Abstract
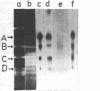
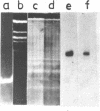
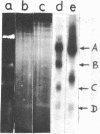
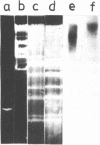
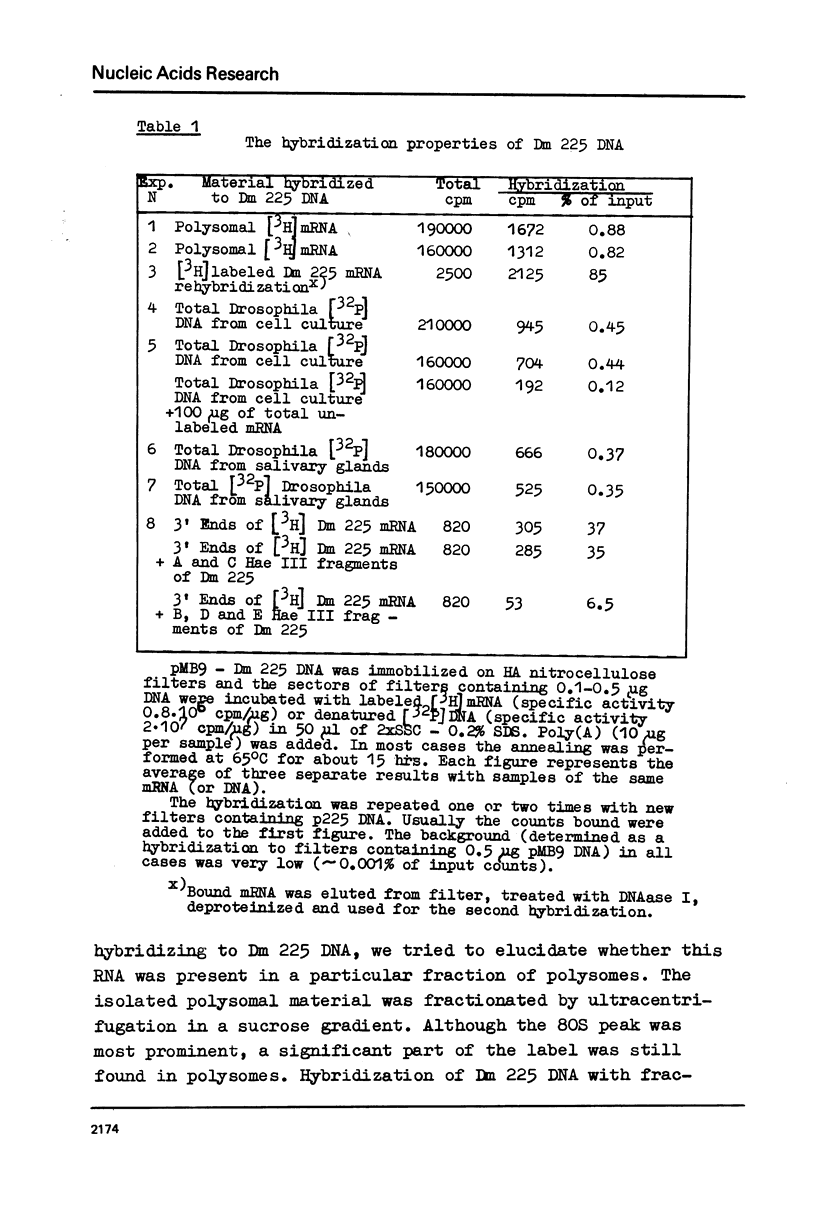
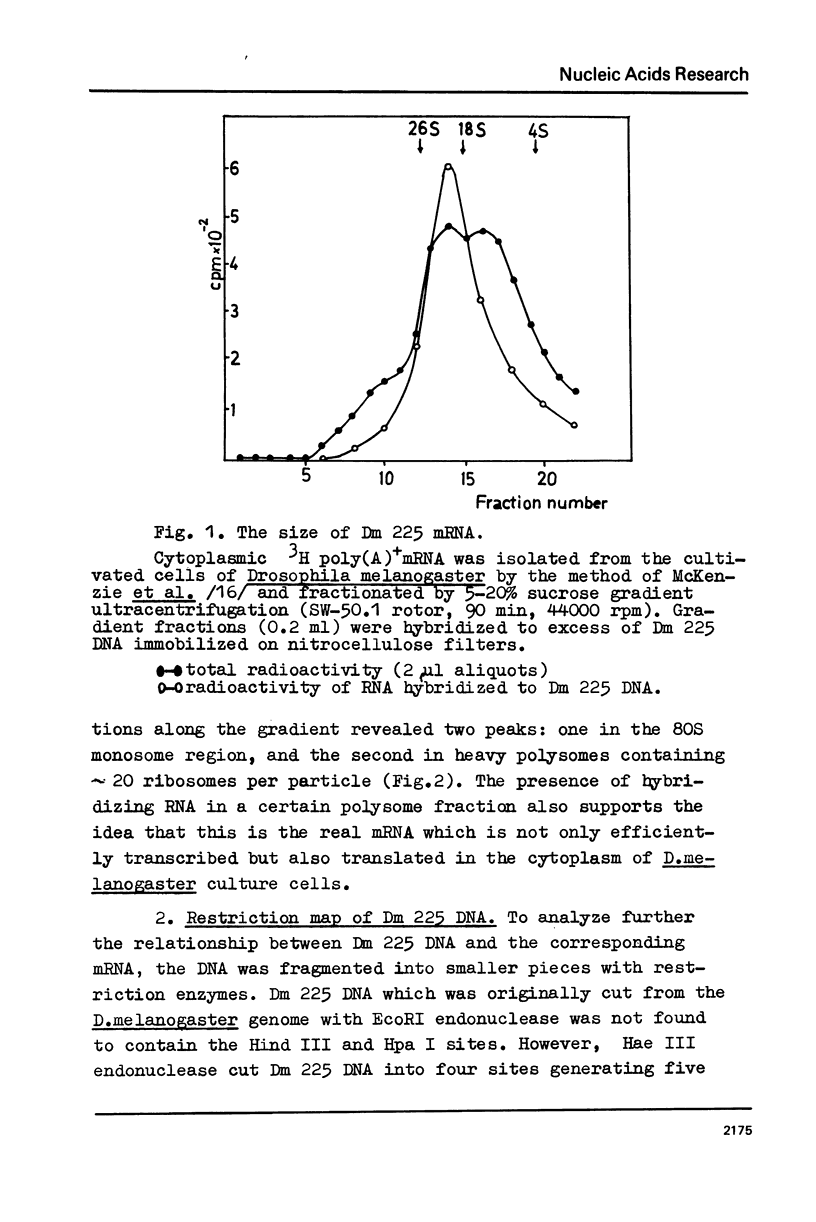
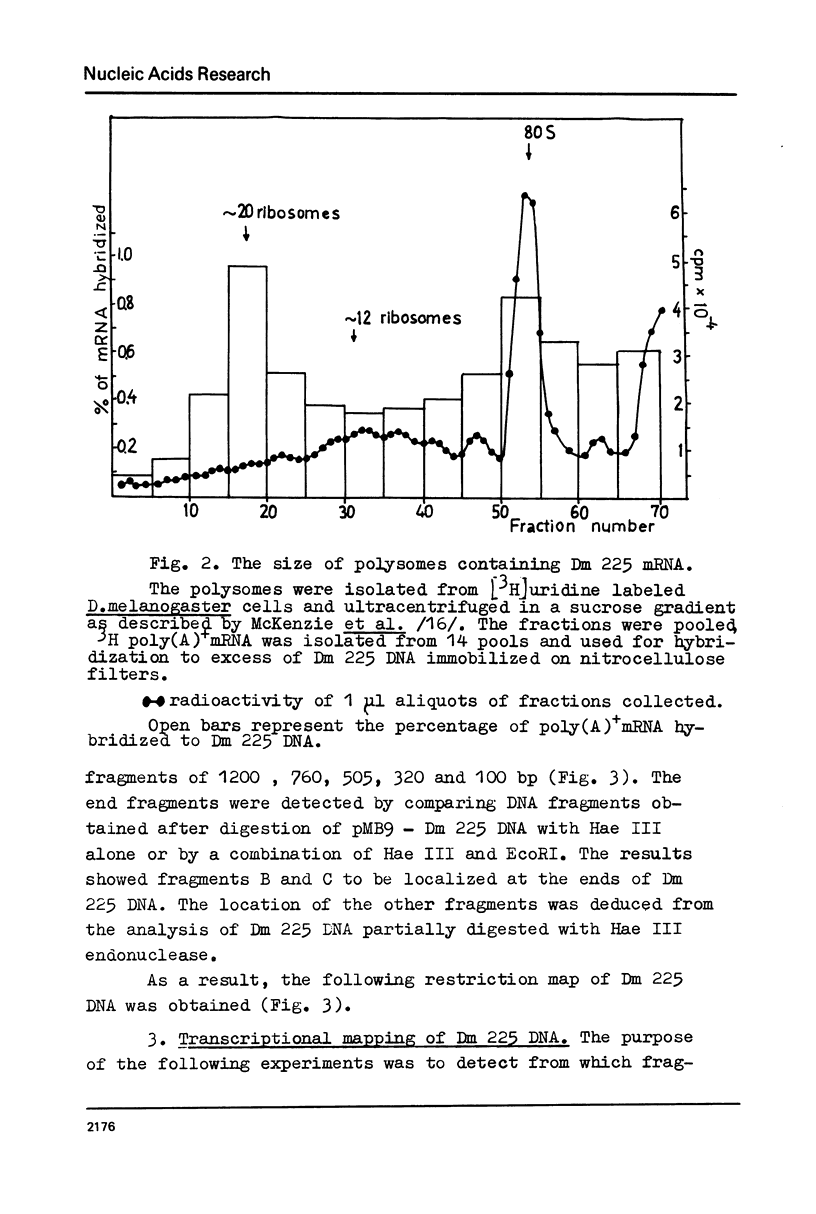
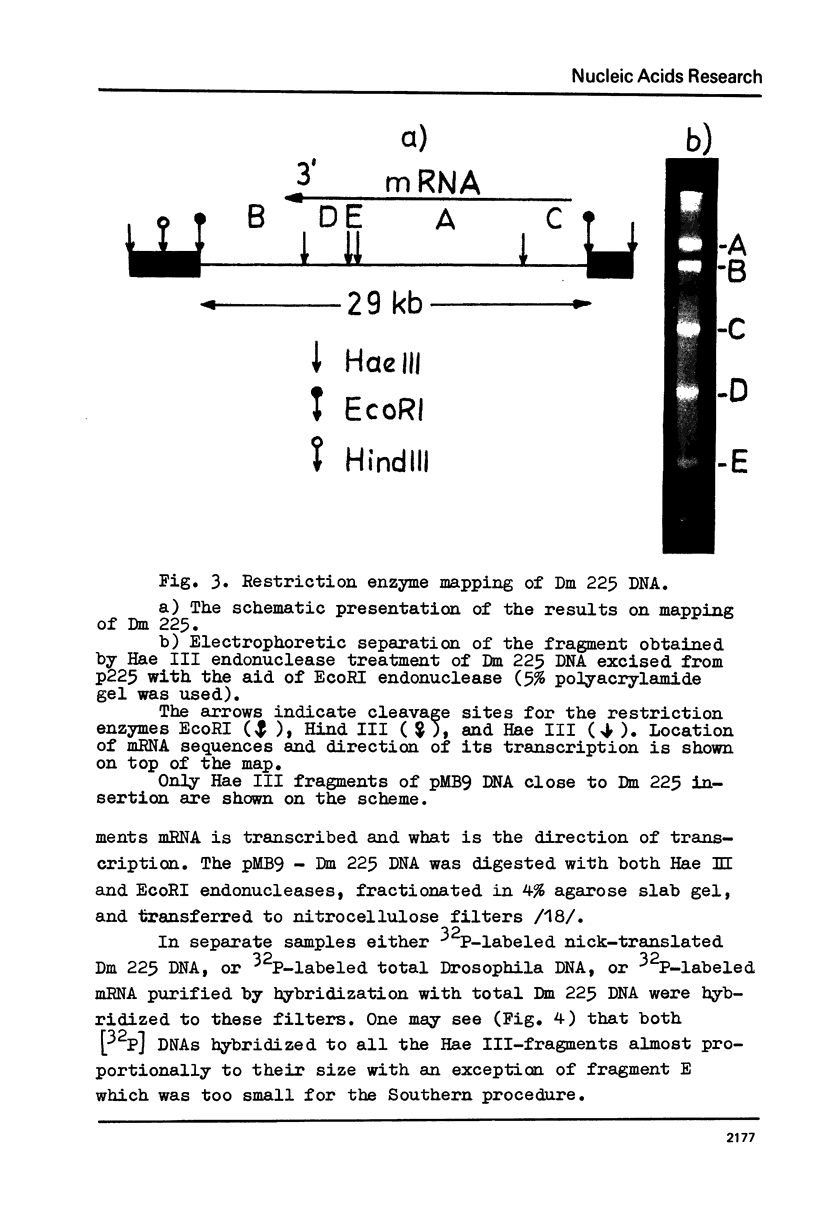
The properties of Dm 225 DNA, a fragment of D.melanogaster genome 2.9 kb in length excised by EcoRI endonuclease and cloned in the λ gt phage or pMB9 plasmid, are described. The DNA hybridizes to a significant portion (0.8%) of total polysomal poly(A)+RNA (mRNA). The size of the hybridizing mRNA is about 2.3 kb (19S); it is present in the fraction of heavy polysomes. Dm 225 DNA fragments obtained with the aid of Hae III endonuclease have been mapped. mRNA hybridizes with all the fragments. In one of the end fragments, the 3′-end of mRNA has been localized and thus the direction of transcription determined. About 250 copies of the gene Dm 225 are present in the haploid genome of D.melanogaster, and all of them have the same size upon restriction with EcoRI endonuclease. On the other hand, the sequences of the genome adjacent to Dm 225 DNA are different and may vary from one cell line to another as evidenced by experiments in which the D.melanogaster DNA was restricted by Hind III endonuclease. In combination with in situ hybridization data /1,2/ the results obtained in this paper demonstrate that the structural gene present in Dm 225 DNA is a representative of a multiple gene family dispersed throughout the whole genome of D.melanogaster.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beatriz L. W., McCarthy B. J. Messenger RNA complexity in Drosophila melanogaster. Biochemistry. 1975 Jun 3;14(11):2440–2446. doi: 10.1021/bi00682a026. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P., Ilyin Y. V., Ryskov A. P., Tchurikov N. A., Yenikolopov G. N., Gvozdev V. A., Ananiev E. V. Isolation of eukaryotic DNA fragments containing structural genes and the adjacent sequences. Science. 1977 Jan 28;195(4276):394–397. doi: 10.1126/science.401545. [DOI] [PubMed] [Google Scholar]
- Locker D., Marrakechi M. Evidence for an excess of rDNA in the testis of Drosophila melanogaster during rDNA magnification. Mol Gen Genet. 1977 Sep 9;154(3):249–254. doi: 10.1007/BF00571279. [DOI] [PubMed] [Google Scholar]
- McKenzie S. L., Henikoff S., Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1117–1121. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., van de Sande J. H., Loewen P. C., Khorana H. G., Raae A. J., Lillehaug J. R., Kleppe K. Physical characterization and simultaneous purification of bacteriophage T4 induced polynucleotide kinase, polynucleotide ligase, and deoxyribonucleic acid polymerase. Biochemistry. 1973 Dec 4;12(25):5045–5050. doi: 10.1021/bi00749a003. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]