Abstract
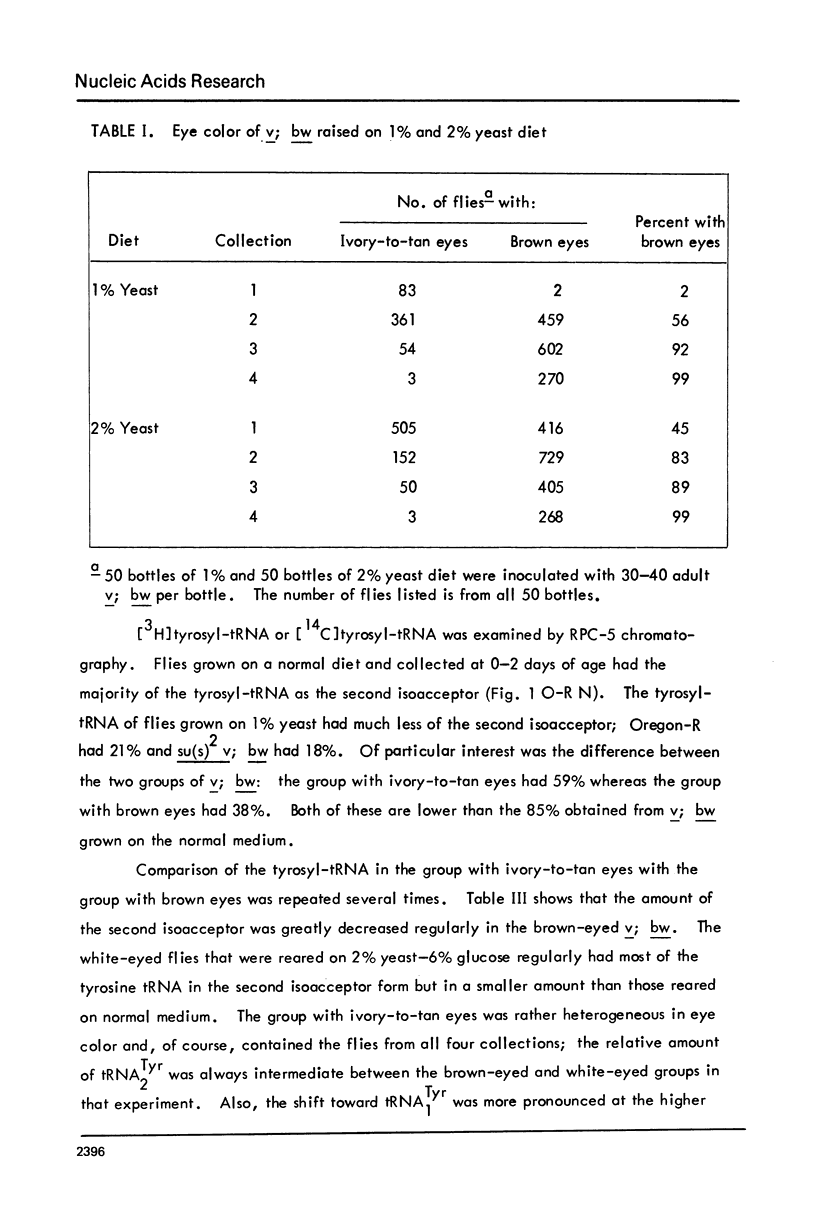
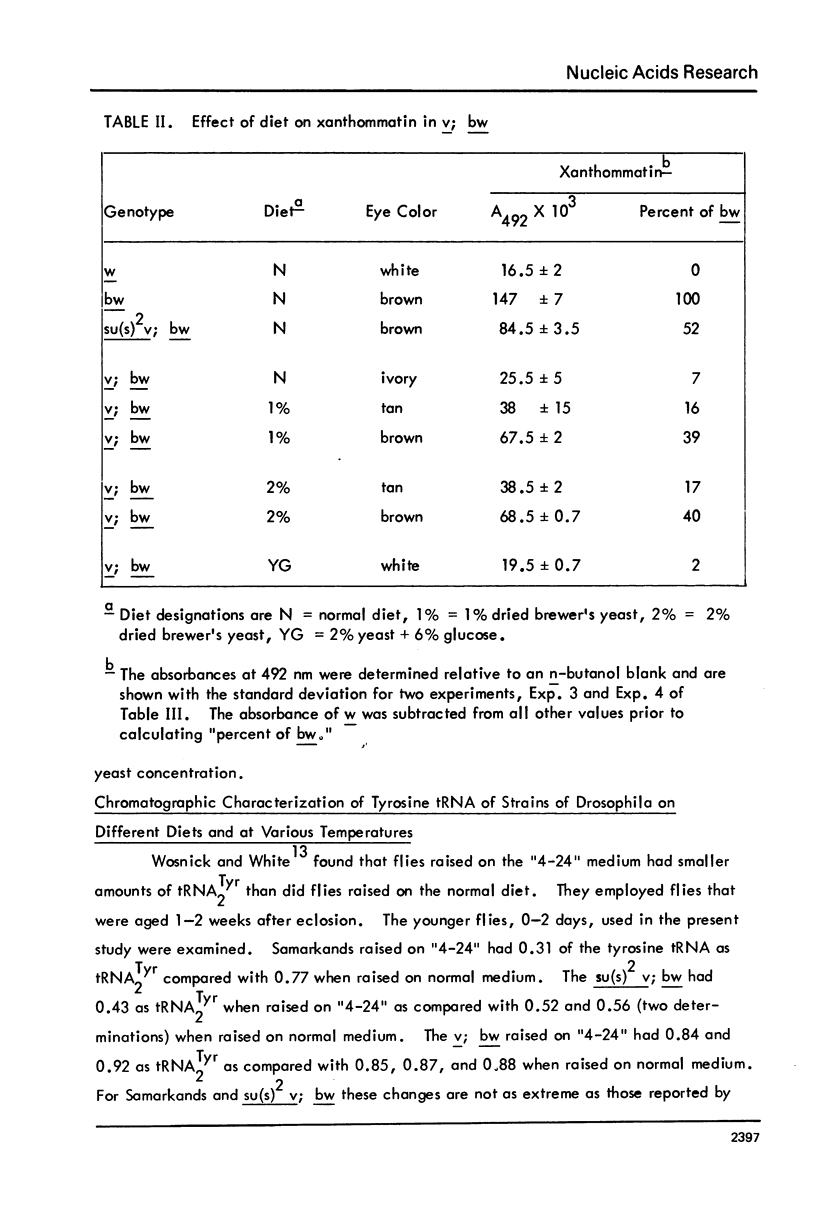
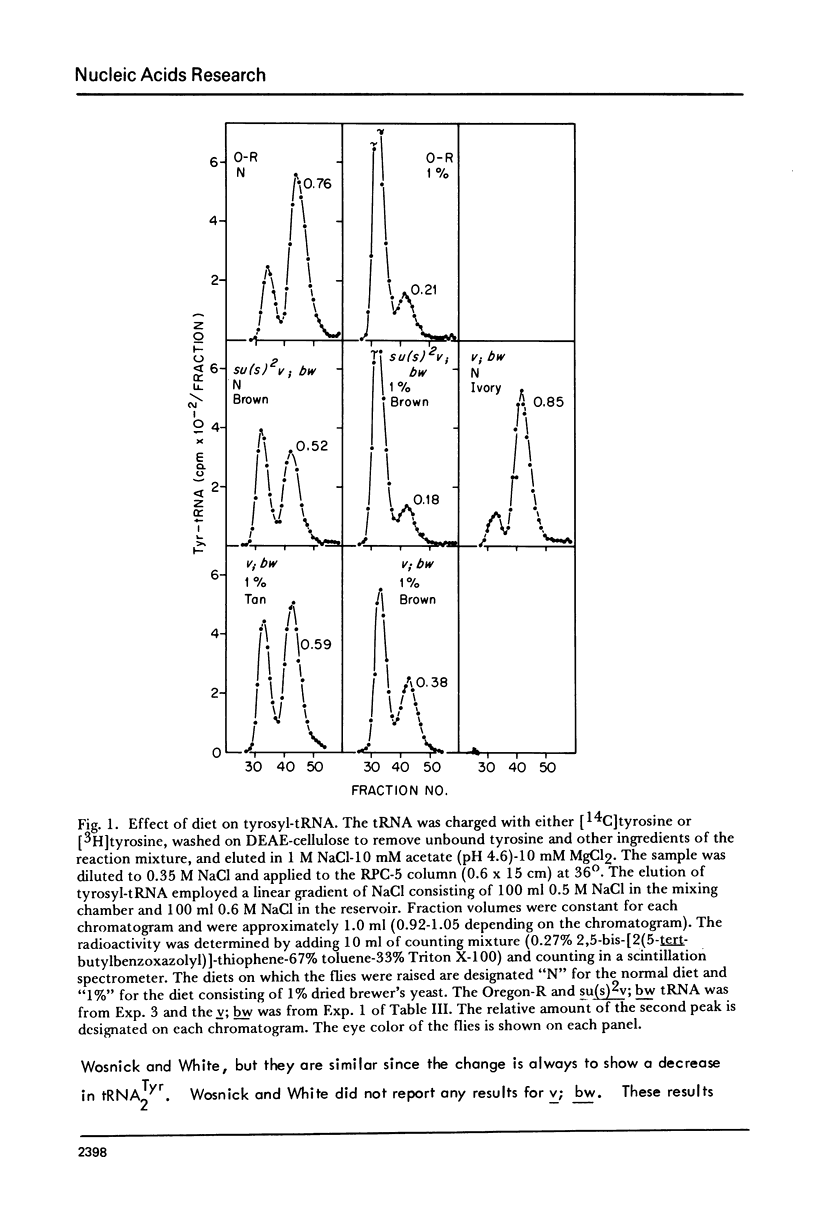
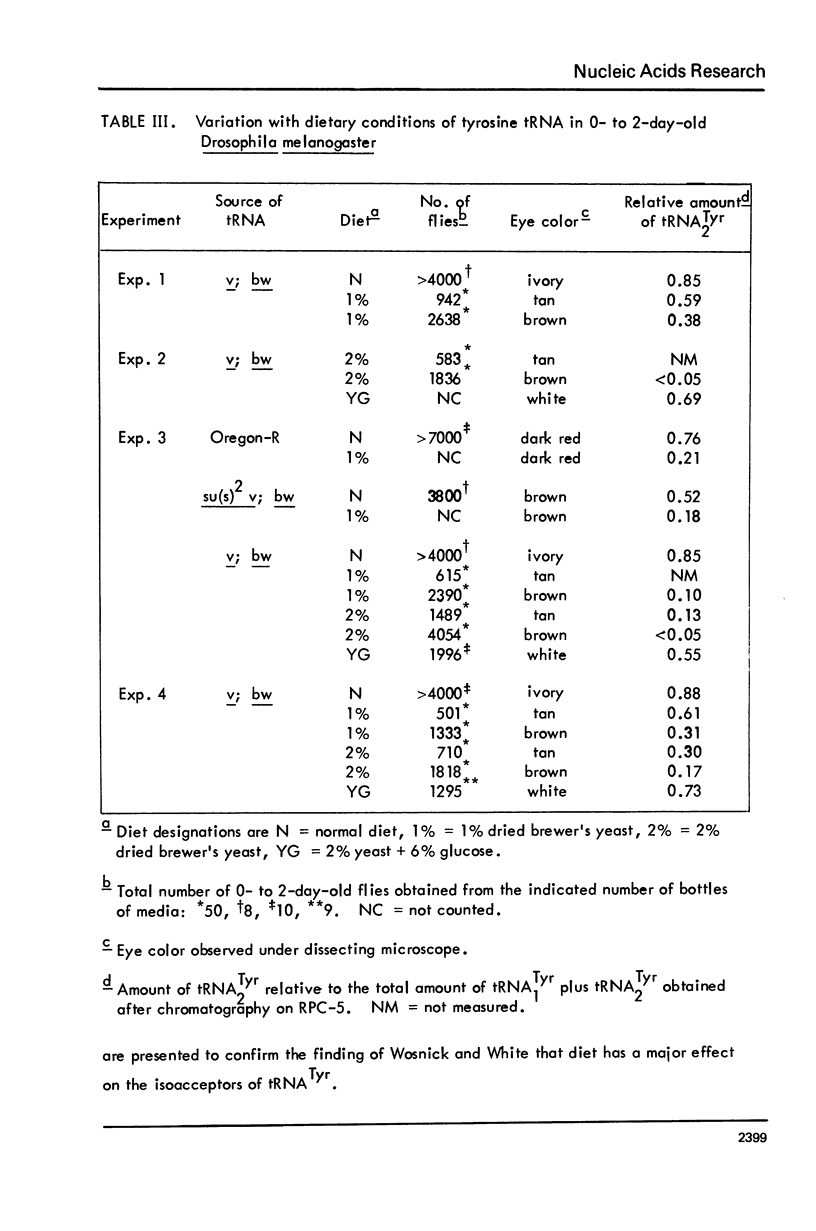
The possibility that tyrosine tRNA modifies the catalytic activity of tryptophan oxygenase that is produced by the vermilion mutant (v) in Drosophila melanogaster is reconsidered. Dietary conditions can modify the ratio of the two major isoacceptors of tyrosine tRNA: one condition allows 85--90% to exist as the second isoacceptor, and another condition allows less than 5% to exist in this form. The function lacking in the vermilion mutant is partially restored when the second isoacceptor of tRNATyr is reduced to low levels (less than 40%), but the function is greatly reduced when this isoacceptor is present as 50% or more of the total. These data support the hypothesis that tRNATyr may be associated with and regulate tryptophan oxygenase. The corresponding isoacceptor of tRNATyr found in a suppressor mutant, su(s)2, should not have any effect on the function of the vermilion gene, and, indeed, it did not. The tRNAs for tyrosine, aspartic acid, and histidine all have one isoacceptor that contains nucleoside Q and all undergo parallel changes in flies raised on the various diets. It appears that these dietary changes affect the ability to synthesize or modify Q or to remove or insert it into tRNA.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTENANDT A., BICKERT E., KUEBLER H., LINZEN B. [On ommochromes. 20. On the distribution of ommatin in animals. New methods of identification and quantitative determination]. Hoppe Seylers Z Physiol Chem. 1960;319:238–256. doi: 10.1515/bchm2.1960.319.1.238. [DOI] [PubMed] [Google Scholar]
- Beadle G. W., Ephrussi B. The Differentiation of Eye Pigments in Drosophila as Studied by Transplantation. Genetics. 1936 May;21(3):225–247. doi: 10.1093/genetics/21.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas W. R., Singh R. D. Guanylation of transfer ribonucleic acid by a cell-free lysate of rabbit reticulocytes. J Biol Chem. 1973 Nov 25;248(22):7780–7785. [PubMed] [Google Scholar]
- Green M. M. Mutant Isoalleles at the Vermilion Locus in Drosophila Melanogaster. Proc Natl Acad Sci U S A. 1952 Apr;38(4):300–305. doi: 10.1073/pnas.38.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. M. PSEUDO-ALLELISM AT THE VERMILION LOCUS IN DROSOPHILA MELANOGASTER. Proc Natl Acad Sci U S A. 1954 Feb;40(2):92–99. doi: 10.1073/pnas.40.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Nishimura S. Possible anticodon sequences of tRNA His , tRNA Asm , and tRNA Asp from Escherichia coli B. Universal presence of nucleoside Q in the first postion of the anticondons of these transfer ribonucleic acids. Biochemistry. 1972 Jan 18;11(2):301–308. doi: 10.1021/bi00752a024. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B., Calviño J. F., Murphy J. B., Warner C. K. Mechanism of suppression in Drosophila. H. Enzymatic discrimination of wild-type and suppressor tyrosine transfer RNA. J Mol Biol. 1975 Mar 25;93(1):89–97. doi: 10.1016/0022-2836(75)90362-9. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B. Role of an isoacceptor transfer ribonucleic acid as an enzyme inhibitor: effect on tryptophan pyrrolase of Drosophila. Nat New Biol. 1971 May 5;231(18):17–19. [PubMed] [Google Scholar]
- Kasai H., Kuchino Y., Nihei K., Nishimura S. Distribution of the modified nucleoside Q and its derivatives in animal and plant transfer RNA's. Nucleic Acids Res. 1975 Oct;2(10):1931–1939. doi: 10.1093/nar/2.10.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluf G. A. Enzymatic studies with the suppressor of vermilion of Drosophila melanogaster. Genetics. 1965 Sep;52(3):503–512. doi: 10.1093/genetics/52.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischke D., Kloetzel P., Schwochau M. Tryptophan pyrrolase activity regulation in Drosophila: role of an isoacceptor tRNA unsettled. Nature. 1975 May 1;255(5503):79–80. doi: 10.1038/255079a0. [DOI] [PubMed] [Google Scholar]
- Okada N., Harada F., Nishimura S. Specific replacement of Q base in the anticodon of tRNA by guanine catalyzed by a cell-free extract of rabbit reticulocytes. Nucleic Acids Res. 1976 Oct;3(10):2593–2603. doi: 10.1093/nar/3.10.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapard P B. A Physiological Study of the Vermilion Eye Color Mutants of Drosophila Melanogaster. Genetics. 1960 Apr;45(4):359–376. doi: 10.1093/genetics/45.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof K. D. Interacting gene systems: I. The regulation of tryptophan pyrrolase by the vermilion-suppressor of vermilion system in Drosophila. Genetics. 1969 Jul;62(3):781–795. [PMC free article] [PubMed] [Google Scholar]
- Twardzik D. R., Grell E. H., Jacobson K. B. Mechanism of suppression in Drosophila: a change in tyrosine transfer RNA. J Mol Biol. 1971 Apr 28;57(2):231–245. doi: 10.1016/0022-2836(71)90343-3. [DOI] [PubMed] [Google Scholar]
- White B. N., Tener G. M. Activity of a transfer RNA modifying enzyme during the development of Drosophila and its relationship to the su(s) locus. J Mol Biol. 1973 Mar 15;74(4):635–651. doi: 10.1016/0022-2836(73)90054-5. [DOI] [PubMed] [Google Scholar]
- Wosnick M. A., White B. N. A doubtful relationship between tyrosine tRNA and suppression of the vermilion mutant in Drosophila. Nucleic Acids Res. 1977 Nov;4(11):3919–3930. doi: 10.1093/nar/4.11.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]


