Abstract
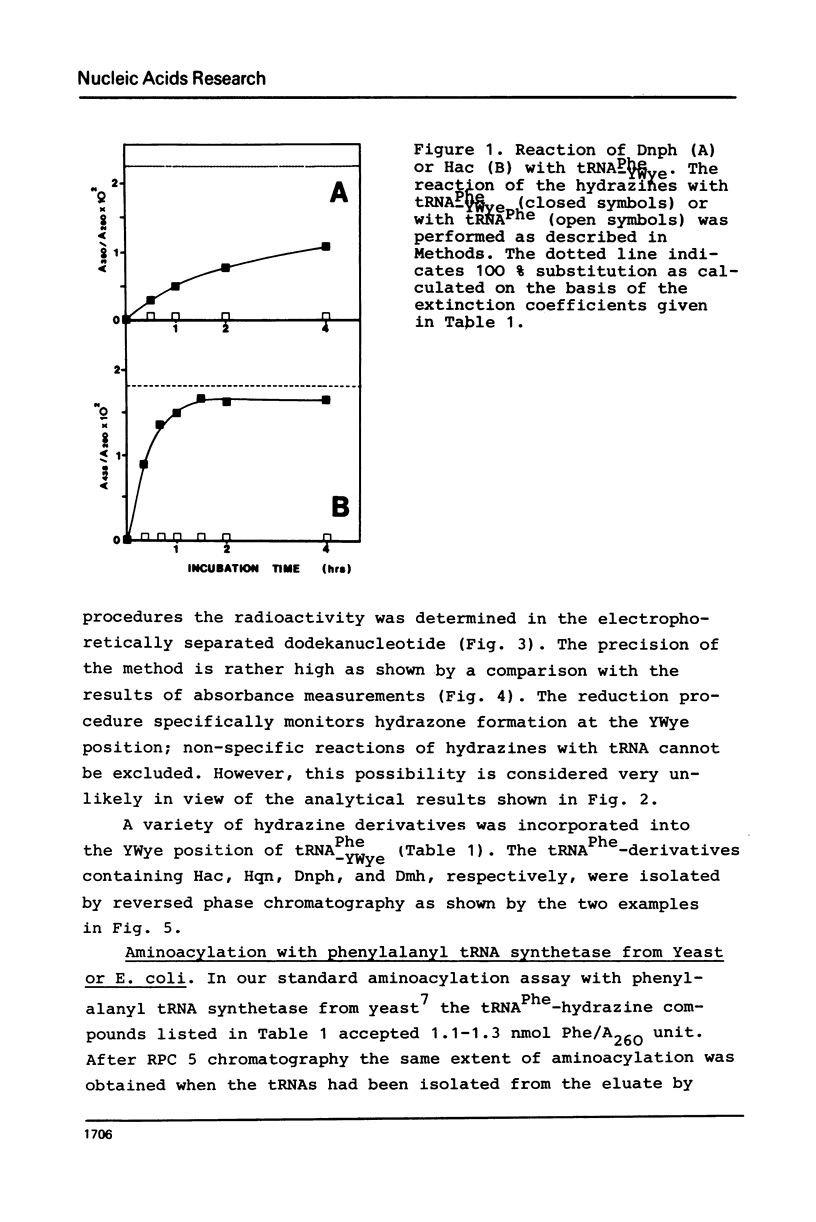
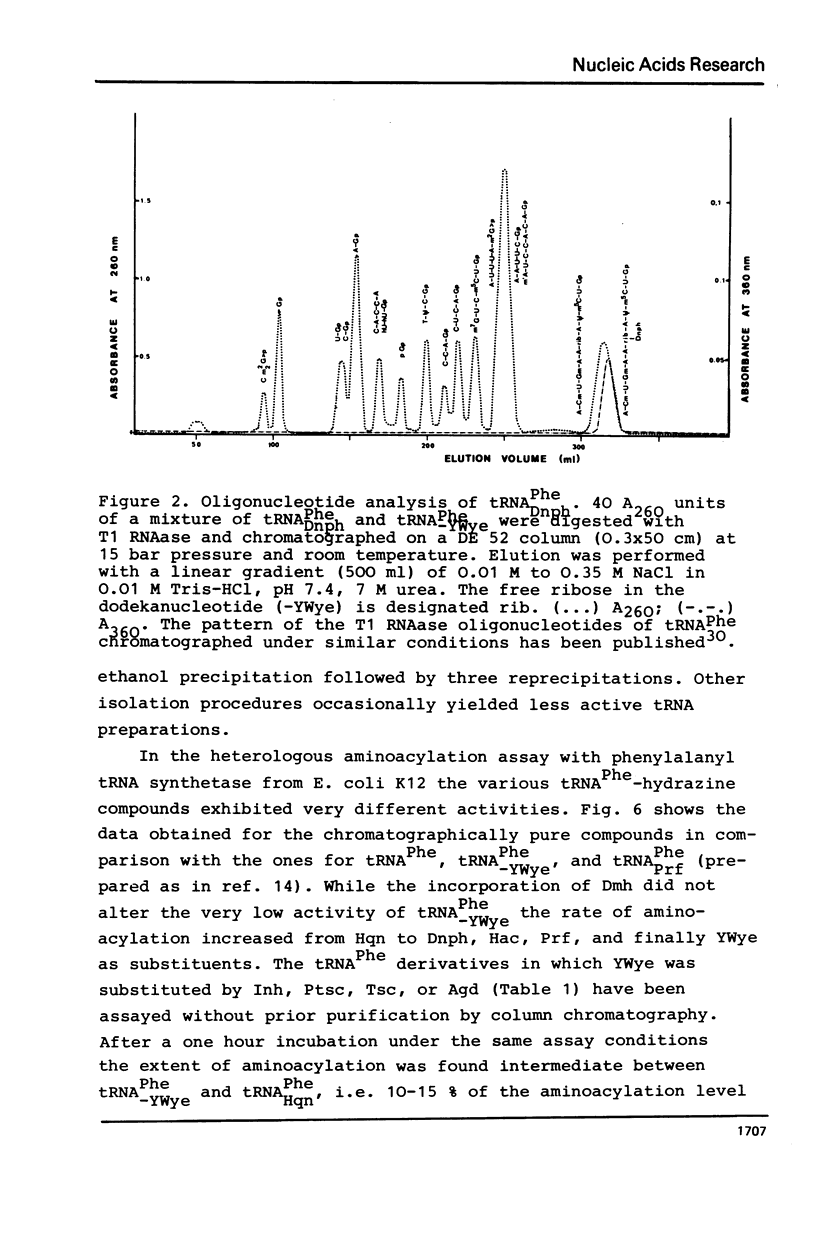
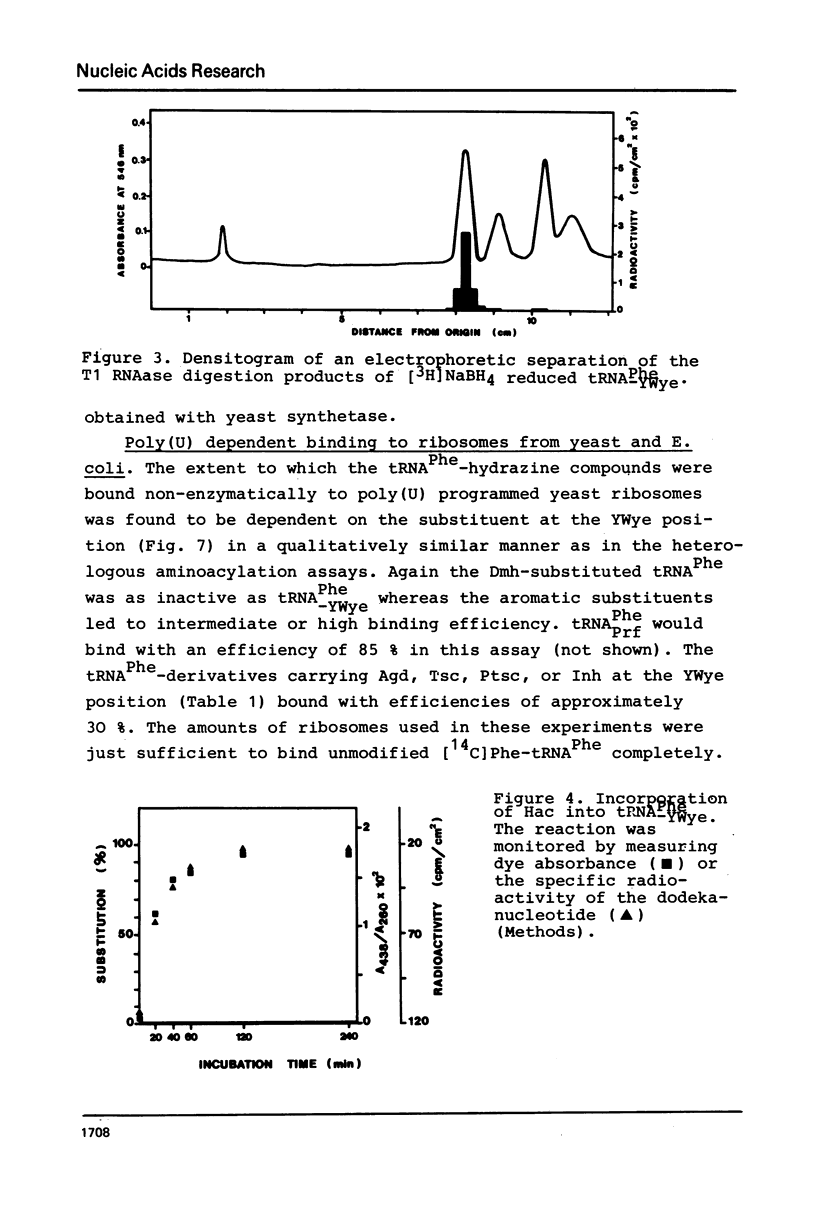
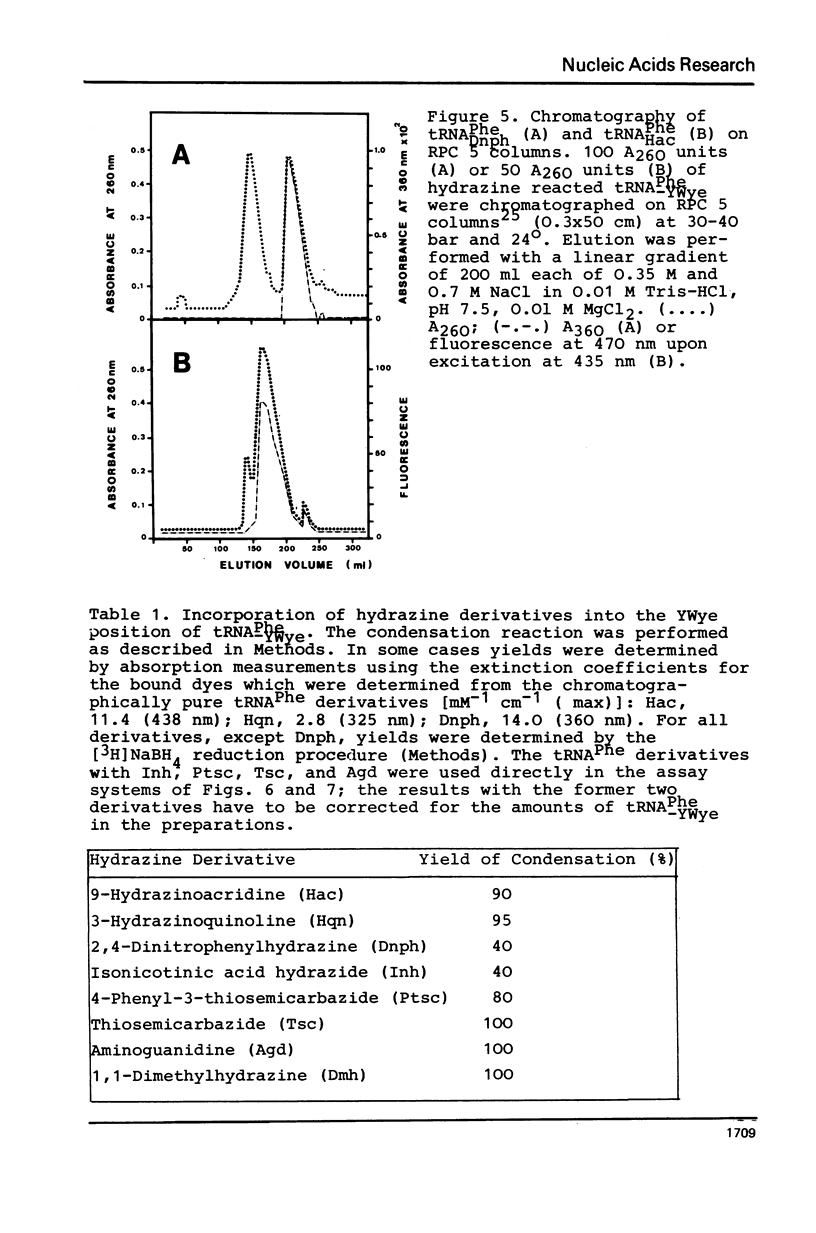
The highly modified base wybutine (YWye) next to the anticodon of yeast tRNAPhe has been replaced by different hydrazine derivatives. The effect of the replacement on the activity of the tRNA has been studied in the heterologous aminoacylation with synthetase from E. coli and in the poly(U) directed binding to ribosomes from both yeast and E. coli. It was found that starting from tRNA-PheYWye the activity increased with increasing size, aromaticity, and stacking tendency of the substituent replacing YWye. It is concluded that YWye by the size of its aromatic system and by its stacking properties is particularly well suited for stabilizing the native conformation of tRNAPhe.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron V., Uhlenbeck O. C. Removal of Y-37 from tRNA phe yeast alters oligomer binding to two loops. Biochem Biophys Res Commun. 1973 Feb 5;50(3):635–640. doi: 10.1016/0006-291x(73)91291-6. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Feuer B., Yamane T. Luminescence and binding studies on tRNA-Phe. Proc Natl Acad Sci U S A. 1970 Mar;65(3):638–644. doi: 10.1073/pnas.65.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M. Y. Minor components in transfer RNA: the location-function relationships. Prog Biophys Mol Biol. 1977;32(1):83–102. [PubMed] [Google Scholar]
- Freist W., Maelicke A., Sprinz M., Cramer F. Incorporation of 3-methyl-2-benzothiazolone hydrazone into the anticodon loop of tRNA Phe from yeast. Eur J Biochem. 1972 Dec 4;31(2):215–220. doi: 10.1111/j.1432-1033.1972.tb02521.x. [DOI] [PubMed] [Google Scholar]
- Hanke T., Bartmann P., Hennecke H., Kosakowski H. M., Jaenicke R., Holler E., Böck A. L-phenylalanyl-tRNA synthetase of Escherichia coli K-10. A reinvestigation of molecular weight and subunit structure. Eur J Biochem. 1974 Apr 16;43(3):601–607. doi: 10.1111/j.1432-1033.1974.tb03447.x. [DOI] [PubMed] [Google Scholar]
- Hirsch R., Zachau H. G. Isolierung und Charakterisierung der Seryl- und Phenylalanyl-tRNA-Synthetase aus Hefe. Hoppe Seylers Z Physiol Chem. 1976 Apr;357(4):509–526. [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Rhodes D., Brown R. S., Klug A. A crystallographic study of metal-binding to yeast phenylalanine transfer RNA. J Mol Biol. 1977 Apr 15;111(3):315–328. doi: 10.1016/s0022-2836(77)80054-5. [DOI] [PubMed] [Google Scholar]
- Kan L. S., Ts'o P. O., von der Haar F., Sprinzl M., Cramer F. Proton magnetic resonance studies on the conformation of the hexanucleotide, GmpApApYpApsiP, and Related fragments from the anticodong loop of baker's yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1975 Jul 15;14(14):3278–3291. doi: 10.1021/bi00685a038. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Nakanishi K., Furutachi N., Funamizu M., Grunberger D., Weinstein I. B. Structure of the fluorescent Y base from yeast phenylalanine transfer ribonucleic acid. J Am Chem Soc. 1970 Dec 30;92(26):7617–7619. doi: 10.1021/ja00729a035. [DOI] [PubMed] [Google Scholar]
- Odom O. W., Hardesty B., Wintermeyer W., Zachau H. G. Efficient polyphenylalanine synthesis with proflavine and ethidium labeled tRNA-Phe from yeast in the reticulocyte ribosomal system. Biochim Biophys Acta. 1975 Jan 6;378(1):159–163. doi: 10.1016/0005-2787(75)90147-1. [DOI] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Zachau G. Partial degradation of transfer RNAs and transfer RNA fragments by spleen phosphodiesterase as studied by disc electrophoretic methods. Biochim Biophys Acta. 1972 Sep 14;277(3):523–538. doi: 10.1016/0005-2787(72)90095-0. [DOI] [PubMed] [Google Scholar]
- Pongs O., Reinwald E. Function of Y in codon-anticodon interaction of tRNA Phe . Biochem Biophys Res Commun. 1973 Jan 23;50(2):357–363. doi: 10.1016/0006-291x(73)90848-6. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Rich A. Structural domains of transfer RNA molecules. Science. 1976 Nov 19;194(4267):796–806. doi: 10.1126/science.790568. [DOI] [PubMed] [Google Scholar]
- RajBhandary U. L., Stuart A., Chang S. H. Studies on polynucleotides. LXXX. Yeast phenylalanine transfer ribonucleic acid: products obtained by degradation with ribonuclease T1. J Biol Chem. 1968 Feb 10;243(3):584–591. [PubMed] [Google Scholar]
- Reines S. A., Cantor C. R. New fluorescent hydrazide reagents for the oxidized 3'-terminus of RNA. Nucleic Acids Res. 1974 Jun;1(6):767–786. doi: 10.1093/nar/1.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. M., Kahan M., Wintermeyer W., Zachau H. G. Interactions of yeast tRNAPhe with ribosomes from yeast and Escherichia coli. A fluorescence spectroscopic study. Eur J Biochem. 1977 Jan 3;72(1):117–125. doi: 10.1111/j.1432-1033.1977.tb11231.x. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Gassen H. G. Codon-dependent rearrangement of the tertiary structure of tRNAPhe from yeast. FEBS Lett. 1977 Jun 15;78(2):267–270. doi: 10.1016/0014-5793(77)80320-7. [DOI] [PubMed] [Google Scholar]
- Thiebe R., Zachau H. G. A specific modification next to the anticodon of phenylalanine transfer ribonucleic acid. Eur J Biochem. 1968 Sep 24;5(4):546–555. doi: 10.1111/j.1432-1033.1968.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Thiebe R., Zachau H. G., Baczynskyj L., Biemann K., Sonnenbichler J. Study on the properties and structure of the modified base Y+ of yeast tRNA Phe . Biochim Biophys Acta. 1971 Jun 30;240(2):163–169. doi: 10.1016/0005-2787(71)90653-8. [DOI] [PubMed] [Google Scholar]
- Thiebe R., Zachau H. G. Further studies on amino acid acceptance and physical properties of tRNA-phe-yeast. Biochim Biophys Acta. 1970 Oct 15;217(2):294–304. [PubMed] [Google Scholar]
- Wintermeyer W., Thiebe R., Zachau H. G. Amin-katalysierte Spaltung von Phenylalanin-spezifischer tRNA nach Baseneliminierung. Hoppe Seylers Z Physiol Chem. 1972 Oct;353(10):1625–1632. [PubMed] [Google Scholar]
- Wintermeyer W., Zachau H. G. Replacement of Y base, dihydrouracil, and 7-methylguanine in tRNA by artificial odd bases. FEBS Lett. 1971 Nov 1;18(2):214–218. doi: 10.1016/0014-5793(71)80447-7. [DOI] [PubMed] [Google Scholar]
- Wintermeyer W., Zachau H. G. Tertiary structure interactions of 7-methylguanosine in yeast tRNA Phe as studied by borohydride reduction. FEBS Lett. 1975 Oct 15;58(1):306–309. doi: 10.1016/0014-5793(75)80285-7. [DOI] [PubMed] [Google Scholar]
- Wong K. L., Kearns D. R., Wintermeyer W., Zachau H. G. NMR investigation of the effect of selective modifications in the anticodon loop on the conformation of yeast transfer RNA-Phe. Biochim Biophys Acta. 1975 Jun 2;395(1):1–4. doi: 10.1016/0005-2787(75)90227-0. [DOI] [PubMed] [Google Scholar]
- Yang C. H., Söll D. Studies of transfer RNA tertiary structure of singlet-singlet energy transfer. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2838–2842. doi: 10.1073/pnas.71.7.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K., Turner D. H., Tinoco I., Jr The kinetics of codon-anticodon interaction in yeast phenylalanine transfer RNA. J Mol Biol. 1975 Dec 25;99(4):507–518. doi: 10.1016/s0022-2836(75)80169-0. [DOI] [PubMed] [Google Scholar]


