Abstract
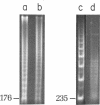
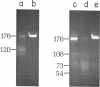
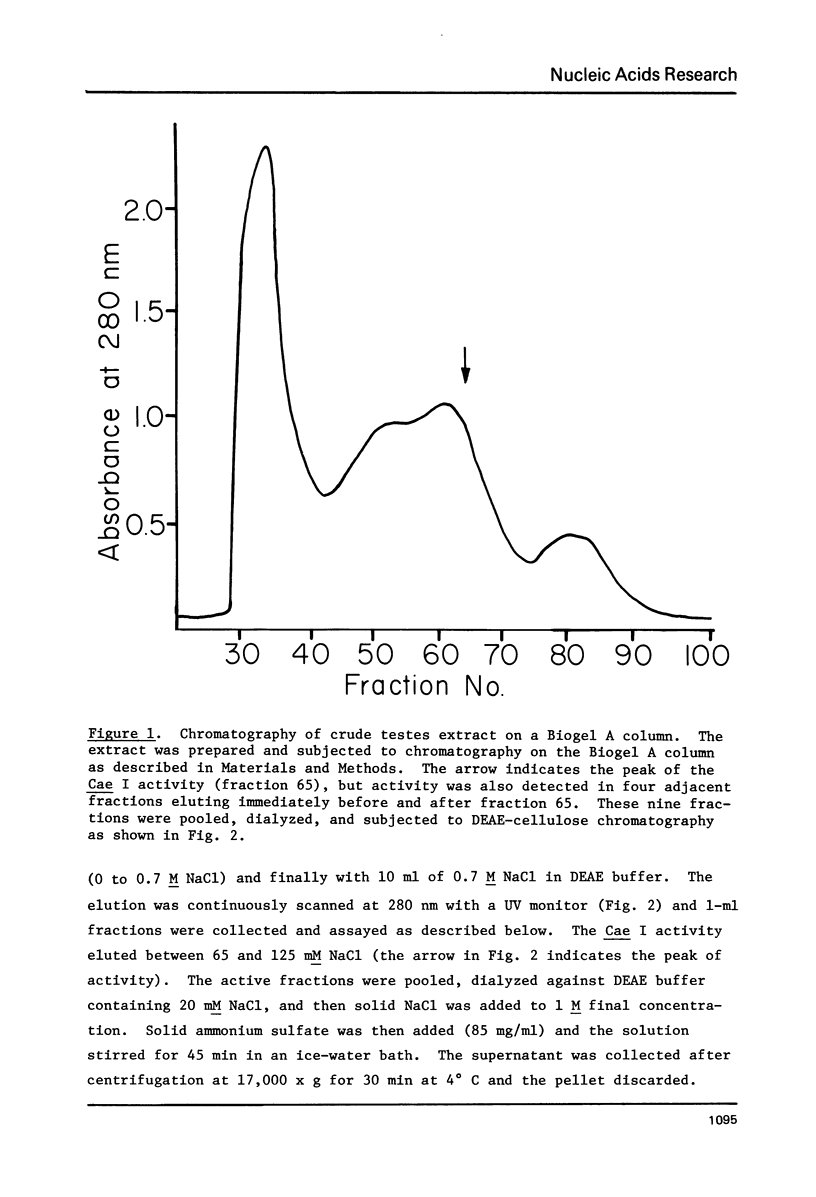
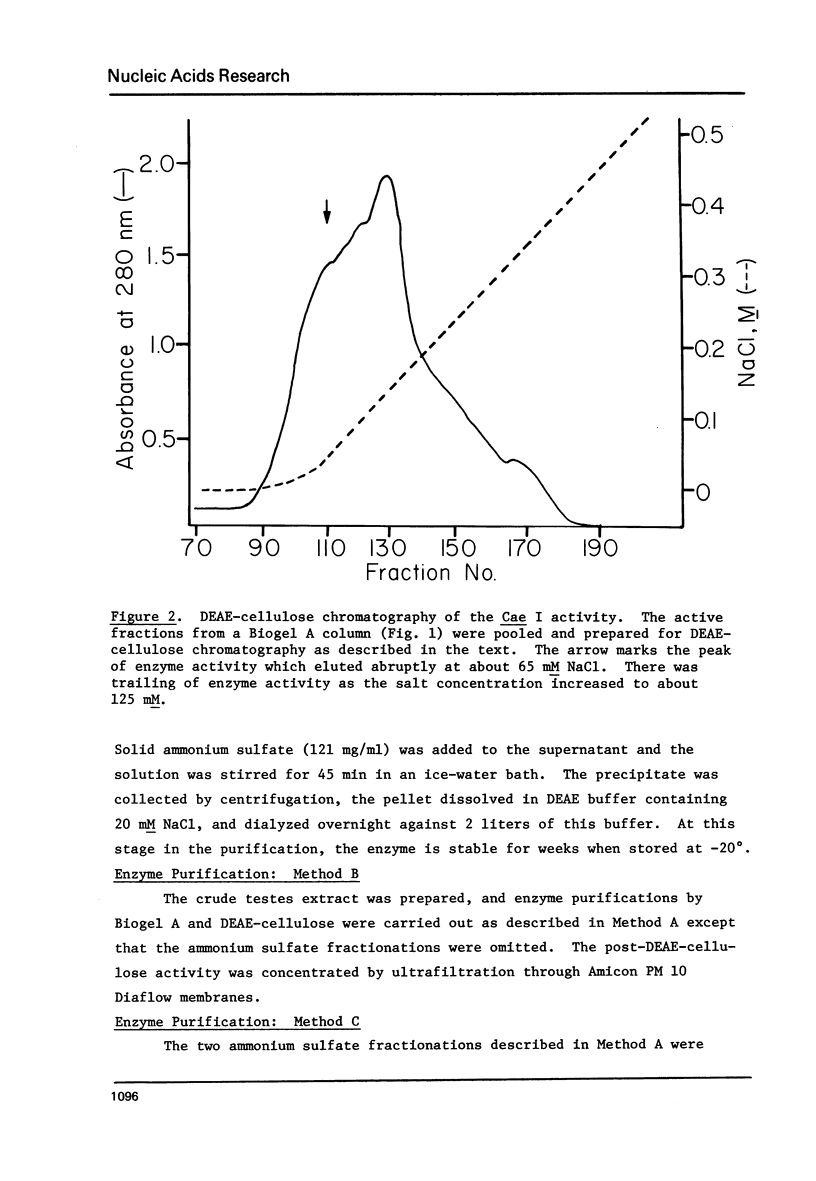
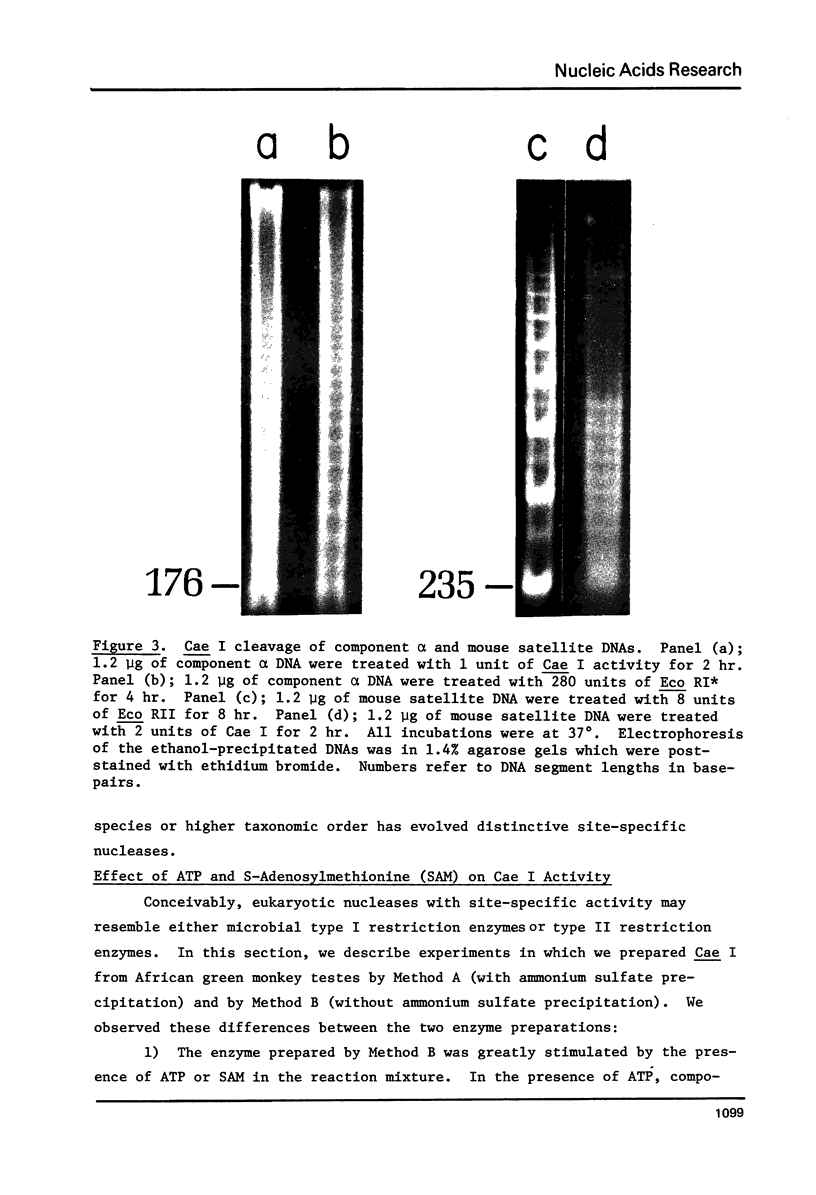
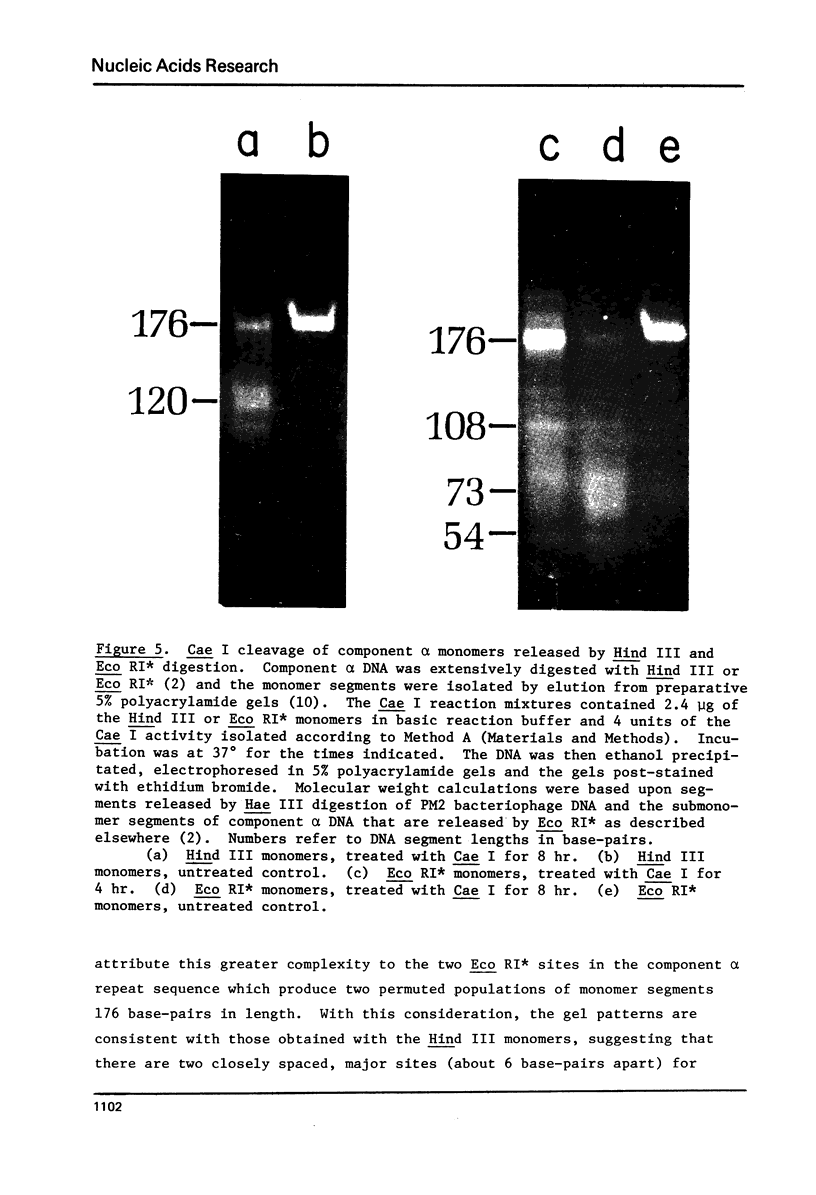
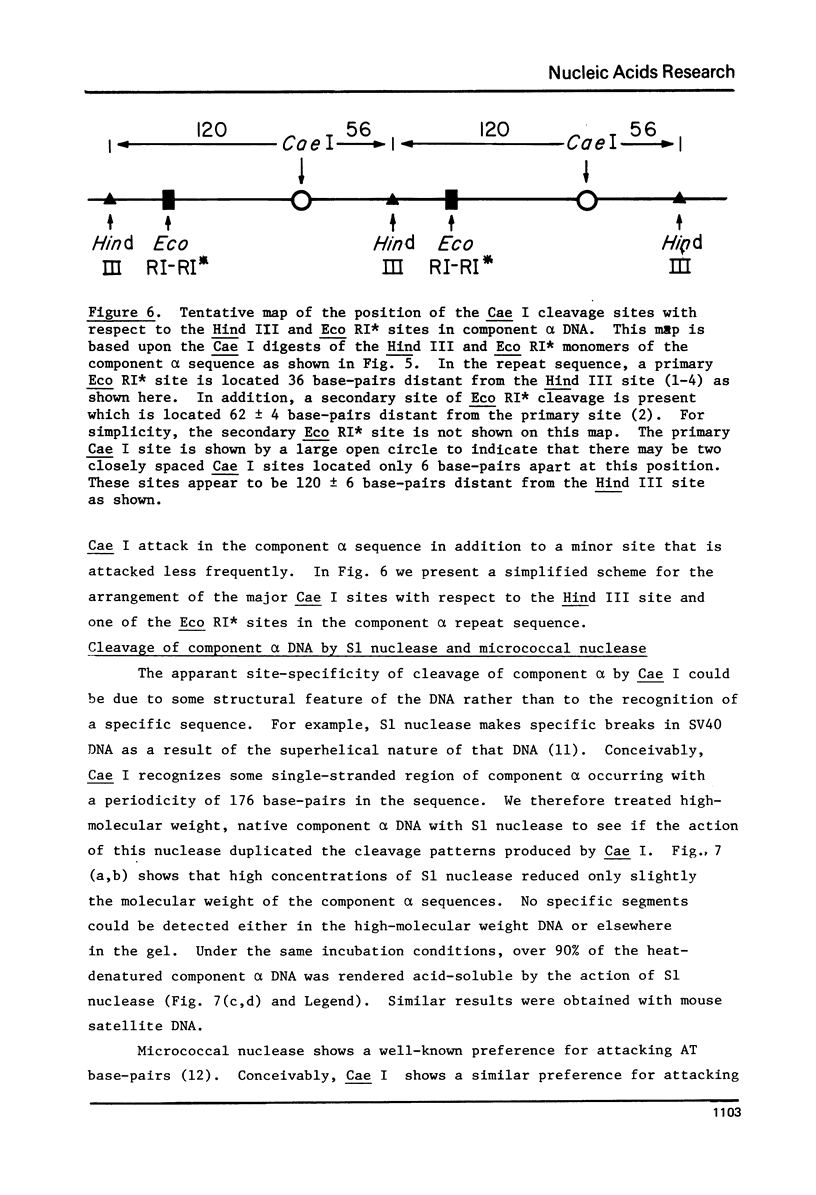
Component alpha DNA is a highly repetitive sequence that comprises nearly a quarter of the African green monkey (Cercopithecus aethiops) genome. A previous microbial restriction enzyme analysis showed that the repeat structure of component alpha DNA is based upon a monomeric unit of 176 +/- 4 base-pairs. An endonuclease, provisionally termed Case I, has been isolated from African green monkey testes that cleaves component alpha DNA into multimeric segments based upon the same repeat periodicity as that revealed by microbial restriction enzymes. The primary sites of Cae I cleavage in the component alpha sequence appear to be 120 +/- 6 base-pairs distant from the Hind III sites and 73 +/- 6 base-pairs distant from the Eco RI* sites. Cae I has been partially characterized with special reference to the effects of ATP and S-adenosylmethionine on the cleavage of component alpha DNA. Cae I may be a member of a class of similar site-specific nucleases present in mammalian cells. Cae I also cleaves mouse satellite DNA into a multimeric series of discrete segments: the periodicity of this series is shorter than that revealed by Eco RII retriction analysis of mouse satellite DNA.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard P., Morrow J. F., Berg P. Cleavage of circular, superhelical simian virus 40 DNA to a linear duplex by S1 nuclease. J Virol. 1973 Dec;12(6):1303–1313. doi: 10.1128/jvi.12.6.1303-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton W. G., Roberts R. J., Myers P. A., Sager R. A site-specific single-strand endonuclease from the eukaryote Chlamydomonas. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2687–2691. doi: 10.1073/pnas.74.7.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnit D. M., Maio J. J. Subnuclear redistribution of DNA species in confluent and growing mammalian cells. Chromosoma. 1973 May 14;42(1):23–36. doi: 10.1007/BF00326328. [DOI] [PubMed] [Google Scholar]
- Kurnit D. M., Shafit B. R., Maio J. J. Multiple satellite deoxyribonucleic acids in the calf and their relation to the sex chromosomes. J Mol Biol. 1973 Dec 15;81(3):273–284. doi: 10.1016/0022-2836(73)90141-1. [DOI] [PubMed] [Google Scholar]
- Maio J. J., Brown F. L., Musich P. R. Subunit structure of chromatin and the organization of eukaryotic highly repetitive DNA: recurrent periodicities and models for the evolutionary origins of repetitive DNA. J Mol Biol. 1977 Dec 15;117(3):637–655. doi: 10.1016/0022-2836(77)90062-6. [DOI] [PubMed] [Google Scholar]
- Maio J. J. DNA strand reassociation and polyribonucleotide binding in the African green monkey, Cercopithecus aethiops. J Mol Biol. 1971 Mar 28;56(3):579–595. doi: 10.1016/0022-2836(71)90403-7. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musich P. R., Brown F. L., Maio J. J. Subunit structure of chromatin and the organization of eukaryotic highly repetitive DNA: nucleosomal proteins associated with a highly repetitive mammalian DNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3297–3301. doi: 10.1073/pnas.74.8.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musich P. R., Maio J. J., Brown F. L. Subunit structure of chromatin and the organization of eukaryotic highly repetitive DNA: indications of a phase relation between restriction sites and chromatin subunits in African green monkey and calf nuclei. J Mol Biol. 1977 Dec 15;117(3):657–677. doi: 10.1016/0022-2836(77)90063-8. [DOI] [PubMed] [Google Scholar]
- ROBERTS W. K., DEKKER C. A., RUSHIZKY G. W., KNIGHT C. A. Studies on the mechanism of action of micrococcal nuclease. 1. Degradation of thymus deoxyribonucleic acid. Biochim Biophys Acta. 1962 May 14;55:664–673. doi: 10.1016/0006-3002(62)90844-2. [DOI] [PubMed] [Google Scholar]
- Sager R., Kitchin R. Selective silencing of eukaryotic DNA. Science. 1975 Aug 8;189(4201):426–433. [PubMed] [Google Scholar]
- Southern E. M. Long range periodicities in mouse satellite DNA. J Mol Biol. 1975 May 5;94(1):51–69. doi: 10.1016/0022-2836(75)90404-0. [DOI] [PubMed] [Google Scholar]
- Tobia A. M., Schildkraut C. L., Maio J. J. DNA replication in synchronized cultured mammalian cells. 3. Relative times of synthesis of mouse satellite and main band DNA. Biochim Biophys Acta. 1971 Aug 26;246(2):258–262. [PubMed] [Google Scholar]
- Wright M., Buttin G., Hurwitz J. The isolation and characterization from Escherichia coli of an adenosine triphosphate-dependent deoxyribonuclease directed by rec B, C genes. J Biol Chem. 1971 Nov;246(21):6543–6555. [PubMed] [Google Scholar]