Abstract
Despite great effort by health organizations worldwide in fighting tuberculosis (TB), morbidity and mortality are not declining as expected. One of the reasons is related to the evolutionary development of Mycobacterium tuberculosis, in particular the Beijing genotype strains. In a previous study, we showed the association between the Beijing genotype and an increased mutation frequency for rifampin resistance. In this study, we use a Beijing genotype strain and an East-African/Indian genotype strain to investigate with our mouse TB model whether the higher mutation frequency observed in a Beijing genotype strain is associated with treatment failure particularly during noncompliance therapy. Both genotype strains showed high virulence in comparison to that of M. tuberculosis strain H37Rv, resulting in a highly progressive infection with a rapid lethal outcome in untreated mice. Compliance treatment was effective without relapse of TB irrespective of the infecting strain, showing similar decreases in the mycobacterial load in infected organs and similar histopathological changes. Noncompliance treatment, simulated by a reduced duration and dosing frequency, resulted in a relapse of infection. Relapse rates were correlated with the level of noncompliance and were identical for Beijing infection and East African/Indian infection. However, only in Beijing-infected mice, isoniazid-resistant mutants were selected at the highest level of noncompliance. This is in line with the substantial selection of isoniazid-resistant mutants in vitro in a wide isoniazid concentration window observed for the Beijing strain and not for the EAI strain. These results suggest that genotype diversity of M. tuberculosis may be involved in emergence of resistance and indicates that genotype-tailor-made treatment should be investigated.
INTRODUCTION
Resistance to antituberculosis drugs is rapidly emerging worldwide (24), with nearly half a million cases of multidrug-resistant tuberculosis (MDR-TB) recorded annually. The majority of MDR-TB is found in former Soviet Union states (FSU) and in Asia (24). The true magnitude of the resistance problem may be much greater, since in most high-TB-prevalence settings the laboratory service is underdeveloped and resistance cannot be adequately detected. Moreover, in almost all countries worldwide, extensively drug-resistant TB (XDR-TB) has also been reported (25), and there are already publications on the emergence of totally drug-resistant TB (TDR-TB), e.g., in Iran and India (23). Because only a minority of the MDR-TB cases can currently be treated according to the guidelines of the WHO, resistant TB may increasingly become an untreatable disease, although there is hope that new drugs may force a major improvement in this situation within a few years (12).
There are multiple known factors that underlie the development of resistance to anti-TB-drugs, such as unregulated availability of anti-TB drugs, poor quality of drugs, unprofessional prescription, noncompliance, malabsorption in certain subpopulations and large interindividual variability in pharmacokinetics of anti-TB drugs, and host genetic factors. The combination and/or relative impact of factors that eventually lead to emergence of resistance are not well understood. However, noncompliance is generally considered to be the most relevant factor leading to resistance in TB treatment (20, 22, 24).
A recent addition to the determinants of resistance to anti-TB drugs is a bacterial one. Certain outbreak-associated strains seem more prone to developing resistance and/or being transmitted as MDR-TB. For example, TB infection caused by the Beijing M. tuberculosis genotype strain is strongly correlated to the development and transmission of MDR-TB and even XDR-TB (11, 19). Regarding transmission of TB in Europe, Beijing strains are significantly associated with transmission of MDR and XDR-TB (8–9). Regarding development of drug-resistant TB, in multiple studies conducted in (for instance) Vietnam, Beijing strains have also been correlated with development of MDR-TB, treatment failures, and relapses after initially curative treatment (2, 10, 17), whereas the East-African/Indian (EAI) genotype strains are not associated with these problems. Still, both Beijing and EAI genotypes are predominant in Vietnam, causing about 40% of the TB cases each. For this reason, in a recent study we selected strains of both these lineages, compared their intrinsic in vitro susceptibilities, and determined their mutation frequencies regarding the development of resistance to four different anti-TB drugs (7). The results revealed that for the Beijing genotype bacteria, a much higher dosage of rifampin was required to achieve 100% killing, and two out of five Beijing strains exhibited a remarkably high frequency of mutations conferring rifampin resistance (7). These data underline the importance of the use of different M. tuberculosis strains in preclinical experimental studies using animal models, as stated by de Groote et al. (3). They recommend the use of different M. tuberculosis strains in the confirmation of treatment efficacy results of novel drugs in the translational phase in order to strengthen the likelihood of success of novel drug regimens that advance into clinical trials (3).
In the present study, we assessed the impact of noncompliance and the role of M. tuberculosis strains as risk factors for the emergence of resistance following TB treatment. More specifically, we investigated, using a well-established mouse TB model (4), whether the consequences of suboptimal treatment, simulating noncompliance, would be more pronounced in the outcome of TB caused by a Beijing genotype strain than for TB caused by an EAI genotype strain. Results obtained may help in better understanding the large differences in treatment success in Vietnam in TB patients infected by Beijing genotype bacteria versus those infected by EAI genotype bacteria.
MATERIALS AND METHODS
Bacterial strains.
The two M. tuberculosis strains used were clinical isolates obtained from Vietnam, one of the Beijing genotype and one of the EAI genotype, as described previously (7). The strains were provided by the National Tuberculosis Reference Laboratory (Bilthoven, the Netherlands), where they were labeled Beijing VN 2002-1585 (Beijing-1585) and EAI VN 2002-1627 (EAI-1627) and were cultured as described previously (5). The MIC of the M. tuberculosis genotype strains was determined using the agar proportion method as described by the Clinical and Laboratory Standards Institute (26). The MICs for both Beijing-1585 and EAI-1627 were 0.125 mg/liter isoniazid and 0.25 mg/liter rifampin. MIC determinations were performed in duplicate.
In order to assess the stability of isoniazid-resistant mutants, the mutants were subcultured five times in MGIT medium without isoniazid, with 0.2 mg/liter isoniazid, or with 1.0 mg/liter isoniazid, and time to positivity within the MGIT Bactec system was used as the outcome parameter.
Infection model.
Specified-pathogen-free female BALB/c mice were obtained from Charles River (Les Oncins, Franc). The experimental protocols adhered to the rules specified in the Dutch Animal Experimentation Act (1977) and the published Guidelines on the Protection of Experimental Animals by the Council of the EC (1986). The Institutional Animal Care and Use Committee of the Erasmus MC Rotterdam approved the present protocols. Mice were infected through intratracheal inoculation followed by inhalation, as described previously (4). The inoculum of M. tuberculosis used for infection contained 1.3 × 105 CFU (range, 1.2 × 105 to 1.3 × 105) of Beijing-1585 and 0.6 × 105 CFU (range, 0.5 × 105 to 0.7 × 105) of EAI-1627.
Drug dosing and anti-TB treatment.
Mice receiving compliance therapy were treated with dosages and schedules of anti-TB drugs derived from current clinical guidelines. The drugs were injected at once. Isoniazid (Hospital Pharmacy, Rotterdam, the Netherlands), rifampin (Rifadin; Aventis Pharma BV, Hoevelaken, the Netherlands), and pyrazinamide (150 mg/kg of body weight, P7136; Sigma Chemical Co, St. Louis, MO) were administered in human pharmacokinetic-equivalent doses, as described previously (4, 13). Treatment was started at 2 weeks after infection. The duration of compliance treatment was 26 weeks (6 months), consisting of a 9-week (2 months) initial phase followed by a 17-week (4 months) continuation phase according to clinical guidelines for TB therapy. During the initial phase, animals received a combination of isoniazid (25 mg/kg), rifampin (10 mg/kg), and pyrazinamide (150 mg/kg). In the continuation phase, animals received isoniazid and rifampin. Agents were administered once daily, 5 days per week, subcutaneously in the neck to avoid potential damage following long-term daily oral gavage. Proper correction was made for the subcutaneous administration in view of the differences in bioavailability of the drugs after oral administration. In the noncompliance treatment, drugs were administered for a treatment duration of only 13 weeks (9 weeks of isoniazid, rifampin, and pyrazinamide, followed by 4 weeks of isoniazid and rifampin), simulating premature discontinuation of treatment in patients (defaulting). Also, underdosing was simulated by applying a reduced frequency of dosing; mice received treatment either 5 days per week, 3 days per week, or once a week. In the noncompliance treatment, the daily doses were identical to the compliance treatment.
Therapeutic efficacy.
Parameters for therapeutic efficacy were the following: (i) M. tuberculosis load in pulmonary and extrapulmonary organs, (ii) emergence of drug resistance, (iii) clinical signs of illness of mice, (iv) relapse of infection during the 13-week posttreatment period, (v) histopathological characterization of the infected organs, and (vi) cytokine concentrations in blood. Treatment success was defined as elimination of the M. tuberculosis load from infected organs, prevention of drug resistance, and prevention of TB relapse at 13 weeks posttreatment.
(i) M. tuberculosis load in pulmonary and extrapulmonary organs and emergence of drug resistance.
At indicated time points, the mycobacterial load in infected organs was assessed. Mice (n = 4 per time point) were sacrificed by CO2 exposure. Subsequently, lung, spleen, and liver were removed aseptically and homogenized in 2 ml (each) phosphate-buffered saline (PBS). Samples were centrifuged and washed with PBS to prevent carryover of anti-TB drugs to the subculture plates. From the undiluted tissue homogenate and 10-fold serial dilutions, samples of 200 μl were plated on 7H10 agar. To detect drug-resistant M. tuberculosis mutants, subcultures were also performed on rifampin-containing plates and on isoniazid-containing plates. The concentrations of rifampin and isoniazid in the subculture plates were 4-fold the “critical concentration,” i.e., 4 mg/liter for rifampin and 0.8 mg/liter for isoniazid (26). Colonies of drug-resistant M. tuberculosis were characterized using the GenoType MTBDRplus assay (Hain Lifescience GmbH, Nehren, Germany) to detect the most common mutations (15).
(ii) Clinical signs of illness of mice.
The behavior of mice was monitored daily, as prescribed by the animal ethics committee. Clinical parameters were the body weight, assessed 3 times per week, and daily evaluation of a discomfort score. Mice that displayed severe signs of illness were euthanized by CO2 exposure.
(iii) Relapse of infection.
The M. tuberculosis load in the lung, spleen, and liver of mice (n = 4) was assessed 13 weeks after termination of TB treatment. Relapse was defined as M. tuberculosis-positive organ cultures, while immediately after termination of treatment, organ cultures were M. tuberculosis negative. Emerging drug-resistant M. tuberculosis mutants were characterized.
(iv) Statistical analysis.
An unpaired Mann-Whitney test was used to analyze differences between the course of infection of the Beijing-infected mice and that of the EAI-infected mice and the therapy efficacies of the different (under dosing) treatment regimens. Differences were considered statistically significant when the P value was ≤0.05.
(v) Histopathological characterization of infected organs.
Lungs, spleens, and livers from sacrificed animals (n = 3) at indicated time points (at weeks 1 and 2 of the untreated infection, at weeks 3, 5, 7, 15, and 28 during treatment, and at 13 weeks posttreatment [weeks 28 and 41]) were processed as described previously (4). In brief, standard 4-μm hematoxylin-eosin-stained sections were prepared from ethanol-fixed, paraffin wax-embedded lung, spleen, and liver tissues. Additionally, a Ziehl-Neelsen staining was performed to detect acid-fast bacilli. Histopathological analysis was performed on tissues from untreated infected mice, from infected mice receiving compliance treatment, and from mice receiving noncompliance treatment once per week for 13 weeks. A pathologist, blinded to the experimental conditions, examined the samples.
(vi) Cytokine concentrations in blood.
Blood samples were obtained by cardiac puncture from mice at week 2 (start of treatment), during compliance treatment at weeks 28 and 41, and during noncompliance treatment (treatment once a week for 13 weeks) at weeks 15 and 28. Plasma samples were prepared from EDTA-blood. Quantification of cytokines was performed using a bead-based flow cytometry technique (xMap; Luminex Corporation, Austin, TX). A milliplex map mouse cytokine, 31-plex, was used (Millipore Corporation, Billerica, MA) consisting of bead-labeled cytokine receptor against the following biomarkers: Eotaxin, granulocyte colony-stimulated factor (G-CSF), granulocyte-macrophage CSF (GM-CSF), gamma interferon (IFN-γ), interleukin 10 (IL-10), IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, IL-1α, IL-1β, IL-2, IL-2, IL-4, IL-5, IL-6, IL-7, IP-10, keratinocyte chemoattractant (KC)-like, leukemia inhibitory factor (LIF), lipopolysaccharide-induced CXC chemokine (LIX), macrophage CSF (M-CSF), monocyte chemoattractant protein 1 (MCP-1), macrophage induced by gamma interferon (MIG), murine macrophage inflammatory protein 1α (MIP-1α), MIP-1β, MIP-2, RANTES, tumor necrosis factor alpha (TNF-α), and vascular endothelial growth factor (VEGF). Tests were performed according the manufacturers' protocol. Samples were tested in duplicate. Results in median fluorescence intensity (MFI) values were converted to pg/ml using the Milliplex Analyst software program (Millipore) and subsequently averaged.
Selection of isoniazid-resistant mutants in vitro.
In order to investigate differences between Beijing-1585 and EAI-1627 in terms of in vitro selection of isoniazid-resistant M. tuberculosis, samples taken after 6 days of exposure to isoniazid at concentrations ranging from 0.015 mg/liter to 256 mg/liter were cultured on drug-free 7H10 agar plates and isoniazid-containing plates. The concentration of isoniazid in the subculture plates was 4-fold the “critical” concentration (26), i.e., 0.8 mg/liter isoniazid. Only isoniazid-resistant M. tuberculosis was able to grow on the drug-containing subculture plates, whereas both susceptible and drug-resistant M. tuberculosis showed growth on the drug-free subculture plates.
RESULTS
Course of untreated TB infection.
As shown in Fig. 1, the course of progressive TB infection caused by Beijing-1585 or EAI-1627 in untreated mice was similar. The M. tuberculosis loads in the lung from week 1 after infection were not significantly different and increased at week 2 (the time point at which therapy was started) up to 2.1 × 108 (range, 1.3 × 108 to 3.1 × 108) CFU Beijng-1585 and 2.7 × 108 (range, 1.8 × 108 to 5.1 × 108) CFU EAI-1627. In the third week of untreated infection caused by either strain, all mice became moribund, as evidenced by their discomfort score and body weight loss (data not shown), and were euthanized.
Fig 1.
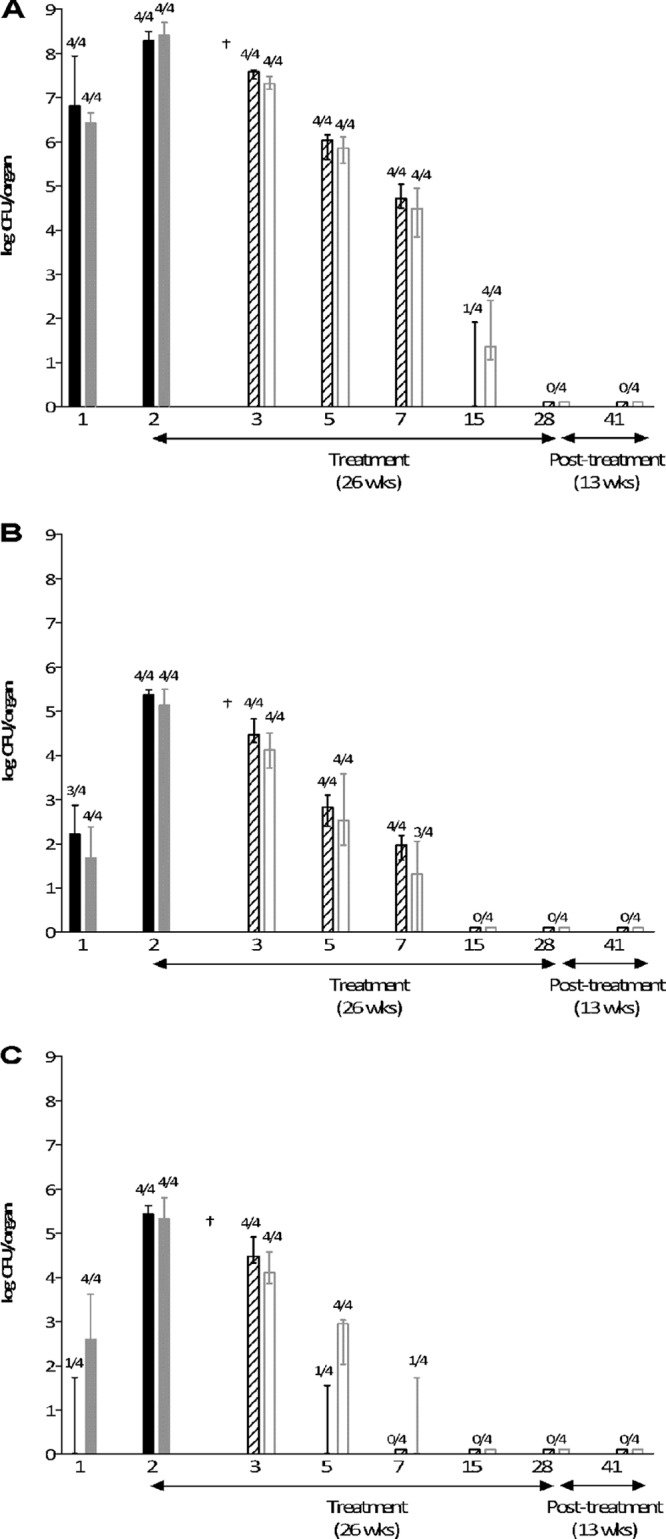
TB infection and efficacy of compliance treatment in mice infected with Beijing-1585 or EAI-1627. The mycobacterial load in lung (A), spleen (B), or liver (C) of the untreated mice, infected with Beijing-1585 (black bars) or EAI-1627 (checkered bars), is shown. Mice receiving therapy started 2 weeks after infection and continued for 26 weeks for BE-1585 (diagonally striped bars) and for EAI-1627 (open bars) are indicated. Results are expressed as median ± range (error bars) of the CFU per organ; n = 4 per time point. Numbers above bars are the numbers of culture-positive mice out of total numbers of mice at that time point. †, 4 out of 4 untreated mice died due to TB infection before this time point.
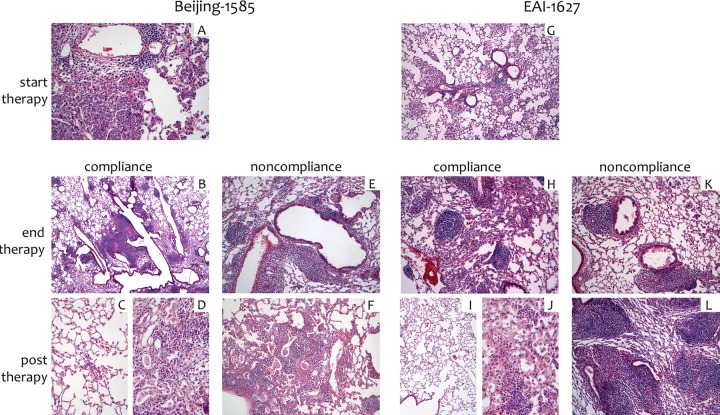
At week 2, histological analysis of lung tissue from untreated mice infected with either strain showed predominantly centrilobular inflammatory infiltrates composed of lymphocytes and histiocytes. The infiltrates were dense and were located within the alveoli, sparing the interstitium (Fig. 2A and F). No differences were observed between the Beijing-1585- and EAI-1627-infected mice at this stage.
Fig 2.
Histology. Lung tissue from mice infected with Beijing-1585 (A to F) or EAI-1627 (G to L) at week 2, start of treatment (A and G), week 28, end of compliance treatment (B and H), week 15, end of noncompliance (once per week) treatment (E and K), week 41, 13 weeks post-compliance treatment (C, D, I, and J), or week 28, 13 weeks post-noncompliance (once per week) treatment (F and L) is shown. (A) Extensive intra-alveolar accumulation of histiocytes is observed, with a peribronchiolar lymphocytic component. Original magnification, ×100. (B) Peribronchiolar infiltrates, predominantly composed of lymphocytes, are seen, admixed with a minor histiocytic component. Magnification, ×25. (C and D) Images from a single lung with highly variable histology, ranging from large areas of normal lung tissue (C) to minor areas of residual densely inflamed lung (D). Magnification, ×50. (E) A moderately dense peribronchiolar predominantly lymphocytic infiltrate is present; no intra-alveolar or interstitial inflammation is seen. Magnification, ×100. (F) A combination of an intra-alveolar histiocytic infiltrate is present with a moderately dense peribronchiolar lymphocytic component; the pattern is similar to but less extensive than the pattern observed in panel A. Magnification, ×100. (G) Intra-alveolar histiocytes with a centrilobular distribution are seen, combined with a minor lymphocytic component. Magnification, ×100. (H) A mild to moderately dense lymphocyte predominant infiltrate is present; a mild increase in alveolar macrophages is also seen. Magnification, ×50. (I and J) Images from a single lung with highly variable histology, ranging from large areas of normal lung tissue (I) (×25) to minor areas of residual densely inflamed lung (J) (×50). (K) A mild to moderately dense peribronchiolar lymphocytic infiltrate is present. Magnification, ×50. (L) A dense lymphohistiocytic infiltrate is present. Magnification, ×25.
Levels of cytokines in plasma were determined in groups of only three mice. The cytokine profile in plasma after 2 weeks of infection revealed only minor differences between the Beijing-1585- and EAI-1627-infected mice. Compared to EAI-1627-infected mice, only the levels of Eotaxin and MCP-1 were higher in Beijing-158- infected mice (see Fig. S1 in the supplemental material), whereas the levels of MIP-1α and MIP-1β were lower in Beijing-1585-infected mice (see Fig. S1). Because untreated mice died in week 3, their cytokine levels could not be assessed at later time points. Therefore, the dynamics of these cytokines in untreated infections could not be assessed further.
Therapeutic efficacy of compliance treatment.
As shown in Fig. 1, the therapeutic efficacies of compliance treatment as measured by decreases in the M. tuberculosis load were similar for the Beijing-1585-infected mice and the EAI-1627-infected mice. The liver was the first organ that became culture negative, followed by the spleen and finally the lung. After 26 weeks of treatment (week 28), lung, spleen, and liver of the Beijing-1585- and EAI-1627-infected mice were culture negative. Importantly, drug-resistant M. tuberculosis mutants were never selected from the mice receiving compliance treatment.
Relapse assessment after the 13-week posttreatment period revealed that relapses of infection never occurred. From 2 to 4 weeks of treatment onwards, the discomfort scores and body weights of both Beijing-1585- and EAI-1627-infected mice were at the levels of those of the uninfected control mice (data not shown).
During compliance treatment, lung tissue from the mice infected with Beijing-1585 initially showed an increase of compact fields of epithelioid histiocytes, which in later stages decreased in intensity (Fig. 2B). Almost complete resolution at week 41 (26 weeks of therapy followed by 13 weeks posttreatment) with only sparse peribronchiolar infiltrates was observed (Fig. 2C and D). Mice infected with EAI-1627 (Fig. 2I and J) showed histopathological characteristics essentially similar to those of the Beijing-1585-infected mice (Fig. 2C and D). At the end of the compliance treatment of EAI-infected mice, the infiltrate diminished to a mild peribronchiolar exclusively lymphocytic pattern (Fig. 2H).
Therapeutic efficacy at treatment noncompliance.
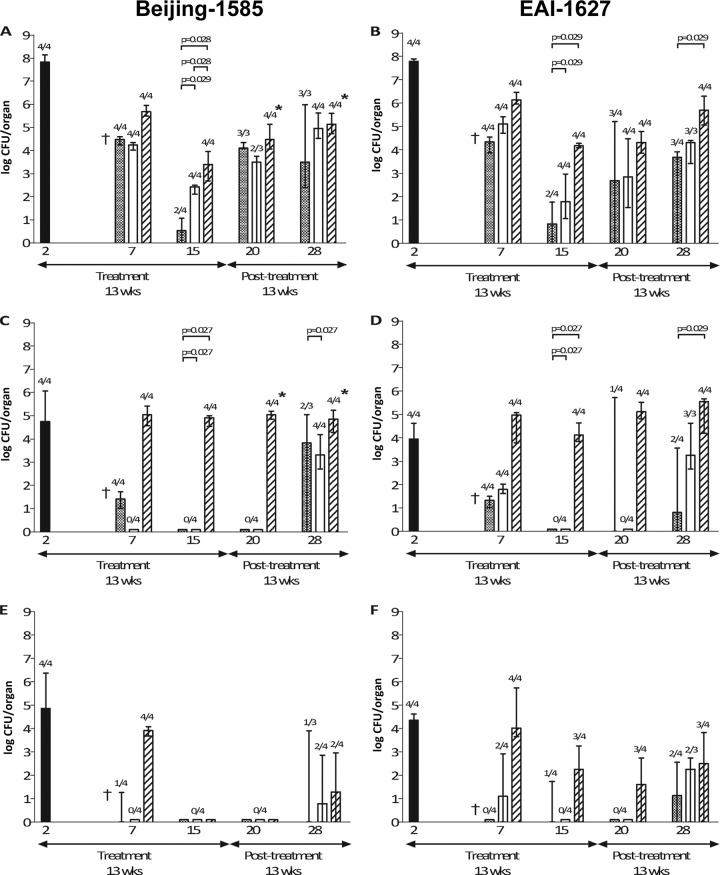
The noncompliance treatment of mice consisted of only 13 weeks of treatment at various dosing frequencies. Results are shown in Fig. 3. At the end of treatment for 5 days per week (week 15), half of the mice were M. tuberculosis culture negative in the lung (two out of four mice for both Beijing-1585 and EAI-1627). However, at 13 weeks posttreatment, all mice relapsed, and the M. tuberculosis load in the lung was 3.1 × 103 (range, 2.4 × 102 to 9.6 × 105) CFU Beijing-1585 and 5.1 × 103 (range, 0 to 8.4 × 103) CFU EAI-1627 at week 28.
Fig 3.
TB infection and efficacy at noncompliance treatment in mice infected with Beijing-1585 (A, C, and E) or EAI-1627 (B, D, and F). Mycobacterial loads in lung (A and B), spleen (C and D), or liver (E and F) of untreated mice (black bars) are shown. Therapy started 2 weeks after infection continued for 13 weeks. Mice received treatment five times per week (checkered bars), three times per week (open bars), or once per week (diagonally striped bars). Results are expressed as medians ± ranges (error bars) of the CFU per organ (n = 4 per time point). Numbers above bars are the numbers of culture-positive mice out of total numbers of mice at that time point. †, 4 out of 4 untreated mice died due to TB before this time point. “*” indicates the presence of isoniazid-resistant mutants. The P values of the statistical evaluation of differences between the noncompliance groups are indicated in the figure if P values were ≤0.05.
Reduction of treatment frequency to 3 days per week resulted in M. tuberculosis culture-positive lungs at the end of treatment (week 15) in all Beijing-1585-infected and all EAI-1627-infected mice, whereas all spleen and liver cultures were negative. The mycobacterial load in the lung at week 15 was 2.7 × 102 (range, 1.3 × 102 to 3.1 × 102) CFU Beijing-1585 and 63 (range, 0.1 × 102 to 9.2 × 102) CFU EAI-1627. At 13 weeks posttreatment, all mice relapsed, and the M. tuberculosis load in the lung was 9.0 × 104 (range, 0.3 × 105 to 4.3 × 105) CFU Beijing-1585 and 2.1 × 104 (range, 0.3 × 104 to 2.5 × 104) CFU EAI-1627 at week 28.
A further reduction in treatment frequency to once per week revealed culture-positive lungs and spleens at the end of treatment (week 15) in all Beijing-1585-infected and all EAI-1627-infected mice. The mycobacterial load in the lung at week 15 was 3.8 × 103 (range, 0.5 × 103 to 9.2 × 103) CFU Beijing-1585 and 1.5 × 104 (range, 1.2 × 104 to 1.9 × 104) CFU EAI-1627. At 13 weeks posttreatment, all mice showed relapse of infection, resulting in a mycobacterial load in the lung of 1.4 × 105 (range, 0.5 × 105 to 4.0 × 105) CFU Beijing-1585 and 6.2 × 105 (range, 0.1 × 106 to 2.0 × 106) CFU EAI-1627 at week 28. Mice receiving the noncompliance treatment once per week showed a very high mycobacterial load throughout the course of infection, especially in the spleen.
Statistical analysis of the efficacy data obtained with the different treatment entities revealed at week 15 a significantly increasing mycobacterial load in the lung and spleen of both Beijing-1585- and EAI-1627-infected mice together with a decreasing treatment frequency (Fig. 3A to D). No significant differences in the efficacy of any treatment modality were observed between Beijing-1585-infected mice and EAI-1627-infected mice.
Remarkably, in only the Beijing-1585-infected mice receiving noncompliance treatment once per week for 13 weeks, isoniazid-resistant M. tuberculosis mutants were selected from lungs and spleens of all mice at week 20 and week 28 (end of therapy). Isoniazid-resistant mutants at week 20 represented a percentage of 2.2 (range, 0.3 to 6.4) of the total mycobacterial load in the lung and a percentage of 1.9 (0.7 to 2.6) of the total mycobacterial load in the spleen. At week 28, isoniazid-resistant M. tuberculosis mutants represented a percentage of 1.7 (range, 1.2 to 3.4) in the lung and a percentage of 5.1 (range, 3.4 to 18.5) in the spleen. In the liver, drug-resistant M. tuberculosis mutants were not found. The selection of resistance was not observed in Beijing-1585-infected mice receiving noncompliance treatment three times per week or five times per week for 13 weeks, nor in any of the EAI-1627-infected mice receiving noncompliance treatment at the three different schedules.
Analysis of the isoniazid-resistant Beijing genotype mutants selected from the lung at week 20 and week 28 showed that the MIC of isoniazid was 128 mg/liter, whereas the strain used to infect the mice exhibited an isoniazid MIC of 0.125 mg/liter. After a five-time subculture of the isoniazid-resistant mutants in isoniazid-free medium, the mutants retained their high MIC of 128 mg/liter. Genotypic analysis of these stable isoniazid-resistant Beijing genotype mutants revealed no mutations in the katG, inhA, kasA, or ahpC gene. Repeated subculture of these resistant isolates in MGIT medium without isoniazid, with 0.2 mg/liter isoniazid, or with 1.0 mg/liter isoniazid showed similar growth rates in all media, as expressed by their similar times to positivity within the MGIT Bactec system (data not shown).
Lung tissue of mice infected with Beijing-1585 and receiving noncompliance treatment once per week initially showed a minor lymphocytic infiltrate (week 15) without epithelioid histiocytes (Fig. 2E), which became more dense at week 28 and was associated with a mild histiocytic component (Fig. 2F). In the EAI-1627-infected mice receiving the same noncompliance treatment, the infiltrate initially decreased at week 15 (Fig. 2K) but became more widespread and denser at week 28 (Fig. 2L).
Cytokine levels in plasma from mice receiving noncompliance treatment (once per week for 13 weeks) showed that in Beijing-1585- as well as EAI-1627-infected mice, the levels of G-CSF, GM-CSF, IFN-γ, IL-13, IL-17, IL-1α, IL-6, IP-10, KC, MIP-1α, MIP-1β, and TNF-α, being increased after 2 weeks of infection, were again decreased after 13 weeks of therapy. Of these cytokines, only the levels of IFN-γ, IP-10, and TNF-α increased again at 13 weeks after termination of therapy, during relapse of infection (see Fig. S1 in the supplemental material).
Selection of isoniazid-resistant mutants in Beijing-1585 and EAI-1627 in vitro.
We observed a difference between the Beijing-1585 strain and the EAI-1627 strain. For Beijing-1585, isoniazid-resistant mutants (ranging from 50 to 420 CFU/ml) were selected up to the concentration of 4 mg/liter isoniazid, whereas for EAI-1627, only a few isoniazid-resistant mutants (≤20 CFU/ml) were found and only up to the concentration of 0.015 mg/liter isoniazid (Fig. 4).
Fig 4.
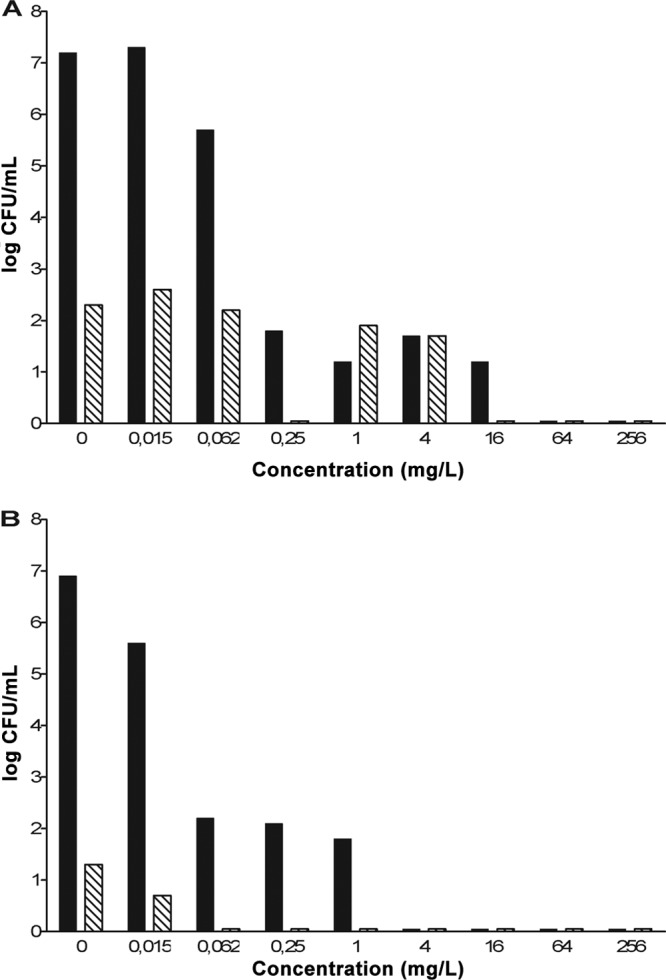
Selection of isoniazid-resistant mutants in vitro. The M. tuberculosis genotype strain Beijing-1585 (A) at a density of 7.7 × 105 CFU/ml or EAI-1627 (B) at a density of 2.3 × 105 CFU/ml was exposed to isoniazid at 4-fold-increasing concentrations for 6 days at 37°C. After 6 days of exposure, quantitative cultures were performed on subculture plates containing 0.4 mg/liter isoniazid (diagonally striped bars) or subculture plates without isoniazid (black bars).
DISCUSSION
From previous studies in Vietnam, it is known that the outcome for TB patients infected with a Beijing genotype strain is relatively poor compared to that for TB patients infected with an EAI genotype strain. However, no satisfactory explanation for this observed phenomenon is yet available. Recently, we have shown in our in vitro studies that Beijing genotype strains have an increased mutation frequency for rifampin resistance compared to that for EAI genotype strains. The mutation frequencies for rifampin in the Beijing-1585 and EAI-1627 strains were 3.7 × 10−3 and 3.5 × 10−6, respectively (7). Also, a relatively large window of rifampin concentrations within which rifampin-resistant M. tuberculosis mutants were selected was observed for the Beijing genotype compared to findings for the EAI genotype. The present study investigated whether the high mutation frequency for rifampin observed in only Beijing genotype strains is associated with treatment failure in Beijing infection. We compared the treatment efficacies of different treatment schedules in a mouse model of TB caused by the Beijing-1585 strain or the EAI-1627 strain. The genotype of M. tuberculosis may influence different aspects of the infection. One hypothesis is that the Beijing genotype strain might exhibit mechanisms that modulate the immune response by the host and as such have an advantage over other M. tuberculosis strains (1, 16). Elaborating on this hypothesis, when mass vaccination with the bacillus Calmette-Guérin (BCG) was introduced in areas to which TB is endemic, the Beijing genotype could use this advantage over other strains and spread more easily (1).
In the present study, in unvaccinated mice, a potential advantage of the Beijing genotype over the EAI genotype was not observed. It was shown that Beijing-1585 and EAI-1627 are equal in pathogenic capacity during the progression of TB. In both infections, the untreated animals died or became moribund within 3 weeks after infection. Also, similar increases in the M. tuberculosis load in lung, spleen, and liver during the first 2 weeks were observed. In addition, the histopathological damage inflicted was equivalent, and the changes in cytokine levels during the course of untreated infection were similar for the two strains. Moreover, the cytokine profiles of both strains were in line with previously described cytokine levels in our model of murine TB caused by the M. tuberculosis strain H37Rv (6). The present study shows that the Beijing-1585 and EAI-1627 strains exhibit an increased virulence reflected by a rapid course of TB compared to TB caused by the H37Rv strain, which did not result in death of mice before 22 weeks after infection, as shown in our previous study (4) The increased virulence of the Beijing genotype is in accordance with findings of previously described studies (14, 18, 19). The high virulence of the EAI genotype in our mouse TB model is also consistent with the fact that EAI strains, similar to the Beijing strains, do very frequently cause disease in Vietnam (40% of TB patients) (2, 17). In contrast to the Beijing strains, the EAI strains in Vietnam are negatively correlated with resistance, and in our previous study (7) and the present study in mice we found clues to explain this.
We observed that treatment efficacies under conditions mimicking compliance are similar for Beijing-1585-infected and EAI-1627-infected mice; both the mycobacterial loads in infected organs and the cytokine levels in blood were equivalent. This finding suggests that if treatment is applied adequately, therapy should be successful and not dependent on the causative infecting strain.
Regarding noncompliance, a strongly dose dependent efficacy of therapy was observed, most likely caused by reduced blood concentrations of the anti-TB drugs and thus a reduced area under the concentration-time curve (AUC) over MIC ratio, which is the pharmacokinetics/pharmacodynamics determinant for therapeutic efficacy. It was demonstrated that an increasing level of noncompliance resulted in decreased M. tuberculosis killing in the lung, as well as in the spleen and liver, at the end of the 13-week treatment period. The results obtained in Beijing-infected and EAI-infected mice in this respect are strikingly identical.
Of the cytokines selected in this study, an increased level of IFN-γ, IP-10, and TNF-α could serve as a biomarker of treatment failure after noncompliance, resulting in relapse of infection. Since these data are obtained from inbred mice, it should be noted that in patients, changes in cytokines may also be influenced by other factors not related to the TB infection. In the TB-infected mice, the cytokine analysis was not helpful in differentiating between the two endemic strains causing TB infection.
Another factor possibly contributing to the emergence of MDR-TB might be variability in pharmacokinetics of anti-TB drugs among patients. Gumbo et al. investigated both bactericidal and sterilizing effects of anti-TB drugs with their hollow-fiber model and predicted that approximately 1% of TB patients following compliance therapy would still develop MDR-TB due to interindividual variability in pharmacokinetics of anti-TB drugs, causing variable and low drug concentrations in a subset of patients (21). Differences in pharmacokinetics of anti-TB drugs are expected to be marginal in inbred mice as used in our TB-model.
The most prominent observation of this study is the selection of isoniazid-resistant mutants exclusively in the Beijing-infected mice and only during the most severe noncompliance treatment (once-per-week treatment), resulting in a relapse of TB. Emergence of resistance is possibly related to a substantial period of low drug concentrations in blood following once-weekly dosing, as such creating a mutant selection window. Remarkably, from week 20 to week 28 (5 and 13 weeks posttreatment, respectively), the percentage of isoniazid-resistant mutants increased in the spleen, whereas the percentage of isoniazid-resistant mutants in the lung remained at the same level. Possibly the spleen serves as a reservoir for these resistant mutants and provides a niche (e.g., the large numbers of macrophages) within which the resistant mutants can divide and thereby reinfect other organs via hematogenic transmission. The stability of these isoniazid-resistant Beijing-genotype mutants was demonstrated by repeated subculture of the mutant in drug-free medium, indicating that isoniazid resistance was conserved even without isoniazid pressure, suggesting that genotypic drug-resistant mutants were selected.
In EAI-infected mice under all noncompliance conditions, resistant M. tuberculosis mutants were never found, although the treatment responses at the various noncompliance conditions were similar in EAI infection and Beijing infection. Similar observational data are available from clinical studies in areas of endemicity (2, 10, 17). As a result, the present model could serve in future studies to further unravel the mechanism(s) by which Beijing genotype strains facilitate the selection of resistant mutants.
In a previous in vitro study, focusing on rifampin, we have shown that Beijing genotype strains exhibit an increased mutation frequency for rifampin resistance (7). However, in the present study, in Beijing infection in vivo, no selection of rifampin-resistant mutants was observed. In contrast, isoniazid-resistant mutants were found only in the Beijing-infected mice during the most severe noncompliance treatment. Given that in patients isoniazid resistance is most often observed and is regarded as a precursor for MDR-TB, our in vivo results are well in line with clinical reality. The fact that isoniazid-resistant mutants were isolated only from the Beijing-infected mice and not from the EAI-infected mice may be explained by our in vitro data. We assessed the time-kill kinetics and selection of isoniazid-resistant mutants using the Beijing-1585 and EAI-1627 strains exposed to isoniazid for 6 days. Whereas for the EAI strain only a few isoniazid-resistant mutants were cultured and could survive isoniazid concentrations of ≥0.015 mg/liter, for the Beijing strain much higher numbers of mutants were found in an isoniazid concentration selection window of 0.015 to 4 mg/liter. In an attempt to understand these observed differences, one could argue that mutations in the DNA repair genes of the Beijing genotype strain resulted in the increased resistance mutant selection range. Possibly, an increase in isoniazid dosage could prevent the selection of isoniazid-resistant Beijing mutants and thereby prevent the formation of MDR-TB. Further studies to explore this hypothesis are needed.
In the present study, we compared only one Beijing genotype strain and one EAI genotype strain. In general, it should be noted that studies with M. tuberculosis genotypes are complex because there are many different outbreak-associated strains from many different geographical locations. This diversity in strains hinders drawing generalized conclusions based on two genotypic strains.
In conclusion, this study shows that the genotypic diversity of M. tuberculosis in terms of selection of resistant mutants occurred not only in vitro but also in vivo in experimental TB in mice. Only isoniazid-resistant M. tuberculosis mutants were selected in Beijing-infected mice during severe noncompliance treatment and not in EAI-infected mice. This might justify genotype-tailor-made therapy in order to prevent the selection of resistance in Beijing-infected patients.
Supplementary Material
ACKNOWLEDGMENT
We thank S. van den Berg (Erasmus MC, University Medical Centre Rotterdam) for technical assistance.
Footnotes
Published ahead of print 16 July 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Abebe F, Bjune G. 2006. The emergence of Beijing family genotypes of Mycobacterium tuberculosis and low-level protection by bacille Calmette-Guerin (BCG) vaccines: is there a link? Clin. Exp. Immunol. 145:389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buu TN, et al. 2009. The Beijing genotype is associated with young age and multidrug-resistant tuberculosis in rural Vietnam. Int. J. Tuberc. Lung Dis. 13:900–906 [PubMed] [Google Scholar]
- 3. De Groote MA, et al. 2012. Importance of confirming in vivo efficacy data of novel antibacterial drug regimens against various strains of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 56:731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Steenwinkel JE, et al. 2009. Immunological parameters to define infection progression and therapy response in a well-defined tuberculosis model in mice. Int. J. Immunopathol. Pharmacol. 22:723–734 [DOI] [PubMed] [Google Scholar]
- 5. de Steenwinkel JE, et al. 2010. Time-kill kinetics of anti-tuberculosis drugs, and emergence of resistance, in relation to metabolic activity of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 65:2582–2589 [DOI] [PubMed] [Google Scholar]
- 6. de Steenwinkel JE, et al. 2012. Dynamics of interferon-gamma release assay and cytokine profiles in blood and respiratory tract specimens from mice with tuberculosis and the effect of therapy. Eur. J. Clin. Microbiol. Infect. Dis. 31:1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Steenwinkel JE, et al. 2012. The worldwide problem of multidrug resistant tuberculosis may be related to lower intrinsic susceptibility of Beijing genotype strains to anti-tuberculosis drugs. Emerg. Infect. Dis. 18:660–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Devaux I, Kremer K, Heersma H, Van Soolingen D. 2009. Clusters of multidrug-resistant Mycobacterium tuberculosis cases, Europe. Emerg. Infect. Dis. 15:1052–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Devaux I, et al. 2010. Surveillance of extensively drug-resistant tuberculosis in Europe, 2003–2007. Euro Surveill. 15:pii=19518 [PubMed] [Google Scholar]
- 10. Duong DA, et al. 2009. Beijing genotype of Mycobacterium tuberculosis is significantly associated with high-level fluoroquinolone resistance in Vietnam. Antimicrob. Agents Chemother. 53:4835–4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. European Concerted Action on New Generation Genetic Markers and Techniques for the Epidemiology and Control of Tuberculosis 2006. Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerg. Infect. Dis. 12:736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ginsberg AM. 2010. Drugs in development for tuberculosis. Drugs 70:2201–2214 [DOI] [PubMed] [Google Scholar]
- 13. Grosset J. 1978. The sterilizing value of rifampicin and pyrazinamide in experimental short-course chemotherapy. Bull. Int. Union Tuberc. 53:5–12 [PubMed] [Google Scholar]
- 14. Hanekom M, et al. 2011. Mycobacterium tuberculosis Beijing genotype: a template for success. Tuberculosis (Edinb.) 91:510–523 [DOI] [PubMed] [Google Scholar]
- 15. Hillemann D, Rusch-Gerdes S, Richter E. 2007. Evaluation of the GenoType MTBDRplus assay for rifampin and isoniazid susceptibility testing of Mycobacterium tuberculosis strains and clinical specimens. J. Clin. Microbiol. 45:2635–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krishnan N, et al. 2011. Mycobacterium tuberculosis lineage influences innate immune response and virulence and is associated with distinct cell envelope lipid profiles. PLoS One 6:e23870 doi:10.1371/journal.pone.0023870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lan NT, Lien HT, Tung LB, Borgdorff MW, Kremer K, van Soolingen D. 2003. Mycobacterium tuberculosis Beijing genotype and risk for treatment failure and relapse, Vietnam. Emerg. Infect. Dis. 9:1633–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nicol MP, Wilkinson RJ. 2008. The clinical consequences of strain diversity in Mycobacterium tuberculosis. Trans. R. Soc. Trop. Med. Hyg. 102:955–965 [DOI] [PubMed] [Google Scholar]
- 19. Parwati I, van Crevel R, van Soolingen D. 2010. Possible underlying mechanisms for successful emergence of the Mycobacterium tuberculosis Beijing genotype strains. Lancet Infect. Dis. 10:103–111 [DOI] [PubMed] [Google Scholar]
- 20. Sharma SK, Mohan A. 2006. Multidrug-resistant tuberculosis: a menace that threatens to destabilize tuberculosis control. Chest 130:261–272 [DOI] [PubMed] [Google Scholar]
- 21. Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. 2011. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J. Infect. Dis. 204:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van den Boogaard J, Boeree MJ, Kibiki GS, Aarnoutse RE. 2011. The complexity of the adherence-response relationship in tuberculosis treatment: why are we still in the dark and how can we get out? Trop. Med. Int. Health 16:693–698 [DOI] [PubMed] [Google Scholar]
- 23. Velayati AA, et al. 2009. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest 136:420–425 [DOI] [PubMed] [Google Scholar]
- 24. WHO 2011. Global tuberculosis control 2011. WHO, Geneva, Switzerland [Google Scholar]
- 25. WHO 2011. Towards universal access to diagnosis and treatment of multidrug-resistant and extensively drug-resistant tuberculosis by 2015; WHO progress report 2011. WHO, Geneva, Switzerland [Google Scholar]
- 26. Woods GL, et al. 2003. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes. Approved standard M24-A, vol. 26, no. 23 National Committee for Clinical Laboratory Standards, Wayne, PA: [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.