Abstract
Glucose regulated protein 78/immunoglobulin binding protein (GRP78/BiP) is an ER chaperone protein and master regulator of the unfolded protein response (UPR). The response of GRP78 to overt pharmacologically induced ER stress is well established, whereas the modulation of GRP78 to physiologic changes is less characterized. In this study, we examined the regulation of GRP78 in response to reduced IGF-1 growth factor signaling, a common consequence of calorie restriction (CR). ER chaperone protein expression was quantified in cell lysates prepared from the livers of calorie restricted (CR) and ad libitum fed mice, as well as MEFs grown in normal medium or serum starved. The requirement of IGF-1 signaling on GRP78 expression was studied using MEFs with IGF-1 receptor overexpression (R+) or deletion (R−), and the regulatory mechanism was examined using mTORC1 and PI3K inhibitors, as well as R− cells with knockdown of transcription factor FOXO1 compared to shRNA control. We observed a 40% reduction in GRP78 protein expression in CR mice and in serum-starved MEF cells. R− cells had drastically reduced AKT phosphorylation and exhibited lower levels of ER chaperones, in particular 80% less GRP78. Despite an 80% reduction in GRP78 expression, R− cells were not under chronic ER stress, but were fully capable of activating the unfolded protein response (UPR). Neither forced expression of FOXO1-AAA nor knockdown of FOXO1 in R− cells affected GRP78 expression. In conclusion, we report that IGF-1 receptor signaling regulates GRP78 expression via the PI3K/AKT/mTORC1 axis independent of the canonical UPR and FOXO1.
Keywords: AKT, FOXO1, Unfolded protein response, GRP78, IGF-1
Introduction
Glucose regulated protein 78 (GRP78), also referred to HSPA5 or BiP, is a chaperone protein highly expressed in the endoplasmic reticulum (ER) which serves as a master regulator of the unfolded protein response (UPR) (Ni and Lee, 2007; Pfaffenbach and Lee, 2011). The UPR is a highly conserved signaling pathway activated when an accumulation of luminal unfolded proteins exceeds the ER folding capacity, a condition termed “ER stress” (Harding et al., 2002; Rutkowski and Kaufman, 2004). The UPR acts initially to suppress protein synthesis, and then subsequently enacts a transcriptional shift whereby proteins, such as GRP78, are upregulated in an attempt to restore ER homeostasis. Thus, GRP78 induction has been widely used as an indicator of ER stress (Mao et al., 2004; Lee, 2005). However, if ER stress persists, the UPR is also capable of inducing apoptosis. To date, most studies on the regulation of UPR proteins either induce ER stress to a non-physiologic level with pharmacologic agents, or study the UPR in the context of an existing pathologic condition. Less studied however, are the molecular mechanisms by which UPR related proteins are modulated outside of the canonical UPR, or in response to the undulating physiologic conditions within a normal homeostatic system (Rutkowski and Hegde, 2010).
There is accumulating evidence which suggests that maintaining ER homeostasis as well as the ability to adequately respond to ER stress is compromised with old age (Erickson et al., 2006; Paz Gavilan et al., 2006; Naidoo et al., 2008; Naidoo, 2009; Salminen and Kaarniranta, 2010). The diminished capacity of the ER to respond to both physiologic and pathologic stress is likely an underlying cause for age associated disease development (Salminen and Kaarniranta, 2010). Indeed, perturbations in ER stress and the UPR, and specifically GRP78, are implicated in the development and progression of a wide array of diseases, including cancer, diabetes, and Alzheimer’s disease (Li and Lee, 2006; Lee, 2007; Wang et al., 2009; Salminen and Kaarniranta, 2010; Pfaffenbach and Lee, 2011; Luo and Lee, 2012), implying that reducing long-term, chronic ER stress may be beneficial in the prevention of age related diseases. Calorie restriction (CR), a reduction in food intake without malnutrition, is established as a potent way to extend life span and prevent the development of age associated diseases across a wide array of organisms and species ranging from yeast to mammals (Spindler, 2001; Masoro, 2005; Fontana et al., 2010; Spindler, 2010; Moore et al., 2011). A key factor responsible for the beneficial effects of long-term CR is a reduction in growth factor signaling, particularly a reduction in insulin-like growth factor-1 (IGF-1) (Fontana et al., 2010). Mice harboring a heterozygous deletion in the IGF-1 receptor (IGF-1R) are healthy, long lived, and resistant to oxidative stress (Holzenberger et al., 2003). A recent study further demonstrated that humans with growth hormone receptor deficiency have significantly lower circulating IGF-1 levels, and also exhibit drastically reduced incidence of cancer, diabetes, and other metabolic disorders compared to age-matched relatives with intact growth hormone receptor (Guevara-Aguirre et al., 2011). Thus, a long-term reduction in IGF-1 signaling appears to prevent age-related loss of function and disease (Shevah and Laron, 2007; Guevara-Aguirre et al., 2011), and the mechanism has been linked to relieving inhibition of the FOXO family of transcription factors and their target genes (Chitnis et al., 2008).
In vertebrates, the IGF-1R promotes growth (Cohen, 2006). Ligands binding to the IGF-1R leads to autophosphorylation and activation of signaling via the phosphatidylinosital-3-kinase (PI3K)/AKT and the mitogen activation protein kinase (MAPK) (LeRoith et al., 1995; Chitnis et al., 2008). Downstream targets of IGF-1R signaling include mammalian target of rapamycin (mTOR) complex 1 (mTORC1), p70 S6 kinase, ribosomal protein S6 (RPS6), the extracellular signal-related kinases (ERK1/2) and c-Myc. Because the ER is a major site for the synthesis and processing of membrane and secretory proteins, growth factor signaling could be coupled to ER chaperone expression. Indeed, previous studies in hematopoietic cells showed induction of Grp78 and Grp94 mRNA levels in response to cytokine stimulation (Brewer et al., 1997); and in NIH3T3 fibroblasts, IGF-1 augmented the ability of an ER stress inducer thapsigargin to upregulate GRP78, thereby associating IGF-1 with increased resistance to ER stress induced apoptosis (Novosyadlyy et al., 2008). Despite the current evidence that CR, growth factor signaling, and ER stress impact ER chaperone expression, little is known about the effect of a reduction in IGF-1 signaling on the expression of chaperone proteins, particularly GRP78, which is key to the protective effects of CR. This study examines how long-term CR affects ER chaperone balance and how IGF-1 signaling regulates GRP78 in the absence of ER stress in model cell systems.
Materials and Methods
Animals and calorie restriction
Male C57BL/6 mice were housed in a temperature and humidity controlled environment, and maintained on a 12 h light/dark cycle. Mice were provided NIH-31/NIA fortified chow ad libitum (AL) from 0-4 mo. At 4 mo, calorie restricted mice were limited to 3 gram/day for 20 mo (40% reduction of AL) compared to age-matched control mice. Mice were overnight fasted prior to sacrifice and collection of liver tissue. Liver tissue was immediately frozen in liquid nitrogen and stored at −80°C. All protocols for animal use and euthanasia were reviewed and approved by the University of Southern California Institutional Animal Care and Use.
Cell culture
Wild type (WT) mouse embryonic fibroblast (MEF) cells were obtained courtesy of Stanley Korsmeyer (Harvard University) (Ye et al., 2010). We also used MEF cells overexpressing the human IGF-1 receptor (R+) and IGF-1 receptor knockout (R−) cells obtained courtesy of Renato Baserga (Thomas Jefferson University) (Sell et al., 1993; Drakas et al., 2004). For FOXO1 knockdown experiments, R+ and R− cells were transduced with lentivirus expressing FOXO1 short hairpin RNA (shFOXO1) (clone ID TRCN0000054880 from Thermo Open Biosystems) or control shRNA (Open Biosystems) using polybrene (final concentration 8 μg/ml). Transduced cells were selected using puromycin (6 μg/ml). Experiments with forced expression of constitutively active FOXO1 were done in 293T cells transfected with pcDNA3 empty vector (2 μg) as a control or FLAG tagged non-phosphorylatable FOXO1-AAA (2 μg) (courtesy of Bangyan Stiles, USC School of Pharmacy) using BioT transfection reagent according to manufacturer’s instructions (Bioland Scientific). All cells were cultured under normal growth conditions, consisting of Dulbecco’s modified Eagle’s medium (DMEM) (4.5 g/L glucose) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C and 5% CO2. For serum starvation experiments, cells were placed in DMEM (4.5 g/L glucose) containing no FBS for 16 h. For the chemical inhibition of PI3K/AKT/mTORC1 signaling, the specific mTORC1 inhibitor rapamycin (20 nM; Cell Signaling) and the specific PI3K inhibitor LY294002 (50 μM; Cell Signaling) were used. To induce ER stress, cells were treated with either tunicamycin (Tu, 1.5 μg/ml; Sigma) or thapsigargin (Tg, 300 nM; Sigma).
Production of lentivirus in 293T cells
Infectious lentivirus was created by cotransfection of plasmid expressing FOXO1 shRNA or control shRNA with pCMVΔR8.91 and pMD.G into human 293T cells. The infection cocktail was added dropwise to 293T cells plated on 100 mm culture dishes and incubated at 37°C overnight. Virus was harvested after 48 h and concentrated with PEG-it virus precipitation solution (System Biosciences). Titers of virus stocks were determined by p24 Elisa Assay Kit (Cell Biolabs, San Diego, CA).
RT-PCR and real time quantitative PCR
Total RNA was extracted using TRI reagent (Sigma-Aldrich) according to the manufacturer’s instructions. cDNA synthesis was carried out by reverse transcriptase using SuperScript II (Invitrogen). For detection of the spliced and non-spliced form of XBP-1, PCR was performed as previously described using the primer for mouse Xbp-1: 5′-GAA CCA GGA GTT AA GAA CAC G-3′ and 5′-AGG CAA CAG TGT CAG AGT CC-3′ (Wang et al., 2010). Real-time quantitative PCR (qPCR) was used to analyze Grp78 mRNA. cDNA samples were analyzed in duplicate with the SYBR Green Supermix (Quanta Biosciences, Gaithersburg, MD) according to manufacturer’s instructions. Primers used for real time qPCR were: Grp78: forward: 5′-CGA CCT GGG GAC CAC CTA CT-3′ and reverse: 5′-TTG GAG GTG AGC TGG TTC TT-3′; 18S RNA forward: 5′-ACG GCC GGT ACA GTG AAA C-3′ and reverse: 5′-GAG GGA GCT CAC CGG G-3′.
Immunoblot analysis
Protein lysates from tissue and cells were extracted using ice-cold radioimmunoprecipitation assay buffer (50 mmol/L Tris-Cl, 150 mmol/L NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS) with added protease and phosphatase inhibitors (Thermo Scientific), and by centrifugation (13,000 rpm for 15 min). Proteins were separated by 8% or 10% SDS-PAGE gel and transferred to nitrocellulose membrane (Pall). Protein expression was determined by Western blot as previously described (Luo and Lee, 2002). The primary antibodies against the following proteins were used: GRP78 (gift of Parkash Gill, Keck School of Medicine of USC), phosphorylated S473-AKT, AKT, phosphorylated ERK1/2, ERK1/2, pSer51-eIF2α, eIF2α, forkhead box protein O1 (FOXO1) (Cell Signaling), phosphorylated ribosomal protein S6 (RPS6), C/EBP homologous protein (CHOP) (Santa Cruz Biotechnology), calreticulin (CRT), calnexin (CNX), protein disulfide isomerase (PDI), GRP94 (Stressgen), c-Myc (Genetex), and β-actin (Sigma). Proteins were detected using either horseradish peroxidase-conjugated secondary antibodies and a chemiluminescence reagent (Thermo Scientific), or IRDye-conjugated secondary antibodies (Li-Cor Biosciences) and the Odyssey infrared Imaging System (Li-Cor Biosciences). Western blot densitometry was analyzed using Quantity One system (Bio-rad) and Odyssey 2.1 software (Li-Cor Biosciences).
Statistical analysis
All pairwise comparisons were made using the two-tailed Student’s t test.
Results
GRP78 expression is selectively down regulated in response to long-term calorie restriction
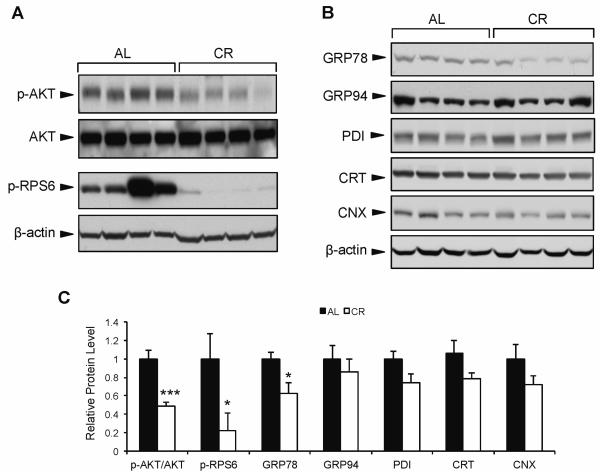
To test the effect of CR on growth signaling and ER chaperone balance, we examined 24 mo old male mice in pure C57/BL6 background to avoid complications due to genetic variation. The mice were either AL fed or placed on a CR diet (3 g/day normal chow) from age 4 to 24 mo. As expected, mice from the CR group demonstrated significantly lower phosphorylation of both AKT and ribosomal protein S6 (RPS6) in liver tissue compared to the AL mice (Fig. 1A,C), indicating a decrease in growth factor signaling in response to calorie restriction. Next, we examined the expression of several ER chaperone proteins in the liver of CR vs. AL mice. While there were no significant differences between CR and AL mice in protein expression of glucose regulated protein 94 (GRP94), protein disulfide isomerase (PDI), calreticulin (CRT), and calnexin (CNX) (Fig. 1B,C), there was a marked (40%) reduction in GRP78 protein level in CR compared to AL mice (Fig. 1B,C). These results suggest that the regulation of hepatic GRP78 is uniquely sensitive to long-term CR compared to other ER chaperones in these mice.
Fig. 1.
Effect of long-term calorie restriction on hepatic AKT signaling and chaperone expression. Mice were subjected to calorie restriction (3 g/day) from age 4-24 mo, or fed ad libitum. A: Representative immunoblots for phosphorylated AKT and RPS6 protein from mouse liver. B: Hepatic expression of ER chaperone proteins GRP78, GRP94, PDI, CRT, and CNX. C: Comparison of the chaperone protein levels in the AL and CR mice. The protein blots were quantitated and the protein levels were normalized against β-actin. The level of each protein in the AL group was set as one. Values are presented as mean ± SE, n=6 mice per group. *p≤0.05 ***p≤0.001.
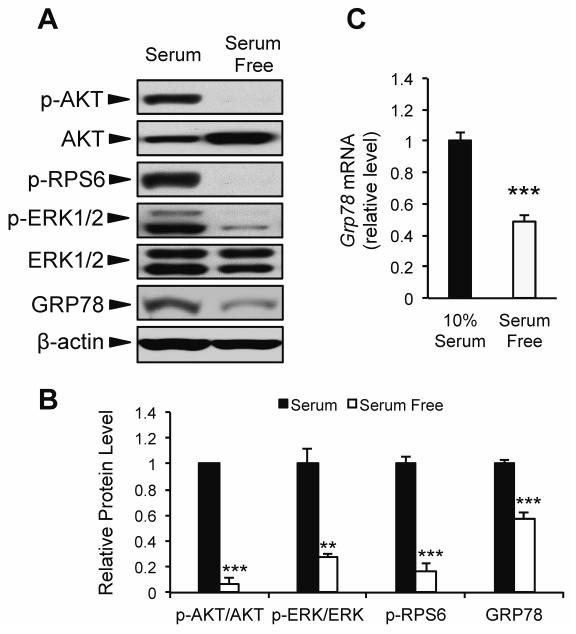
To further examine whether acute changes in growth factor signaling modulate GRP78 expression, we utilized an in vitro model system of mouse embryonic fibroblast cells (MEF) subjected to serum starvation for 16 h. As expected, serum starvation lead to decreased growth factor signaling demonstrated by reduced phosphorylation of AKT, RPS6, and ERK1/2 (Fig. 2A,B). Further, both Grp78 mRNA and protein level were significantly reduced (51% and 43%, respectively) in response to serum starvation (Fig. 2A-C). Taken together with the data from CR mice, these findings suggest that GRP78 expression can be modulated by both long-term and acute changes in growth factor signaling.
Fig. 2.
Effect of short-term serum starvation on GRP78 expression. Mouse embryonic fibroblast (MEF) cells were cultured in growth medium containing either 4.5 g/L glucose and 10% FBS (10% serum), or 4.5 g/L glucose absent of FBS (serum free) for 16 h. A: Representative immunoblots for growth factor signaling and GRP78 protein expression. B: Quantitation of relative protein levels from Western blots. C: Grp78 mRNA expression determined using quantitative real time PCR. Values are presented as mean ± SE, n=3-6, **p≤0.01 ***p≤0.001.
Basal expression of GRP78 expression is uniquely dependent on integrity of the IGF-1R pathway
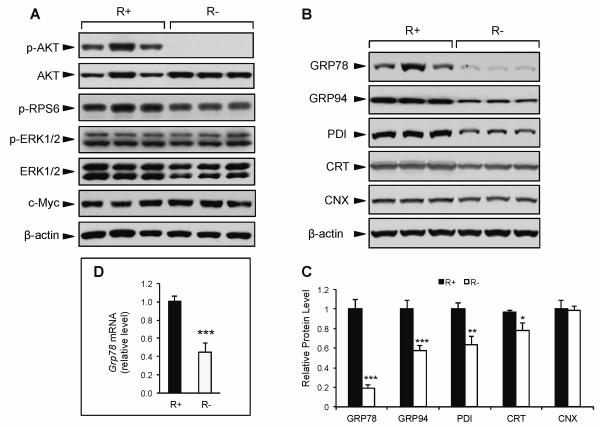
Among growth factors, IGF-1 is a major effector of the beneficial effects of CR. To examine the role of IGF-1 in regulating ER chaperone balance, we utilized MEF cells overexpressing human IGF-1R (R+) and IGF-1R knockout cells (R−) (Sell et al., 1993; Drakas et al., 2004). Consistent with previous reports, and despite the lack of IGF-1R, R− cells were viable and grew at a comparable rate as R+ cells under normal culture conditions (4.5 g/L glucose, 10% FBS) (data not shown). Compared to R+ cells, the R− cells displayed drastically reduced phosphorylation of AKT (Fig. 3A). Strikingly, there were no differences in the phosphorylation of RPS6, ERK1/2, or c-Myc levels, suggesting that compensatory signaling from other growth pathways contribute to sustain protein synthesis and viability in the R− cells (Fig. 3A).
Fig. 3.
Effect of genetic modulation of IGF-1R on growth signaling and ER chaperone expression. IGF-1R overexpressing (R+) and knockout cells (R−) cells were cultured in growth medium containing 4.5 g/L glucose and 10% FBS. A: Representative immunoblots for phospho-AKT, total AKT, phospho-RPS6, phospho-ERK1/2, total ERK1/2, c-Myc, and β-actin as the loading control. B: Representative immunoblots for ER chaperone proteins GRP78, GRP94, PDI, CRT, CNX, and β-actin as the loading control. C: Quantitation of relative protein levels of UPR chaperones. D: Expression levels for Grp78 mRNA using quantitative real time PCR. The values are presented as mean ± SE, n=6-10, *p≤0.05 **p≤0.01 ***p≤0.001.
Upon analyzing the expression level of several major chaperone proteins in these cells, we observed that under normal culture conditions R− cells exhibited 80% lower GRP78 protein expression compared to R+ cells (Fig. 3B,C). Grp78 mRNA expression was also significantly lower in the R− cells (Fig. 3D). While other ER chaperone proteins (GRP94, PDI, and CRT) also showed reduced expression (42%, 36%, and 22%, respectively) in the R− compared to R+ cells, the magnitude of reduction was considerably less than that of GRP78, and for calnexin (CNX), no difference was observed between the R− and R+ cells (Fig. 3B,C). These results imply that basal GRP78 protein and mRNA expression are uniquely dependent on the integrity of IGF-1R and AKT activation.
GRP78 regulation in the absence of IGF-1R is independent of GRP78 induction in response to ER stress
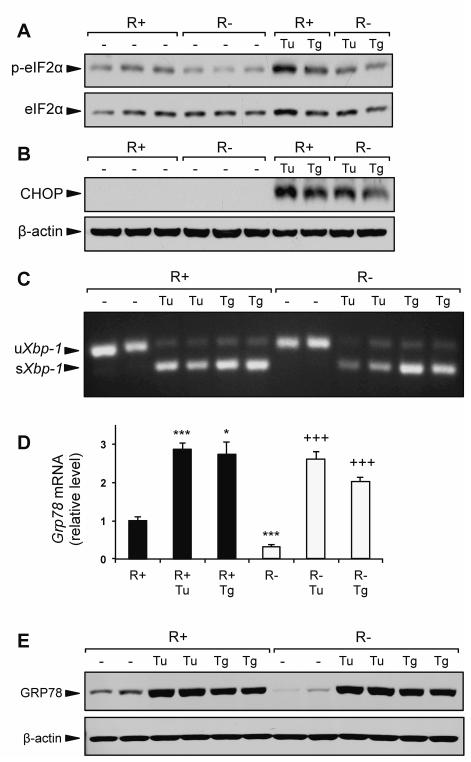
The UPR is activated in response to ER stress, a condition where ER protein load exceeds the ER capacity to fold and process nascent proteins. In non-stressed cells, GRP78 binds to the luminal domains of the proximal UPR sensors and maintains them in an inactive form (Ma and Hendershot, 2004; Lee, 2005; Wang et al., 2009). Thus, an 80% decrease in basal GRP78 level in the R− cells might lead to ER stress, as was previously observed in cells with GRP78 knockdown by siRNA (Li et al., 2008). However, R− cells did not demonstrate increased activation of several classic ER stress markers compared to R+ cells. Under basal conditions, we did not observe increased eIF2α phosphorylation, elevated CHOP protein expression, or the presence of spliced Xbp-1 mRNA (Fig. 4A-C). Interestingly, eIF2α phosphorylation was modestly elevated in the R+ cells compared to R− cells (Fig. 4A), which could be a feedback mechanism to modulate protein synthesis due to the overexpression of the IGF-1R in these cells.
Fig. 4.
IGF-1R knockout does not affect UPR signaling. R+ cells and R− cells were cultured in growth medium containing 4.5 g/L glucose and 10% FBS alone, or growth medium containing ER stress inducing agents tunicamycin (Tu, 1.5 μg/ml) or thapsigargin (Tg, 300nM) for 16 h. A,B: Representative immunoblots for eIF2α phosphorylation and CHOP protein expression. C: Detection of spliced Xbp-1 mRNA by RT-PCR. D: Expression levels for Grp78 mRNA using quantitative real time PCR. E: Representative immunoblot for GRP78 protein induction by Tu and Tg. Values are presented as mean ± SE, n=2-4 *p≤0.05, ***p≤0.001 compared to untreated R+ group. +++p≤0.001 compared to untreated R− group.
When treated with the pharmacologic ER stress inducers tunicamycin (Tu) and thapsigargin (Tg), R− cells were capable of activating the UPR. In response to both Tu and Tg, induction of eIF2α phosphorylation, CHOP protein, and spliced Xbp-1 mRNA were observed in both R+ and R− cells (Fig. 4A-C). Furthermore, despite normally expressing ~60-70% less Grp78 mRNA and ~80% less GRP78 protein compared to R+ cells, the induction of both GRP78 protein and mRNA in R− cells treated with Tu and Tg matched the level observed in treated R+ cells (Fig. 4D,E). Taken together, these results demonstrate that IGF-1R signaling is not required for the UPR, and that ER stress induction of GRP78 is not impaired in IGF-1R deficient cells.
GRP78 is regulated by IGF-1R signaling via the AKT/mTORC1 signaling axis
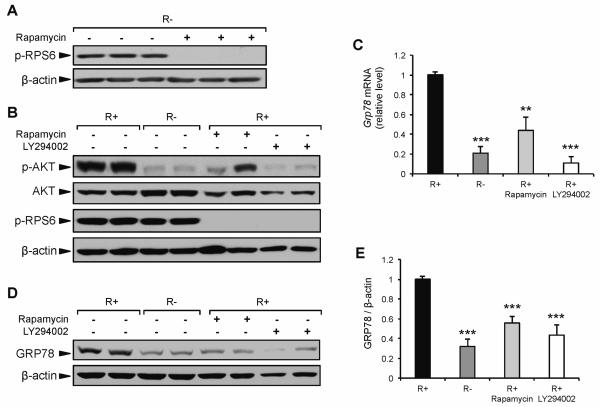
One of the major downstream components of the activated IGF-1R is AKT, which controls the activation of multiple transcription factors (Chitnis et al., 2008). AKT also plays an important role in the initiation of protein translation via the activation of mTOR complex 1 (mTORC1) (Sarbassov et al., 2005). As demonstrated above, there was lower AKT phosphorylation in response to CR, serum starvation, and IGF-1R knockout cells. Thus, AKT and/or mTORC1 may be critical signaling components by which GRP78 expression is regulated under all three conditions. To dissect the role of the AKT/mTORC1 pathway in regulating GRP78 expression through IGF1R signaling, R+ and R− cells were cultured in medium containing either rapamycin or LY294002. Rapamycin is a specific inhibitor of mTORC1, whereas LY294002 is a specific inhibitor of PI3K used to block the activation of AKT. The phosphorylation of ribosomal protein S6, a direct target of p70 S6 kinase, is sensitive to mTORC1 activation and is used as a marker for mTORC1 activity (Chung et al., 1992). As shown in Figure 3A, the level of phospho-RPS6 was similar between R+ and R− cells, suggesting that compensatory growth pathways outside of AKT are responsible for maintaining protein synthesis in R− cells. Previous reports have demonstrated that the ERK1/2 pathway can directly activate mTORC1 (Carriere et al., 2011), as well as directly phosphorylate RPS6 independent of mTORC1 (Roux et al., 2007). To examine whether mTORC1 activity in R− cells was responsible for RPS6 phosphorylation, R− cells were incubated in medium containing rapamycin. The phosphorylation of RPS6 was completely mitigated by rapamycin, demonstrating that under normal growth conditions mTORC1 is active in R− cells and that the low basal level of GRP78 expression observed in R− cells was not due to a reduction in mTORC1 activation (Fig. 5A). To further examine the role of AKT and mTORC1 in the regulation of GRP78, R+ cells were cultured in medium containing either rapamycin or LY294003 (Fig. 5B). Grp78 mRNA expression in R+ cells was reduced 57% and 89% in response to 24 h treatment with rapamycin or LY294002, respectively (Fig. 5C). Importantly, LY294002 reduced Grp78 mRNA to the same level as R− cells, which are characterized by a marked reduction in phospho-AKT under basal conditions. Further, the observation that rapamycin significantly reduced Grp78 mRNA demonstrates a role for mTORC1 and/or its downstream components in the regulation of GRP78 when an intact IGF-1R pathway is present. Western blot analysis at the 24 h time point demonstrated a similar reduction in GRP78 protein level in R+ cells treated with rapamycin or LY294002 compared to R− cells (Fig. 5D,E). These results demonstrate that in the absence of IGF-1R, basal GRP78 expression is not regulated by the mTORC1 pathway or ERK1/2, but is likely modulated by the PI3K/AKT pathway. Additionally, under normal growth conditions, and in the presence of IGF-1R, basal GRP78 expression can be modulated through PI3K/AKT/mTORC1 signaling. Our proposed model for IGF-1R regulation of GRP78 expression is summarized in Figure 6.
Fig. 5.
Effect of rapamycin and LY294002 on GRP78 expression. A: Representative immunoblot for change in RPS6 phosphorylation in response to 16 h exposure to rapamycin (20 nM) in R− cells. B: Representative immunoblots for AKT and RPS6 phosphorylation in response to 24 h exposure to either rapamycin or LY294002 in R+ cells compared to non-treated R+ and R− cells. C: Grp78 mRNA expression in R+ cells treated with rapamycin or LY294002 for 24 h, determined using quantitative real time PCR. D,E: Representative immunoblot and quantitation for GRP78 protein expression in R+ cells treated with rapamycin or LY294002 compared to non-treated R+ and R− cells. The values are presented as mean ± SE, n=3-9, **p≤0.01, ***p≤0.001 compared to untreated R+ group.
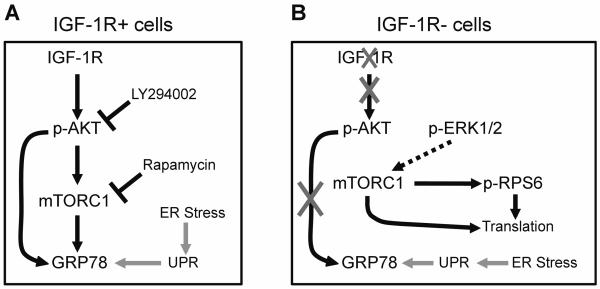
Fig. 6.
Schematic representation of proposed GRP78 regulation by the IGF-1/ AKT/mTORC1 signaling axis in the absence of ER stress, in MEF cells either overexpressing (R+) or lacking (R−) IGF-1 receptor. A: In the presence of intact IGF-1R signaling, GRP78 expression is regulated by PI3K/AKT and mTORC1, and is downregulated in response to inhibitors of both. B: In the absence of IGF-1R, there is dramatically reduced AKT phosphorylation and GRP78 expression compared to R+ cells. However, protein translation is maintained via mTORC1 activity, likely driven by p-ERK1/2, which is maintained in the R− cells. Induction of the unfolded protein response (UPR) and GRP78 by ER stress is intact in both cell types.
Reduction of GRP78 expression in IGF-1R deficient cells is independent of the transcription factor FOXO1
A critical downstream protein that is regulated by AKT phosphorylation is the forkhead box protein O1 (FOXO1) (Kim et al., 2008; Maiese et al., 2009; Yamaza et al., 2010). Phospho-AKT prevents the nuclear translocation of FOXO1 via phosphorylation (Brunet et al., 1999; Rena et al., 1999). However, in the absence of active AKT, non-phosphorylated FOXO1 can enter the nucleus and act as a transcription factor regulating the expression of a diverse array of metabolic genes (Huang and Tindall, 2007). Thus, FOXO1 presents a potential transcriptional repressor which may be key for the regulation of GRP78 expression by IGF-1/AKT signaling, such that under conditions where IGF-1 receptor signaling is activated, phospho-AKT phosphorylates FOXO1, prevents its nuclear translocation, and relieves FOXO1 inhibition on Grp78 transcription. Conversely, in the absence of IGF-1 receptor signaling, without AKT phosphorylation, FOXO1 would be active and able to translocate to the nucleus and suppress Grp78 transcription. In support of this hypothesis, a previous report demonstrated that FOXO1 phosphorylation was significantly lower, and that FOXO1 activity was significantly higher, in the R− vs R+ cells (Guevara-Aguirre et al., 2011).
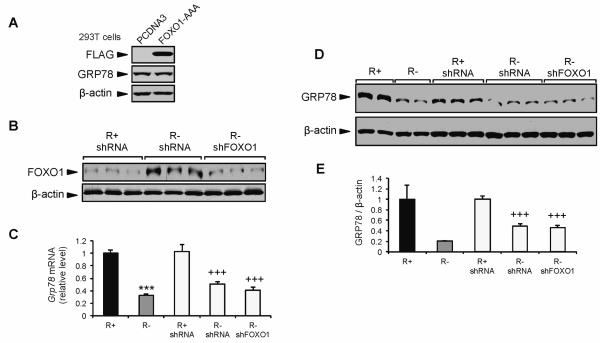
To directly test whether FOXO1 is a suppressor of GRP78 expression, we expressed a constitutively active form of FOXO1 (FOXO1-AAA) in 293T cells cultured under normal growth conditions. However, the forced expression of FOXO1-AAA in 293T cells failed to suppress GRP78 protein expression (Fig. 7A). Next, we knocked down FOXO1 in R− cells using lentiviral shRNA against FOXO1 (Fig. 7B). The low level of total FOXO1 observed in R+ shRNA control cells was consistent with previous studies using this cell type (Guevara-Aguirre et al., 2011). The transduced R− cells showed an 80% knockdown of FOXO1 protein compared to controls (Fig. 7B). If FOXO1 negatively regulates Grp78 transcription, then knocking down FOXO1 protein would alleviate FOXO1 suppression of GRP78, leading to increased expression of GRP78 protein and mRNA. However, neither Grp78 mRNA nor GRP78 protein expression was altered following FOXO1 knockdown in R− cells (Fig. 7C-E). Collectively, our results show that in the context of these cellular model systems, the PI3K/AKT/mTORC1 pathway modulates GRP78 expression by IGF-1R signaling. However, the alleviation of FOXO1 as a repressor of GRP78 expression downstream of the IGF-1R and PI3K/AKT signaling in non-stressed cells does not appear to be directly involved in this regulation.
Fig. 7.
Suppression of GRP78 expression in IGF-1R deficient cells under basal growth conditions is independent of the transcription factor FOXO1. A: Representative immunoblots for endogenous GRP78 protein, FLAG-tag, and β-actin in 293T cells transfected with the empty vector PCDNA or vector expressing constitutively active FLAG-tagged FOXO1-AAA. B: Representative immunoblot for FOXO1 protein expression in R+ and R− cells transduced with lentivirus expressing control shRNA or FOXO1 shRNA (shFOXO1). C: Grp78 mRNA expression measured in R+ and R− cells transduced with lentivirus expressing FOXO1 or control shRNA by quantitative real-time RT-PCR. D,E: Representative immunoblot and quantitation for GRP78 protein expression in R+ and R− cells transduced with lentivirus expressing FOXO1 or control shRNA. The values are presented as mean ± SE, n=2-6, ***p≤0.001 compared to untreated R+ group, +++p≤0.001 compared to R+ shRNA group.
Discussion
While the molecular mechanisms leading to the induction of GRP78 by pharmacological agents invoking ER stress have been well studied (Roy and Lee, 1999; Lee, 2001; Baumeister et al., 2005), how GRP78 is regulated by growth factor signaling is not well understood. In this study, we examined how CR modulates the ER chaperone balance and dissected the molecular mechanisms whereby the IGF-1 pathway regulates the expression of GRP78 in a non-stressed, physiologic context. Here we demonstrate that GRP78 is selectively downregulated in the liver of aged, long-term CR C57/B6 mice compared to AL fed controls. Additionally, we found that GRP78 expression at both the mRNA and protein levels were reduced in response to acute serum starvation. Using cells with IGF-1R overexpression or knockout, we observed that IGF-1 signaling via the AKT pathway directly modulates the expression of GRP78 and that this regulation functions independently from the unfolded protein response. Finally, we demonstrated that the AKT modulation of GRP78 acts through a mechanism independent of the transcription factor FOXO1. Our studies reveal several new observations that could have important implications in understanding the role of aging, CR and growth factor deprivation in health and disease.
With regard to the effect of CR on ER chaperone balance, previous reports using female mice of hybrid strain (C3B10RF) showed a general decrease in several ER chaperone proteins in the liver of energy restricted mice (Dhahbi et al., 1997; Cao et al., 2001; Dhahbi et al., 2001), whereas in our study using genetically pure male C57/B6 mice, we primarily observed significant reduction in GRP78. These differences suggest that effects of long-term CR could be modulated by host factors such as male vs. female and the genetic background, which may also be applicable in humans. Importantly, in these and other studies in rats (Heydari et al., 1995), among members of the heat shock protein family, GRP78 expression is consistently and potently suppressed by CR. This may seem paradoxical to the beneficial effects of CR given that a decrease in GRP78 function and activity with aging is expected to be detrimental (Erickson et al., 2006). Nonetheless, since GRP78 is a sensor of ER stress, a reduction in GRP78 by long-term CR could be a reflection of improved ER homeostasis as a result of CR-mediated reduction in growth factor signaling. Furthermore, it has been proposed that CR decreased ER chaperone content and concomitantly increased the rate and efficiency of serum protein secretion, thereby promoting serum protein turnover and reducing the circulating level of damaged, glycated serum proteins (Dhahbi et al., 2001). However, to date, the studies regarding long-term CR and ER chaperone expression have been largely observational, and thus the mechanism(s) by which GRP78 is regulated under conditions of CR are unresolved.
A reduction in growth factor signaling, particularly via IGF-1R signaling, is well accepted as one of the main beneficial effect of CR on a variety of diseases (Cohen et al., 2009; Fontana et al., 2010). Here, we examined GRP78 expression in response to acute serum starvation, as well as in IGF-1R overexpressing (R+) and knockout cells (R−) cells and uncovered that IGF-1R signaling regulates basal expression of GRP78 through the PI3K/AKT/mTORC1 axis. Previously, in hematopoietic cell lines that are dependent for cytokines for growth and survival, an acute deprivation of the cytokine IL-3 caused a reduction in both Grp78 and Grp94 mRNA levels, and the re-stimulation of Grp78 mRNA expression by IL3 is independent of protein synthesis, ER protein load and ER stress (Brewer et al., 1997). Nonetheless, in these studies, IL-3 stimulation of GRP78 biosynthesis did not result in a change in GRP78 protein level, likely due to transient nature of the cytokine stimulation and the long half life of GRP78 (Brewer et al., 1997). Here, in our studies to dissect the molecular mechanisms whereby IGF-1 regulates GRP78, we utilized a matched paired of MEFs genetically altered in IGF1-R signaling thereby allowing us to examine the long-term effect of IGF-1 depletion. Another point of note is that the R− cells are not analogous to cells subjected to CR or general depletion of growth factors, because they were grown in 10% serum, and showed activation of ERK and c-Myc. The robust level of phospho-RPS6, sensitivity to rapamycin, and low level of p-eIF2α in the R− cells further suggest normal protein synthesis and ER protein load. Therefore, the R− cells offer a unique model system to isolate the downstream targets of IGF-1 signaling in the presence of other growth signaling pathways in a growth competent cell line. Our finding that GRP78 is reduced in R− cells and that this effect is replicated in R+ cells treated with the PI3K inhibitor LY294002 provides evidence that GRP78 is a downstream target of IGF-1/PI3K/AKT signaling. In the case of R− cells, the regulation of GRP78 expression appears to be at the transcriptional level, as evidenced by the reduction in Grp78 mRNA expression with no apparent reduction in protein synthesis. In addition to the regulation of GRP78 expression by PI3K/AKT in R+ cells, both GRP78 protein and mRNA were reduced after exposure to rapamycin, demonstrating a significant role for the mTORC1 signaling when the IGF-1R pathway is fully intact. However, the exact mechanism by which rapamycin suppresses GRP78 expression requires further study. The observation that the R− cells were capable of activating the UPR, including full GRP78 activation, in response to pharmacological ER stress inducers clearly shows that the regulation of GRP78 by IGF-1 is distinct from its regulation by ER stress (Fig. 6). This finding is consistent with the report that IGF-1 was able to augment the expression of GRP78 induced by thapsigargin (Novosyadlyy et al., 2008).
FOXO1 is a downstream target of IGF-1/AKT signaling and phosphorylated AKT prevents its nuclear localization. However, in the absence of IGF-1/AKT signaling, non-phosphorylated FOXO1 enters the nucleus and can modulate a wide array of genes implicated in oxidative stress resistance, longevity, and metabolism (Huang and Tindall, 2007; Partridge and Bruning, 2008). Thus, FOXO1 may be a key mediator for the beneficial effects resulting from a reduction in IGF-1 signaling, analogously to the effect of forkhead transcription factor DAF-16 (FOXO homologue) in promoting longevity and increased stress resistance in worms (Kim et al., 2008; Yamaza et al., 2010; Guevara-Aguirre et al., 2011). Thus, lower phospho-FOXO1 previously reported in R− cells might be responsible for the suppressed GRP78 expression. However, neither forced expression of activated FOXO1 in 293T cells nor knockdown of FOXO1 in R− cells had an effect on GRP78 gene or protein expression. AKT/FOXO3a has been reported to suppress the expression of HSP70, a major stress-inducible cytosolic chaperone (Kim et al., 2005). Grp78 and Hsp70 share common features in their transcriptional regulatory machinery (Zhou and Lee, 1998) and two putative FOXO1 binding sites are located in the human Grp78 promoter which are conserved in the mouse Grp78 promoter (Furuyama et al., 2000). Nonetheless, FOXO1 does not appear to play a role in the repression of GRP78 in the absence of IGF-1R, suggesting other pathways downstream of PI3K/AKT are involved. Interestingly, while in this report we show that GRP78 expression is a downstream target of growth factors, other reports suggest that GRP78 may also be an upstream regulator of PI3K/AKT signaling under certain conditions. In cancer cell lines and cancer mouse models, knockdown of GRP78 suppressed the conversion of PIP2 to PIP3 at the cell surface, and reduced AKT phosphorylation (Fu et al., 2008; Wey et al., 2012). Furthermore, cell surface GRP78 was required for Cripto activated PI3K/AKT and MAPK signaling in NCCIT cells (Kelber et al., 2009). Clearly, GRP78 expression is sensitive to growth factor signaling, and these regulatory loops may contribute to calibrate the optimal level of GRP78 depending on cellular conditions.
In summary, in the present study we report that hepatic GRP78 is selectively downregulated in the liver of long-term CR mice. We also demonstrated that in MEF cells GRP78 expression is suppressed in response to reduced growth factor signaling, specifically via the IGF-1/AKT signaling axis. Further, the IGF-1 regulation of GRP78 occurred independently of GRP78 regulation by the UPR pathway. Given the important roles that CR, reduced IGF-1 signaling, and reduced ER stress play in the prevention of age associated disease (Pfaffenbach and Lee, 2011; Luo and Lee, 2012), the current study lends increased understanding into how a critical component of ER homeostasis is regulated in response to physiologic changes, and identifies potential modulators for therapeutic targeting.
Acknowledgments
We thank Drs. S. Korsmeyer, R. Baserga, P. Gill, B. Stiles and Z. Borok for the gift of cell lines and reagents. This work was supported in part by grants to A.S.L. from the National Institutes of Health, AG034906 and CA027607. The first author was a recipient of a postdoctoral fellowship from the National Institutes of Health, 5T32 CA009320 and a USC Norris Comprehensive Cancer Center Postdoctoral Supplemental Award. The sixth author was supported by an American Thoracic Society/Coalition for Pulmonary Fibrosis/Pulmonary Fibrosis Foundation Research Grant.
Footnotes
Conflict of Interest None
Literature Cited
- Baumeister P, Luo S, Skarnes WC, Sui G, Seto E, Shi Y, Lee AS. Endoplasmic reticulum stress induction of the Grp78/BiP promoter: activating mechanisms mediated by YY1 and its interactive chromatin modifiers. Mol Cell Biol. 2005;25:4529–4540. doi: 10.1128/MCB.25.11.4529-4540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JW, Cleveland JL, Hendershot LM. A pathway distinct from the mammalian unfolded protein response regulates expression of endoplasmic reticulum chaperones in non-stressed cells. EMBO J. 1997;16:7207–7216. doi: 10.1093/emboj/16.23.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Cao SX, Dhahbi JM, Mote PL, Spindler SR. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci USA. 2001;98:10630–10635. doi: 10.1073/pnas.191313598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere A, Romeo Y, Acosta-Jaquez HA, Moreau J, Bonneil E, Thibault P, Fingar DC, Roux PP. ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1) J Biol Chem. 2011;286:567–577. doi: 10.1074/jbc.M110.159046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–6370. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- Chung J, Kuo CJ, Crabtree GR, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, Masliah E, Dillin A. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139:1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. Overview of the IGF-I system. Horm Res. 2006;65(Suppl 1):3–8. doi: 10.1159/000090640. [DOI] [PubMed] [Google Scholar]
- Dhahbi JM, Cao SX, Tillman JB, Mote PL, Madore M, Walford RL, Spindler SR. Chaperone-mediated regulation of hepatic protein secretion by caloric restriction. Biochem Biophys Res Commun. 2001;284:335–339. doi: 10.1006/bbrc.2001.4972. [DOI] [PubMed] [Google Scholar]
- Dhahbi JM, Mote PL, Tillman JB, Walford RL, Spindler SR. Dietary energy tissue-specifically regulates endoplasmic reticulum chaperone gene expression in the liver of mice. J Nutr. 1997;127:1758–1764. doi: 10.1093/jn/127.9.1758. [DOI] [PubMed] [Google Scholar]
- Drakas R, Tu X, Baserga R. Control of cell size through phosphorylation of upstream binding factor 1 by nuclear phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 2004;101:9272–9276. doi: 10.1073/pnas.0403328101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RR, Dunning LM, Holtzman JL. The effect of aging on the chaperone concentrations in the hepatic, endoplasmic reticulum of male rats: the possible role of protein misfolding due to the loss of chaperones in the decline in physiological function seen with age. J Gerontol A Biol Sci Med Sci. 2006;61:435–443. doi: 10.1093/gerona/61.5.435. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Wey S, Wang M, Ye R, Liao CP, Roy-Burman P, Lee AS. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci USA. 2008;105:19444–19449. doi: 10.1073/pnas.0807691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- Heydari AR, Conrad CC, Richardson A. Expression of heat shock genes in hepatocytes is affected by age and food restriction in rats. J Nutr. 1995;125:410–418. doi: 10.1093/jn/125.3.410. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- Kelber JA, Panopoulos AD, Shani G, Booker EC, Belmonte JC, Vale WW, Gray PC. Blockade of Cripto binding to cell surface GRP78 inhibits oncogenic Cripto signaling via MAPK/PI3K and Smad2/3 pathways. Oncogene. 2009;28:2324–2336. doi: 10.1038/onc.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Kim JY, Yu BP, Chung HY. The activation of NF-kappaB through Akt-induced FOXO1 phosphorylation during aging and its modulation by calorie restriction. Biogerontology. 2008;9:33–47. doi: 10.1007/s10522-007-9114-6. [DOI] [PubMed] [Google Scholar]
- Kim HS, Skurk C, Maatz H, Shiojima I, Ivashchenko Y, Yoon SW, Park YB, Walsh K. Akt/FOXO3a signaling modulates the endothelial stress response through regulation of heat shock protein 70 expression. FASEB J. 2005;19:1042–1044. doi: 10.1096/fj.04-2841fje. [DOI] [PubMed] [Google Scholar]
- Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–1471. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anti-cancer therapies. Oncogene. 2012 doi: 10.1038/onc.2012.130. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Lee AS. Requirement of the p38 MAPK signaling pathway for the induction of Grp78/BiP by azetidine stress: ATF6 as a target for stress-induced phosphorylation. Biochem J. 2002;366:787–795. doi: 10.1042/BJ20011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. ER chaperone functions during normal and stress conditions. J Chem Neuroanat. 2004;28:51–65. doi: 10.1016/j.jchemneu.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, Hou J. A “FOXO” in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009;29:395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C, Dong D, Little E, Luo S, Lee AS. Transgenic mouse models for monitoring endoplasmic reticulum stress in vivo. Nat Med. 2004;10:1013–1014. doi: 10.1038/nm1004-1013. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Moore T, Checkley LA, Digiovanni J. Dietary energy balance modulation of epithelial carcinogenesis: a role for IGF-1 receptor signaling and crosstalk. Ann N Y Acad Sci. 2011;1229:7–17. doi: 10.1111/j.1749-6632.2011.06099.x. [DOI] [PubMed] [Google Scholar]
- Naidoo N. ER and aging-Protein folding and the ER stress response. Ageing Res Rev. 2009;8:150–159. doi: 10.1016/j.arr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci. 2008;28:6539–6548. doi: 10.1523/JNEUROSCI.5685-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novosyadlyy R, Kurshan N, Lann D, Vijayakumar A, Yakar S, LeRoith D. Insulin-like growth factor-I protects cells from ER stress-induced apoptosis via enhancement of the adaptive capacity of endoplasmic reticulum. Cell Death Differ. 2008;15:1304–1317. doi: 10.1038/cdd.2008.52. [DOI] [PubMed] [Google Scholar]
- Partridge L, Bruning JC. Forkhead transcription factors and ageing. Oncogene. 2008;27:2351–2363. doi: 10.1038/onc.2008.28. [DOI] [PubMed] [Google Scholar]
- Paz Gavilan M, Vela J, Castano A, Ramos B, del Rio JC, Vitorica J, Ruano D. Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiol Aging. 2006;27:973–982. doi: 10.1016/j.neurobiolaging.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Pfaffenbach KT, Lee AS. The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol. 2011;23:150–156. doi: 10.1016/j.ceb.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem. 1999;274:17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282:14056–14064. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B, Lee AS. The mammalian endoplasmic reticulum stress response element consists of an evolutionarily conserved tripartite structure and interacts with a novel stress-inducible complex. Nucleic Acids Res. 1999;27:1437–1443. doi: 10.1093/nar/27.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol. 2010;189:783–794. doi: 10.1083/jcb.201003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K. ER stress and hormetic regulation of the aging process. Ageing Res Rev. 2010;9:211–217. doi: 10.1016/j.arr.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Sell C, Rubini M, Rubin R, Liu JP, Efstratiadis A, Baserga R. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci USA. 1993;90:11217–11221. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res. 2007;17:54–57. doi: 10.1016/j.ghir.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Spindler SR. Calorie restriction enhances the expression of key metabolic enzymes associated with protein renewal during aging. Ann N Y Acad Sci. 2001;928:296–304. doi: 10.1111/j.1749-6632.2001.tb05659.x. [DOI] [PubMed] [Google Scholar]
- Spindler SR. Caloric restriction: from soup to nuts. Ageing Res Rev. 2010;9:324–353. doi: 10.1016/j.arr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ye R, Barron E, Baumeister P, Mao C, Luo S, Fu Y, Luo B, Dubeau L, Hinton DR, Lee AS. Essential role of the unfolded protein response regulator GRP78/BiP in protection from neuronal apoptosis. Cell Death Differ. 2010;17:488–498. doi: 10.1038/cdd.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wey S, Luo B, Tseng CC, Ni M, Zhou H, Fu Y, Bhojwani D, Carroll WL, Lee AS. Inducible knockout of GRP78/BiP in the hematopoietic system suppresses Pten-null leukemogenesis and AKT oncogenic signaling. Blood. 2012;119:817–825. doi: 10.1182/blood-2011-06-357384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaza H, Komatsu T, Wakita S, Kijogi C, Park S, Hayashi H, Chiba T, Mori R, Furuyama T, Mori N, Shimokawa I. FoxO1 is involved in the antineoplastic effect of calorie restriction. Aging Cell. 2010;9:372–382. doi: 10.1111/j.1474-9726.2010.00563.x. [DOI] [PubMed] [Google Scholar]
- Ye R, Jung DY, Jun JY, Li J, Luo S, Ko HJ, Kim JK, Lee AS. Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes. 2010;59:6–16. doi: 10.2337/db09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Lee AS. Mechanism for the suppression of the mammalian stress response by genistein, an anticancer phytoestrogen from soy. J Natl Cancer Inst. 1998;90:381–388. doi: 10.1093/jnci/90.5.381. [DOI] [PubMed] [Google Scholar]