transporter PepT1. Am J Physiol Gastrointest Liver Physiol. 2011;300:G895–G902. doi: 10.1152/ajpgi.00521.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TJ, Feinle-Bisset C. Effects of dietary fat on appetite and energy intake in health and obesity – oral and gastrointestinal sensory contributions. Physiol Behav. 2011a;104:613–620. doi: 10.1016/j.physbeh.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Little TJ, Feinle-Bisset C. Oral and gastrointestinal sensing of dietary fat and appetite regulation in humans: modification by diet and obesity. Front Neurosci. 2011b;4:1–9. doi: 10.3389/fnins.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TJ, Doran S, Meyer JH, Smout AJ, O'Donovan DG, Wu KL, et al. The release of GLP-1 and ghrelin, but not GIP and CCK, by glucose is dependent upon the length of small intestine exposed. Am J Physiol Endocrinol Metab. 2006;291:E647–E655. doi: 10.1152/ajpendo.00099.2006. [DOI] [PubMed] [Google Scholar]
- Little TJ, Gopinath A, Patel E, McGlone A, Lassman DJ, d'amato M, et al. Gastric emptying of hexose sugars: role of osmolality, molecular structure and the CCK receptor. Neurogastroenterol Motil. 2010;22:1183–1190. doi: 10.1111/j.1365-2982.2010.01552.x. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111–121. doi: 10.1016/j.neuroscience.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CM, Ma L, Zhang DM, Lee R, Qin A, Liu M, et al. Mechanism of the induction of brain c-Fos-positive neurons by lipid absorption. Am J Physiol Regul Integr Comp Physiol. 2007;292:R268–R273. doi: 10.1152/ajpregu.00334.2006. [DOI] [PubMed] [Google Scholar]
- Lo CM, King A, Samuelson LC, Kindel TL, Rider T, Jandacek RJ, et al. Cholecystokinin knockout mice are resistant to high-fat diet-induced obesity. Gastroenterology. 2011;138:1997–2005. doi: 10.1053/j.gastro.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz DN. Effects of gastric filling and vagotomy on ingestion, nipple attachment, and weight gain by suckling rats. Dev Psychobiol. 1983;16:469–483. doi: 10.1002/dev.420160603. [DOI] [PubMed] [Google Scholar]
- Luo J, Liu Z, Liu J, Eugene C. Distribution pattern of GPRC6A mRNA in mouse tissue by in situ hybridization. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:1–10. doi: 10.3969/j.issn.1672-7347.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Ma D, Tao B, Warashina S, Kotani S, Lu L, Kaplamadzhiev D, et al. Expression of free fatty acid receptor GPR40 in the central nervous system of adult monkeys. Neurosci Res. 2007;58:394–401. doi: 10.1016/j.neures.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen AN, Hansen G, Paulsen SJ, Lykkegaard K, Tang-Christensen M, Hansen HS, et al. Long-term characterization of the diet-induced obese and diet-resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J Endocrinol. 2011;206:287–296. doi: 10.1677/JOE-10-0004. [DOI] [PubMed] [Google Scholar]
- Makoff A, Lelchuk R, Oxer M, Harrington K, Emson P. Molecular characterization and localization of human metabotropic glutamate receptor type 4. Brain Res Mol Brain Res. 1996;37:239–248. doi: 10.1016/0169-328x(95)00321-i. [DOI] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, Blackstone C, Huganir R, Price D. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992;9:259–270. doi: 10.1016/0896-6273(92)90165-a. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Miki T, Jhomori T, Gonoi T, Seino S. Possible role of PEPT1 in gastrointestinal hormone secretion. Biochem Biophys Res Commun. 2005;336:1028–1032. doi: 10.1016/j.bbrc.2005.08.259. [DOI] [PubMed] [Google Scholar]
- Matsumura S, Mizushige T, Yoneda T, Iwanaga T, Tsuzuki S, Inoue K, et al. GPR expression in the rat taste bud relating to fatty acid sensing. Biomed Res. 2007;28:49–55. doi: 10.2220/biomedres.28.49. [DOI] [PubMed] [Google Scholar]
- Matzinger D, Degen L, Drewe J, Meuli J, Duebendorfer R, Ruckstuhl N, et al. The role of long chain fatty acids in regulating food intake and cholecystokinin release in humans. Gut. 2000;46:688–693. doi: 10.1136/gut.46.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mau M, Mielenz M, Südekum K, Obukhov A. Expression of GPR30 and GPR43 in oral tissues: deriving new hypotheses on the role of diet in animal physiology and the development of oral cancers. J Anim Physiol Anim Nutr (Berl) 2011;95:280–285. doi: 10.1111/j.1439-0396.2010.01052.x. [DOI] [PubMed] [Google Scholar]
- McIntyre A, Vincent RM, Perkins AC, Spiller RC. Effect of bran, ispaghula, and inert plastic particles on gastric emptying and small bowel transit in humans: the role of physical factors. Gut. 1997;40:223–227. doi: 10.1136/gut.40.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, Grazia Luca M, Jones MN, D'Amato M, Dockray GJ, Thompson DG. Fatty acid chain length determines cholecystokinin secretion and effect on human gastric motility. Gastroenterology. 1999;116:46–53. doi: 10.1016/s0016-5085(99)70227-1. [DOI] [PubMed] [Google Scholar]
- Mei N. Vagal glucoreceptors in the small intestine of the cat. J Physiol. 1978;282:485–506. doi: 10.1113/jphysiol.1978.sp012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei N, Garnier L. Osmosensitive vagal receptors in the small intestine of the cat. J Auton Nerv Syst. 1986;16:159–170. doi: 10.1016/0165-1838(86)90022-6. [DOI] [PubMed] [Google Scholar]
- Mei N, Perrin J, Crousillat J, Boyer A. Comparison between the properties of the vagal and splanchnic glucoreceptors of the small intestine. Involvement in insulin release. J Auton Nerv Syst. 1984;10:275–278. doi: 10.1016/0165-1838(84)90024-9. [DOI] [PubMed] [Google Scholar]
- Meier JJ, Goetze O, Anstipp J, Hagemann D, Holst JJ, Schmidt WE, et al. Gastric inhibitory polypeptide does not inhibit gastric emptying in humans. Am J Physiol Endocrinol Metab. 2004;286:E621–E625. doi: 10.1152/ajpendo.00499.2003. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Miyauchi S, Hirasawa A, Iga T, Liu N, Itsubo C, Sadakane K, et al. Distribution and regulation of protein expression of the free fatty acid receptor GPR120. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:427–434. doi: 10.1007/s00210-008-0390-8. [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8:738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- Mondal MS, Yamaguchi H, Date Y, Shimbara T, Toshinai K, Shimomura Y, et al. A role for neuropeptide W in the regulation of feeding behavior. Endocrinology. 2003;144:4729–4733. doi: 10.1210/en.2003-0536. [DOI] [PubMed] [Google Scholar]
- Mondal MS, Yamaguchi H, Date Y, Toshinai K, Kawagoe T, Tsuruta T, et al. Neuropeptide W is present in antral G cells of rat, mouse, and human stomach. J Endocrinol. 2006;188:49–57. doi: 10.1677/joe.1.06195. [DOI] [PubMed] [Google Scholar]
- Morales M, Wang SD. Differential composition of 5-hydroxytryptamine3 receptors synthesized in the rat CNS and peripheral nervous system. J Neurosci. 2002;22:6732–6741. doi: 10.1523/JNEUROSCI.22-15-06732.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Daly K, et al. Expression of Na+/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Br J Nutr. 2010a;104:637–646. doi: 10.1017/S0007114510000917. [DOI] [PubMed] [Google Scholar]
- Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Ionescu C, et al. Expression of Na+/glucose co-transporter 1 (SGLT1) in the intestine of piglets weaned to different concentrations of dietary carbohydrate. Br J Nutr. 2010b;104:647–655. doi: 10.1017/S0007114510000954. [DOI] [PubMed] [Google Scholar]
- Moran TH, Smith GP, Hostetler AM, McHugh PR. Transport of cholecystokinin (CCK) binding sites in subdiaphragmatic vagal branches. Brain Res. 1987;415:149–152. doi: 10.1016/0006-8993(87)90278-2. [DOI] [PubMed] [Google Scholar]
- Moran TH, Ameglio PJ, Schwartz GJ, McHugh PR. Blockade of type A, not type B, CCK receptors attenuates satiety actions of exogenous and endogenous CCK. Am J Physiol. 1992;262:R46–R50. doi: 10.1152/ajpregu.1992.262.1.R46. [DOI] [PubMed] [Google Scholar]
- Moran TH, Kornbluh R, Moore K, Schwartz GJ. Cholecystokinin inhibits gastric emptying and contracts the pyloric sphincter in rats by interacting with low affinity CCK receptor sites. Regul Pept. 1994;52:165–172. doi: 10.1016/0167-0115(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Morley JE, Alshaher MM, Farr SA, Flood JF, Kumar VB. Leptin and neuropeptide Y (NPY) modulate nitric oxide synthase: further evidence for a role of nitric oxide in feeding. Peptides. 1999;20:595–600. doi: 10.1016/s0196-9781(99)00012-1. [DOI] [PubMed] [Google Scholar]
- Morley JE, Farr SA, Sell RL, Hileman SM, Banks WA. Nitric oxide is a central component in neuropeptide regulation of appetite. Peptides. 2011;32:776–780. doi: 10.1016/j.peptides.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Nagell CF, Wettergren A, Ørskov C, Holst JJ. Inhibitory effect of GLP-1 on gastric motility persists after vagal deafferentation in pigs. Scand J Gastroenterol. 2006;41:667–672. doi: 10.1080/00365520500408253. [DOI] [PubMed] [Google Scholar]
- Nakabayashi M, Suzuki T, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, et al. Orexin-A expression in human peripheral tissues. Mol Cell Endocrinol. 2003;205:43–50. doi: 10.1016/s0303-7207(03)00206-5. [DOI] [PubMed] [Google Scholar]
- Nakade Y, Tsukamoto K, Pappas TN, Takahashi T. Central glucagon like peptide-1 delays solid gastric emptying via central CRF and peripheral sympathetic pathway in rats. Brain Res. 2006;1111:117–121. doi: 10.1016/j.brainres.2006.06.090. [DOI] [PubMed] [Google Scholar]
- Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, et al. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE. 2009;4:e5106. doi: 10.1371/journal.pone.0005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Hassani H, Canals JM, Ekstrand AJ, Larefalk A, Chhajlani V, et al. Normal feeding behavior, body weight and leptin response require the neuropeptide Y Y2 receptor. Nat Med. 1999;5:1188–1193. doi: 10.1038/13514. [DOI] [PubMed] [Google Scholar]
- Neary NM, Small CJ, Druce MR, Park AJ, Ellis SM, Semjonous NM, et al. Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology. 2005;146:5120–5127. doi: 10.1210/en.2005-0237. [DOI] [PubMed] [Google Scholar]
- Nefti W, Chaumontet C, Fromentin G, Tome D, Darcel N. A high-fat diet attenuates the central response to within-meal satiation signals and modifies the receptor expression of vagal afferents in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1681–R1686. doi: 10.1152/ajpregu.90733.2008. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL. Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J Auton Nerv Syst. 1987;20:243–255. doi: 10.1016/0165-1838(87)90153-6. [DOI] [PubMed] [Google Scholar]
- Nilsson N, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047–1052. doi: 10.1016/s0006-291x(03)00488-1. [DOI] [PubMed] [Google Scholar]
- Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. 2010;11:19–30. doi: 10.1111/j.1467-789X.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Nakabayashi H, Uchida K, Nakagawa A, Niijima A. The hepatic vagal nerve is receptive to incretin hormone glucagon-like peptide-1, but not to glucose-dependent insulinotropic polypeptide, in the portal vein. J Auton Nerv Syst. 1996;61:149–154. doi: 10.1016/s0165-1838(96)00071-9. [DOI] [PubMed] [Google Scholar]
- Oh D, Yoon J, Moon M, Hwang J, Choe H, Lee J, et al. Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92. J Biol Chem. 2008;283:21054–21064. doi: 10.1074/jbc.M708908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton H, Babbs A, Doel S, Fyfe M, Gardner L, Griffin G, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Gebhart GF. Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1449–G1459. doi: 10.1152/ajpgi.2001.281.6.G1449. [DOI] [PubMed] [Google Scholar]
- Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol (Lond) 1998;512:907–916. doi: 10.1111/j.1469-7793.1998.907bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002;87:2095–2103. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, et al. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Slattery JA, Milte C, Laker R, O'Donnell TA, Dorian C, et al. Ghrelin selectively reduces mechanosensitivity of upper gastrointestinal vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1376–G1384. doi: 10.1152/ajpgi.00536.2006. [DOI] [PubMed] [Google Scholar]
- Page AJ, O'Donnell TA, Cooper NJ, Young RL, Blackshaw LA. Nitric oxide as an endogenous peripheral modulator of visceral sensory neuronal function. J Neurosci. 2009;29:7246–7255. doi: 10.1523/JNEUROSCI.6099-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009;52:289–298. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partosoedarso ER, Young RL, Blackshaw LA. GABA(B) receptors on vagal afferent pathways: peripheral and central inhibition. Am J Physiol Gastrointest Liver Physiol. 2001;280:G658–G668. doi: 10.1152/ajpgi.2001.280.4.G658. [DOI] [PubMed] [Google Scholar]
- Paschetta E, Hvalryg M, Musso G. Glucose-dependent insulinotropic polypeptide: from pathophysiology to therapeutic opportunities in obesity-associated disorders. Obes Rev. 2011;12:813–828. doi: 10.1111/j.1467-789X.2011.00897.x. [DOI] [PubMed] [Google Scholar]
- Perrin J, Crousillat J, Mei N. Assessment of true splanchnic glucoreceptors in the jejuno-ileum of the cat. Brain Res Bull. 1981;7:625–628. doi: 10.1016/0361-9230(81)90108-8. [DOI] [PubMed] [Google Scholar]
- Peters JH, McKay BM, Simasko SM, Ritter RC. Leptin-induced satiation mediated by abdominal vagal afferents. Am J Physiol Regul Integr Comp Physiol. 2005;288:R879–R884. doi: 10.1152/ajpregu.00716.2004. [DOI] [PubMed] [Google Scholar]
- Phifer CB, Berthoud HR. Duodenal nutrient infusions differentially affect sham feeding and Fos expression in rat brain stem. Am J Physiol. 1998;274:R1725–R1733. doi: 10.1152/ajpregu.1998.274.6.R1725. [DOI] [PubMed] [Google Scholar]
- Pilichiewicz AN, Chaikomin R, Brennan IM, Wishart JM, Rayner CK, Jones KL, et al. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab. 2007a;293:E743–E753. doi: 10.1152/ajpendo.00159.2007. [DOI] [PubMed] [Google Scholar]
- Pilichiewicz AN, Papadopoulos P, Brennan IM, Little TJ, Meyer JH, Wishart JM, et al. Load-dependent effects of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am J Physiol Regul Integr Comp Physiol. 2007b;293:R2170–R2178. doi: 10.1152/ajpregu.00511.2007. [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Vagal intramuscular array afferents form complexes with interstitial cells of Cajal in gastrointestinal smooth muscle: analogues of muscle spindle organs? Neuroscience. 2011;186:188–200. doi: 10.1016/j.neuroscience.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punjabi M, Arnold M, Geary N, Langhans W, Pacheco-Lopez G. Peripheral glucagon-like peptide-1 (GLP-1) and satiation. Physiol Behav. 2011;105:71–76. doi: 10.1016/j.physbeh.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Raliou M, Boucher Y, Wiencis A, Bézirard V, Pernollet J, Trotier D, et al. Tas1R1-Tas1R3 taste receptor variants in human fungiform papillae. Neurosci Lett. 2009;451:217–221. doi: 10.1016/j.neulet.2008.12.060. [DOI] [PubMed] [Google Scholar]
- Ranieri F, Mei N, Crousillat J. Splanchnic afferents arising from gastro-intestinal and peritoneal mechanoreceptors. Exp Brain Res. 1973;16:276–290. doi: 10.1007/BF00233331. [DOI] [PubMed] [Google Scholar]
- Ray J, Squires P, Curtis S, Meloche M, Buchan A. Expression of the calcium-sensing receptor on human antral gastrin cells in culture. J Clin Invest. 1997;99:2328–2333. doi: 10.1172/JCI119413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE. Does your gut taste? Sensory transduction in the gastrointestinal tract. News Physiol Sci. 1998;13:275–280. doi: 10.1152/physiologyonline.1998.13.6.275. [DOI] [PubMed] [Google Scholar]
- Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci. 2009;153:41–46. doi: 10.1016/j.autneu.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE, Holzer H. Dual capsaicin-sensitive afferent pathways mediate inhibition of gastric emptying in rat induced by intestinal carbohydrate. Neurosci Lett. 1992;141:236–238. doi: 10.1016/0304-3940(92)90902-j. [DOI] [PubMed] [Google Scholar]
- Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, et al. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2003;284:G367–G372. doi: 10.1152/ajpgi.00292.2001. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem. 1978;253:4022–4030. [PubMed] [Google Scholar]
- Reidelberger RD, Hernandez J, Fritzsch B, Hulce M. Abdominal vagal mediation of the satiety effects of CCK in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1005–R1012. doi: 10.1152/ajpregu.00646.2003. [DOI] [PubMed] [Google Scholar]
- Reimann F. Molecular mechanisms underlying nutrient detection by incretin-secreting cells. Int Dairy J. 2010;20:236–242. doi: 10.1016/j.idairyj.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci. 2009;3:1–15. doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw D, Batterham RL. Peptide YY: a potential therapy for obesity. Curr Drug Targets. 2005;6:171–179. doi: 10.2174/1389450053174523. [DOI] [PubMed] [Google Scholar]
- Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The ‘normal’ endocrine cell of the gut: changing concepts and new evidences. Ann N Y Acad Sci. 2004;1014:1–12. doi: 10.1196/annals.1294.001. [DOI] [PubMed] [Google Scholar]
- Ritter RC. Gastrointestinal mechanisms of satiation for food. Physiol Behav. 2004;81:249–273. doi: 10.1016/j.physbeh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- Roze C, Couturier D, Chariot J, Debray C. Inhibition of gastric electrical and mechanical activity by intraduodenal agents in pigs and the effects of vagotomy. Digestion. 1977;15:526–539. doi: 10.1159/000198043. [DOI] [PubMed] [Google Scholar]
- Ruat M, Molliver M, Snowman A, Snyder S. Calcium sensing receptor: molecular cloning in rat and localization to nerve terminals. Proc Natl Acad Sci U S A. 1995;92:3161–3165. doi: 10.1073/pnas.92.8.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393–411. doi: 10.1146/annurev.med.051308.105148. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Inoue H, Kawakami S, Miyawaki K, Miyamoto T, Mizuta K, et al. Expression and distribution of Gpr119 in the pancreatic islets of mice and rats: predominant localization in pancreatic polypeptide-secreting PP-cells. Biochem Biophys Res Commun. 2006;351:474–480. doi: 10.1016/j.bbrc.2006.10.076. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Samuel B, Shaito A, Motoike T, Rey F, Backhed F, Manchester J, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Gabriel A, Maekawa T, Uneyama H, Yoshie S, Torii K. mGluR1 in the fundic glands of rat stomach. FEBS Lett. 2007;581:1119–1123. doi: 10.1016/j.febslet.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Savastano DM, Carelle M, Covasa M. Serotonin-type 3 receptors mediate intestinal Polycose- and glucose-induced suppression of intake. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1499–R1508. doi: 10.1152/ajpregu.00745.2004. [DOI] [PubMed] [Google Scholar]
- Savastano DM, Hayes MR, Covasa M. Serotonin-type 3 receptors mediate intestinal lipid-induced satiation and Fos-like immunoreactivity in the dorsal hindbrain. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1063–R1070. doi: 10.1152/ajpregu.00699.2006. [DOI] [PubMed] [Google Scholar]
- Schick RR, Harty GJ, Yaksh TL, Go VL. Sites in the brain at which cholecystokinin octapeptide (CCK-8) acts to suppress feeding in rats: a mapping study. Neuropharmacology. 1990;29:109–118. doi: 10.1016/0028-3908(90)90050-2. [DOI] [PubMed] [Google Scholar]
- Schirra J, Goke B. The physiological role of GLP-1 in human: incretin, ileal brake or more? Regul Pept. 2005;128:109–115. doi: 10.1016/j.regpep.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Schoepp D, Goldsworthy J, Johnson B, Salhoff C, Baker S. 3,5-Dihydroxyphenylglycine is a highly selective agonist for phosphoinositide-linked metabotropic glutamate receptors in the rat hippocampus. J Neurochem. 1994;63:769–772. doi: 10.1046/j.1471-4159.1994.63020769.x. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Berkow G, McHugh PR, Moran TH. Gastric branch vagotomy blocks nutrient and cholecystokinin-induced suppression of gastric emptying. Am J Physiol. 1993;264:R630–R637. doi: 10.1152/ajpregu.1993.264.3.R630. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Whitney A, Skoglund C, Castonguay TW, Moran TH. Decreased responsiveness to dietary fat in Otsuka Long-Evans Tokushima fatty rats lacking CCK-A receptors. Am J Physiol. 1999;277:R1144–R1151. doi: 10.1152/ajpregu.1999.277.4.R1144. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K, Schwartz GJ. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol Behav. 2003;78:285–294. doi: 10.1016/s0031-9384(02)00968-x. [DOI] [PubMed] [Google Scholar]
- Scott V, Kimura N, Stark JA, Luckman SM. Intravenous peptide YY3-36 and Y2 receptor antagonism in the rat: effects on feeding behaviour. J Neuroendocrinol. 2005;17:452–457. doi: 10.1111/j.1365-2826.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- Shen T, Kaya N, Zhao FL, Lu SG, Cao Y, Herness S. Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules alpha-gustducin and T1R2 in rat taste receptor cells. Neuroscience. 2005;130:229–238. doi: 10.1016/j.neuroscience.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Harada M, Goto M, Sugo T, Matsumoto Y, Abe M, et al. Identification of neuropeptide W as the endogenous ligand for orphan G-protein-coupled receptors GPR7 and GPR8. J Biol Chem. 2002;277:35826–35832. doi: 10.1074/jbc.M205337200. [DOI] [PubMed] [Google Scholar]
- Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985;249:R638–R641. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau JP, et al. Leptin secretion and leptin receptor in the human stomach. Gut. 2000;47:178–183. doi: 10.1136/gut.47.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani I, Buyse M, Goiot H, Weber N, Laigneau JP, Henin D, et al. Vagal stimulation rapidly increases leptin secretion in human stomach. Gastroenterology. 2002;122:259–263. doi: 10.1053/gast.2002.31385. [DOI] [PubMed] [Google Scholar]
- Soga T, Ohishi T, Matsui T, Saito T, Matsumoto M, Takasaki J, et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem Biophys Res Commun. 2005;326:744–751. doi: 10.1016/j.bbrc.2004.11.120. [DOI] [PubMed] [Google Scholar]
- Spannagel AW, Nakano I, Tawil T, Chey WY, Liddle RA, Green GM. Adaptation to fat markedly increases pancreatic secretory response to intraduodenal fat in rats. Am J Physiol. 1996;270:G128–G135. doi: 10.1152/ajpgi.1996.270.1.G128. [DOI] [PubMed] [Google Scholar]
- Steinert R, Beglinger C. Nutrient sensing in the gut: interactions between chemosensory cells, visceral afferents and the secretion of satiation peptides. Physiol Behav. 2011;105:62–70. doi: 10.1016/j.physbeh.2011.02.039. [DOI] [PubMed] [Google Scholar]
- Stephan D, Bon C, Holzwarth J, Galvan M, Pruss R. Human metabotropic glutamate receptor 1: mRNA distribution, chromosome localization and functional expression of two splice variants. Neuropharmacology. 1996;35:1649–1660. doi: 10.1016/s0028-3908(96)00108-6. [DOI] [PubMed] [Google Scholar]
- Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–191. doi: 10.1053/j.gastro.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Sullivan CN, Raboin SJ, Gulley S, Sinzobahamvya NT, Green GM, Reeve JJR, et al. Endogenous cholecystokinin reduces food intake and increases Fos-like immunoreactivity in the dorsal vagal complex but not in the myenteric plexus by CCK1 receptor in the adult rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1071–R1080. doi: 10.1152/ajpregu.00490.2006. [DOI] [PubMed] [Google Scholar]
- Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- Sutherland K, Brierley SM, Horowitz M, Rayner CK, Blackshaw LA, Young RL. Sweet taste transduction molecules are expressed in the upper gastrointestinal tract in humans. Gastroenterology. 2007;132:A587. doi: 10.1136/gut.2008.148932. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Okumura T, Yamada H, Kohgo Y. Stimulation of gastric acid secretion by centrally administered orexin-A in conscious rats. Biochem Biophys Res Commun. 1999;254:623–627. doi: 10.1006/bbrc.1998.9994. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Nomura A, Masu M, Shigemoto R, Mizuno N, Nakanishi S. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J Neurosci. 1993;13:1372–1378. doi: 10.1523/JNEUROSCI.13-04-01372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Yoshida T, Miyamoto N, Motoike T, Kurosu H, Shibata K, et al. Characterization of a family of endogenous neuropeptide ligands for the G protein-coupled receptors GPR7 and GPR8. Proc Natl Acad Sci U S A. 2003;100:6251–6256. doi: 10.1073/pnas.0837789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Katsuma S, Adachi T, Koshimizu T, Hirasawa A, Tsujimoto G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch Pharmacol. 2007;377:523–527. doi: 10.1007/s00210-007-0200-8. [DOI] [PubMed] [Google Scholar]
- Taylor AI, Irwin N, McKillop AM, Patterson S, Flatt PR, Gault VA. Evaluation of the degradation and metabolic effects of the gut peptide xenin on insulin secretion, glycaemic control and satiety. J Endocrinol. 2011;207:87–93. doi: 10.1677/JOE-10-0085. [DOI] [PubMed] [Google Scholar]
- Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M, et al. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res. 2009;30:149–156. doi: 10.2220/biomedres.30.149. [DOI] [PubMed] [Google Scholar]
- Testa C, Standaert D, Young A, Penney J. Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C, Suzdak P. Serine-O-phosphate has affinity for type IV, but not type I, metabotropic glutamate receptor. Neuroreport. 1993;4:1099–1101. [PubMed] [Google Scholar]
- Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol. 2009;587:27–32. doi: 10.1113/jphysiol.2008.164012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas E, Wood JA, Stanojevic V, Habener JF. Glucagon-like peptide-1(9-36)amide metabolite inhibits weight gain and attenuates diabetes and hepatic steatosis in diet-induced obese mice. Diabetes Obes Metab. 2011;13:26–33. doi: 10.1111/j.1463-1326.2010.01316.x. [DOI] [PubMed] [Google Scholar]
- Toouli J, Collins J, Wray N, Billington C, Knudson M, Pulling C, et al. Vagal blocking for obesity control (VBLOC): effects on excess weight loss, calorie intake, satiation and satiety. Obes Surg. 2007;17:1043–1043. [Google Scholar]
- Toyono T, Seta Y, Kataoka S, Kawano S, Shigemoto R, Toyoshima K. Expression of metabotropic glutamate receptor group I in rat gustatory papillae. Cell Tissue Res. 2003;313:29–35. doi: 10.1007/s00441-003-0740-2. [DOI] [PubMed] [Google Scholar]
- Tuleu C, Andrieux C, Boy P, Chaumeil JC. Gastrointestinal transit of pellets in rats: effect of size and density. Int J Pharm. 1999;180:123–131. doi: 10.1016/s0378-5173(98)00400-1. [DOI] [PubMed] [Google Scholar]
- Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965–4973. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- Vrang N, Madsen AN, Tang-Christensen M, Hansen G, Larsen PJ. PYY(3-36) reduces food intake and body weight and improves insulin sensitivity in rodent models of diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2006;291:R367–R375. doi: 10.1152/ajpregu.00726.2005. [DOI] [PubMed] [Google Scholar]
- Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res. 2007;1149:118–126. doi: 10.1016/j.brainres.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12:151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- Walls EK, Phillips RJ, Wang FB, Holst MC, Powley TL. Suppression of meal size by intestinal nutrients is eliminated by celiac vagal deafferentation. Am J Physiol. 1995;269:R1410–R1419. doi: 10.1152/ajpregu.1995.269.6.R1410. [DOI] [PubMed] [Google Scholar]
- Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, et al. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci. 2008;28:4957–4966. doi: 10.1523/JNEUROSCI.5398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wu X, Simonavicius N, Tian H, Ling L. Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J Biol Chem. 2006;281:34457–34464. doi: 10.1074/jbc.M608019200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chandra R, Samsa L, Gooch B, Fee B, Cook J, et al. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol Gastrointest Liver Physiol. 2011;300:G528–G537. doi: 10.1152/ajpgi.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellendorph P, Bräuner-Osborne H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene. 2004;335:37–46. doi: 10.1016/j.gene.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Hansen K, Balsgaard A, Greenwood J, Egebjerg J, Bräuner-Osborne H. Deorphanization of GPRC6A: a promiscuous L-alpha-amino acid receptor with preference for basic amino acids. Mol Pharmacol. 2005;67:589–597. doi: 10.1124/mol.104.007559. [DOI] [PubMed] [Google Scholar]
- West DB, Fey D, Woods SC. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am J Physiol. 1984;246:R776–R787. doi: 10.1152/ajpregu.1984.246.5.R776. [DOI] [PubMed] [Google Scholar]
- Whited KL, Thao D, Lloyd KCK, Kopin AS, Raybould HE. Targeted disruption of the murine CCK1 receptor gene reduces intestinal lipid-induced feedback inhibition of gastric function. Am J Physiol Gastrointest Liver Physiol. 2006;291:G156–G162. doi: 10.1152/ajpgi.00569.2005. [DOI] [PubMed] [Google Scholar]
- Wilkinson AR, Johnston D. Effect of truncal, selective and highly selective vagotomy on gastric emptying and intestinal transit of a food-barium meal in man. Ann Surg. 1973;178:190–193. doi: 10.1097/00000658-197308000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150:1680–1687. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G. Orexins: a newly discovered family of hypothalamic regulators of food intake. Nutr Rev. 1998;56:172–173. doi: 10.1111/j.1753-4887.1998.tb06131.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2011. Obesity and Overweight. Fact Sheet No. 311, updated March 2011. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/
- Wu XY, Zhu JX, Gao J, Owyang C, Li Y. Neurochemical phenotype of vagal afferent neurons activated to express C-FOS in response to luminal stimulation in the rat. Neuroscience. 2005;130:757–767. doi: 10.1016/j.neuroscience.2004.09.060. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Miyamoto N, Shibata K, Valasek M, Motoike T, Kedzierski R, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Emson P, Hokfelt T. Immunohistochemical mapping of nitric oxide synthase in the rat hypothalamus and colocalization with neuropeptides. J Chem Neuroanat. 1996;10:295–316. doi: 10.1016/0891-0618(96)00133-0. [DOI] [PubMed] [Google Scholar]
- Yip RG, Boylan MO, Kieffer TJ, Wolfe MM. Functional GIP receptors are present on adipocytes. Endocrinology. 1998;139:4004–4007. doi: 10.1210/endo.139.9.6288. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Doucet E, Drapeau V, Dionne I, Tremblay A. Combined effects of red pepper and caffeine consumption on 24 h energy balance in subjects given free access to foods. Br J Nutr. 2001;85:203–211. doi: 10.1079/bjn2000224. [DOI] [PubMed] [Google Scholar]
- Young RL. Sensing via intestinal sweet taste pathways. Front Neurosci. 2011;5:1–13. doi: 10.3389/fnins.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RL, Sutherland K, Pezos N, Brierley SM, Ma J, Rayner CK, et al. Control of intestinal sweet taste receptor expression is impaired in type 2 diabetes. Neurogastroenterol Motil. 2010;22(Suppl 1):31. [Google Scholar]
- Yox DP, Ritter RC. Capsaicin attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol. 1988;255:R569–R574. doi: 10.1152/ajpregu.1988.255.4.R569. [DOI] [PubMed] [Google Scholar]
- Yox DP, Brenner L, Ritter RC. CCK-receptor antagonists attenuate suppression of sham feeding by intestinal nutrients. Am J Physiol. 1992;262:R554–R561. doi: 10.1152/ajpregu.1992.262.4.R554. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk V, Chen B, Brookes S. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaibi M, Stocker C, O'Dowd J, Davies A, Bellahcene M, Cawthorne M, et al. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010;584:2381–2386. doi: 10.1016/j.febslet.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ji RR, Arvidsson J, Lundberg JM, Bartfai T, Bedecs K, et al. Expression of peptides, nitric oxide synthase and NPY receptor in trigeminal and nodose ganglia after nerve lesions. Exp Brain Res. 1996;111:393–404. doi: 10.1007/BF00228728. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shi T, Holmberg K, Landry M, Huang W, Xiao H, et al. Expression and regulation of the neuropeptide Y Y2 receptor in sensory and autonomic ganglia. Proc Natl Acad Sci U S A. 1997;94:729–734. doi: 10.1073/pnas.94.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhu X, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


