Abstract
Resistance to available therapeutic agents has been a common problem thwarting progress in treatment of castrate-resistant and metastatic prostate cancer (PCa). Overexpression of the Bcl-2 family members, including Mcl-1, in PCa cells is known to inhibit intracellular mitochondrial-dependent apoptosis. Here we report the development of a novel transgenic mouse model that spontaneously develops prostatic intraepithelial neoplasia and adenocarcinoma by the inducible, conditional knockout of transforming growth factor β receptor type II in stromal fibroblastic cells (Tgfbr2ColTKO). The Tgfbr2ColTKO prostate epithelia demonstrated down-regulation of luminal and basal differentiation markers, as well as Pten expression and up-regulation of Mcl-1. However, unlike in men, Tgfbr2ColTKO prostates exhibited no regression acutely after castration. The administration of Sabutoclax (BI-97C1), a pan-active Bcl-2 protein family antagonist mediated apoptosis in castrate-resistant PCa cells of Tgfbr2ColTKO mice and human subcutaneous, orthotopic, and intratibial xenograft PCa models. Interestingly, Sabutoclax had little apoptotic effect on benign prostate tissue in Tgfbr2ColTKO and wild-type mice. Sabutoclax was able to block c-Met activation, a critical axis in PCa metastatic progression. Further, Sabutoclax synergistically sensitized PC-3 cells to the cytotoxic effects of docetaxel (Taxotere). Together, these data suggest that Sabutoclax inhibits castrate-resistant PCa alone at the primary and bone metastatic site as well as support sensitivity to docetaxel treatment.
Introduction
Prostate cancer (PCa) remains the second leading cause of cancer-related death among American men [1]. Traditional therapy for PCa removal of the prostate (prostatectomy) remains the basis for primary PCa treatment. Ablative radiation therapies and cytotoxic chemotherapeutic agents in numerous clinical trials and in practice have an has not changed substantially over the last few decades in that surgical overall limited treatment efficacy [2]. Androgen-deprivation therapy after prostatectomy of primary PCa, although generally efficacious in the short term, fails when a subset of the carcinoma cells becomes castrate-resistant (insensitive to withdrawal of androgen and/or androgen signaling blockade by administration of androgen antagonists, such as bicalutamide) [3]. The generation of new molecularly targeted therapies is required to improve PCa patient care, treatment, and prognosis. Evasion of apoptosis (programmed cell death to prevent uncontrolled growth) is a known hallmark of cancer progression and a means by which cancers develop resistance to chemotherapeutic treatments [4]. Castrate-resistant PCa (CRPC) may be overcome by either sensitizing cancer cells to apoptotic stimuli or circumventing upstream blockages in apoptotic signaling [5,6]. Such restoration of apoptosis in PCa cells would counterbalance the proliferative and invasive burdens imposed on the host by localized primary and disseminated metastatic tumor cells.
Analyses of human prostate tumors over the last decade have shed much light on the molecular factors and mechanisms contributing to PCa initiation and progression. Loss of at least one allele of PTEN, and hence reduction or loss of expression of the PTEN protein, frequently occurs in up to 70% of human PCa specimens and considered a contributing factor in CRPC [7,8]. The unrestrained PI3K/ AKT antiapoptotic activity caused by the loss of PTEN expression is also attributed to CRPC development of Pten knockout mice [9]. Insensitivity to transforming growth factor β (TGF-β)is also commonly observed and is mediated in human PCa by heterogeneous loss of type II TGF-β receptor expression in stromal fibroblastic cells [10]. Knockout of the gene encoding type II TGF-β receptor (Tgfbr2) in prostate stromal fibroblasts in Tgfbr2fspKO mice resulted in the development of prostatic intraepithelial neoplasia (PIN) lesions and subsequent prostate adenocarcinoma [10,11]. This loss of stromal TGF-β responsiveness in Tgfbr2fspKO mice led to CRPC progression associated with elevated stromal release of proproliferative cytokines including hepatocyte growth factor (HGF) [11,12] and Wnts [10], proinflammatory cytokines including CCL2 [13], and proinvasive cytokines such as SDF-1 [14,15]. Similar induction of cytokine secretion by the stromal cells has been reported in studies of Tgfbr2fspKO mammary gland tumors [12,16,17]. However, the early lethality of Tgfbr2fspKO model limited prostate tumor progression analysis. Here, we describe the development of novel prostate adenocarcinoma model with an inducible stromal fibroblastic knockout of Tgfbr2, with viability allowing for testing of therapeutic targets.
Overexpression of Mcl-1 is frequently associated with advanced human PCa (high-Gleason-grade primary tumors and metastases) [18], as well as other carcinomas and leukemia [19]. Mcl-1 is a highly regulated antiapoptotic member of the Bcl-2 protein family known to antagonize the function of many proapoptotic BH and BH3-only proteins [20]. Elevated expression of Bcl-2 family of proteins is a key mechanism resulting in evasion of mitochondrial-dependent apoptosis by cancer cells [19]. Previous studies from our laboratories and from others [21–23] have reported that the natural product Gossypol is a potent inhibitor of Bcl-2, Bcl-XL and Mcl-1, functioning as a BH3 mimic, is currently in clinical trials, displaying single-agent antitumor activity in patients with advanced malignancies [22]. However, we anticipated that the two reactive aldehyde groups could render Gossypol intrinsically toxic and was thus eliminated to lead to the compound Apogossypol [24–26]. Further modifications on Apogossypol were made to improve potency and efficacy [27]. These studies culminated in Sabutoclax [27,28], with increased potency in vitro against Bcl-2 family proteins [28–30]. Here we report the use of Sabutoclax (SBX, also known as BI-97C1) to inhibit prostate tumor progression. We used multiple PCa models to specifically test late stage disease that can involve castrate resistance, bone metastasis, and docetaxel resistance. In our studies, Sabutoclax caused the regression of CRPC transgenic and xenograft models at both primary and bone microenvironments. A mediator of PCa castrate resistance and metastasis, the HGF/c-Met signaling, was downregulated by Sabutoclax treatment in in vitro and in vivo models. Sabutoclax restored sensitivity of PCa epithelial cells to intracellular apoptotic signaling, both alone and with docetaxel, resulting in substantial reduction in tumor progression.
Materials and Methods
In Vivo Efficacy Testing of Sabutoclax in Xenograft and Transgenic Mouse Models of PCa
The Tgfbr2ColTKO mice (C57BL/6) express a tamoxifen-inducible Cre recombinase under the control of a COL1A2 proximal promoter [31]. Intraperitoneal (ip) injection of tamoxifen (1 mg dose in 90% vegetable oil and 10% ethanol vehicle; Sigma-Aldrich, St Louis, MO) in lactating dams induced Cre-mediated fibroblastic Tgfbr2 conditional knockout of nursing pups (1, 3, and 5 days postpartum). Recombination of the Tgfbr2 locus was confirmed at 3 weeks of age by genotyping polymerase chain reaction (PCR) as previously described [11]. Genotyping for Cre, floxed Tgfbr2, and Rosa26 were performed by PCR using primers as described [12]. Tgfbr2ColTKO (36 weeks old) and control C57BL/6 male mice were treated ip with Sabutoclax (5 mg/kg) or phosphate-buffered saline (PBS) vehicle three times per week.
A subcutaneous tumor model was established by injecting 8- to 10-week-old male Balb/c Nu/Nu nude mice subcutaneously with 2 x 106 C4-2 human PCa cells per site (four sites/mouse). The C4-2 cells were routinely cultured as previously described [32], harvested by trypsinization, and suspended in sterile PBS for injection. When tumors were visible (day 20), Sabutoclax (5 mg/kg) or vehicle were injected ip every other day for 1 week. Body weights and tumor volumes were measured before each injection. Data were expressed as relative ratios to day 0 (the day drug injection was initiated).
A prostatic bone growth model was established by intratibial injection of 1 x 106 ARCaPM-luc (luciferase expressing) PCa epithelia suspended in PBS into both legs of male Nu/Nu nude mice. The ARCaPM cells were cultured as previously described [33]. A week after injection, the mice were treated with vehicle or with 2.5 mg/kg of Sabutoclax ip for 5 days. Tumor progression was measured using luciferase imaging on a Xenogen system (Caliper, Hopkinton, MA).
In vivo efficacy studies for Sabutoclax and docetaxel, as single agents and in combination, were conducted by subcutaneously implanting PC-3 cells (2 x 106) in the hind flank of male athymic nude mice using 100 µl of a 50:50 solution of Matrigel and RPMI-1640 media. Tumor growth was monitored until it reached a mean tumor volume of 150 to 200 mm3 at which point the mice were distributed into groups of six animals each. Sabutoclax was dosed at 2, 5, or 10 mg/kg intravenously three times weekly. Docetaxel was administered at 12.5 mg/kg ip once weekly. Tumor volumes were measured in two dimensions and determined by the formula of w x h2 x 1/2. Animal body weights were tracked throughout the experiment.
Institutional Animal Care and Use Committees of Vanderbilt University, Sanford-Burnham Medical Research Institute, and Cedars-Sinai Medical Center approved all animal procedures.
In Vitro Synergy Analysis of Sabutoclax and Docetaxel in PC-3 Cells
PC-3 cells were grown in RPMI-1640 medium containing 5 mM Glutamax (Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (Invitrogen) and 1x antibiotic/antimycotic (Invitrogen) at 37°C in a humidified incubator with 5% CO2. Cells were maintained at 40% to 80% confluence for a minimum of two passages before testing. The activity of the compounds individually and in combination was determined using the ATP-Lite 1-Step assay (PerkinElmer, Waltham, MA). Cells were seeded in 96-well plates flat-bottom white plates (Greiner, Monroe, NC) at a density of 5000 cells per well in RPMI-1640 medium with 5 mM Glutamax (Invitrogen) supplemented with 5% fetal bovine serum (Invitrogen) and 1x antimitotic/antimycotic (Invitrogen). After 24 hours, the medium was removed, and fresh RPMI-1640 medium, supplemented as above, was added. At this time, cells were treated with Sabutoclax (dose range of 30–3000 nM) and/or docetaxel (dose range of 0.3–300 nM). Each treatment was performed in triplicate with a final dimethyl sulfoxide (DMSO) concentration of 0.3%. For synergy analyses, a constant Sabutoclax/docetaxel ratio of 10:1 was established across the dosing range. Each sample plate was incubated at 37°C in a 5% CO2 environment for 72 hours. Cell viability was evaluated using ATP-LITE reagent (PerkinElmer), and luminescence measurements were obtained on a Victor 2030 Explorer plate reader (PerkinElmer). Data were normalized to DMSO control-treated cells, and ED50 values were calculated using GraphPad Prism 5.2 (GraphPad Software, Inc). The synergy tests were performed three times, and all data represent the mean ± SEM of tests. Combination index (CI) values for quantification of synergy were calculated using CalcuSyn (Biosoft, Cambridge, United Kingdom).
Immunohistochemistry
Histochemical staining was performed on mouse tissue that was fixed with either 4% paraformaldehyde or 10% neutral buffered formalin, paraffin-embedded, and sectioned (5 µm thick), essentially as described [10]. After antigen retrieval, using 1:100 diluted antigen unmasking solution (Vector Laboratories, Burlingame, CA), the sections were incubated sequentially with primary antibody (overnight) and visualized using appropriate horseradish peroxidase-conjugated secondary antibody systems (Dako, Carpinteria, CA). Antibodies and concentrations used for histochemical staining included Ki-67 1:200 (Abcam, Cambridge, MA), phosphorylated Smad2 1:1000 (Cell Signaling, Danvers, MA), PTEN 1:50 (Cell Signaling), phosphorylated histone H3 1:500 (Upstate, Billerica, MA), CD31 1:50 (Abcam), phosphorylated Mcl-1 1:500 (Santa Cruz Biotechnology, Santa Cruz, CA). β-Galactosidase was stained histochemically using proteinase K (20 µg/ml for 10 minutes at 37°C) treatment of hydrated tissue sections for antigen retrieval and using β-galactosidase antibody 1:2000 (Abcam). Terminal uridine deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed using the ApopTag Peroxidase In situ Apoptosis Detection kit (Chemicon, Billerica, MA) to detect apoptotic cells. Statistical significance was determined by two-tailed Student's t tests (P ≤ .05).
Results
Tgfbr2ColTKO Mice Exhibit Progression of Prostatic Tumorigenesis Useful for Evaluating Experimental Therapeutics
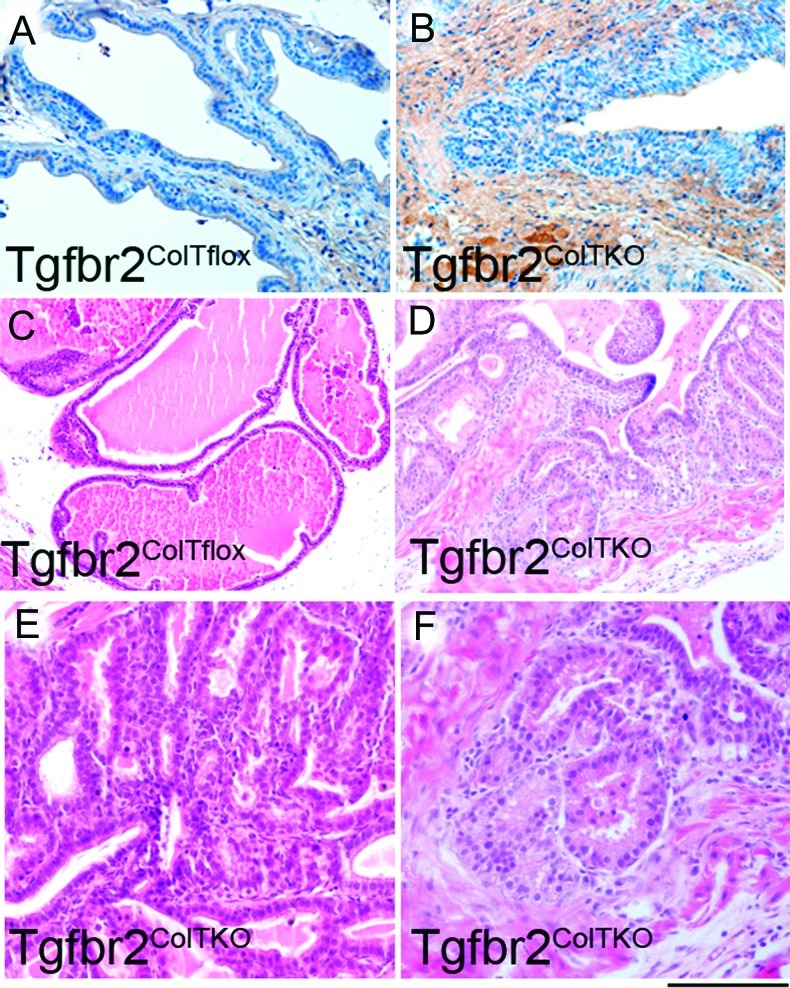
The development of new murine models that more adequately recapitulate the characteristics of human disease is needed to advance preclinical testing of novel therapeutic agents and to increase the rate of translation of new treatment regimens into validated clinical protocols. However, no single model can mimic all facets of human disease. Thus, multiple models are used in this study to test the efficacy of Sabutoclax in late-stage PCa. Transgenic mouse models have several advantages to xenograft models in that mouse hosts have an intact immune system and tumors result from stromal/epithelial interactions of the same species. Therefore, a new long-term model of PCa progression was established by generating mice lacking stromal responsiveness to TGF-β by tamoxifen-inducible conditional stromal knockout of Tgfbr2 (Tgfbr2ColTKO). These mice develop normally without the early lethality observed previously in Tgfbr2fspKO mice [34]. β-Galactosidase staining (Figure 1) and Tgfbr2 recombination PCR (data not shown) demonstrated, respectively, the activation of Cre-mediated recombination of the Rosa26 and floxed Tgfbr2 loci in these mice. By 17 weeks of age, the Tgfbr2ColTKO mouse prostates develop PIN lesions (Figure 1) throughout each of the anterior and dorsolateral lobes with 100% penetrance. Examination of hematoxylin and eosin (H&E)-stained sections revealed the presence of focal areas of prostate adenocarcinoma in these mice (Figure 1). Areas of adenocarcinoma expanded throughout the life of Tgfbr2ColTKO mice as indicated by H&E stained sections from 58-week-old mice. These data suggest that Tgfbr2ColTKO mice, mimicking the stromal loss of TGF-β responsiveness found in human disease, develop PCa through a paracrine manner.
Figure 1.
Establishment of a prostate adenocarcinoma mouse model by stromal knockout of Tgfbr2. Compared with (A) control Tgfbr2floxE2/floxE2/ColCre/Rosa26 (Tgfbr2ColTflox) control mice, β-galactosidase staining of (B) Tgfbr2ColTKO/Rosa26 mouse prostate stroma indicated Cre-mediated recombination of Tgfbr2. Representative images of H&E-stained paraffin-embedded tissue sections from Tgfbr2ColTflox and Tgfbr2ColTKO mice indicate (C, D) development of widespread PIN lesions with 100% penetrance by 17 weeks of age in Tgfbr2ColTKO mice. (E) PIN lesions in 17-week-old Tgfbr2ColTKO mice can be better appreciated at higher power. (F) By 58 weeks of age, 70% of Tgfbr2ColTKO mice develop further progression to focal lesions of adenocarcinoma. Scale bar, 20 µm (A–C) and 10 µm (D–F).
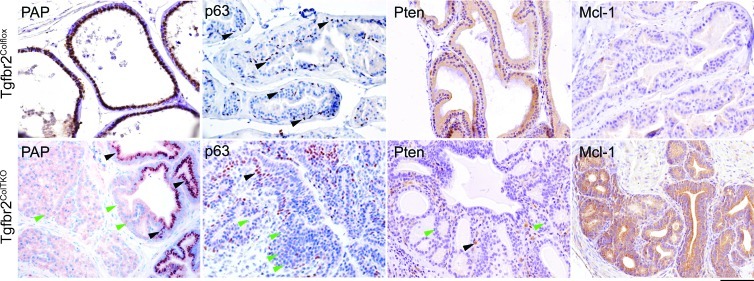
A number of molecular features were examined in the Tgfbr2ColTKO mice to determine the extent to which the progression of PCa in these mice phenocopies human disease. Heterogeneous expression of phosphorylated Smad2 was observed histochemically in the prostatic stromal compartment of Tgfbr2ColTKO mice (Figure W1), similar to our previous observations of Tgfbr2fspKO mice and human PCa specimens [15,35]. There was a pronounced loss of the differentiation marker, prostatic acid phosphatase (PAP), in areas of transformation of Tgfbr2ColTKO prostates (Figure 2). Disorganization and depletion of the basal cell layer was demonstrated through p63 localization, a differentiation marker for basal cells. Further, Tgfbr2ColTKO mice had a demonstrable loss of Pten expression in regions of epithelial hyperplasia. As a control, Tgfbr2ColTFlox prostates expressed PAP and Pten in the luminal epithelia and p63 in the basal epithelia (Figure 2). The loss of Pten expression suggested the potential for castrate resistance [36]. To test for the castrate response of the Tgfbr2ColTKO mice, the proliferative potential of the prostate glands were examined 4 days after castration. Immediately after castration, the Tgfbr2ColTKO prostate luminal epithelia maintained a high mitotic index (P = .01) and low apoptosis levels (P = .03) as measured by the expression of phosphorylated histone H3 and TUNEL, respectively, in the luminal epithelial cells compared with Tgfbr2ColTFlox mice (Figure 3A). Unlike in men, the Tgfbr2ColTKO prostates maintain a high rate of proliferation independent of castration status, modeling a paracrine castration resistance model without a phase of regression. Although the etiology of CRPC development in Tgfbr2ColTKO mice may not be found in man, the histologic and molecular changes in the stromal and epithelial compartments mimicked human CRPC.
Figure 2.
Human hallmarks of PCa are identified in the Tgfbr2ColTKO mouse prostate glands. The Tgfbr2ColTKO mice have immunohistochemical evidence of the loss of PAP expression in the epithelia, disorganization of the basal cell layer as indicated by p63 staining, and epithelial down-regulation of Pten, compared with control Tgfbr2ColTflox mice. Further, Mcl-1 expression was localized in the epithelia of Tgfbr2ColTKO prostates and was absent in Tgfbr2ColTflox tissues. Black and green arrowheads indicate cells expressing or not expressing the indicated markers, respectively. Scale bar, 10 µm.
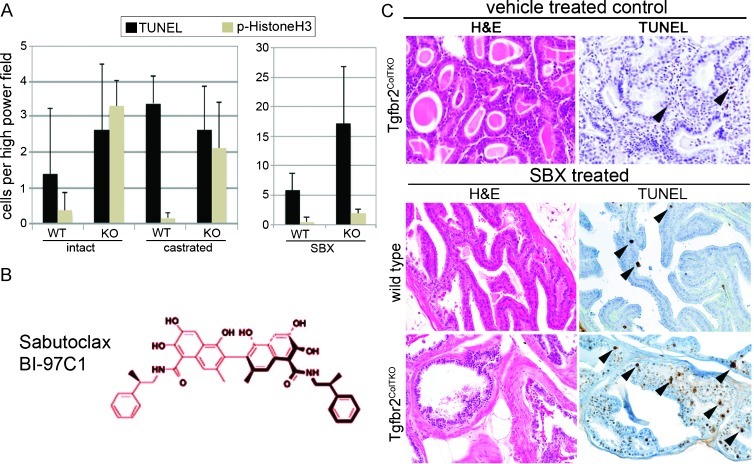
Figure 3.
Sabutoclax, a novel broad-spectrum Bcl-2 family antagonist limits the prostate tumor and PIN phenotype in castrate-resistant Tgfbr2ColTKO mice. (A) Quantitation of positively stained cells undergoing mitosis (by histone H3 localization) and apoptosis (by TUNEL localization) in wild-type (WT) and Tgfbr2ColTKO (KO) mice that were left intact, castrated for 4 days, or treated with Sabutoclax (SBX, n = 20) are indicated. Significant differences were observed in the following comparisons: proliferative index of intact WT and KO prostates in each of the conditions (P < .01), as well as the apoptotic index of the KO prostates comparing SBX treatment to either intact or castrate conditions (P < .0001). Elevated apoptosis was observed in SBX-treated KO compared with WT prostates (P = .00006). (B) The chemical structure of SBX is indicated. (C) Tgfbr2ColTKO mice (36 weeks old), as well as similarly aged wild-type C57BL/6 control mice, were treated with vehicle or SBX for 2 weeks. H&E staining suggested that the Tgfbr2ColTKO prostate tumors were more differentiated in the SBX-treated mice compared with vehicle control-treated mice. TUNEL-positive apoptotic prostate cells were more abundant in SBX-treated Tgfbr2ColTKO compared with similarly treated Tgfbr2ColTFlox and vehicle-treated mice. Little change was observed in morphology or apoptosis in vehicle-and SBX-treated wild-type C57BL/6 mice.
Sabutoclax Treatment Restored Differentiation and Reduced Tumor Size in Models of PCa
Sabutoclax is a recently developed Mcl-1 antagonist currently undergoing preclinical testing in several laboratories (see structure, Figure 3B). To determine whether this drug would have efficacy on CRPC, 36-week-old male Tgfbr2ColTKO mice were treated with Sabutoclax or vehicle. The H&E-stained sections of Tgfbr2ColTKO prostate treated with Sabutoclax revealed increased differentiation as exhibited by reduction in the extent of PCa lesions and a more normal glandular architecture (Figure 3C). TUNEL staining further indicated the presence of significant apoptosis of prostatic epithelia in Sabutoclax-treated Tgfbr2ColTKO mice compared with vehicle treatment (P ≤ .0001) and significantly greater than wild-type C57BL/6 control mice as determined by TUNEL positivity in the prostate (P ≤ .0001; Figure 3, A and C). Interestingly, Sabutoclax had no significant effect on the mitotic index of either wild-type or Tgfbr2ColTKO prostate. The data demonstrated that Sabutoclax inhibited and reversed the PCa progression phenotype of Tgfbr2ColTKO mice where castration had limited effect.
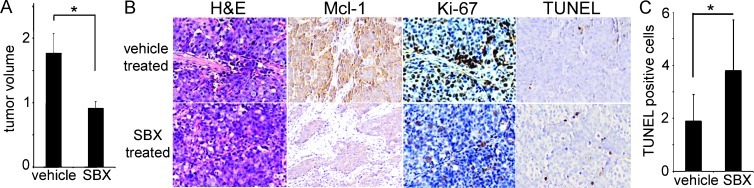
Human PCa xenograft models were used to test Sabutoclax efficacy on castrate-resistant tumor growth. The castrate-resistant C4-2 PCa cells were injected subcutaneously into nude mice and allowed to form palpable tumors by 20 days after injection. Treatment of C4-2 tumor-bearing mice with Sabutoclax for 1 week resulted in a significant reduction in tumor volume compared with vehicle-treated mice without a significant change in mouse body weight (Figure 4A). Histologic examination of these xenografts did not suggest changes in differentiation of the C4-2 xenografts other than gross size. However, the immunohistochemical staining suggested reduced expression of Mcl-1 and the proliferation marker, Ki-67 (Figure 4B). Further, quantitation of TUNEL staining indicated a significant increase in apoptosis after Sabutoclax treatment (P = .027; Figure 4C). Together, transgenic mouse and human xenograft CRPC models provided complementary support on the role of Sabutoclax in reducing tumor burden.
Figure 4.
Sabutoclax reduced the size of established xenografted human C4-2 PCa xenograft tumors. (A) Significant tumor volume reduction of the subcutaneous xenografts was observed by Sabutoclax (SBX) treatment compared with vehicle-treated control mice, without any significant impact on mouse's body weight (n = 8, P = .03). (B) By immunohistochemical staining, the tumors showed reduction in phosphorylated Mcl-1, Ki-67, and TUNEL expression after SBX treatment compared with vehicle control. (C) Quantitation of TUNEL staining indicated a significant elevation of apoptosis with SBX treatment compared with vehicle control (P = .027).
Sabutoclax Treatment Reduced c-Met Signaling and PCa Xenografts Growth in Bone
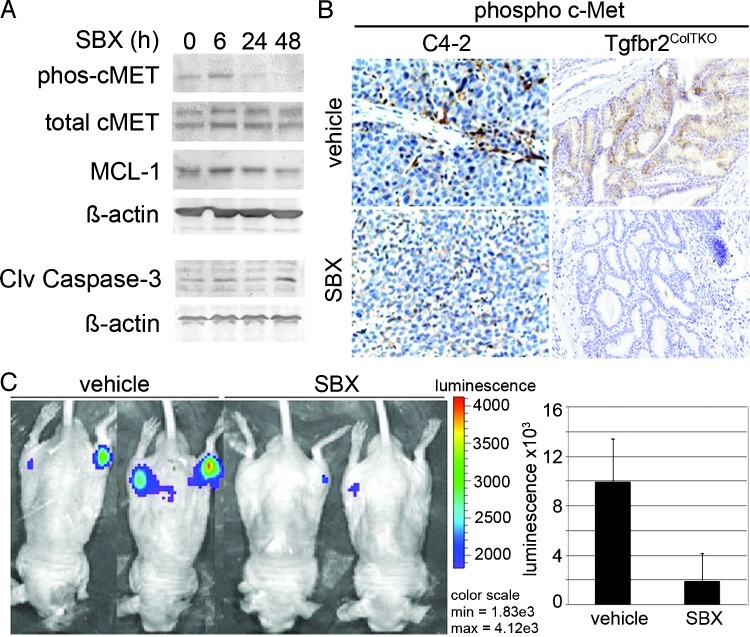
HGF/c-Met signaling is demonstrated to regulate Mcl-1 expression and associated with PCa metastatic bone growth [37,38]. So, to test whether Sabutoclax affects c-Met signaling, we initially examined its role on human bone metastatic ARCAPM cells, previously reported to have autocrine c-Met activity [39]. Western blot analysis suggested the loss in phosphorylated c-Met expression in ARCAPM cells by 24 hours of treatment, with little change in total c-Met in the same time frame (Figure 5A). Forty-eight hours of Sabutoclax treatment reduced total Mcl-1 expression by 50% of untreated control. Concomitant up-regulation of cleaved caspase 3 provided an independent measure apoptosis induced by Sabutoclax in the same period. Phosphorylated c-Met histochemical localization was performed on longer-term Sabutoclax treatment of Tgfbr2ColTKO mice and C4-2 subcutaneous xenografts in nude mice. Sabutoclax reduced phosphorylated c-Met expression in Tgfbr2ColTKO prostatic tissues and the C4-2 xenografts compared with control mouse tissues (Figure 5B). Observed decrease in c-Met activation, concurrent with Sabutoclax treatment, suggested autocrine and paracrine regulation of c-Met signaling by Mcl-1.
Figure 5.
Reduction in phosphorylated c-Met expression and ARCaPM prostate tumor growth in bone by Sabutoclax treatment. (A) Western blot of ARCaPM cells treated with Sabutoclax (SBX) for a time course of 0 to 48 hours indicating the expression of phosphorylated and total c-Met, Mcl-1, and β-actin. In the same time frame, elevated cleaved caspase 3 expression was observed with SBX treatment. (B) Immunohistochemical staining of Tgfbr2ColTKO prostates and C4-2 subcutaneous xenografts indicated a reduction in phosphorylated c-Met expression compared with vehicle-treated control tissues. (C) Intratibial inoculation of ARCaPM-LUC cells were detected by in vivo luciferase imaging. The representative images shown were taken after 2 weeks of treatment with vehicle or SBX. Significant reduction of luciferase signal was observed with SBX treatment (n =6, P = .0006). Postacquisition histochemical analysis confirmed reduced tumor size and repair of the bone lesion.
Effects of Sabutoclax on c-Met signaling, commonly associated with in human metastatic PCa, supported further testing in a model of PCa in a common metastatic site—the bone. A xenograft model of PCa growth in bone was established by bilateral intratibial injection of ARCaPM-luc PCa cells into male nude mice. Longitudinal luciferase imaging revealed the establishment and growth of the ARCaPM-luc tumors. Treatment with Sabutoclax after 1 week significantly reduced the size of the bone lesion compared with mice treated with vehicle alone (P = .0006). Reduction in the extent of the bone tumors was verified by in vivo luciferase detection of the ARCaPM-luc bone xenografts (Figure 5C). Thus, Sabutoclax downregulated proliferation of both the C4-2 and ARCaPM xenografts, concomitant with increased apoptosis, as evidenced by dramatic tumor size reduction, similar to that observed in Tgfbr2ColTKO transgenic mice. Together, it suggested that antagonism of Mcl-1 and other antiapoptotic Bcl-2 family proteins by Sabutoclax may be effective to target the growth of both primary and metastatic CRPCs.
Sabutoclax Sensitized PC-3 Cells to Docetaxel-Induced Apoptosis In Vitro and In Vivo
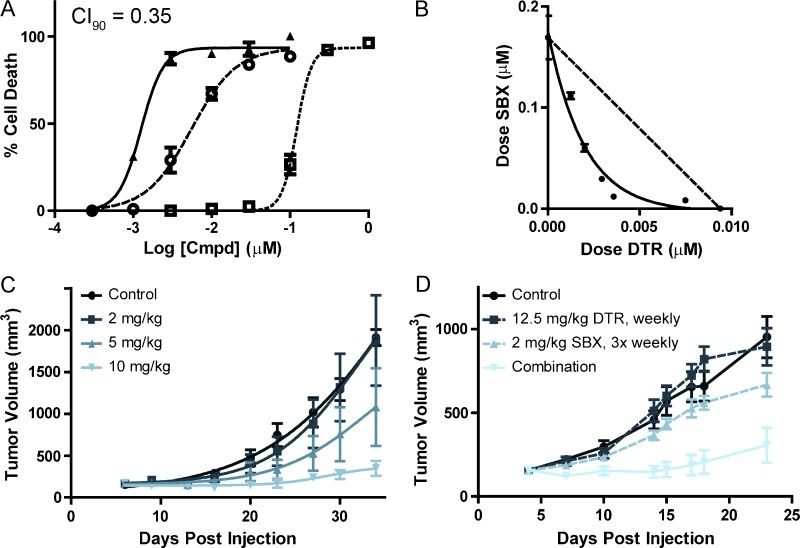
Mortality from PCa most commonly results from the resistance to chemotherapy with docetaxel. To evaluate the ability of the pan-active Bcl-2 inhibitor Sabutoclax to synergize with docetaxel for the treatment of human PCa, we used the Chou-Talalay method for synergy analysis [28,40]. This model is able to take into account the nonuniform dose-response curves that we had previously observed in single-agent dosing experiments [30]. For these tests, we plated a three-log order dose-response curve for each of the compounds individually as well as in a ratio combination on a single 96-well plate with each dose collected in triplicate. At 72 hours after administration of the drug to the plated androgen receptor-deficient PC-3 cells, the relative ATP concentration in each well was measured with respect to an internal DMSO control as a proxy for cell viability. The resultant dose-response curves (Figure 6A) were used to identify the respective ED50 and ED90 values for each drug individually and in combination. In addition, these curves were analyzed to calculate the CI value for each cell line [41]. Using the CI model of drug combination, CI = 1 indicates an additive effect whereas CI < 1 indicates synergy and CI > 1 indicates antisynergy. Our analysis identified strong synergy of Sabutoclax and docetaxel treatment (Figure 6B).
Figure 6.
Synergistic cytotoxic activity of Sabutoclax (SBX) in combination with docetaxel (DTR). (A) In vitro dose-response curves of SBX (□), DTR (○), and their 10:1 SBX/DTR combination (▲) exhibited ED50/ED90 values of 122 ± 12/196 ± 55 nM, 5.5 ± 0.4/23 ± 4 nM, and 1.3 ± 0.1/2.6 ± 0.3 nM, respectively. CI50 and CI90 values in CalcuSyn were 0.45 and 0.35, respectively. (B) ED50 isobologram of SBX and DTR synergy. The ED50 values for seven combinations of SBX and DTR show a significant (P < .0001) deviation below the additive dotted line, which is indicative of a synergistic combination. (C) In vivo SBX efficacy. Tumor growth curves show a dose-dependent reduction in tumor volume to treatment with SBX 3 times weekly at 2, 5, and 10 mg/kg versus control. Each point is the mean ± SEM (n = 5) of the measured tumor volume. The curves for the 5 and 10 mg/kg were each significantly different from the control (P = .0084 and P < .0001, respectively). (D) In vivo efficacy of SBX and DTR combination. Tumor growth curves show the mean ± SEM (n = 6) of subcutaneous PC-3 xenografts after treatment with DTR (12.5 mg/kg, weekly), SBX (2.0 mg/kg, three times weekly), and their combination. The combination is significantly different (P < .0001) from either of the other three growth curves.
To determine whether the synergy observed in vitro could be replicated in vivo, we tested the compounds individually and in combination for their ability to suppress growth of PC-3 mouse xenograft tumors. We first established the dose dependence of Sabutoclax (2, 5, and 10 mg/kg three times weekly) in the PC-3 xenograft model. The three groups of mice had a strong dose-dependent response (Figure 6C) and exhibited minimal weight loss over the course of the study. From this initial analysis, we chose a three-time weekly dose of 2 mg/kg Sabutoclax and a once-weekly dose of 12.5 mg/kg docetaxel for the combination study. Control injections without drug were administered to the control group and to each of the single-agent groups to simulate stress from the total number of injections administered to the combination group. Mouse weight and tumor size was tracked during the course of the treatment (Figure 6D). A significant difference in tumor size was observed between the Sabutoclax or docetaxel arms and the combination arm (P < .0001). The observed in vitro and in vivo synergism resulting from the combination of Sabutoclax with docetaxel treatment established Sabutoclax as an effective drug that should be further evaluated in clinical trials aimed at improving treatments for chemotherapeutically resistant PCa.
Discussion
Treatment of PCa with cytotoxic chemotherapeutic agents to date has largely been ineffective because of prevalent cellular resistance. Inhibition of apoptosis by the overexpression of Mcl-1- and other antiapoptotic Bcl-2-related proteins constitutes a common mechanism for PCa resistance to therapy [6]. Gossypol derivatives have been identified and developed over the last several years as strong antagonists of Bcl-2- related proteins, blocking their ability to inappropriately sequester proapoptotic proteins when overexpressed [42,43]. Initial pharmacodynamics and pharmacokinetic studies for Sabutoclax involving an extensive panel of cancer cell lines have been previously published and support the efficacy of Sabutoclax as both a single and combined therapeutic agent [28,30,44]. Administration of Bcl-2 family antagonists, like Sabutoclax, may alleviate resistance by either directly activating mitochondrial-dependent apoptosis or restoring PCa sensitivity to conventional therapeutic agents when used in combination. Indeed, initial pharmacodynamic and pharmacokinetic studies for Sabutoclax involving an extensive panel of cancer cell lines have been previously published and support the efficacy as a single and combined therapeutic agent [28,44]. In short, the data from these experiments demonstrate for the first time that Sabutoclax is effective at inhibiting tumor progression in transgenic, subcutaneous, and orthotopic mouse models of human PCa. Further, Sabutoclax increased sensitivity to docetaxel when used in combination.
We tested the hypothesis that treatment of prostate tumors with Sabutoclax would result in inhibition of castrate-resistant prostate tumor progression and perhaps contribute to tumor regression. The development of new stromally targeted transgenic mouse models for studying PCa was important in testing Sabutoclax efficacy on CRPC and extends our understanding of the complex roles of the tumor microenvironment on the progression of PCa [45]. Previously, we demonstrated that Tgfbr2fspKO mice develop PIN lesions that, after tissue rescue, could progress to adenocarcinoma [10,11]. However, the Tgfbr2fspKO mice had limited usefulness in studies of PCa progression as they died at 6 to 8 weeks of age [11,34]. In contrast, untreated and tamoxifen-induced Tgfbr2ColTKO mice are healthy and live to at least 60 weeks of age, which is advantageous for their use in long-term studies of PCa progression and therapeutic investigation in aging adult mice. Tgfbr2ColTKO mice develop widespread PIN and foci of prostatic adenocarcinoma, with castrate-resistant growth. Importantly, unlike other models of castrate-resistant growth (or that generally observed in men), the Tgfbr2ColTKO mice do not exhibit an initial prostate regression after castration. Thus, surgical castration of Tgfbr2ColTKO mice is not necessary to push the prostatic lesions to castrate resistance (providing a potential savings in time and labor in future preclinical murine studies of CRPC) as well as enabling the use of these mice to model the effects of the entire range of clinically used androgen-deprivation therapies (chemical and/or surgical castration). The Tgfbr2ColTKO mice do exhibit other characteristics of human PCa as exemplified by heterogeneous expression of stromal phosphorylated Smad2, similar to our reported observations in Tgfbr2fspKO mice and human PCa tissues [15]. Further, the loss of PTEN expression in areas of epithelial hyperplasia of Tgfbr2ColTKO prostatic glands recapitulates a key aspect of human PCa progression, demonstrated to be a prognostic predictor for the development of CRPC (as defined by PSA biochemical recurrence) in patients [46]. Unlike epithelial-targeted PCa models, the transgenic targeting of stromal cells allows for heterogeneous development of multifocal tumors, to arguably better mimic human PCa. The Tgfbr2ColTKO model supports the concept that castrate-resistant prostate cells may reside in the absence of androgen receptor antagonism [36]. Together, these data validate the use of Tgfbr2ColTKO mice as an in vivo model system for testing therapeutic agents targeting CRPC.
The tumor-bearing mouse models of human PCa used in this study had diminished measures of cancer progression in Sabutoclax-treated animals. Administration of Sabutoclax reduced the severity of the PIN phenotype and restored a more normal prostatic glandular architecture to the prostates of Tgfbr2ColTKO transgenic mice, yet had a lesser apoptotic effect on benign tissues from C57BL/6 mice. The restricted specificity of action for Sabutoclax on early-initiated and progressed prostatic tumors is attributed to the selective over expression of Mcl-1 and related antiapoptotic Bcl-2 family proteins in cancer tissues. The mixture of benign and heterogeneous cancer tissues in Tgfbr2ColTKO prostates acted as an internal control. Sabutoclax was also effective in reducing the size and volume of subcutaneous tumors grown from human C4-2 PCa cells in nude mice, while being well tolerated by these mice without appreciable weight loss, as previously suggested in other tumor bearing mouse models [24,30]. The regression of the C4-2 tumors is readily attributed to the combined decrease in proliferation (Ki-67 staining) and increased apoptosis sensitivity (MCL-1 staining) caused by Sabutoclax treatment. Finally, Sabutoclax was effective in reducing the growth and extent of tibial bone destruction by ARCaPM orthotopic xenografts. A similar reduction in bone lesions was reported in early results from ongoing clinical studies that used the c-Met/VEGFR2 antagonist, XL184 [47]. Inhibition of tumor progression by Sabutoclax for both C4-2 and ARCaPM human prostatic tumor xenografts subcutaneously and intratibially is known to grow in a castrate-resistant manner [48,49], to support Sabutoclax efficacy as a single agent in the clinical treatment of advanced and metastatic PCa.
Owing to the recent characterization of Mcl-1 as a key regulator of apoptosis during mitotic progression [50], we sought to determine whether an Mcl-1-targeting therapeutic agent, such as Sabutoclax, could potentiate docetaxel action. Docetaxel is a known microtubule stabilizer and first-line chemotherapeutic agent for the treatment of PCa. PC-3 cells are known to be temporally insensitive to docetaxel in that they undergo growth arrest with a significant delay in onset of cell death [51]. Our studies with Sabutoclax as single agent and in combination with docetaxel demonstrated a significant synergistic benefit for the treatment of androgen-resistant, docetaxel-refractive PC-3 cells in culture and xenograft models. These results are supported by a recent study in which melanoma was sensitized synergistically to apoptotic cell death by treatment with Sabutoclax and Ad-mda7/IL-24 [30,52]. These data support Sabutoclax as a powerful component of combinatorial therapy for PCa. We noted that phosphorylation of c-Met was reduced in tumors treated with Sabutoclax, correlating with increased apoptosis. The mechanisms underlying these observations remain to be determined but could reflect a downstream consequence of Sabutoclax-mediated caspase activation or other elements of the apoptotic program in reversing both docetaxel resistance and c-Met signaling. However, we cannot exclude the possibility that Sabutoclax may in fact be a direct regulator of the activation pathway of c-Met—the receptor for HGF. The inhibition of c-Met phosphorylation by Sabutoclax in Tgfbr2ColTKO mice suggests an inhibition of paracrine HGF signaling, whereas that observed in ARCAPM cells is autocrine. The relationship between Mcl-1 and c-Met signaling, and hence whether Mcl-1 impinges on stromal TGF-β signaling, which is known to negatively regulate HGF production [11,12], deserves further exploration in future biochemical and signal transduction studies.
In summary, administration of the pan-active Bcl-2 family antagonist, Sabutoclax, was sufficient to inhibit tumor progression in models of advanced PCa. The data support a potential role in blocking the feedback loop between Mcl-1 and c-Met signaling. Synergy of Sabutoclax with docetaxel supports future testing of its efficacy in combination therapies for multiple advanced cancers, besides PCa, that develop taxol resistance.
Supplementary Material
Acknowledgments
The authors thank Matthew Smail for technical support.
Abbreviations
- CRPC
castrate-resistant prostate cancer
- ip
intraperitoneal
- PCa
prostate cancer
- PIN
prostatic intraepithelial neoplasia
- TGF-β
transforming growth factor β
- Tgfbr2ColTKO
TGF-β receptor type II conditional fibroblastic cell knockout induced by tamoxifen
- Tgfbr2Colflox
TGF-β receptor type II floxed mice not induced to be knocked out by tamoxifen
- TUNEL
terminal uridine deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
This work was supported by Department of Defense Prostate Cancer Research Program Postdoctoral Fellowship W81XWH-08-1-0542 (R.S.J.), as well as National Institutes of Health/National Cancer Institute grants CA113318 (J.C.R., M.P.), CA149668 (M.P.), CA098912 (L.W.C.), and CA108646 (N.A.B.). J.C.R. and M.P. have licensed Sabutoclax to Oncothyreon.
This article refers to supplementary material, which is designated by Figure W1 and is available online at www.neoplasia.com.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Shepard DR, Raghavan D. Innovations in the systemic therapy of prostate cancer. Nat Rev Clin Oncol. 2010;7:13–21. doi: 10.1038/nrclinonc.2009.187. [DOI] [PubMed] [Google Scholar]
- 3.Niu Y, Chang TM, Yeh S, Ma WL, Wang YZ, Chang C. Differential androgen receptor signals in different cells explain why androgen-deprivation therapy of prostate cancer fails. Oncogene. 2010;29:3593–3604. doi: 10.1038/onc.2010.121. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Uzzo RG, Haas NB, Crispen PL, Kolenko VM. Mechanisms of apoptosis resistance and treatment strategies to overcome them in hormone-refractory prostate cancer. Cancer. 2008;112:1660–1671. doi: 10.1002/cncr.23318. [DOI] [PubMed] [Google Scholar]
- 6.Karnak D, Xu L. Chemosensitization of prostate cancer by modulating Bcl-2 family proteins. Curr Drug Targets. 2010;11:699–707. doi: 10.2174/138945010791170888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van Dyke T, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao H, Ouyang X, Banach-Petrosky WA, Shen MM, Abate-Shen C. Emergence of androgen independence at early stages of prostate cancer progression in Nkx3.1;Pten mice. Cancer Res. 2006;66:7929–7933. doi: 10.1158/0008-5472.CAN-06-1637. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Placencio V, Iturregui JM, Uwamariya C, Sharif-Afshar AR, Koyama T, Hayward SW, Bhowmick NA. Prostate tumor progression is mediated by a paracrine TGF-β/Wnt3a signaling axis. Oncogene. 2008;27:7118–7130. doi: 10.1038/onc.2008.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 12.Cheng N, Bhowmick NA, Chytil A, Gorksa AE, Brown KA, Muraoka R, Arteaga CL, Neilson EG, Hayward SW, Moses HL. Loss of TGF-β type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-α-, MSP- and HGF-mediated signaling networks. Oncogene. 2005;24:5053–5068. doi: 10.1038/sj.onc.1208685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bierie B, Moses HL. Transforming growth factor β (TGF-β) and inflammation in cancer. Cytokine Growth Factor Rev. 2010;21:49–59. doi: 10.1016/j.cytogfr.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67:4244–4253. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- 15.Kiskowski MA, Jackson RS II, Banerjee J, Li X, Kang M, Iturregui JM, Franco OE, Hayward SW, Bhowmick NA. Role for stromal heterogeneity in prostate tumorigenesis. Cancer Res. 2011;71:3459–3470. doi: 10.1158/0008-5472.CAN-10-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng N, Chytil A, Shyr Y, Joly A, Moses HL. Transforming growth factor-β signaling-deficient fibroblasts enhance hepatocyte growth factor signaling in mammary carcinoma cells to promote scattering and invasion. Mol Cancer Res. 2008;6:1521–1533. doi: 10.1158/1541-7786.MCR-07-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hembruff SL, Jokar I, Yang L, Cheng N. Loss of transforming growth factor-β signaling in mammary fibroblasts enhances CCL2 secretion to promote mammary tumor progression through macrophage-dependent and -independent mechanisms. Neoplasia. 2010;12:425–433. doi: 10.1593/neo.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei J, Kitada S, Stebbins JL, Placzek W, Zhai D, Wu B, Rega MF, Zhang Z, Cellitti J, Yang L, et al. Synthesis and biological evaluation of Apogossypolone derivatives as pan-active inhibitors of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2010;53:8000–8011. doi: 10.1021/jm100746q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci. 2009;66:1326–1336. doi: 10.1007/s00018-008-8637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010;584:2981–2989. doi: 10.1016/j.febslet.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 21.Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003;46:4259–4264. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 22.Wang G, Nikolovska-Coleska Z, Yang CY, Wang R, Tang G, Guo J, Shangary S, Qiu S, Gao W, Yang D, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–6142. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 23.Wei J, Rega MF, Kitada S, Yuan H, Zhai D, Risbood P, Seltzman HH, Twine CE, Reed JC, Pellecchia M. Synthesis and evaluation of Apogossypol atropisomers as potential Bcl-XL antagonists. Cancer Lett. 2009;273:107–113. doi: 10.1016/j.canlet.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitada S, Kress CL, Krajewska M, Jia L, Pellecchia M, Reed JC. Bcl-2 antagonist Apogossypol (NSC736630) displays single-agent activity in Bcl-2-transgenic mice and has superior efficacy with less toxicity compared with gossypol (NSC19048) Blood. 2008;111:3211–3219. doi: 10.1182/blood-2007-09-113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becattini B, Kitada S, Leone M, Monosov E, Chandler S, Zhai D, Kipps TJ, Reed JC, Pellecchia M. Rational design and real time, in-cell detection of the proapoptotic activity of a novel compound targeting Bcl-X(L) Chem Biol. 2004;11:389–395. doi: 10.1016/j.chembiol.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Coward L, Gorman G, Noker P, Kerstner-Wood C, Pellecchia M, Reed JC, Jia L. Quantitative determination of Apogossypol, a pro-apoptotic analog of gossypol, in mouse plasma using LC/MS/MS. J Pharm Biomed Analysis. 2006;42:581–586. doi: 10.1016/j.jpba.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Wei J, Kitada S, Rega MF, Emdadi A, Yuan H, Cellitti J, Stebbins JL, Zhai D, Sun J, Yang L, et al. Apogossypol derivatives as antagonists of antiapoptotic Bcl-2 family proteins. Mol Cancer Ther. 2009;8:904–913. doi: 10.1158/1535-7163.MCT-08-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei J, Stebbins JL, Kitada S, Dash R, Placzek W, Rega MF, Wu B, Cellitti J, Zhai D, Yang L, et al. BI-97C1, an optically pure Apogossypol derivative as pan-active inhibitor of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2010;53:4166–4176. doi: 10.1021/jm1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azab B, Dash R, Das SK, Bhutia SK, Shen XN, Quinn BA, Sarkar S, Wang XY, Hedvat M, Dmitriev IP, et al. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) in combination with the Apogossypol derivative BI-97C1 (Sabutoclax) improves therapeutic efficacy in low CAR colorectal cancer cells. J Cell Physiol. 2012;227:2145–2153. doi: 10.1002/jcp.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dash R, Azab B, Quinn BA, Shen X, Wang XY, Das SK, Rahmani M, Wei J, Hedvat M, Dent P, et al. Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proc Natl Acad Sci USA. 2011;108:8785–8790. doi: 10.1073/pnas.1100769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Ferrer M, Afshar-Sherif AR, Uwamariya C, de Crombrugghe B, Davidson JM, Bhowmick NA. Dermal transforming growth factor-β responsiveness mediates wound contraction and epithelial closure. Am J Pathol. 2010;176:98–107. doi: 10.2353/ajpath.2010.090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang SM, Ozen M, Pathak S, Chung LW. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000;44:91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 33.Zhau HE, Odero-Marah V, Lue HW, Nomura T, Wang R, Chu G, Liu ZR, Zhou BP, Huang WC, Chung LW. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clin Exp Metastasis. 2008;25:601–610. doi: 10.1007/s10585-008-9183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boomershine CS, Chamberlain A, Kendall P, Afshar-Sharif AR, Huang H, Washington MK, Lawson WE, Thomas JW, Blackwell TS, Bhowmick NA. Autoimmune pancreatitis results from loss of TGFβ signalling in S100A4-positive dendritic cells. Gut. 2009;58:1267–1274. doi: 10.1136/gut.2008.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franco OE, Jiang M, Strand DW, Peacock J, Fernandez S, Jackson RS II, Revelo MP, Bhowmick NA, Hayward SW. Altered TGF-β signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;71:1272–1281. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abate-Shen C, Banach-Petrosky WA, Sun X, Economides KD, Desai N, Gregg JP, Borowsky AD, Cardiff RD, Shen MM. Nkx3.1; Pten mutant mice develop invasive prostate adenocarcinoma and lymph node metastases. Cancer Res. 2003;63:3886–3890. [PubMed] [Google Scholar]
- 37.Zhang S, Zhau HE, Osunkoya AO, Iqbal S, Yang X, Fan S, Chen Z, Wang R, Marshall FF, Chung LW, et al. Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells. Mol Cancer. 2010;9:9. doi: 10.1186/1476-4598-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knudsen BS, Gmyrek GA, Inra J, Scherr DS, Vaughan ED, Nanus DM, Kattan MW, Gerald WL, Vande Woude GF. High expression of the Met receptor in prostate cancer metastasis to bone. Urology. 2002;60:1113–1117. doi: 10.1016/s0090-4295(02)01954-4. [DOI] [PubMed] [Google Scholar]
- 39.Zhau HY, Chang SM, Chen BQ, Wang Y, Zhang H, Kao C, Sang QA, Pathak SJ, Chung LW. Androgen-repressed phenotype in human prostate cancer. Proc Natl Acad Sci USA. 1996;93:15152–15157. doi: 10.1073/pnas.93.26.15152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 41.Chou TC, Hayball MP. CalcuSyn for Windows: Multiple-Drug Dose-Effect Analyzer and Manual. Cambridge, UK: Biosoft; 1997. [Google Scholar]
- 42.Rega MF, Reed JC, Pellecchia M. Robust lanthanide-based assays for the detection of anti-apoptotic Bcl-2-family protein antagonists. Bioorg Chem. 2007;35:113–120. doi: 10.1016/j.bioorg.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Wei J, Kitada S, Rega MF, Stebbins JL, Zhai D, Cellitti J, Yuan H, Emdadi A, Dahl R, Zhang Z, et al. Apogossypol derivatives as pan-active inhibitors of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2009;52:4511–4523. doi: 10.1021/jm900472s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Placzek WJ, Wei J, Kitada S, Zhai D, Reed JC, Pellecchia M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 2010;1:e40. doi: 10.1038/cddis.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson RS II, Franco OE, Bhowmick NA. Gene targeting to the stroma of the prostate and bone. Differentiation. 2008;76:606–623. doi: 10.1111/j.1432-0436.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang J, Perry SR, Labrot ES, Wu X, Lis R, et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470:269–273. doi: 10.1038/nature09677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y, Rosenberg JE, Taplin ME. Novel agents and new therapeutics in castration-resistant prostate cancer. Curr Opin Oncol. 2011;23:290–296. doi: 10.1097/CCO.0b013e3283449400. [DOI] [PubMed] [Google Scholar]
- 48.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994;57:406–412. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 49.Xu J, Wang R, Xie ZH, Odero-Marah V, Pathak S, Multani A, Chung LW, Zhau HE. Prostate cancer metastasis: role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate. 2006;66:1664–1673. doi: 10.1002/pros.20488. [DOI] [PubMed] [Google Scholar]
- 50.Harley ME, Allan LA, Sanderson HS, Clarke PR. Phosphorylation of Mcl-1 by CDK1-cyclin B1 initiates its Cdc20-dependent destruction during mitotic arrest. EMBO J. 2010;29:2407–2420. doi: 10.1038/emboj.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makarovskiy AN, Siryaporn E, Hixson DC, Akerley W. Survival of docetaxel-resistant prostate cancer cells in vitro depends on phenotype alterations and continuity of drug exposure. Cell Mol Life Sci. 2002;59:1198–1211. doi: 10.1007/s00018-002-8498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dash R, Richards JE, Su ZZ, Bhutia SK, Azab B, Rahmani M, Dasmahapatra G, Yacoub A, Dent P, Dmitriev IP, et al. Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine. Cancer Res. 2010;70:5034–5045. doi: 10.1158/0008-5472.CAN-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.