Abstract
Proton magnetic resonance spectroscopy (1H-MRS) has been used to demonstrate metabolic changes in the visual cortex on visual stimulation. Small (2% to 11%) but significant stimulation induced increases in lactate, glutamate, and glutathione were observed along with decreases in aspartate, glutamine, and glycine, using 1H-MRS at 7 T during single and repeated visual stimulation. In addition, decreases in glucose and increases in γ-aminobutyric acid (GABA) were seen but did not reach significance. Changes in glutamate and aspartate are indicative of increased activity of the malate–aspartate shuttle, which taken together with the opposite changes in glucose and lactate, reflect the expected increase in brain energy metabolism. These results are in agreement with those of Mangia et al. In addition, increases in glutamate and GABA coupled with the decrease in glutamine can be interpreted in terms of increased activity of the neurotransmitter cycles. An entirely new observation is the increase of glutathione during prolonged visual stimuli. The similarity of its time course to that of glutamate suggests that it may be a response to the increased release of glutamate or to the increased production of reactive oxygen species. Together, these observations constitute the most detailed analysis to date of functional changes in human brain metabolites.
Keywords: antioxidants, energy metabolism, lactate, MR spectroscopy, neurotransmitters
Introduction
Proton magnetic resonance spectroscopy (1H-MRS) is a powerful tool for investigating human metabolism in vivo and has been used to demonstrate metabolic changes in the visual cortex on visual stimulation (Prichard et al, 1991; Frahm et al, 1996; Mangia et al, 2003, 2007b; Tuunanen et al, 2006). These previous studies focused mainly on lactate (Lac) changes. However, the increased spectral resolution and SNR (signal-to-noise ratio) available at 7 T enables improved discrimination and quantification of other brain metabolites (Tkáč et al, 2001; Tkáč and Gruetter, 2005). Mangia et al (2007a) used a visual checkerboard stimulus to investigate metabolic changes at 7 T during prolonged periods of visual stimulation lasting 5.3 and 10.6 minutes: a new steady state of increased oxidative metabolism was observed, Lac increased by 23%±5%, glutamate (Glu) increased by 3%±1%, aspartate (Asp) decreased by 15%±6%, and glucose (Glc) showed a tendency to decrease, but the changes did not reach significance. The decrease in (intracellular) Glc was interpreted as driving an increased flux of Glc into the cell, the increase in Lac as reflecting an increase in pyruvate (Pyr) and hence, an increased flux into the tricarboxylic acid (TCA) cycle. This is sustained by an increase in the activity of the malate–aspartate shuttle (MAS), reflected in the increase in Glu and decrease in Asp concentrations as a consequence of the rate-limiting Glu–Asp antiporter at the inner mitochondrial membrane (McKenna et al, 2006). This work has significantly improved our understanding of how brain energy metabolism underpins brain function. However, the metabolite changes observed due to visual stimulation were small. We therefore decided to use a long, intense visual stimulus, designed to retain attention, to try to elicit maximal changes in the levels of Glc, Lac, Glu, and Asp and to investigate the time course of poststimulus recovery.
Given that at 7 T, the principal excitatory and inhibitory neurotransmitters (Glu and γ-aminobutyric acid (GABA), respectively) and glutamine (Gln) are well resolved (Tkáč et al, 2001), we also set out to establish whether there was evidence for stimulus-related changes in neurotransmitter levels. One reason for believing there could be, is the knowledge that vesicular Glu released into the synaptic cleft is taken up by surrounding glial cells, converted to Gln and returned to neurons where it is converted back to Glu. The first magnetic resonance (MR) observation of this Glu–Gln cycle was by Badar-Goffer et al (1990) using 13C-labeled acetate in studies of superfused brain slices. Subsequently, in vivo 13C-labeled Glc studies in humans enabled the cycling rate to be accurately measured (Chhina et al, 2001). Measuring changes in this rate during visual stimulation has proven to be a challenge in humans (Morris and Bachelard, 2003). However, in animal studies, it has been possible to demonstrate that almost all of this cycling is associated with neurotransmitter cycling (Sibson et al, 1998). A similar GABA–Glu–Gln cycle exists for the recycling of GABA. If recycling rates of Glu and/or GABA are increased, it is possible that there will be a relative change in the proportions of the neurotransmitters and of Gln. Only a small proportion of the total Glu is involved as neurotransmitter, and changes in GABA and Gln may therefore provide less equivocal evidence of changes in neurotransmitter activity.
We undertook two studies: the first consisted of 6.6 minutes baseline followed by 13.2 minutes of visual stimulation and 19.8 minutes recovery; the second involved two 9.9 minutes rest periods interleaved with two 9.9 minutes stimulation periods. Our results broadly confirmed the results of Mangia et al (2007a), in particular with respect to possible changes in MAS activity. However, our studies demonstrated a peak in Lac levels followed by a subsequent decline toward baseline during the stimulus, and a failure to rise again on subsequent stimulation. Interestingly, we also observed a highly reproducible but unexpected increase in glutathione (GSH) levels, with an equivalent decrease in glycine (Gly) during stimulation, as well as changes in the relative levels of Gln and the principal neurotransmitters Glu and GABA.
Materials and methods
Human Subjects
Ten healthy subjects (7M, 3F, age=25±3 years) without history of neurologic disorders were involved in the study, which was conducted over a 3-month period. Informed written consent was obtained from all subjects according to the procedures approved by the University of Nottingham Medical School Ethics Committee. Nine subjects participated in each paradigm (single stimulus and double stimulus), including eight who participated in both. Subsequently, the data for one subject was removed from all analyses of the double stimulus paradigm due to significant stimulated echo artifacts affecting the spectral region around 3 to 4 p.p.m. This subject did not participate in the single stimulus paradigm.
Visual Stimulation
The visual stimulus was projected, with a 7.6° visual angle, onto a screen using a LED projector, coupled to a PC, and was viewed with prism spectacles. A small cross in the center of the field of view was used as fixation point. A median gray background was presented during rest periods and the stimulation paradigm comprised contrast-defined wedges, moving toward or away from the fixation cross and randomized by the computer (see Figure 1A(i)). This stimulation paradigm has been shown to lead to less neural adaptation over time compared with a checkerboard (Wandell et al, 2005).
Figure 1.
(A) (i) One frame of the randomly varying visual stimulus presented to subjects. (ii) Representative example of blood oxygen level-dependent (BOLD) response with the voxel position for magnetic resonance spectroscopy (MRS) indicated by a white square (the yellow box shows the shim volume). (B) 1H-MRS spectra for the single and double stimulation paradigm using data from 17 subjects (group analysis) during (i) rest and (ii) stimulation. Difference spectrum before (iii) and after compensation for the BOLD effect (iv). Peaks ascribed to lactate (Lac) (1.33 p.p.m.), glutamate (Glu) (2.35/3.75 p.p.m.), and glutathione (GSH) (2.92/2.97/3.78 p.p.m.) are clearly evident in the compensated difference spectrum. The simulation, obtained by summation of the LCModel spectra of the metabolites for which significant changes were detected, clearly reproduces all the major features of the compensated difference spectrum. It provides convincing explanations as to why some resonances associated with a particular metabolite are visible, whereas others, for example, the GSH resonances at 2.54 and 2.16 p.p.m., are not. Asp, aspartate; GABA, γ-aminobutyric acid; Gln, glutamine; Gly, glycine; NAA, N-acetylaspartate; PCr, phosphocreatine; PE, phosphorylethanolamine. The color reproduction of this figure is available on the Journal of Cerebral Blood Flow and Metabolism journal online.
Magnetic Resonance Imaging and Spectroscopy
All MR measurements were acquired using a Philips Achieva 7 T MR system (Philips Healthcare, Best, Netherlands) with reception employing a 16-channel SENSE head coil and transmission a surrounding volume coil. First- and second-order shim terms were automatically adjusted using FASTMAP with EPI readout (Gruetter and Tkáč, 2000). Head movement was minimized by positioning two pieces of foam surrounding the subject's head.
Functional Magnetic Resonance Imaging Measurement
An initial functional magnetic resonance imaging scan (EPI, echo time/repetition time (TE/TR)=26/2,200 milliseconds, spatial resolution=2 × 2 × 2 mm3 isotropic voxels, functional paradigm: 4.4 seconds stimulation and 28.6 seconds rest, eight repeats) was performed before spectroscopic acquisition. Functional magnetic resonance imaging data were analyzed in real-time using IViewBOLD (Philips Healthcare, Best, Netherlands) to determine the region of maximum activation in the visual cortex.
Magnetic Resonance Spectroscopy Measurement
A cubic volume of interest of 2 × 2 × 2 cm3 was positioned inside the region exhibiting significant BOLD (blood oxygen level-dependent) change on stimulation for functional MRS acquisition (see Figure 1A(ii)). A STEAM (STimulated Echo Acquisition Mode) sequence (TE/mixing time/TR=15/17/3,000 milliseconds, spectral width 4,000 Hz, 4,096 time points) was used for acquiring the MRS data. Spectra were collected in blocks of 32 averages with one acquisition without water suppression acquired at the beginning of every block (99 seconds per block). Water suppression was performed using MOIST (Multiply Optimized Insensitive Suppression Train) (Tarasów et al, 2003). Two different visual stimulation protocols were employed. The first functional paradigm comprised a 6.6-minutes resting period, before 13.2 minutes of visual stimulation followed by 19.8 minutes recovery, according to an acquisition protocol: OFF(4 blocks)–ON(8 blocks)–OFF(12 blocks). The second functional paradigm consisted of two 9.9 minutes rest periods interleaved with two 9.9 minutes stimulation periods, according to an acquisition protocol: OFF(6 blocks)–ON(6 blocks)–OFF(6 blocks)–ON(6 blocks). The total duration for each functional session was 39.6 minutes.
Magnetic Resonance Imaging Measurement
A 3D T1-weighted magnetization prepared rapid gradient echo (MPRAGE) image was acquired (field of view=192 × 192 × 52.5 mm3 with 1.5 mm isotropic voxels, TE/inversion time (TI)/TR=1.5/965/14 milliseconds) to allow the tissue content of the MRS voxel to be calculated. The anatomical image was first masked to show only image voxels contained within the MRS voxel. The tissue content was then assessed by segmenting the MRS voxel based on the image intensity (threshold, manually chosen by an observer, set to the upper threshold for cerebrospinal fluid tissue).
Postprocessing
Spectra were saved separately and were frequency aligned to the N-acetylaspartate (NAA) peak at ∼2.02 p.p.m. using an inhouse built program in MATLAB (Mathworks, Natick, MA, USA) before phase correction in jMRUI (A van den Boogaart, Katholieke Universiteit Leuven, Leuven, Belgium). Spectra were then averaged into groups for further analysis in LCModel (Provencher, 1993). For the single visual stimulation paradigm, spectra from each subject acquired during 6.6 minutes rest (4 blocks), 13.2 minutes stimulation (8 blocks), and 19.8 minutes recovery (12 blocks) were averaged across each of the conditions. In addition, spectra were averaged into groups of four blocks for each subject. For the double stimulation paradigm, all stimulation spectra were averaged as were all rest spectra for each subject. In addition, spectra were averaged across the individual rest and stimulation periods for each subject. Finally, time courses of metabolites for both functional paradigms were achieved separately by quantifying every spectrum (time resolution of 99 seconds) for each subject, and averaged intersubject trajectories of metabolites were calculated from all subjects.
A group difference spectrum was generated from the single and double visual stimulation paradigm using spectra from 17 subjects (one subject was excluded due to the noise within the chemical shift range 3.0 to 4.0 p.p.m.). The group averaged stimulation spectrum subtracted from the group averaged rest period spectrum. In all, 0.5 Hz Lorentzian line broadening was added to the group stimulation spectrum to compensate for BOLD changes in linewidth. This resulted in a ‘BOLD-free' difference spectrum.
Metabolite Quantification
The LCModel (Provencher, 1993) basis data set includes the simulated spectra of 20 metabolites (Mekle et al, 2009): Ala (alanine), Asp, PCho (phosphorylcholine), Cr (creatine), PCr (phosphocreatine), GABA, Gln, Glu, GSH, Gly, Ins (myo-inositol), Lac, NAA, scyllo-Ins (scyllo-inositol), Tau (taurine), Asc (ascorbate), Glc, NAAG (N-acetylaspartylglutamate), GPC (glycerophosphorylcholine), and PE (phosphorylethanolamine). Spectra were simulated for an STEAM sequence using previously published chemical shifts and coupling constants (Govindaraju et al, 2000) with TE/mixing time values identical to those used for data acquisition. A macromolecule spectrum, experimentally measured from the human visual cortex using a metabolite nulling inversion-recovery sequence with TR=2,000 milliseconds and TI=0.675 seconds (Tkáč and Gruetter, 2005) was also included in the basis set (see Figure 2). Before insertion into the LCModel basis data set, the residual signals from NAA and total Cr (tCr) were removed from the macromolecule spectrum using an HLSVD (Hankel Lanczos Squares Singular Values Decomposition) filter (Cabanes et al, 2001) in jMRUI. LCModel analysis was performed on all spectra within the chemical shift range 0.2 to 4.2 p.p.m. Resulting outputs were rejected if the %s.d. from LCModel was >20% (Glc was quantified with %s.d. <30%). The unsuppressed water signal was used as an internal reference for metabolite quantification and for eddy current correction using LCModel. The absolute concentration values for every cerebral metabolite were determined as μmol per gram of wet tissue, assuming a brain water content of 80%. Measured concentrations for all metabolites, excluding Lac (Eckstein et al, 2008), Glc (Dougherty and Roth, 1986), Gln (Eckstein et al, 2008), and GSH (Kannan et al, 1992), which exist in significant concentrations in the cerebrospinal fluid, were corrected for the volume of interest tissue fraction, with the MPRAGE anatomical image.
Figure 2.
1H 7 T magnetic resonance spectroscopy (MRS) spectrum (STEAM (STimulated Echo Acquisition Mode) sequence: echo time/mixing time/repetition time (TE/TM/TR)=15/17/3,000 milliseconds, BW 4,000 Hz, 4,096 points, NSA 32) obtained from a 2 × 2 × 2 cm3 volume in the visual cortex (uppermost spectrum), LCModel fit (second spectrum from top) in the range 0.2 to 4.2 p.p.m. and residual (third spectrum from top). The LCModel basis components, macromolecule, and baseline contributions to the fit are shown in the lower traces. Asp, aspartate; GABA, γ-aminobutyric acid; Gln, glutamine; Glu, glutamate; Gly, glycine; GPC, glycerophosphorylcholine; GSH, glutathione; Lac, lactate; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; PCr, phosphocreatine; PE, phosphorylethanolamine; scyllo-Ins, scyllo-inositol.
Statistical Analysis
A Wilcoxon signed rank test was conducted to compare the metabolite changes during different time scales of stimulation versus resting periods for both functional paradigms. Values are presented as mean±s.d. and P<0.05 is considered as statistically significant.
Results
Spectral Analysis
Figure 1B depicts the group difference spectrum between rest (Figure 1B(i)) and stimulation (Figure 1B(ii)) obtained from both the single and double stimulation paradigms, using data from 17 subjects (group analysis). The peaks seen at 1.33 p.p.m. (Lac), 2.01 p.p.m. (NAA), 2.35 p.p.m. (Glu), 2.92 and 2.97 p.p.m. (GSH), 3.03 p.p.m. (tCr), 3.21 p.p.m. (Cho), 3.78 p.p.m. (GSH) in the difference spectrum (Figure 1B(iii)) are attributed to linewidth changes due to the BOLD effect as well as concentration changes of metabolites (Mangia et al, 2007a). When the spectrum acquired during stimulation was line broadened by 0.5 Hz to match the linewidth of the spectrum acquired at rest, to eliminate the linewidth changes, only Lac (1.33 p.p.m.), Glu (2.35 p.p.m.), and GSH (2.92/2.97/3.78 p.p.m.) survived the procedure, revealing stimulus-related changes in these metabolites. However, to avoid the uncertainties associated with this procedure, we chose not to use difference spectra for the subsequent analysis, but rather used LCModel on the raw data to determine absolute concentrations and performed statistical tests to determine changes on activation.
Quantitative Analysis of Metabolites During a Single 13.2-Minute Visual Stimulation
Shimming resulted in typical water linewidths of 10.3 to 11.2 Hz and typical NAA SNR values for a spectrum of 32 averages were between 43 and 58. More than 15 metabolites were reliably quantified from in vivo 1H-MRS spectra of the human brain measured at 7 T using LCModel. Figure 2 illustrates the high quality of the fit as evidenced by the absence of spectral features in the residual (raw spectrum—fitted spectrum) and the smoothness of the baseline. Figure 3A shows the comparison of neurochemical profiles for the 20 metabolites determined during 6.6 minutes rest, 13.2 minutes stimulation, and 19.8 minutes recovery periods (mean±s.d., N=9). The significant changes are summarized in Table 1. In good agreement with previously published results at 7 T (Mangia et al, 2007a), during stimulation Glu was found to be increased by 2%±1% (equivalent to 0.22 μmol/g, P=0.011), Asp decreased by 9%±8% (equivalent to 0.32 μmol/g, P=0.038), Lac increased by 10%±6% (equivalent to 0.1 μmol/g, P=0.044), and Glc showed a tendency to decrease by 22%±18% (equivalent to 0.40 μmol/g) but did not reach statistical significance because of the higher s.d. for this metabolite. Separating the 13.2-minute stimulation into two 6.6-minute blocks allows further insight into the time course of these changes. It can be seen that the elevation in Lac (26%±7%, ∼0.2 μmol/g, P=0.038), is only evident during the first 6.6 minutes of activation, with a tendency to return toward baseline thereafter, despite the ongoing visual stimulation (Figure 3B). Interestingly, during the 13.2 minutes of visual stimulation, the brain GSH concentration was found to be elevated by 7%±4% (equivalent to 0.17 μmol/g, P=0.021), in parallel with decreased brain Gln and Gly, by 8%±5% (equivalent to 0.18 μmol/g, P=0.038) and 11%±6% (equivalent to 0.20 μmol/g, P=0.015), respectively. γ-Aminobutyric acid exhibited a tendency to increase throughout the period of stimulation, but the increase did not reach statistical significance (Figure 3A, see also Figure 5A). All metabolite levels returned to their baseline levels during the 19.8-minute recovery period.
Figure 3.
(A) LCModel quantification of metabolite levels for the single visual stimulation paradigm during rest (6.6 minutes), stimulation (13.2 minutes), and recovery (19.8 minutes). (B) For the metabolites showing significant change on stimulation, the data have been further analyzed in blocks of 6.6 minutes duration: rest, stimulation, recovery, 1, 2, and 3 blocks, respectively. All data (mean±s.d.) are shown for metabolites for which the CRLB were lower than specified limits (N=9 for all metabolites except N (alanine (Ala))=5, N (sI, lactate (Lac), N-acetylaspartylglutamate (NAAG), glucose (Glc))=6, N (Phosphorylethanolamine (PE))=7). Asp, aspartate; GABA, γ-aminobutyric acid; Gln, glutamine; Glu, glutamate; Gly, glycine; GSH, glutathione; NAA, N-acetylaspartate; PCr, phosphocreatine; scyllo-Ins, scyllo-inositol; tCr, total Cr.
Table 1. Absolute concentrations of metabolites and their percentage changes on stimulation.
| Metabolite |
Single stimulus |
Double stimulus |
||||||
|---|---|---|---|---|---|---|---|---|
|
Rest (6.6 minutes) |
Stimulation (13.2 minutes) |
Stimulation-rest |
First stimulation (9.9 minutes)–first rest (9.9 minutes) |
Second stimulation (9.9 minutes)–first rest (9.9 minutes) |
||||
| Concentration±s.d. (μmol/g) | Change±s.d. (μmol/g) | Change±s.d. (%) | Change±s.d. (μmol/g) | Change±s.d. (%) | Change±s.d. (μmol/g) | Change±s.d. (%) | ||
| Asp* | 3.52±0.28 | 3.20±0.28 | −0.32±0.28 | +9%±8% | +0.4±0.23 | +12%±7% | −0.33±0.26 | −9%±7% |
| Gln* | 2.25±0.10 | 2.07±0.10 | −0.18±0.11 | −8%±5% | −0.21±0.10 | −8%±4% | −0.29±0.10 | −11%±4% |
| Glu* | 11.28±0.11 | 11.50±0.11 | +0.22±0.11 | +2%±1% | +0.20±0.10 | +2%±1% | +0.35±0.10 | +3%±1% |
| GSH* | 2.28±0.1 | 2.45±0.1 | +0.17±0.1 | +7%±4% | +0.26±0.07 | +7%±2% | +0.36±0.07 | +10%±2% |
| Gly* | 1.85±0.11 | 1.65±0.11 | −0.20±0.11 | +11%±6% | −0.23±0.14 | +11%±6% | −0.32±0.14 | −15%±6% |
| Lac* | 0.79±0.05 | 0.89±0.05 | +0.1±0.05 | +10%±6% | +0.21±0.05 | +30%±7% | — | — |
Asp, aspartate; Gln, glutamine; Glu, glutamate; Gly, glycine; GSH, glutathione; Lac, lactate.
*P<0.05.
Quantitative Analysis of Metabolites During Repeated 9.9 Minutes Visual Stimulations
The second experimental paradigm involved two periods of rest (each of 9.9 minutes duration) interleaved with two 9.9-minute stimulation periods. It was undertaken to confirm the pattern of metabolic changes observed in the single 13.2-minute stimulation paradigm, and to investigate whether the responses to repeated periods of stimulation are identical or differ from the initial response. Figure 4A shows the comparison of neurochemical profiles for the 20 metabolites measured (mean±s.d., N=8). The significant changes are summarized in Table 1. In good agreement with our previous findings for the single stimulation, significant stimulation-related changes were found in Glu (increased by 3%±1%, P=0.021), Asp (decreased by 10%±7%, P=0.044), Lac (increased by 9%±7%, P=0.015), GSH (increased by 8%±2%, P=0.011), Gln (decreased by 6%±4%, P=0.044), and Gly (decreased by 12%±6%, P=0.038). Again, Glc exhibited a tendency to decrease (by 30%±19%) and GABA showed a slight increase during stimulation but neither effect reached significance.
Figure 4.
(A) LCModel quantification of metabolite levels for the repeated (double) visual stimulation paradigm, comparing rest (two 9.9-minute periods) and stimulation (two 9.9-minute periods). (B) Data for individual rest and stimulation blocks for metabolites demonstrating significant change on activation. All data (mean±s.d.) are shown for metabolites for which the CRLB were lower than specified limits (N=8 for all metabolites except N (alanine (Ala))=5, N (sI, phosphorylethanolamine (PE))=7, N (lactate (Lac))=6, N (glucose (Glc))=4). Asp, aspartate; GABA, γ-aminobutyric acid; Gln, glutamine; Glu, glutamate; Gly, glycine; GSH, glutathione; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; PCr, phosphocreatine; scyllo-Ins, scyllo-inositol; tCr, total Cr.
Changes in Asp, Glu, Gln, GSH, and Gly during the second 9.9-minute stimulation period were comparable to those found during the first 9.9-minute stimulation period (Figure 4B). However, Lac only showed significant elevation in the first 9.9-minute stimulation (30%±7%, equivalent to 0.21 μmol/g, P=0.038) with no significant accumulation evident in the second 9.9-minute stimulation (Table 1). In addition, Glu and GSH showed a significantly enhanced increase in the second stimulation period, by 1%±1% (equivalent to 0.15 μmol/g, P=0.021) and 3%±2% (equivalent to 0.1 μmol/g, P=0.011), respectively. A possible reason for the increased elevation in Glu and GSH in the second phase of activation might be their slow recovery rate, such that their levels had not returned to baseline during the second 9.9-minute resting period. In support of this, we find significant elevation of Glu (by 1%±1% equivalent to 0.12 μmol/g, P=0.021) and GSH (by 5%±3% equivalent to 0.15 μmol/g, P=0.038), in the second compared with the first resting period.
Metabolic Time Courses
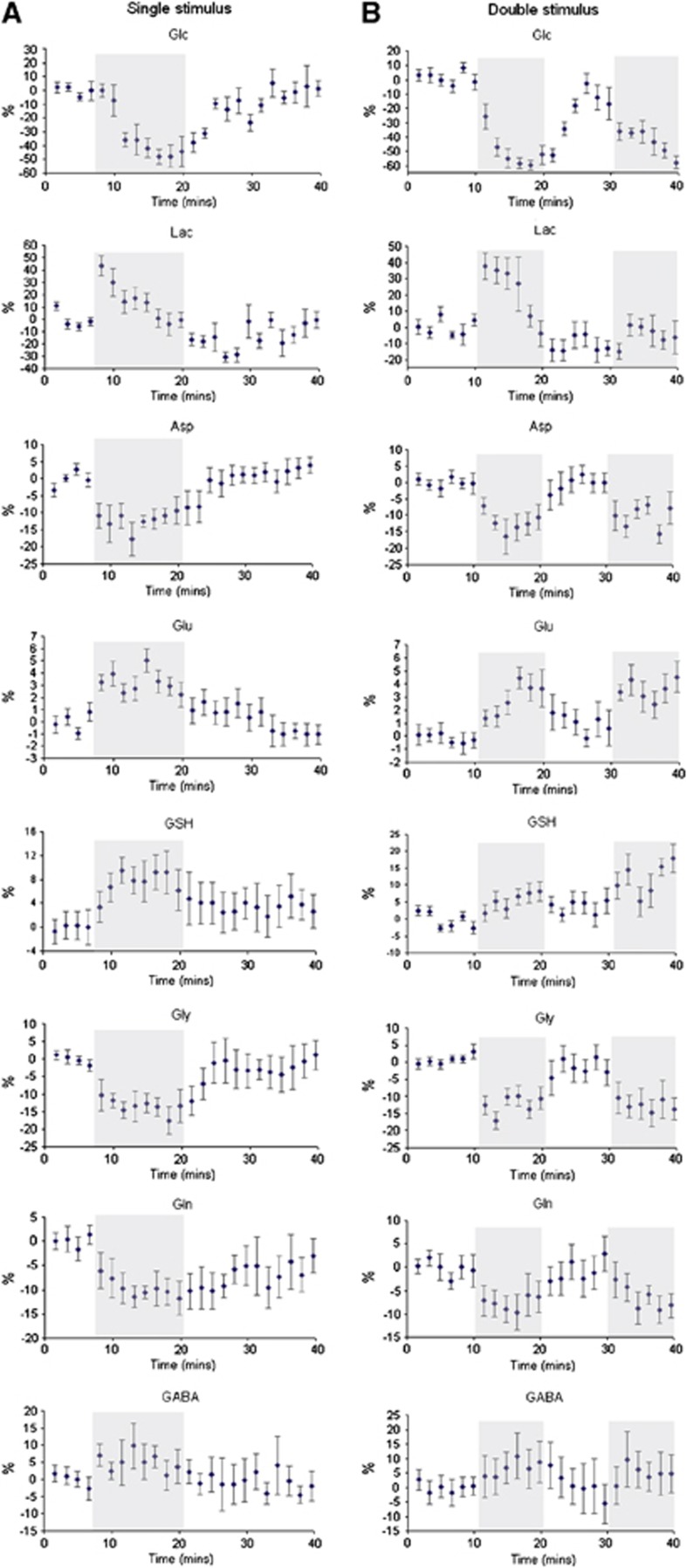
Metabolic time courses with a 99-second time resolution are shown for the single (A) and repeated (B) visual stimulation paradigms in Figure 5. They reveal that Lac increases to a maximum level within the first 99 seconds of activation, followed by a decrease toward the basal level even during ongoing stimulation. After cessation of the first stimulus, the averaged Lac level was slightly below baseline, and interestingly failed to show a response to a second visual stimulus. In contrast, Glc was reduced from baseline levels for the entire duration of both visual stimuli.
Figure 5.
Time courses of metabolites during the single (A) and double (B) visual stimulation paradigms. Concentration changes are expressed as a percentage relative to the average concentration during the first rest period, temporal resolution is 99 seconds. Data are mean±s.e.m. (with the number of subjects included in each time course the same as in Figures 3 and 4 for the single and double stimulus, respectively). The shaded areas show the periods of visual stimulation. Asp, aspartate; GABA, γ-aminobutyric acid; Glc, glucose; Gln, glutamine; Glu, glutamate; Gly, glycine; GSH, glutathione; Lac, lactate.
During stimulation, there was a small but statistically significant increase in Glu. Recovery toward baseline levels was relatively slow and was incomplete at the time of a second stimulus, which resulted in a significantly higher Glu level as noted above. Glutathione levels followed a similar time course to Glu. Changes in Gln, Gly, and Asp were opposite to those of Glu and GSH, that is, decreased in response to the stimuli. Asp and Gly showed a response to both stimuli, recovering during the resting periods, whereas Gln recovered more slowly, remaining below baseline levels for a significant period poststimulation. γ-Aminobutyric acid exhibited a tendency to increase during visual stimulation in both single and repeated stimulation paradigms, and to normalize to baseline levels poststimulation, but as noted above the changes did not achieve significance.
Discussion
Spectral Acquisition at 7 T
This study shows that reliable metabolite quantification can be achieved with reasonable temporal resolution using a 16-channel SENSE head coil. This is significant because it demonstrates that similar studies are possible in subcortical regions of the brain and in those cortical areas less amenable to surface coil study. A penalty is, of course, paid in SNR; see for comparison, the surface coil study of Mangia et al (2007a). However, in future studies, this could be mitigated by appropriately weighting the signal contributions of the individual coil elements, effectively synthesizing the most appropriate coil for the region of interest selected. The expected increase in spectral resolution at 7 T, relative to lower field strengths, is realized, enabling many more metabolites to be quantified (Figures 1, 2, 3, 4, 5), including those associated with energy metabolism (Glc, Lac, Asp), neurotransmitter activity (Glu, GABA, and Gln), and neuroprotection (GSH). As previously reported, the BOLD effect, due to the decreased susceptibility effects resulting from the local hyperoxygenation of blood during activation, increases the T2* of both water and metabolite signals during stimulation. The observed line narrowing effect is small (∼0.5 Hz at 7 T) and is easily discernible only for the strongest singlets in the spectrum (NAA and tCr, see Figure 1B(iii)). Subtraction of the resting spectrum from the spectrum acquired during stimulation results in small peaks in the difference spectrum at 2.01 p.p.m., 3.03 p.p.m. and 3.12 p.p.m., bordered by negative sidelobes. This effect is largely removed by adding 0.5 Hz line broadening to the spectrum acquired during stimulation to match the linewidth of the spectrum acquired at rest. This reveals stimulus-related changes in several metabolites, including Lac (1.33 p.p.m.), Glu (2.35/3.75 p.p.m.), and GSH (2.92/2.97/3.78 p.p.m.) (Figure 1B(iv)). It is to be expected that the BOLD-induced T2* changes should have little effect on quantification if a short TE sequence is used, and indeed, no change is observed between rest and stimulation for NAA concentration (Figures 3 and 4), lending confidence to this assertion. It also suggests that the TR we have chosen (3 seconds) is sufficiently long for any stimulation-induced changes in T1 values to be negligible. There is one proviso, namely, that if stimulation leads to movement of metabolites between compartments with substantially different relaxation behaviors, this may not be the case.
Stimulation-Induced Changes in Brain Energy Metabolism
The brain's energy requirement is met primarily by Glc, delivered from plasma via the Glc transporter, which is reversible (Gruetter et al, 1998). The increased Glc consumption, required to meet the increased energy demand during visual stimulation, leads to a decrease in brain Glc concentration, slowing down reverse transport until a new steady state is reached, in which net Glc influx is equal to CMRglc (Mangia et al, 2007a). In our experiments, basal Glc levels in the visual cortex (1.7±0.3 μmol/g) were reduced by ∼32%±8% throughout the stimulation periods (Figure 5).
Although the trends in the Glc time courses are clear, the changes did not reach statistical significance. This is a result of a higher s.d. associated with the Glc quantitation, probably because >80% of the signal intensity of Glc is in the 3.2 to 3.9 p.p.m. spectral region (Gruetter et al, 1992), where there is overlap with the much stronger resonances of Glu, Gln, Ins, and Tau. The observed decrease in Glc under stimulation in our experiments is in good agreement with previous reports (Mangia et al, 2007a), lending weight to the idea that decreased tissue Glc does indeed reflect increased CMRglc during visual stimulation.
While there is general agreement with the increase in CMRglc on visual stimulation, there has been considerable debate as to whether this increase is primarily aerobic or anaerobic. Early PET (positron emission tomography) studies by Fox et al (1988) found an uncoupling between oxygen and Glc consumption, suggesting that, whereas basal metabolism is primarily aerobic, the stimulus-related increase is essentially anaerobic. However, studies using 13C-MRS (Chen et al, 2001; Chhina et al, 2001) have found large (tens of %) increases in TCA cycle activity, suggesting that the increase is primarily in aerobic metabolism. Later PET studies have suggested a greater role for aerobic metabolism, but a discrepancy remains and it is therefore of interest to know whether there is accumulation of Lac, suggestive of anaerobic metabolism.
Several 1H-MRS studies have reported elevated brain Lac during visual stimulation and we too observe increases above baseline of up to 0.2 μmol/g (26%±7%) in the early stage of stimulation (Figures 3B, 4B, and 5). In contrast to the studies by Mangia et al (2007a), which show an increase to a new steady state during a prolonged stimulus, comparable in duration to ours, we observed a transient increase, with a subsequent return toward baseline (Figure 5). This is consistent with the first report of Lac increase during sustained visual stimulation (Prichard et al, 1991), later confirmed by Frahm et al (1996). It is possible that differences in Lac response may reflect differences in the neuronal populations excited. In the present study, the stimulus consisted of achromatic moving wedges, which are thought to preferentially activate neurons in the interblob regions of the primary visual cortex (Tootell et al, 1988), where cytochrome oxidase levels are relatively low. In contrast, Mangia et al (2007a) utilized a red/black full field flickering checkerboard which would have activated neighboring blob regions with high levels of cytochrome oxidase and hence a higher capacity for oxidative metabolism. However, the feasibility of measuring Lac changes under stimulation has been challenged by other authors: Tuunanen et al (2006) could not detect any Lac accumulation during several kinds of visual stimulation; Boucard et al (2005) also failed to detect the Lac signal during visual stimulation. The reasons for these differences in Lac response, measured by 1H-MRS, are unclear. It is possible that the transient responses reflect adaptation or a decrease in attention. However, we specifically chose a visual stimulus designed to minimize these effects (Wandell et al, 2005). It is also possible that different acquisition sequences or experimental protocols have different sensitivities to possible changes in relaxation times associated with changes in intracellular milieu or movement between compartments. Again, we chose a short TE acquisition sequence to try to minimize these effects.
The initial increase in Lac during the early stimulation period most probably reflects an increase in anaerobic glycolysis. It also implies an increase in Pyr (because of the dynamic equilibrium between Lac and Pyr mediated by Lac dehydrogenase (LDH)). This rise in Pyr has been suggested to stimulate activation of pyruvate dehydrogenase, leading to an increased flux into the oxidative pathway (Hawkins et al, 1973). Mangia et al (2007a) argue that a new steady state of increased Lac sustains this increased flux into the TCA cycle. However, it could also be argued that an increased flux through pyruvate dehydrogenase could lead to a decrease in Lac as we observe. Because of the permeability of the blood–brain barrier to Lac, the increased brain Lac concentration during stimulation could also lead to an increased Lac efflux to blood, accounting for the decreased brain Lac level we observe in the later stages of the visual stimulation. Lac export to the extracellular compartment is believed to occur ∼30 seconds to 1 minute after the start of stimulation (Boer et al, 1991). The observed peak in the brain Lac then represents a balance between production and efflux, which is likely to depend strongly on the nature of the stimulation paradigm. It is possible that the changes in Lac we observe reflect changes in its utilization as energy substrate by neurons as suggested by several authors (Hu and Wilson, 1997). However, as noted by Mangia et al (2007a), modeling of this Lac shuttle hypothesis suggests that activation should result in a sustained increase in Lac, rather than a transient increase as noted above (Frahm et al, 1996). It is also possible, though unlikely in our case, that the changes we see reflect movement of Lac between compartments with radically different relaxation times.
Of great interest is the lack of Lac response to a second period of visual stimulation (Figures 4B and 5B). Although an attenuated Lac response due to repeated stimuli has been previously reported (Mangia et al, 2007b), we believe this study is the first to demonstrate complete suppression. We suggest that processes, such as increased Lac efflux, switched on during the first period of visual stimulation, remain active or ‘primed' during the ensuing rest period. This observation is reminiscent of previously reported ischemic conditioning effects (Niemann et al, 2005), and merits further investigation regarding its origin and longevity. This is the subject of further studies in our laboratory.
Our findings of decreased Asp and increased Glu during single and repeated periods of visual stimulation are in good agreement with Mangia et al (2007a), who interpreted them as an increase in the activity of the MAS, which has an important role in maintaining the cytosolic redox potential NADH/NAD+ (NADH and NAD+ are the reduced and oxidized forms of nicotinamide adenine dinucleotide), required for the oxidative metabolism of Glc and for synthesis of brain neurotransmitters (McKenna et al, 2006). The rate-limiting step in this shuttle is the Glu–Asp antiporter located in the inner mitochondrial membrane (Mangia et al, 2007a) and an increase in shuttle activity would therefore be expected to lead to the changes observed by Mangia et al (2007a) and confirmed in our studies.
Glutathione and Its Response to Visual Stimulation
To the best of our knowledge, no prior 1H-MRS studies have reported GSH responses to visual stimulation, whereas a significant activation induced increase of 7% to 8% is a clear finding in this work (Figures 3, 4, 5; Table 1). This difference may be due to the nature of our stimulus, which is intense, prolonged, and designed to minimize adaptation. Despite its lower concentration, the Cramer-Rao lower bound (CRLB) of GSH was much lower than that of Gln (Table 1). This can be ascribed to the fact that the spin system of GSH includes a singlet at 3.77 p.p.m.—the ‘Gly moiety'—which does not experience signal loss due to J-modulation.
The human brain constitutes only 2% of the body's weight yet consumes 20% of the oxygen utilized by the body (Clarke and Sokoloff, 1999). Further, visual stimulation leads to large increases (approaching 50%) in the cerebral metabolic rate in the visual cortex, and though still a matter of contention, this increase is likely to be primarily aerobic (Chhina et al, 2001). The increase in mitochondrial respiration is inevitably accompanied by an increase in the generation of ROS (reactive oxygen species), such as the superoxide anion (O2−), peroxynitrite (ONOO−), and hydrogen peroxide, which cause oxidative damage of nucleic acids, lipids, carbohydrates, and proteins (Dringen, 2000). The body's mainline of defense against ROS is GSH (Muller, 2000), and so it is reasonable to expect that GSH would be present in significant amounts in the brain, and also that prolonged periods of visual stimulation might result in an increase in its level in visual cortex as we observe. During detoxification, GSH is oxidized to GSH disulfide (GSSG). However, GSSG does not accumulate, because it is rapidly reduced back to GSH by GSH reductase, and in normal brain, GSSG constitutes only ∼1% of total GSH. Maintenance of a highly reduced GSH pool is important to protect against excessive free radical formation. Glutathione is a tripeptide, made up of the amino acids Glu, Cys (cysteine), and Gly. It is interesting to note that the increase in GSH during visual stimulation is mirrored by a decrease in Gly, one of its metabolic precursors, as might be expected.
An alternative hypothesis for the observed increase in GSH during stimulation relates to the clearance of the increased Glu generated during the intense neuronal activation (Glu is also a metabolic precursor of GSH). Further, activation of the Glu NMDA (N-methyl-D-aspartate) channels requires simultaneous binding of both Glu and its coagonist Gly (Kuryatov et al, 1994). The increased GSH synthesis during prolonged intense visual stimulation will deplete pools of both agonists, and could therefore reduce activation of the NMDA receptor protecting against Glu-induced excitotoxicity (activation of the NMDA receptor results in the Ca2+-induced activation of nitric oxide synthase, which in turn leads to NO production, inhibition of Gln synthetase, and accumulation of Glu (Rao et al, 2003)). The failure to observe a stimulus-related increase in GSH (and decrease in Gly) in the oxidatively more active blob neurons stimulated in the studies by Mangia et al (2007a, 2007b) favors this alternative explanation.
Stimulus-Driven Changes in Neurotransmitter Cycling
The principal excitatory neurotransmitter in the cortex of the human brain is Glu. It is released from synaptic vesicles in the presynaptic terminal into the synaptic cleft where it activates receptors in the postsynaptic membrane. Glutamate is then transported, mainly into surrounding astrocytes, where it is first converted into Gln before being returned to the neurons and converted back into Glu. This Glu/Gln cycle sustains neural activity, and the flux through it would be expected to increase on neural stimulation—something that has been demonstrated in animal studies using 13C-MRS (Sibson et al, 1997) but is difficult to measure in humans (Morris and Bachelard, 2003). The concomitant changes in Glu and Gln levels depend on the rate-limiting processes in the cycle, but we might surmise that an increased rate of cycling could lead to an increase in neurotransmitter Glu, mirrored by a decrease in Gln. The improved spectral dispersion at 7 T allows clear separation and quantification of Glu and Gln resonances, and, on visual activation, we do indeed observe a modest increase in Glu (2% to 3%) and decrease (6% to 8%) in Gln. In addition to sustaining brain activity, the increased Glu/Gln neurotransmitter cycling flux may have an important role in preventing extracellular Glu levels from reaching excitotoxic levels as the blood–brain barrier is impermeable to Glu, even at high concentrations (Hawkins, 2009). Several factors other than increased Glu/Gln cycling may contribute to a change in the concentration of Glu on stimulation, including, as discussed above, an increased activity of the MAS and synthesis of GSH, as well as increased synthesis via anaplerosis or loss through oxidation. The concentration changes reported in Table 1 are quantitatively consistent (within the quoted errors) with the suggested changes in Glu/Gln cycling, MAS activity, and GSH synthesis (based on the changes in associated metabolites (Gln, Asp, GSH). There is thus no evidence for a significant anaplerotic flux, but given the small size of the changes observed, and the proportionately large errors, this remains a possibility.
The inhibitory neurotransmitter GABA is formed by decarboxylation of Glu and is recycled via Gln in a Gln/Glu/GABA. Again we might surmise that stimulation would lead to an increase in flux through this cycle. In our experiments, GABA, like Glu, exhibited a tendency to increase throughout both the single (Figure 5A) and double (Figure 5B) stimulation periods. However, we emphasize that this change did not reach statistical significance.
Conclusions
Using a novel visual stimulation paradigm and 1H-MRS at 7 T, we have been able to observe the time course of the metabolic response to activation. In agreement with previous findings (Mangia et al, 2007a), we observed changes in Glc and Lac consistent with increased energy metabolism due to neuronal activation, and this is supported by the changes in Glu and Asp indicative of an increase in MAS activity. However, the changes we observed in Lac were transient in nature and muted in response to a second stimulus. Our observation of increased Glu with decreased Gln during stimulation can be interpreted as a stimulus driven increase in excitatory neurotransmitter cycling. We also observed similar changes in GABA, but these did not achieve significance. The elevated GSH in the visual cortex in response to visual stimulation is a new observation. Possible explanations for this include detoxification of ROS or clearance of the increased Glu generated during the intense neuronal activation. Glycine, a precursor of GSH, shows a significant decrease on activation, which is consistent with increased GSH synthesis.
Acknowledgments
The authors are grateful to Sam Wharton and Olivier E Mougin for their assistance in MATLAB programming, to Denis Schluppeck for providing the visual stimulus and to the Centre d'Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, and EPFL, Switzerland, for making available to us their LCModel basis data set. The authors also gratefully acknowledge the Medical Research Council for Programme Grand Support and for a Dorothy Hodgkin Postgraduate Award (YL) in partnership with GlaxoSmithKline.
The authors declare no conflict of interest.
References
- Badar-Goffer RS, Bachelard HS, Morris PG. Cerebral metabolism of acetate and glucose studied by C-13-NMR spectroscopy. A technique for investigating metabolic compartmentation in the brain. Biochem J. 1990;266:133–139. doi: 10.1042/bj2660133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer J, Postema F, Plijter-Groendijk H, Korf J. Continuous monitoring of extracellular lactate concentration by microdialysis lactography for the study of rat muscle metabolism in vivo. Pflügers Archiv Eur J Physiol. 1991;419:1–6. doi: 10.1007/BF00373739. [DOI] [PubMed] [Google Scholar]
- Boucard CC, Mostert JP, Cornelissen FW, De Keyser J, Oudkerk M, Sijens PE. Visual stimulation, 1H MR spectroscopy and fMRI of the human visual pathways. Eur Radiol. 2005;15:47–52. doi: 10.1007/s00330-004-2494-y. [DOI] [PubMed] [Google Scholar]
- Cabanes E, Confort-Gouny S, Le Fur Y, Simond G, Cozzone PJ. Optimization of residual water signal removal by HLSVD on simulated short echo time proton MR spectra of the human brain. J Magn Reson. 2001;150:116–125. doi: 10.1006/jmre.2001.2318. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhu X-H, Gruetter R, Seaquist ER, Adriany G, Ugurbil K. Study of tricarboxylic acid cycle flux changes in human visual cortex during hemifield visual stimulation using 1H-{13C} MRS and fMRI. Magn Reson Med. 2001;45:349–355. doi: 10.1002/1522-2594(200103)45:3<349::aid-mrm1045>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Chhina N, Kuestermann E, Halliday J, Simpson LJ, Macdonald IA, Bachelard HS, Morris PG. Measurement of human tricarboxylic acid cycle rates during visual activation by 13C magnetic resonance spectroscopy. J Neurosci Res. 2001;66:737–746. doi: 10.1002/jnr.10053. [DOI] [PubMed] [Google Scholar]
- Clarke DD, Sokoloff L.1999Circulation and energy metabolism of the brain Basic Neurochemistry: Molecular, Cellular and Medical Aspects(Siegel GJ, Agranoff BW, eds) 6th ed,Lippincott-Raven: Philadelphia; 637–669. [Google Scholar]
- Dougherty JM, Roth RM. Cerebral spinal fluid. Emerg Med Clin North Am. 1986;4:281–297. [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Eckstein JA, Ammerman GM, Reveles JM, Ackermann BL. Analysis of glutamine, glutamate, pyroglutamate, and GABA in cerebrospinal fluid using ion pairing HPLC with positive electrospray LC/MS/MS. J Neurosci Methods. 2008;171:190–196. doi: 10.1016/j.jneumeth.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Frahm J, Kruger G, Merboldt KD, Kleinschmidt A. Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal brain activation in man. Magn Reson Med. 1996;35:143–148. doi: 10.1002/mrm.1910350202. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Rothman DL, Novotny EJ, Shulman GI, Prichard JW, Shulman RG. Detection and assignment of the glucose signal in H-1-NMR difference spectra of the human brain. Magn Reson Med. 1992;27:183–188. doi: 10.1002/mrm.1910270118. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Tkáč I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43:319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Ugurbil K, Seaquist ER. Steady-state cerebral glucose concentrations and transport in the human brain. J Neurochem. 1998;70:397–408. doi: 10.1046/j.1471-4159.1998.70010397.x. [DOI] [PubMed] [Google Scholar]
- Hawkins RA. The blood-brain barrier and glutamate. Am J Clin Nutr. 2009;90:867S–8874. doi: 10.3945/ajcn.2009.27462BB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RA, Miller AL, Nielsen RC, Veech RL. Acute action of ammonia on rat brain metabolism in vivo. Biochem J. 1973;134:1001–1008. doi: 10.1042/bj1341001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Wilson GS. A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J Neurochem. 1997;69:1484–1490. doi: 10.1046/j.1471-4159.1997.69041484.x. [DOI] [PubMed] [Google Scholar]
- Kannan R, Kuhlenkamp JF, Ookhtens M, Kaplowitz N. Transport of glutathione at blood-brain-barrier of the rat: inhibiton by glutathione analogs and age-dependence. J Pharmacol Exp Ther. 1992;263:964–970. [PubMed] [Google Scholar]
- Kuryatov A, Laube B, Betz H, Kuhse J. Mutational analysis of the glycine-binding site of the NMDA receptor-structural similarity with bacterial amino acid-binding proteins. Neuron. 1994;12:1291–1300. doi: 10.1016/0896-6273(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Mangia S, Garreffa G, Bianciardi M, Giove F, Di Salle F, Maraviglia B. The aerobic brain: lactate decrease at the onset of neural activity. Neuroscience. 2003;118:7–10. doi: 10.1016/s0306-4522(02)00792-3. [DOI] [PubMed] [Google Scholar]
- Mangia S, Tkáč I, Gruetter R, Van de Moortele PF, Maraviglia B, Ugurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from H-1 NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab. 2007a;27:1055–1063. doi: 10.1038/sj.jcbfm.9600401. [DOI] [PubMed] [Google Scholar]
- Mangia S, Tkáč I, Logothetis NK, Gruetter R, Van de Moortele PF, Ugurbil K. Dynamics of lactate concentration and blood oxygen level-dependent effect in the human visual cortex during repeated identical stimuli. J Neurosci Res. 2007b;85:3340–3346. doi: 10.1002/jnr.21371. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Waagepetersen HS, Schousboe A, Sonnewald U. Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: current evidence and pharmacological tools. Biochem Pharmacol. 2006;71:399–407. doi: 10.1016/j.bcp.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Mekle R, Mlynarik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced singla intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med. 2009;61:1279–1285. doi: 10.1002/mrm.21961. [DOI] [PubMed] [Google Scholar]
- Morris P, Bachelard H. Reflections on the application of 13C-MRS to research on brain metabolism. NMR Biomed. 2003;16:303–312. doi: 10.1002/nbm.844. [DOI] [PubMed] [Google Scholar]
- Muller F. The nature and mechanism of superoxide production by the electron transport chain: its relevance to aging. AGE. 2000;23:227–253. doi: 10.1007/s11357-000-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann CU, Hirose R, Liu T, Behrends M, Brown JL, Kominsky DF, Roberts JP, Serkova N. Ischemic preconditioning improves energy state and transplantation survival in obese zucker rat livers. Anesth Analg. 2005;101:1577–1583. doi: 10.1213/01.ANE.0000184897.53609.2A. [DOI] [PubMed] [Google Scholar]
- Prichard J, Rothman D, Novotny E, Petroff O, Kuwabara T, Avison M, Howseman A, Hanstock C, Shulman R. Lactate rise detected by H-1-NMR in human visual cortex during physiological stimulation. Proc Natl Acad Sci USA. 1991;88:5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Rao SD, Yin HZ, Weiss JH. Disruption of glial glutamate transport by reactive oxygen species produced in motor neurons. J Neurosci. 2003;23:2627–2633. doi: 10.1523/JNEUROSCI.23-07-02627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Behar KL, Rothman DL, Shulman RG. In vivo C-13 NMR measurements of cerebral glutamine synthesis as evidence for glutamate-glutamine cycling. Proc Natl Acad Sci USA. 1997;94:2699–2704. doi: 10.1073/pnas.94.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasów E, Wiercińska-Drapało A, Kubas B, Dzienis W, Orzechowska-Bobkiewicz A, Prokopowicz D, Walecki J. Cerebral MR spectroscopy in neurologically asymptomatic HIV-infected patients. Acta Radiol. 2003;44:206–212. doi: 10.1080/j.1600-0455.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Tkáč I, Andersen P, Adriany G, Merkle H, Urbil K, Gruetter R. In vivo1H NMR spectroscopy of the human brain at 7T. Mag Reson Med. 2001;46:451–456. doi: 10.1002/mrm.1213. [DOI] [PubMed] [Google Scholar]
- Tkáč I, Gruetter R. Methodology of H-1 NMR spectroscopy of the human brain at very high magnetic fields. Appl Magn Reson. 2005;29:139–157. doi: 10.1007/BF03166960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RBH, Switkes E, Silverman MS, Hamilton SL. Functional-anatomy of macaque striate cortex. 2. Retinotopic organization. J Neurosci. 1988;8:1531–1568. doi: 10.1523/JNEUROSCI.08-05-01531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuunanen PI, Vidyasagar R, Kauppinen RA. Effects of mild hypoxic hypoxia on poststimulus undershoot of blood-oxygenation-level-dependent fMRI signal in the human visual cortex. Magn Reson Imaging. 2006;24:993–999. doi: 10.1016/j.mri.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Brewer AA, Dougherty RF. Visual field map clusters in human cortex. Philos Trans R Soc B Biol Sci. 2005;360:693–707. doi: 10.1098/rstb.2005.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]