Abstract
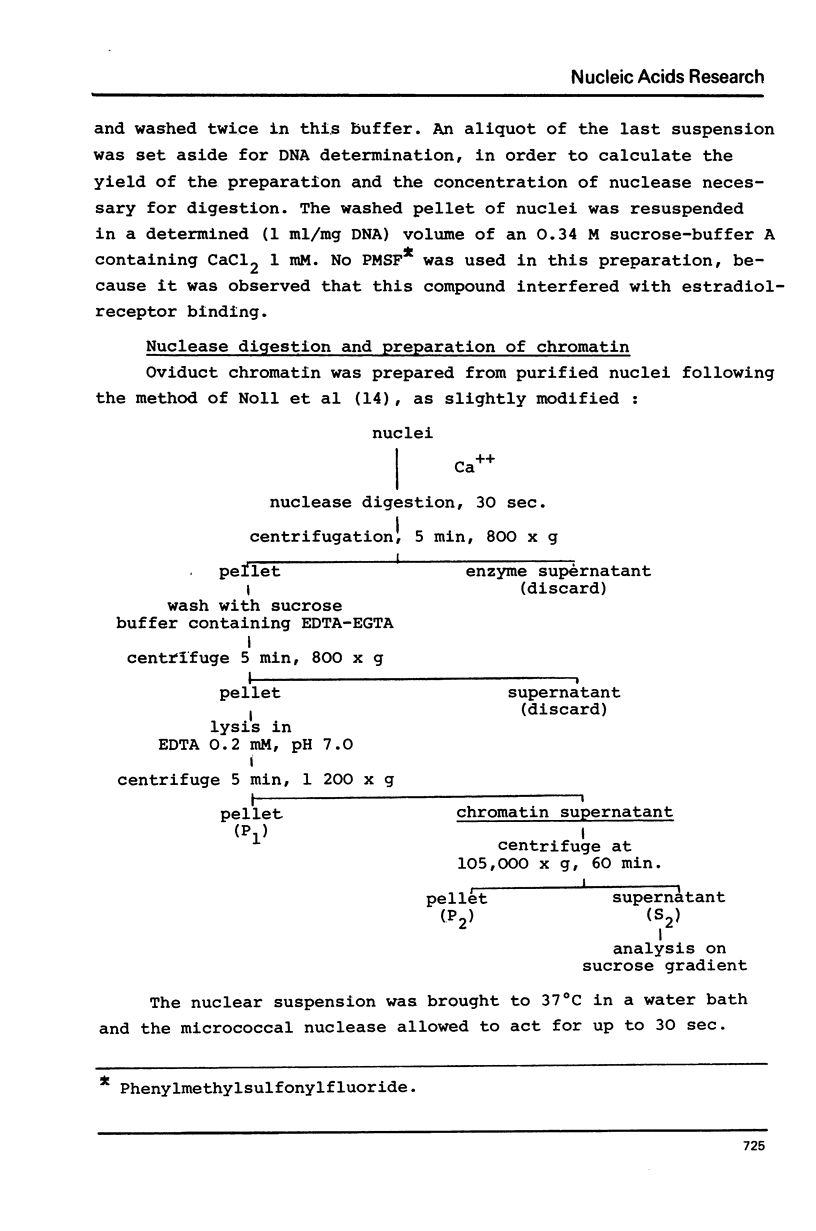
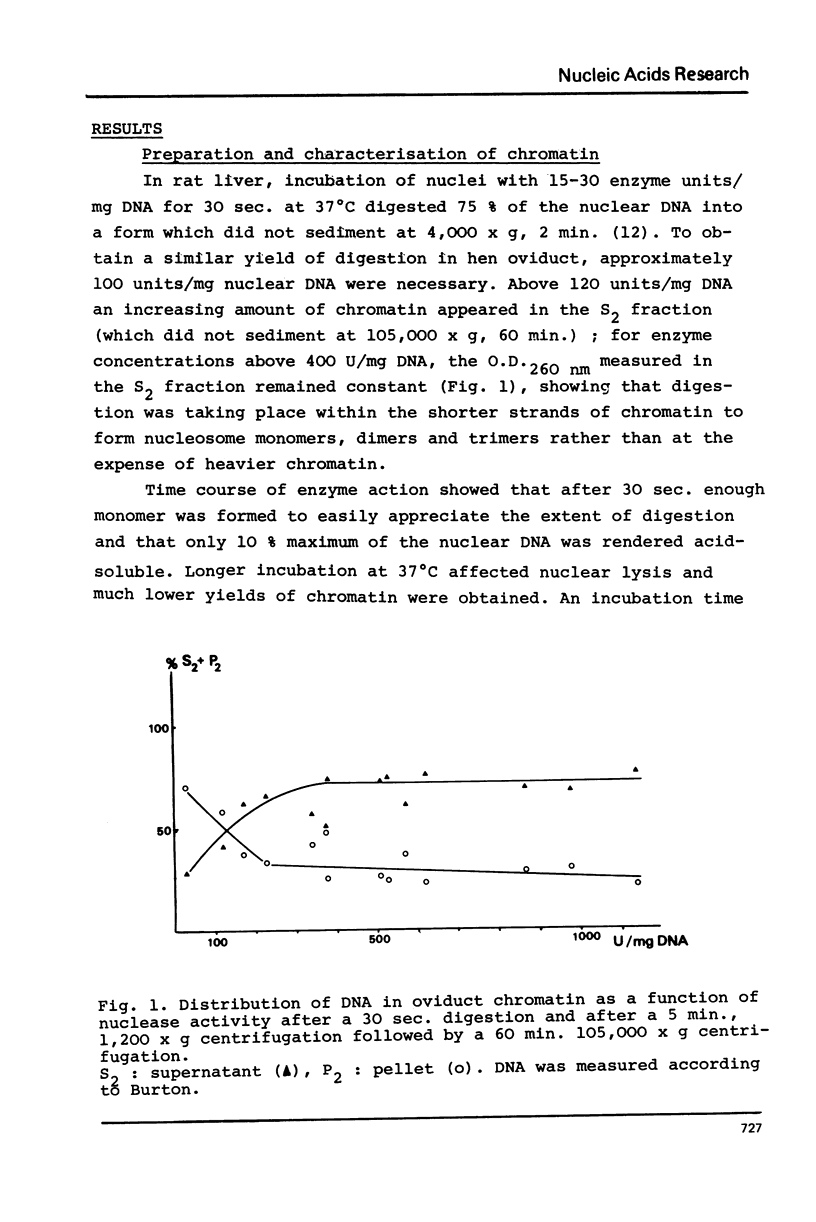
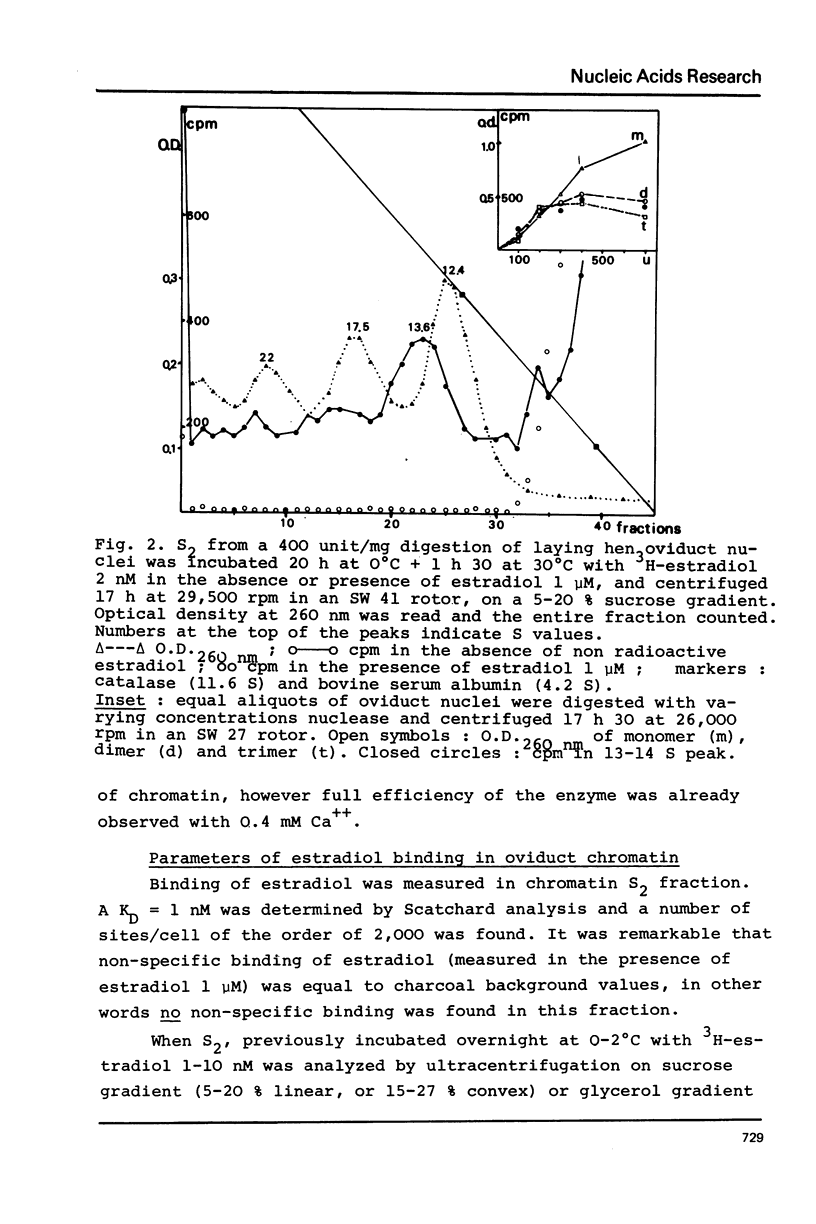
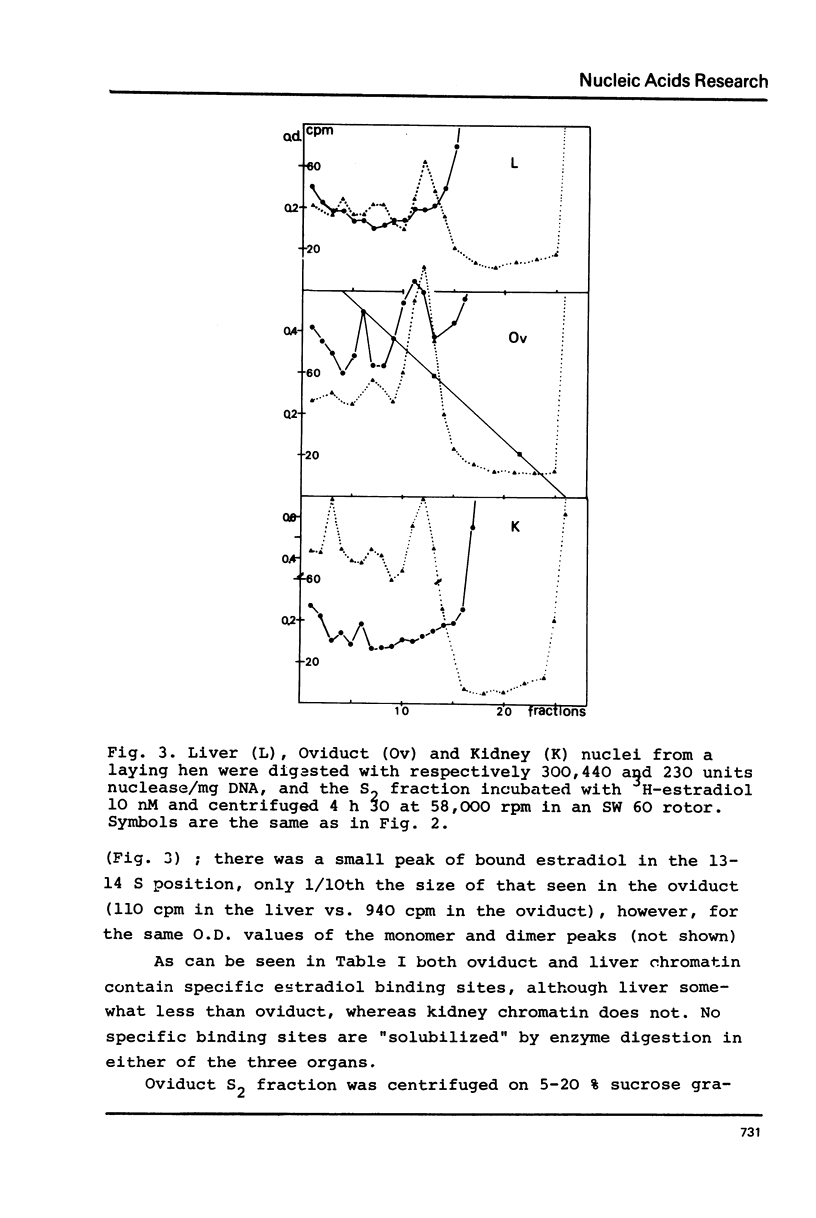
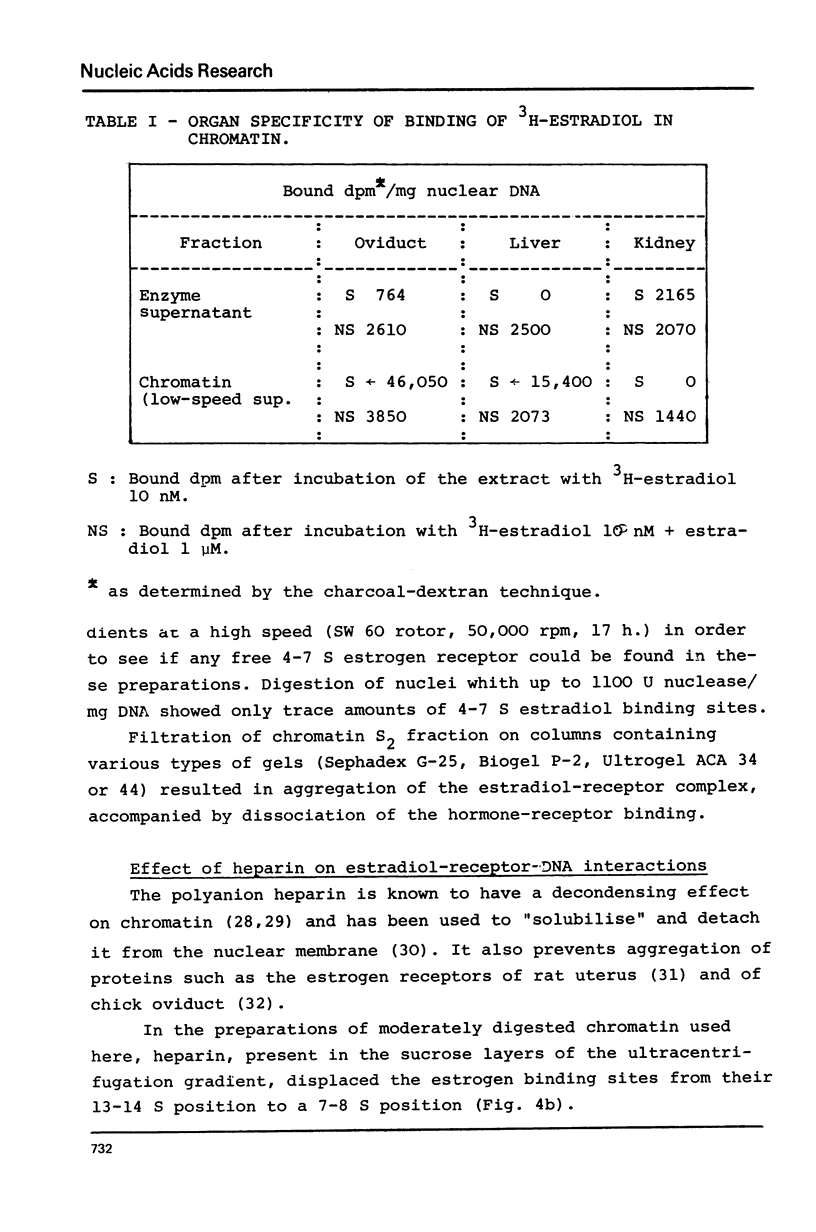
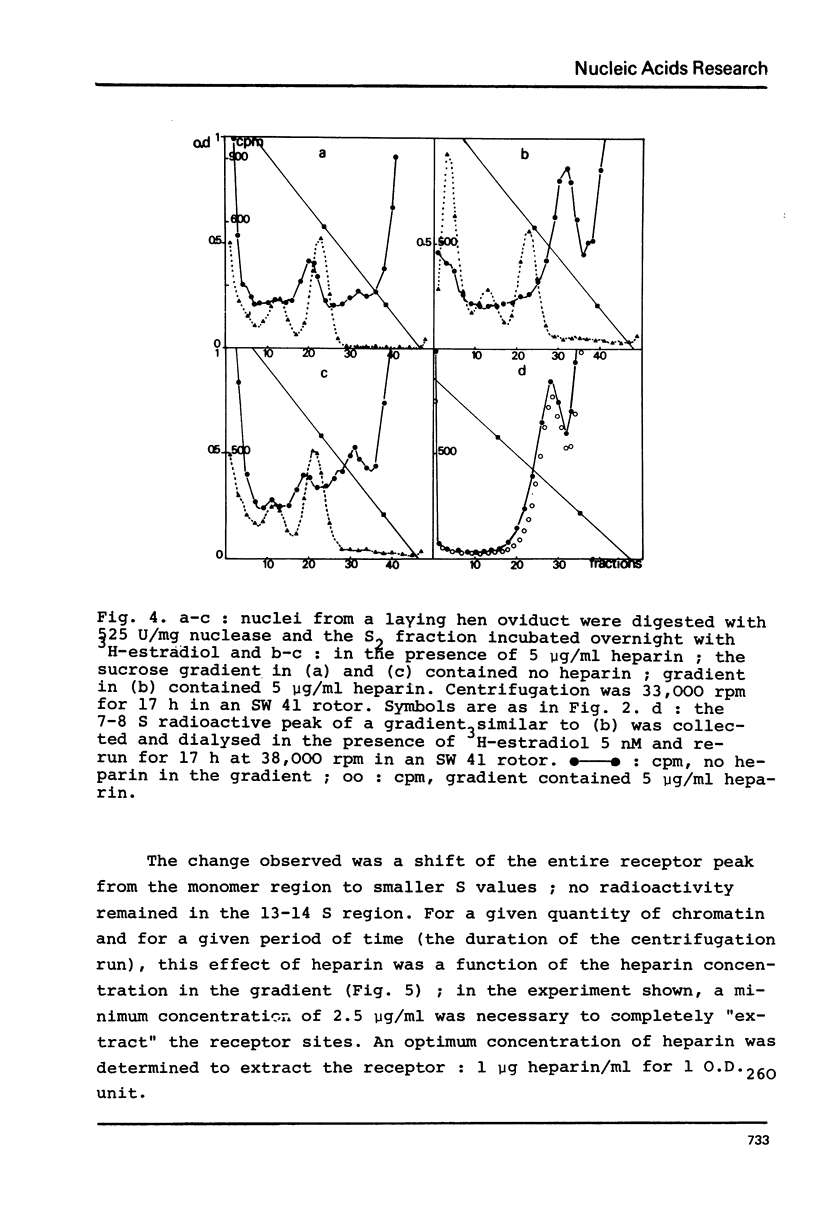
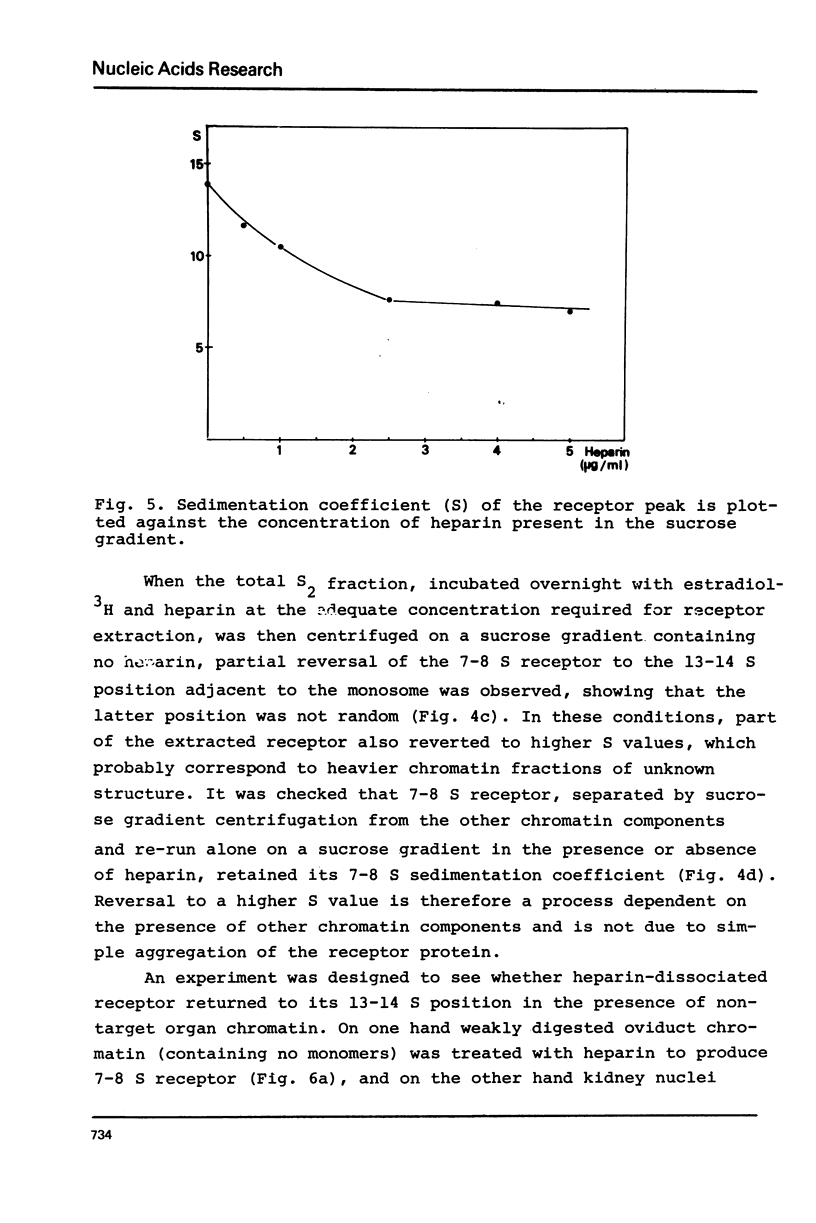
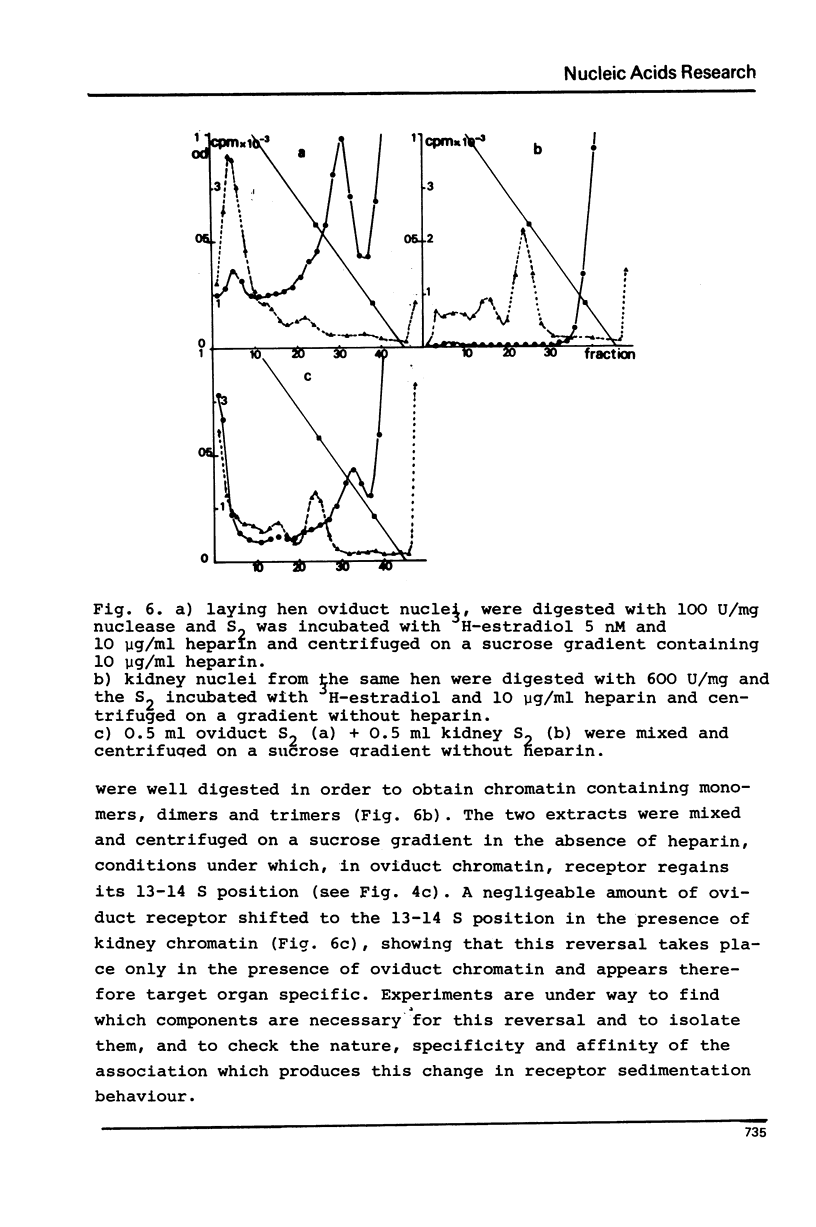
Nuclei from laying hen oviduct were prepared according to Hewish and Burgoyne i.e. in the presence of spermine and spermidine and in the absence of divalent cations and were then moderately digested by micrococcal nuclease. When the resulting chromatin was analysed by ultracentrifugation on a sucrose gradient, a peak of specific estradiol-binding sites was observed, sedimenting slightly faster (13-14 S) than the mononucleosomes (12 S). When the chromatin was centrifuged on a gradient containing heparin (5 microngram/ml) the sedimentation coefficient of the estradiol receptor peak shifted to 7-8 S; it returned to the 13-14 S position in the absence of heparin, when target organ chromatin was also present in the gradient. The preparation of the chromatin is described and the validity of the method to explore receptor localisation is discussed, as is the specificity of the receptor-DNA interaction.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenburger W., Hörz W., Zachau H. G. Nuclease cleavage of chromatin at 100-nucleotide pair intervals. Nature. 1976 Dec 9;264(5586):517–522. doi: 10.1038/264517a0. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornens M. Letter: Action of heparin on nuclei: solubilization of chromatin enabling the isolation of nuclear membranes. Nature. 1973 Jul 6;244(5410):28–30. doi: 10.1038/244028a0. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Carpenter B. G., Rattle H. W. Magnetic resonance studies of deoxyribonucleoprotein. Nature. 1973 Jan 12;241(5385):123–126. doi: 10.1038/241123a0. [DOI] [PubMed] [Google Scholar]
- Chamness G. C., McGuire W. L. Estrogen receptor in the rat uterus. Physiological forms and artifacts. Biochemistry. 1972 Jun 20;11(13):2466–2472. doi: 10.1021/bi00763a013. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., Butler P. J. Structure of transcriptionally-active chromatin subunits. Nucleic Acids Res. 1977 Sep;4(9):3155–3173. doi: 10.1093/nar/4.9.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., Garrard W. T., Bagi G., Wilson R. F., Bonner J. Partial purification of the template-active fraction of chromatin: a preliminary report. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2193–2197. doi: 10.1073/pnas.71.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., Murphy R. F., Bonner J. Structure of transcriptionally active chromatin. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4404–4408. doi: 10.1073/pnas.72.11.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G. S. Nature of oestrogen specific binding sites in the nuclei of mouse uteri. Nat New Biol. 1971 Jun 23;231(25):246–248. doi: 10.1038/newbio231246a0. [DOI] [PubMed] [Google Scholar]
- Harrison R. W., Toft D. O. Estrogen receptors in the chick oviduct. Endocrinology. 1975 Jan;96(1):199–205. doi: 10.1210/endo-96-1-199. [DOI] [PubMed] [Google Scholar]
- Hemminki K. Differential distribution of oestrogen receptors in subfractions of oviduct chromatin. Acta Endocrinol (Copenh) 1977 Jan;84(1):215–224. doi: 10.1530/acta.0.0840215. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Honda B. M., Baillie D. L., Candido E. P. Properties of chromatin subunits from developing trout testis. J Biol Chem. 1975 Jun 25;250(12):4643–4647. [PubMed] [Google Scholar]
- Kornberg R. D. Chromatin structure: a repeating unit of histones and DNA. Science. 1974 May 24;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Kraemer R. J., Coffey D. S. The interaction of natural and synthetic polyanions with mammalian nucleo. I. DNA synthesis. Biochim Biophys Acta. 1970 Dec 14;224(2):553–567. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levy B., Baxter J. D. Distribution of thyroid and glucocorticoid hormone receptors in transcriptionally active and inactive chromatin. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1045–1051. doi: 10.1016/0006-291x(76)90301-6. [DOI] [PubMed] [Google Scholar]
- Mester J., Baulieu E. E. Nuclear estrogen receptor of chick liver. Biochim Biophys Acta. 1972 Jan 28;261(1):236–244. doi: 10.1016/0304-4165(72)90334-0. [DOI] [PubMed] [Google Scholar]
- Milgrom E., Baulieu E. E. A method for studying binding proteins, based upon differential dissociation of small ligand. Biochim Biophys Acta. 1969 Dec 23;194(2):602–605. doi: 10.1016/0005-2795(69)90124-x. [DOI] [PubMed] [Google Scholar]
- Noll M., Thomas J. O., Kornberg R. D. Preparation of native chromatin and damage caused by shearing. Science. 1975 Mar 28;187(4182):1203–1206. doi: 10.1126/science.187.4182.1203. [DOI] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Saiga H., Kinoshita S. Changes of chromatin structure induced by acid mucopolysaccharides. Exp Cell Res. 1976 Oct 1;102(1):143–152. doi: 10.1016/0014-4827(76)90309-8. [DOI] [PubMed] [Google Scholar]
- Shaw B. R., Herman T. M., Kovacic R. T., Beaudreau G. S., Van Holde K. E. Analysis of subunit organization in chicken erythrocyte chromatin. Proc Natl Acad Sci U S A. 1976 Feb;73(2):505–509. doi: 10.1073/pnas.73.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner-Webb B., Felsenfeld G. A comparison of the digestion of nuclei and chromatin by staphylococcal nuclease. Biochemistry. 1975 Jul;14(13):2915–2920. doi: 10.1021/bi00684a019. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C. Nuclear binding of progesterone in chick oviduct. Multiple binding sites in vivo and transcriptional response. Biochem J. 1976 May 15;156(2):391–398. doi: 10.1042/bj1560391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., De Troy B., Roberts R. J., Sambrook J. Agarose slab-gel electrophoresis equipment. Anal Biochem. 1975 Sep;68(1):36–46. doi: 10.1016/0003-2697(75)90676-4. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Holde K. E., Sahasrabuddhe C. G., Shaw B. R. A model for particulate structure in chromatin. Nucleic Acids Res. 1974 Nov;1(11):1579–1586. doi: 10.1093/nar/1.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]


