Abstract
We previously reported the first detection of simian picobirnaviruses (PBVs) by polyacrylamide gel electrophoresis in fecal specimens of two monkeys with diarrhea in China. We now report the detection of genogroup I PBVs in 48% (44/92) of the fecal specimens by reverse transcriptase PCR (RT-PCR) and amplicon sequencing using primers specific for the RNA-dependent RNA polymerase (RDRP) gene. Molecular characterization of these 44 strains demonstrated both sequence conservation and diversity among simian PBVs and among simian, porcine, and human PBVs. We further determined full-length sequences of segment 2 of the two simian PBV strains, monkey/CHN-14/2002 and monkey/CHN-49/2002, and demonstrated 52.5% to 54.2% nucleotide sequence similarity to the corresponding gene of the bovine strain RUBV and the prototype human strain 1-CHN-97 of genogroup I PBVs and an even lower similarity (38.4%) to segment 2 of the prototype human genogroup II strain 4-GA-91. Further studies are needed to investigate the epidemiology and pathogenesis of PBVs in animals and humans.
TEXT
Picobirnaviruses (PBVs) belong to the family Picobirnaviridae. They are small, nonenveloped viruses that are 35 nm in diameter and consist of a simple capsid with an indistinct surface structure (14). PBVs possess a bisegmented double-stranded RNA (dsRNA) genome. The large genome segment (segment 1) is 2.3 to 2.6 kb in size and encodes the capsid protein and a polypeptide of unknown function. The small genome segment (segment 2) is 1.5 to 1.9 kb and encodes the viral RNA-dependent RNA polymerase (RDRP). Based on the sequences of the small segment, PBVs are classified into genogroup I (prototype strain, 1-CHN-97) and II (prototype strain, 4-GA-91) (1, 15).
PBVs were first detected in fecal specimens from humans and rats in 1988 (13, 14). Subsequently, the viruses have been detected in fecal samples from pigs (4, 11), calves (19), foals (3), rabbits (10), giant anteaters (6), birds (9), dogs and snakes (5), and monkeys (20). Since PBVs have been found in humans and animals with diarrhea and in healthy people and animals (12, 21) and in samples with or without the presence of other known enteric pathogens, the role of PBVs in gastroenteritis has not been established. PBVs have been commonly identified by the characteristic migration profiles of the bisegmented dsRNA by the use of polyacrylamide gel electrophoresis (PAGE) and silver staining. However, this method is relatively insensitive and time-consuming. The availability of a reverse transcriptase PCR (RT-PCR) greatly improved the sensitivity of PBV detection using two pairs of primers specific to segment 2 sequences (15). Amplification with these primers gave an amplicon of 201 to 207 bp for genogroup I and ∼369 bp for genogroup II PBVs. Sequence analysis of the shorter amplicons has enabled us to perform molecular characterization and demonstrate extensive genetic diversity of PBVs within and across mammalian and avian species in geographic locations worldwide. However, the limited partial-sequence data and the lack of full-length sequences make it difficult to establish a firm linkage between human and animal strains. Thus, sequence information from more animal PBVs is needed to fully understand the epidemiology and evolution of this group of viruses.
We recently reported the detection of PBVs by PAGE and electron microscopy in fecal specimens from two monkeys with diarrhea in a primate colony outside Beijing, China (20). However, due to the use of insensitive diagnostic tests, we neither reported true prevalence nor performed molecular characterization of these simian PBVs. In the present study, we applied molecular methods of greater sensitivity and demonstrated high prevalence and diversity of PBVs in this primate colony.
A total of 92 fecal or rectal swab specimens were collected from monkeys between February 2002 and January 2003. All monkeys had diarrhea at the time of specimen collection. Monkey species included 79 rhesus, 10 cynomolgus, and 3 pigtailed macaques, with ages ranging from 18 months to 14 years. All specimens were stored at −70°C before being shipped and tested at the U.S. CDC in Atlanta, GA.
Fecal specimens were diluted 1:10 in phosphate-buffered saline (PBS), and the suspensions were clarified by centrifugation at 10,000 × g for 2 min in an Eppendorf bench-top centrifuge (model 5415; Westbury, NY). Aliquots (100 μl) of the clarified supernatants were transferred to a 1.7-ml Eppendorf tube and mixed with an equal volume of NucliSens lysis buffer (bioMérieux, Inc., Durham, NC), followed by addition of 200 μl of 100% ethanol. The mixtures were then loaded to a HiBind RNA minicolumn (Omega Bio Tek, Norcross, GA) and centrifuged at 20,000 × g for 1 min. After being washed with 75% ethanol, RNA was eluted from the column with 50 μl of NucliSens elution buffer (bioMérieux).
RNA samples were subjected to amplification by RT-PCR using a primer set (PicoB25 and PicoB43) specific to segment 2 of genogroup I PBV (15). Briefly, 2 μl of nuclease-free H2O and 1.5 μl of the primers PicoB25 and PicoB43 (each at 20 μM) were added to 5 μl of viral RNA, followed by heat denaturation for 5 min at 97°C and quenching on ice. Subsequently, 40 μl of RT mix from a Qiagen One-Step RT-PCR kit was added. The RT-PCR conditions were as follows: 45°C for 1 h, 95°C for 15 min, and 35 cycles at 94°C for 30 s, 45°C for 30 s, and 72°C for 1 min, followed by a final extension at 72°C for 7 min. The PCR products were analyzed in a 2% agarose gel and stained with GelRed Nucleic Acid Gel Stain (Biotium, Inc., Hayward, CA). The bands with the expected size (∼200 bp) were cut and purified with a Qiagen Gel Extraction kit (Qiagen, Inc., Valencia, CA) according to the manufacturer's protocol. To confirm that the bands were actually amplified from genogroup I PBVs, PCR amplicons were sequenced using primers PicoB25 and PicoB43 and a BigDye v3.1 cycle sequencing kit and an ABI 3100XL automated sequencer (Applied Biosystems, Inc., Foster City, CA).
Two PBV strains with clear RNA profiles were selected for full-length segment 2 characterization using a modified single-primer amplification method (8). Briefly, oligonucleotide linker 1 was ligated to the 3′ end of viral dsRNA with T4 RNA ligase (New England BioLabs, Ipswich, MA). Free oligonucleotides were removed by column purification with a Qiagen miniElute Gel Extraction kit (Qiagen) according to the manufacturer's protocol. Two overlapping fragments spanning all of segment 2 were amplified by a combination of oligonucleotide linker 2 (complementary to linker 1) and primer PicoB43 or another internal primer, PicoB14 (GAT GGC GTG GAC AGG AAG), specific to segment 2 of simian PBV. The nucleotide sequence of the entire segment, including the 5′ and 3′ termini, was determined by sequencing with PCR primers and newly designed internal primers. Nucleotide sequences obtained from simian PBV strains were first cleaned and assembled in Sequencher 4.8 (Gene Codes, Ann Arbor, MI). Multisequence alignments were performed by ClustalW within BioEdit version 7.0.0 (Carlsbad, CA) (7). Pairwise nucleotide and amino acid sequence similarities of partial gene sequences as well as the full-length open reading frames were determined in Mega 5 software (17). Phylogenetic trees of short sequences were constructed by the neighbor-joining method and p-distance using Mega 5. Bootstrap resampling analysis of 500 replicates was included.
Nucleotide sequence accession numbers.
All sequences determined in this study were deposited in GenBank under accession numbers JQ710464 to JQ710507.
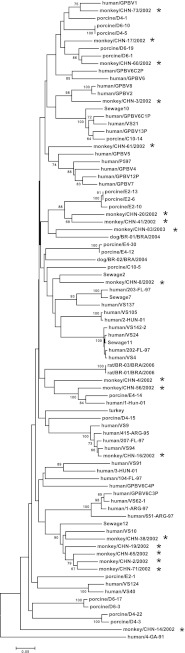
We examined 92 fecal specimens for PBV genogroup I by RT-PCR with primers PicoB25 and PicoB43 and observed a band of the expected size in 59 samples. We sought to sequence the amplicons from all 59 positive samples and obtained high-quality nucleotide sequences from 44 PCR products. The remaining 15 samples showed multiple nucleotide sequences. Of the 44 sequence-confirmed specimens, 41 were from 79 rhesus and 3 from 10 cynomolgus macaques. None of the 3 pigtail macaques gave positive results. The rate of detection did not vary with the age and gender of the monkeys. These 44 sequences (170 to 176 bp) were divided into 18 clusters; those in the same cluster shared ≥95% nucleotide sequence identity, and some strains had sequences that were 100% identical (data not shown). To examine the genetic relatedness of simian PBVs to those from other animals, representative sequences from each of the 18 simian PBV clusters were compared with the corresponding PBV gene sequences from avian, canine, murine, porcine, bovine, and human strains as well as from wastewater (Fig. 1). Five simian strains, monkey/CHN-38/2002, monkey/CHN-19/2002, monkey/CHN-65/2002, monkey/CHN-2/2002, and monkey/CHN-71/2002, representatives of PBVs from 16 monkeys, fell into the same lineage as a strain from raw sewage water in the United States (strain Sewage 12′ in the tree) and a human strain (strain VS10) recently reported in the Netherlands (16, 18). The 13 other strains, representatives of 13 simian PBV clusters, formed clades of various levels of genetic identity with animal and human PBVs, raising the issue of potential interspecies or zoonotic transmission. Of note, the two strains (monkey/CHN-14/2002 and monkey/CHN-49/2002) that were detected in samples from rhesus macaques that were collected on dates more than 4 months apart shared identical nucleotide sequences but were relatively distantly related (50% to 60% nucleotide identity) to other simian strains of this study and to all the genogroup I strains reported in GenBank (47.1% to 56.5% nucleotide identity).
Fig 1.
Phylogenetic tree based on 168 bp of gene segment 2 of representative simian PBVs and the cognate stretch of other genogroup I strains. The origin of host species is indicated. Simian PBV strains are indicated by the character “*.” Environmental samples are labeled “Sewage.” The genogroup II human strain 4-GA-91 is included as an outlier.
The complete nucleotide sequences of segment 2 of strains monkey/CHN-14/2002 and monkey/CHN-49/2002 were determined to be 1,784 bp in length and were almost identical, with only one nucleotide difference at position 906. Nucleotide and deduced amino acid sequences of the whole protein coding region of monkey/CHN-49/2002 were compared with the corresponding and comparable lengths of segment 2 of published sequences (Table 1). The simian PBV strain shows nucleotide (52.5% to 54.2%) and amino acid (47.8% to 50.1%) similarities to 1 bovine and 3 human strains of genogroup I PBV but lower similarities to the prototype genogroup II human 4-GA-1 strain. Nucleotide sequence analysis indicated that the five nucleotides at the 5′ (GUAAA) or 3′ (ACUGC) end were conserved among simian, bovine, and human genogroup I strains (data not shown). Amino acid alignments of 7 strains (2 simian, 1 bovine, 3 human genogroup I, and 1 human genogroup II) demonstrated that the simian strains retained all three conserved RDRP motifs that are characteristic of dsRNA viruses (data not shown). In addition, the alignments showed 15 conserved proline residues among all six genogroup I strains but only 7 conserved proline residues in common with genogroup II strain 4-GA-91. Four conserved cysteine residues were found among genogroup I strains, but none were seen with genogroup II strain 4-GA-91.
Table 1.
Nucleotide and amino acid sequence similarity of the whole-protein coding region of strain monkey/CHN-49 to those of reference strains with comparable lengths of genomic segment 2
| Genogroup | Reference strain | Percent sequence identity |
|
|---|---|---|---|
| Nucleotide | Amino acid | ||
| I | Bovine/RUBV-P | 52.5 | 49.3 |
| Human/1-CHN-97 | 54.2 | 50.1 | |
| Human/Hy005102 | 52.6 | 47.8 | |
| Human/VS10 | 52.7 | 49.1 | |
| II | Human/4-GA-1 | 38.4 | 20.3 |
This report is the first from a study of the prevalence and molecular characterization of simian PBVs. The rate of detection (48%) of PBVs by RT-PCR and sequencing sharply contrasts with the 2% rate detected by RNA PAGE in our previous study. Most of the PBV-positive macaques in this study also harbored other viruses, such as rotavirus and adenovirus, that are known to cause gastroenteritis (20). Thus, the role of PBVs in diarrheal disease among monkeys remains to be determined. A recent study from Argentina (12) demonstrated that PBVs caused repeated and persistent infection in pigs without any sign of diarrhea or any other illness. Instead, the researchers found that PBV infection was linked to a particular physiological status (i.e., lactation and pregnancy) and was more common in piglets than in adult male pigs. In the present study, we observed no significant differences in PBV prevalence in different age or sex groups of monkeys. Except for two PBV strains (monkey/CHN-14/2002 and monkey/CHN-49/2002) in fecal specimens that had a clear dsRNA genome profile by PAGE, indicating active replication and abundant virus shedding, other simian PBVs were detectable only by RT-PCR. Whether these PBVs established infection in monkeys or simply were transient passengers as a result of ingestion of contaminated food or water by those monkeys remains unknown. Previous studies reported that not all PBVs found in the gut were specific to the host (5, 14, 21).
Our findings of a high degree of genetic diversity within the same colony agree with published reports of wide-range intraspecies diversity of PBVs (2, 5). On the other hand, high sequence identities among some simian PBVs and between simian PBVs and other genogroup I human and porcine PBV strains in GenBank suggest that (i) PBVs readily transmitted among species of the Chinese monkey colony and (ii) simian PBVs had potential for interspecies transmission. A study of American wastewater samples had demonstrated that genogroup I PBV RNA could be detected in 100% of raw sewage water samples and in 33% of treated effluent samples (16). The extreme stability and resistance to treatment of PBVs make these viruses ubiquitous. Thus, a waterborne route of PBV acquisition could explain how the closely related strains are acquired by different host species in the same setting.
Despite the availability of partial sequences of the RDRP gene from various animal species and humans, very few full-length segment 2 and even fewer segment 1 sequences of PBVs are available in GenBank. Further studies are needed to find a conserved region and develop robust RT-PCR assays for the detection and analysis of gene segment 1 of PBVs, which would allow us to identify possible serotypes and understand if reassortment plays a role in the epidemiology and evolution of PBVs in animals and humans.
ACKNOWLEDGMENTS
We thank Mathew Esona and Kim Foytich for assistance in DNA sequencing and sequence analysis.
K.B. is supported by the Hungarian Academy of Sciences (Momentum Initiative).
The findings and conclusions in this report are ours and do not necessarily represent the views of CDC.
Footnotes
Published ahead of print 23 May 2012
REFERENCES
- 1. Bányai K, et al. 2003. Sequence heterogeneity among human picobirnaviruses detected in a gastroenteritis outbreak. Arch. Virol. 148:2281–2291 [DOI] [PubMed] [Google Scholar]
- 2. Bányai K, et al. 2008. Genogroup I picobirnaviruses in pigs: evidence for genetic diversity and relatedness to human strains. J. Gen. Virol. 89:534–539 [DOI] [PubMed] [Google Scholar]
- 3. Browning GF, et al. 1991. The prevalence of enteric pathogens in diarrheic thoroughbred foals in Britain and Ireland. Equine Vet. J. 23:405–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chasey D. 1990. Porcine picobirnavirus in UK? Vet. Rec. 126:465. [PubMed] [Google Scholar]
- 5. Fregolente MC, et al. 2009. Molecular characterization of picobirnaviruses from new hosts. Virus Res. 143:134–136 [DOI] [PubMed] [Google Scholar]
- 6. Haga IR, et al. 1999. Identification of a bisegmented double-stranded RNA virus (Picobirnavirus) in faeces of giant anteaters (Myrmecophaga tridactyla). Vet. J. 158:234–236 [DOI] [PubMed] [Google Scholar]
- 7. Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. (Oxf.). 41:95–98 [Google Scholar]
- 8. Lambden PR, Cooke SJ, Caul EO, Clarke IN. 1992. Cloning of noncultivatable human rotavirus by single primer amplification. J. Virol. 66:1817–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leite JP, Monteiro SP, Fialho AM, Pereira HG. 1990. A novel avian virus with trisegmented double-stranded RNA and further observations on previously described similar viruses with bisegmented genome. Virus Res. 16:119–126 [DOI] [PubMed] [Google Scholar]
- 10. Ludert JE, Abdul-Latiff L, Liprandi A, Liprandi F. 1995. Identification of picobirnavirus, viruses with bisegmented double stranded RNA, in rabbit faeces. Res. Vet. Sci. 59:222–225 [DOI] [PubMed] [Google Scholar]
- 11. Ludert JE, Hidalgo M, Gil F, Liprandi F. 1991. Identification in porcine faeces of a novel virus with a bisegmented double stranded RNA genome. Arch. Virol. 117:97–107 [DOI] [PubMed] [Google Scholar]
- 12. Martínez LC, et al. 2010. Picobirnavirus causes persistent infection in pigs. Infect. Genet. Evol. 10:984–988 [DOI] [PubMed] [Google Scholar]
- 13. Pereira HG, Fialho AM, Flewett TH, Teixeira JM, Andrade ZP. 1988. Novel viruses in human faeces. Lancet ii:103–104 [DOI] [PubMed] [Google Scholar]
- 14. Pereira HG, Flewett TH, Candeias JA, Barth OM. 1988. A virus with a bisegmented double-stranded RNA genome in rat (Oryzomys nigripes) intestines. J. Gen. Virol. 69(Pt. 11):2749–2754 [DOI] [PubMed] [Google Scholar]
- 15. Rosen BI, Fang ZY, Glass RI, Monroe SS. 2000. Cloning of human picobirnavirus genomic segments and development of an RT-PCR detection assay. Virology 277:316–329 [DOI] [PubMed] [Google Scholar]
- 16. Symonds EM, Griffin DW, Breitbart M. 2009. Eukaryotic viruses in wastewater samples from the United States. Appl. Environ. Microbiol. 75:1402–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamura K, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Leeuwen M, et al. 2010. Human picobirnaviruses identified by molecular screening of diarrhea samples. J. Clin. Microbiol. 48:1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vanopdenbosch E, Wellemans G. 1990. Bovine birna type virus—a new etiologic agent of neonatal calf diarrhea. Vlaams Diergen. Tijds. 59:137–140 [Google Scholar]
- 20. Wang Y, et al. 2007. Detection of viral agents in fecal specimens of monkeys with diarrhea. J. Med. Primatol. 36:101–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang T, et al. 2006. RNA viral community in human feces: prevalence of plant pathogenic viruses. PLoS Biol. 4:108–118 doi:10.1371/journal.pbio.0040003 [DOI] [PMC free article] [PubMed] [Google Scholar]