Abstract
The  -function and the
-function and the  -function are phenomenological models that are widely used in the context of timing interceptive actions and collision avoidance, respectively. Both models were previously considered to be unrelated to each other:
-function are phenomenological models that are widely used in the context of timing interceptive actions and collision avoidance, respectively. Both models were previously considered to be unrelated to each other:  is a decreasing function that provides an estimation of time-to-contact (ttc) in the early phase of an object approach; in contrast,
is a decreasing function that provides an estimation of time-to-contact (ttc) in the early phase of an object approach; in contrast, 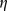 has a maximum before ttc. Furthermore, it is not clear how both functions could be implemented at the neuronal level in a biophysically plausible fashion. Here we propose a new framework – the corrected modified Tau function – capable of predicting both
has a maximum before ttc. Furthermore, it is not clear how both functions could be implemented at the neuronal level in a biophysically plausible fashion. Here we propose a new framework – the corrected modified Tau function – capable of predicting both  -type (“
-type (“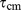 ”) and
”) and 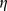 -type (“
-type (“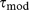 ”) responses. The outstanding property of our new framework is its resilience to noise. We show that
”) responses. The outstanding property of our new framework is its resilience to noise. We show that 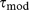 can be derived from a firing rate equation, and, as
can be derived from a firing rate equation, and, as 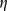 , serves to describe the response curves of collision sensitive neurons. Furthermore, we show that
, serves to describe the response curves of collision sensitive neurons. Furthermore, we show that 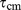 predicts the psychophysical performance of subjects determining ttc. Our new framework is thus validated successfully against published and novel experimental data. Within the framework, links between
predicts the psychophysical performance of subjects determining ttc. Our new framework is thus validated successfully against published and novel experimental data. Within the framework, links between  -type and
-type and 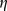 -type neurons are established. Therefore, it could possibly serve as a model for explaining the co-occurrence of such neurons in the brain.
-type neurons are established. Therefore, it could possibly serve as a model for explaining the co-occurrence of such neurons in the brain.
Author Summary
In 1957, Sir Fred Hoyle published a science fiction novel in which he described humanity's encounter with an extraterrestrial life form. It came in the shape of a huge black cloud which approached the earth. Hoyle proposed a formula (“ ”) for computing the remaining time until contact (“ttc”) of the cloud with the earth. Nowadays in real science,
”) for computing the remaining time until contact (“ttc”) of the cloud with the earth. Nowadays in real science,  serves as a model for ttc -perception for animals and humans, although it is not entirely undisputed. For instance,
serves as a model for ttc -perception for animals and humans, although it is not entirely undisputed. For instance,  seems to be incompatible with a collision-sensitive neuron in locusts (the Lobula Giant Movement Detector or LGMD neuron). LGMD neurons are instead better described by the
seems to be incompatible with a collision-sensitive neuron in locusts (the Lobula Giant Movement Detector or LGMD neuron). LGMD neurons are instead better described by the 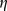 -function, which differs from
-function, which differs from  . Here we propose a generic model (“
. Here we propose a generic model (“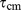 ”) that contains
”) that contains  and
and 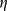 as special cases. The validity of the
as special cases. The validity of the 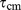 model was confirmed with a psychophysical experiment. Also, we fitted many published response curves of LGMD neurons with our new model and with the
model was confirmed with a psychophysical experiment. Also, we fitted many published response curves of LGMD neurons with our new model and with the 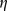 -function. Both models fit these response curves well, and we thus can conclude that
-function. Both models fit these response curves well, and we thus can conclude that  and
and 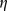 possibly result from a generic neuronal circuit template such as it is described by
possibly result from a generic neuronal circuit template such as it is described by 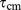 .
.
Introduction
Monocular presentation of a looming object elicits escape or avoidance reactions in many species, including humans [1]–[4]. When a planar object travels perpendicular to a surface toward an observer (i.e. the object approaches the observer on a direct collision course), it projects a symmetrically expanding image on the retina. Notice that in the present paper we only focus on a subset of approaches where the approaching object eventually collides with the observer. We assume that collision happens at time 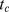 (time to contact, “ttc”). At time
(time to contact, “ttc”). At time 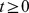 before
before 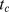 , the image subtends an angle
, the image subtends an angle 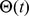 , and its outer contours expand with angular velocity
, and its outer contours expand with angular velocity 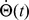 . Both angular variables grow nearly exponentially with decreasing distance
. Both angular variables grow nearly exponentially with decreasing distance 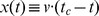 between object and eye (assuming a constant velocity
between object and eye (assuming a constant velocity 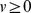 ). With knowledge of a predator's or object's typical size [5], it is therefore possible to trigger a behavioral response as soon as
). With knowledge of a predator's or object's typical size [5], it is therefore possible to trigger a behavioral response as soon as  or
or 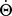 , respectively, crosses a threshold [1], [6], [7].
, respectively, crosses a threshold [1], [6], [7].
The visual systems of various species are also known to “compute” functions of 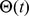 and
and 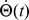 (see e.g. [8] for a recent review). The Tau-function (“
(see e.g. [8] for a recent review). The Tau-function (“ ”) is defined by
”) is defined by 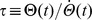 . Under the assumption that the object is a rigid sphere that approaches with
. Under the assumption that the object is a rigid sphere that approaches with 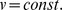 ,
,  has several interesting properties [9], [10]: First,
has several interesting properties [9], [10]: First,  provides a running estimation of ttc during the approach. Second, the ttc estimation is largely independent of physical object size, provided that
provides a running estimation of ttc during the approach. Second, the ttc estimation is largely independent of physical object size, provided that  and
and  are noise-free. Third,
are noise-free. Third,  decreases approximately linearly with time with a constant slope of
decreases approximately linearly with time with a constant slope of 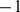 , but eventually linearity is compromised, as
, but eventually linearity is compromised, as  has a minimum shortly before ttc. It therefore would be comparatively easy to track the remaining time
has a minimum shortly before ttc. It therefore would be comparatively easy to track the remaining time  until impact, and to precisely time avoidance reactions, for example as soon as
until impact, and to precisely time avoidance reactions, for example as soon as 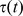 is below a certain threshold value.
is below a certain threshold value.
These three properties, however, are valid only for “sufficiently small” angular sizes 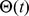 . Any quantitative criterion for “sufficiently small” implicates an error threshold for the deviation of
. Any quantitative criterion for “sufficiently small” implicates an error threshold for the deviation of  from linearity, that is
from linearity, that is 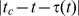 . For example, according to Text S6 a corresponding threshold for the visual angle can be defined as
. For example, according to Text S6 a corresponding threshold for the visual angle can be defined as 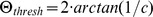 with some constant
with some constant 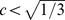 . Notice that the
. Notice that the 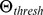 -criterion is independent from stimulus parameters such as object diameter or approach velocity.
-criterion is independent from stimulus parameters such as object diameter or approach velocity.
Because  is well suited for the estimation of
is well suited for the estimation of 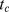 , it could in principal serve as a universal mechanism for guiding motor actions during object approaches or during self-motion towards static objects. Indeed, several studies related
, it could in principal serve as a universal mechanism for guiding motor actions during object approaches or during self-motion towards static objects. Indeed, several studies related  to behavioral responses in this context, thus asserting that many organisms, including humans, rely on
to behavioral responses in this context, thus asserting that many organisms, including humans, rely on  for their timing of motor actions (e.g. [10]–[12]). But a critical re-evaluation of the
for their timing of motor actions (e.g. [10]–[12]). But a critical re-evaluation of the  -hypothesis arrived at the conclusion that
-hypothesis arrived at the conclusion that  does not necessarily play a unique role for ttc estimation [13], [14]. For example, humans also rely on the rate of change of relative disparity, particularly in the late phase of an approach, for small object sizes [15]–[18], for low speeds [19], [20], or if knowledge of object size is available [7]. In addition, the task at hand (e.g. catching a ball or eluding a meteorite) seems to dictate the information that will eventually be used for action timing [14], [18], [21]. Further inconsistencies with respect to
does not necessarily play a unique role for ttc estimation [13], [14]. For example, humans also rely on the rate of change of relative disparity, particularly in the late phase of an approach, for small object sizes [15]–[18], for low speeds [19], [20], or if knowledge of object size is available [7]. In addition, the task at hand (e.g. catching a ball or eluding a meteorite) seems to dictate the information that will eventually be used for action timing [14], [18], [21]. Further inconsistencies with respect to  were reported with psychophysical results, where
were reported with psychophysical results, where 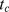 tended to be underestimated [16]. In addition, ttc -estimation reveals a certain dependence on object size [22], which is also not predicted by
tended to be underestimated [16]. In addition, ttc -estimation reveals a certain dependence on object size [22], which is also not predicted by  at “sufficiently small” angular sizes.
at “sufficiently small” angular sizes.
The Tau-function is often studied in the context of ttc -estimation. It appears, however, that in order to describe the responses of collision-sensitive neurons in certain species  is inadequate. For example, the Lobula Giant Movement Detector (LGMD) neuron in locusts responds with increasing activity to a stimulation with a symmetrically expanding image, if the expansion pattern is consistent with an approaching object [23], [24]. The response curve of the LGMD neuron gradually increases to a maximum and then abruptly ceases (often to a nonzero baseline response). Because
is inadequate. For example, the Lobula Giant Movement Detector (LGMD) neuron in locusts responds with increasing activity to a stimulation with a symmetrically expanding image, if the expansion pattern is consistent with an approaching object [23], [24]. The response curve of the LGMD neuron gradually increases to a maximum and then abruptly ceases (often to a nonzero baseline response). Because  does not have a maximum, a different function has been proposed for modeling LGMD responses: The Eta-function (“
does not have a maximum, a different function has been proposed for modeling LGMD responses: The Eta-function (“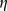 ”). It is defined as
”). It is defined as 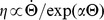 , with a constant
, with a constant  [25]. Theoretically, the time when the activity peak occurs depends linearly on the ratio
[25]. Theoretically, the time when the activity peak occurs depends linearly on the ratio  of object half-size
of object half-size 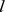 to object velocity
to object velocity  . The peak will shift closer to
. The peak will shift closer to 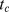 for smaller or faster objects, and always occurs at angular size
for smaller or faster objects, and always occurs at angular size 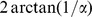 , independent of
, independent of  [26]. The LGMD activity peak could in principle signal a critical angular size for escaping. Indeed, a recent study with freely behaving locust suggests that the time of peak firing rate of the Descending Contralateral Movement Detector (DCMD) predicts that of jump [27] (each LGMD spike triggers a spike in the postsynaptic DCMD as well, because the LGMD is strongly coupled to the DCMD by a combined electrical and chemical synapse [28], [29]).
[26]. The LGMD activity peak could in principle signal a critical angular size for escaping. Indeed, a recent study with freely behaving locust suggests that the time of peak firing rate of the Descending Contralateral Movement Detector (DCMD) predicts that of jump [27] (each LGMD spike triggers a spike in the postsynaptic DCMD as well, because the LGMD is strongly coupled to the DCMD by a combined electrical and chemical synapse [28], [29]).
It has nevertheless been argued that – in some ecologically meaningful situations (small  ) – there is no guarantee for the peak to occur before
) – there is no guarantee for the peak to occur before
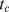 [2], [5]. This statement may be true to the extent that in freely behaving locusts, a reliable escape jump is triggered before collision only in the range of
[2], [5]. This statement may be true to the extent that in freely behaving locusts, a reliable escape jump is triggered before collision only in the range of 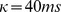 to
to 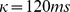 [30]. For
[30]. For 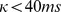 , the jump would occur after projected collision, and this value thus may reflect the typical sizes and speeds of predators.
, the jump would occur after projected collision, and this value thus may reflect the typical sizes and speeds of predators.
Apart from the locust, other species have collision-sensitive neurons with 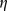 -like properties, for instance fruitflies [31] and bullfrogs [32]. In pigeons, the response properties of one of three classes of neurons in the dorsal posterior zone of the nucleus rotundus also seems to be compatible with the
-like properties, for instance fruitflies [31] and bullfrogs [32]. In pigeons, the response properties of one of three classes of neurons in the dorsal posterior zone of the nucleus rotundus also seems to be compatible with the 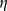 -function [1]. (The two remaining classes seem to compute
-function [1]. (The two remaining classes seem to compute 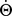 and
and  , respectively). In the goldfish, responses of the M-cell to looming stimuli also appear to follow a version of the
, respectively). In the goldfish, responses of the M-cell to looming stimuli also appear to follow a version of the 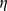 -function, in which
-function, in which  replaces
replaces 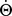 , such that the new function does only depend on
, such that the new function does only depend on  [33].
[33].
The Tau-function and the Eta-function are the two prevailing models for studying ttc -perception and (interceptive) action timing on the one hand, and escape behavior and collision avoidance on the other. In other words, we have two different models for two seemingly separated contexts. Each model brings about some hitherto unresolved issues, which are subsequently described.
From a computational point of view,  is numerically unstable: In the presence of noise, we have to reckon with the fact that
is numerically unstable: In the presence of noise, we have to reckon with the fact that 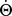 can get very small – or even reach zero – at certain instants during the initial phase of the approach (cf. [17]). As a consequence, fluctuations of
can get very small – or even reach zero – at certain instants during the initial phase of the approach (cf. [17]). As a consequence, fluctuations of  with large amplitudes may occur. If, however, noise levels are constant in time, and noise is not multiplicative, the signal to noise ratio continuously improves as
with large amplitudes may occur. If, however, noise levels are constant in time, and noise is not multiplicative, the signal to noise ratio continuously improves as 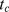 is approached. It is furthermore not entirely clear how
is approached. It is furthermore not entirely clear how  could be biophysically implemented in a neuron.
could be biophysically implemented in a neuron.
As for the 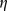 -function, the LGMD neuron seems to bypass a direct multiplication or division by computing
-function, the LGMD neuron seems to bypass a direct multiplication or division by computing 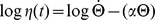 with subsequent exponentiation of the result [34]. From a mathematical viewpoint, however, taking the logarithm introduces an instability for
with subsequent exponentiation of the result [34]. From a mathematical viewpoint, however, taking the logarithm introduces an instability for 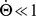 , although neuronal circuits with divisive inhibition can be adjusted such that no stability problems occur [35]. Moreover, Gabbiani et al.
[34] found that a third-order power law fitted the mean instantaneous firing rate of the LGMD better than an exponential or a linear function (see also reference [36]).
, although neuronal circuits with divisive inhibition can be adjusted such that no stability problems occur [35]. Moreover, Gabbiani et al.
[34] found that a third-order power law fitted the mean instantaneous firing rate of the LGMD better than an exponential or a linear function (see also reference [36]).
Our original motivation was to improve the stability of  with a simple modification. This modification led us to the modified Tau function
with a simple modification. This modification led us to the modified Tau function 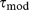 . Similar to the
. Similar to the 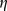 -function, the
-function, the 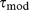 -function also reveals a maximum before ttc. We were able to fit the response curves of
-function also reveals a maximum before ttc. We were able to fit the response curves of 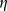 -type neurons with
-type neurons with 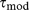 (Text S4). Our
(Text S4). Our 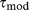 -function represents the equilibrium solution of an equation for describing neuronal firing rate. Because of this,
-function represents the equilibrium solution of an equation for describing neuronal firing rate. Because of this, 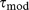 is based on a biophysically plausible mechanism.
is based on a biophysically plausible mechanism.
But 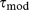 comes with a disadvantage: Unlike
comes with a disadvantage: Unlike  , it no longer provides a running value of ttc. In order to recover the ttc prediction, we needed to add a correction term to
, it no longer provides a running value of ttc. In order to recover the ttc prediction, we needed to add a correction term to 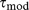 . This so-defined corrected modified Tau function (
. This so-defined corrected modified Tau function (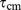 ) recovers the ttc prediction of the original
) recovers the ttc prediction of the original  -function, but suppresses noise better than
-function, but suppresses noise better than  . Most importantly, the corrected m-Tau function predicts the results of a psychophysical experiment, requiring subjects to estimate ttc.
. Most importantly, the corrected m-Tau function predicts the results of a psychophysical experiment, requiring subjects to estimate ttc.
Theoretically, we therefore can explain  -type and
-type and 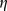 -type responses within the
-type responses within the 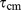 framework, which contains
framework, which contains 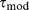 (but also
(but also  !) as a special case. Until now,
!) as a special case. Until now,  and
and 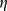 did not have any obvious relationship with each other (although we show in Text S6 how
did not have any obvious relationship with each other (although we show in Text S6 how 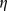 could formally be related to
could formally be related to 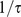 ). The
). The 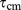 -function could thus serve to explain why
-function could thus serve to explain why  -type and
-type and 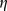 -type neurons could be found alongside each other in the pigeon brain [1].
-type neurons could be found alongside each other in the pigeon brain [1].
Results
The corrected modified Tau function “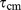 ” (equation 5) contains the modified Tau function “
” (equation 5) contains the modified Tau function “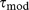 ” (equation 1) as a special case. We nevertheless first introduce the
” (equation 1) as a special case. We nevertheless first introduce the 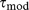 model, as this makes its relation to the original
model, as this makes its relation to the original  -function much easier understood.
-function much easier understood.
The modified  model (“
model (“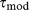 ”)
”)
Behavioral and neural responses to optical variables (e.g.,  ,
, 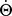 ,
,  ,
, 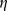 ) in the initial part of a trajectory are very noisy signals. Signal fluctuations may occur as a consequence of the discrete structure of the retinal photoreceptor array and its limited spatial resolution. The signal-to-noise ratio continuously improves as ttc is approached (Text S3).
) in the initial part of a trajectory are very noisy signals. Signal fluctuations may occur as a consequence of the discrete structure of the retinal photoreceptor array and its limited spatial resolution. The signal-to-noise ratio continuously improves as ttc is approached (Text S3).
Our first step adds computational stability to the  model. Let
model. Let 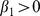 be a constant (in units of
be a constant (in units of 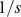 ). The modified Tau model is defined as:
). The modified Tau model is defined as:
| (1) |
Biophysically, 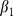 can be interpreted as leakage conductance (equation S2 in Text S1). According to equation (1),
can be interpreted as leakage conductance (equation S2 in Text S1). According to equation (1), 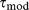 can formally be expressed in terms of
can formally be expressed in terms of 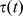 multiplied with a gain control factor
multiplied with a gain control factor 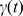 , which depends only on angular velocity. Notice, however, that the multiplicative version “
, which depends only on angular velocity. Notice, however, that the multiplicative version “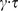 ” would again compromise stability, because
” would again compromise stability, because  appears as one of the factors in the product. Figure 1 a
juxtaposes
appears as one of the factors in the product. Figure 1 a
juxtaposes 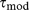 and the factors
and the factors 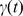 and
and 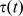 , respectively.
, respectively.
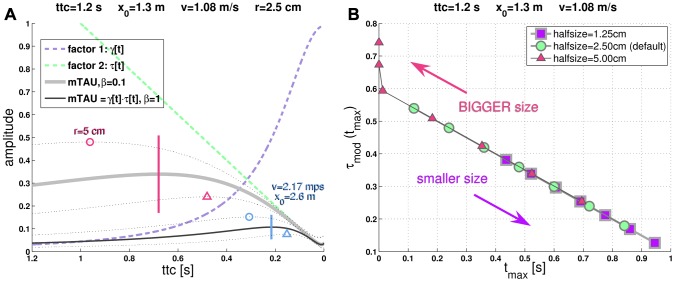
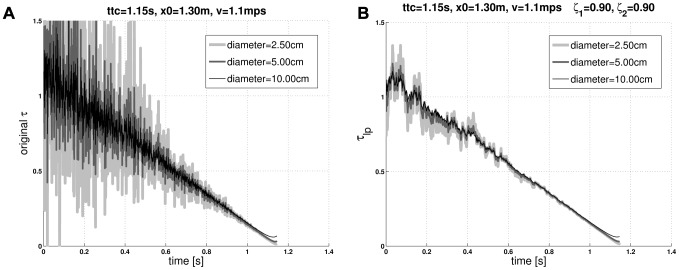
Figure 1. The modified Tau function (“m-Tau”).
(a) The figure shows two m-Tau functions which are distinguished by 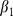 (with values
(with values  and
and 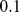 , see legend). The horizontal bars denote their respective maxima for the default stimulus values (
, see legend). The horizontal bars denote their respective maxima for the default stimulus values (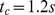 ,
, 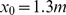 ,
, 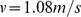 ,
, 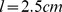 ). The maxima shift to the left (circles) upon doubling the object radius
). The maxima shift to the left (circles) upon doubling the object radius 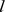 to
to 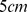 (“size effect”). They shift in the opposite direction (triangles) upon doubling both the approach velocity
(“size effect”). They shift in the opposite direction (triangles) upon doubling both the approach velocity  and the initial distance
and the initial distance 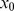 (“velocity effect”), such that
(“velocity effect”), such that 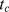 remains unchanged (
remains unchanged (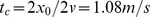 ). The thin dotted lines (not identified in the legend) show the m-Tau functions with correspondingly doubled values. For the m-Tau function with
). The thin dotted lines (not identified in the legend) show the m-Tau functions with correspondingly doubled values. For the m-Tau function with 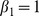 , the two factors
, the two factors 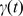 and
and 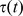 are furthermore plotted, see equation (1). The shift directions of the maxima are identical with the corresponding shifts observed with the
are furthermore plotted, see equation (1). The shift directions of the maxima are identical with the corresponding shifts observed with the 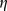 -function, see Text S1. (b) Here it is shown how the maxima of seven m-Tau functions shift when the object diameter is halved or doubled with respect to its default value
-function, see Text S1. (b) Here it is shown how the maxima of seven m-Tau functions shift when the object diameter is halved or doubled with respect to its default value 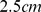 . Each point indicates
. Each point indicates 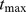 (time of maximum) along with its corresponding amplitude
(time of maximum) along with its corresponding amplitude 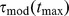 . Circular symbols represent the default case with
. Circular symbols represent the default case with 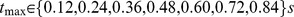 . All maxima lie on a line. With a smaller object diameter all maxima shift to the right (towards
. All maxima lie on a line. With a smaller object diameter all maxima shift to the right (towards 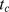 ), and an increase in object size causes a shift of all maxima to the left (away from
), and an increase in object size causes a shift of all maxima to the left (away from 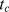 ). All shifts proceed along the same straight line. Notice that some artifacts occur for the two leftmost points, because all maxima were computed numerically. The velocity effect is illustrated in Text S1.
). All shifts proceed along the same straight line. Notice that some artifacts occur for the two leftmost points, because all maxima were computed numerically. The velocity effect is illustrated in Text S1.
Let the initial distance between the eye and a circular object (diameter 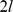 ) be denoted by
) be denoted by 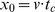 . Then, choosing
. Then, choosing 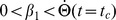 will create a maximum of
will create a maximum of 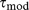 at time
at time 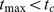 (i.e., a maximum before
(i.e., a maximum before
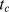 ):
):
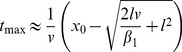 |
(2) |
(the previous equation is derived in the Methods Section). The time 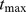 when
when  assumes its maximum can thus be controlled by specifying
assumes its maximum can thus be controlled by specifying 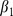 , where bigger values will place the maximum closer to
, where bigger values will place the maximum closer to 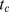 . The maximum depends as follows on approach velocity and object diameter, respectively.
. The maximum depends as follows on approach velocity and object diameter, respectively.
Assume fixed values for 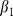 and
and 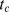 . Then,
. Then, 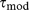 will have an activity maximum at
will have an activity maximum at 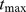 (default case). Now increase approach velocity and initial distance, such that
(default case). Now increase approach velocity and initial distance, such that 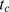 remains constant. As a consequence, the peak will shift closer to
remains constant. As a consequence, the peak will shift closer to 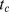 with respect to the default case (triangle symbols in Figure 1a
; further figures in Text S2). This is the velocity effect.
with respect to the default case (triangle symbols in Figure 1a
; further figures in Text S2). This is the velocity effect.
Now increase the object diameter. The maximum of 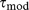 will then occur earlier compared to the default case (circle symbols in Figure 1). This is the size effect.
will then occur earlier compared to the default case (circle symbols in Figure 1). This is the size effect.
Assuming that the peak signals an imminent collision, this shifting behavior is consistent with larger objects being perceived to have an earlier ttc than smaller ones [22]. Note that the original  -function (i.e.
-function (i.e. 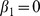 and noise-free angular variables) does not show a strong dependence on object size where
and noise-free angular variables) does not show a strong dependence on object size where 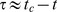 holds (but see Text S6).
holds (but see Text S6).
The 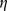 -function is the prevailing model for describing responses from collision sensitive neurons to object approaches with constant velocity. Its characteristic feature is its maximum. Because
-function is the prevailing model for describing responses from collision sensitive neurons to object approaches with constant velocity. Its characteristic feature is its maximum. Because 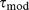 also has a maximum, we fit
also has a maximum, we fit 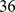 previously published neuronal response curves with the
previously published neuronal response curves with the 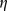 -function and
-function and 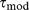 (Text S4). Figure 2 summarizes these fits by comparing the response maxima of the experimental curves (“
(Text S4). Figure 2 summarizes these fits by comparing the response maxima of the experimental curves (“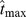 ”) with the maxima predicted by the best fits achieved with the two functions (“
”) with the maxima predicted by the best fits achieved with the two functions (“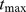 ”). Predictions of
”). Predictions of 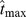 are slightly better with
are slightly better with 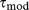 -fits, both in terms of mean and median of absolute differences (
-fits, both in terms of mean and median of absolute differences (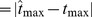 ). With respect to goodness of fit measures (root-mean-square-errors,
). With respect to goodness of fit measures (root-mean-square-errors, 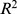 , F-statistics), both functions perform again on par with each other. Therefore, both
, F-statistics), both functions perform again on par with each other. Therefore, both 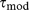 and the
and the 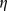 -function describe neuronal responses of object approaches with constant velocity.
-function describe neuronal responses of object approaches with constant velocity.
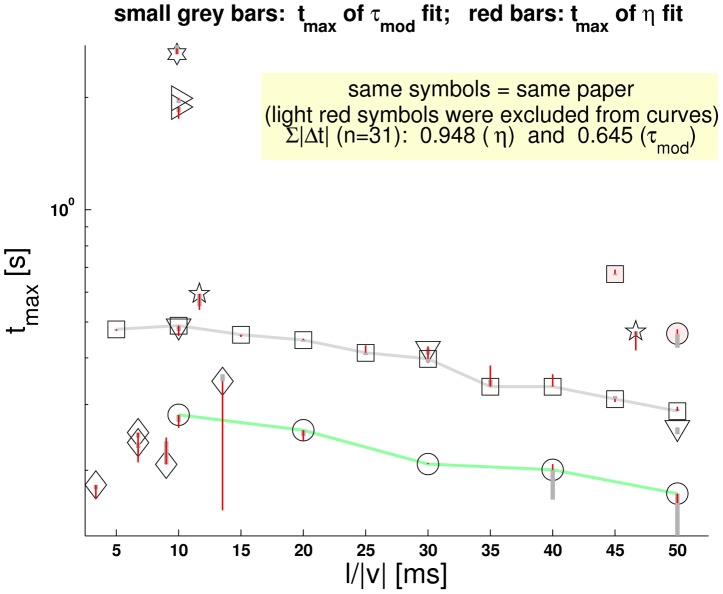
Figure 2.
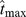 from experiments (symbols) compared to fitted
from experiments (symbols) compared to fitted 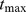 (bars).
(bars).
All symbols indicate the maxima 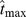 in the neuronal recording data as a function of
in the neuronal recording data as a function of 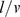 (with
(with 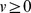 ). These data were manually resampled from previously published studies (see Text S4 for further details). The line ends (lines start at the center of each symbol) denote where the fitted functions
). These data were manually resampled from previously published studies (see Text S4 for further details). The line ends (lines start at the center of each symbol) denote where the fitted functions 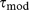 (thick gray bars) and
(thick gray bars) and 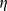 (thin and red bars) have their respective maxima. Thus, the longer a bar, the bigger the difference between the predicted maxima and that of the neuronal data. The respective sum of absolute differences is indicated in the inset. The mean (
(thin and red bars) have their respective maxima. Thus, the longer a bar, the bigger the difference between the predicted maxima and that of the neuronal data. The respective sum of absolute differences is indicated in the inset. The mean (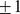 s.d.,
s.d., 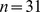 ) of absolute differences is
) of absolute differences is 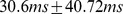 (median
(median 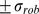 :
: 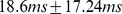 ) for the
) for the 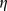 -function, and
-function, and 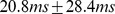 (median
(median 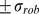 :
: 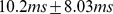 ) for
) for 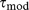 . The two continuous lines connect the data for a series of
. The two continuous lines connect the data for a series of 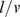 values from the same paper (light gray: reference [26]; green: reference [39]; first figure in Text S4: all references.)
values from the same paper (light gray: reference [26]; green: reference [39]; first figure in Text S4: all references.)
The experimental maxima at time 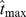 depend linearly on
depend linearly on 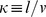 [26]. The
[26]. The 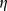 -function predicts this linear relationship (equation S5 in Text S2), where slope is identified by
-function predicts this linear relationship (equation S5 in Text S2), where slope is identified by  , and intercept by a temporal delay
, and intercept by a temporal delay  of corresponding line fits (Figure 3a
).
of corresponding line fits (Figure 3a
).
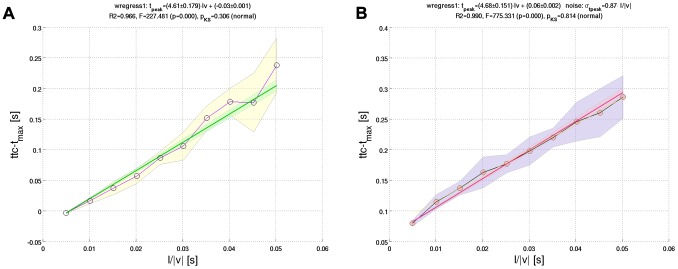
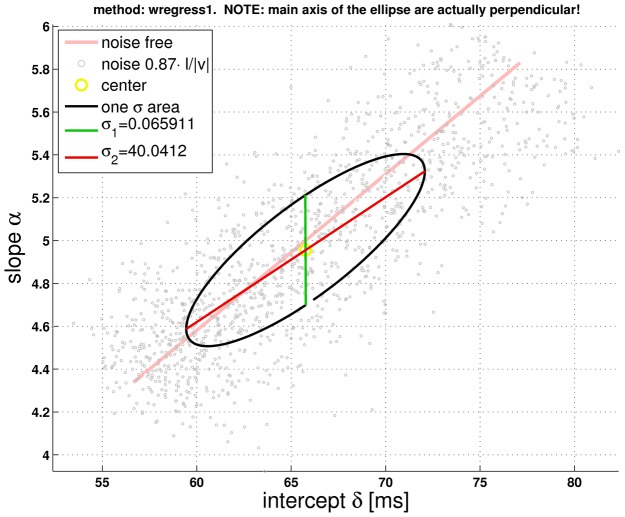
Figure 3. Masking of the m-Tau nonlinearity by noise.
The experimental data from Gabbiani et al.
[26] suggest a linear relationship between relative time of peak firing rate 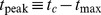 and the half-size to velocity ratio
and the half-size to velocity ratio 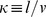 . The big shaded areas indicate one standard deviation
. The big shaded areas indicate one standard deviation  from the mean value of
from the mean value of 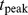 . Notice the increase in
. Notice the increase in  with increasing
with increasing  . (a) Resampled Figure 4a from Reference [26] (p. 1128). The locusts were stimulated by approaching dark squares with different sizes and velocities, such that various values of
. (a) Resampled Figure 4a from Reference [26] (p. 1128). The locusts were stimulated by approaching dark squares with different sizes and velocities, such that various values of  were covered. The circle symbol for each
were covered. The circle symbol for each  represents the mean
represents the mean 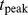 of neuronal response curves across
of neuronal response curves across 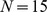 DCMD neurons. The result of a weighted least square regression fit reported by Gabbiani et al. had slope
DCMD neurons. The result of a weighted least square regression fit reported by Gabbiani et al. had slope 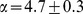 and intercept
and intercept 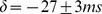 . With the manually resampled data points shown here, we obtained
. With the manually resampled data points shown here, we obtained 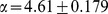 and
and 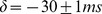 , respectively. The light green shaded area indicates one standard deviation of slope. Additional statistical parameters of our weighted least square fit are shown above the figure. (b) An example of fitting a straight line to
, respectively. The light green shaded area indicates one standard deviation of slope. Additional statistical parameters of our weighted least square fit are shown above the figure. (b) An example of fitting a straight line to  averaged random trials of the “noisified” equation (2) with
averaged random trials of the “noisified” equation (2) with 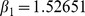 . “Noisifying” means that Gaussian noise with standard deviation
. “Noisifying” means that Gaussian noise with standard deviation 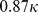 was added to
was added to 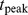 (according to equation 8, page 1129 in [26]). The noise blurs the nonlinear character of the m-Tau function and makes it appear linear. The light red shaded area indicates one standard deviation of slope. Further simulation results are presented in Text S2.
(according to equation 8, page 1129 in [26]). The noise blurs the nonlinear character of the m-Tau function and makes it appear linear. The light red shaded area indicates one standard deviation of slope. Further simulation results are presented in Text S2.
The maximum of the 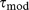 -function
-function 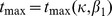 , however, depends in a nonlinear way on
, however, depends in a nonlinear way on  (equation 2 & equation S6 in Text S2; illustration: Figure 4). (Nonlinearity means that the slope depends on
(equation 2 & equation S6 in Text S2; illustration: Figure 4). (Nonlinearity means that the slope depends on  , and linearity means that it does not). Linearity is approached with increasing values of
, and linearity means that it does not). Linearity is approached with increasing values of 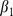 , eventually reaching a slope of one for
, eventually reaching a slope of one for 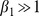 (equation S9 in Text S2). This is nevertheless inconsistent with experimental evidence, as the experimental values for
(equation S9 in Text S2). This is nevertheless inconsistent with experimental evidence, as the experimental values for  are underestimated (typically
are underestimated (typically 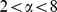 ).
).
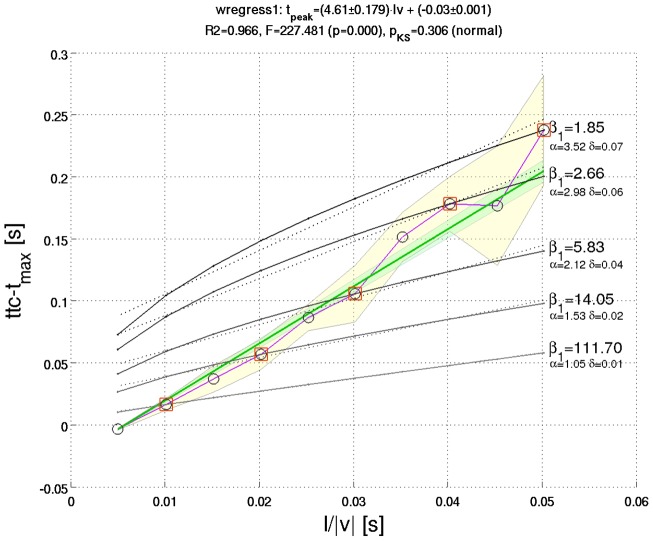
Figure 4. Illustration of nonlinear dependence of m-Tau maxima.
The red square symbols denote data points 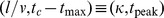 , according to Figure 3a
from reference [26]). In order to illustrate the nonlinear behavior of m-Tau, for each of these points an instance of m-Tau was created, such that the peaks of the
, according to Figure 3a
from reference [26]). In order to illustrate the nonlinear behavior of m-Tau, for each of these points an instance of m-Tau was created, such that the peaks of the 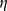 -function and the m-Tau function coincide. The corresponding values of
-function and the m-Tau function coincide. The corresponding values of 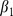 were computed with equation S7 in Text S2, and are indicated in the figure. Along with the
were computed with equation S7 in Text S2, and are indicated in the figure. Along with the 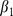 , the values of
, the values of  and
and  are shown in small font size. The latter two values were obtained by “brute-force” fitting a straight line to the nonlinear m-Tau curves. We observe that: (i) the curvature of m-Tau (equation S6 in Text S2) increases with decreasing values of
are shown in small font size. The latter two values were obtained by “brute-force” fitting a straight line to the nonlinear m-Tau curves. We observe that: (i) the curvature of m-Tau (equation S6 in Text S2) increases with decreasing values of 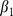 . (ii) All “slopes” of the “brute-force” line-fit to m-Tau are smaller than suggested by the data from Gabbiani et al., who reported
. (ii) All “slopes” of the “brute-force” line-fit to m-Tau are smaller than suggested by the data from Gabbiani et al., who reported 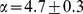 (our fit of their re-sampled data is indicated by the green line and yielded
(our fit of their re-sampled data is indicated by the green line and yielded 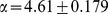 ; see figure headline).
; see figure headline).
We thus explored a different possibility: Can the nonlinear function 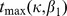 be hidden by noise? Figure 3b
suggests that it nearly can, as seen when fitting a line to a version of
be hidden by noise? Figure 3b
suggests that it nearly can, as seen when fitting a line to a version of 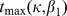 with additive Gaussian noise. Noise levels were set as reported in [26]. This hide-and-seek works quite well, and the fitting statistics (
with additive Gaussian noise. Noise levels were set as reported in [26]. This hide-and-seek works quite well, and the fitting statistics (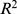 , KS-test on residuals, F-statistics) are consistent with linearity in many random trials (detailed analysis: Text S2).
, KS-test on residuals, F-statistics) are consistent with linearity in many random trials (detailed analysis: Text S2).
Figure 4 suggests a correlation between intercept and slope of line fits for different values of 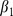 . We thus fit lines to the noisified version of
. We thus fit lines to the noisified version of 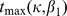 for various values of
for various values of 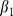 . As before, noise levels were set as reported, and we again identified intercept and slope of the line fits to
. As before, noise levels were set as reported, and we again identified intercept and slope of the line fits to 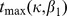 with
with  and
and  , respectively. The result of this procedure is shown in Figure 5, and agrees well with Figure 4 in [26]. Thus,
, respectively. The result of this procedure is shown in Figure 5, and agrees well with Figure 4 in [26]. Thus, 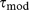 consistently predicts a good correlation between intercepts and slopes both in the presence and in the absence of noise.
consistently predicts a good correlation between intercepts and slopes both in the presence and in the absence of noise.
Figure 5. Simulation of Figure 4b from Reference [26] (p. 1128).
For compiling this figure, a value of 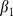 was first selected. Then,
was first selected. Then,  noisified curves
noisified curves 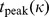 (
(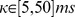 ) were generated and averaged, assuming a noise level of
) were generated and averaged, assuming a noise level of 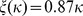 in equation S10 in Text S2
[26]. A pair of intercept and slope values (
in equation S10 in Text S2
[26]. A pair of intercept and slope values ( and
and  , respectively) were obtained from a weighted linear regression fit to the average curve (weights
, respectively) were obtained from a weighted linear regression fit to the average curve (weights 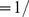 variance). Now,
variance). Now, 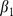 was parsed from
was parsed from 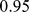 to
to 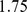 in steps of
in steps of 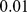 (totaling
(totaling 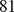 values). For each value of
values). For each value of 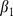 , the weighted linear regression fit to the averaged
, the weighted linear regression fit to the averaged 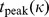 -curves was repeated
-curves was repeated 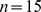 times. The small grey circles represent the mean value of these
times. The small grey circles represent the mean value of these 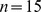 intercept-slope pairs. Statistical parameters for each fit were also recorded, and the corresponding figures are included in Text S2. The main axis of the ellipse are in the direction of the eigenvectors of the covariance matrix. The matrix was computed from all intercept-slope pairs (i.e.
intercept-slope pairs. Statistical parameters for each fit were also recorded, and the corresponding figures are included in Text S2. The main axis of the ellipse are in the direction of the eigenvectors of the covariance matrix. The matrix was computed from all intercept-slope pairs (i.e. 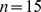 samples for each
samples for each 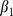 ). The lengths of the eigenvectors were scaled with the square root of their associated eigenvalues. The area enclosed by the ellipse thus corresponds to one standard deviation (legend:
). The lengths of the eigenvectors were scaled with the square root of their associated eigenvalues. The area enclosed by the ellipse thus corresponds to one standard deviation (legend: 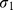 and
and 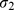 ). (Note that the ellipse shown in Figure 4b from Gabbiani et al. denotes instead a
). (Note that the ellipse shown in Figure 4b from Gabbiani et al. denotes instead a 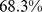 confidence region for intercept and slope). The noise-free correlation is indicated by the straight line. Notice that the abscissa values are defined up to an arbitrary additive constant.
confidence region for intercept and slope). The noise-free correlation is indicated by the straight line. Notice that the abscissa values are defined up to an arbitrary additive constant.
The corrected modified  model (“
model (“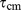 ”)
”)
Maximum detection of 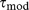 in the initial phase of an object approach (i.e., for small values of
in the initial phase of an object approach (i.e., for small values of 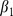 ) is problematic, due to the signal's poor signal-to-noise ratio and the rather “shallow” curvature around the maximum. The situation gets progressively better if we place the maximum closer to
) is problematic, due to the signal's poor signal-to-noise ratio and the rather “shallow” curvature around the maximum. The situation gets progressively better if we place the maximum closer to 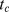 , that is for bigger values of
, that is for bigger values of 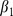 : The signal-to-noise ratio is better, and curvature is higher. With
: The signal-to-noise ratio is better, and curvature is higher. With 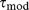 , however, we fell short of explaining the results of our psychophysical experiment (which is below described further). This led us to modify
, however, we fell short of explaining the results of our psychophysical experiment (which is below described further). This led us to modify 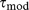 as follows.
as follows.
Observe that 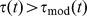 for all
for all 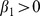 , and thus
, and thus
| (3) |
is a positive correction factor to 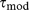 , such that
, such that 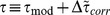 . As with
. As with  , the correction factor
, the correction factor 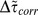 per se is again susceptible to fluctuations in the angular variable
per se is again susceptible to fluctuations in the angular variable 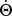 , and we would have gained no improvement by simply adding it to
, and we would have gained no improvement by simply adding it to 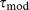 .
.
Now, the crucial idea is to render 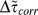 insensitive to such fluctuations. This is achieved with a first order low-pass filter (a short introduction is given in Text S8). Low-pass filtering of
insensitive to such fluctuations. This is achieved with a first order low-pass filter (a short introduction is given in Text S8). Low-pass filtering of  and
and 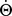 transforms
transforms 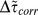 into a slowly varying signal, which is eventually added to
into a slowly varying signal, which is eventually added to 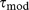 :
:
| (4) |
 and
and 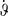 are low-pass filtered visual angle and angular velocity, respectively, and
are low-pass filtered visual angle and angular velocity, respectively, and 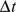 is the system's integration time constant. In order to avoid initial filter transients, the filter variables were initialized with
is the system's integration time constant. In order to avoid initial filter transients, the filter variables were initialized with 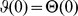 and
and 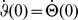 , respectively. The
, respectively. The 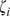 are filter memory coefficients with
are filter memory coefficients with 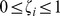 for
for 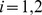 . No filtering would take place for
. No filtering would take place for 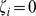 (no memory), and the filters would never change their initial state for
(no memory), and the filters would never change their initial state for 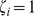 (infinite memory).
(infinite memory).
The corrected, modified
 model (“corrected m-Tau”) is then defined as:
model (“corrected m-Tau”) is then defined as:
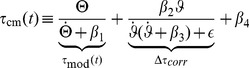 |
(5) |
where 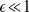 is a small constant, such that possible division-by-zero errors are avoided in the simulation. Nevertheless,in the presence of noise, division-by-zero errors do not typically represent a problem during an approach with
is a small constant, such that possible division-by-zero errors are avoided in the simulation. Nevertheless,in the presence of noise, division-by-zero errors do not typically represent a problem during an approach with 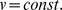 , because
, because 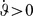 if the following two conditions hold: (i) appropriate initialization of
if the following two conditions hold: (i) appropriate initialization of 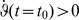 , and (ii) “sufficiently strong” lowpass filtering. The offset
, and (ii) “sufficiently strong” lowpass filtering. The offset 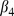 is included for the sake of completeness. It was only considered for simulating our psychophysical experiment (described below), where it turned out to be negligibly small. In general, therefore, it is safe to assume
is included for the sake of completeness. It was only considered for simulating our psychophysical experiment (described below), where it turned out to be negligibly small. In general, therefore, it is safe to assume 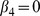 .
.
Similar to  , the new corrected m-Tau-model also computes an estimation of ttc for “sufficiently small” angular sizes. But the principal advantage of
, the new corrected m-Tau-model also computes an estimation of ttc for “sufficiently small” angular sizes. But the principal advantage of 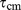 over
over  is that it is less sensitive to noise. The noise suppression of the corrected m-Tau-model is constrained by the noise suppression performance of two “limit functions”, which are approached dependent on the values of
is that it is less sensitive to noise. The noise suppression of the corrected m-Tau-model is constrained by the noise suppression performance of two “limit functions”, which are approached dependent on the values of 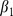 ,
, 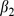 , and
, and 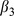 (Figure 6). For the derivation of these limit functions, assume (to simplify matters) that in equation (5)
(Figure 6). For the derivation of these limit functions, assume (to simplify matters) that in equation (5)
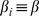 with
with 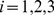 (and
(and 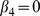 ). Then, as we will show subsequently, the constraining functions are the ordinary
). Then, as we will show subsequently, the constraining functions are the ordinary  function for
function for 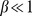 , on the one hand (equation 6), and for
, on the one hand (equation 6), and for 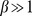 a version of
a version of  with lowpass-filtered angular variables, on the other (equation 8). Thus,
with lowpass-filtered angular variables, on the other (equation 8). Thus,  , where
, where 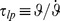 , provided that we exclude the case
, provided that we exclude the case 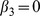 ,
, 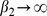 , which would imply that
, which would imply that 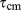 is unbounded.
is unbounded.
Figure 6. Limit functions of the corrected m-Tau function.
The corrected m-Tau -function 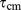 responds similar to
responds similar to  , but with an improved noise suppression performance, as long as parameter values
, but with an improved noise suppression performance, as long as parameter values 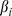 (
(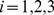 and
and 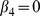 ) are suitably chosen. More precisely,
) are suitably chosen. More precisely, 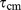 is constrained by the limit functions
is constrained by the limit functions  and
and 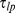 . This means that corrected m-Tau can approach the former or the latter function for the corresponding (extreme) values of
. This means that corrected m-Tau can approach the former or the latter function for the corresponding (extreme) values of 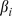 , but typically
, but typically 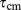 will perform somewhere between the two limit functions. For the simulations shown in this figure, uncorrelated normal-distributed noise was added to the angular variables
will perform somewhere between the two limit functions. For the simulations shown in this figure, uncorrelated normal-distributed noise was added to the angular variables  and
and 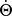 . Each curve represents a typical random trial, where noise was identical for all curves. The different shades of gray indicate different object diameters, as indicated in the legends. (a) “Normal”
. Each curve represents a typical random trial, where noise was identical for all curves. The different shades of gray indicate different object diameters, as indicated in the legends. (a) “Normal”  function, which is the limit function approached by
function, which is the limit function approached by 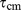 for
for 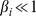 . Noise suppression is poor. Notice that the displayed range has been truncated so as to match it to the range of the figure on the right-hand side. (b) The
. Noise suppression is poor. Notice that the displayed range has been truncated so as to match it to the range of the figure on the right-hand side. (b) The 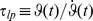 function is the limit function that is approached for
function is the limit function that is approached for 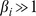 . It has an excellent noise suppression performance, owing to lowpass filtering of angular variables (
. It has an excellent noise suppression performance, owing to lowpass filtering of angular variables (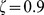 , c.f. equation 4). Further details are presented in Text S3.
, c.f. equation 4). Further details are presented in Text S3.
Case I: 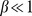
For very small 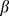 (more precisely
(more precisely 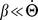 ), the first term of the equation (5) is approximately
), the first term of the equation (5) is approximately
| (6) |
which is just the ordinary  function. For the second term
function. For the second term 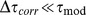 , which implies that it can be neglected because its denominator is approximately equal to
, which implies that it can be neglected because its denominator is approximately equal to 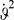 . Furthermore, during an object approach with constant velocity, angular size
. Furthermore, during an object approach with constant velocity, angular size  and angular velocity
and angular velocity 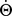 are increasing, and
are increasing, and 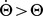 , as
, as 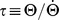 is monotonically decreasing (except at times very close to ttc, see Text S6). The last arguments hold also for
is monotonically decreasing (except at times very close to ttc, see Text S6). The last arguments hold also for  and
and 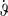 , respectively, which are the lowpass-filtered optical variables, where
, respectively, which are the lowpass-filtered optical variables, where 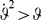 . We eventually arrive at the approximation
. We eventually arrive at the approximation
| (7) |
Summarizing the above, if 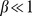 , then the noise suppression performance of the corrected m-Tau -model is comparable with that of ordinary
, then the noise suppression performance of the corrected m-Tau -model is comparable with that of ordinary  (Figure 6a
).
(Figure 6a
).
Case II: 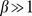
For 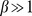 (more precisely
(more precisely 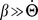 ), the situation is just the opposite of Case I. The first term of equation (5) can be neglected, because
), the situation is just the opposite of Case I. The first term of equation (5) can be neglected, because 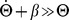 . Given that
. Given that 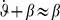 in the denominator of the
in the denominator of the 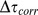 term, we obtain
term, we obtain
| (8) |
This is the ordinary  -function but with lowpass filtered optical variables (“
-function but with lowpass filtered optical variables (“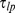 ”, Figure 6b
).
”, Figure 6b
).
Predicting psychophysical performance
Details on our psychophysical experiment are spelled out in the Methods Section. In a nutshell, subjects viewed approaching balls on a monitor. The balls had two different sizes (big & small, corresponding to object diameters 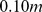 &
& 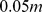 , respectively), and disappeared after
, respectively), and disappeared after 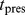 (presentation time) until
(presentation time) until 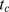 . A beep sounded always at the same time,
. A beep sounded always at the same time, 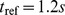 , in order to indicate a reference time to the subjects. Approaches with different values of
, in order to indicate a reference time to the subjects. Approaches with different values of 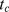 were presented, where
were presented, where 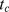 could occur before or after
could occur before or after 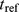 . Subjects were asked to judge whether they were hit by the ball before or after
. Subjects were asked to judge whether they were hit by the ball before or after 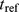 . Responses were pooled, and the “proportion of later responses” for each presentation time (corresponding to “ball hit me after
. Responses were pooled, and the “proportion of later responses” for each presentation time (corresponding to “ball hit me after 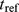 ”) was computed as a function of ttc. Figure 7a
shows the corresponding data points for
”) was computed as a function of ttc. Figure 7a
shows the corresponding data points for 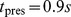 , along with the best matching Gaussian cumulative density function (“GCDF”-fit) for each object diameter. The GCDF-fits represent an estimate of the underlying psychometric curves or psychometric functions, respectively. Figure 7b
suggests that subjects did not respond to the average of the stimulus set, because the mean of the distribution (point of subjective simultaneity) shifted with presentation time. In addition, the variance of the distribution decreased with increasing presentation time. The small object diameter is furthermore associated with a higher variance than the big one.
, along with the best matching Gaussian cumulative density function (“GCDF”-fit) for each object diameter. The GCDF-fits represent an estimate of the underlying psychometric curves or psychometric functions, respectively. Figure 7b
suggests that subjects did not respond to the average of the stimulus set, because the mean of the distribution (point of subjective simultaneity) shifted with presentation time. In addition, the variance of the distribution decreased with increasing presentation time. The small object diameter is furthermore associated with a higher variance than the big one.
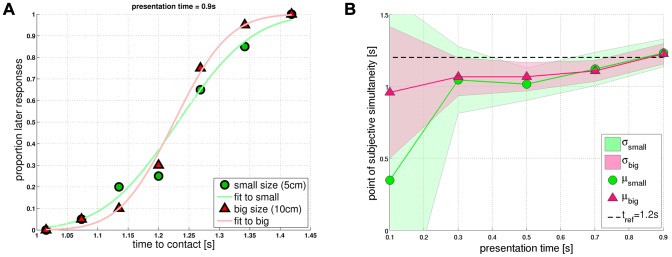
Figure 7. Psychometric functions.
(a) Psychophysical data points 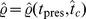 for “proportion of later responses” are shown for the presentation time
for “proportion of later responses” are shown for the presentation time 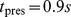 and object diameters big (triangle symbols) and small (circle symbols), respectively. Each sigmoid curve represents a fit of a Gaussian cumulative density function (“GCDF” with mean
and object diameters big (triangle symbols) and small (circle symbols), respectively. Each sigmoid curve represents a fit of a Gaussian cumulative density function (“GCDF” with mean 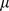 and standard deviation
and standard deviation  ) to the data points of the respective object diameter. The GCDF-fits approximate the underlying psychometric functions, with the mean
) to the data points of the respective object diameter. The GCDF-fits approximate the underlying psychometric functions, with the mean 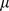 indicating the time point of subjective simultaneity. (b) The curves show how
indicating the time point of subjective simultaneity. (b) The curves show how 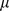 and
and  depend on presentation time and object diameter. Each point represents the result of a GCDF-fit to the psychophysical data. If subjects responded correctly, the point of subjective simultaneity would coincide with
depend on presentation time and object diameter. Each point represents the result of a GCDF-fit to the psychophysical data. If subjects responded correctly, the point of subjective simultaneity would coincide with  (
( is indicated by the dashed horizontal line).
is indicated by the dashed horizontal line).
The full set of data points is shown in Figure 8, where each figure panel corresponds to a different presentation time (small object size: circles; big: triangles). The curves shown in Figure 8 do not represent GCDF-fits (as in Figure 7a
), but rather display simulation results from the 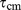 -model. For short presentation times, subjects show near-random performance across ttc (Figure 8a, b
), thereby revealing a bias towards later responses (i.e. “ball hit me after
-model. For short presentation times, subjects show near-random performance across ttc (Figure 8a, b
), thereby revealing a bias towards later responses (i.e. “ball hit me after 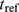 ”). The GCDF-fits reveal a higher bias for the small object diameter (Figure 7b
). The corresponding psychometric functions (not shown) and
”). The GCDF-fits reveal a higher bias for the small object diameter (Figure 7b
). The corresponding psychometric functions (not shown) and 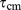 -predictions for the shortest presentation time (
-predictions for the shortest presentation time (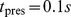 ; Figure 8a
) are thus rather flat and noisy. This bias is progressively reduced with increasing
; Figure 8a
) are thus rather flat and noisy. This bias is progressively reduced with increasing 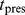 , indicating improving performance: For
, indicating improving performance: For 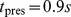 , the point of subjective simultaneity approaches
, the point of subjective simultaneity approaches 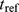 for both object diameters, and psychometric functions get closer to a step-wise increase at
for both object diameters, and psychometric functions get closer to a step-wise increase at 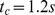 (Figure 7a
).
(Figure 7a
).
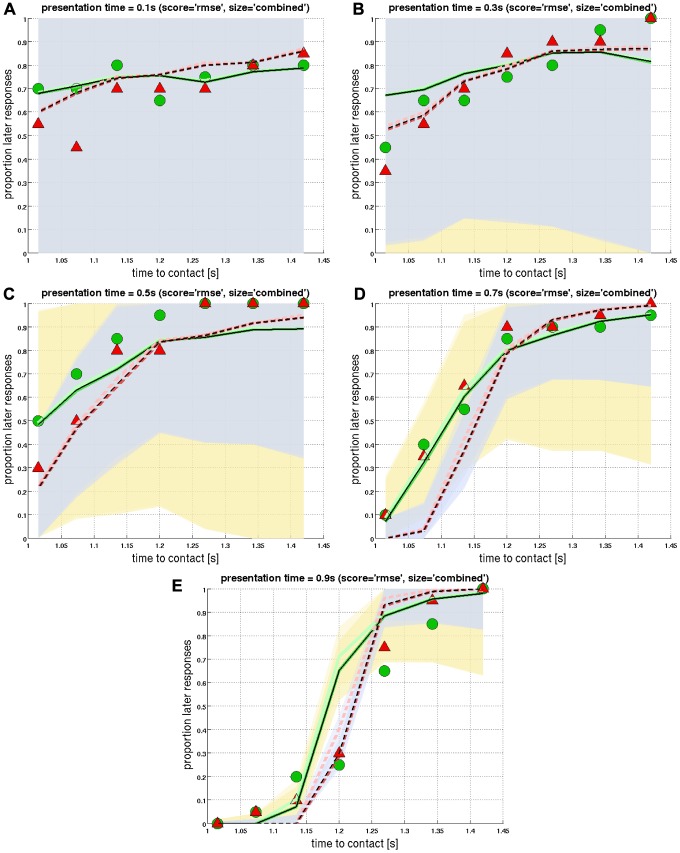
Figure 8.
Corrected m-Tau predictions (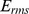 score; combined diameter).
score; combined diameter).
The proportion of later responses (i.e. subjects perceived ttc after 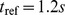 ) are shown as a function of ttc for different presentation times
) are shown as a function of ttc for different presentation times 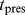 : (a)
: (a) 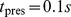 , (b)
, (b) 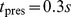 , (c)
, (c) 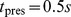 , (d)
, (d) 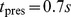 , and (e)
, and (e) 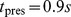 . Psychophysical results
. Psychophysical results 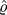 were pooled across subjects and are denoted by circles (small object diameter
were pooled across subjects and are denoted by circles (small object diameter 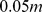 ) and triangles (big object diameter
) and triangles (big object diameter 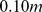 ), respectively. Predictions
), respectively. Predictions  of the corrected m-Tau -model “
of the corrected m-Tau -model “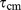 ” are represented by curves. In this figure, the prediction performance of
” are represented by curves. In this figure, the prediction performance of 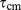 was measured according to the root mean square error (“
was measured according to the root mean square error (“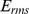 -score”). Corrected m-Tau -predictions with the three best performing parameter sets are juxtaposed (i.e. first three rows in Table S3 in Text S5 with smallest
-score”). Corrected m-Tau -predictions with the three best performing parameter sets are juxtaposed (i.e. first three rows in Table S3 in Text S5 with smallest 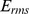 -score). Thinner and darker lines represent a better prediction performance. Furthermore, continuous curves are the
-score). Thinner and darker lines represent a better prediction performance. Furthermore, continuous curves are the 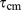 -predictions for small (thus should match the circles), while dashed curves correspond to big (should match the triangles). Here, the same set of
-predictions for small (thus should match the circles), while dashed curves correspond to big (should match the triangles). Here, the same set of 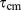 -parameters was used for both object diameters (“combined diameter”). The light-shaded areas correspond to the variability of simulated responses (
-parameters was used for both object diameters (“combined diameter”). The light-shaded areas correspond to the variability of simulated responses (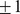 SD, see Methods Section): Yellowish shading for small, and bluish shading for big.
SD, see Methods Section): Yellowish shading for small, and bluish shading for big.
We already mentioned that we simulated the psychometric functions with the corrected m-Tau -model (equation 5), at which we added noise to angular size and angular velocity (equation 9). By assuming a constant approach velocity, one could compute an estimation of ttc with equation (12). Note that this estimation should be constant throughout the approach in a noise-free situation and for “sufficiently small” angular sizes. As a consequence of having noise, however, the ttc estimation fluctuates. We therefore computed an average estimation with equation (14), by taking the mean value across a time interval (the interval contained the last 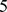 estimates). The average ttc estimation was evaluated at presentation time
estimates). The average ttc estimation was evaluated at presentation time 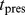 , and compared with the reference
, and compared with the reference 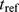 . With a total number
. With a total number 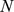 of such trials, we then counted
of such trials, we then counted 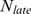 occurrences where the average estimate occurred after
occurrences where the average estimate occurred after 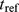 . The simulated proportion of later responses is then obtained by dividing
. The simulated proportion of later responses is then obtained by dividing 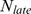 by
by 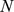 (equation 13).
(equation 13).
In order to find the appropriate 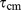 -parameters for predicting psychophysical performance, the error between
-parameters for predicting psychophysical performance, the error between 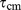 -predictions and psychophysical data points was minimized. We refer to this procedure as optimization. Optimization was carried out separately for object diameters big and small. The first step of the optimization procedure consisted in parsing the parameter space, and recording the error associated with each set of
-predictions and psychophysical data points was minimized. We refer to this procedure as optimization. Optimization was carried out separately for object diameters big and small. The first step of the optimization procedure consisted in parsing the parameter space, and recording the error associated with each set of 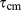 -parameters. The error was determined with two measures (“score measures”): The root mean square error (
-parameters. The error was determined with two measures (“score measures”): The root mean square error (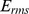 ), and an outlier-insensitive robust error (
), and an outlier-insensitive robust error (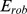 ). In the second step, the parameter sets were sorted in ascending order with respect to their associated score measure. Sorting took place separately for
). In the second step, the parameter sets were sorted in ascending order with respect to their associated score measure. Sorting took place separately for 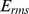 and
and 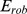 , leading to corresponding tables where the best set of parameters was assigned rank one (1st table row), the second best rank two (2nd table row), and so on (Tables S1 & S2 in Text S5).
, leading to corresponding tables where the best set of parameters was assigned rank one (1st table row), the second best rank two (2nd table row), and so on (Tables S1 & S2 in Text S5).
A third table of 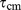 -parameters was then computed which was optimal for both object diameters simultaneously (combined; Table S3 in Text S5). This could be done in a straightforward way, simply by averaging the score measures of big and small of corresponding parameter sets, and subsequently sorting the averaged errors (more details on finding parameters are given in Text S5).
-parameters was then computed which was optimal for both object diameters simultaneously (combined; Table S3 in Text S5). This could be done in a straightforward way, simply by averaging the score measures of big and small of corresponding parameter sets, and subsequently sorting the averaged errors (more details on finding parameters are given in Text S5).
For the computation of 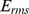 and
and 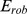 , all psychophysical data points that represent the proportion of later responses entered equivalently, in the sense that no weighting coefficients were used to bias the optimization process toward longer presentation times (as GCDF-fits at longer presentation times have a smaller variance, see Figure 7b
). Notice that parameter optimization for the combined diameter naturally implicates a trade off – the errors with respect to big and small will be bigger compared to individual parameter optimization.
, all psychophysical data points that represent the proportion of later responses entered equivalently, in the sense that no weighting coefficients were used to bias the optimization process toward longer presentation times (as GCDF-fits at longer presentation times have a smaller variance, see Figure 7b
). Notice that parameter optimization for the combined diameter naturally implicates a trade off – the errors with respect to big and small will be bigger compared to individual parameter optimization.
Figure 8 shows that the corrected m-Tau -model adjusts fairly well to the psychophysical data of both object diameters. Nevertheless, the 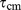 -predictions for
-predictions for 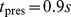 are somewhat worse with the combined parameter optimization (Figure 8e
) when compared to a separate optimization for big and small (corresponding figures in Text S7). The most likely explanation for this discrepancy (individual versus combined parametrizations) is that each object size is associated with a different noise level (noise levels are represented by the
are somewhat worse with the combined parameter optimization (Figure 8e
) when compared to a separate optimization for big and small (corresponding figures in Text S7). The most likely explanation for this discrepancy (individual versus combined parametrizations) is that each object size is associated with a different noise level (noise levels are represented by the 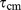 -parameters
-parameters 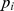 with
with 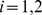 ; see equation 9). We investigated this hypothesis by comparing the corresponding values of
; see equation 9). We investigated this hypothesis by comparing the corresponding values of 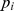 for big and small, as a function of their rank. Figure 9 shows that the
for big and small, as a function of their rank. Figure 9 shows that the 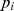 for small are consistently higher than for big. Therefore, the corrected m-Tau -model generally supports the notion that smaller object diameters imply higher noise levels in angular size and angular velocity, respectively.
for small are consistently higher than for big. Therefore, the corrected m-Tau -model generally supports the notion that smaller object diameters imply higher noise levels in angular size and angular velocity, respectively.
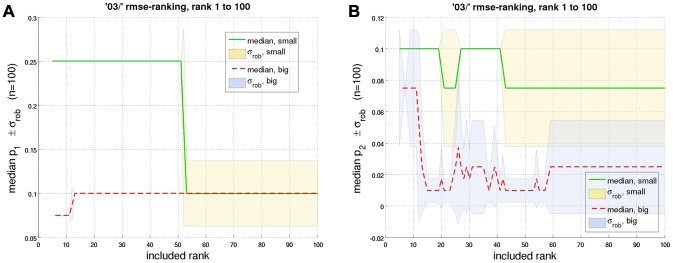
Figure 9. Median value of noise probabilities as a function of 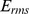 -rank.
-rank.
In order to predict psychophysical performance with the corrected m-Tau -model, its parameters were optimized. Prediction performance was measured with a score measure, either the root mean square error (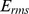 , shown here), or an outlier-insensitive robust error (
, shown here), or an outlier-insensitive robust error (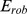 ; shown in Text S5). The
; shown in Text S5). The 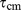 -parameter set with which the best prediction was achieved was assigned rank one, the second best rank two, and so on. Thus, rank one corresponds to the parameter set with the smallest score measure. The figure shows the median value of the noise probability equation (9) of: (a) angular size
-parameter set with which the best prediction was achieved was assigned rank one, the second best rank two, and so on. Thus, rank one corresponds to the parameter set with the smallest score measure. The figure shows the median value of the noise probability equation (9) of: (a) angular size 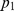 , and (b) angular velocity
, and (b) angular velocity  , as a function of rank. Abscissa values of
, as a function of rank. Abscissa values of  ,
,  , etc. signify that the median value across the first
, etc. signify that the median value across the first  , first
, first 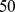 , etc. values of
, etc. values of 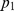 and
and 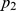 , respectively, was computed, according to “
, respectively, was computed, according to “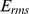 -ranking”. Shaded areas indicate
-ranking”. Shaded areas indicate 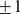 of the corresponding robust estimation of standard deviation
of the corresponding robust estimation of standard deviation 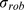 . The continuous curves were computed with the
. The continuous curves were computed with the 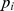 -values optimized for the small object diameter (listed in Table S1 in Text S5), and broken curves denote corresponding values for the big diameter (Table S2 in Text S5). The curves shown here suggest that the small object diameter is associated with a higher noise level. This conclusion is valid for
-values optimized for the small object diameter (listed in Table S1 in Text S5), and broken curves denote corresponding values for the big diameter (Table S2 in Text S5). The curves shown here suggest that the small object diameter is associated with a higher noise level. This conclusion is valid for 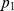 until rank
until rank 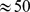 (curves become indistinguishable beyond that value), and for
(curves become indistinguishable beyond that value), and for 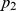 until rank ten: For ranks bigger than ten,
until rank ten: For ranks bigger than ten, 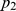 reveals a certain dependence on the score measure and the averaging procedure (not visible in this plot, but see corresponding figures in Text S5).
reveals a certain dependence on the score measure and the averaging procedure (not visible in this plot, but see corresponding figures in Text S5).
We also studied two models with less degrees of freedom than corrected m-Tau : The first was 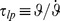 , and the second was
, and the second was 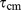 with
with 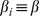 for
for 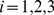 (
(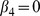 ). The best (i.e. smallest) score measures achieved with these reduced models were consistently higher than the best values achieved by the corrected m-Tau -model (Text S5), and their best-ranked parameter sets resulted in psychometric curve predictions that were also inferior by visual inspection (not shown).
). The best (i.e. smallest) score measures achieved with these reduced models were consistently higher than the best values achieved by the corrected m-Tau -model (Text S5), and their best-ranked parameter sets resulted in psychometric curve predictions that were also inferior by visual inspection (not shown).
Discussion
With the corrected m-Tau -model equation (5), we proposed a general framework that comprises the  -function and several properties of the
-function and several properties of the 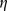 -function. By means of adjusting only a single parameter (
-function. By means of adjusting only a single parameter (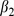 ), the corrected m-Tau -model can approximate
), the corrected m-Tau -model can approximate  and
and 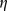 , respectively. Moreover, the
, respectively. Moreover, the  -approximation is less sensitive to noise than the original
-approximation is less sensitive to noise than the original  -function, and accounts well for the performance of the psychophysical experiment that we carried out.
-function, and accounts well for the performance of the psychophysical experiment that we carried out.
In the experiment, subjects had to decide whether a (displayed) ball reached them before or after a reference signal at time 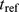 . However, balls were only presented until
. However, balls were only presented until 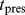 , and disappeared afterwards. In other words, subjects had to estimate
, and disappeared afterwards. In other words, subjects had to estimate 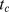 (
(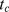 could occur before or after
could occur before or after 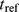 ). With respect to our experiment, the corrected m-Tau -model suggests the following conclusions:
). With respect to our experiment, the corrected m-Tau -model suggests the following conclusions:
Subjects relied on a
 -based mechanism for judging ttc (
-based mechanism for judging ttc (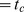 ). We use the term “
). We use the term “ -based” as a synonym for any timing-based mechanism. The full corrected m-Tau -model better predicted our psychophysical results than any of the two alternative models that we considered (
-based” as a synonym for any timing-based mechanism. The full corrected m-Tau -model better predicted our psychophysical results than any of the two alternative models that we considered ( and
and 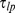 ).
).The decision about whether perceived
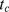 occurs before or after
occurs before or after 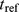 is based on information at (or immediately around)
is based on information at (or immediately around) 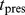 , as the only information used was from
, as the only information used was from  until
until 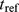 for predicting ttc (see equation 14).
for predicting ttc (see equation 14).Subjects' performance improves with increasing
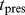 , indicating that the signal-to-noise ratio (SNR) that is associated with the computation of (perceived)
, indicating that the signal-to-noise ratio (SNR) that is associated with the computation of (perceived) 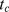 improves during an object approach. Such an improvement can be brought about by two mechanisms. First, the noise level is signal-independent and thus stays the same during an object approach. As angular size
improves during an object approach. Such an improvement can be brought about by two mechanisms. First, the noise level is signal-independent and thus stays the same during an object approach. As angular size  and angular velocity
and angular velocity 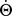 increase monotonically with time, the SNR would improve accordingly. Second, noise may increase with the signal [37], but is concurrently suppressed by low-pass filtering. Low-pass filtering may be adaptive, such that it adjusts to signal variability in each moment. We are not aware of any such signal-dependent noise suppression, and we therefore deem the first mechanism to be the more likely. Accordingly, we propose that approaching objects with smaller size lead to decreased SNRs in the signals that represent
increase monotonically with time, the SNR would improve accordingly. Second, noise may increase with the signal [37], but is concurrently suppressed by low-pass filtering. Low-pass filtering may be adaptive, such that it adjusts to signal variability in each moment. We are not aware of any such signal-dependent noise suppression, and we therefore deem the first mechanism to be the more likely. Accordingly, we propose that approaching objects with smaller size lead to decreased SNRs in the signals that represent  and
and 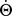 , respectively.
, respectively.The perception of ttc in humans reveals a certain dependence on object size [22]. Thus, one might argue that
 -based mechanisms are not an adequate model for ttc perception, because they are largely object-size-independent in the early phase of an object approach when
-based mechanisms are not an adequate model for ttc perception, because they are largely object-size-independent in the early phase of an object approach when  is still “sufficiently small”. However, this argument ignores noise. As long as the noise-induced fluctuations in
is still “sufficiently small”. However, this argument ignores noise. As long as the noise-induced fluctuations in  and
and 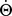 do not cancel (“correlated noise”), the SNR of
do not cancel (“correlated noise”), the SNR of  will depend on object size (Figure 6). Therefore, any decision based on computing
will depend on object size (Figure 6). Therefore, any decision based on computing 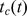 with a
with a  -based mechanisms will be limited by the SNR at time
-based mechanisms will be limited by the SNR at time  (ttc can be computed by adding
(ttc can be computed by adding  to
to 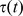 , because in the early phase of an approach
, because in the early phase of an approach 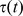 decreases linearly with time for
decreases linearly with time for 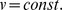 , see equation 12). The SNR improves with increasing object size and with decreasing (initial) distance between object and observer. Thus, bigger objects will imply better accuracy in estimating
, see equation 12). The SNR improves with increasing object size and with decreasing (initial) distance between object and observer. Thus, bigger objects will imply better accuracy in estimating 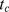 . Similarly, smaller distances will imply better estimation accuracy. Both effects are observed in our psychophysical experiment, where a better “estimation accuracy” translates into psychometric curves that adjust better to a step-wise increase from zero to one at
. Similarly, smaller distances will imply better estimation accuracy. Both effects are observed in our psychophysical experiment, where a better “estimation accuracy” translates into psychometric curves that adjust better to a step-wise increase from zero to one at 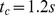 (because of
(because of 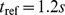 ; Figure 7a
). Without noise, however,
; Figure 7a
). Without noise, however,  -based mechanisms cannot predict such dependence on object size for small angular sizes.
-based mechanisms cannot predict such dependence on object size for small angular sizes.
The modified  -model (“
-model (“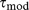 ”) constitutes a special case of
”) constitutes a special case of 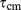 . It is obtained from equation (5) for
. It is obtained from equation (5) for 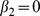 (
(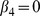 by default). Its distinguishing feature is a maximum before
by default). Its distinguishing feature is a maximum before 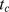 , which can be shifted via
, which can be shifted via 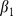 (equation 2). The
(equation 2). The 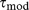 -maximum decreases as it is positioned closer to
-maximum decreases as it is positioned closer to 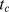 , because this implies bigger values of
, because this implies bigger values of 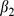 . The time
. The time 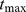 of the
of the 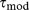 -maximum depends furthermore on size and velocity (Figure 1). The curve shape of
-maximum depends furthermore on size and velocity (Figure 1). The curve shape of 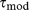 is reminiscent of the
is reminiscent of the 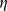 -function, since both functions have a maximum. We thus decided to fit previously published response curves from collision sensitive neurons to both functions, and observed that both functions fit the neural curves well in terms of goodness-of-fit criteria (Text S4). We must not forget, however, two important differences between
-function, since both functions have a maximum. We thus decided to fit previously published response curves from collision sensitive neurons to both functions, and observed that both functions fit the neural curves well in terms of goodness-of-fit criteria (Text S4). We must not forget, however, two important differences between 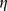 and
and 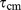 .
.
First, since  reveals a minimum shortly before
reveals a minimum shortly before 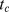 (Text S6) and
(Text S6) and 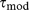 derives from
derives from  , the
, the 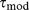 -response is more precisely biphasic. The biphasic structure gets pronounced in some of the curve fits, especially when
-response is more precisely biphasic. The biphasic structure gets pronounced in some of the curve fits, especially when 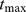 is close to
is close to 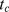 (see corresponding figures in Text S4). Then, the amplitude of the
(see corresponding figures in Text S4). Then, the amplitude of the 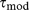 -maximum is small, and consequently the fitting algorithm has to scale it to the maximum of the neuronal recording data. In this way, the minimum is also scaled.
-maximum is small, and consequently the fitting algorithm has to scale it to the maximum of the neuronal recording data. In this way, the minimum is also scaled.
Second, 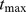 depends in a nonlinear way on the size-to-velocity ratio
depends in a nonlinear way on the size-to-velocity ratio 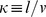 (see Figure 4 for an illustration). This is contradictory to several studies that found a linear dependence. A linear dependence is also predicted by the
(see Figure 4 for an illustration). This is contradictory to several studies that found a linear dependence. A linear dependence is also predicted by the 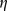 -function (equation S5 in Text S2). The contradiction can be alleviated by adding noise to relative time of the
-function (equation S5 in Text S2). The contradiction can be alleviated by adding noise to relative time of the 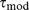 -maximum (
-maximum (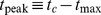 ; equation S10 in Text S2), with noise amplitudes as reported in [26]. As a consequence of noise, the nonlinear relationship can be literally hidden (Figure 3), such that statistical tests would affirm an underlying linear process (Text S2). Masking by noise is more effective for bigger values of
; equation S10 in Text S2), with noise amplitudes as reported in [26]. As a consequence of noise, the nonlinear relationship can be literally hidden (Figure 3), such that statistical tests would affirm an underlying linear process (Text S2). Masking by noise is more effective for bigger values of  , because the noise level is proportional to
, because the noise level is proportional to  .
.
The 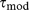 -function in its original form cannot explain the neuronal response curves for an approach with
-function in its original form cannot explain the neuronal response curves for an approach with 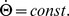 (“linear approach”) [25]: Rather than predicting a decreasing response with time, the
(“linear approach”) [25]: Rather than predicting a decreasing response with time, the 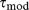 -function would linearly increase. In contrast, the
-function would linearly increase. In contrast, the 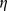 -function makes correct predictions. Correct predictions with
-function makes correct predictions. Correct predictions with 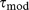 can nevertheless be made by including an additional inhibitory process in the firing rate equation of
can nevertheless be made by including an additional inhibitory process in the firing rate equation of 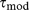 (equation S3 in Text S1, where a full proof of concept is described). Important, this extension of
(equation S3 in Text S1, where a full proof of concept is described). Important, this extension of 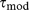 (i) is based on a power function with an exponent between
(i) is based on a power function with an exponent between  and
and 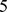 , but not on an exponential function as with
, but not on an exponential function as with 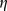 , and in this regard it may hence be considered as being biophysically more plausible than
, and in this regard it may hence be considered as being biophysically more plausible than 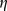 (see also reference [36]); (ii) does not interfere with the “normal”
(see also reference [36]); (ii) does not interfere with the “normal” 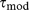 behavior (i.e. normal object approaches are not affected); and (iii) tolerates high noise levels (i.e., the mechanism is robust).
behavior (i.e. normal object approaches are not affected); and (iii) tolerates high noise levels (i.e., the mechanism is robust).
What about alternative models which also have a response peak? In Text S6 we studied two such functions, namely “inverse  ” (
” (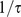 ), and angular acceleration (
), and angular acceleration (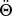 ). Both of them reveal a linear dependence of
). Both of them reveal a linear dependence of 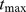 on
on  (equations S24 & S26, respectively, in Text S6). The maximum of
(equations S24 & S26, respectively, in Text S6). The maximum of 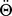 always precedes that of
always precedes that of 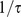 . However,
. However, 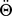 does not make correct predictions for the “linear approach”, as we would obtain
does not make correct predictions for the “linear approach”, as we would obtain 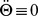 ab initio for
ab initio for 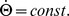 (although a dynamical version may predict the decreasing LGMD-activity on the basis of temporal filtering effects).
(although a dynamical version may predict the decreasing LGMD-activity on the basis of temporal filtering effects).
In contrast, 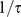 would make consistent predictions in that case. Without further modifications, though, neither
would make consistent predictions in that case. Without further modifications, though, neither 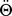 nor
nor 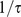 seems to be adequate for fitting the response curves of collision sensitive neurons, because there is no free model parameter to shift their respective maximum. Although the occurrence of their maxima could principally be controlled by a global shift of the time scale
seems to be adequate for fitting the response curves of collision sensitive neurons, because there is no free model parameter to shift their respective maximum. Although the occurrence of their maxima could principally be controlled by a global shift of the time scale  , the corresponding values (obtained by fitting the neuronal response curves) would overestimate experimental values (Text S6). Similarly, when “fitting” the
, the corresponding values (obtained by fitting the neuronal response curves) would overestimate experimental values (Text S6). Similarly, when “fitting” the 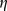 -function to
-function to 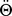 and
and 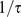 the so obtained values of
the so obtained values of  would underestimate experimental values: The
would underestimate experimental values: The 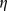 -maximum would coincide with the maximum of
-maximum would coincide with the maximum of  for
for 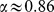 , and with the maximum of
, and with the maximum of 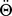 for
for 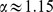 .
.
In conclusion, 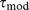 is no replacement for the
is no replacement for the 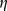 -function, at least for describing neuronal responses of collision sensitive neurons in insects. However, in the nucleus rotundus of pigeons three classes of neurons were reported [1], [38]. They conform to
-function, at least for describing neuronal responses of collision sensitive neurons in insects. However, in the nucleus rotundus of pigeons three classes of neurons were reported [1], [38]. They conform to 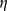 -like,
-like, 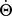 -like, and
-like, and  -like responses. The fact that
-like responses. The fact that 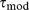 is just a special case of
is just a special case of 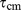 could possibly explain why neurons with
could possibly explain why neurons with 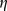 -like and
-like and  -like properties can be found in a single brain. Within the
-like properties can be found in a single brain. Within the 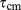 -framework, the
-framework, the 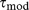 function corresponds to
function corresponds to 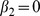 , and
, and 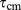 is obtained for choosing
is obtained for choosing 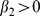 . Thus, the adjustment of only a single weight (
. Thus, the adjustment of only a single weight (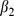 ) is necessary to go from one function to the other. The corrected m-Tau -framework could thus offer a parsimonious yet full-fledged explanation of the implementation of
) is necessary to go from one function to the other. The corrected m-Tau -framework could thus offer a parsimonious yet full-fledged explanation of the implementation of 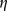 -like and
-like and  -like neurons at the circuit level.
-like neurons at the circuit level.
Methods
Psychophysical experiment
Subjects
Four subjects that were members of the Basic Psychology Department of the University of Barcelona participated in the experiment. All had normal or corrected-to-normal vision and were naive with respect to the aims of the experiment. Two of the subjects were well-trained psychophysical subjects in similar tasks. None of the subjects was stereo blind (StereoFly test, Stereo Optical Co.). They all signed an informed consent. The psychophysical experiment was approved by the Ethics Committee of the Faculty for Psychology of the University of Barcelona, in agreement with the ethical guidelines of the Declaration of Helsinki in 1954.
Stimuli and apparatus
Stimuli were displayed on a Phillips 22 inch monitor (Brilliance 202P4) at a refresh rate of 118 Hz and a screen resolution of 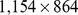 pixels. A 3Dlabs VP870 video card controlled the stereo shutter spectacles (CristalEyes). Simulated targets were uniform disks that moved on a collision trajectory along a line that passed the midpoint between the subjects' eyes. The screen was at one meter distance from subjects' eyes.
pixels. A 3Dlabs VP870 video card controlled the stereo shutter spectacles (CristalEyes). Simulated targets were uniform disks that moved on a collision trajectory along a line that passed the midpoint between the subjects' eyes. The screen was at one meter distance from subjects' eyes.
Seven time-to-contact values (experimentally fixed values 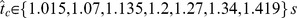 ) were combined with two different object sizes (diameter
) were combined with two different object sizes (diameter 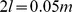 and
and 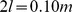 ), and five presentation times (
), and five presentation times (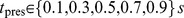 ), totaling
), totaling  different combinations. In order to ensure that the subjects used the judged time to contact rather than some other correlated measure, we varied the initial simulated starting distances (from
different combinations. In order to ensure that the subjects used the judged time to contact rather than some other correlated measure, we varied the initial simulated starting distances (from 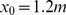 to
to 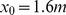 ), and set velocities
), and set velocities  to
to 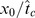 .
.
Procedure
Each simulated object appeared at its initial distance 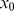 on the monitor. After one second, the object started approaching the observer at the designated constant velocity
on the monitor. After one second, the object started approaching the observer at the designated constant velocity  , and was visible until
, and was visible until 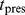 (presentation time). The reference time
(presentation time). The reference time 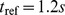 was indicated to subjects with an acoustic signal (beep) [16]. The reference time remained unchanged throughout the experiment. Subjects were instructed to press one of two buttons to indicate whether they thought being hit by the object before or after
was indicated to subjects with an acoustic signal (beep) [16]. The reference time remained unchanged throughout the experiment. Subjects were instructed to press one of two buttons to indicate whether they thought being hit by the object before or after 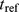 . In each session, the complete set of
. In each session, the complete set of 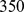 stimuli was shown to subjects in random order (five repetitions times the
stimuli was shown to subjects in random order (five repetitions times the 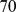 combinations). Each subject took part in five sessions. Feedback on incorrect responses was provided after each trial.
combinations). Each subject took part in five sessions. Feedback on incorrect responses was provided after each trial.
Simulation of our psychophysical experiment
We simulated our psychophysical experiment with the corrected m-Tau -model (equation 3), where we plugged in noisified versions of the optical variables (i.e. 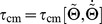 ),
),
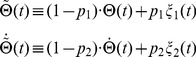 |
(9) |
with noise probabilities 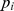 (
(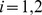 ), and with the dot denoting the time derivative. The
), and with the dot denoting the time derivative. The 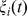 are random variables, which at each instant
are random variables, which at each instant  return a value that is drawn from a centered normal distribution. In the last equations, we used the explicit expression for angular size,
return a value that is drawn from a centered normal distribution. In the last equations, we used the explicit expression for angular size,
| (10) |
and angular velocity ( rate of expansion)
rate of expansion)
| (11) |
with 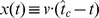 and
and 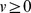 . The values of
. The values of 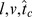 and
and 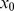 are the psychophysical stimulus parameters. Simulations were carried out with a temporal resolution of
are the psychophysical stimulus parameters. Simulations were carried out with a temporal resolution of 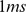 .
.
The corrected m-Tau -model is constrained by two limit functions: Ordinary  on the one hand (equation 6), and
on the one hand (equation 6), and 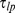 on the other (equation 8). Both limit functions decrease approximately as
on the other (equation 8). Both limit functions decrease approximately as 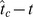 (illustration: Figure 6). Thus, a ttc estimation at time
(illustration: Figure 6). Thus, a ttc estimation at time  can be computed as
can be computed as
| (12) |
(Nomenclature: 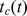 is the model prediction for ttc at time
is the model prediction for ttc at time  , and
, and 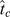 is the experimentally set parameter). In the psychophysical study, subjects were asked to estimate whether they were hit by the approaching object before or after
is the experimentally set parameter). In the psychophysical study, subjects were asked to estimate whether they were hit by the approaching object before or after 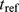 . We accordingly define their proportion of later responses
. We accordingly define their proportion of later responses
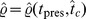 as the number of trials
as the number of trials 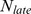 (where subjects responded with being struck after
(where subjects responded with being struck after 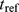 ) divided by the total number of trials
) divided by the total number of trials 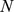 :
:
| (13) |
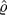 is represented by circle and triangle symbols in Figure 7 and 8. The corresponding predictions from the model are denoted by
is represented by circle and triangle symbols in Figure 7 and 8. The corresponding predictions from the model are denoted by  . Specifically,
. Specifically, 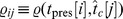 with
with 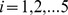 and
and 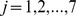 , and analogous for
, and analogous for 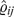 . Computation of
. Computation of 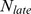 is required for
is required for 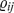 , which we did with equation (12) as per
, which we did with equation (12) as per
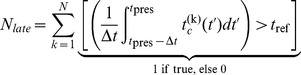 |
(14) |
Notice that, due to noise (equation 9), 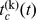 will be subjected to random jitter with each trial
will be subjected to random jitter with each trial 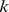 . Therefore, in order to obtain a more robust estimate of ttc , we do not use only
. Therefore, in order to obtain a more robust estimate of ttc , we do not use only 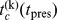 : The integral in the last equation computes – in the discrete case – the mean value across the last
: The integral in the last equation computes – in the discrete case – the mean value across the last  time steps until
time steps until  (typically
(typically  , what amounts to a time interval for averaging of
, what amounts to a time interval for averaging of 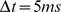 , cf. first figure in Text S7). In order to illustrate the noise level at each
, cf. first figure in Text S7). In order to illustrate the noise level at each 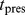 , we also computed the standard deviation
, we also computed the standard deviation  of the
of the 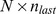 last values of
last values of 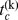 . The shaded areas in the figures which visualize
. The shaded areas in the figures which visualize  &
& 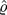 correspond to
correspond to 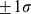 . Predictions of the corrected m-Tau -model are shown as curves in Figure 8, as well as in Text S7.
. Predictions of the corrected m-Tau -model are shown as curves in Figure 8, as well as in Text S7.
Parameters of the corrected m-Tau model
The corrected m-Tau -model has eight free parameters: 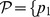 ,
, 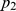 ,
, 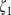 ,
, 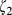 ,
, 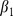 ,
, 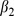 ,
, 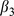 ,
, 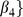 . The parameter space was parsed with constant step widths. For each set of parameter values
. The parameter space was parsed with constant step widths. For each set of parameter values 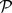 ,
, 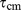 -predictions for the proportion-of-later-response curves were computed according to the procedure described in the previous section. The corresponding “goodness of prediction” (or “prediction performance”) was evaluated with the root mean square error (rmse,
-predictions for the proportion-of-later-response curves were computed according to the procedure described in the previous section. The corresponding “goodness of prediction” (or “prediction performance”) was evaluated with the root mean square error (rmse, 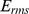 ), and the outlier insensitive, robust error (robe,
), and the outlier insensitive, robust error (robe, 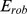 ), see equation S18 in Text S5. The “goodness of prediction” measures are referred to as score-measures (rmse-scores & robe-scores, respectively). Parameter values were sorted according to their scores. In this way we ended up with several score tables, which list the best set of parameters, according to object size: Table S1 in Text S5 for small object diameter (
), see equation S18 in Text S5. The “goodness of prediction” measures are referred to as score-measures (rmse-scores & robe-scores, respectively). Parameter values were sorted according to their scores. In this way we ended up with several score tables, which list the best set of parameters, according to object size: Table S1 in Text S5 for small object diameter (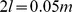 ), Table S2 in Text S5 for big object diameter (
), Table S2 in Text S5 for big object diameter (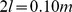 ), & Table S3 in Text S5 for combined object diameter. The scores for the combined size were computed by averaging the scores of big & small for corresponding parameter values, and then sorting the averaged scores in ascending order. More details on parameter finding and analysis are given in Text S5.
), & Table S3 in Text S5 for combined object diameter. The scores for the combined size were computed by averaging the scores of big & small for corresponding parameter values, and then sorting the averaged scores in ascending order. More details on parameter finding and analysis are given in Text S5.
Derivation of Equation 2
Consider a rigid sphere (object radius or half-size 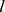 ) that approaches an observer on a direct collision course. If the approach proceeds at a constant velocity
) that approaches an observer on a direct collision course. If the approach proceeds at a constant velocity 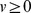 , the object-observer distance at time
, the object-observer distance at time  is
is 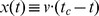 . Thus, the initial distance is
. Thus, the initial distance is 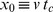 .
.
Now, consider the gain control factor 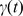 from equation (1)
from equation (1)
| (15) |
where we plug in the explicit expression for angular velocity equation (11) and obtain
| (16) |
Especially in the initial phase of the approach, when visual angle and angular velocity are sufficiently small,  decreases approximately linearly with time (cf. Text S6),
decreases approximately linearly with time (cf. Text S6),
| (17) |
Because of 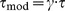 , the m-Tau function becomes approximately
, the m-Tau function becomes approximately
| (18) |
A maximum of the m-Tau function implies that its first time derivative is zero. We define 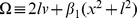 . The first time derivative of the (approximate) m-Tau function
. The first time derivative of the (approximate) m-Tau function
| (19) |
disappears if 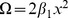 , or
, or
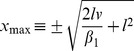 |
(20) |
The last equation is the distance  (positive sign) where the approximated m-Tau function attains its maximum during an object approach. Thus, the time
(positive sign) where the approximated m-Tau function attains its maximum during an object approach. Thus, the time  when the
when the  -maximum occurs is
-maximum occurs is
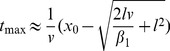 |
(21) |
Supporting Information
Properties and extension of modified Tau.
Text S1 presents additional mathematical details of the  -function. Specifically, it is shown how the
-function. Specifically, it is shown how the  -function could be extended to a model which predicts the so-called “linear approach” data. Corresponding simulation results from this model are also shown.
-function could be extended to a model which predicts the so-called “linear approach” data. Corresponding simulation results from this model are also shown.
(PDF)
Nonlinearity of the m-Tau function.
Text S2 is dedicated to the nonlinear character of  and how it could be successfully hidden behind noise. The section presents additional figures with random trials (analogous to Figure 3b
), and corresponding scatter plots with goodness-of-fit measures as a function of
and how it could be successfully hidden behind noise. The section presents additional figures with random trials (analogous to Figure 3b
), and corresponding scatter plots with goodness-of-fit measures as a function of 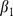 .
.
(PDF)
Noise suppression.
Text S3 considers the numerical robustness of  ,
, 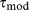 ,
, 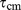 and
and 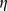 , by adding correlated and uncorrelated noise to the angular variables. Similar to Figure 6, it is shown how noise affects these functions (e.g. bigger object diameters are associated with correspondingly less fluctuations), and thus the results presented in this section help to understand the simulation and the interpretation of our psychophysical experiment.
, by adding correlated and uncorrelated noise to the angular variables. Similar to Figure 6, it is shown how noise affects these functions (e.g. bigger object diameters are associated with correspondingly less fluctuations), and thus the results presented in this section help to understand the simulation and the interpretation of our psychophysical experiment.
(PDF)
Fitting m-Tau and
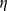 -function to neuronal recordings.
Text S4 juxtaposes the individual fitting results of
-function to neuronal recordings.
Text S4 juxtaposes the individual fitting results of 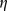 and
and 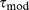 to a variety of previously published neural recording data, which served to compile Figure 2. Further summary results are presented along with fitting results of individual recording traces.
to a variety of previously published neural recording data, which served to compile Figure 2. Further summary results are presented along with fitting results of individual recording traces.
(PDF)
Finding parameter values for
corrected m-Tau
.
Text S5 describes the optimization procedure for the corrected m-Tau -model “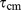 ”, with which we obtained the parameter values for the simulation of our psychophysical experiment (e.g. Figure 8). The
”, with which we obtained the parameter values for the simulation of our psychophysical experiment (e.g. Figure 8). The 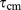 -parameters were optimized in three different ways: For achieving a good prediction performance of the psychophysical data corresponding to (i) the small object diameter (Table S1 in Text S5), (ii) the big object diameter (Table S2 in Text S5), and (iii) both diameters at the same time (combined; Table S3 in Text S5). The best ten values are listed in their respective tables according to their psychophysical prediction performance (as quantified by score measures
-parameters were optimized in three different ways: For achieving a good prediction performance of the psychophysical data corresponding to (i) the small object diameter (Table S1 in Text S5), (ii) the big object diameter (Table S2 in Text S5), and (iii) both diameters at the same time (combined; Table S3 in Text S5). The best ten values are listed in their respective tables according to their psychophysical prediction performance (as quantified by score measures 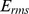 and
and 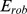 , respectively): The best parameter set (smallest score measure) was assigned rank one, the second best rank two, etc. Several figures were compiled that show an additional analysis of the parameter ranking.
, respectively): The best parameter set (smallest score measure) was assigned rank one, the second best rank two, etc. Several figures were compiled that show an additional analysis of the parameter ranking.
(PDF)
Time to contact approximation of “Tau” and
 .
Text S6 presents a comprehensive analysis of two alternative functions which have a maximum before ttc, namely “inverse
.
Text S6 presents a comprehensive analysis of two alternative functions which have a maximum before ttc, namely “inverse  ” (
” (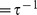 ) and “angular acceleration” (
) and “angular acceleration” (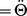 ). The two functions were also fitted to the neuronal recording data (they turn out to be inadequate), and compared to the maximum of the
). The two functions were also fitted to the neuronal recording data (they turn out to be inadequate), and compared to the maximum of the  -function. This section also provides insights into the biphasic nature of
-function. This section also provides insights into the biphasic nature of 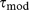 , because as
, because as 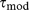 approaches ttc, it gets more similar to
approaches ttc, it gets more similar to  , and thus reveals a minimum.
, and thus reveals a minimum.
(PDF)
Predictions of
corrected m-Tau
for the psychophysical experiment.
Text S7 shows the full set of figures with simulation results of our psychophysical experiment. Whereas Figure 8 shows 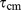 -predictions that were obtained with the parameter set optimized for the “combined” object diameter according to
-predictions that were obtained with the parameter set optimized for the “combined” object diameter according to 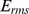 -score, Text S7 shows analogous figures for the remaining parameter optimizations (big and small object diameter, and
-score, Text S7 shows analogous figures for the remaining parameter optimizations (big and small object diameter, and 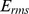 and
and 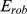 -scores, respectively).
-scores, respectively).
(PDF)
First order temporal low-pass filter (
Equation 4
).
Text S8 gives a short introduction to the temporal low-pass filter that forms a part of the 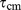 -model (equation 4), and is also used for the extension of the
-model (equation 4), and is also used for the extension of the 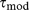 -model described in Text S1).
-model described in Text S1).
(PDF)
Acknowledgments
The authors like to thank Eric Johnson for his help in proofreading our manuscript.
Funding Statement
MSK acknowledges support from a Ramon & Cajal grant from the Ministry of Science and Innovation of the Spanish government, and from the national grant DPI2010-21513. JLM acknowledges support by grant PSI2010-15867 from the Ministry of Science and Innovation of the Spanish government and an ICREA Academia Distinguished Professorship award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sun H, Frost B (1998) Computation of different optical variables of looming objects in pigeon nucleus rotundus neurons. Nat Neurosci 1: 296–303. [DOI] [PubMed] [Google Scholar]
- 2. Rind F, Simmons P (1999) Seeing what is coming: building collision-sensitive neurones. Trends Neurosci 22: 215–220. [DOI] [PubMed] [Google Scholar]
- 3. Yonas A, Bechthold A, Frankel D, Gordon F, McRoberts G, et al. (1977) Development of sensitivity to information for impending collision. Percept Psychophys 21: 97–104. [Google Scholar]
- 4. Schiff W, Caviness J, Gibson J (1962) Persistent fear responses in rhesus monkeys to optical stimulus of looming. Science 136: 982–983. [DOI] [PubMed] [Google Scholar]
- 5. Rind F, Santer D (2004) Collision avoidance and a looming sensitive neuron: size matters but biggest is not necessarily best. Proc R Soc Lond B Biol Sci 271: S27–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robertson R, Johnson A (1993) Collision avoidance of ying locusts: steering torques and behaviour. J Exp Biol 183: 35–60. [Google Scholar]
- 7. López-Moliner J, Field D, Wann J (2007) Interceptive timing: Prior knowledge matters. J Vis 7: 11. [DOI] [PubMed] [Google Scholar]
- 8. Fotowat H, Gabbiani F (2011) Collision detection as a model for sensory-motor integration. Annu Rev Neurosci 34: 1–19. [DOI] [PubMed] [Google Scholar]
- 9.Hoyle F (1957) The Black Cloud. London: Pinguin Books. 26–29 pp.
- 10. Lee D (1976) A theory of visual control of braking based on information about time-to-collision. Perception 5: 437–459. [DOI] [PubMed] [Google Scholar]
- 11. Lee D, Reddish P (1981) Plummeting gannets: a paradigm of ecological optics. Nature 293: 293–294. [Google Scholar]
- 12. Regan D, Hamstra S (1993) Dissociation of discrimination thresholds for time to contact and for rate of angular expansion. Vision Res 33: 447–462. [DOI] [PubMed] [Google Scholar]
- 13. Wann J (1979) Anticipating arrival: is the tau-margin a specious theory? J Exp Psychol Hum Percept Perform 22: 1031–1048. [DOI] [PubMed] [Google Scholar]
- 14. Tresilian J (1999) Visually timed action: time-out for ‘tau’? Trends Cogn Sci 3: 1999. [DOI] [PubMed] [Google Scholar]
- 15. Regan D, Beverley K (1979) Binocular and monocular stimuli for motion in depth: changing disparity and changing-size feed the same motion-in-depth stage. Vision Res 19: 1331–1342. [DOI] [PubMed] [Google Scholar]
- 16. Gray R, Regan D (1998) Accuracy of estimating time to collision using binocular and monocular information. Vision Res 38: 499–512. [DOI] [PubMed] [Google Scholar]
- 17. Rushton S, Wann J (1999) Weighted combination of size and disparity: a computational model for timing ball catch. Nat Neurosci 2: 186–190. [DOI] [PubMed] [Google Scholar]
- 18. Regan D, Gray R (2000) Visually guided collision avoidance and collision achievement. Trends Cogn Sci 4: 99–107. [DOI] [PubMed] [Google Scholar]
- 19. Regan D, Beverley K (1973) Some dynamic features of depth perception. Vision Res 13: 2369–2379. [DOI] [PubMed] [Google Scholar]
- 20. McKee S, Watamaniuk S, Harris J, Smallman H, Taylor D (1997) Is stereopsis e_ective in breaking camouage for moving targets? Vision Res 37: 2047–2055. [DOI] [PubMed] [Google Scholar]
- 21. Tresilian J (1994) Approximation of information sources and perceptual variables in interceptive timing. J Exp Psychol Hum Percept Perform 20: 154–173. [Google Scholar]
- 22. DeLucia P, Warren R (1994) Pictorial and motion-based depth information during active control of self-motion: size-arrival effects on collision avoidance. J Exp Psychol Hum Percept Perform 20: 783–798. [DOI] [PubMed] [Google Scholar]
- 23. Judge S, Rind F (1997) The locust DCMD, a movement-detecting neurone tightly tuned to collision trajectories. J Exp Biol 200: 2209–2216. [DOI] [PubMed] [Google Scholar]
- 24. Rind F, Simmons P (1997) Signaling of object approach by the DCMD neuron of the locust. J Neurophysiol 77: 1029–1033. [DOI] [PubMed] [Google Scholar]
- 25. Hatsopoulos N, Gabbiani F, Laurent G (1995) Elementary computation of object approach by a wide-field visual neuron. Science 270: 1000–1003. [DOI] [PubMed] [Google Scholar]
- 26. Gabbiani F, Krapp H, Laurent G (1999) Computation of object approach by a wide-field, motion sensitive neuron. J Neurosci 19: 1122–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fotowat H, Harrison R, Gabbiani F (2011) Multiplexing of motor information in the discharge of a collision detecting neuron during escape behaviors. Neuron 69: 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Shea M, Rowell C (1975) A spike-transmitting electrical synapse between visual interneurons in the locust movement detector system. J Comp Physiol A 97: 143–158. [Google Scholar]
- 29. Rind F (1984) A chemical synapse between two motion detecting neurones in the locust brain. J Exp Biol 110: 143–167. [DOI] [PubMed] [Google Scholar]
- 30. Fotowat H, Gabbiani F (2007) Relationship between the phases of sensory and motor activity during a looming-evoked multistage escape behavior. J Neurosci 27: 10047–10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fotowat H, Fayyazuddin A, Bellen H, Gabbiani F (2009) A novel neuronal pathway for visually guided escape in drosophila melanogaster. J Neurophysiol 102: 875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakagawa H, Hongjian K (2010) Collision-sensitive neurons in the optic tectum of the bullfrog, rana catesbeiana. J Neurophysiol 104: 2487–2499. [DOI] [PubMed] [Google Scholar]
- 33. Preuss T, Osei-Bonsu P, Weiss S, Wang C, Faber D (2006) Neural representation of object approach in a decision-making motor circuit. J Neurosci 26: 3454–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gabbiani F, Krapp H, Koch C, Laurent G (2002) Multiplicative computation in a visual neuron sensitive to looming. Nature 420: 320–324. [DOI] [PubMed] [Google Scholar]
- 35. Grossberg S, Repin D (2003) A neural model of how the brain represents and compares multi-digit numbers: spatial and categorical processes. Neural Netw 16: 1107–1140. [DOI] [PubMed] [Google Scholar]
- 36.Keil M (2011) Emergence of multiplication in a biophysical model of a wide-field visual neuron for computing object approaches: Dynamics, peaks, & fits. Neural Information Processing Systems (NIPS) foundation. Available: http://books.nips.cc. http://arxiv.org/abs/1110.0433.
- 37. Harris C, Wolpert D (1998) Signal-dependent noise determines motor planning. Nature 394: 780–794. [DOI] [PubMed] [Google Scholar]
- 38.Frost B, Sun H (2003) The biological bases of time-to-collision computation. In: Hecht H, JPSavelsbergh G, editors, Time-to-Contact (Advances in Psychology Series). Amsterdam - North Holland: Elsevier. pp. 13–38.
- 39. Gabbiani F, Mo C, Laurent G (2001) Invariance of angular threshold computation in a wide-field looming-sensitive neuron. J Neurosci 21: 314–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Properties and extension of modified Tau.
Text S1 presents additional mathematical details of the 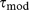 -function. Specifically, it is shown how the
-function. Specifically, it is shown how the 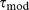 -function could be extended to a model which predicts the so-called “linear approach” data. Corresponding simulation results from this model are also shown.
-function could be extended to a model which predicts the so-called “linear approach” data. Corresponding simulation results from this model are also shown.
(PDF)
Nonlinearity of the m-Tau function.
Text S2 is dedicated to the nonlinear character of 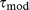 and how it could be successfully hidden behind noise. The section presents additional figures with random trials (analogous to Figure 3b
), and corresponding scatter plots with goodness-of-fit measures as a function of
and how it could be successfully hidden behind noise. The section presents additional figures with random trials (analogous to Figure 3b
), and corresponding scatter plots with goodness-of-fit measures as a function of 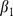 .
.
(PDF)
Noise suppression.
Text S3 considers the numerical robustness of  ,
, 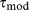 ,
, 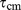 and
and 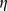 , by adding correlated and uncorrelated noise to the angular variables. Similar to Figure 6, it is shown how noise affects these functions (e.g. bigger object diameters are associated with correspondingly less fluctuations), and thus the results presented in this section help to understand the simulation and the interpretation of our psychophysical experiment.
, by adding correlated and uncorrelated noise to the angular variables. Similar to Figure 6, it is shown how noise affects these functions (e.g. bigger object diameters are associated with correspondingly less fluctuations), and thus the results presented in this section help to understand the simulation and the interpretation of our psychophysical experiment.
(PDF)
Fitting m-Tau and
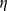 -function to neuronal recordings.
Text S4 juxtaposes the individual fitting results of
-function to neuronal recordings.
Text S4 juxtaposes the individual fitting results of 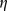 and
and 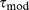 to a variety of previously published neural recording data, which served to compile Figure 2. Further summary results are presented along with fitting results of individual recording traces.
to a variety of previously published neural recording data, which served to compile Figure 2. Further summary results are presented along with fitting results of individual recording traces.
(PDF)
Finding parameter values for
corrected m-Tau
.
Text S5 describes the optimization procedure for the corrected m-Tau -model “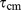 ”, with which we obtained the parameter values for the simulation of our psychophysical experiment (e.g. Figure 8). The
”, with which we obtained the parameter values for the simulation of our psychophysical experiment (e.g. Figure 8). The 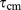 -parameters were optimized in three different ways: For achieving a good prediction performance of the psychophysical data corresponding to (i) the small object diameter (Table S1 in Text S5), (ii) the big object diameter (Table S2 in Text S5), and (iii) both diameters at the same time (combined; Table S3 in Text S5). The best ten values are listed in their respective tables according to their psychophysical prediction performance (as quantified by score measures
-parameters were optimized in three different ways: For achieving a good prediction performance of the psychophysical data corresponding to (i) the small object diameter (Table S1 in Text S5), (ii) the big object diameter (Table S2 in Text S5), and (iii) both diameters at the same time (combined; Table S3 in Text S5). The best ten values are listed in their respective tables according to their psychophysical prediction performance (as quantified by score measures 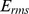 and
and 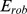 , respectively): The best parameter set (smallest score measure) was assigned rank one, the second best rank two, etc. Several figures were compiled that show an additional analysis of the parameter ranking.
, respectively): The best parameter set (smallest score measure) was assigned rank one, the second best rank two, etc. Several figures were compiled that show an additional analysis of the parameter ranking.
(PDF)
Time to contact approximation of “Tau” and
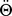 .
Text S6 presents a comprehensive analysis of two alternative functions which have a maximum before ttc, namely “inverse
.
Text S6 presents a comprehensive analysis of two alternative functions which have a maximum before ttc, namely “inverse  ” (
” (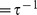 ) and “angular acceleration” (
) and “angular acceleration” (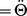 ). The two functions were also fitted to the neuronal recording data (they turn out to be inadequate), and compared to the maximum of the
). The two functions were also fitted to the neuronal recording data (they turn out to be inadequate), and compared to the maximum of the 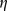 -function. This section also provides insights into the biphasic nature of
-function. This section also provides insights into the biphasic nature of 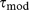 , because as
, because as 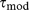 approaches ttc, it gets more similar to
approaches ttc, it gets more similar to  , and thus reveals a minimum.
, and thus reveals a minimum.
(PDF)
Predictions of
corrected m-Tau
for the psychophysical experiment.
Text S7 shows the full set of figures with simulation results of our psychophysical experiment. Whereas Figure 8 shows 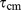 -predictions that were obtained with the parameter set optimized for the “combined” object diameter according to
-predictions that were obtained with the parameter set optimized for the “combined” object diameter according to 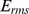 -score, Text S7 shows analogous figures for the remaining parameter optimizations (big and small object diameter, and
-score, Text S7 shows analogous figures for the remaining parameter optimizations (big and small object diameter, and 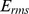 and
and 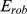 -scores, respectively).
-scores, respectively).
(PDF)
First order temporal low-pass filter (
Equation 4
).
Text S8 gives a short introduction to the temporal low-pass filter that forms a part of the 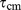 -model (equation 4), and is also used for the extension of the
-model (equation 4), and is also used for the extension of the 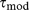 -model described in Text S1).
-model described in Text S1).
(PDF)