Abstract
Studies of striatal physiology and motor control have increasingly relied on the use of bacterial artificial chromosome (BAC) transgenic mice expressing fluorophores or other genes under the control of genetic regulatory elements for the dopamine D1 receptor (D1R) or dopamine D2 receptor (D2R). Three recent studies have compared wild-type, D1R, and D2R BAC transgenic mice, and found significant differences in physiology and behavior, calling into question the use of these mice in studies of normal circuit function. We repeated the behavioral portions of these studies in wild-type C57BL/6 mice and hemizygous Drd1a-td Tomato (D1-Tmt), Drd1a-eGFP (D1-GFP), and Drd2-eGFP (D2-GFP) mice backcrossed into the C57BL/6 background. Our three laboratories independently found that open-field locomotion, acute locomotor responses to cocaine (20 mg/kg), locomotor sensitization to 5 d of daily injections of cocaine (15 mg/kg) or amphetamine (3 mg/kg), cocaine (20 mg/kg) conditioned place preference, and active avoidance learning to paired light and footshock were indistinguishable in these four mouse lines. These results suggest that while it is crucial to screen new transgenic mouse lines for abnormal behavior and physiology, these BAC transgenic mouse lines remain extremely valuable tools for evaluating the cellular, synaptic, and circuit basis of striatal motor control and associative learning.
Introduction
In recent years, numerous transgenic mouse lines have been generated using bacterial artificial chromosome (BAC) technology (Gong et al., 2003, 2007) to express fluorophores or other genes in specific subsets of neurons. This method has allowed neurophysiologists to selectively target subpopulations of neurons in brain slices (Wang et al., 2006; Kreitzer and Malenka, 2007). In particular, basal ganglia neurophysiology has been limited by the fact that striatal principal neurons, medium spiny neurons (MSNs), are divided into two functionally distinct but intermixed populations. MSNs expressing the D1 dopamine receptor form the direct pathway and striatonigral projection, whereas MSNs expressing the D2 dopamine receptor form the indirect pathway and striatopallidal projection (Gerfen et al., 1990). These two populations of MSNs are thought to have complementary if not opposing effects on movement (DeLong, 1990) and associative learning (Bach et al., 2008; Lobo et al., 2010; Boschen et al., 2011) so distinguishing them is essential. Historically, these neurons could not be distinguished in physiological recordings without the use of single-cell PCR (Surmeier et al., 1996; Wang et al., 2006), retrograde tracing (Waszczak et al., 1998; Gertler et al., 2008; Planert et al., 2010), or by filling them with a marker followed by post hoc immunohistochemistry (Bagetta et al., 2011). By permitting prospective identification of cell types and cell type-specific molecular manipulations, BAC transgenic technology has been the foundation for many recent studies elucidating the properties of different populations of striatal neurons, thus enabling a greater understanding of how basal ganglia circuits function in health and disease.
Despite the rapid adoption of these transgenic mouse lines, three recent studies have identified concerning behavioral abnormalities in D1 and D2 BAC transgenic mice (Ade et al., 2011; Bagetta et al., 2011; Kramer et al., 2011). Mice from a D2-GFP (Drd2-eGFP) mouse line, which express GFP in indirect pathway MSNs, showed changes in dopamine receptor expression, dopamine sensitivity, and open-field behavior compared with wild-type and D1-GFP (Drd1a-eGFP) mice (Kramer et al., 2011). Abnormal open-field behavior was shown in the same mouse line by another laboratory (Ade et al., 2011). A third laboratory found differences in striatal synaptic plasticity, open-field locomotion, and active avoidance in D1-GFP mice (Bagetta et al., 2011). These studies appropriately call into question the use of BAC transgenic mouse lines for both striatal physiology and behavior.
We have used similar mouse lines in our three independent laboratories for several years, and therefore sought to determine whether these abnormalities were specific to the mouse colonies examined, or were a general feature of these BAC transgenic lines. We report that in assays of open-field behavior, acute and chronic responses to psychostimulants, conditioned place preference (CPP), and active avoidance learning, hemizygous BAC transgenic D1-Tmt (Drd1a-tdomato), D1-GFP, and D2-GFP mouse lines are indistinguishable from wild-type C57BL/6 mice. Thus the previously reported abnormalities in similar BAC transgenic lines cannot be attributed to the presence of the BAC transgenes, and these lines remain a valuable tool for study of striatal circuits in adaptive and pathological behavior.
Materials and Methods
Animals
Mice were bred and tested in three different laboratories, located at the Gladstone Institute, Stanford University, and the University of Geneva. Hemizygous mice of either sex were used in all experiments. D2-GFP mice were originally obtained from X. William Yang at the University of California, Los Angeles (Kreitzer and Malenka laboratories) or Paul Greengard and Jean-Antoine Girault at The Rockefeller University (New York, NY) and University of Pierre and Marie Curie (Paris, France), respectively (Luscher laboratory), and were back-crossed to C57BL/6 mice for >10 generations. D1-GFP mice were back-crossed to C57BL/6 mice for 4–5 generations. D1-Tmt line 5 mice were obtained from Nicole Calakos (Duke University, Durham, NC; Shuen et al., 2008). Mice from all three lines were bred against C57BL/6 mice (Jackson Labs) to obtain hemizygous mice for behavioral experiments. Nontransgenic littermates or wild-type C57BL/6 mice (Jackson Labs) were used as controls. For open-field behavior, acute responses to cocaine, psychostimulant sensitization, and active avoidance learning, wild-type C57BL/6 (Jackson Labs) were used as controls. For acute locomotor responses to cocaine, cocaine sensitization, and cocaine conditioned place preference, nontransgenic littermates were used as an additional control group. All mice were 6–10 weeks of age at the time of behavioral measurements. Mice were housed on a 7:00 A.M.–7:00 P.M. light cycle, and testing was performed during daytime hours.
Drugs
Amphetamine and cocaine (Sigma-Aldrich) were dissolved in normal saline and administered intraperitoneally. Amphetamine (0.6 mg/ml) was administered at a dose of 3 mg/kg. Cocaine (2 mg/ml) was administered at a dose of 20 mg/kg for acute locomotor responses and cocaine CPP, and 15 mg/kg (1.5 mg/ml) for assessment of sensitization. Equivalent volumes of saline were injected in control animals.
Behavior
Open-field behavior.
Naive animals were placed in square polycarbonate cages with opaque white walls measuring 41 cm on a side for a period of 10 min. Open-field locomotion was recorded and analyzed using video-tracking software (Noldus). Analysis was subdivided into time spent and distance traveled in peripheral areas (2.5 cm or less from the walls of the cage) versus the center, following the method of Bagetta et al. (2011).
Acute locomotor effects of cocaine.
Naive animals were placed in a behavioral chamber (a circular corridor with total diameter 19 cm, width 4 cm) after being injected with either normal saline (0.9%) or cocaine (20 mg/kg). Open-field locomotion was monitored using video-tracking software (Any-maze, Stoelting) for a total of 1 h after the injection, and distance traveled was divided into 5 min bins.
Amphetamine sensitization.
Animals were habituated to square polycarbonate cages for 1 h daily for 2 d before behavioral testing. Open-field locomotion was recorded and analyzed using video-tracking software (Noldus) for 1 h before and 1 h after administration of amphetamine (3 mg/kg dissolved in normal saline, intraperitoneal injection) or normal saline (intraperitoneal injection). Injections were given and behavioral responses recorded for 5 consecutive days.
Cocaine sensitization.
Animals were placed into a polycarbonate cage fitted with a beam-break movement-detection system (Med Associates), then injected with either cocaine (15 mg/kg) or normal saline (intraperitoneal injection), and returned to the polycarbonate cage for 15 min. This procedure was repeated for 5 consecutive days.
Active avoidance learning.
Animals were placed in a behavioral apparatus with two chambers and an open intervening door fitted with electrified steel floor beams (Gemini Avoidance System, San Diego Instruments). For five daily sessions (60 trials per session), after a 30 s dark acclimation period, each trial began with illumination of the chamber in which the mouse was located. After 4 s of light, a 0.2 mA shock was applied to the floor of that chamber until the mouse left the chamber, for a maximum of 25 s. When the mouse left the chamber, light and shock were coterminated. If the mouse exited <4 s after the light began, no shock was delivered. A randomly generated intertrial interval of 5–25 s separated trials. Conditioned responses were defined as trials during which the mouse left the chamber after the light came on, but before the shock being delivered (≤4 s).
Conditioned place preference.
Animals were placed in a behavioral apparatus (PanLab) consisting of two compartments distinguished by different floor and wall patterns, separated by a neutral chamber. Video tracking software (Any-maze, Stoelting) was used to determine time spent in each of the chambers. The CPP protocol was as follows. (1) In the preconditioning test (day 1), mice were placed in the neutral area, and the time spent in each compartment was measured for 15 min. (2) For conditioning (days 2–7), after injection of cocaine (20 mg/kg, days 2,4, 6 or 3, 5, and 7) or saline (days 2,4, 6 or 3, 5, and 7), mice were alternatively confined in each compartment for 20 min. Control mice received saline every day. (3) In the postconditioning test (day 8), mice had free access to both compartments, and the time spent in each compartment was measured as in the preconditioning test. Place preference (CPP score) was calculated for each mouse as the difference between postconditioning and preconditioning time spent in drug-paired compartment.
Statistics
Values cited in the text and figures are mean ± SEM. We performed power calculations to ensure adequate sample size for each behavioral test. Based on a predicted effect size of 1.5 SDs (similar to the effect sizes reported by Bagetta et al., 2011 and Kramer et al., 2011 for differences between BAC transgenic and wild-type mice), a sample size of 10 mice per group has a 99% likelihood of detecting a difference between groups using a 2-factor ANOVA with three groups (for example, interaction of genotype and treatment).
Basic locomotor activity measurements and conditioned place preference were compared using the one- or two-factor ANOVA. Acute responses to cocaine, psychostimulant sensitization and active avoidance learning in the three groups were assessed using the repeated-measures ANOVA. Post hoc comparisons were performed using the Tukey test. Statistical calculations were made using OpenStat software.
Results
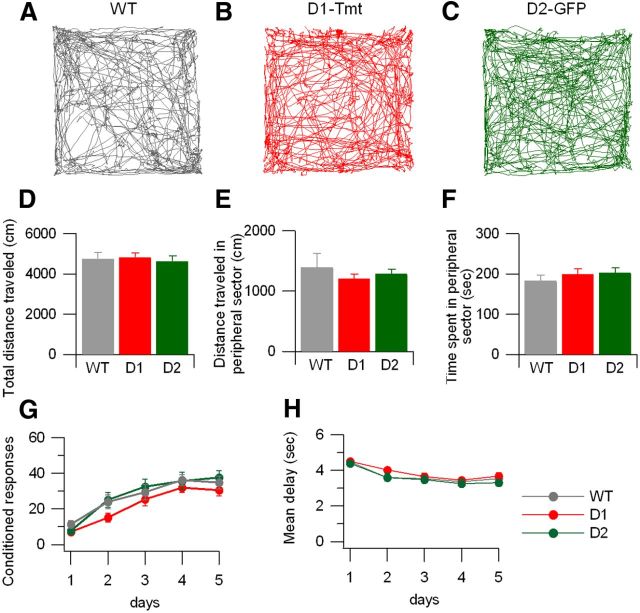
Following the same breeding strategy used for electrophysiology and behavioral studies in our laboratories, we have back-crossed BAC transgenic mice with wild-type C57BL/6 mice to generate transgenic mice in a C57BL/6 background. We used hemizygous D2-GFP, D1-GFP or D1-Tmt mice for all experiments. Controls were commercially available C57BL/6 mice from The Jackson Laboratory for all behavior other than conditioned place preference. We first measured open-field behavior in naive mice. Representative tracks for the 10 min session are shown for each of the mouse lines (Fig. 1A–C). There was no significant difference between the total distance traveled across groups (Fig. 1D, ANOVA: F(2,30) = 0.12, p = 0.89). As two previous studies had shown a difference between locomotion in the peripheral versus center portion of the open field in D1 or D2 BAC transgenic mice (Ade et al., 2011; Bagetta et al., 2011), we subdivided the session into distance traveled and time spent in peripheral versus central areas. We found no significant differences between mouse lines in either distance traveled (Fig. 1E, ANOVA: F(2,30) = 0.43, p = 0.65) or time spent in the peripheral sector (Fig. 1F, ANOVA: F(2,30) = 0.60, p = 0.55).
Figure 1.
Open-field locomotor activity and active avoidance learning are similar in wild-type, D1-Tmt, and D2-GFP mouse lines. A–C, Example traces of locomotor activity recorded in a novel cage for 10 min in each mouse line. D–F, Summary of locomotor activity in each session (n = 10 wild-type mice, n = 10 D1-Tmt mice, and n = 12 D2-GFP mice). D, Total distance traveled. E, Total distance traveled in the peripheral sector. F, Time spent in the peripheral sector. G, H, Active avoidance behavioral training (n = 15 wild-type, n = 10 D1-Tmt, and n = 12 D2-GFP mouse lines). G, Number of conditioned responses per 60-trial session on each of 5 consecutive training days. H, Mean delay time between the onset of the light cue and the mouse leaving the chamber on each training day. In all panels, wild-type is shown with gray symbols, D1-Tmt with red symbols, and D2-GFP with green symbols.
We also assessed more complex associative behavior in the BAC transgenic lines by administering a two-way active avoidance test, which has been shown to depend on the striatum (Vécsei and Beal, 1991), as well as on dopamine signaling (Zis et al., 1974; Koob et al., 1984). In this task, animals learn to avoid an aversive stimulus by forming an association between a predictive sensory cue and the aversive stimulus. A previous study showed that BAC Drd1a-eGFP reporter mice had deficits in this task (Bagetta et al., 2011). C57BL/6 as well as D1-Tmt and D2-GFP hemizygote mice were trained on the active avoidance task with five daily sessions of 60 trials each. During each trial, a light (conditioned stimulus) signaled to the mouse that a footshock would occur in 4 s. Mice learn to leave the chamber before the footshock (the conditioned response). Initially, mice display few conditioned responses, instead escaping when the shock is delivered, but over time they form the association and show more conditioned responses (Fig. 1G, repeated-measures ANOVA: F(4,148) = 88, p < 0.0001 for effect across days). In addition, the delay between onset of the conditioned stimulus (light) and the conditioned response decreased steadily over the training sessions (Fig. 1H, repeated-measures ANOVA: F(4,148) = 40, p < 0.0001 for effect across days). Neither of these measures of learning were significantly different between wild-type, D1-Tmt and D2-GFP mice (repeated-measures ANOVA: F(8,148) = 0.838, p = 0.5711 and F(8,148) = 0.585, p = 0.79, for interactions between genotype and day).
Next we examined the acute locomotor response to cocaine. Cocaine blocks the reuptake of dopamine at dopaminergic axon terminals, thus amplifying any existing dopamine signaling, which typically results in increased locomotion. A prior study showed a near-absent response to acute cocaine administration in homozygous D2-GFP mice (Kramer et al., 2011). In contrast, we found that wild-type C57BL/6, hemizygous D1-GFP and D2-GFP mice all responded to cocaine with a brisk increase in locomotion (Fig. 2A–C; repeated-measures ANOVA: F(10,750) = 94; p < 0.0001), which was significantly greater than in saline-injected mice. The responses to cocaine in all three groups were statistically indistinguishable (Fig. 2A–C, repeated-measures ANOVA: F(20,750) = 0.65, p = 0.52). These findings demonstrate that BAC transgenic animals show an acute response to cocaine comparable to that seen in wild-type animals.
Figure 2.
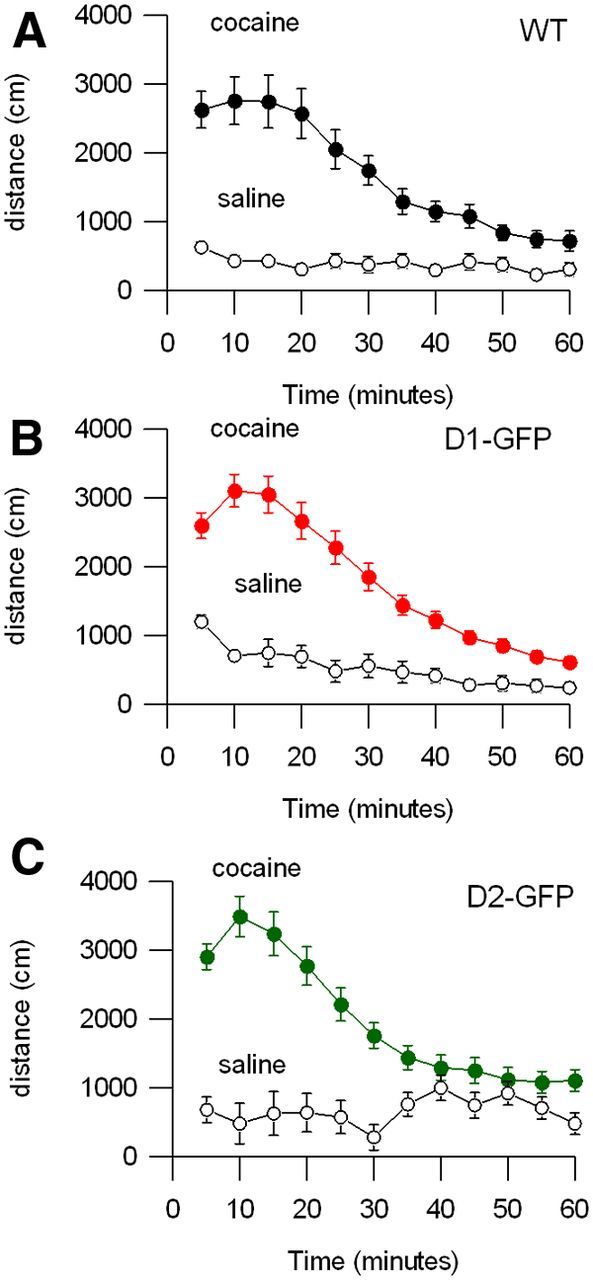
Acute locomotor responses to cocaine are similar in wild-type, D1-GFP, and D2-GFP mouse lines. A–C, Total distance traveled following intraperitoneal injection of saline (open symbols) or cocaine (filled symbols) in wild-type C57BL/6 (n = 12 saline and 12 cocaine), D1-GFP+/− mice (n = 4 saline and n = 23 cocaine), D2-GFP+/− mice (n = 6 saline and n = 22 cocaine).
Psychostimulant sensitization and conditioned place preference are commonly used as a model for certain features of reward learning and addiction (Robinson et al., 1998; Tzschentke, 1998; Vezina and Leyton, 2009). Both phenomena are thought to involve modulation of midbrain dopamine circuitry and its striatal targets (Anderson and Pierce, 2005; Hikida et al., 2010; Wolf and Ferrario, 2010). In cocaine sensitization, the locomotor response to a fixed dose of psychostimulant is enhanced on each subsequent day of exposure, until a plateau is reached. This behavior was impaired in a prior study of D2-GFP homozygotes (Kramer et al., 2011). We performed cocaine sensitization by giving one group of mice daily intraperitoneal injections of saline, the other daily injections of cocaine (15 mg/kg), and measuring the locomotor response in each. Wild-type animals injected with cocaine showed both an acute increase in locomotion and subsequent robust increases in locomotion over the course of 5 d (Fig. 3A,F), while saline-injected animals moved a similar distance each day. D1-Tmt, D2-GFP, and wild-type mice showed strong locomotor sensitization (repeated-measures ANOVA: F(4,104) = 23.8, p < 0.0001 for effect across days). To confirm this behavior using a different dopaminergic drug, we examined amphetamine locomotor sensitization as well. Wild-type, D1-Tmt, and D2-GFP mice all showed robust levels of locomotor sensitization in response to the daily amphetamine treatments (Fig. 3E–G; repeated-measures ANOVA: F(4,96) = 94.4, p < 0.0001 for effect across days, summarized in Fig. 3H). The lack of differences between wild-type, D1-Tmt and D2-GFP mice in either cocaine (repeated-measures ANOVA: F(8,104) = 0.671, p = 0.72 for interaction between genotype and day) or amphetamine locomotor sensitization (repeated-measures ANOVA: F(8,96) = 1.44, p = 0.19 for interaction between genotype and day) demonstrate that this extensively studied form of behavioral plasticity is normal in these BAC transgenic lines.
Figure 3.
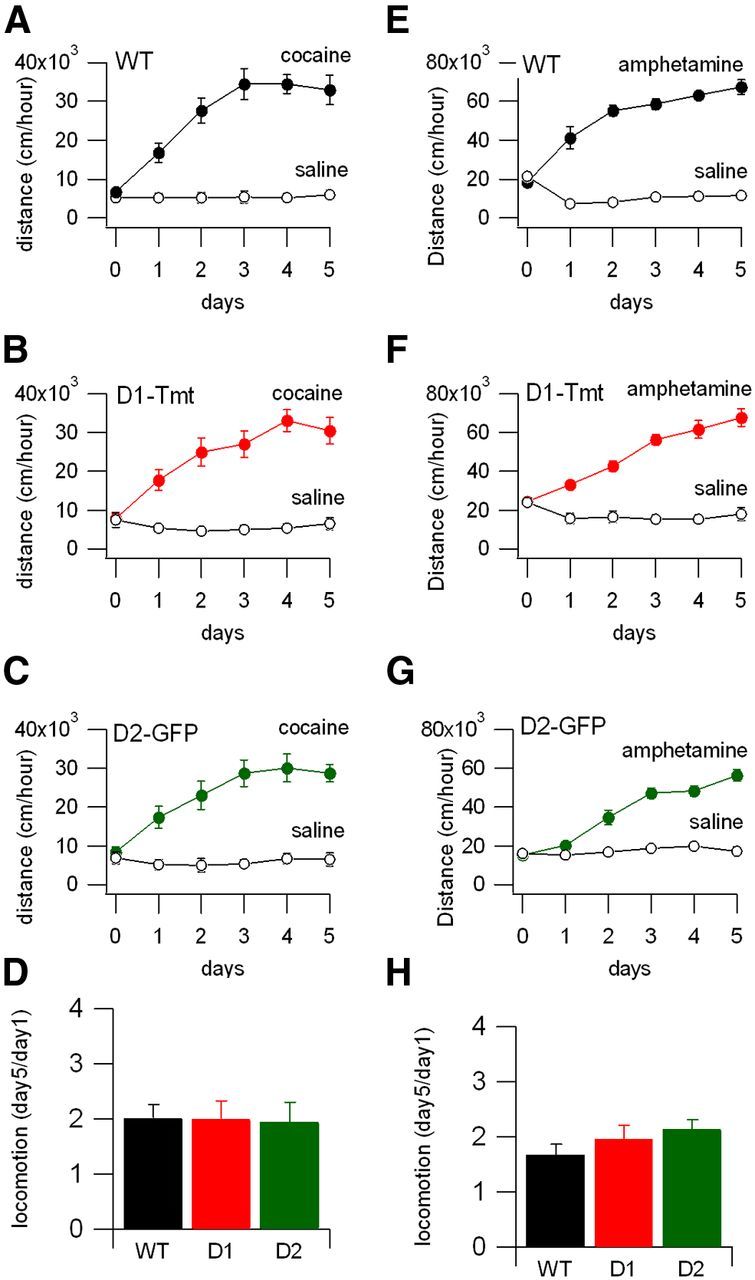
Psychostimulant locomotor sensitization is similar in wild-type, D1-Tmt, and D2-GFP mouse lines. A–C, Total distance traveled following intraperitoneal injection of saline (open symbols) or cocaine (filled symbols, 15 mg/kg) in wild-type C57BL/6 (n = 7 saline and n = 10 cocaine), D1-Tmt (n = 6 saline and n = 8 cocaine), and D2-GFP (n = 6 saline and n = 8 cocaine) mice. D, Summary of ratio of locomotion on day 5 to day 1 of cocaine for each of the mouse lines. E–G, Total distance traveled following intraperitoneal injection of saline (open symbols) versus amphetamine (filled symbols, 3 mg/kg) in wild-type C57BL/6, D1-Tmt+/−, and D2-GFP+/− mice (n = 4 saline and n = 8 cocaine for each group). H, Summary of ratios of locomotion on day 5 to day 1 of amphetamine for each of the mouse lines.
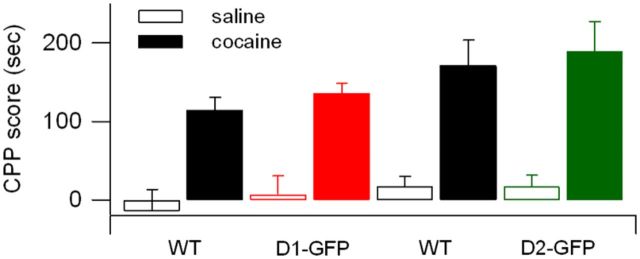
Finally, to further examine whether dopamine-dependent learning was normal in these mouse lines, we evaluated D1-GFP and D2-GFP hemizygotes and wild-type littermates for their ability to develop cocaine-induced CPP. In this task, which was developed as an assay of drug-seeking behavior (Tzschentke, 1998), animals were exposed to cocaine in one behavioral context and to saline in another, over several learning sessions, then assayed for their preference for the cocaine-paired chamber (CPP score). Experiments were performed in four groups of mice: D1-GFP hemizygotes and their nontransgenic littermates, and D2-GFP hemizygotes and their nontransgenic littermates. All groups showed a significant difference between saline and cocaine CPP scores (Fig. 4; ANOVA: F(1,32) = 55, p < 0.0001 for saline vs cocaine), which was indistinguishable across groups (ANOVA: F(2,32) = 1.33, p = 0.28 for interaction between genotype and drug treatment), demonstrating that both BAC transgenic lines tested have intact and normal cocaine CPP. These results showing intact cocaine CPP further support the hypothesis that BAC transgenic mouse lines have normal striatal- and dopamine-dependent behavior.
Figure 4.
Cocaine conditioned place preference is similar in D1-GFP, and D2-GFP hemizygotes and littermate controls. CPP score in wild-type (D1-GFP−/−) littermates (n = 4 saline, n = 7 cocaine); D1-GFP+/− (n = 7 saline, n = 10 cocaine), wild-type (D2-GFP−/−) littermates (n = 4 saline, n = 6 cocaine); and D2-GFP+/− (n = 5 saline, n = 6 cocaine).
Discussion
Using mice from three separate colonies and using different behavioral measurement techniques, we found that open-field behavior, acute response to cocaine, and three types of striatal-dependent behaviors are indistinguishable in wild-type and hemizygous D1- and D2-BAC transgenic mice. These findings contradict recent reports that D1 and D2 BAC transgenic mice display abnormal behavior and strongly suggest that the BAC transgene insertion does not account for the observed behavioral changes. An important feature of our studies was the use of commercially available wild-type controls, which increase the ability to detect the effects of genetic background and/or inbreeding of mice, in addition to the effects of transgenic material. Using both wild-type and littermate controls in our cohorts of mice, we found no differences in behavior across mouse lines.
BAC transgenic mouse lines are increasingly critical tools for study of neural circuits, particularly those involving the striatum. Given that behavioral abnormalities were reported in several of these lines, we performed several well established behavioral assays which reflect dopamine signaling and striatal function. These tests assessed motor and limbic aspects of basal ganglia function, as well as experience-dependent behavioral plasticity (Redgrave et al., 2010). Normal behavioral results in our cohorts strongly suggest that neural circuits mediating these behaviors are also normal. There are several possible explanations for the discrepancy between our results and those of the other groups using D1 and D2 BAC transgenic lines: (1) the use of hemizygous versus homozygous animals, (2) differences in breeding strategy, and/or (3) differences in genetic background. Our use of hemizygous mice which have been outbred over many generations to C57BL/6 mice can reduce the impact of within-colony breeding. In contrast, generating homozygous mice involves some degree of inbreeding, which can increase the likelihood of maintaining inbred copy number variations of the BAC transgenes and may also promote the expression of additional genetic polymorphisms that affect behavior. The more subtle differences in behavior seen in D1-GFP and D2-GFP hemizygotes compared with D2-GFP homozygotes (Ade et al., 2011; Bagetta et al., 2011) support this hypothesis. Furthermore, genetic background may interact with BAC transgenes to attenuate or enhance behavioral abnormalities (Chan et al., 2012).
Our results suggest that BAC transgenic mouse lines used to study striatal function remain valuable tools and, in contrast to previous reports, do not necessarily display abnormal behaviors in common assays of striatal function. However, together with these previous reports, our results suggest that there are likely to be additional factors beyond the presence of the transgene(s) that determine behavior. One particularly important factor may be the breeding strategy that is used to generate the animals. Moreover, using C57BL/6 mice as strain controls should enhance the sensitivity to any abnormal behavior. The continued use of these experimentally powerful BAC transgenic mouse lines will have to be monitored carefully to avoid contamination of results by the additional effects that transgenic manipulation may cause and conclusions will always need to be supported by the judicious use of control subjects.
Footnotes
The work in this article was funded by the A.P. Giannini Research Fellowship (A.B.N.), NINDS Grant R01 NS064984 (A.C.K.), NIDA Grant P01 DA008227 (R.C.M.), and a grant of the Swiss National Science Foundation and National Center of Competence in Research (C.L.).
References
- Ade KK, Wan Y, Chen M, Gloss B, Calakos N. An improved BAC transgenic fluorescent reporter line for sensitive and specific identification of striatonigral medium spiny neurons. Front Syst Neurosci. 2011;5:32. doi: 10.3389/fnsys.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Bach ME, Simpson EH, Kahn L, Marshall JJ, Kandel ER, Kellendonk C. Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning. Proc Natl Acad Sci U S A. 2008;105:16027–16032. doi: 10.1073/pnas.0807746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagetta V, Picconi B, Marinucci S, Sgobio C, Pendolino V, Ghiglieri V, Fusco FR, Giampà C, Calabresi P. Dopamine-dependent long-term depression is expressed in striatal spiny neurons of both direct and indirect pathways: implications for Parkinson's disease. J Neurosci. 2011;31:12513–12522. doi: 10.1523/JNEUROSCI.2236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschen SL, Wietzikoski EC, Winn P, Da Cunha C. The role of nucleus accumbens and dorsolateral striatal D2 receptors in active avoidance conditioning. Neurobiol Learn Mem. 2011;96:254–262. doi: 10.1016/j.nlm.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Chan CS, Peterson JD, Gertler TS, Glajch KE, Quintana RE, Cui Q, Sebel LE, Plotkin JL, Shen W, Heiman M, Heintz N, Greengard P, Surmeier DJ. Strain-specific regulation of striatal phenotype in Drd2-eGFP bacterial artificial chromosome transgenic mice. J Neurosci. 2012;32:9124–9132. doi: 10.1523/JNEUROSCI.0229-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Koob GF, Simon H, Herman JP, Le Moal M. Neuroleptic-like disruption of the conditioned avoidance response requires destruction of both the mesolimbic and nigrostriatal dopamine systems. Brain Res. 1984;303:319–329. doi: 10.1016/0006-8993(84)91218-6. [DOI] [PubMed] [Google Scholar]
- Kramer PF, Christensen CH, Hazelwood LA, Dobi A, Bock R, Sibley DR, Mateo Y, Alvarez VA. Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice. J Neurosci. 2011;31:126–132. doi: 10.1523/JNEUROSCI.4287-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planert H, Szydlowski SN, Hjorth JJ, Grillner S, Silberberg G. Dynamics of synaptic transmission between fast-spiking interneurons and striatal projection neurons of the direct and indirect pathways. J Neurosci. 2010;30:3499–3507. doi: 10.1523/JNEUROSCI.5139-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Shuen JA, Chen M, Gloss B, Calakos N. Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J Neurosci. 2008;28:2681–2685. doi: 10.1523/JNEUROSCI.5492-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Vécsei L, Beal MF. Comparative behavioral and neurochemical studies with striatal kainic acid- or quinolinic acid-lesioned rats. Pharmacol Biochem Behav. 1991;39:473–478. doi: 10.1016/0091-3057(91)90211-j. [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56(Suppl 1):160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Waszczak BL, Martin LP, Greif GJ, Freedman JE. Expression of a dopamine D2 receptor-activated K+ channel on identified striatopallidal and striatonigral neurons. Proc Natl Acad Sci U S A. 1998;95:11440–11444. doi: 10.1073/pnas.95.19.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zis AP, Fibiger HC, Phillips AG. Reversal by l-dopa of impaired learning due to destruction of the dopaminergic nigro-neostriatal projection. Science. 1974;185:960–962. doi: 10.1126/science.185.4155.960. [DOI] [PubMed] [Google Scholar]