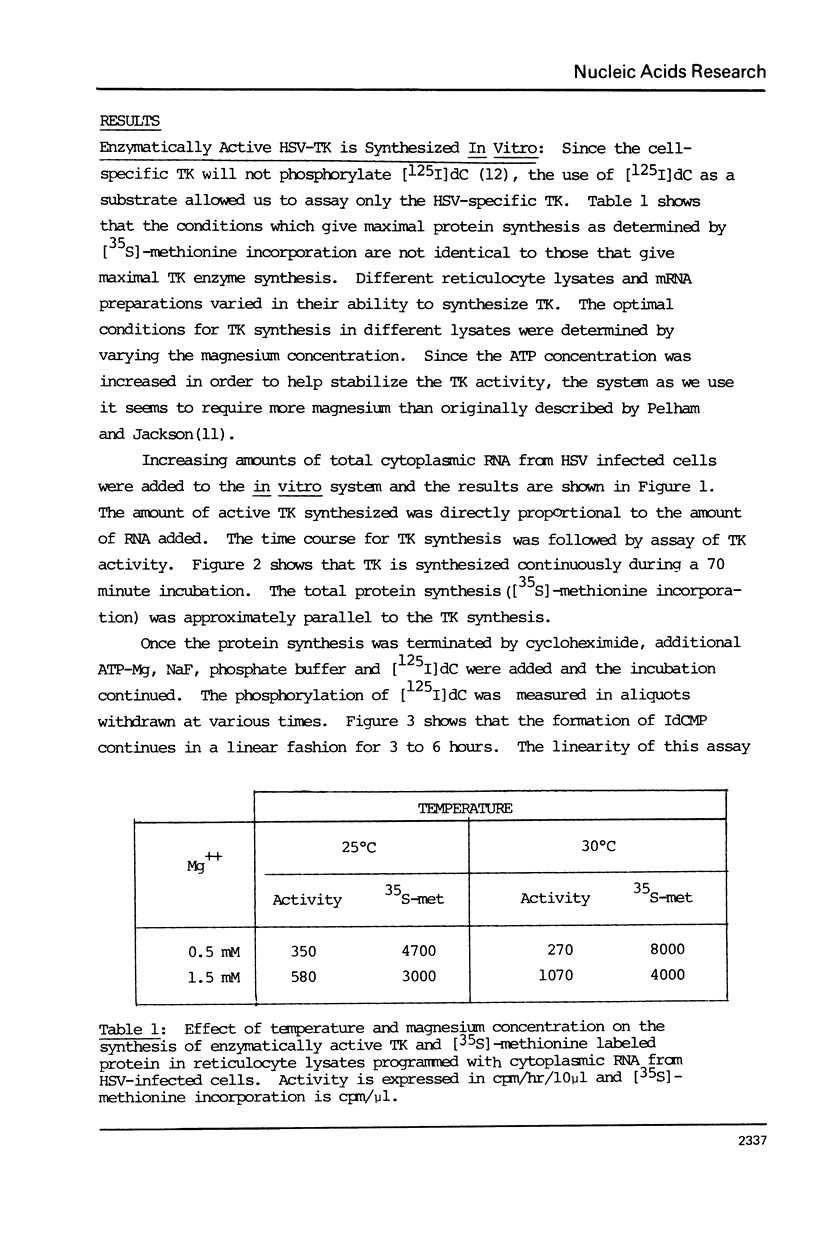
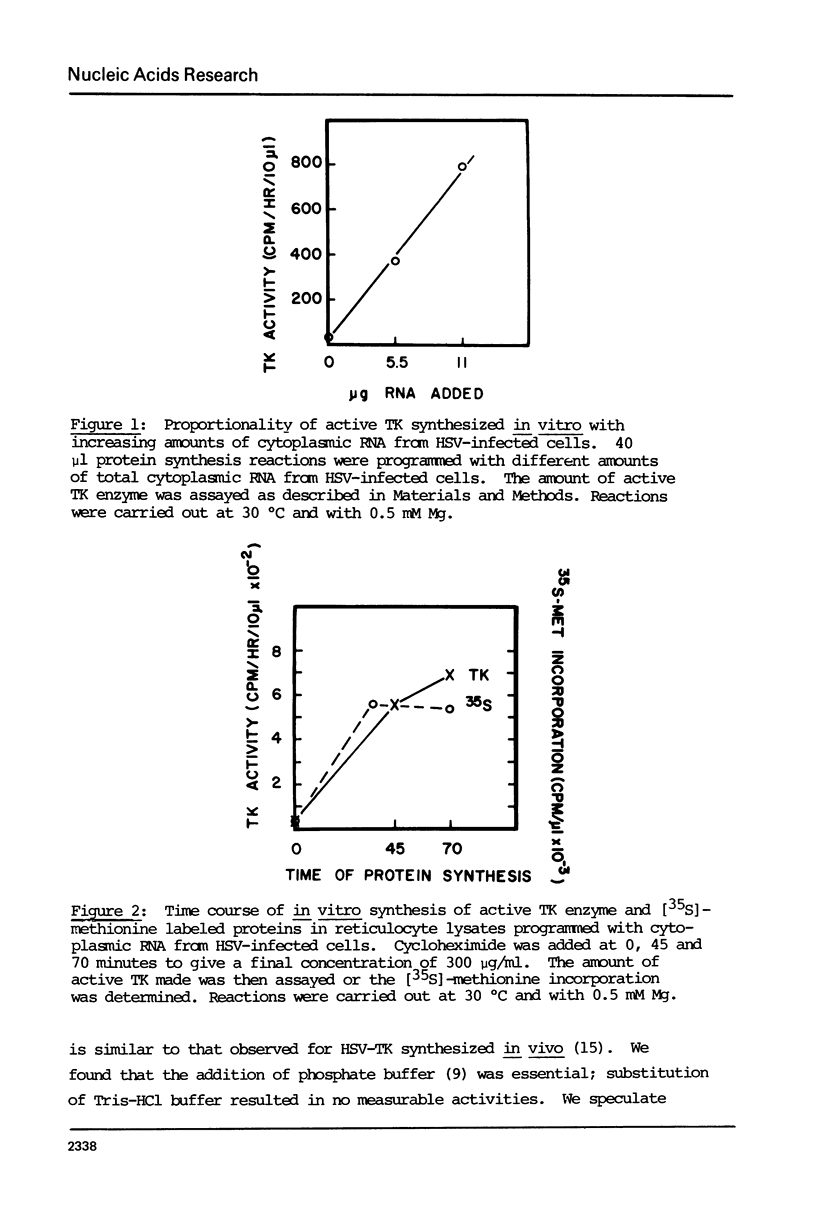
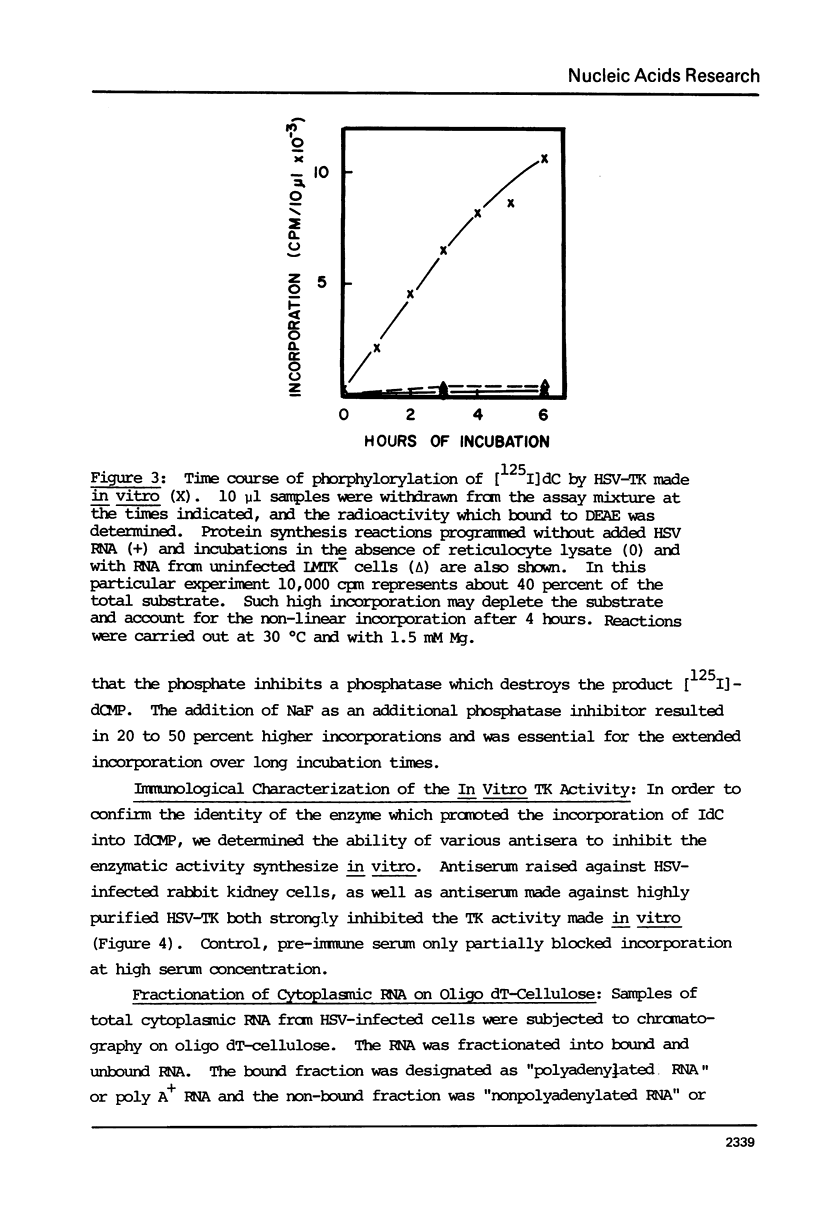
Abstract
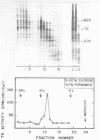
The cytoplasmic mRNA which codes for the herpes simplex virus specific thymidine kinase (TK) is polyadenylated and is 14.5s in size. This corresponds to an RNA of 1400 nucleotides. The TK polypeptide is about 42,000 daltons, which requires 1100 nucleotide. We conclude that the cytoplasmic mRNA is monocistronic. The reticulocyte lysate cell-free translation system synthesizes enzymatically active HSV-TK which can be assayed with high specificity and sensitivity by use of 125I-iododeoxycytidine as a substrate.
Full text
PDF



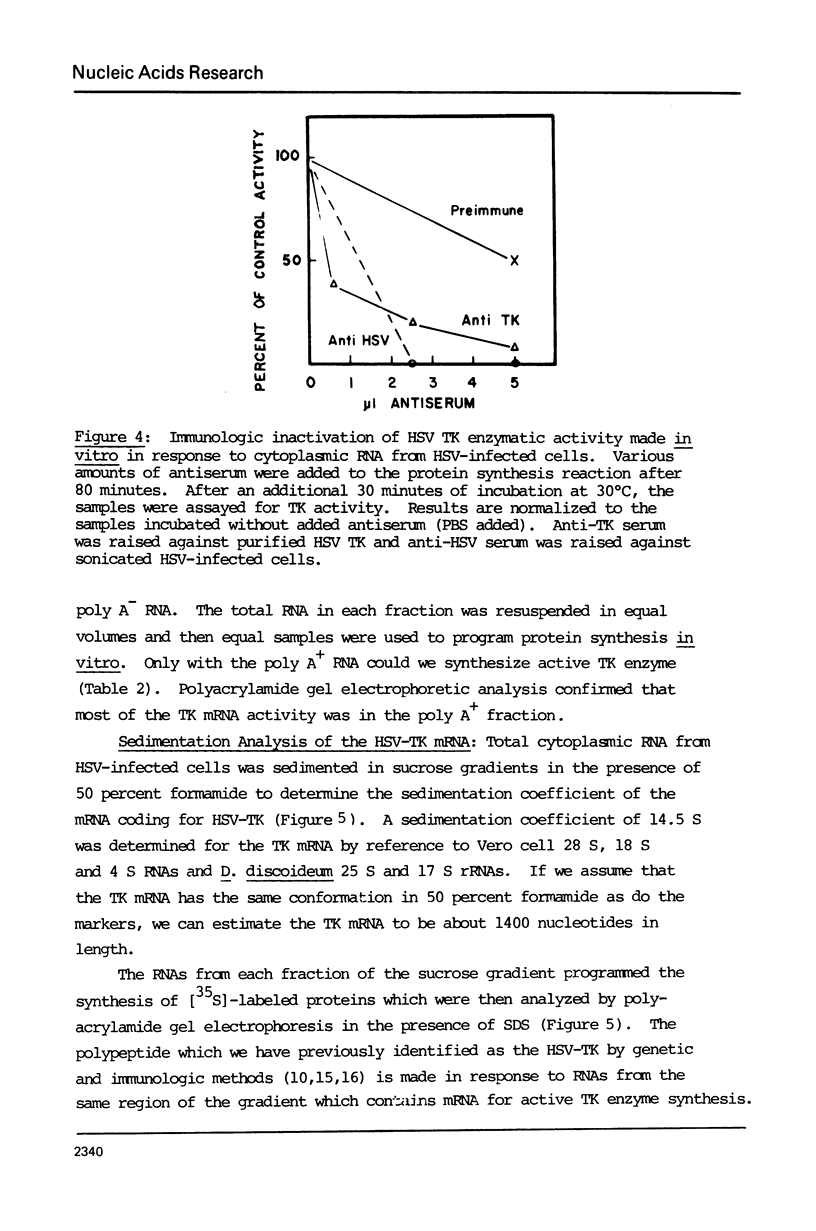

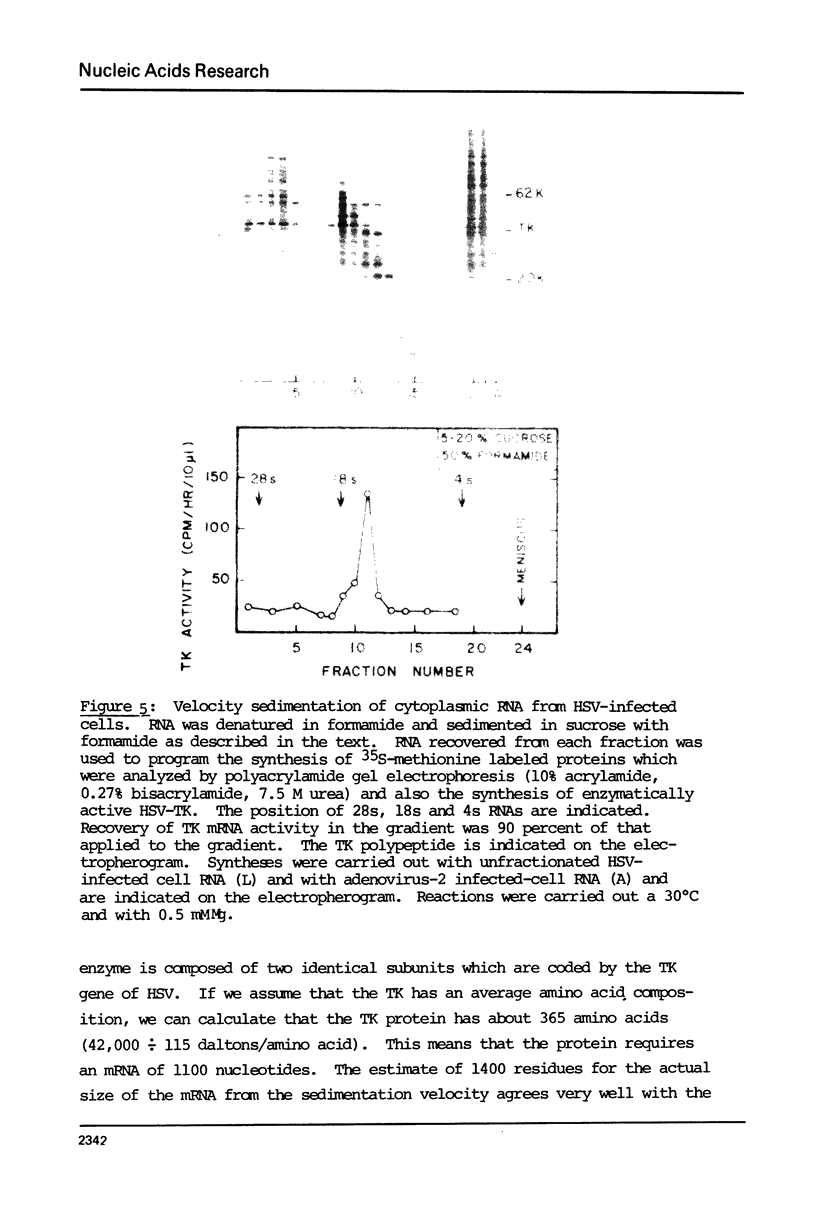


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Lewis J. B., Atkins J. F., Gesteland R. F. Cell-free synthesis of adenovirus 2 proteins programmed by fractionated messenger RNA: a comparison of polypeptide products and messenger RNA lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2756–2760. doi: 10.1073/pnas.71.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. B., Smith A. E. Monomer molecular weight of T antigen from simian virus 40-infected and transformed cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2254–2258. doi: 10.1073/pnas.73.7.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri R. L., Havell E. A., Vilcek J., Pestka S. Synthesis of human interferon by Xenopus laevis oocytes: two structural genes for interferons in human cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3287–3291. doi: 10.1073/pnas.74.8.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Chadha K. C., Hughes R. G., Jr Biochemical and immunological characterization of deoxythymidine kinase of thymidine kinaseless HeLa cells biochemically transformed by herpes simplex virus type. Infect Immun. 1977 May;16(2):486–492. doi: 10.1128/iai.16.2.486-492.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer K. J., Summers W. C., Gesteland R. F. Cell-free synthesis of herpes simplex virus proteins. J Virol. 1977 Jun;22(3):750–757. doi: 10.1128/jvi.22.3.750-757.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Absence of a requirement for host polypeptides in the herpes virus thymidine kinase. J Gen Virol. 1974 Jul;24(1):215–220. doi: 10.1099/0022-1317-24-1-215. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Anderson C. W., Atkins J. F. Further mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Cell. 1977 Sep;12(1):37–44. doi: 10.1016/0092-8674(77)90183-0. [DOI] [PubMed] [Google Scholar]
- Maitland N. J., McDougall J. K. Biochemical transformation of mouse cells by fragments of herpes simplex virus DNA. Cell. 1977 May;11(1):233–241. doi: 10.1016/0092-8674(77)90334-8. [DOI] [PubMed] [Google Scholar]
- Pawson T., Harvey R., Smith A. E. The size of Rous sarcoma virus mRNAs active in cell-free translation. Nature. 1977 Aug 4;268(5619):416–420. doi: 10.1038/268416a0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Cell-free synthesis of herpes simplex virus-coded pyrimidine deoxyribonucleoside kinase enzyme. J Virol. 1977 Sep;23(3):455–460. doi: 10.1128/jvi.23.3.455-460.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J. Adenovirus amazes at Cold Spring Harbor. Nature. 1977 Jul 14;268(5616):101–104. doi: 10.1038/268101a0. [DOI] [PubMed] [Google Scholar]
- Silvertien S., Millette R., Jones P., Roizman B. RNA synthesis in cells infected with herpes simplex virus. XII. Sequence complexity and properties of RNA differing in extent of adenylation. J Virol. 1976 Jun;18(3):977–991. doi: 10.1128/jvi.18.3.977-991.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer J. R., Holland L. E., Swanstrom R. I., Pivo K., Wagner E. K. Quantitation of herpes simplex virus type 1 RNA in infected HeLa cells. J Virol. 1977 Mar;21(3):889–901. doi: 10.1128/jvi.21.3.889-901.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C., Summers W. P. [125I]deoxycytidine used in a rapid, sensitive, and specific assay for herpes simplex virus type 1 thymidine kinase. J Virol. 1977 Oct;24(1):314–318. doi: 10.1128/jvi.24.1.314-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. P., Wagner M., Summers W. C. Possible peptide chain termination mutants in thymide kinase gene of a mammalian virus, herpes simplex virus. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4081–4084. doi: 10.1073/pnas.72.10.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Seidman J. G., Peterlin B. M., Sullivan M., Maizel J. V., Leder P. Intervening sequence of DNA identified in the structural portion of a mouse beta-globin gene. Proc Natl Acad Sci U S A. 1978 Feb;75(2):725–729. doi: 10.1073/pnas.75.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Woo S. L., Chandra T., Means A. R., O'Malley B. W. Ovalbumin gene: purification of the coding strand. Biochemistry. 1977 Dec 27;16(26):5670–5676. doi: 10.1021/bi00645a003. [DOI] [PubMed] [Google Scholar]