INTRODUCTION
Heart failure (HF) is a major worldwide public health problem. One in five persons aged 40 years in the United States will develop HF during their lifetime1 and HF remains the leading cause for hospitalization among the elderly2. While age and sex-specific HF incidence is not increasing3, overall HF survival has improved and the number of persons over age 65 is rapidly increasing. Thus, the absolute number of patients with HF will continue to increase. Half of patients with HF have a preserved ejection fraction (HFpEF) and the remainder display reduced ejection fraction (HFrEF)4–6. The proportion of patients with normal EF is steadily increasing, due to increased incidence and/or increasing physician recognition of the syndrome4. Resource utilization associated with HF is high in both the inpatient and outpatient settings, regardless of EF.
HF is a syndrome that can be defined clinically by a collection of symptoms (dyspnea, fatigue, exertional intolerance) and signs (edema, gallop, rales) that are attributable to a cardiac disorder2. HF may also be defined hemodynamically, by an inability to provide adequate cardiac output to the body at rest or with exertion, or to do so only in the setting of elevated cardiac filling pressures. The cardiovascular system responds to a wide variety of insults (e.g. myocardial disease, ischemia, valvular or pericardial disease) in a finite number of ways, both hemodynamically (elevated filling pressures, depressed output) and symptomatically (dyspnea, fatigue, angina). However, these similarities in clinical expression do not indicate that the underlying mechanisms of disease are the same, or that treatment will be similar. For example, a headache may be noted with a migraine or brain tumor, dyspnea may be reported with HF, emphysema, or neuromuscular disease, and diarrhea may be observed with infection, dysmotility or sprue. In each case, common treatments (analgesics, oxygen, and rehydration) will improve symptoms, but only unique interventions targeted to the specific insults causing each disease will be effective to modify long term outcomes.
HFpEF and HFrEF share the same clinical phenotype. Signs, symptoms, exercise intolerance, hemodynamics, and outcomes may be identical or highly similar in each form of HF5–11, but this does not indicate that these disorders are due to a common etiology or that they should be treated in the same way. Indeed, the principal rationale to taxonomically distinguish diseases is based upon cause and treatment. In this review we shall examine the wealth of evidence proving that despite multiple similarities in clinical expression, HFpEF and HFrEF represent two distinct disorders in the heart failure spectrum, and as such, should be studied and treated separately.
CONCLUSIVE EVIDENCE THAT HFpEF AND HFrEF ARE DISTINCT DISEASES
Among patients with clinical diagnosis of HF, the distribution of EF is bimodal
If HFpEF and HFrEF are part of the same disease process, one would expect to observe a unimodal distribution of EF within HF populations. In an analysis of data from patients enrolled in the CHARM-Program, Solomon and colleagues observed such a unimodal distribution of EF12. This has been interpreted to support the notion that HFpEF and HFrEF are part of the same disease spectrum13. However, as pointed out by Gaasch et al., the CHARM program enrolled more patients with HFrEF than HFpEF, which may skew the distribution, and analysis of two other HF trials that did not pre-specify EF enrollment criteria revealed bimodal distributions of EF14. These data are limited by selection bias, as the populations examined were referred or selected for a clinical trial, but community-based data shows similar findings. Data from the OPTIMIZE registry of >30,000 patients admitted for acutely decompensated heart failure has also shown a bimodal distribution of EF among HF patients9. We analyzed all consecutive patients admitted with HF to our own institution over a 16 year period (from previously published data)4 (Figure 1). This plot clearly shows a bimodal EF distribution. Inspection of the EF histogram stratified by gender further shows a greater female preponderance in HFpEF, as has been shown in numerous studies. These data provide strong a priori evidence that HFpEF and HFrEF represent two distinct disease processes.
Figure 1.
Bimodal distribution of ejection fraction in heart failure.
Therapies with Proven Benefit in HFrEF have failed to improve outcome in HFpEF
If HFpEF and HFrEF were part of the same HF disease spectrum, they would be expected to respond similarly to treatment. However, medications which have been shown to produce unequivocal improvements in HFrEF have not produced similar beneficial effects in HFpEF (Figure 2). While survival for patients with HFrEF has improved over the past two decades, there has been no improvement in HFpEF survival4. The CHARM-Preserved study (n=3023) compared the angiotensin receptor blocker (ARB) candesartan versus placebo in patients with HF and EF>40% and did not evidence a significant reduction in the composite outcome of death and cardiovascular hospitalization15. There was a trend toward benefit overall, but this study included a large proportion of patients with mild systolic dysfunction (EF 40–49%) and more patients with coronary disease and male gender than are typically noted in community-based HFpEF populations. The larger I-PRESERVE trial (n=4128) similarly showed no reduction in death or hospitalization with the ARB irbesartan over 4 years of followup16. Angiotensin converting enzyme inhibitors (ACEI) have also failed to show benefit in HFpEF. The PEP-CHF trial (n=850) randomized HFpEF patients aged ≥70 years to perindopril or placebo and found over the 3 year study period there was no reduction in mortality or HF hospitalizations17. A recent trial of enalapril in elderly patients with HFpEF reported no improvement in exercise capacity, aortic distensibility or neurohormonal profile compared with placebo18.
Figure 2.
Differential response to treatment in HFpEF and HFrEF. Summary of hazard ratios observed in trials or registries studying patients with HFpEF and HFrEF.
Observational data from the OPTIMIZE registry has failed to demonstrate reduced hazard of mortality and hospitalization in association with discharge ACEI/ARB use in HFpEF, in striking contrast to reductions in events observed in HFrEF9. The unique disease-specific responses to anti-angiotensin therapies is further highlighted by a recent ancillary analysis of the very large ALLHAT Trial (n=42,418), where chlorthalidone reduced incidence of both HFpEF and HFrEF compared with amlodipine and doxazosin; yet lisinopril was only effective in reducing incident HFrEF, with no benefit in HFpEF incidence compared with the other agents19.
The efficacy of beta blockers (BB) in HFpEF remains unresolved, though they remain one of the most prescribed medications in this population9. Observational studies from OPTIMIZE observed no reduction in morbidity and mortality in short term9 or long term20 followup in HFpEF, in contrast to HFrEF where significant reductions in maladaptive remodeling, HF hospitalizations and mortality are observed with BB in both registry9, 20 and trial data2. Ancillary analysis from the SENIORS Trial suggested the benefits of the beta blocker nebivolol were also observed in the patients with preserved EF21, though few patients in the trial had EF>50–55%. A recent observational study noted that women with HFpEF (EF>50%) discharged on beta blockers had higher 6 month rehospitalization rates compared with those not prescribed beta blockers22, and it is speculated that this could be related to deleterious effects of heart rate reduction in normal to small sized ventricles in HFpEF where chronotropic incompetence is common23–25. The effects of BB on cardiomyocytes appear to differ in HFpEF and HFrEF, with higher resting tension observed in HFpEF patients treated with BB, but no apparent BB effect on myocyte stiffness in HFrEF26.
In an ancillary analysis of 988 patients with HFpEF (EF>45%) enrolled in the DIG trial, Ahmed et al. found that while digoxin did lower HF hospitalization27, this benefit was over-come by an equivalent increase in coronary syndrome hospitalizations28. Other therapies with proven benefit in HFrEF, such as aldosterone antagonists or devices, are less investigated in HFpEF. Myocardial ischemia and infarction cause systolic and diastolic dysfunction, and revascularization for triple vessel disease among patients with reduced EF is associated with improved survival29. The role of revascularization is less well-studied in HFpEF, though a case series from Little and colleagues found that episodes of pulmonary edema tend to recur despite revascularization in this setting30.
HFpEF and HFrEF display unique patterns of Ventricular and Cellular Remodeling
While increased LV mass is characteristic of most forms of HF, the patterns of ventricular remodeling in HFpEF and HFrEF are highly distinct10. Left ventricular chamber dilation is a defining characteristic of HFrEF. Indeed, in chronic, compensated HFrEF, the EF is reduced because the chamber size (denominator of EF equation) is larger, while the stroke volume (numerator) is typically similar to normal controls7, 10. Chamber dilation in HFrEF is coupled with pathologic electrical remodeling, as left bundle branch block is much more common in HFrEF compared with HFpEF31, 32. In contrast, most6, 7, 25, 33–40, though not all41, 42 studies have reported that ventricular chamber size is normal or near-normal in HFpEF, with increased wall thickness, greater ratio of wall thickness to chamber dimension, and increased ratio of ventricular mass to chamber volume when compared with HFrEF and healthy controls. These changes are similar to those observed with chronic pressure overload due to arterial hypertension43, and indeed, abundant data suggests that HFpEF develops as a progression from asymptomatic hypertensive heart disease25, 33, 34, 44. In contrast, while hypertension is a potent risk factor for all forms of HF45, it is an uncommon solitary cause of HFrEF46.
These disparate ventricular structural changes in HFpEF and HFrEF are associated with diametrically opposing effects on ventricular-arterial interaction, particularly involving the end-systolic pressure-volume relationship or end-systolic elastance (Ees)47. Ees is markedly reduced in HFrEF, and as a result HFrEF patients respond very favorably to arterial vasodilators, with minimal drop in blood pressure and substantial improvement in stroke volume47, 48. In contrast, Ees is elevated in HFpEF patients33, 44, 49. This leads to more exaggerated drops in blood pressure with vasodilator therapy in HFpEF, while similarly promoting more marked increases in blood pressures during stress48. These fundamental differences in clinical response to alterations in ventricular loading may partially explain the failure of vasodilators to improve outcomes in clinical trials for HFpEF15–18. Remodeling in both forms of HF may be associated with mechanical dyssynchrony2, 50, though the type of dyssynchrony that is amenable to device therapy51 (bundle branch block) is much more common in HFrEF, where eccentric remodeling predominates31, 32.
Differences between HFrEF and HFpEF extend to the level of the interstitium and cardiomyocyte. The balance of matrix metalloproteinases and their inhibitors differs in HFrEF and HFpEF, and this difference is hypothesized to contribute to the distinct patterns of chamber remodeling observed in these diseases52. Histopathologic studies from Paulus and colleagues have shown that the cardiomyocyte is narrow and elongated in HFrEF, with reduced myofibrillar density, whereas myocyte diameter and resting tension are both increased in HFpEF40, 53, particularly among diabetics54. Furthermore, the mechanisms responsible for increased myocyte stiffness appear to differ in diabetic HFrEF, where enhanced deposition and cross-linking of advanced glycation end-products predominate, in contrast to HFpEF where higher cardiomyocyte resting tension has been observed, presumably related to sarcomeric protein phosphorylation status54. There is an increased ratio of the stiffer isoform of the macromolecule titin in HFpEF compared with HFrEF, which may contribute to higher resting tension and the larger drop in tension in response to phosphorylation40.
SUGGESTIVE EVIDENCE THAT HFpEF AND HFrEF ARE DISTINCT DISEASES
HFpEF and HFrEF tend to arise among different patient populations
Community-based studies have demonstrated that HFpEF patients differ from HFrEF in a number of characteristic ways, though there is considerable overlap when large community-based studies are examined4–6, 8, 9, 31, 32, 36 (see Supplemental table and references). Most studies have found that HFpEF patients are older (weighted average 74 vs 70 years), more often hypertensive (weighted average 74 vs 65 %) and less likely to have coronary disease (weighted average 46 vs 58 %). The most robust difference is female gender (weighted average 63 vs 38 %) possibly related to less coronary disease, enhanced concentric remodeling and age-related vascular stiffening in women55, 56. These differences in age, gender, hypertensive history and coronary disease become more prominent when defining HFpEF more stringently by EF≥50–55%9, 57, providing further evidence that these represent two distinct disease processes.
Arterial loading conditions differ in HFpEF and HFrEF: vasoconstriction is common to both forms of HF, but pulse pressure is higher in HFpEF, and this vascular stiffening produces greater blood pressure lability with changes in preload, afterload and stress in HFpEF48, 49, 57.
Pathogenesis and Disease Progression in HFpEF and HFrEF Appear Distinct
Hypertension is the single largest risk factor for development of HF, regardless of EF45, and while prevalence of hypertension is greater in HFpEF, it is common to both forms of HF. Changes in cardiovascular structure and function in arterial hypertension are similar to those seen with normal maturation, leading some to refer to hypertension as “accelerated aging”43. The dominant risk factors for HFpEF are age and hypertension, and many of the pathophysiologic derangements in HFpEF present as a continuum with asymptomatic hypertensive heart disease25, 33, 34, 44, suggesting that HFpEF may be a form of “accelerated hypertension”. Age, hypertensive and in some cases diabetes related ventricular remodeling thus create the slowly progressive substrate upon which HFpEF is formed (Figure 3), and recent evidence suggests that progression of a number of abnormalities in cardiovascular function may promote the transition to overt HFpEF, including loss of contractile reserve, diastolic reserve, chronotropy, vasodilation and endothelial function23–25, 58. In contrast, HFrEF most commonly develops in response to distinct pathophysiologic perturbations leading to accelerated and larger-scale myocyte loss/dysfunction, with the most common etiologies including acute myocardial infarction, genetic abnormalities, myocarditis or toxin effects (e.g. alcohol or chemotherapy)2. These more distinct processes may occur in younger patients where they predominate or later in life on a background of age/hypertension/diabetic remodeling (e.g. anterior myocardial infarction in an elderly woman with hypertension), leading to the appearance of greater overlap between the clinical phenotype. However, the common appearance of some characteristics such as this in both forms of HF should not be taken as evidence that they represent the same underlying disease process.
Figure 3.
Distinct pathophysiology of HFpEF and HFrEF.
It has been suggested that HFpEF may “progress” to HFrEF, consistent with the notion that the two diagnoses exist in a continuum. However, in the absence of coronary disease/myocardial infarction (the leading cause of HFrEF), there is little evidence that this “transition” occurs59. Increased LV mass is indeed a risk factor for the development of depressed EF, but this relationship is observed principally in the setting of eccentric hypertrophy, not in concentric remodeling as is typical of HFpEF60. These longitudinal data provide further evidence that HFpEF and HFrEF develop in two distinct mechanistic pathways—eccentric remodeling in the latter and concentric in the former.
FEATURES WHICH ARE SHARED BY HFpEF AND HFrEF YET DO NOT PROVE A COMMON DISEASE PROCESS
Ventricular and Vascular Dysfunction are common to all HF
Diastolic dysfunction is characteristic of both forms of HF and is evidenced clinically by the presence of elevated filling pressures, abnormal relaxation and increased chamber stiffness10, 35. Systolic dysfunction had traditionally been considered to be unique to HFrEF38, but a number of recent studies have shown that regional and chamber-level systolic dysfunction are also common in HFpEF10, 25, 44, though not as severe as in HFrEF. Systolic dysfunction becomes more apparent and limiting during the stress of exercise in HFpEF23, 25, where it is potently associated with depressed functional capacity25, possibly because mild systolic dysfunction has more severe consequences in the absence of chamber dilation. Chronotropic incompetence is also common in both HFpEF and HFrEF, likely related to autonomic dysfunction and/or β-receptor desensitization23–25, 61, processes which are common to all HF. Abnormal vasorelaxation and endothelium-dependent vasodilation are observed in both HFpEF and HFrEF, both in the systemic circulation23, 25, 62 and the pulmonary vasculature63, 64. Each of these abnormalities may exacerbate ventricular dysfunction in either form of HF. However, the presence of many common abnormalities in ventricular-vascular functional response to HF does not indicate that HFpEF and HFrEF share the same initial or predominant pathogenic mechanism.
Neurohormonal Activation and Renal Dysfunction are common to all HF
Pathologic activation of the renin-angiotensin-aldosterone axis, natriuretic peptides and the sympathetic nervous system are characteristic of both HFrEF and HFpEF, but have been studied much more extensively in HFrEF. Norepinephrine levels are similarly elevated in HFpEF and HFrEF7. Natriuretic peptide levels are elevated in both forms of HF, though they are elevated to a greater extent in HFrEF7, 65, 66. This is not surprising, as the stimulus for myocardial BNP release is wall stress, which varies directly with filling pressures (elevated in both HFpEF and HFrEF) and chamber size (elevated in HFrEF but normal in HFpEF)65. Aldosterone levels are similar in HFrEF and HFpEF66, but other neurohormones such as plasma renin activity, angiotensin II and vasopressin have not been compared in these groups.
Renal dysfunction is similarly problematic in HFpEF and HFrEF4–6, 8, but recent evidence suggests that patients with HFpEF may be more vulnerable to the development of renal dysfunction during the course of treatment for HF decompensation57, and renal-associated mortality was higher in HFpEF in a post-hoc analysis from the DIG trial67. Regardless of the similarities in neurohormonal activation and renal dysfunction in both forms of HF, these maladaptations are fundamentally a common response to hemodynamic derangements (elevated filling pressures and low output) which are shared by both HFpEF and HFrEF but that do not indicate that the disease processes are the same.
NATURAL HISTORY OF HFpEF - IMPLICATIONS FOR DIAGNOSIS AND MODE OF DEATH
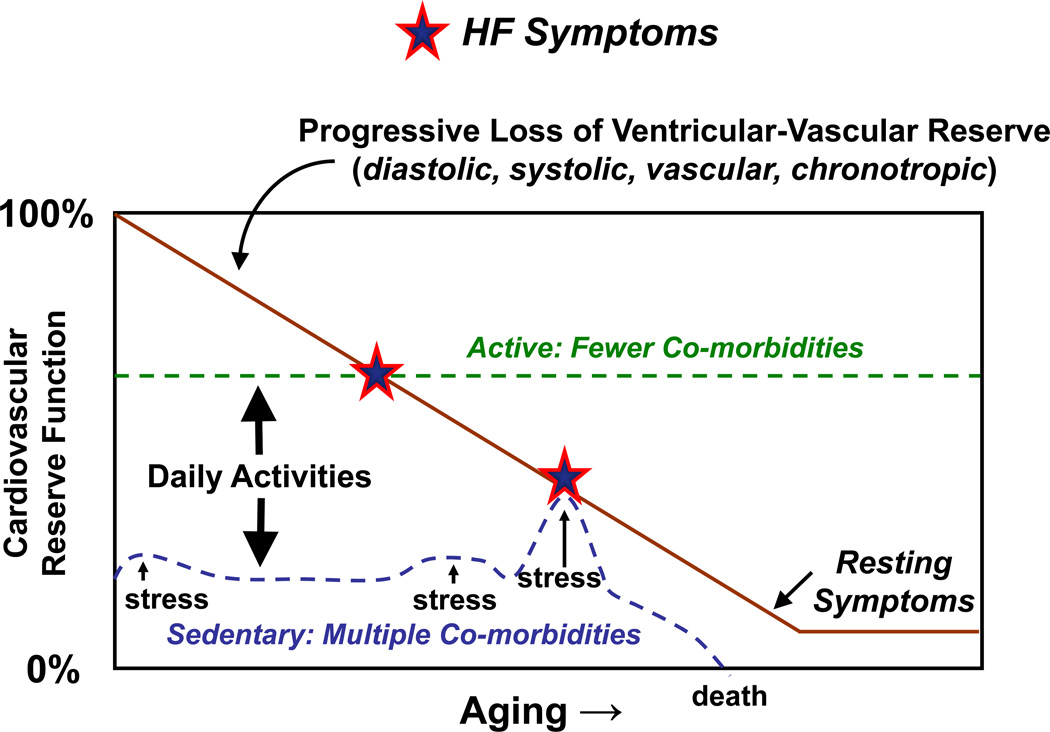
Exertional dyspnea and fatigue are common symptoms in patients with HF, particularly among the elderly. When echocardiography demonstrates a depressed EF, these symptoms are usually ascribed to HFrEF. However, when symptoms of exertional intolerance are noted in a patient with preserved EF, diagnosis is more challenging and symptoms may be related to deconditioning, obesity, pulmonary disease, pulmonary vascular disease or HFpEF. In relation to a lack of awareness and/or a lack of established treatment options, the diagnosis of HFpEF is often not entertained, even among cardiovascular specialists. Many patients with earlier-stage HFpEF may be younger, more active or have fewer comorbidities and present with predominantly exertional (NYHA functional class II) symptoms at a time when the severity of the underlying cardiovascular remodeling and dysfunction is less severe and potentially, still amenable to treatment (Figure 4). Indeed, recent studies have shown that despite normal examination, echocardiography, and resting hemodynamics, patients with early-stage HFpEF may display hemodynamic abnormalities (elevated filling pressures) exclusively during the stress of exercise58.
Figure 4.
Variable natural history in HFpEF.
Earlier recognition of HFpEF may enable investigation of treatment and preventive strategies where the potential for benefit is increased. In contrast, elderly patients who are inactive and have many co-morbidites may present with more severe symptoms (NYHA III-IV) during an episode of hemodynamic stress (often due to a co-morbidity) and at a time when pathologic abnormalities may be less reversible (Figure 4). In many such patients, their subsequent clinical course may be more driven by the comorbidity than their HF. Indeed, patients with HFpEF are more likely to die from non-cardiovascular causes compared with HFrEF15, 68, 69. Importantly, a recent-community based study directly comparing mode of death in HFpEF and HFrEF found that the greater rate of non-cardiovascular death in HFpEF was primarily attributable to fewer coronary disease deaths in HFpEF with similar rates of HF death and otherwise comparable comorbidity scores in the two groups70.
THE PROBLEM OF THE “INTERMEDIATE GROUP” (EF 40–50%)
The definition of “preserved EF” has varied considerably between studies, ranging from ≥35% to ≥55%. Lumping together patients with mildly depressed EF with truly normal EF may lead to the appearance of a continuous spectrum while also producing confusing results when attempting to properly interpret the trial data. As discussed above, recent studies have suggested that this intermediate group has many features more typical of HFrEF—greater male predominance, more coronary disease, less hypertension, more chamber dilation and greater risk of dying from cardiovascular causes, compared with more stringently defined HFpEF (>50–55%). It is most likely that this intermediate EF group is populated by patients with either mild or well-treated HFrEF, rather than patients whose EF is gradually diminishing—in either case, these patients would be more appropriately treated according to established HFrEF guidelines. We would propose that “definite” HFpEF be defined by EF>50%, and that this intermediate group be included in HFrEF. At this time there is insufficient rationale to alter the established EF-based nosology to distinguish the two forms of HF. While EF is not synonymous with contractility44, it is easy to conceptualize, measure, and is universally available—making it a useful marker to distinguish the two forms of HF.
CONCLUSIONS
Great strides have been made to better understand the pathophysiology of HFpEF and HFrEF, but important questions remain unanswered. The two forms of HF differ fundamentally in the acuity and extent of myocardial loss/dysfunction, the pattern of remodeling at the chamber and ultrastructural level, and the response to therapeutic interventions. Progression to HFpEF is gradual and tends to develops in concert with typical age-acquired comorbidities, particularly hypertension with concentric remodeling. In contrast, HFrEF may develop acutely or indolently, but typically in response to a larger-scale myocardial insult. Drug and device therapies which target maladaptive eccentric ventricular remodeling improve outcome in HFrEF, because these are the processes that drive the pathogenesis. In contrast, the pathophysiologic derangements in HFpEF include concentric remodeling, ventricular-vascular stiffening and loss of ventricular-vascular reserve function, so it is perhaps not surprising that therapies targeting HFrEF pathophysiology have not improved outcome in HFpEF. Future basic and clinical research should separate these two distinct forms of HF so as to better understand its unique mechanisms of disease and define optimal treatment strategies.
Supplementary Material
Acknowledgments
FUNDING SOURCE: BAB is supported by the Marie Ingalls Career Development Award in Cardiovascular Research and an American Heart Association NCRP award. MMR is supported by NIH Grants HL84907, HL76611 and HL63281.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT of INTEREST DISCLOSURES:
None.
References
- 1.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D, Framingham Heart S. Lifetime risk for developing congestive heart failure: the Framingham Heart Study.[see comment] Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 4.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England journal of medicine. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. The New England journal of medicine. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 6.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 7.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. Journal of the American College of Cardiology. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. Journal of the American College of Cardiology. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 10.Fukuta H, Little WC. Contribution of systolic and diastolic abnormalities to heart failure with a normal and a reduced ejection fraction. Progress in cardiovascular diseases. 2007;49:229–240. doi: 10.1016/j.pcad.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM, Jr, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 13.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. European heart journal. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 14.Gaasch WH, Delorey DE, Kueffer FJ, Zile MR. Distribution of left ventricular ejection fraction in patients with ischemic and hypertensive heart disease and chronic heart failure. The American journal of cardiology. 2009;104:1413–1415. doi: 10.1016/j.amjcard.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 16.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. The New England journal of medicine. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 17.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. European heart journal. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 18.Kitzman DW, Hundley WG, Brubaker PH, Morgan T, Moore JB, Stewart KP, Little WC. A Randomized, Double-Blinded Trial of Enalapril in Older Patients with Heart Failure and Preserved Ejection Fraction: Effects on Exercise Tolerance and Arterial Distensibility. Circ Heart Fail. 2010 doi: 10.1161/CIRCHEARTFAILURE.109.898916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, Farber MA, Ford CE, Levy D, Massie BM, Nawaz S. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118:2259–2267. doi: 10.1161/CIRCULATIONAHA.107.762229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez AF, Hammill BG, O'Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. Journal of the American College of Cardiology. 2009;53:184–192. doi: 10.1016/j.jacc.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Veldhuisen DJ, Cohen-Solal A, Bohm M, Anker SD, Babalis D, Roughton M, Coats AJ, Poole-Wilson PA, Flather MD. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: Data From SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure) Journal of the American College of Cardiology. 2009;53:2150–2158. doi: 10.1016/j.jacc.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 22.Farasat SM, Bolger DT, Shetty V, Menachery EP, Gerstenblith G, Kasper EK, Najjar SS. Effect of Beta-blocker therapy on rehospitalization rates in women versus men with heart failure and preserved ejection fraction. The American journal of cardiology. 105:229–234. doi: 10.1016/j.amjcard.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 24.Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–89. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global Cardiovascular Reserve Dysfunction in Heart Failure with Preserved Ejection Fraction. J Am Coll Card. 2010 doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamdani N, Paulus WJ, van Heerebeek L, Borbely A, Boontje NM, Zuidwijk MJ, Bronzwaer JG, Simonides WS, Niessen HW, Stienen GJ, van der Velden J. Distinct myocardial effects of beta-blocker therapy in heart failure with normal and reduced left ventricular ejection fraction. European heart journal. 2009;30:1863–1872. doi: 10.1093/eurheartj/ehp189. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF, Jr, Gheorghiade M. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The effect of digoxin on mortality and morbidity in patients with heart failure. The Digitalis Investigation Group. The New England journal of medicine. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 29.Passamani E, Davis KB, Gillespie MJ, Killip T. A randomized trial of coronary artery bypass surgery. Survival of patients with a low ejection fraction. The New England journal of medicine. 1985;312:1665–1671. doi: 10.1056/NEJM198506273122603. [DOI] [PubMed] [Google Scholar]
- 30.Kramer K, Kirkman P, Kitzman D, Little WC. Flash pulmonary edema: Association with hypertension and reoccurrence despite coronary revascularization. American heart journal. 2000;140:451–455. doi: 10.1067/mhj.2000.108828. [DOI] [PubMed] [Google Scholar]
- 31.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–2289. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 32.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. Journal of the American College of Cardiology. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 35.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. The New England journal of medicine. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 36.Devereux RB, Roman MJ, Liu JE, Welty TK, Lee ET, Rodeheffer R, Fabsitz RR, Howard BV. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study. The American journal of cardiology. 2000;86:1090–1096. doi: 10.1016/s0002-9149(00)01165-6. [DOI] [PubMed] [Google Scholar]
- 37.Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, Kalman J, Phillips RA, Steingart R, Brown EJ, Jr, Berkowitz R, Moskowitz R, Soni A, Mancini D, Bijou R, Sehhat K, Varshneya N, Kukin M, Katz SD, Sleeper LA, Le Jemtel TH. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. Journal of the American College of Cardiology. 2004;43:1432–1438. doi: 10.1016/j.jacc.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 38.Baicu CF, Zile MR, Aurigemma GP, Gaasch WH. Left ventricular systolic performance, function, and contractility in patients with diastolic heart failure. Circulation. 2005;111:2306–2312. doi: 10.1161/01.CIR.0000164273.57823.26. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. European heart journal. 2008 doi: 10.1093/eurheartj/ehn141. [DOI] [PubMed] [Google Scholar]
- 40.van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ, Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 41.Maurer MS, El Khoury Rumbarger L, King DL. Ventricular volume and length in hypertensive diastolic heart failure. J Am Soc Echocardiogr. 2005;18:1051–1057. doi: 10.1016/j.echo.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Maurer MS, Burkhoff D, Fried LP, Gottdiener J, King DL, Kitzman DW. Ventricular structure and function in hypertensive participants with heart failure and a normal ejection fraction: the Cardiovascular Health Study. Journal of the American College of Cardiology. 2007;49:972–981. doi: 10.1016/j.jacc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 43.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiological Reviews. 1993;73:413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- 44.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. Journal of the American College of Cardiology. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure.[see comment] JAMA. 1996;275(20):1557–1562. [PubMed] [Google Scholar]
- 46.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. The New England journal of medicine. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 47.Kass DA, Maughan WL. From 'Emax' to pressure-volume relations: A broader view. Circulation. 1988;77:1203–1212. doi: 10.1161/01.cir.77.6.1203. [DOI] [PubMed] [Google Scholar]
- 48.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin. 2008;4:23–36. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Kurrelmeyer KM, Torre-Amione G, Nagueh SF. Systolic and diastolic dyssynchrony in patients with diastolic heart failure and the effect of medical therapy. Journal of the American College of Cardiology. 2007;49:88–96. doi: 10.1016/j.jacc.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 51.Kass DA. An epidemic of dyssynchrony: but what does it mean? Journal of the American College of Cardiology. 2008;51:12–17. doi: 10.1016/j.jacc.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 52.Lopez B, Gonzalez A, Querejeta R, Larman M, Diez J. Alterations in the pattern of collagen deposition may contribute to the deterioration of systolic function in hypertensive patients with heart failure. Journal of the American College of Cardiology. 2006;48:89–96. doi: 10.1016/j.jacc.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 53.Borbely A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111(6):774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 54.van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbely A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 55.Douglas PS, Katz SE, Weinberg EO, Chen MH, Bishop SP, Lorell BH. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. Journal of the American College of Cardiology. 1998;32:1118–1125. doi: 10.1016/s0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- 56.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 57.Sweitzer NK, Lopatin M, Yancy CW, Mills RM, Stevenson LW. Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (>or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. The American journal of cardiology. 2008;101:1151–1156. doi: 10.1016/j.amjcard.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise Hemodynamics Enhance Diagnosis of Early Heart Failure with Preserved Ejection Fraction. Circ Heart Fail. 2010 doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rame JE, Ramilo M, Spencer N, Blewett C, Mehta SK, Dries DL, Drazner MH. Development of a depressed left ventricular ejection fraction in patients with left ventricular hypertrophy and a normal ejection fraction. The American journal of cardiology. 2004;93:234–237. doi: 10.1016/j.amjcard.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 60.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, Manolio TA, Dries DL, Siscovick DS. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. Journal of the American College of Cardiology. 2004;43:2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 61.Colucci WS, Ribeiro JP, Rocco MB, Quigg RJ, Creager MA, Marsh JD, Gauthier DF, Hartley LH. Impaired chronotropic response to exercise in patients with congestive heart failure. Role of postsynaptic beta-adrenergic desensitization. Circulation. 1989;80:314–323. doi: 10.1161/01.cir.80.2.314. [DOI] [PubMed] [Google Scholar]
- 62.Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84:1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]
- 63.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. Journal of the American College of Cardiology. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shapiro BP, McGoon MD, Redfield MM. Unexplained pulmonary hypertension in elderly patients. Chest. 2007;131:94–100. doi: 10.1378/chest.06-1571. [DOI] [PubMed] [Google Scholar]
- 65.Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. Journal of the American College of Cardiology. 2006;47:742–748. doi: 10.1016/j.jacc.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 66.Guder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, Angermann CE, Stork S. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007;115:1754–1761. doi: 10.1161/CIRCULATIONAHA.106.653964. [DOI] [PubMed] [Google Scholar]
- 67.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. The American journal of cardiology. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones RC, Francis GS, Lauer MS. Predictors of mortality in patients with heart failure and preserved systolic function in the Digitalis Investigation Group trial. Journal of the American College of Cardiology. 2004;44:1025–1029. doi: 10.1016/j.jacc.2004.05.077. [DOI] [PubMed] [Google Scholar]
- 69.Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez-Sendon J, Teerlink JR, White M, McMurray JJ, Komajda M, McKelvie R, Ptaszynska A, Hetzel SJ, Massie BM, Carson PE. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121:1393–1405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 70.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91–97. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.