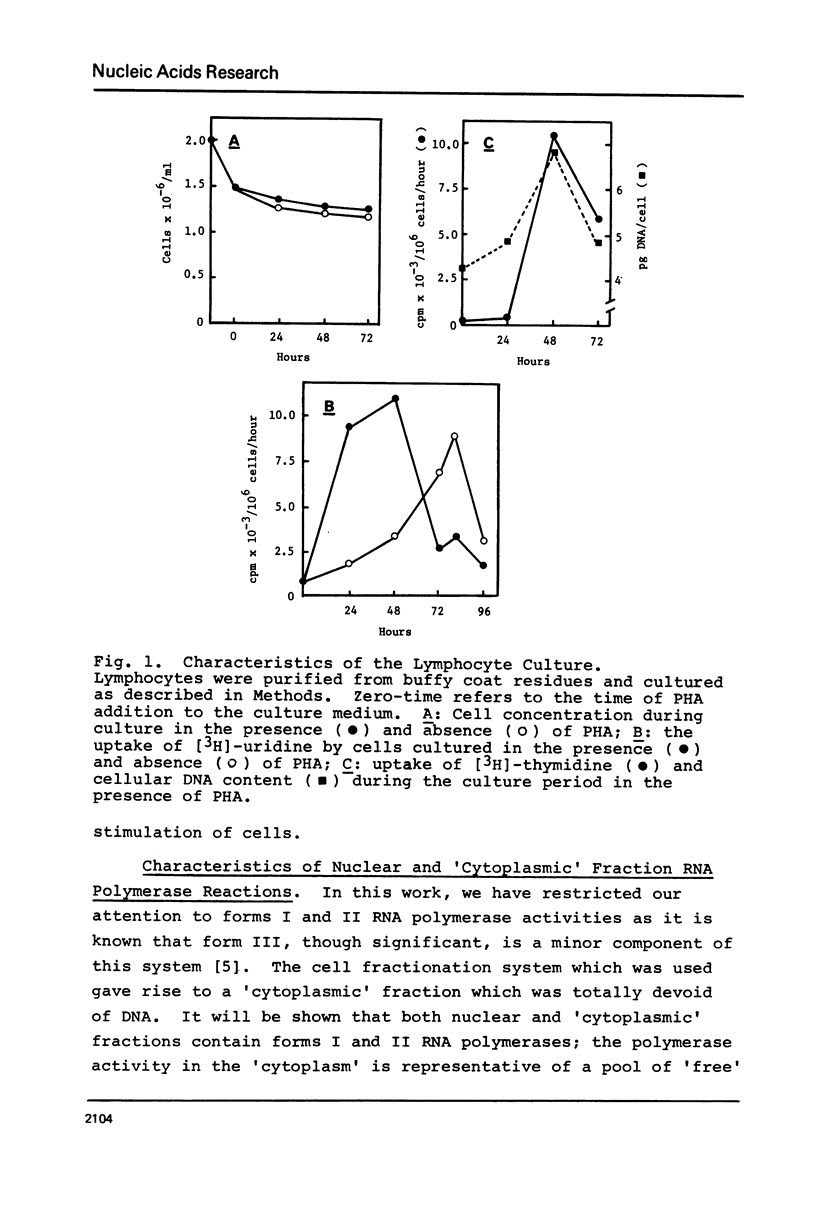
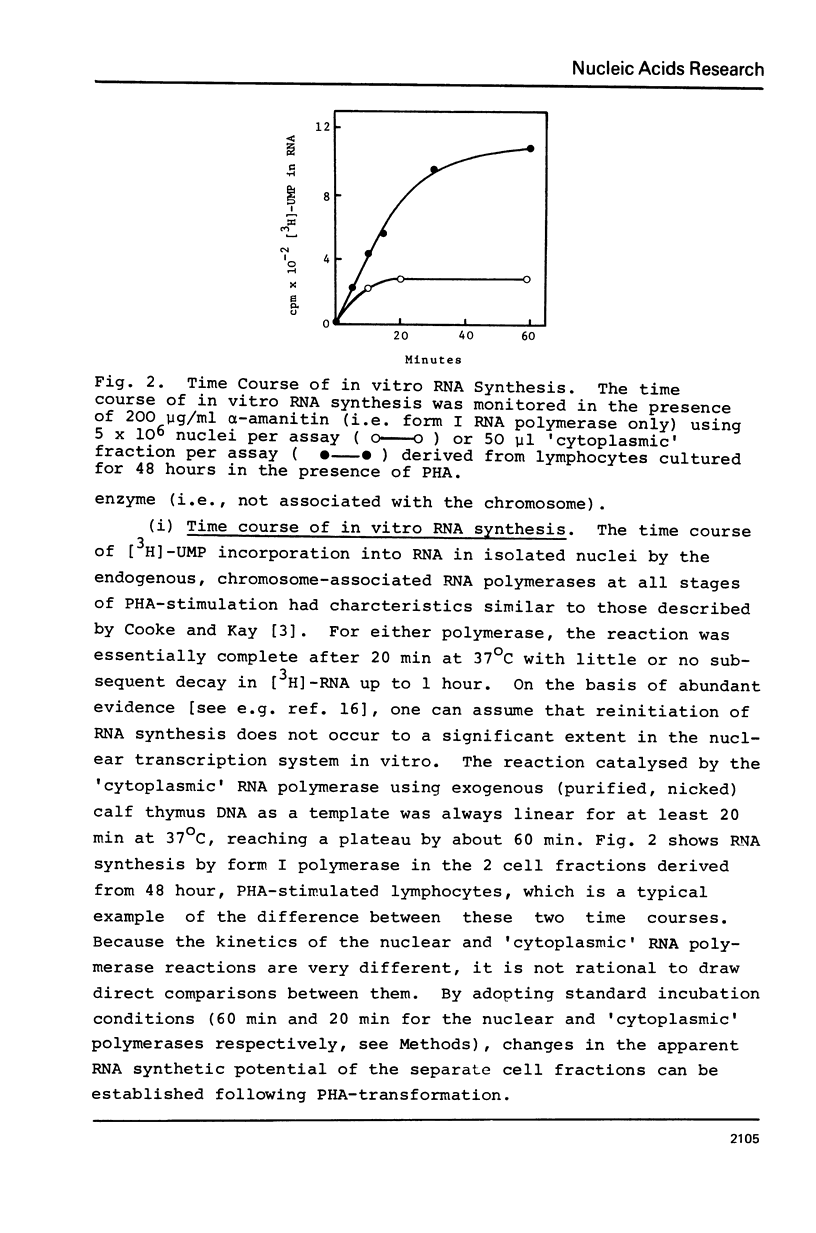
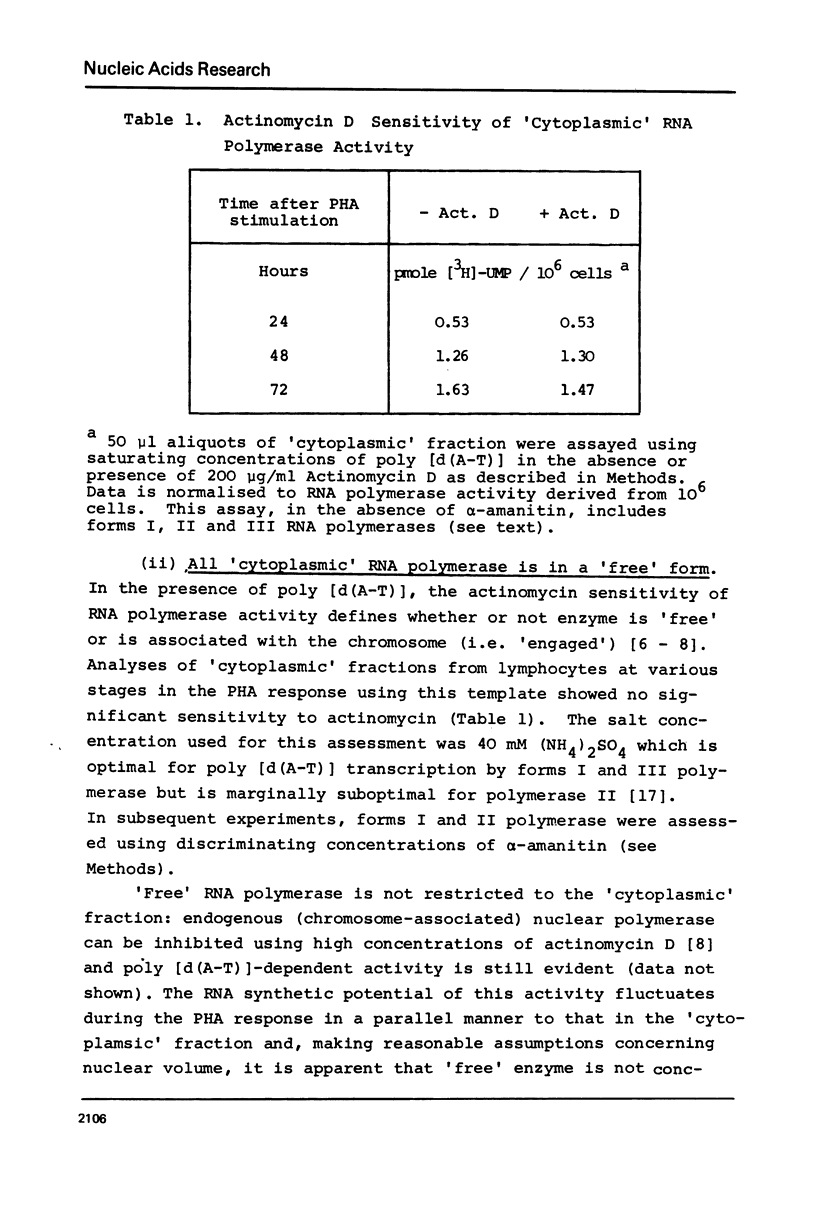
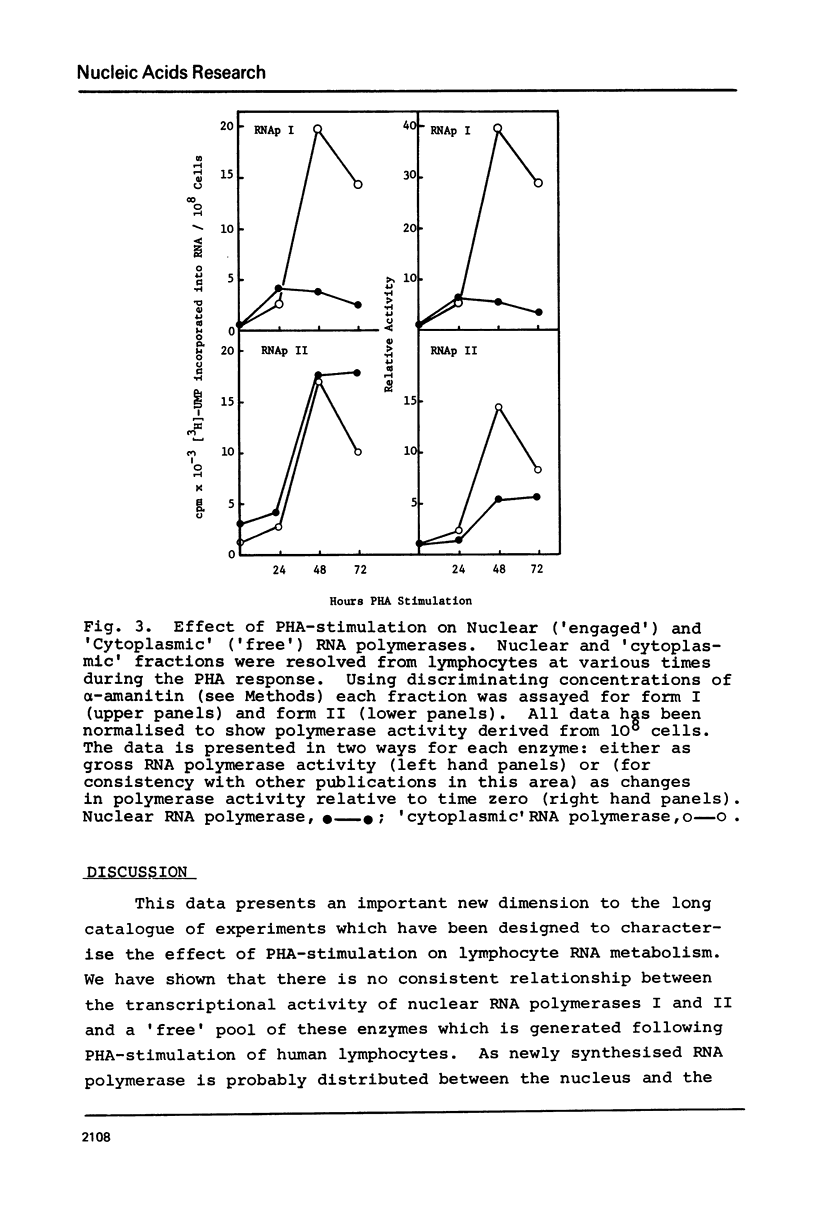
Abstract
Following PHA-stimulation of lymphocytes in culture, it is known that nuclear RNA synthesis and the amount of extractable RNA polymerase activity rise in these cells. The relationship between these two phenomena has been examined. Using an in vitro assay system which discriminates between polymerase activity which is "engaged" in nuclear RNA synthesis and a pool of "free" enzyme, the data suggest that the factors regulating the interaction between these two pools of enzyme activity are different for forms I and II RNA polymerases.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Coutinho A., Melchers F., Watanabe T. Growth and maturation of single clones of normal murine T and B lymphocytes in vitro. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):227–236. doi: 10.1101/sqb.1977.041.01.028. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Franze-Fernández M. T. Ehrlich ascites cells DNA-dependent RNA polymerases: effect of amino acids and protein synthesis inhibition. FEBS Lett. 1974 Apr 15;41(1):161–165. doi: 10.1016/0014-5793(74)80978-6. [DOI] [PubMed] [Google Scholar]
- Chambon P. Eukaryotic nuclear RNA polymerases. Annu Rev Biochem. 1975;44:613–638. doi: 10.1146/annurev.bi.44.070175.003145. [DOI] [PubMed] [Google Scholar]
- Chesterton C. J., Butterworth P. H. Selective extraction of form I DNA dependent RNA polymerase from rat liver nuclei and its separation into two species. Eur J Biochem. 1971 Mar 11;19(2):232–241. doi: 10.1111/j.1432-1033.1971.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Cooke A., Brown M. Stimulation of the activities of solubilized pig lymphocyte RNA polymerases by phytohaemagglutinin. Biochem Biophys Res Commun. 1973 Apr 16;51(4):1042–1047. doi: 10.1016/0006-291x(73)90032-6. [DOI] [PubMed] [Google Scholar]
- Cooke A., Kay J. E. Effect of phytohaemagglutinin on the nuclear RNA polymerase activity of human lymphocytes. Exp Cell Res. 1973 Apr;79(1):179–185. [PubMed] [Google Scholar]
- Cooper H. L. Ribosomal ribonucleic acid wastage in resting and growing lymphocytes. J Biol Chem. 1969 Oct 25;244(20):5590–5596. [PubMed] [Google Scholar]
- Cooper H. L. Ribsomal ribonucleic acid production and growth regulation in human lymphocytes. J Biol Chem. 1969 Apr 10;244(7):1946–1952. [PubMed] [Google Scholar]
- Dauphinais C., Waithe W. I. Phytohaemagglutinin stimulation of human lymphocytes during amino-acid deprivation. RNA polymerase I activity of isolated nuclei. Eur J Biochem. 1977 Aug 15;78(1):189–194. doi: 10.1111/j.1432-1033.1977.tb11729.x. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Soreq H., Aviv H. Initiation of RNA synthesis in isolated nuclei. Eur J Biochem. 1977 Jul 15;77(2):393–400. doi: 10.1111/j.1432-1033.1977.tb11679.x. [DOI] [PubMed] [Google Scholar]
- Gross K. J., Pogo A. O. Control mechanism of ribonucleic acid synthesis in eukaryotes. The effect of amino acid and glucose starvation and cycloheximide on yeast deoxyribonucleic acid-dependent ribonucleic acid polymerases. J Biol Chem. 1974 Jan 25;249(2):568–576. [PubMed] [Google Scholar]
- Jaehning J. A., Stewart C. C., Roeder R. G. DNA-dependent RNA polymerase levels during the response of human peripheral lymphocytes to phytohemagglutinin. Cell. 1975 Jan;4(1):51–57. doi: 10.1016/0092-8674(75)90133-6. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Ahern T., Lindsay V. J., Sampson J. The control of protein synthesis during the stimulation of lymphocytes by phytohaemagglutinin. III. Poly(U) translation and the rate of polypeptide chain elongation. Biochim Biophys Acta. 1975 Jan 20;378(2):241–250. doi: 10.1016/0005-2787(75)90112-4. [DOI] [PubMed] [Google Scholar]
- Kellas B. L., Austoker J. L., Beebee T. J., Butterworth P. H. Forms AI and AII DNA-dependent RNA polymerases as components of two defined pools of polymerase activity in mammalian cells. Eur J Biochem. 1977 Feb;72(3):583–594. doi: 10.1111/j.1432-1033.1977.tb11281.x. [DOI] [PubMed] [Google Scholar]
- Lampert A., Feigelson P. A short lived polypeptide component of one of two discrete functional pools of hepatic nuclear alpha-amanitin resistant RNA polymerases. Biochem Biophys Res Commun. 1974 Jun 18;58(4):1030–1038. doi: 10.1016/s0006-291x(74)80247-0. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Mingari M. C., Moretta A., Webb S. R. Subpopulations of human T cells identified by receptors for immunoglobulins and mitogen responsiveness. J Immunol. 1976 Dec;117(6):2171–2174. [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G. Early events in lymphocyte transformation by phytohemagglutinin. I. DNA-dependent RNA polymerase activities in isolated lymphocyte nuclei. J Cell Biol. 1972 Jun;53(3):635–641. doi: 10.1083/jcb.53.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder R. G. Multiple forms of deoxyribonucleic acid-dependent ribonucleic acid polymerase in Xenopus laevis. Levels of activity during oocyte and embryonic development. J Biol Chem. 1974 Jan 10;249(1):249–256. [PubMed] [Google Scholar]
- Schwartz L. B., Sklar V. E., Jaehning J. A., Weinmann R., Roeder R. G. Isolation and partial characterization of the multiple forms of deoxyribonucleic acid-dependent ribonucleic acid polymerase in the mouse myeloma, MOPC 315. J Biol Chem. 1974 Sep 25;249(18):5889–5897. [PubMed] [Google Scholar]
- Somers D. G., Pearson M. L., Ingles C. J. Regulation of RNA polymerase II activity in a mutant rat myoblast cell line resistant to alpha-amanitin. Nature. 1975 Jan 31;253(5490):372–374. doi: 10.1038/253372a0. [DOI] [PubMed] [Google Scholar]
- Stewart C. C., Cramer S. F., Steward P. G. The response of human peripheral blood lymphocytes to phytohemagglutinin: determination of cell numbers. Cell Immunol. 1975 Apr;16(2):237–250. doi: 10.1016/0008-8749(75)90115-x. [DOI] [PubMed] [Google Scholar]
- Wolff J. S., 3rd, Langstaff J. A., Weinberg G., Abell C. W. DNA dependent RNA polymerase activity of nuclei isolated from human peripheral blood lymphocytes. Biochem Biophys Res Commun. 1967 Feb 8;26(3):366–371. doi: 10.1016/0006-291x(67)90133-7. [DOI] [PubMed] [Google Scholar]
- Yu F. L. An improved method for the quantitative isolation of rat liver nuclear RNA polymerases. Biochim Biophys Acta. 1975 Jul 7;395(3):329–336. doi: 10.1016/0005-2787(75)90204-x. [DOI] [PubMed] [Google Scholar]
- Yu F. L. Mechanism of aflatoxin B1 inhibition of rat hepatic nuclear RNA synthesis. J Biol Chem. 1977 May 25;252(10):3245–3251. [PubMed] [Google Scholar]
- Yu F. L. Two functional states of the RNA polymerases in the rat hepatic nuclear and nucleolar fractions. Nature. 1974 Sep 27;251(5473):344–346. doi: 10.1038/251344a0. [DOI] [PubMed] [Google Scholar]


