Abstract
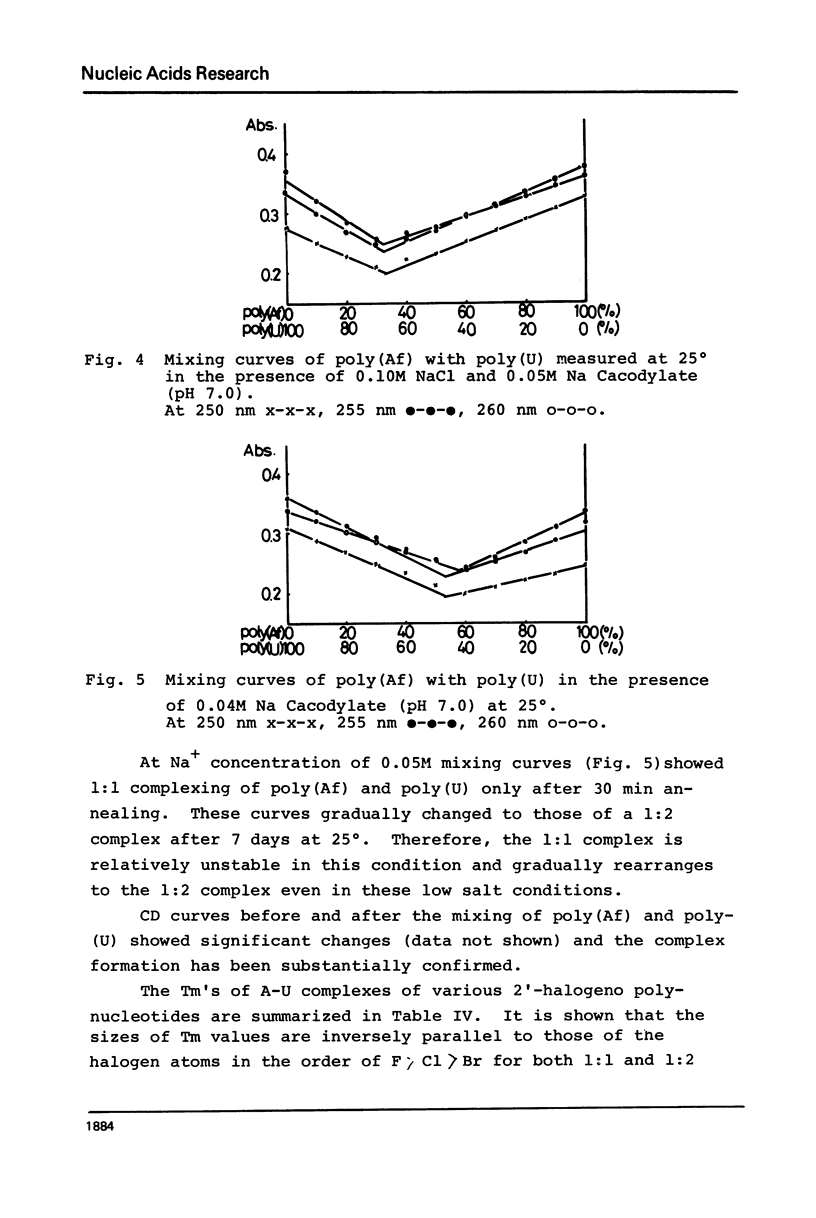
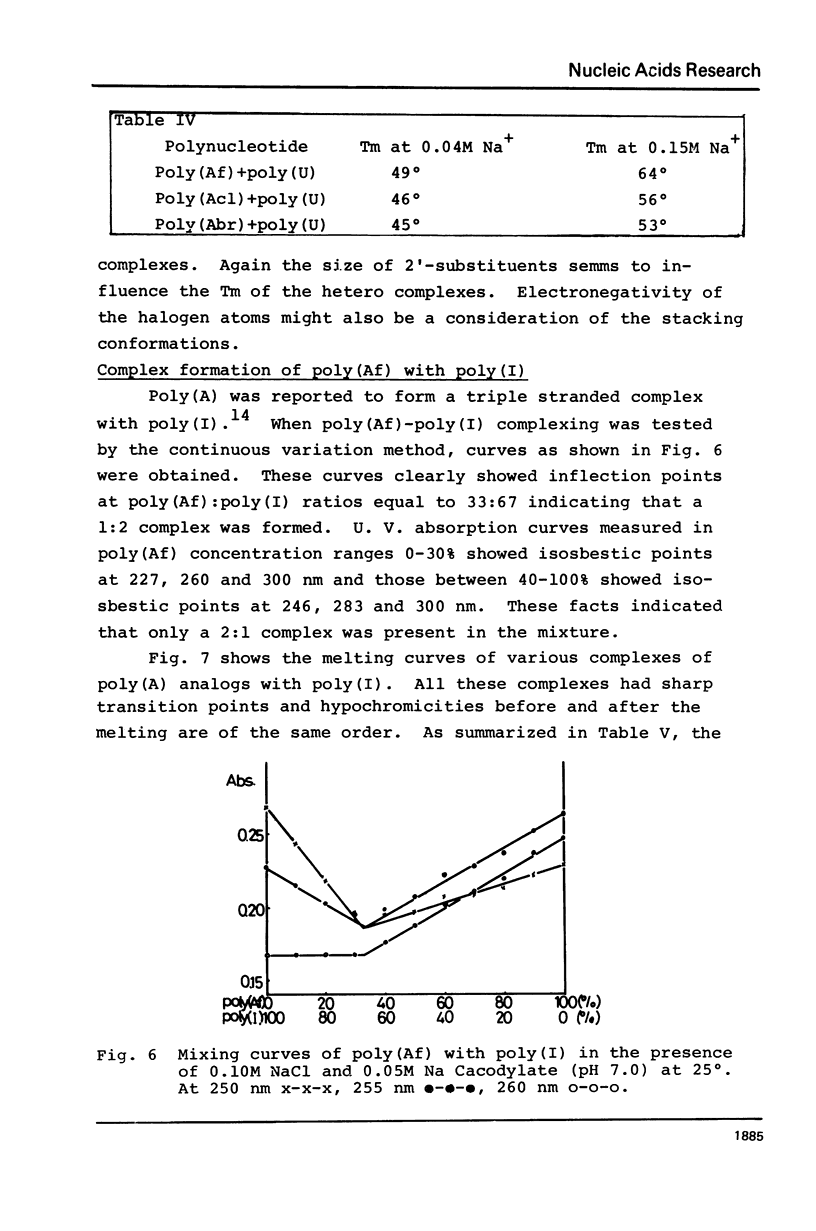
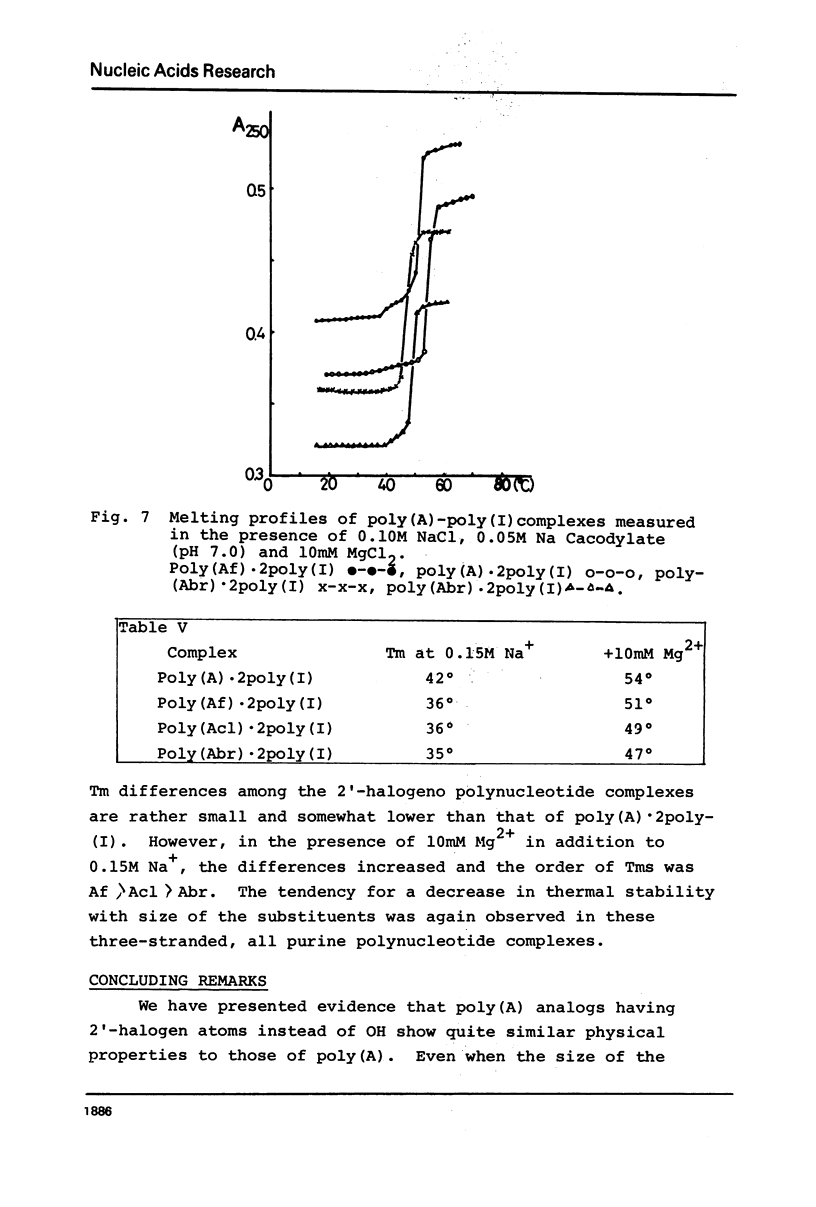
2′-Deoxy-2′-fluoroadenosine was chemically transformed to its 5′-diphosphate and polymerized with polynucleotide phosphorylase to give poly(2′-deoxy-2′-fluoroadenylic acid) [poly(Af)]. Polymerization proceeded smoothly as in the case of poly(A) and the yield of the polymerization was 55%. The UV absorption spectra of poly(Af) closely resembled those of poly(A) and the hypochromicity was 32% at pH 7.0. The CD profile at 25° and neutrality showed similar pattern to that of other poly(2′-deoxy-2′-halogenoadenylic acids) with somewhat larger [θ] values both in the positive and negative maxima. Acid titration of poly(Af) showed a transition point at pH 5.2 and the Tm of the acid form was 37° which was significantly lower than that of poly(A), but similar to that of poly(2′-azido-2′-deoxyadenylic acid). Poly(Af) formed 1:1 and 1:2 complexes with poly-(U) having Tm of 49° and 62° at 0.04M and 0.15M Na+ concentration, respectively. Poly(Af) also formed a 1:2 complex with poly(I) and its Tm was 36° at 0.05M Na+ concentration. These data showed that poly(Af) has rather similar properties to those of poly(A), but not to poly(dA).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton P. H., Kearns D. R. Hydrogen bonding of the 2' OH in RNA. Biochim Biophys Acta. 1978 Feb 16;517(2):329–337. doi: 10.1016/0005-2787(78)90199-5. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Singer M. F. Deoxyadenosine diphosphate as a substrate and inhibitor of polynucleotide phosphorylase of Micrococcus luteus. I. Deoxyadenosine diphosphate as a substrate for polymerization and the exchange reaction with inorganic 32 P. J Biol Chem. 1971 Dec 25;246(24):7486–7496. [PubMed] [Google Scholar]
- Fukui T., Kakiuchi N., Ikehara M. Polynucleotides. XLV Synthesis and properties of poly(2'-azido-2'-deoxyinosinic acid). Nucleic Acids Res. 1977 Aug;4(8):2629–2639. doi: 10.1093/nar/4.8.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara M., Fukui T., Kakiuchi N. Polynucleotides. L. Synthesis and properties of poly (2'-chloro-2'-deoxyadenylic acid) and poly (2'-bromo-2'-deoxyadenylic acid). Nucleic Acids Res. 1977 Dec;4(12):4249–4260. doi: 10.1093/nar/4.12.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara M., Fukui T., Kakiuchi N. Polynucleotides. XL. Synthesis and properties of poly 2'-azido-2'-deoxyadenylic acid. Nucleic Acids Res. 1976 Aug;3(8):2089–2099. doi: 10.1093/nar/3.8.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICH A., DAVIES D. R., CRICK F. H., WATSON J. D. The molecular structure of polyadenylic acid. J Mol Biol. 1961 Feb;3:71–86. doi: 10.1016/s0022-2836(61)80009-0. [DOI] [PubMed] [Google Scholar]


