Abstract
As members of the indigenous human microbiota found on several mucosal tissues, Methanobrevibacter smithii and Methanosphaera stadtmanae are exposed to the effects of antimicrobial peptides (AMPs) secreted by these epithelia. Although antimicrobial and molecular effects of AMPs on bacteria are well described, data for archaea are not available yet. Besides, it is not clear whether AMPs affect them as the archaeal cell envelope differs profoundly in terms of chemical composition and structure from that of bacteria. The effects of different synthetic AMPs on growth of M. smithii, M. stadtmanae, and Methanosarcina mazei were tested using a microtiter plate assay adapted to their anaerobic growth requirements. All three tested methanoarchaea were highly sensitive against derivatives of human cathelicidin, of porcine lysin, and a synthetic antilipopolysaccharide peptide (Lpep); however, sensitivities differed markedly among the methanoarchaeal strains. The potent AMP concentrations affecting growth were below 10 μM, whereas growth of Escherichia coli WBB01 was not affected at peptide concentrations up to 10 μM under the same anaerobic growth conditions. Atomic force microscopy and transmission electron microscopy revealed that the structural integrity of the methanoarchaeal cells is destroyed within 4 h after incubation with AMPs. The disruption of the cell envelope of M. smithii, M. stadtmanae, and M. mazei within a few minutes of exposure was verified by using LIVE/DEAD staining. Our results strongly suggest that the release of AMPs by eukaryotic epithelial cells is a potent defense mechanism targeting not only bacteria, but also methanoarchaea.
INTRODUCTION
Members of the domain Archaea, the third domain of life, are ubiquitous and show a wide phylogenetic diversity (9, 10). During the last 3 decades, several strictly anaerobic archaea were found to be associated with the human mucosa (11, 12, 56, 57). Methane breath studies, cultivation of isolates, and PCR-based detection assays demonstrated the presence of methanoarchaea in the human gut (11, 12, 25, 56, 57). Here, a crucial role of those is the modulation of the fermentation balance by converting bacterial primary fermentation products, like acetate, methanol, hydrogen, and carbon dioxide, to methane (54, 64). The first species identified as part of the human indigenous gut microflora was Methanobrevibacter smithii, whose genomic adaptations to the human intestine are apparently the reason for its predominance compared to the later detected species Methanosphaera stadtmanae (25, 65).
Besides competition for nutrients and niches with their habitat mates, all members of the human microflora are exposed to various epithelial defense mechanisms to prevent invasion or colonization of organs and to maintain homeostasis (53). One line of epithelial defense is the secretion of antimicrobial peptides (AMPs) that are considered to be an essential part of the innate immunity of eukaryotes (15, 16, 73). AMPs are derived from different origins and have diverse structural motifs (74). They are assumed to exert their antimicrobial mechanism via interaction with the negatively charged membrane of bacteria, fungi, protozoa, and viruses, thereby disturbing membrane integrity (33, 43, 46, 50). The biochemical effects of various AMPs on bacterial cell membranes and their properties are well characterized. In contrast to the bacterial membrane lipids consisting of fatty acid esters, archaeal membrane lipids are built from glycerol diethers of isoprenoid alcohols arranged as a bilayer or in several archaea also from glycerol tetraethers that form monolamellar membrane patches (14, 22). In addition, the compounds of the archaeal cell wall polymer are structurally highly diverse, ranging from pseudomurein, surface-layer (S-layer) proteins to methanochondroitin (47). Due to the unique composition and biochemical structure of the archaeal cell envelope, if and how AMPs affect their cell membrane was not evident, nor have such data been available until now.
Thus, we examined the effects of several AMPs with regard to growth inhibition and morphological changes of the methanoarchaeal strains M. smithii and M. stadtmanae, which are considered to be inhabitants of the human gut (66), and of one member of the Methanosarcinales, Methanosarcina mazei strain Gö1. To determine inhibitory concentrations of the AMPs, the establishment of a system to reproducibly analyze the anaerobic growth of these strains in parallel small culture volumes was essential. Furthermore, the present study included a time-dependent examination of AMP-induced killing of archaea and an ultrastructural analysis of AMP-treated methanoarchaeal strains by atomic force microscopy (AFM) and electron microscopy.
MATERIALS AND METHODS
Strains and growth media of methanoarchaea.
M. mazei strain Gö1 (DSM 3647), M. stadtmanae (DSM 3091), and M. smithii (DSM 861) were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ; Braunschweig, Germany). All strains were grown at 37°C in minimal medium under strict anaerobic conditions, as described for M. mazei (32) with minor modifications. The reductant Na2S was completely omitted; instead the medium was solely reduced with cysteine (2 mM). The gas phase used for M. mazei cultures was N2-CO2 (80/20 [vol/vol]), whereas M. stadtmanae and M. smithii were grown with 1.5 atm H2-CO2 (80/20 [vol/vol]). A 150 mM concentration of methanol was added as the carbon and energy source for M. mazei and M. stadtmanae, whereas H2-CO2 of the gas phase served as sole carbon and energy source for M. smithii. To prevent bacterial contamination, the medium for all strains was in general supplemented with 100 μg/ml ampicillin.
Bacterial strain.
E. coli strain WBB01 (17) was grown in LB (Luria-Bertani) medium or in the minimal medium that was used for the methanoarchaea, except that 20 mM glucose was added instead of methanol as the carbon and energy source. Cultures were grown in microtiter plates with constant shaking at 37°C for aerobic conditions and at 37°C without shaking for anaerobic conditions.
AMPs.
The AMPs tested in this study were derivatives of the human cathelicidin LL37 (LL20 and LL32) (41), derivatives of porcine NK-lysin (NK2 and C7S) (8), and a synthetic antilipopolysaccharide (anti-LPS) peptide, Lpep 19-2.5 (5). (All were purified from chemical peptide synthesis and kindly provided by O. Holst, Division of Structural Biochemistry, and J. Andrä, Division of Biophysics, Research Center Borstel, Borstel, Germany.) Peptides were stored at −20°C and diluted in anaerobic aquadest prior use.
Microtiter plate assay for AMP susceptibility test.
The antimicrobial activity of the peptides was determined by growth inhibition of M. mazei, M. stadtmanae, and M. smithii. Thus, the commonly used microtiter plate assay for antimicrobial testing (6) was adapted to the anaerobic growth conditions required for the methanoarchaeal strains. A microtiter plate reader (Sunrise; Tecan Group, Ltd., Crailsheim, Germany) was set up in an anaerobic chamber with an atmosphere of N2-CO2-H2 (78/20/2 [vol/vol/vol]), and the microtiter plates for the AMP susceptibility tests were prepared under anaerobic conditions. To maintain at least semisterile conditions within the chamber, the gas atmosphere was constantly circled over a 0.3-μm filter system (Coy Laboratory Products, Inc., Grass Lake, MI).
Precultures of the methanoarchaeal strains were grown in 50 ml anaerobic medium as described above. Growth of precultures was monitored by the optical density at 600 nm (OD600), and cell numbers were generally determined by microscopic counting using a Thoma counting chamber during the growth period (at least three technical replicates). For AMP susceptibility tests, 1 × 107 cells from the mid-exponential growth phase of precultures were inoculated into 250 μl minimal medium in U-bottom polystyrene microtiter plates (Microlon; Greiner Bio-One GmbH, Frickenhausen, Germany) supplemented with different AMP concentrations. Microtiter plates were covered with gas-permeable membranes (Carl Roth GmbH and Co., KG, Karlsruhe, Germany), and in the case of M. stadtmanae and M. smithii, transferred to an anaerobic jar with a side tube for manipulation of the gas phase (Schuett-Biotec GmbH, Göttingen, Germany) to continuously supply H2-CO2. Growth was monitored in constant intervals until the stationary phase was reached. To monitor microbial contamination under the semisterile working conditions in the anaerobic chamber during experiments, a control containing minimal medium was routinely included and the autofluorescence of the potential contaminating cells was monitored by fluorescence microscopy following the growth measurements. All values or data obtained are in general based on three independent experiments, each performed at least in duplicate.
LIVE/DEAD staining.
LIVE/DEAD staining was performed with the LIVE/DEAD BacLight bacterial viability kit (Invitrogen GmbH, Darmstadt, Germany). Cultures were grown to mid-exponential growth phase under anaerobic conditions, kit reagents were added according to the manufacturer's protocol, and 1-ml aliquots of cultures supplemented with reagents were dispensed to anaerobic Hungate tubes. AMPs were supplemented to different final concentrations, as given in the Results section. At different time points of incubation, cells were microscopically examined at a ×1,000 magnification using an Axio Lab microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany) supplied with a digital camera (AxioCam Mr5, Carl Zeiss MicroImaging GmbH, Jena, Germany). Fluorescent micrographs with filter sets for propidium iodide and SYTO 9 fluorescence and phase-contrast micrographs in the same visual fields were captured using the digital image analysis software AxioVision Release 4.7.1 (Carl Zeiss MicroImaging GmbH, Jena, Germany).
Atomic force microscopy.
Cultures were grown to the mid-exponential growth phase as described above and adjusted to 1 × 107 cells in fresh minimal medium. The indicated AMP concentrations were supplemented, and the cells were further incubated for 4 h at 37°C. After brief agitation, 20 μl of each preparation was dropped on glass slides coated with mica (29) and dried in an anaerobic chamber. Samples were washed twice with water to remove the large amounts of salt in the sample preparation. Dried archaea were imaged with an MFP-3D atomic force microscope (Asylum Research, Santa Barbara, CA). Images were taken under normal atmosphere in contact mode using a CSG11-A cantilever (k = 0.1 N/m; NT-MDT, Moscow, Russia). The set point was adjusted to guarantee applying minimal forces to the sample. Further image editing (flattening) was done with the MFP-3D software under IGOR Pro (Lake Oswego, OR). The images shown are representative for the overall measurements.
Electron microscopy.
Transmission electron microscopy (TEM) analysis was performed as described previously (8). Ten milliliters of mid-exponential growth-phase cultures was incubated in the presence of the indicated AMP concentrations for 4 h under anaerobic conditions, while the following procedures were done under aerobic conditions. Preparations were centrifuged for 30 min at 3,200 × g, and the supernatant was discarded. Five milliliters of 10% LB agar was then added to each tube, and the mixture was centrifuged at 4°C for 15 min at 3,200 × g to concentrate cells in the setting agar. Shown images are representative for the respective samples.
RESULTS
The aim of this study was to examine the effects of several AMPs with regard to growth inhibition and morphological changes of the methanoarchaeal strains M. smithii, M. stadtmanae, and M. mazei.
Establishment of growth analysis of methanoarchaea in microtiter plates under anaerobic conditions.
The broth microdilution assay routinely used for antimicrobial testing of AMPs and other antimicrobial active substances with bacterial strains (6) was assigned to meet the growth requirements of methanoarchaea. Strains had to be grown under reducing conditions in minimal medium supplied with the respective carbon and energy sources and gas atmospheres (according to Materials and Methods) to enable methanogenesis. The culture volume was reduced to a minimum of 250 μl. Optimal and reproducible growth was enabled in U-bottom polystyrene microtiter plates covered with a gas-permeable membrane. Under these conditions, M. mazei was able to grow from an inoculum of 1 × 106 cells per 250 μl, whereas growth of M. stadtmanae and M. smithii was not observed until an initial cell number of 1 × 107 cells per 250 μl. Growth progression at 37°C was recorded up to 96 h and confirmed that the growth of all methanoarchaeal strains as well as of Escherichia coli WBB01 was comparable to growth in higher culture volumes using Hungate tubes or closed bottles. Independent from inoculated cell numbers, cultures of methanoarchaea reached their maximum OD600 after 50 h (M. mazei) to 96 h (M. stadtmanae and M. smithii) but resulted in different optical turbidities at the end of cultivation due to different sedimentation behaviors (data not shown). A starting cell number of 1 × 107 cells per 250 μl medium was chosen for all methanoarchaeal and bacterial strains in the study presented here.
Inhibitory effects of AMPs on methanoarchaea.
Cathelicidin derivatives LL32 and LL20 (41), NK-lysine derivatives NK2 and C7S (8), and the synthetic peptide Lpep 19-2.5 originating from Limulus anti-lipopolysaccharide (anti-LPS) factor (LALF) (5) were used to study their antimicrobial effects on the methanoarchaea M. mazei, M. stadtmanae, and M. smithii. The minimal peptide concentrations necessary to inhibit archaeal growth (MICs) were determined with the microdilution assay described above in at least three independent experiments. The percentage of growth was calculated from the optical densities in the stationary phase compared to that of a noninhibited control. Except for a certain strain-dependent manner, all peptides showed activity against M. mazei, M. stadtmanae, and M. smithii at concentrations below 10 μM (summarized in Table 1). Concerning the three methanoarchaeal strains, the AMPs LL20, LL32, NK2, C7S, and Lpep 19-2.5 are most effective against M. mazei, with MICs between 0.5 and 4 μM. Against M. stadtmanae and M. smithii, they display only moderate effectiveness, with the exception of LL32 and Lpep 19-2.5 being similarly effective against M. smithii as against M. mazei (Table 1, Fig. 1A and C, and Fig. 2A).
Table 1.
Antimicrobial activities of various AMPs against methanogenic archaea and E. coli strain WBB01 in minimal medium under anaerobic conditions
| Organism | MIC (μM) of: |
||||
|---|---|---|---|---|---|
| LL32 | LL20 | NK2 | C7S | Lpep 19-2.5 | |
| M. mazei | 1 | 4 | 0.5 | 0.5 | 1 |
| M. stadtmanae | 5 | >10 | 10 | >10 | >10 |
| M. smithii | 1 | >10 | 3 | >10 | 3 |
| E. coli WBB01 | >10 | >10 | >10 | >10 | >10 |
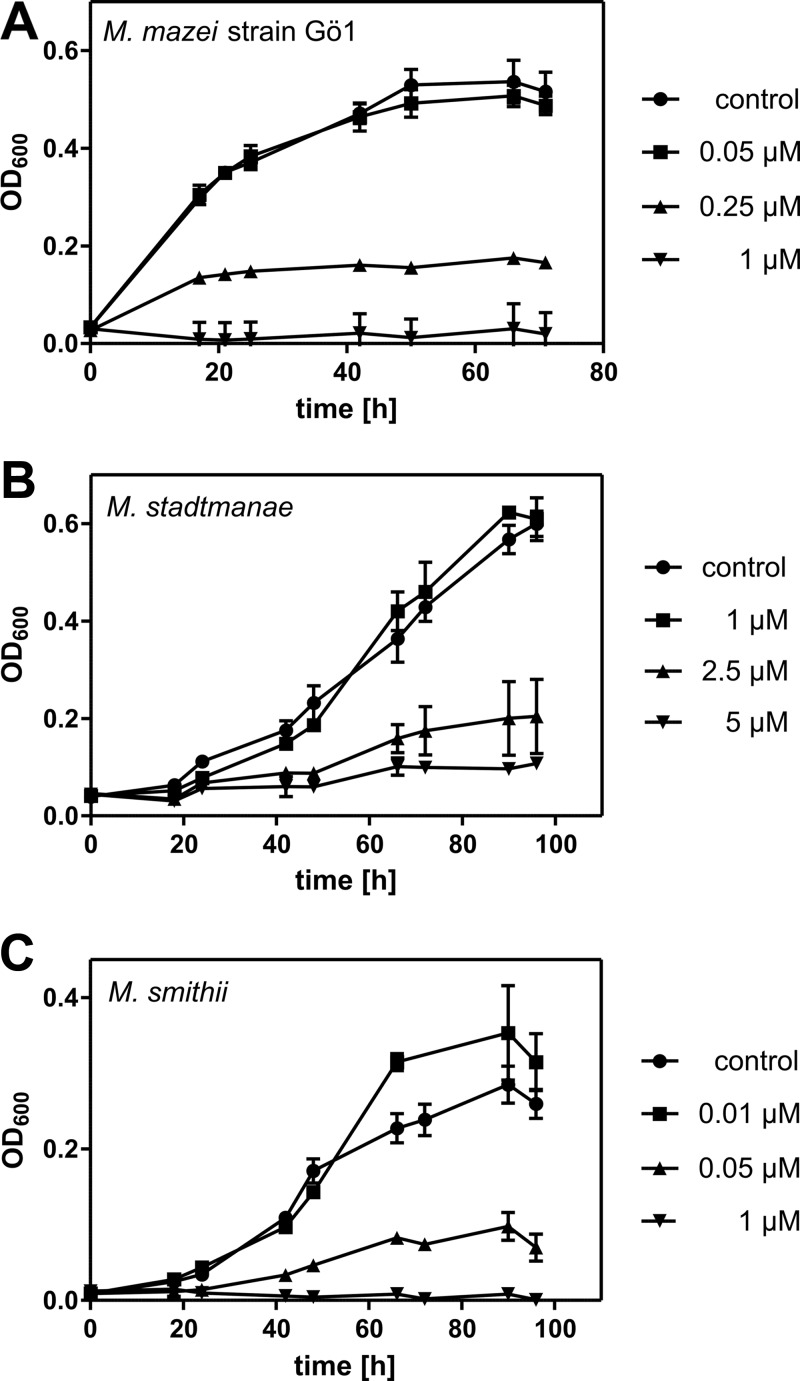
Fig 1.
Growth inhibition of M. mazei, M. stadtmanae, and M. smithii by LL32. A total of 1 × 107 cells were incubated with the indicated concentrations of the human cathelicidin derivative LL32 at 37°C in 250 μl minimal medium under anaerobic conditions. Turbidity of cultures at 600 nm (OD600) was measured at different time points. Error bars represent standard deviations of three parallel cultures in one experimental setup. (A) M. mazei; (B) M. stadtmanae; (C) M. smithii.
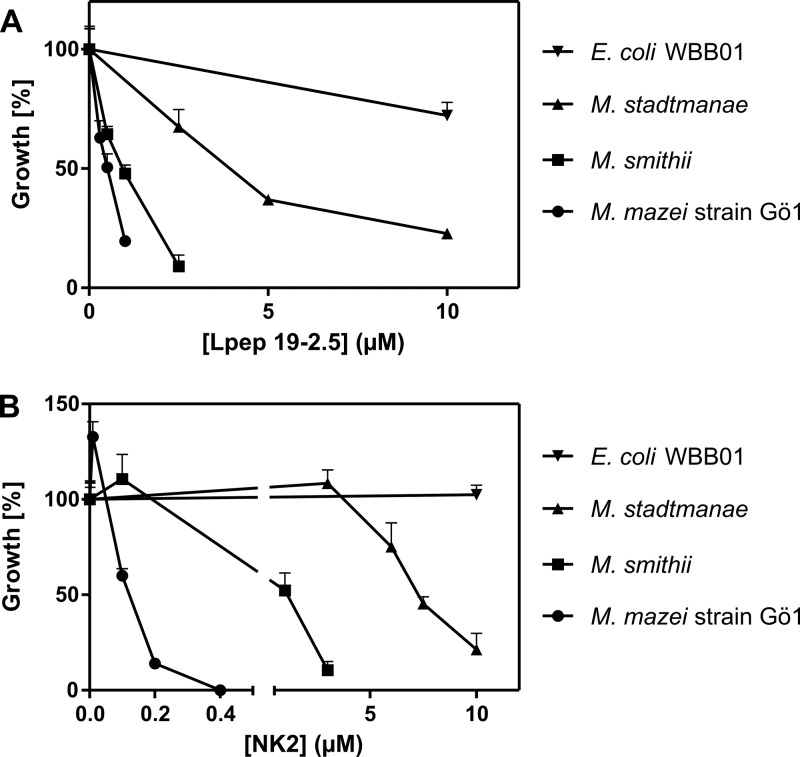
Fig 2.
Growth inhibition of M. mazei, M. stadtmanae, M. smithii, and E. coli WBB01 in cultures containing Lpep 19-2.5 and NK2. A tota1 of 1 × 107 cells were incubated with different concentrations of the peptide Lpep 19-2.5 (A) or NK2 (B) at 37°C in 250 μl minimal medium. The turbidity of control cultures at 600 nm (OD600) in the stationary phase (M. mazei, 50 h; M. stadtmanae, 72 h; M. smithii, 96 h; E. coli WBB01, 6 h) was set to 100%. Error bars represent standard abbreviations of three parallel cultures in one experimental setup.
M. stadtmanae was able to grow in medium containing higher concentrations of cathelicidins (Fig. 1B) and lysins (Fig. 2B), but growth was also inhibited at low concentrations of Lpep 19-2.5 (Fig. 2A). Interestingly, only in assays containing NK2 did the relative growth of methanoarchaea appear to be promoted by the peptide at low concentrations compared to that in the nontreated controls (Fig. 2B). Microscopic evaluation of cultures at the end of cultivation revealed coagulation of cells to different extents due to AMP treatments.
Except for Lpep 19-2.5, the growth of E. coli WBB01 was not affected by the peptides, tested under the set experimental conditions using 1 × 107 cells and an anaerobic gas atmosphere (Table 1 and Fig. 2). However, functionality of the peptides was verified using E. coli WBB01 and a starting cell number of 1 × 105, as described for the general broth microdilution assay under aerobic conditions using Luria-Bertani medium (data not shown).
Confirmation of the effects of AMPs on methanoarchaea using LIVE/DEAD staining.
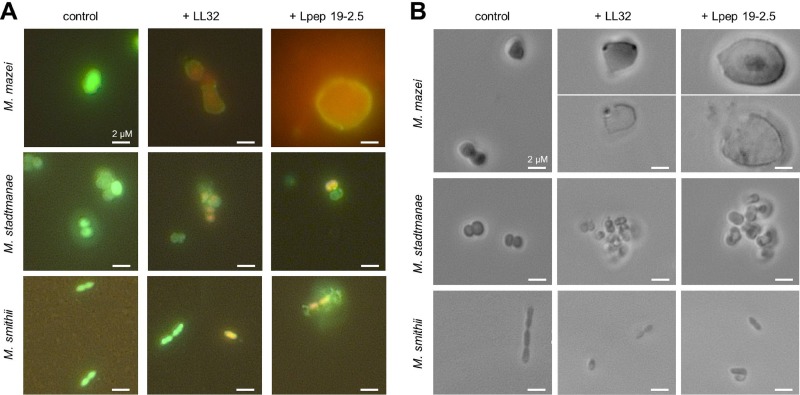
Evaluation of partly inhibited cultures after cultivation in AMP-containing medium by light microscopy demonstrated coagulation of cells for all methanoarchaeal strains. As this coagulation might result from sedimentation in microtiter plates, the short-term effects of AMPs at their MICs on the three methanoarchaeal strains were studied with the LIVE/DEAD staining assay. This assay contains two fluorescent dyes, propidium iodide, which penetrates cells with damaged membranes, and SYTO 9, which accumulates only in living cells. Thus, unaffected archaeal cells with intact membranes show the green fluorescence of SYTO 9, while cells with damaged membranes show the red fluorescence of propidium iodide.
LL32 and Lpep 19-2.5 were added to cell cultures at the mid-exponential growth phase containing the LIVE/DEAD staining components, and aliquots were examined under the microscope after 10, 30, 60, and 120 min in comparison to those of untreated controls. The tested methanoarchaeal strains reacted differently on the application of AMPs. First effects became visible for all strains after 10 min of incubation with AMPs (Fig. 3). About 1/3 of M. mazei cells were enlarged after the addition of 1 μM Lpep 19-2.5 or 1 μM LL32. The highest enlargement of cells (about 5- to 10-fold increase in cell diameter) could be observed in cultures containing Lpep 19-2.5 (Fig. 3). Increased cell size led to bursting of cell membranes, which could also be observed during microscopic examination (Fig. 3B). In contrast, LIVE/DEAD staining of M. stadtmanae and M. smithii treated with both peptides revealed no obvious increase in cell size before destruction (Fig. 3). Addition of 5 μM LL32 and 10 μM Lpep 19-2.5 to cultures of M. stadtmanae increased cell coagulation, whereas such an effect was not visible for cultures of M. mazei or M. smithii (Fig. 3B).
Fig 3.
Microscopic analysis of methanoarchaea after treatment with LL32 and Lpep 19-2.5. Cultures of M. mazei, M. stadtmanae, and M. smithii were grown to the mid-exponential growth phase, LIVE/DEAD staining kit reagents were added according to the manufacturer's protocol, and 1-ml aliquots of cultures were dispensed to anaerobic Hungate tubes. AMPs were added at the following concentrations: M. mazei, 1 μM LL32 or 1 μM Lpep 19-2.5; M. stadtmanae, 5 μM LL32 or 10 μM Lpep 19-2.5; M. smithii, 1 μM LL32 or 3 μM Lpep 19-2.5. (A) Fluorescent micrographs with filter sets for propidium iodide and SYTO 9 fluorescence taken after 10 min of incubation in the presence of AMPs (or in the absence for control cultures). (B) Phase-contrast micrographs taken after 60 min of incubation in the presence of AMPs (or in the absence for control cultures). Pictures are representative for the respective sample.
Influence of peptides on the ultrastructure of methanoarchaea.
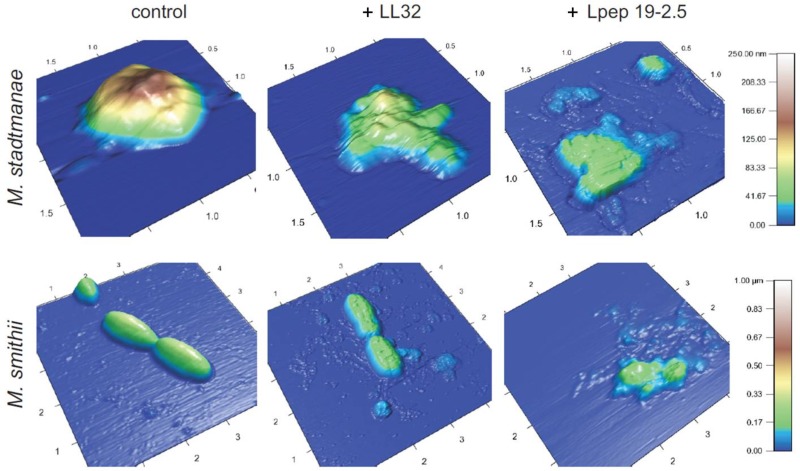
The morphological changes of the methanoarchaea upon treatment with LL32 and Lpep 19-2.5 were visualized by atomic force microscopy (AFM) and transmission electron microscopy (TEM). Mid-exponential-phase cultures were treated with AMPs at the respective MICs for 4 h and compared to controls, as described in Materials and Methods. As depicted in Fig. 4, both AMPs had a dramatic impact on the structural integrity of M. stadtmanae and M. smithii compared to untreated controls. Cells of both strains showed different sensitivities against the used AMPs within a given culture and were therefore not affected to the same extent. The addition of LL32 to M. smithii increased surface roughness but appeared not to disturb the strain's cellular morphology, whereas M. stadtmanae cells lost their typical spherical shape. Lpep 19-2.5 destroyed the cell morphology of both strains. In general, adhesion of cells of M. stadtmanae and M. smithii to the mica plates used for AFM decreased significantly upon peptide treatment. The adhesion of M. mazei cells to mica plates upon peptide treatment was reduced to a much higher extent, making AFM analysis impossible.
Fig 4.
Effects of LL32 and Lpep 19.2-5 treatment on the morphology of M. stadtmanae and M. smithii examined by AFM. AFM images of untreated cultures (controls) and of cultures incubated with respective MICs in the presence of 5 μM LL32 or 10 μM Lpep 19-2.5 for M. stadtmanae and 1 μM LL32 or 3 μM Lpep 19-2.5 for M. smithii. Cultures were grown until the mid-exponential phase at 37°C, adjusted to 1 × 107 cells in fresh minimal medium, and incubated in the presence of AMPs for 4 h. Images were taken in air in alternating current (AC) (tapping) mode and are representative of the samples examined. The length (scale in μm) and height (color code) are indicated.
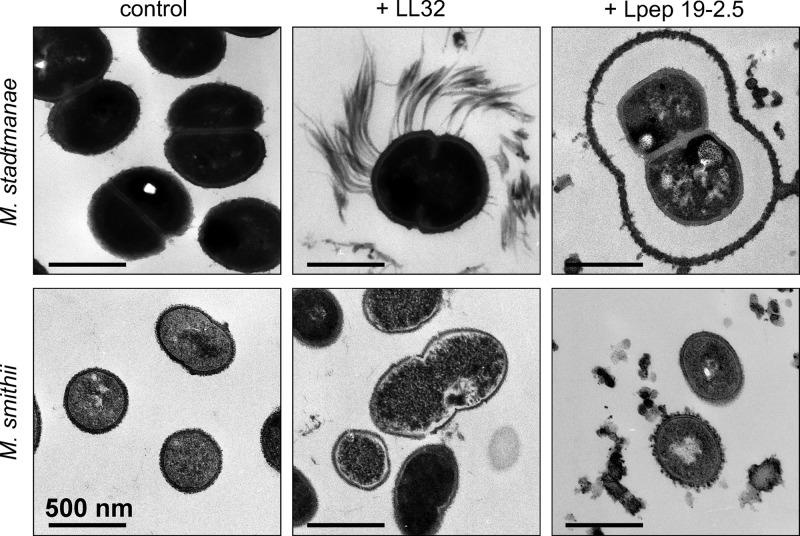
Using TEM, it was possible to obtain a more detailed picture of the morphological changes resulting from AMP treatment of M. stadtmanae and M. smithii. As was already observed by AFM, not all cells of a culture were affected to the same extent, indicating heterogeneity of the methanoarchaeal cultures. Probably due to the preparation procedures, transmission electron micrographs of cells of M. mazei could not be obtained.
For M. smithii, similar morphological effects of both tested peptides were observed. LL32 induced membrane ruffling and membrane detachment, which in strongly affected cells appeared to lead to release of cytoplasmic material from the cells of M. smithii (Fig. 5). On the other hand, when treated with Lpep 19-2.5, only membrane ruffling and partial membrane destruction was observed. M. smithii cells from untreated controls showed a dense cell wall that is surrounded by a secondary diffuse layer. In contrast to control cells, the cell wall of AMP-treated cells seemed to be less dense and the outer layer was diminished (after treatment with LL32) or even more diffuse (after treatment with Lpep 19-2.5) than in control cells.
Fig 5.
Effects of LL32 and Lpep 19.2-5 treatment on the morphology of M. stadtamanae and M. smithii examined by transmission electron microscopy (TEM). Shown are the TEM results from untreated cultures (controls) and cultures that were incubated with their respective MICs: 5 μM LL32 or 10 μM Lpep 19-2.5 for M. stadtmanae and 1 μM LL32 or 3 μM Lpep 19-2.5 for M. smithii. Cultures were grown until the mid-exponential phase at 37°C, adjusted to 1 × 107 cells in fresh minimal medium, and incubated with AMPs for 4 h. Images are representative of the respective samples.
TEM micrographs of M. stadtmanae illustrated a composition of the cell envelope similar to that observed for M. smithii, consisting of a bilayer membrane, a cell wall, and an outer layer (Fig. 5, control). Treatment of M. stadtmanae with LL32 and Lpep 19-2.5 revealed different effects of the peptides on cell morphology. For LL32-treated cells, filamentous structures obviously originating from the outer layer were observed. Cell size, cell membrane, and cellular organization appeared not to be affected after 4 h of incubation (Fig. 5). However, after Lpep 19-2.5 treatment, a part of the outer layer was detached from the cell wall and expanded. For some cells, this layer was already destroyed while their cell wall was still intact. In general, in all cells only a minor increase in cell size was observed; however, their membranes were ruffled to different extents.
DISCUSSION
The fact that archaea not only occupy extreme environments but are also permanent part of the human microbiota (11, 12, 30, 49, 57, 72) has become accepted in the last decade (19, 31, 51). However, since their abundance and diversity in humans are far below those reported for bacteria (30, 51, 55, 60, 62, 69) and obviously no archaeal pathogen has been reported so far (19, 31), archaea were omitted or overlooked in many studies dealing with microbial composition and antimicrobial susceptibility. Only a few studies have been reported on the efficiency of clinically relevant antibiotics on archaea (26). Moreover, to date research on the efficiency of AMPs has been mainly focused on bacteria. This attracted us to study the effects of several AMPs with regard to growth inhibition and morphological changes of two methanoarchaeal strains that are considered to be inhabitants of the human gut (66).
Growth analysis of methanoarchaea in anaerobic microtiter plates.
The effects of AMPs are generally tested by counting CFU on agar plates or using a broth microdilution assay (39, 44). Both assays conflict with the growth requirements of the methanoarchaea tested in this study: besides the anoxic atmosphere, the inability to grow on agar plates and the need for a specific gas atmosphere containing gases like hydrogen and carbon dioxide as growth substrates further complicate such tests. Using the respectively adapted microdilution assays for methanoarchaea, we were able to demonstrate that structurally different AMPs, like cathelicidin and NK-lysin derivatives, as well as a synthetic antilipopolysaccharide peptide (Lpep), affect methanoarchaea. Due to the higher starting cell number that has to be used for the anaerobic cultivation of methanoarchaea, the MICs of the AMPs determined here are not directly comparable to MICs reported for bacteria (5, 8). However, under test conditions comparable to that used for the methanoarchaea, growth of E. coli WBB01 was only slightly influenced by the presence of the tested AMPs in concentrations inhibitory for methanoarchaea. Since peptides were functionally active against E. coli WBB01 under common test conditions using 1 × 105 cells in 250 μl, the results presented here argue for an AMP sensitivity of methanoarchaea comparable to or even higher than that reported for bacteria, which are well inhibited or killed by AMPs in the nanomolar concentration (20- to 200-nM) range, as described for LL37 (70). Collaterally, the insensitivity of E. coli under the chosen test conditions might probably result from the amount of peptides sequestered by more bacterial lipopolysaccharide (LPS) due to the higher cell number (7) and/or a changed net charge, structural order, or fluidity of the E. coli inner membrane under the changed physiological conditions during anaerobic growth in minimal medium. The latter growth conditions have been described by other groups to be variables influencing antimicrobial efficiency of AMPs (18, 23, 42, 58).
Inhibitory effects of AMPs on methanoarchaea.
Although all methanoarchaeal strains tested were sensitive against the tested AMPs, M. mazei showed the highest susceptibility. Microscopic examination using LIVE/DEAD staining confirmed a tremendous increase in cell size, followed by cell disruption already after short incubation times, probably due to the osmotic stress proposed by the membrane lytic activity of the tested peptides. This sensitivity may be rooted to the cell wall and cell membrane chemistry of M. mazei. The strain lacks the pseudomurein cell wall of M. stadtmanae and M. smithii. Instead, its cell wall is composed of a porous proteinaceous surface layer (S-layer) covered with methanochondroitin (2, 47), an acidic heteropolysaccharide similar to the eukaryotic chondroitin, which is essential for the formation of the sarcinal clusters (63). The S-layer of M. mazei is composed of an unusual high number of glycosylated and nonglycosylated proteins that result in a weaker or not crystalline behavior, as is typical for most S-layers composed of only two proteins (35). This might facilitate the passage of the peptides to the cell membrane.
Beyond probable intracellular modes of action, which have already been described for some AMPs (18, 45), the first event interfering with cellular functions and viability is the interaction of a peptide with the microbial cell membrane. The M. mazei cell membrane has a different composition related to the cytoplasmic membranes of M. smithii and M. stadtmanae, consisting only of archaeols and hydroxyarchaeols that constitute a bilayer membrane (48, 67). The membranes of M. smithii and M. stadtmanae are composed of substantial amounts of caldarcheols (13 to 40%), the monolayer-forming tetraether lipids (68) that influence the ordered structure of the membrane (1, 40, 71). A dependence of bacterial AMP susceptibility on the structural order of membrane lipids has been shown for the Gram-positive bacterium Staphylococcus aureus being less sensitive against human defensins when carotenoids increase rigidity of its cell membrane (58). The archaeols and hydroxyarchaeols of M. mazei are composed of the phosphatidylserine, ethanolamine, glycerol, and inositol derivatives of the isoprenyl ethers (48, 67). Especially phosphatidylserine and phosphatidylglycerol, which also occur as hydrophilic head groups in the fatty acid esters of bacterial membranes and make up the majority of polar head groups of M. mazei (67), are responsible for the negative membrane charge and thus increase the influence of the interaction of the cationic charged peptide with the membrane (4, 21, 24). Furthermore, in contrast to M. mazei, the membrane lipids of M. stadtmanae and M. smithii are composed of nonpolar phosphoglycolipids to a great extent: ∼80% in M. stadtmanae and ∼50% in M. smithii (68). Phosphoglycolipids are described to stabilize the archaeal membrane structure (71). Thus, the higher AMP sensitivity of M. mazei compared to those of M. smithii and M. stadtmanae might result from a more disordered, less rigid, and a more negatively charged cytoplasmic membrane. Besides, it should also be considered that membrane proteins modify the structure and fluidity of the membrane (27) and will thereby contribute to the interactions of membrane compounds with AMPs. To verify these assumptions and to gain an insight into the modes of action of the AMPs, biophysical studies on planar membranes and membrane vesicles built from isolated membrane compounds of the methanoarchaeal strains are under way.
Influence of peptides on the ultrastructure of methanoarchaea.
The peptides LL32 and Lpep 19-2.5 show distinct MICs against M. smithii and M. stadtmanae, and in addition, AFM and TEM micrographs underline different modes of action on the ultrastructure of cells of both strains. In accordance with other descriptions, LL32 as a derivative of the human peptide LL37 is proposed to have a membrane lytic effect (28, 61), which is also clearly visible for Lpep 19-2.5 on cells of M. smithii. The increased roughness of the outermost layer of M. smithii cells induced by the synthetic peptide points toward an additional interaction of the peptide with polysaccharides of the cell envelope, as described for the interaction of AMPs with anionic heparan sulfate of mammalian surfaces (45). Both strains of M. smithii and M. stadtmanae possess the genetic equipment to vary their capsular polysaccharides according to the host environment (36, 65) as has been shown for M. smithii in mouse colonization experiments (64).
A weak membrane lytic effect of both peptides has also been observed on cells of M. stadtmanae, although TEM micrographs exhibit a more pronounced effect on the cell wall of this archaeal strain. The appendages on M. stadtmanae cells caused by treatment with LL32 could be either part of the cell wall polymer dismantled from the cell's surface or aggregated pili (R. Rachel, University of Regensburg, personal communication). Type IV pili and archaeal flagella with diameters of ∼10 to 20 nm are known from several archaea to fulfill different tasks (3). In the hyperthermophile crenarcheaon Sulfolobus solfataricus, type IV pili are induced by environmental stress and formation of pili appears to be a highly dynamic reaction occurring within 1.5 h (37, 38). However, no pili have been detected on the surface of M. stadtmanae so far, and the genetic machinery necessary to build these structures has not been detected in its genome (36). On the other hand, M. stadtmanae cells easily aggregate in biofilms (C. Ehlers, University of Kiel, personal communication). Whether this process is supported by exopolysaccharide disposal or by formation of pili is not known, but both processes are accompanied by cellular aggregation. The effect obtained after treatment with Lpep 19-2.5 clearly demonstrates its severe interaction with capsular polysaccharides of M. stadtmanae, leading to a swelling of the surrounding layer, which is detached from the cell wall and almost twice as thick as the layer without treatment. The interaction of AMPs (α-defensins, LL37, and hBD3) with bacterial exopolysaccharides of lung pathogens has already been described (13, 34, 52). Although this was found to reduce the activity of the AMPs on E. coli, an about 3-fold enlargement of the exopolysaccharide fibers has been observed using AFM (34). Further experiments might show if the extended polysaccharide layer could also be a result of a rapid response of M. stadtmanae by induced polysaccharide production. Such studies might be hampered by our observation that not all cells in a certain growth phase of M. stadtmanae and M. smithii appeared to be equally sensitive to the peptides and thus resulted in differently affected cells detected by TEM. Those observed differences might result from a different metabolic or cell cycle state of the cells. This is also evident by constantly observed variations of the intrinsic fluorescence caused by cofactor F420, an electron carrier in methanogenesis and thus a marker for metabolically active cells during cultivation. This observed heterogeneity within the methanoarchaeal cultures may be responsible for the inhomogeneous effects of the AMPs and could thus be a survival strategy with regard to their natural living habitats. Additionally it is likely that cells in a culture do not necessarily have a homogenous membrane architecture, since, e.g., the tetraether lipids are also discussed to influence membrane bending (59) and proteins are assembled to the membrane in a specific manner, thereby influencing the membrane structure and properties (27).
Conclusions and outlook.
Our studies clearly show that the methanoarchaea M. stadtmanae and M. smithii as a part of the human gut microbiota are, like bacteria and eukaryotic cells, prone to the lytic effects of AMPs, whose release is an essential eukaryotic epithelial defense mechanism. Whether the effects of the AMPs could be mainly ascribed to the membrane interaction with the structurally different ether lipids or an additional intracellular mode of action has to be further studied using biophysical and molecular biological methods. Furthermore, evaluation of the efficiency of human-derived AMPs secreted by gut epithelial cells (e.g., α- and β-defensins), against methanoarchaeal strains in comparison to bacteria is compulsory in order to speculate if and how those AMPs might influence the community structure of the human gut flora.
ACKNOWLEDGMENTS
We dedicate this article to Gerhard Gottschalk on the occasion of his 77th birthday.
This work was financially supported by the Deutsche Forschungsgemeinschaft (DFG) (SCHM1051/11-1).
We gratefully acknowledge Otto Holst and Jörg Andrä (Research Center Borstel) for providing the synthetic AMPs and Reinhard Rachel (University of Regensburg) for fruitful discussions about the electron micrographs.
Footnotes
Published ahead of print 14 May 2012
REFERENCES
- 1. Albers SV, van de Vossenberg JL, Driessen AJ, Konings WN. 2000. Adaptations of the archaeal cell membrane to heat stress. Front. Biosci. 5:D813–D820 [DOI] [PubMed] [Google Scholar]
- 2. Albers S-V, Meyer BH. 2011. The archaeal cell envelope. Nat. Rev. Microbiol. 9:414–426 [DOI] [PubMed] [Google Scholar]
- 3. Albers S-V, Pohlschröder M. 2009. Diversity of archaeal type IV pilin-like structures. Extremophiles 13:403–410 [DOI] [PubMed] [Google Scholar]
- 4. Andrä J, Goldmann T, Ernst CM, Peschel A, Gutsmann T. 2011. Multiple peptide resistance factor (MprF)-mediated resistance of Staphylococcus aureus against antimicrobial peptides coincides with a modulated peptide interaction with artificial membranes comprising lysyl-phosphatidylglycerol. J. Biol. Chem. 286:18692–18700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andrä J, et al. 2007. Mechanism of interaction of optimized Limulus-derived cyclic peptides with endotoxins: thermodynamic, biophysical and microbiological analysis. Biochem. J. 406:297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrä J, et al. 2008. Structure and mode of action of the antimicrobial peptide arenicin. Biochem. J. 410:113–122 [DOI] [PubMed] [Google Scholar]
- 7. Andrä J, et al. 2004. Cyclic antimicrobial peptides based on Limulus anti-lipopolysaccharide factor for neutralization of lipopolysaccharide. Biochem. Pharmacol. 68:1297–1307 [DOI] [PubMed] [Google Scholar]
- 8. Andrä J, et al. 2007. Rationale for the design of shortened derivatives of the NK-lysin-derived antimicrobial peptide NK-2 with improved activity against Gram-negative pathogens. J. Biol. Chem. 282:14719–14728 [DOI] [PubMed] [Google Scholar]
- 9. Barns SM, Delwiche CF, Palmer JD, Pace NR. 1996. Perspectives on archaeal diversity, thermophily and monophyly from environmental rRNA sequences. Proc. Natl. Acad. Sci. U. S. A. 93:9188–9193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barns SM, Fundyga RE, Jeffries MW, Pace NR. 1994. Remarkable archaeal diversity detected in a Yellowstone National Park hot spring environment. Proc. Natl. Acad. Sci. U. S. A. 91:1609–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Belay N, Johnson R, Rajagopal BS, Conway de Macario E, Daniels L. 1988. Methanogenic bacteria from human dental plaque. Appl. Environ. Microbiol. 54:600–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belay N, Mukhopadhyay B, Conway de Macario E, Galask R, Daniels L. 1990. Methanogenic bacteria in human vaginal samples. J. Clin. Microbiol. 28:1666–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benincasa M, et al. 2009. Activity of antimicrobial peptides in the presence of polysaccharides produced by pulmonary pathogens. J. Peptide Sci. 15:595–600 [DOI] [PubMed] [Google Scholar]
- 14. Beveridge TJ, Choquet CG, Patel GB, Sprott GD. 1993. Freeze-fracture planes of methanogen membranes correlate with the content of tetraether lipids. J. Bacteriol. 175:1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boman HG. 1994. Antimicrobial peptides. Chairman's opening remarks. Ciba Found. Symp. 186:1–4 [PubMed] [Google Scholar]
- 16. Boman HG. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61–92 [DOI] [PubMed] [Google Scholar]
- 17. Brabetz W, Müller-Loennies S, Holst O, Brade H. 1997. Deletion of the heptosyltransferase genes rfaC and rfaF in Escherichia coli K-12 results in an Re-type lipopolysaccharide with a high degree of 2-aminoethanol phosphate substitution. Eur. J. Biochem. 247:716–724 [DOI] [PubMed] [Google Scholar]
- 18. Brogden KA. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238–250 [DOI] [PubMed] [Google Scholar]
- 19. Cavicchioli R, Curmi PMG, Saunders N, Thomas T. 2003. Pathogenic archaea: do they exist? Bioessays 25:1119–1128 [DOI] [PubMed] [Google Scholar]
- 20. Reference deleted.
- 21. Clausell A, Pujol M, Alsina MA, Cajal Y. 2003. Influence of polymyxins on the structural dynamics of Escherichia coli lipid membranes. Talanta 60:225–234 [DOI] [PubMed] [Google Scholar]
- 22. De Rosa M, Gambacorta A, Gliozzi A. 1986. Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol. Rev. 50:70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dorschner Ra, et al. 2006. The mammalian ionic environment dictates microbial susceptibility to antimicrobial defense peptides. FASEB J. 20:35–42 [DOI] [PubMed] [Google Scholar]
- 24. Dowhan W. 1997. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu. Rev. Biochem. 66:199–232 [DOI] [PubMed] [Google Scholar]
- 25. Dridi B, Henry M, El Khéchine A, Raoult D, Drancourt M. 2009. High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS One 4:e7063 doi:10.1371/journal.pone.0007063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dridi B, Raoult D, Drancourt M. 2011. Archaea as emerging organisms in complex human microbiomes. Anaerobe 17:56–63 [DOI] [PubMed] [Google Scholar]
- 27. Dumas F, Lebrun MC, Tocanne JF. 1999. Is the protein/lipid hydrophobic matching principle relevant to membrane organization and functions? FEBS Lett. 458:271–277 [DOI] [PubMed] [Google Scholar]
- 28. Dürr UHN, Sudheendra US, Ramamoorthy A. 2006. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 1758:1408–1425 [DOI] [PubMed] [Google Scholar]
- 29. Eastman T, Zhu D-M. 1996. Adhesion forces between surface-modified AFM tips and a mica surface. Langmuir 12:2859–2862 [Google Scholar]
- 30. Eckburg PB, et al. 2005. Diversity of the human intestinal microbial flora. Science 308:1635–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eckburg PB, Lepp PW, Relman DA. 2003. Archaea and their potential role in human disease. Infect. Immun. 71:591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ehlers C, Grabbe R, Veit K, Schmitz RA. 2002. Characterization of GlnK1 from Methanosarcina mazei strain Gö1: complementation of an Escherichia coli glnK mutant strain by GlnK1. J. Bacteriol. 184:1028–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Epand RM, Vogel HJ. 1999. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1462:11–28 [DOI] [PubMed] [Google Scholar]
- 34. Foschiatti M, Cescutti P, Tossi A, Rizzo R. 2009. Inhibition of cathelicidin activity by bacterial exopolysaccharides. Mol. Microbiol. 72:1137–1146 [DOI] [PubMed] [Google Scholar]
- 35. Francoleon DR, et al. 2009. S-layer, surface-accessible, and concanavalin A binding proteins of Methanosarcina acetivorans and Methanosarcina mazei. J. Proteome Res. 8:1972–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fricke WF, et al. 2006. The genome sequence of Methanosphaera stadtmanae reveals why this human intestinal archaeon is restricted to methanol and H2 for methane formation and ATP synthesis. J. Bacteriol. 188:642–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fröls S, et al. 2008. UV-inducible cellular aggregation of the hyperthermophilic archaeon Sulfolobus solfataricus is mediated by pili formation. Mol. Microbiol. 70:938–952 [DOI] [PubMed] [Google Scholar]
- 38. Fröls S, et al. 2007. Response of the hyperthermophilic archaeon Sulfolobus solfataricus to UV damage. J. Bacteriol. 189:8708–8718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giacometti A, et al. 2000. Combination studies between polycationic peptides and clinically used antibiotics against Gram-positive and Gram-negative bacteria. Peptides 21:1155–1160 [DOI] [PubMed] [Google Scholar]
- 40. Gliozzi A, Relini A, Chong PL-G. 2002. Structure and permeability properties of biomimetic membranes of bolaform archaeal tetraether lipids. J. Membr. Sci. 206:131–147 [Google Scholar]
- 41. Gutsmann T, Hagge SO, Larrick JW, Seydel U, Wiese A. 2001. Interaction of CAP18-derived peptides with membranes made from endotoxins or phospholipids. Biophys. J. 80:2935–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gutsmann T, et al. 2005. Lipid-mediated resistance of Gram-negative bacteria against various pore-forming antimicrobial peptides. J. Endotoxin Res. 11:167–173 [DOI] [PubMed] [Google Scholar]
- 43. Hancock RE, Chapple DS. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hecht DW, et al. 2007. In vitro activities of 15 antimicrobial agents against 110 toxigenic clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob. Agents Chemother. 51:2716–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jenssen H, Hamill P, Hancock REW. 2006. Peptide antimicrobial agents. Clin. Microbiol. Rev. 19:491–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kagan BL, Sokolov Y. 1994. Use of lipid bilayer membranes to detect pore formation by toxins. Methods Enzymol. 235:691–705 [DOI] [PubMed] [Google Scholar]
- 47. Kandler O, König H. 1998. Cell wall polymers in Archaea (Archaebacteria). Cell. Mol. Life Sci. 54:305–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Koga Y, Morii H, Akagawa-Matsushita M, Ohga M. 1998. Correlation of polar lipid composition with 16S rRNA phylogeny in methanogens. Further analysis of lipid component parts. Biosci. Biotechnol. Biochem. 62:230–236 [DOI] [PubMed] [Google Scholar]
- 49. Kulik EM, Sandmeier H, Hinni K, Meyer J. 2001. Identification of archaeal rDNA from subgingival dental plaque by PCR amplification and sequence analysis. FEMS Microbiol. Lett. 196:129–133 [DOI] [PubMed] [Google Scholar]
- 50. Lehrer RI, Ganz T. 1999. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 11:23–27 [DOI] [PubMed] [Google Scholar]
- 51. Ley RE, Peterson DA, Gordon JI. 2006. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124:837–848 [DOI] [PubMed] [Google Scholar]
- 52. Llobet E, Tomás JM, Bengoechea JA. 2008. Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology (Reading, England) 154:3877–3886 [DOI] [PubMed] [Google Scholar]
- 53. Lotz M, Ménard S, Hornef M. 2007. Innate immune recognition on the intestinal mucosa. Int. J. Med. Microbiol. 297:379–392 [DOI] [PubMed] [Google Scholar]
- 54. McNeil NI. 1984. The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr. 39:338–342 [DOI] [PubMed] [Google Scholar]
- 55. Mihajlovski A, Alric M, Brugère J-F. 2008. A putative new order of methanogenic Archaea inhabiting the human gut, as revealed by molecular analyses of the mcrA gene. Res. Microbiol. 159:516–521 [DOI] [PubMed] [Google Scholar]
- 56. Miller TL, Weaver GA, Wolin MJ. 1984. Methanogens and anaerobes in a colon segment isolated from the normal fecal stream. Appl. Environ. Microbiol. 48:449–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miller TL, Wolin MJ, Conway de Macario E, Macario AJ. 1982. Isolation of Methanobrevibacter smithii from human feces. Appl. Environ. Microbiol. 43:227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mishra NN, et al. 2011. Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob. Agents Chemother. 55:526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mykytczuk NCS, Trevors JT, Leduc LG, Ferroni GD. 2007. Fluorescence polarization in studies of bacterial cytoplasmic membrane fluidity under environmental stress. Prog. Biophys. Mol. Biol. 95:60–82 [DOI] [PubMed] [Google Scholar]
- 60. Oxley AP, et al. 2010. Halophilic archaea in the human intestinal mucosa. Environ. Microbiol. 12:2398–2410 [DOI] [PubMed] [Google Scholar]
- 61. Porcelli F, et al. 2008. NMR structure of the cathelicidin-derived human antimicrobial peptide LL-37 in dodecylphosphocholine micelles. Biochemistry 47:5565–5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rieu-Lesme F, Delbès C, Sollelis L. 2005. Recovery of partial 16S rDNA sequences suggests the presence of Crenarchaeota in the human digestive ecosystem. Curr. Microbiol. 51:317–321 [DOI] [PubMed] [Google Scholar]
- 63. Robinson RW, Aldrich HC, Hurst SF, Bleiweis AS. 1985. Role of the cell surface of Methanosarcina mazei in cell aggregation. Appl. Environ. Microbiol. 49:321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Samuel BS, Gordon JI. 2006. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc. Natl. Acad. Sci. U. S. A. 103:10011–10016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Samuel BS, et al. 2007. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc. Natl. Acad. Sci. U. S. A. 104:10643–10648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Scanlan PD, Shanahan F, Marchesi JR. 2008. Human methanogen diversity and incidence in healthy and diseased colonic groups using mcrA gene analysis. BMC Microbiol. 8:79 doi:10.1186/1471-2180-8-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sprott GD, Dicaire CJ, Patel GB. 1994. The ether lipids of Methanosarcina mazei and other Methanosarcina species, compared by fast atom bombardment mass spectrometry. Can. J. Microbiol. 40:837–843 [Google Scholar]
- 68. Sprott GD, et al. 1999. A structural comparison of the total polar lipids from the human archaea Methanobrevibacter smithii and Methanosphaera stadtmanae and its relevance to the adjuvant activities of their liposomes. Biochim. Biophys. Acta 1440:275–288 [DOI] [PubMed] [Google Scholar]
- 69. Stewart JA, Chadwick VS, Murray A. 2006. Carriage, quantification, and predominance of methanogens and sulfate-reducing bacteria in faecal samples. Lett. Appl. Microbiol. 43:58–63 [DOI] [PubMed] [Google Scholar]
- 70. Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI. 1998. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 42:2206–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ulrih NP, Gmajner D, Raspor P. 2009. Structural and physicochemical properties of polar lipids from thermophilic archaea. Appl. Microbiol. Biotechnol. 84:249–260 [DOI] [PubMed] [Google Scholar]
- 72. Vianna ME, Conrads G, Gomes BP, Horz HP. 2006. Identification and quantification of archaea involved in primary endodontic infections. J. Clin. Microbiol. 44:1274–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zasloff M. 1992. Antibiotic peptides as mediators of innate immunity. Curr. Opin. Immunol. 4:3–7 [DOI] [PubMed] [Google Scholar]
- 74. Zasloff M. 2002. Antimicrobial peptides in health and disease. New Engl. J. Med. 347:1199–1200 [DOI] [PubMed] [Google Scholar]