Abstract
Oxygen deprivation limits the energy available for cellular processes and yet no comprehensive ATP budget has been reported for any plant species under O2 deprivation, including Oryza sativa. Using 3-d-old coleoptiles of a cultivar of O. sativa tolerant to flooding at germination, (i) rates of ATP regeneration in coleoptiles grown under normoxia (aerated solution), hypoxia (3% O2), and anoxia (N2) and (ii) rates of synthesis of proteins, lipids, nucleic acids, and cell walls, as well as K+ transport, were determined. Based on published bioenergetics data, the cost of synthesizing each class of polymer and the proportion of available ATP allocated to each process were then compared. Protein synthesis consumed the largest proportion of ATP synthesized under all three oxygen regimes, with the proportion of ATP allocated to protein synthesis in anoxia (52%) more than double that in normoxic coleoptiles (19%). Energy allocation to cell wall synthesis was undiminished in hypoxia, consistent with preferential elongation typical of submerged coleoptiles. Lipid synthesis was also conserved strongly in O2 deficits, suggesting that membrane integrity was maintained under anoxia, thus allowing K+ to be retained within coleoptile cells. Rates of protein synthesis in coleoptiles from rice cultivars with contrasting tolerance to oxygen deficits (including mutants deficient in fermentative enzymes) confirmed that synthesis and turnover of proteins always accounted for most of the ATP consumed under anoxia. It is concluded that successful establishment of rice seedlings under water is largely due to the capacity of coleoptiles to allocate energy to vital processes, particularly protein synthesis.
Keywords: Anoxia, ATP utilization, hypoxia, Oryza sativa, rice
Introduction
Rice (Orzya sativa L.) is a key food crop, providing a dietary staple for more than two billion people (FAO, 2004). Uniquely amongst major food crops, some rice cultivars have an extraordinary tolerance to anoxia at germination (Atwell et al., 1982; Kawano et al., 2002; Magneschi and Perata, 2009) and an ability to survive various periods of submergence up to maturity (Gibbs and Greenway, 2003; Xu et al., 2006).
There are marked changes in rice seedling morphology in response to oxygen deprivation. During germination in normoxic conditions, roots and coleoptiles emerge almost simultaneously. Leaves then emerge from the protective sheath of the coleoptile (Alpi and Beevers, 1983). Under severely hypoxic conditions only coleoptiles emerge from seeds, elongating rapidly to reach the surface of the water (Kordan, 1974) while, in anoxia, preferential coleoptile elongation proceeds slowly, acting as a ‘snorkel’ for O2 transport from air to a basal meristem if the water surface is breached (Kordan, 1974; Jackson, 1985). Re-oxygenation allows normal seedling development to ensue (Alpi and Beevers, 1983; Perata and Alpi, 1993).
The developmental differences described above are accompanied by biochemical and physiological phenomena that confer variation in anoxia tolerance between rice cultivars during germination (Atwell et al., 1982; Setter et al., 1994, 1997). These responses include the synthesis of the classic anaerobic response proteins (Sachs et al., 1980; Mujer et al., 1993; Matsumura et al., 1998), higher rates of glycolysis in anoxia than in normoxia (the ‘Pasteur Effect’) (Crawford, 1977; Gibbs et al., 2000; Gibbs and Greenway, 2003), and the high activity of fermentative enzymes (Gibbs et al., 2000; Saika et al., 2006). Despite these responses, ATP synthesis rates are at least 3-fold lower in anoxia compared with normoxia (reviewed by Gibbs and Greenway, 2003). Atkinson (1968) proposed a general hypothesis that lowering energy status in cells (e.g. in anoxia: Raymond et al., 1985) should elicit two broad complementary responses in order to stabilize energy charge. First, ATP-regenerating pathways such as glycolysis become de-repressed to maximize energy production while ATP-utilizing pathways should decline, thereby conserving ATP. Such ATP-conserving events have been reported in protein synthesis (Mocquot et al., 1981; Ishizawa et al., 1999; Chang et al., 2000; Geigenburger et al., 2000), lipid synthesis (Rawyler et al., 1999; Geigenburger et al., 2000), and ion transport (Zhang and Greenway, 1995; Colmer et al., 2001) but fine regulation of gene expression is only now being elucidated (Lasanthi-Kudahettige et al., 2007; Narsai et al., 2009). For example, diversity in the promoter region of six vacuolar pyrophosphatases in rice causes just one homologue to be preferentially transcribed (Liu et al., 2010). Based on an analysis of biomass transfer from seed to seedling, Fox et al. (1994) made estimates of total ATP requirements for the growth of aerobic and anaerobic rice seedlings. However, a global picture of the co-ordination of energy allocation to individual ATP-consuming processes has not been reported for a single organ. Analysis of energy allocation to major processes leads us to the hypothesis that there is a hierarchical down-regulation of ATP consumption during periods of ATP shortage, as proposed by Atwell et al. (1982) and Greenway and Gibbs (2003). To test this hypothesis, an ATP budget was developed using O. sativa cv. ‘Amaroo’, a relatively submergence-tolerant cultivar grown commercially in Australia. This required detailed measurements of fermentation and respiration, fluxes of substrates into key biosynthetic pathways, and estimates of transport processes. Using bioenergetics data and theoretical estimates of ATP cost of various ATP-utilizing processes (Penning de Vries et al., 1974; Amthor, 2000), the energy made in coleoptiles under steady-state oxygen deficits was apportioned to growth requirements. This question was further addressed by using radioactive tracers to estimate gross rates of protein biosynthesis in coleoptiles of another six rice genotypes (see the Materials and methods). Across all genotypes, our data suggest that protein synthesis becomes the predominant sink for ATP consumption in anoxic coleoptiles at the cost of almost all other biosynthetic processes.
Materials and methods
Plant material
Seven O. sativa genotypes were examined: Amaroo, an anoxia-tolerant line grown commercially in Australia; Khaiyan, an anoxia-tolerant line from Bangladesh; Khao Hlan On (KHO), an anoxia-tolerant line from the Myanmar; rad, a reduced alcohol dehydrogenase mutant (Matsumura et al., 1998; Saika et al., 2006); Kinmaze (the rad parent line); a pyruvate decarboxylase (PDC) T-DNA insertional knockout mutant (T-181-8-6-4) obtained from Dr Narayana Upadhyaya (CSIRO Division of Plant Industry, Canberra), and Nipponbare, the PDC mutant parent line.
Seeds were de-hulled and surface-sterilized [70% ethanol (v/v) for 1 min, washed in distilled H2O for 1 min, 25% bleach (v/v) for 10 min, washed ×3 in sterile distilled H2O for 1 min, 0.1% mercuric chloride (w/v) for 3.5 min, washed ×5 in sterile distilled H2O for 1 min]. Seeds (excepting rad) then were grown in 2.0 l of solution containing 0.8 mM KH2PO4 and 0.5 mM CaCl2 with air (normoxia), 3% O2 in N2 (v/v) (hypoxia) or N2 (anoxia) bubbled through the solution at ∼0.2 l h−1. To grow coleoptiles of rad in hypoxia/anoxia, seeds were pre-germinated at 28 °C in Petri dishes containing moistened tissue paper. They were then transferred to the oxygen treatments as previously listed.
Growth measurements
Coleoptiles were collected from each treatment at 24 h periods, beginning 2 d after imbibition. Coleoptile lengths were measured (Fig. 1a) and then coleoptiles were excised and fresh weight determined (Fig. 1b). Coleoptiles were dried for 2 d at 60 °C and weighed to determine dry mass (Table 1). Dried coleoptiles were placed in 80% ethanol (v/v) and heated to 80 °C for 10 min to remove free amino acids. The ethanol was removed and samples were again dried at 60 °C for 24 h. Dried coleoptiles were weighed again to determine the ethanol-insoluble dry mass (Table 1).
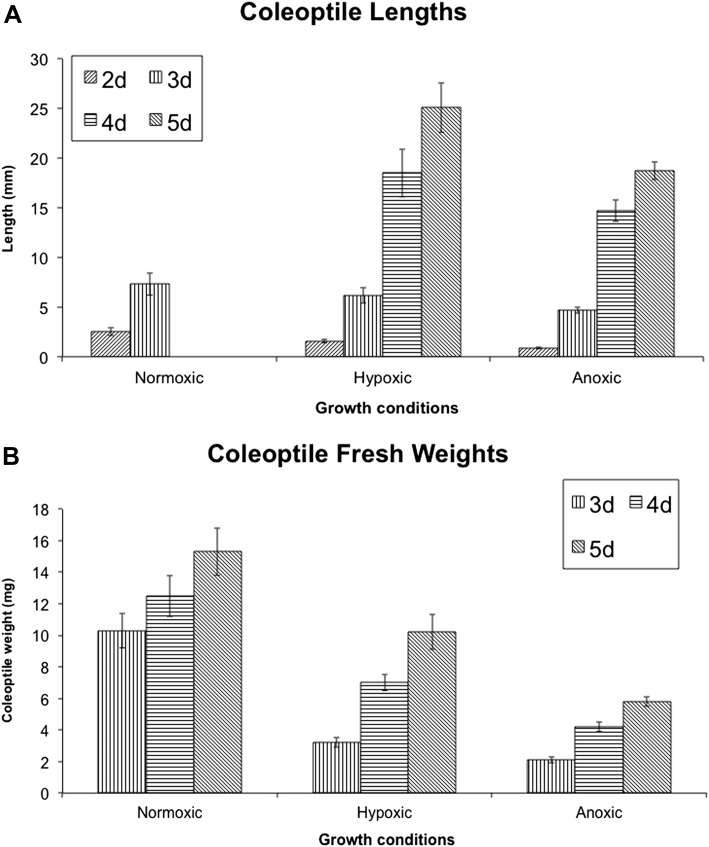
Fig. 1.
Coleoptile lengths (A) and fresh weights (B) of rice seeds germinated in normoxic (aerated), hypoxic (3% O2), and anoxic (N2) solution. Coleoptiles (30–40) were collected from each treatment 2, 3, 4, and 5 d after imbibition. Lengths and fresh weights were determined. By 3 d after imbibition the coleoptiles of the normoxic seeds had begun to senesce. Length measurements were therefore halted at 3 d after imbibition in normoxic coleoptiles. Data are mean and SEM (n ≥3).
Table 1.
Growth characteristics of coleoptiles grown in normoxic (aerated), hypoxic (3% O2), and anoxic (N2) solution Coleoptiles were collected at 3, 4, and 5 d after imbibition and ethanol-insoluble dry weights (EiDW) were determined. Protein and free amino acid levels were determined by amino acid hydrolysis and water extraction, respectively, and were analysed via ultra-high performance liquid chromatography. Total DNA was extracted with phenol–chloroform and total amounts determined spectrophotometrically. Total lipids were determined by extracting powdered, freeze-dried material with hexane/ethanol (95:5%, v/v). (ND, not determined.) ‘col’ refers to ‘coleoptile’. Data are mean and SEM (n ≥3 for all samples). Statistically different data across treatments (Tukey-Kramer, α=0.05) are indicated by different letters.
| Normoxic | Hypoxic | Anoxic | ||||
| Time (d) | 3 | 4 | 3 | 4 | 3 | 4 |
| Dry weight (mg col−1) | 1.2±0.2 a | 1.4±0.2 a | 0.4±0.1 b | 0.5±0.1 b | 0.3±0.1 b | 0.3±0.1 b |
| Ethanol-insoluble dry | 0.61±0.22 a | 0.92 ±0.21 a | 0.28±0.07 b | 0.31±0.09 b | 0.11±0.04 c | 0.14±0.02 c |
| weight (mg col−1) | ||||||
| Protein | 137±5 a | 142±8 a | 152±13 a | 157±18 a | 138±9 a | 143±11 a |
| (mg g−1 DW) | ||||||
| Free amino acids | 16.1±0.8 b | ND | 62.9±6.8 b | ND | 41.2±2.8 b | ND |
| (mg g-1 DW) | ||||||
| DNA (μg col−1) | 1.2±0.6 a | ND | 1.1±0.3 a | ND | 0.8±0.1 a | ND |
| Lipid | 109±16 a | 93±12 a,b | 122±22 a | 88±9 b | 106±19 a | 81±13 a,b |
| (mg g−1 DW) | ||||||
Determination of protein content and amino acid analysis
Estimates of protein on a fresh weight basis were made in 3-d-old and 4-d-old coleoptiles of all cultivars by a phenol/chloroform extraction of total protein (Wang et al, 2003) and levels quantified by the Bradford assay (Pierce) as per the manufacturer’s instructions. Nitrogen analysis was also made on 2–3 mg samples of 3-, 4-, and 5-d-old freeze-dried coleoptiles using a LECO CHN-900 to confirm the protein analyses above. Total protein by mass in each dried sample was estimated by a conversion factor from nitrogen to protein of 5.95 (Merrill and Watt, 1973). These data were used to estimate N demand by the growing coleoptile.
Analysis of total protein amino acid make-up and free amino acid levels in cv. Amaroo were determined from the same samples analysed above by reversed-phase UPLC performed by the Australian Proteome Analysis Facility (APAF, www.proteome.org.au). Results are shown in Supplementary Tables S1 and S2 at JXB online.
Determination of lipid content
Three- and 4-d-old coleoptiles from each treatment were excised, weighed, and freeze-dried. Total lipids in each sample were determined according to Lilitchan et al. (2008) with the exception that hexane:ethanol (19:1, v/v) was used to increase the extraction efficiency of phospholipids. Lipid content of each sample was determined by comparison with a standard curve of rice-bran oil dissolved in hexane:ethanol (19:1, v/v). Results are shown in Table 1.
Determination of nucleic acid content
Three- and 4-d-old coleoptiles were collected from each treatment and weighed. Total nucleic acids were extracted from coleoptiles (Kang and Yang, 2004). RNA and DNA were separated by the use of LiCl (Raha et al., 1990). RNA/DNA quality and levels were determined spectrophotometrically. The results are shown in Table 1.
Using the known genome size of O. sativa (403 Mbp) and the GC/AT ratio (Goff et al., 2002) the mass of a single copy of the rice genome was calculated. To estimate cell numbers per coleoptile, total DNA content was divided by the theoretical estimate of DNA content per cell (see Supplementary Table S3 at JXB online). Total cell numbers were also estimated from previously published rice coleoptile cell dimensions (Wada, 1961) and measurements of coleoptile widths measured with micrometers (see Supplementary Table S3 at JXB online).
Estimations of cell number from coleoptile DNA content and the cell packing method were similar across all treatments (see Supplementary Table S3 at JXB online).
Oxygen utilization rates
Oxygen electrodes (Rank Brothers) were set up according to the manufacturer’s instructions. To calibrate the electrodes, 5 ml of growth solution was placed into the electrode cuvette, the magnetic stirrer was engaged and the stable level achieved taken to be full saturation at 21% O2. A small quantity of sodium dithionite was added to zero the electrode. This solution was removed and the electrode washed three times with fresh growth solution. Fresh solution that had been equilibrated for 4 h with either air or 3% O2 (depending on the treatment) was used as the initial solution in the cuvette.
Three-, 4-, and 5-d-old coleoptiles (10–15 coleoptiles per replicate, three replicates per treatment) were excised, weighed, and placed into the electrode cuvette. Oxygen depletion curves were generated for each treatment and used to determine rates of O2 utilization by coleoptiles in each treatment (Table 2 for Amaroo, Table 8 for other cultivars).
Table 2.
Rates of O2 respiration, ethanol fermentation, and estimated ATP synthesis by rice coleoptiles grown in normoxic (aerated), hypoxic (3% O2), and anoxic (N2) solution Coleoptiles were collected at 2, 3, and 4 d after imbibition. Coleoptiles from each treatment (10–15) were excised, weighed, and oxygen-use curves generated using an oxygen electrode. Rates of ethanol usage were determined for 3-d-old coleoptiles from each growth condition. Rates are given in the following units: O2 consumption in nmol (O2) g−1 (FW) min−1; ethanol synthesis in μmol (ethanol) g−1 (FW) h−1; ATP synthesis nmol (ATP) g−1 (FW) min−1. Data are mean and SEM (n ≥3) for all samples. Statistically different data across time (O2 consumption) and treatments (ethanol synthesis) using the Tukey–Kramer test (α=0.05) are indicated by different letters.
| Treatment | O2 consumption Age of coleoptile (d) | Ethanol synthesis | Estimation of ATP synthesis (3 d) | ||
| 2 | 3 | 4 | |||
| Normoxic | 590±66 a | 490±49 a | 470±57 a | 0.9±0.2 a | 2471±249 |
| Hypoxic | 395±43 a | 270±42 b | 155±22 c | 5.2±0.2 b | 1520±213 |
| Anoxic | ND | ND | ND | 9.1±0.8 c | 302±27 |
Table 8.
Coleoptile length and fresh weight of 3-d-old Khaiyan, KHO, rad, PDC-insertional mutant, Kinmaze, and Nipponbare coleoptiles grown in normoxic (aerated), hypoxic (3% O2), and anoxic (N2) solution Coleoptiles (30–40) were collected from each cultivar/treatment combination 3 d after imbibition. Lengths and fresh weights were determined. Data are mean and SEM (n ≥3). Statistically different data across genotypes within a single O2 treatment (Tukey–Kramer, α=0.05) are indicated by different letters.
| Normoxic | Hypoxic | Anoxic | ||||
| Genotype | Length (mm) | Weight (mg) | Length (mm) | Weight (mg) | Length (mm) | Weight (mg) |
| Khaiyan | 16.3±0.5 a | 8.8±1.4 a | 36.8 ±2.1 a | 4.6 ±0.6 a | 41.2±2.0 a | 5.4±1.1 a |
| KHO | 16.5±0.9 a | 9.1±0.9 a | 37.0±1.6 a | 5.6±1.2 a | 38.4±4.6 a | 4.7±1.3 a |
| Nipponbare | 22.8±5.3 a | 10.5±1.2 a | 20.6±3.8 b | 6.7±0.4 a | 19.2±2.8 b | 3.6±0.8 a |
| Kinmaze | 22.0±2.8 a | 7.8±0.9 a | 19.2±2.6 b | 6.2±0.8 a | 15.6±2.5 b | 3.8±0.9 a |
| PDC-insertional mutant | 20.8±1.7 a | 9.6±1.8 a | 15.0±1.7 b | 5.5±0.4 a | 10.2±2.2 b | 1.5±0.3 b |
| rad | 21.8±5.3 a | 6.4±1.3 a | 4.8±0.4 c | 0.9±0.3 b | 3.6±0.3 c | 1.0±0.4 b |
Determination of rates of ethanol fermentation
Three-day-old coleoptiles were excised from seedlings for each growth condition, weighed, and washed in distilled H2O to remove residual ethanol. Groups of coleoptiles (∼10 coleoptiles per group, three groups per treatment per biological replicate) were placed into 2 ml of distilled water in 5 ml Wheaton vials that previously had been previously been equilibrated for 4 h in an appropriate gaseous atmosphere (i.e. air, 3% O2 or N2) and were sealed for 1 h under each atmosphere. The O2 concentration in the 3 ml headspace of the vials was sufficient to maintain a stable O2 concentration in the bathing solution. 660 μl of 30% (w/v) HClO4 was added to the coleoptiles, which were left for 10 min on ice to allow ethanol to leach from the them. The solution was neutralized with 500 μl of 69% (w/v) K2CO3. Liquid was removed to Eppendorf tubes and spun at 5000 g for 2 min to precipitate KClO4. An aliquot (0.5 ml) of the supernatant was added to 2 ml of 0.15 M Na4P2O7 (sodium pyrophosphate)+0.15 M semicarbazide HCl+0.45 M glycine pH 9.0, 0.5 ml distilled H2O, and 0.05 ml of 20 mg ml−1 NAD+. The A340 of samples was measured, and then 2.5 IU of ADH was added to each sample. Samples were placed at 37 °C for 60 min and A340 measured again. Amounts of ethanol present in each sample were determined by comparison with a standard curve and were used to calculate rates of ethanol synthesis for each treatment on a fresh-mass basis (Table 2 for Amaroo, Table 9 for the other cultivars).
Table 9.
Rates of O2 consumption, ethanol fermentation, and estimated ATP synthesis by rice coleoptiles of anoxia tolerant (*) and intolerant (**) cultivars grown in normoxic (aerated), hypoxic (3% O2), and anoxic (N2) solution Coleoptiles from each cultivar and treatment (10–15) were excised, weighed, and oxygen-use curves generated using an oxygen electrode. Rates of ethanol usage were determined for 3-d-old coleoptiles from each growth condition. Rates are given in the following units: O2 consumption in nmol (O2) g−1 (FW) min−1; ethanol production in μmol (ethanol) g−1 (FW) h−1; ATP synthesis nmol (ATP) g−1 (FW) min−1. Data are mean and SEM (n >3) for all samples. Statistically different data across genotypes within a single set of measurements (Tukey–Kramer, α=0.05) are indicated by different letters.
| O2 consumption | Ethanol production | ATP production | ||||||
| Cultivar | Normoxic | Hypoxic | Normoxic | Hypoxic | Anoxic | Normoxic | Hypoxic | Anoxic |
| Khaiyan* | 716±26 a | 253±21 a | 1.2±0.5 a | 11.0±2.3 a | 11.1±2.1 a | 3600±146 | 1448±143 | 370±70 |
| KHO* | 590±29 a | 244±32 a | 1.1±0.4 a | 10.1±0.4 a | 10.5±2.5 a | 2968±152 | 1388±167 | 350±83 |
| Nipponbare | 427±29 b | 273±65 a | 0.9±0.3 a | 7.9±0.4 a | 6.8±0.2 b | 2138±151 | 1470±325 | 226±7 |
| Kinmaze (rad parent line) | 666±56 a | 201±18 a | 1.0±0.4 a | 8.3±2.3 a | 9.7±1.9 a | 3346±287 | 1143±94 | 323±63 |
| PDC-insertional mutant** | 406±19 b | 244±66 a | 0.8±0.3 a | 7.6±1.1 a | 4.3±1.2 c | 2043±101 | 1346±327 | 143±40 |
| rad** | 403±38 b | 180±29 a | 0.2±0.2 b | 2.3±0.6 b | 2.3±0.4 d | 2018±206 | 938±155 | 76±13 |
Estimation of rates of ATP synthesis
To estimate the rates of ATP synthesis, the rates determined above (oxygen consumption and ethanol synthesis) were converted into rates of ATP production. It was assumed that, in normoxic and hypoxic coleoptiles, the ATP:O2 ratio was 5, based on rates of phosphorylation thought to be achieved in mitochondria (Gibbs and Greenway, 2003). In addition, each mole of ethanol produced was assumed to generate one mole of ATP in normoxia and hypoxia (Gibbs and Greenway, 2003) and two moles of ATP in anoxia through the engagement of PPi metabolism (Igamberdiev and Kleczkowski, 2011).
Using tracers to estimate ‘instantaneous’ rates of polymer synthesis
The choice of 4 h as a labelling period was made as a compromise between the need to label internal pools substantially while not allowing significant release of labelled product through degradation. Shorter labelling periods resulted in too few counts, while after 4 h, the most heavily labelled pool was the protein pool in normoxia where one-fifth of the endogenous pool was labelled. In the case of all other treatments and products, the 4 h exposure to tracers labelled a much smaller proportion of the endogenous pool. Therefore, it is concluded that instantaneous (gross) synthesis of these important polymers has been estimated rather than net synthesis in the steady-state.
14C/3H amino acid tracer experiments
Three-day-old seedlings were placed in 10 ml of the appropriate growth solution that had been equilibrated for 4 h with a continuous gas supply specific to each treatment (air for normoxia, 3% O2 for hypoxia, N2 for anoxia) containing 5 mM of cold label and 10 nCi ml−1 of 14C/3H label as a tracer (∼10 seedlings per vial, three vials per replicate, with three replicates per treatment per tracer). Seedlings were left to label in this solution for 4 h under the appropriate gas supply. Seedlings were removed from the labelling solution, washed three times with distilled H2O and coleoptiles excised. Fresh weights for each replicate were determined and the excised coleoptiles were placed in solution containing 5 mM cold label overnight at 4 °C. The cold label was removed, coleoptiles were snap frozen in liquid N2 and then freeze-dried. Freeze-dried coleoptiles were placed in 10 ml of 80% ethanol (v/v) and heated to 80 °C for 10 min to extract free amino acids. Ethanol was removed and coleoptiles dried in an oven at 60 °C for 60 min. 500 μl of 1 N NaOH was added to each sample to solubilize the protein and the mixture left overnight at 60 °C. 500 μl of 1 N HCl was added to each sample to neutralize the 1 N NaOH. Ten ml of BCS scintillation cocktail (Amersham) was added to each sample and total 14C/3H activity determined using a scintillation counter (Packard 2100TR-LSC). Total 14C/3H incorporation was calculated for each treatment. Using the known amino acid composition of proteins in each treatment (see Supplementary Table S2 at JXB online), total rates of amino acid incorporation into protein were estimated for each treatment. Samples were also taken for scintillation counting at the following stages in the experiment: from the stock solution; from the labelling solution after labelling was complete; from each of the washes, from the cold label when it was removed and from the ethanol after heating to determine leakage of 14C/3H activity during the extraction.
[3H] thymidine tracer experiments
Three-day-old Amaroo seedlings were placed in 10 ml of the growth solution equilibrated for 4 h as above containing 1 mM of thymidine and 10 nCi ml−1 of [3H] thymidine as a tracer (∼10 seedlings per vial, three vials per replicate, with three replicates per treatment per tracer). Seedlings were left to label in this solution for 4 h in the appropriate gas supply. Seedlings were removed from the labelling solution, washed three times with distilled H2O and coleoptiles excised. Fresh masses for each replicate were determined and the excised coleoptiles placed in solution containing 5 mM thymidine overnight at 4 °C. Total DNA was isolated as described previously. 3H incorporation was determined for each treatment by scintillation counting (Packard 2100TR-LSC). Total rates of DNA synthesis for each treatment were estimated by allowing for the known GC/AT ratio of the O. sativa genome.
[14C] sucrose tracer experiments
Three-day-old cv. Amaroo seedlings were placed into 10 ml of growth solution, equilibrated for 4 h as above, and supplemented with sucrose to a final concentration of 50 mM and 10 nCi ml−1 of [14C] sucrose (∼10 seedlings per vial, three vials per replicate, with three replicates for each treatment). Seedlings were left to label in this solution for 4 h in the appropriate gas supply. Seedlings were removed from the labelling solution, washed three times in distilled H2O and coleoptiles excised. Fresh masses for each replicate were determined and the coleoptiles placed in solution containing 50 mM sucrose cold label overnight at 4 °C. The cold label was removed, coleoptiles were snap frozen in liquid N2, and freeze-dried. Free amino acids, extractable proteins, cell wall mass, lipid and nucleic acid content was determined as described above. 200 μl of each extracted sample (except cell walls) were added to 800 μl of distilled H2O. Ten ml of BCS scintillation cocktail (Amersham) was added to each sample and 14C activity measured using a scintillation counter (Packard 2100TR-LSC). 14C activity was converted to quantities of sucrose for each sample and rates of incorporation into each pool were determined.
Determination of K+ uptake rates
Experiments were conducted to determine the rates of K+/Rb+ uptake by normoxic, hypoxic and anoxic cv. Amaroo coleoptiles.
Fresh boxes containing growth solution were prepared and gas (air, 3% O2 or N2) bubbled through them for 4 h. Three-day-old seedlings were transferred to this fresh solution containing 0.5 mM CaCl2 and 0.25 mM KH2PO4. A lower external K+ concentration than in the standard growth solution was used in order to preclude passive K+ uptake. Two 50 ml samples were taken from the growth solution at the beginning of the experiment and each hour afterwards for 4 h. The coleoptiles for each treatment were then harvested and weighed. Total K+ levels in each sample were determined using a flame photometer (Jenway PFP7) and rates of K+ uptake by coleoptiles was determined by depletion of K+ from the growth solution.
To determine Rb+ uptake, a similar experiment was conducted but with the substitution of K+ by adding 0.25 mM RbCl to the growth solution. Samples were taken every hour for 4 h and the coleoptiles harvested and weighed. Total Rb+ levels in each sample were determined by flame photometry and rates of Rb+ uptake by coleoptiles was determined by depletion of Rb+ from the growth solution.
Statistical analysis
The raw data for Tables 1−6 and 8−10 were analysed with a single-factor ANOVA (across oxygen treatments for Tables 1−6 and across genotypes for Tables 8−10). The results of these ANOVAs were used to perform a Tukey–Kramer analysis (α=0.05) to determine significant differences.
Table 6.
Rates of Rb+ and K+ influx for coleoptiles grown in normoxic (aerated), hypoxic (3% O2), and anoxic (N2) solution Three-day-old seedlings were transferred to fresh solution containing 0.25 mM of Rb+ or K+. Triplicate 50 ml samples were taken from the growth solution every hour for 4 h. Coleoptiles were excised and weighed. Total amounts of Rb+/K+ uptake were determined using a flame photometer. Data are mean and SEM (n=3). Statistically different data across treatments (Tukey–Kramer, α=0.05) are indicated by different letters.
| Treatment | Rubidium influx (μmol Rb+ g−1 FW h−1) | Net potassium influx (μmol K+ g−1 FW h−1) |
| Normoxic | 3.9±0.2 a | 3.0±0.3 a |
| Hypoxic | 3.6±0.3 a | 2.6±0.1 a |
| Anoxic | 0.8±0.2 b | 0.9±0.2 b |
Table 10.
Rates of amino acid incorporation into protein by normoxic (aerated), hypoxic (3% O2), and anoxic solution-grown coleoptiles of anoxia-tolerant (*) and -intolerant (**) lines as estimated by [14C]leucine tracer experiments Three-day-old seedlings (10–15) were labelled in solution containing 5 mM of cold label and 10 nCi ml−1−of [14C]leucine as a tracer. Labelling period was 4 h. Total 14C incorporation was determined for each treatment. Data are mean and SEM (n=3). Statistically different data across genotypes within a single O2 treatment (Tukey-Kramer, α=0.05) are indicated by different letters.
| Cultivar | Normoxic | Hypoxic | Anoxic |
| Khaiyan* | 102±9 a | 44±8 a | 41±6 a |
| KHO* | 98±12 a | 51±5 a | 35±6 a |
| Nipponbare | 72±11 a | 38±4 a | 31±6 a |
| Kinmaze (rad parent line) | 63±3 a | 35±7 a | 26±8 a |
| PDC-insertional mutant** | 69±9 a | 31±7 a,b | 12±6 b |
| rad** | 39±6 b | 22±4 b | 8±6 b |
Developing an ATP budget
To examine ATP utilization under different O2 regimes, the various biosynthetic rates determined above were incorporated into an ATP budget.
Since it was not possible to measure rates of carbohydrate import from the seed to the coleoptile directly, a minimal estimate for carbohydrate import requirements was developed. It was assumed that the increase in EiDW between 3 d and 4 d (on a coleoptile basis) was wholly due to an increase in structural biomass such as cell walls. This increase would require an equivalent amount (by weight) of sucrose which was then converted to a rate of sucrose required for each treatment [in nmol (sucrose) g−1 (FW) min−1]. For carbohydrate consumption by glycolysis, it was assumed that it was the sum of one-quarter of the rate of ethanol fermentation (one sucrose is consumed per four ethanol produced: Igamberdiev and Kleczkowski, 2011) plus one-twelfth of the rate of O2 consumption (12 O2 molecules are required to oxidize a single sucrose molecule completely: Fernie et al., 2004). It was assumed that each unit of sucrose loaded into the coleoptile consumed 1 ATP and that unloading was symplastic (Scofield et al., 2007).
Rates of amino acid synthesis were estimated by calculating the average rate of synthesis needed to supply the increase in soluble protein content seen in all treatments between 3 d and 4 d. Amounts of nitrate import needed to support this synthesis were estimated using the assumptions of a Jones Factor of 5.95 for rice (Merrill and Watt, 1973), that each nitrogen required was supplied by a single nitrate (NO3 −) and that the ATP cost for nitrate transport was 1 ATP per nitrate (Lin et al., 2000).
In developing this budget, a number of assumptions were made. (i) The cells of the coleoptiles were considered as a homogeneous mass, with no accounting made for the differences in ATP utilization between epidermal and cortical cells. (ii) Rates of biosynthesis estimated over 4 h labelling periods were assumed to represent steady-state rates in each treatment. (iii) In spite of a range of ATP costs associated with each biosynthetic process (e.g. longer lipids require greater amounts of ATP to synthesize) a single ATP cost was assumed for each process and was assumed to be invariant across treatments. (iv) Because of the differences in mass between coleoptiles in the three treatments, ATP utilization was normalized by converting all data to a rate of utilization per gram of fresh mass per minute.
ATP costs for each process in terms of moles of ATP consumed per mole of product synthesized were derived from the following sources: protein synthesis (per peptide bond formed and incorporating estimates of the ATP cost of protein breakdown and turnover)—5 moles ATP (Amthor, 2000); cell wall materials—3 moles ATP (Penning de Vries et al., 1974); lipid synthesis (to produce palmitic acid)—21 moles ATP (Penning de Vries et al., 1974; Goodwin and Mercer, 1985), and nucleic acid—4 moles ATP (Penning de Vries et al., 1974).
Results
Coleoptile growth
Increases in length and fresh weight of Amaroo coleoptiles grown in normoxia, hypoxia or anoxia were measured from 2 d after imbibition (Fig. 1A, B). Hypoxic coleoptiles elongated approximately twice as fast as anoxic coleoptiles between 2 d and 5 d after imbibition (0.4 mm h−1 cf. 0.2 mm h−1, respectively). Fresh weights were greatly reduced by oxygen deprivation, with a 2-fold difference in the increase in fresh weight for hypoxic compared to anoxic coleoptiles [0.15 mg (FW) h−1 cf. 0.08 mg (FW) h−1, respectively].
Table 1 summarizes the effect of O2 supply on the composition of Amaroo coleoptiles. The ethanol-insoluble dry weight (EiDW) of individual normoxic coleoptiles was approximately double that of hypoxic coleoptiles and 6–8-fold greater than that of anoxic coleoptiles. However, there was no significant difference between the three treatments in levels of protein, DNA or lipid on a dry weight basis. By contrast, anoxic and hypoxic coleoptiles had three to four times higher levels of free amino acids than normoxic coleoptiles, with alanine approximately 25-fold and 40-fold higher in anoxic and hypoxic tissues, respectively, than in normoxic coleoptiles (see Supplementary Table S1 at JXB online).
Normoxic coleoptiles, unlike hypoxic or anoxic coleoptiles, began to senesce 4 d after imbibition as shown by Kawai and Uchimiya (2000), determining that the detailed ATP budgets should be derived from coleoptiles 3 d after imbibition.
Estimating ATP synthesis by respiration and fermentation
Rates of O2 uptake and ethanol synthesis of excised normoxic, hypoxic, and anoxic coleoptiles were measured in order to estimate rates of ATP synthesis in each treatment (Table 2). Rates of O2 consumption decreased in both normoxic and hypoxic coleoptiles between the second and fourth day after imbibition, declining by approximately 20% in normoxic coleoptiles [590±66 to 470±57 nmol O2 g−1 (FW) min−1, respectively]) and by approximately 60% in hypoxic coleoptiles measured at 3% O2 [395±75 to 155±39 nmol O2 g−1 (FW) min−1, respectively] over this period.
Rates of ethanol synthesis were higher in anoxic tissue [8.46±0.76 μmol (ethanol) g−1 (FW) h−1] than either hypoxic [(5.15±0.20 μmol (ethanol) g−1 (FW) h−1] or normoxic tissue [0.86±0.21 μmol (ethanol) g−1 (FW) h−1]. A measurable but very low rate of ethanol synthesis during constant aeration suggested a failure of pyruvate to be fully oxidized in the TCA cycle and may be an indicator of localized hypoxia within normoxic coleoptiles or an imbalance between glycolysis and mitochondrial activity.
Estimated rates of ATP production [(nmol (ATP synthesized) g−1 (FW) min−1] were 2464±249 (normoxic), 1520±213 (hypoxic), and 302±27 (anoxic). These values represent a 2-fold and an 8-fold decrease in ATP available under hypoxia and anoxia, respectively, compared with normoxia. The experiments described in the following paragraphs were used to derive estimates of total ATP consumption during normoxic, hypoxic, and anoxic conditions. By assuming that ATP production and consumption reach equilibrium in each steady-state O2 treatment, energy costs could be ascribed to the major endergonic processes required for growth.
Estimating rates of protein synthesis
Estimates of the rates of protein turnover in normoxic, hypoxic and anoxic coleoptiles were made 3 d after imbibition (Table 3). Normoxic coleoptiles incorporated all radioactive amino acids two to three times faster than in hypoxic and anoxic coleoptiles [93±6 nmol amino acids g−1 (FW) min−1 in normoxic coleoptiles versus 41±2 and 32±2 nmol amino acids g−1 (FW) min−1 in hypoxic and anoxic coleoptiles, respectively]. The observed decrease in protein synthesis rates in anoxic coleoptiles compared with normoxic coleoptiles was proportionately less than the decrease in the estimated rates of ATP synthesis in anoxia, suggesting that a greater proportion of available ATP was directed into protein synthesis during an O2 deficit.
Table 3.
Rates of amino acid incorporation into protein by normoxic (aerated), hypoxic (3% O2), and anoxic (N2) grown coleoptiles as determined by [14C/3H]amino acid tracer experiments Three-day-old seedlings (10–15) were labelled in solution containing 5 mM of cold label and 10 nCi ml−1 of [14C/3H] label as a tracer. Labelling period was 4 h. Total 14C/3H incorporation was determined for each treatment. Using the known amino acid composition of proteins in each treatment (see Supplementary Table S1 at JXB online), total rates of amino acid incorporation into protein were estimated for each treatment. Data are mean and SEM (n=3 for each label). Statistically different data across treatments (Tukey–Kramer, α=0.05) are indicated by different letters.
| Label | Estimated incorporation of amino acid into protein (nmol aa g−1 FW min−1) | ||
| Normoxic | Hypoxic | Anoxic | |
| [14C]valine | 83±12 a | 40±6 b | 30±4 b |
| [14C]isoleucine | 92±11 a | 39±5 b | 31±5 b |
| [3H]leucine | 105±15 a | 45±7 b | 35±6 b |
| Mean | 93±6 | 41± 2 | 32±2 |
Estimating DNA synthesis rates by [3H] thymidine incorporation
Experiments were conducted to determine rates of DNA synthesis via [3H] thymidine incorporation (Table 4). Rates of DNA synthesis in hypoxic and anoxic coleoptiles were not significantly different and were approximately half those observed in normoxic coleoptiles [42±9, 51±17, and 90±6 ng (DNA) g−1 (FW) min−1, respectively]. It should be emphasized that tissues of the first leaf, in which cell division was rapid, were removed from all coleoptile samples.
Table 4.
Rates of DNA synthesis in normoxic (aerated), hypoxic (3% O2), and anoxic (N2) coleoptiles as estimated by [3H]thymidine incorporation Three-day-old seedlings (10–15) were placed in labelling solution containing 1 mM of thymidine and 1 nCi ml−1 of [3H] thymidine as a tracer. Labelling period was 4 h. Total [3H] thymidine incorporation was determined for each treatment. Using the known GC/AT ratio of the O. sativa genome, total rates of DNA synthesis were estimated for each treatment. Data are mean and SEM (n=3). Statistically different data across treatments (Tukey–Kramer, α=0.05) are indicated by different letters.
| Rate of [3H]thymidine incorporation(ng DNA g−1 FW min−1) | |
| Normoxic | 90±6 a |
| Hypoxic | 42±9 b |
| Anoxic | 51±17 b |
Incorporation of [14C] sucrose to estimate biosynthesis
Experiments were conducted to estimate the rates of de novo synthesis of a range of metabolites from the dominant carbon source, using [14C] sucrose as a tracer. Rates of incorporation of sucrose into free amino acids, proteins, lipids, nucleic acids, and cell wall materials were determined (Table 5).
Table 5.
Incorporation of sucrose into various biopolymer pools of normoxic (aerated), hypoxic (3% O2), and anoxic (N2) rice coleoptiles using a [14C] sucrose tracer Three-day-old seedlings (10–15) were placed into growth solution supplemented with 50 mM sucrose and 10 nCi ml−1 [14C] sucrose. The labelling period was 4 h. Coleoptiles were frozen in liquid N2 then freeze-dried. Free amino acids, extractable proteins, lipids, nucleic acids, and cell walls were extracted as described in the Materials and methods. 14C activity was measured and converted to quantities of sucrose for each sample. Rates of incorporation into each pool were determined. Rates are in nmol (sucrose incorporated) g−1 (FW) min−1. Data are mean and SEM (n=3). Statistically different data across treatments (Tukey–Kramer, α=0.05) are indicated by different letters.
| Biopolymer pool | Normoxic | Hypoxic | Anoxic |
| Free amino acids | 42.4±3.7 a | 27.9±1.0 b | 4.8±0.8 c |
| Extractable protein | 35.4±4.0 a | 19.9±2.2 b | 4.4±0.9 c |
| Lipids | 3.8±1.0 a | 2.7±0.7 b | 2.4±0.1 b |
| Nucleic acids | 3.8±1.0 a | 1.7±0.3 b | 2.2±0.3 b |
| Cell walls | 24.4±3.0 a | 25.3±5.9 a | 5.0±1.2 |
Rates of [14C] sucrose incorporation into both free amino acids and soluble protein were greatly reduced in hypoxic and anoxic coleoptiles compared with normoxic coleoptiles [42.4±3.7 and 35.4±4.0 (normoxic); 27.9±1.0 and 19.9±2.2 (hypoxic); 4.8±0.8 and 4.4±0.9 (anoxic) nmol (sucrose incorporated) g−1 (FW) min−1]. [14C] sucrose incorporation into lipids on a fresh weight basis was ∼30% lower in both hypoxic and anoxic treatments compared with normoxic coleoptiles, although there was no significant difference between hypoxic and anoxic coleoptiles [2.7±0.7 and 2.4±0.1 versus 3.8±1.0 nmol (sucrose incorporated) g−1 (FW) min−1, respectively].
[14C] sucrose incorporation into total nucleic acids (RNA and DNA) was halved in hypoxic and anoxic treatments [1.7±0.3 and 2.2±0.3 nmol (sucrose incorporated) g−1 (FW) min−1] compared with normoxic coleoptiles [3.8 nmol (sucrose incorporated) g−1 (FW) min−1].
There was no significant difference in the amounts of [14C] sucrose incorporated into cell walls between the normoxic and hypoxic coleoptiles [(24.4±3.0 and 25.3±5.9 nmol (sucrose incorporated) g−1 (FW) min−1, respectively]. Rates of incorporation in anoxic coleoptiles [(5.0 ± 1.2 nmol (sucrose incorporated) g−1 (FW) min−1)] were ∼20% of the rates in the presence of O2.
Estimating costs of ion uptake using K+/Rb+ uptake
Experiments were conducted to estimate the costs of potassium acquisition by measuring the rates of K+/Rb+ uptake (Table 6). Both normoxic and hypoxic coleoptiles maintained a higher net uptake of K+ [3.0±0.1 and 2.6±0.1 μmol K+ g−1 (FW) h−1] than anoxic coleoptiles [0.9±0.2 μmol K+ g−1 (FW) h−1]. Because K+ uptake was measured by depletion, the contribution of K+ efflux to net uptake was accounted for by substituting Rb+ for K+ within the medium. This allowed us to estimate gross K+ influx because Rb+ mimics K+ by entering through K+ transporters (Etherton, 1967) but is not significantly effluxed in the 4 h time-course of the experiment. Rates of Rb+ influx for normoxic and hypoxic coleoptiles were between four and five times greater than in anoxic coleoptiles [3.9±0.2, 3.6±0.3, and 0.8±0.2 μmol Rb+ g−1 (FW) h−1, respectively]. Low levels of efflux occurred when O2 was present but not in anoxic coleoptiles.
Devising a budget of ATP consumption by rice coleoptiles
Significant differences were calculated in both the amounts and proportions of ATP allocated to each of the major ATP-consuming processes between the three O2 treatments (Table 7). Key anabolic processes were analysed using substrates to estimate instantaneous rates of synthesis. Energy that was not accounted for by this reconciliation is identified in the last line of Table 7.
Table 7.
Breakdown of ATP costs associated with various biosynthetic processes for 3-d-old coleoptiles grown in normoxic (aerated), hypoxic (3% O2), and anoxic (N2) solution
Rates associated with each process are taken from Tables 2–6. The estimated costs for each process (given as moles of ATP consumed to produce one mole of product) were taken from the following: protein synthesis, Amthor (2000); cell wall synthesis, Penning de Vries (1974); lipid synthesis, Penning de Vries (1974) and Goodwin and Mercer (1985); nucleic acid synthesis, Penning de Vries (1974). ATP production via oxidative phosphorylation assumes an ATP:O2 of 5 for normoxic and hypoxic coleoptiles, respectively (Gibbs and Greenway, 2003). Total rates of ATP generation/utilization were calculated by multiplying the rate of the process in each treatment by its associated ATP cost and are expressed on the basis of nmol (ATP consumed) g−1 (FW) min−1. Carbohydrate import is expressed as sucrose equivalents. Nitrogen import is expressed as nitrate equivalent. Rates shown are the mean calculated rates for each process (n ≥3 for all rates).
| Normoxic | Hypoxic | Anoxic | |||||
| ATP-generating processes | ATP generated by processa | Rate of process in tissueb | Total ATP generatedc | Rate of process in tissueb | Total ATP generatedc | Rate of process in tissueb | Total ATP generatedc |
| Respiration (ATP:O2) | 5 | 490 | 2450±245 | 270 | 1350±210 | N/A | N/A |
| Ethanol fermentation (ATP:ethanol) | 1 (hypoxia) or 2 (anoxia) | 14 | 14±3 | 85 | 85±3 | 151 | 302±26 |
| Total ATP generated | 2464 | 1435 | 302 | ||||
| ATP-consuming processes | ATP cost of processa | Rate of process in tissueb | Total ATP costc | Rate of process in tissueb | Total ATP costc | Rate of process in tissueb | Total ATP costc |
| Protein | 5 | 93 | 465±30 | 41 | 205±10 | 32 | 160±10 |
| Cell wall | 3 | 24 | 72±9 | 25 | 75±18 | 5 | 15±4 |
| Lipid | 18 | 4 | 72±18 | 3 | 54±13 | 2 | 36±2 |
| Nucleic acid | 4 | 6 | 24±4 | 2 | 8±1 | 1 | 4±1 |
| K+ influx | 1 | 44 | 44±3 | 45 | 45±4 | 15 | 15±6 |
| Carbohydrate (sucrose) import (from seed) | 1 | 100 | 100±34 | 51 | 51±11 | 46 | 46±9 |
| Nitrogen (nitrate) import (from seed) | 1 | 22 | 22±8 | 24 | 24±7 | 9 | 9±2 |
| Amino acid synthesis | 4 | 17 | 68±15 | 18 | 72±12 | 7 | 28±6 |
| Total ATP consumed | 867±122 | 534±76 | 313±40 | ||||
| ATP generation/consumption | 1597 | 901 | –11 | ||||
a Units are mol (ATP produced/consumed) mol−1 (process product).
b Units are nmol (process product) g−1 (FW) min−1.
c Units are nmol (ATP produced/consumed) g−1 (FW) min−1.
Protein synthesis consumed the largest proportion of available ATP in all three treatments [465, 205, and 160 nmol (ATP consumed) g−1 (FW) min−1 for normoxic, hypoxic, and anoxic coleoptiles, respectively]. Proportionally, however, the ATP utilized by protein synthesis in anoxic coleoptiles (∼52%) was more then double that in normoxic coleoptiles (∼19%) with a slightly smaller allocation observed in hypoxic coleoptiles (∼14%).
There was no statistically significant reduction in the absolute rate of ATP consumed by cell wall synthesis in hypoxic vs. normoxic coleoptiles [72 and 75 nmol (ATP consumed) g−1 (FW) min−1, respectively]. However, the rate of ATP consumption by cell wall synthesis in anoxic coleoptiles was 15 nmol (ATP consumed) g−1 (FW) min−1, one-fifth of that in the normoxic and hypoxic tissues.
ATP consumption by lipid synthesis was ∼25% lower in hypoxic and anoxic compared to normoxic coleoptiles [54, 54, and 72 nmol (ATP consumed) g−1 (FW) min−1, respectively]). Proportional to ATP production, lipid synthesis consumed approximately five times more ATP in anoxic coleoptiles compared to normoxic coleoptiles (14% and 3%, respectively).
ATP consumption by nucleic acid synthesis in hypoxic and anoxic coleoptiles was approximately one-third or one-sixth of that in normoxic coleoptiles [(8, 4 and 24 nmol (ATP consumed) g−1 (FW) min−1, respectively]. However, there was no significant difference in the proportion of ATP allocated to nucleic acid synthesis amongst the three treatments and only a tiny proportion of the available ATP pool was expended making DNA.
Estimates of ATP consumption required to energize Rb+ influx were similar between normoxic and hypoxic coleoptiles but significantly lower in anoxic coleoptiles [44, 45, and 15 nmol (ATP consumed) g−1 (FW) min−1, respectively].
ATP was consumed in transporting carbohydrates from seeds to coleoptiles. ATP consumption estimates for transport were similar in all three treatments [157 to 187 nmoles (ATP consumed) g−1 (FW) min−1]. ATP was also required to synthesize amino acids by assuming all N arrived in coleoptiles as nitrate ions. This component of ATP consumption fell dramatically in anoxic coleoptiles compared to hypoxic and normoxic coleoptiles [28, 72, and 68 nmol (ATP consumed) g−1 (FW) min−1, respectively].
ATP production and consumption in anoxia-tolerant and -intolerant rice cultivars: using genetic variation in flood tolerance to assess cost of protein synthesis
Based on the dominant contribution of protein synthesis to ATP demand in anoxia (Table 7), further experiments were conducted to examine the cost of protein synthesis in coleoptiles of diverse anoxia-tolerant and -intolerant genotypes grown in normoxia, hypoxia, and anoxia. The six genotypes described in the Materials and methods were examined: Khaiyan, Khao Hlan On (KHO), rad (reduced alcohol dehydrogenase mutant), Kinmaze (the rad parent line), a pyruvate decarboxylase (PDC) T-DNA knockout mutant, and Nipponbare, the PDC-mutant parent line.
Table 8 shows there were no significant differences in the lengths or fresh weights of the coleoptiles of the six genotypes in normoxia. In hypoxia and anoxia, the two anoxia-tolerant lines (Khaiyan and KHO) produced coleoptiles that were significantly longer and weighed more than the less anoxia-tolerant lines (Kinmaze and Nipponbare). The genotypes with lesions in their fermentative pathways produced coleoptiles whose growth was significantly retarded compared with their parent lines.
Table 9 reports O2 consumption, ethanol synthesis, and estimated rates of ATP production for the six genotypes. For the non-mutated lines (Khaiyan, KHO, Kinmaze, and Nipponbare), patterns of respiration and fermentation were broadly the same. The two genotypes with mutations in the fermentation pathway (rad and the PDC-knockout line) had significantly impaired ATP production because of low activities of ADH and PDC, respectively (Table 9, and Supplementary Table S4 at JXB online, respectively). The energy deficit in rad was approximately twice as severe as that in the PDC-knockout line.
Incorporation of [14C] leucine was used to the assess rates of protein synthesis in the six genotypes (Table 10) based on preliminary experiments with several labelled amino acid species. Even in aerated solution, rad coleoptiles had low rates of protein synthesis, reflecting its intolerance to submergence. In anoxia, lesions in the fermentative pathway had a clear effect, reducing absolute rates of protein synthesis to less than 50% of those in non-mutated genotypes. These data give rise to an energy budget for protein synthesis (Table 11).
Table 11.
ATP consumption ascribed to protein synthesis in rice coleoptiles of a variety of anoxia-tolerant (*) and -intolerant (**) cultivars grown in normoxic (aerated), hypoxic (3% O2), or anoxic (N2) solutions ATP cost is given as nmol (ATP consumed) g−1 (FW) min−1. Per cent available ATP was calculated as the estimated cost of protein synthesis divided by the total available ATP for each condition.
| Normoxic | Hypoxic | Anoxic | ||||
| Cultivar | ATP cost | % Available ATP | ATP cost | % Available ATP | ATP cost | % Available ATP |
| Khaiyan* | 510 | 14.1 | 220 | 15.1 | 205 | 55.4 |
| KHO* | 490 | 16.5 | 255 | 18.4 | 175 | 50.0 |
| Nipponbare | 360 | 16.8 | 190 | 12.9 | 155 | 68.5 |
| Kinmaze (rad parent line) | 315 | 9.4 | 155 | 13.5 | 130 | 40.2 |
| PDC-insertional mutant** | 345 | 16.9 | 155 | 11.5 | 60 | 41.9 |
| rad** | 195 | 9.6 | 110 | 11.7 | 40 | 52.6 |
Discussion
During an O2 deficit, alcoholic fermentation in anoxic tissues produces ATP at a rate that is, at best, one-third that of aerobic tissues (Gibbs and Greenway, 2003). This dramatic decrease in ATP production must be matched by an equivalent decrease in ATP consumption. Atkinson (1968) enunciated the concept of ‘energy equilibrium’, a general theory of ATP regeneration being up-regulated during an energy deficit and ATP utilization being simultaneously down-regulated such that the two reach a new equilibrium. If the sensitivity of each anabolic process to ATP supply were identical, ATP consumption might be expected to decrease uniformly as each process succumbs to anoxia. In reality, the single curves for ‘ATP regeneration’ and ‘ATP utilization’ (Atkinson, 1968) are composites of individual responses of processes to cell energy status. This postulate will be tested below.
In our study, ATP production decreased by one-half under hypoxia and by ∼88% in anoxia when compared with normoxia. This detailed analysis of ATP consumption by Amaroo coleoptiles suggests that key processes accounted for about half of the available ATP in the presence of O2 but all available ATP in anoxic coleoptiles. That is, all ATP produced in anoxia can be accounted for by the synthesis and turnover of just four polymer classes and potassium influx. Fox et al. (1994) calculated ATP requirements of 50.2 and 4.7 μmol ATP d−1 seedling−1 to sustain seedling growth in aerobic and anaerobic seedlings, respectively. From Table 7, total ATP production by coleoptiles in these conditions was calculated as 35.3±3.5 and 2.7±0.2 μmol ATP d−1 coleoptile−1. As Fox et al. (1994) was estimating ATP utilization based on biomass transfer from the seed to seedling, the concordance of these findings is remarkable.
Protein synthesis was strongly inhibited by hypoxia and anoxia, as reported earlier for rice seedlings in hypoxia (Mohanty and Ong, 2003) and coleoptiles in anoxia (Alpi and Beevers, 1983). However, the proportion of ATP estimated to support the synthesis and turnover of protein was at least three times greater in anoxic coleoptiles compared with those in the presence of O2. Indeed, the gross rates of protein synthesis estimated to occur in anoxia (Table 3) could replace the entire protein complement approximately every 2.5 d. This accords with previously published work, suggesting that sustained gene expression and the synthesis of new proteins is critical for the acclimation of rice coleoptiles to prolonged O2 deficits (Sachs et al., 1980; Umeda et al., 1994; Huang et al., 2005; Lasanthi-Kudahettige et al., 2007). The allocation of 52% of available ATP to a restricted complement of proteins under anoxia (Sachs et al., 1980; Mujer et al., 1993; Matsumura et al., 1999) is clearly part of the acclimation response typical of rice coleoptiles.
Complex changes in transcriptional/translational patterns have been shown through studies on anaerobic rice coleoptiles. Lasanthi-Kudahettige et al. (2007) identified 3134 probe sets in microarray experiments that showed changed levels of expression (1364 increased, 1770 decreased) in anoxia compared with normoxia. Huang et al. (2005) similarly identified a number of unknown proteins by two-dimensional electrophoresis that were more abundant in anoxic coleoptiles than in normoxic controls. Of those that were identified, one vacuolar H+-pyrophosphatase was highly expressed in coleoptiles of the flood-tolerant cultivar Amaroo when exposed to anoxia (Liu et al., 2010). Similarly, the over-expression of Sub1-A, an ethylene response factor known to be induced by flooding in the highly flood-tolerant cultivar FR13A, conferred increased flood tolerance to transgenic lines compared with their flooding-intolerant parent lines (Xu et al., 2006). The current study of ATP allocation, viewed alongside the strong induction of individual genes by anoxia, suggests that there may be a broader base of anoxically induced proteins than previously thought.
Further evidence for a shift in energy allocation can be seen from lipid synthesis, where the proportion of ATP directed to membrane synthesis doubles in anoxia compared with allocation in the presence of O2. Membrane integrity is vital for survival in anoxia (Atwell et al., 1982; Greenway and Gibbs, 2003; Felle, 2005). While free fatty acids increased in anoxic potato cell cultures (Rawyler et al., 1999) indicating membrane damage (Crawford and Braendle, 1996), lipid metabolism in rice tissues is more robust under anoxia (Generosova and Vartapetian, 2005). However, unsaturated fatty acids were not synthesized in anoxic rice coleoptiles (Brown and Beevers, 1987), presumably because of the O2 requirement of desaturase enzymes. Thus, even though the lipid pool was found to be preferentially synthesized in anoxic rice coleoptiles under an energy deficit, membrane composition could have been compromised.
ATP consumption based on estimates of carbohydrate transport from seeds to coleoptiles was similar in all three treatments, indicating that anoxia did not impede either carbohydrate transport or unloading. A Pasteur Effect is typically seen in rice coleoptiles, accounting for more rapid carbohydrate catabolism in anoxia (Setter et al., 1987). In maize root tips, Saglio (1985) also showed that long-distance carbohydrate transport was not impaired by anoxia. If sugars were delivered to rapidly expanding coleoptile cells by a symplastic pathway (Scofield et al., 2007), unloading could be expected to be relatively insensitive to anoxia.
Coleoptile growth was maintained even in anoxia, notwithstanding the ∼50% fewer cells than in normoxic coleoptiles (Opik, 1973; see Supplementary Table S3 at JXB online). DNA synthesis was reduced in hypoxic as well as in anoxic coleoptiles. By contrast, cell elongation was rapid in hypoxia, as shown by a disproportionate length to biomass ratios (compare Fig. 1 with Table 1). Overall, this suggests that the energy cost of cell elongation is trivial compared with cell division.
Absolute rates of cell wall synthesis were identical in normoxic and hypoxic coleoptiles but were ∼80% lower in anoxia (Table 5). Estimates of net K+ import and K+ influx using Rb+ followed a similar response to O2 as for cell wall synthesis, leading to the conclusion that turgor maintenance and coleoptile extension is robustly maintained in severe hypoxia, driving rapid elongation. The maintenance of tissue K+ during growth in anoxia, and thus turgor pressure (Atwell et al., 1982), relies, in part, upon the import of K+ from seed reserves. This potentially lowers the costs of K+ uptake from the bathing medium.
The energetics of coleoptile growth reflect the functional requirements of this organ. The lower ATP cost of synthesizing cell walls (cf. protein and lipid) and K+ import, enable rapid coleoptile elongation, even when structural dry weight gain is compromised (Fig. 1a; Table 1). In turn, the rapid elongation of coleoptile cells (Wada, 1961) underpins the morphology classically known as the ‘Snorkel Effect’ (Kordan, 1974). In flooded rice beds, the decline in O2 concentration is gradual and, therefore, maintenance of cell wall synthesis in severe hypoxia improves the chances of survival during subsequent anoxia (suggested by Atwell et al., 1982). Even anoxic coleoptiles elongated at around 80% of the rates seen in hypoxic coleoptiles (Fig. 1), in spite of 80% less carbohydrate being directed to cell wall synthesis (Table 5). Reduced biosynthesis would conserve carbohydrates for accelerated glycolysis (the Pasteur Effect), sustaining the ATP production and turnover essential to survival in anoxia. By contrast, ATP requirements for maintenance in hypoxic and normoxic coleoptiles are easily accounted for within the 50% of ATP of unknown fate (Table 7).
To test the relationship between ATP production and consumption in O. sativa coleoptiles more rigorously, a range of genotypes with contrasting tolerance to anoxia was examined during germination and early seedling growth. By varying ethanol production the question of preferential ATP allocation could be tested more deeply.
Coleoptiles of all the genotypes without a lesion in energy production grew substantially in hypoxia/anoxia. The cultivars with the highest rates of ethanol synthesis (Khaiyan>KHO>Kinmaze>Amaroo) were also those that displayed the fastest growth in these conditions (Amaroo, Fig. 1; others, Table 8). Conversely the growth of coleoptiles of rad and the PDC-insertional mutant were compromised in anoxia (Table 8), with the rad seeds not even developing a coleoptile unless first pre-treated with 12 h of normoxia (see Materials and methods). These genetic contrasts afford an opportunity to test how a reduction in ATP consumption was achieved.
While absolute rates of protein synthesis halved in hypoxia compared with normoxia, the proportion of ATP dedicated to protein synthesis remained steady. By contrast, in anoxia the allocation of ATP to protein synthesis rose to 40–70% in all cultivars (Table 11). This establishes the preferential allocation of energy to protein synthesis during anoxia across many O. sativa genotypes regardless of submergence tolerance.
Notwithstanding these findings, those genotypes with compromised fermentative capacity (rad and PDC-insertional mutant) down-regulated protein synthesis as predicted by the Atkinson model (1968). While protein synthesis is obviously a high priority for survival in anoxia, cell maintenance processes which require ATP may be even more critical than protein synthesis.
The theoretical ATP deficit in anoxic coleoptiles suggests that there may be other unaccounted-for processes at work in the anoxic coleoptiles. These include the production of ATP by other processes within the tissue and the utilization of pyrophosphate (PPi) as an alternative energy currency during anoxic conditions. For example, Stoimenova et al. (2007) reported that mitochondria from rice roots were able to utilize nitrite as an alternative electron acceptor and produce ATP under anoxic conditions. This may explain the improvement in growth when anoxic seedlings were supplied with exogenous nitrate/nitrite (Trought and Drew, 1981; Prioul and Guyot, 1985). If nitrate were available from seed reserves (Reggiani et al., 1995), it could act as a potential electron acceptor in rice coleoptiles.
Utilization of PPi as an alternate energy currency would also alleviate the anoxic ATP deficit reported in Table 7. Huang et al. (2008) suggested that PPi, produced by a variety of cellular processes including protein synthesis, cell wall synthesis, and nucleic acid synthesis (Maeshima, 2000), would decrease energy stress in anoxic rice coleoptiles by relieving demand on the ATP pool. A series of ATP-dependent enzymes can be functionally substituted by PPi-driven analogues during anoxia (Plaxton, 1996; Huang et al., 2005; Lasanthi-Kudahettige et al., 2007; Liu et al., 2010). Igamberdiev and Kleczkowski (2011) reported that, at pH 7.2, the lower end of the cytosolic pH range in anoxic rice coleoptiles (Kulichikhin et al., 2009), the ΔG0 of PPi is about 60% of that of ATP. In the ‘anoxic’ energy budget reported in Table 7, even assuming the higher efficiency of four ATPs per glucose molecule consumed (Igamberdiev and Kleczkowski, 2011), all available energy from fermentation was accounted for. If the claim of Plaxton (1996) of a yield of five ATPs per glucose through PPi metabolism were accepted, energy yield would very slightly exceed utilization. Within the bounds of error, the budget shows that anoxic coleoptiles illustrate clearly the principle of balance between energy regeneration and utilization (Atkinson, 1968).
The bioenergetics of rice coleoptiles appears to be a paradigm for anoxia tolerance in higher plants. Bailey-Serres and Voesenek (2008) noted that plant responses to prolonged anoxia fall within two broad strategies: escape by rapid elongation to an O2 source and quiescence, where energy allocation to maintenance dominates over growth. The cultivars Khaiyan and KHO employ the escape strategy, directing scarce energy resources in anoxia to the synthesis of key proteins, many of which remain unidentified. Coleoptiles of those genotypes with compromised fermentation scarcely grow in anoxia, thus employing the quiescence strategy. Their low rates of protein synthesis reflect this ecological characterization.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Table S1. Amino acid makeup of proteins of rice coleoptiles grown in normoxic (aerated), hypoxic (3% O2), and anoxic (N2) solution.
Supplementary Table S2. Free amino acid content of rice coleoptiles grown in normoxic (aerated), hypoxic (3% O2), and anoxic (N2) solution.
Supplementary Table S3. Total DNA content and estimates of cell number of 3-d-old coleoptiles grown in normoxic (aerated), hypoxic (3% O2), and anoxic (N2) solution.
Supplementary Table S4. Pyruvate decarboxylase activity in the O. sativa PDC mutant and its parent line (cv. Nipponbare).
Acknowledgments
The authors acknowledge the help of the following individuals and groups: Mr Michael Joseph (Sydney University) for the generous gift of isotopes; Dr Abdelbagi Ismail (International Rice Research Institute) for seed of Khaiyan and Khao Hlan On; Dr Narayana Upadhyaya (CSIRO) for seed of the PDC mutant; and Professor Mikio Nakazono (Nagoya University) and Professor Hideo Matsumura (Shinshu University) for seed of rad and Kinmaze. We also thank Dr Hank Greenway for comments on the experiments, Dr Jan Gebicki for reviewing an early draft of the paper, and Denise Thomas from the Australian Proteome Analysis Facility (APAF). Work conducted at APAF was facilitated using infrastructure provided by the Australian Government through the National Collaborative Research Infrastructure Strategy (NCRIS).
References
- Alpi A, Beevers H. Effects of O2 concentration on rice seedlings. Plant Physiology. 1983;71:30–34. doi: 10.1104/pp.71.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor JS. The McCree–de Wit–Penning de Vries–Thornley respiration paradigms: 30 years later. Annals of Botany. 2000;86:1–20. [Google Scholar]
- Atkinson DE. Energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968;7:4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Atwell BJ, Waters I, Greenway H. The effect of oxygen and turbulence on elongation of coleoptiles of submergence-tolerant and -intolerant rice cultivars. Journal of Experimental Botany. 1982;33:1030–1044. [Google Scholar]
- Bailey-Serres J, Voesenek L. Flooding stress: acclimations and genetic diversity. Annual Review of Plant Biology. 2008;59:313–339. doi: 10.1146/annurev.arplant.59.032607.092752. [DOI] [PubMed] [Google Scholar]
- Brown DJ, Beevers H. Fatty acids of rice coleoptiles in air and anoxia. Plant Physiology. 1987;84:555–559. doi: 10.1104/pp.84.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WWP, Huang L, Shen M, Webster C, Burlingame AL, Roberts JKM. Patterns of protein synthesis and tolerance of anoxia in root tips of maize seedlings acclimated to a low-oxygen environment, and identification of proteins by mass spectrometry. Plant Physiology. 2000;122:295–318. doi: 10.1104/pp.122.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD, Huang S, Greenway H. Evidence for down-regulation of ethanolic fermentation and K+ effluxes in the coleoptile of rice seedlings during prolonged anoxia. Journal of Experimental Botany. 2001;52:1507–1517. doi: 10.1093/jexbot/52.360.1507. [DOI] [PubMed] [Google Scholar]
- Crawford RMM. Tolerance of anoxia and ethanol metabolism in germinating seeds. New Phytologist. 1977;79:511–517. [Google Scholar]
- Crawford RMM, Braendle R. Oxygen deprivation stress in a changing environment. Journal of Experimental Botany. 1996;47:145–159. [Google Scholar]
- Etherton B. Steady-state sodium and rubidium effluxes in Pisum sativum roots. Plant Physiology. 1967;42:685–690. doi: 10.1104/pp.42.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO. The state of food insecurity in the world. Rome: FAO; 2004. [Google Scholar]
- Felle HH. pH regulation in anoxic plants. Annals of Botany. 2005;96:519–532. doi: 10.1093/aob/mci207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Carrari F, Sweetlove LJ. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Current Opinion in Plant Biology. 2004;7:254–261. doi: 10.1016/j.pbi.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Fox TC, Kennedy RA, Rumpho ME. Energetics of plant growth under anoxia: metabolic adaptations of Oryza sativa and Echinochloa phyllopogon. Annals of Botany. 1994;74:445–455. [Google Scholar]
- Geigenburger P, Fernie AR, Gibon Y, Christ A, Stitt M. Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biological Chemistry. 2000;381:723–740. doi: 10.1515/BC.2000.093. [DOI] [PubMed] [Google Scholar]
- Generosova IP, Vartapetian BB. On the physiological role of anaerobically synthesized lipids in Oryza sativa seedlings. Russian Journal of Plant Physiology. 2005;52:481–488. [Google Scholar]
- Gibbs J, Greenway H. Review: mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology. 2003;30:353–353. doi: 10.1071/PP98095_ER. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Morrell S, Valdez A, Setter T, Greenway H. Regulation of alcoholic fermentation in coleoptiles of two rice cultivars differing in tolerance to anoxia. Journal of Experimental Botany. 2000;51:785–796. [PubMed] [Google Scholar]
- Goodwin TW, Mercer EI. Introduction to plant biochemistry. New York, New York State, USA: Pergamon Press; 1985. [Google Scholar]
- Goff SA, Ricke D, Lan TH, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica) Science. 2002;296:92–100. doi: 10.1126/science.1068275. [DOI] [PubMed] [Google Scholar]
- Greenway H, Gibbs J. Review: mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Functional Plant Biology. 2003;30:999–1036. doi: 10.1071/PP98096. [DOI] [PubMed] [Google Scholar]
- Huang S, Colmer TD, Millar AH. Does anoxia tolerance involve altering the energy currency towards PPi? Trends in Plant Science. 2008;13:221–227. doi: 10.1016/j.tplants.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Huang S, Greenway H, Colmer TD, Millar AH. Protein synthesis by rice coleoptiles during prolonged anoxia: implications for glycolysis, growth and energy utilization. Annals of Botany. 2005;96:703–715. doi: 10.1093/aob/mci222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igamberdiev AU, Kleczkowski LA. Magnesium and cell energetics in plants under anoxia. Biochemical Journal. 2011;437:373–379. doi: 10.1042/BJ20110213. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Murakami S, Kawakami Y, Kuramochi H. Growth and energy status of arrowhead tubers, pondweed turions and rice seedlings under anoxic conditions. Plant, Cell and Environment. 1999;22:505–514. [Google Scholar]
- Jackson MB. Ethylene and responses of plants to soil waterlogging and submergence. Annual Review of Plant Physiology. 1985;36:145–174. [Google Scholar]
- Kang TJ, Yang MS. Rapid and reliable extraction of genomic DNA from various wild-type and transgenic plants. BMC Biotechnology. 2004;4:20. doi: 10.1186/1472-6750-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Uchimiya H. Coleoptile senescence in rice (Oryza sativa L.) Annals of Botany. 2000;58:405–414. [Google Scholar]
- Kawano N, Ella E, Ito O, Yamauchi Y, Tanaka K. Metabolic changes in rice seedlings with different submergence tolerance after desubmergence. Environmental and Experimental Biology. 2002;47:195–203. [Google Scholar]
- Kordan H. The rice shoot in relation to oxygen supply and root growth in seedlings germinating under water. New Phytologist. 1974;73:695–697. [Google Scholar]
- Kulichikhin KY, Greenway H, Byrne L, Colmer TD. Regulation of intracellular pH during anoxia in rice coleoptiles in acidic and near neutral conditions. Journal of Experimental Biology. 2009;60:2119–2128. doi: 10.1093/jxb/erp090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasanthi-Kudahettige R, Magneschi L, Loreti E, Gonzali S, Licausi F, Novi G, Beretta O, Vitulli F, Alpi A, Perata P. Transcript profiling of the anoxic rice coleoptile. Plant Physiology. 2007;144:218–231. doi: 10.1104/pp.106.093997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilitchan S, Tangprawat C, Aryusuk K, Krisnangkura S, Chokmoh S, Krisnangkura K. Partial extraction method for the rapid analysis of total lipids and [gamma]-oryzanol contents in rice bran. Food Chemistry. 2008;106:752–759. [Google Scholar]
- Lin C-M, Koh S, Yu S-M, Lin T-Y, Tsay Y F. Cloning and functional characterization of a constitutively expressed nitrate transporter gene, OsNRT1, from rice. Plant Physiology. 2000;112:379–388. doi: 10.1104/pp.122.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zhang Q, Burton RA, Shirley NJ, Atwell BJ. Expression of vacuolar H+-pyrophosphatase (OVP3) is under control of an anoxia-inducible promoter in rice. Plant Molecular Biology. 2010;72:47–60. doi: 10.1007/s11103-009-9549-z. [DOI] [PubMed] [Google Scholar]
- Maeshima M. Vacuolar H+-pyrophosphatase. Biochimica et Biophysica Acta: Biomembranes. 2000;1465:37–51. doi: 10.1016/s0005-2736(00)00130-9. [DOI] [PubMed] [Google Scholar]
- Magneschi L, Perata P. Rice germination and seedling growth in the absence of oxygen. Annals of Botany. 2009;103:181–196. doi: 10.1093/aob/mcn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H, Nirasawa S, Terauchi R. Transcript profiling in rice (Oryza sativa L.) seedlings using serial analysis of gene expression (SAGE) The Plant Journal. 1999;20:719–726. doi: 10.1046/j.1365-313x.1999.00640.x. [DOI] [PubMed] [Google Scholar]
- Matsumura H, Takano T, Takeda G, Uchimiya H. Adh1 is transcriptionally active but its translational product is reduced in a rad mutant of rice (Oryza sativa L.), which is vulnerable to submergence stress. Theoretical and Applied Genetics. 1998;97:1197–1203. [Google Scholar]
- Merrill AL, Watt BK. Energy value of foods. Agriculture Handbook No. 74. United States Department of Agriculture. 1973 [Google Scholar]
- Mocquot B, Prat C, Mouches C, Pradet A. Effect of anoxia on energy charge and protein synthesis in rice embryo. Plant Physiology. 1981;68:636–640. doi: 10.1104/pp.68.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty B, Ong BEEL. Contrasting effects of submergence in light and dark on pyruvate decarboxylase activity in roots of rice lines differing in submergence tolerance. Annals of Botany. 2003;91:291–300. doi: 10.1093/aob/mcf050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujer CV, Rumpho ME, Lin JJ, Kennedy RA. Constitutive and inducible aerobic and anaerobic stress proteins in the Echinochloa complex and rice. Plant Physiology. 1993;101:217–226. doi: 10.1104/pp.101.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsai R, Howell KA, Carroll A, Ivanova A, Millar AH, Whelan J. Defining core metabolic and transcriptomic responses to oxygen availability in rice embryos and young seedlings. Plant Physiology. 2009;151:306–322. doi: 10.1104/pp.109.142026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öpik H. Effect of anaerobiosis on respiratory rate, cytochrome oxidase activity and mitochondrial structures in coleoptiles of rice (Oryza sativa L.) Journal of Cell Science. 1973;12:725–739. doi: 10.1242/jcs.12.3.725. [DOI] [PubMed] [Google Scholar]
- Penning de Vries VFW, Brunsting A, Van Laar H. Products, requirements and efficiency of biosynthesis: a quantitative approach. Journal of Theoretical Biology. 1974;45:339–377. doi: 10.1016/0022-5193(74)90119-2. [DOI] [PubMed] [Google Scholar]
- Perata P, Alpi A. Plant responses to anaerobiosis. Plant Science. 1993;93:1–17. [Google Scholar]
- Plaxton WC. The organisation and regulation of plant glycolysis. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:185–214. doi: 10.1146/annurev.arplant.47.1.185. [DOI] [PubMed] [Google Scholar]
- Prioul JL, Guyot C. Role of oxygen transport and nitrate metabolism in the adaptation of wheat plant to root anaerobiosis. Physiologie Végétale. 1985;23:175–185. [Google Scholar]
- Raha S, Merante F, Proteau G, Reed JK. Simultaneous isolation of total cellular RNA and DNA from tissue culture cells using phenol and lithium chloride. Gene Analysis Techniques. 1990;7:173–177. doi: 10.1016/0735-0651(90)90022-8. [DOI] [PubMed] [Google Scholar]
- Rawyler A, Pavelic D, Gianinazzi C, Oberson J, Braendle R. Membrane lipid integrity relies on a threshold of ATP production rate in potato cell cultures submitted to anoxia. Plant Physiology. 1999;120:293–300. doi: 10.1104/pp.120.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond P, Al-Ani A, Pradet A. ATP production by respiration and fermentation, and energy charge during aerobiosis and anaerobiosis in twelve fatty and starchy germinating seeds. Plant Physiology. 1985;79:879–884. doi: 10.1104/pp.79.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiani R, Bertini F, Mattana M. Incorporation of nitrate nitrogen into amino acids during the anaerobic germination of rice. Amino Acids. 1995;9:385–390. doi: 10.1007/BF00807275. [DOI] [PubMed] [Google Scholar]
- Sachs MM, Freeling M, Okimoto R. The anaerobic proteins of maize. Cell. 1980;20:761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Saglio PH. Effect of path or sink anoxia on sugar translocation in roots of maize seedlings. Plant Physiology. 1985;77:285–290. doi: 10.1104/pp.77.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika H, Matsumura H, Takano T, Tsutsumi N, Nakazono M. A point mutation of ADH1 gene is involved in the repression of coleoptile elongation under submergence in rice. Breeding Science. 2006;56:69–74. [Google Scholar]
- Scofield GN, Hirose T, Aoki N, Furbank RT. Involvement of the sucrose transporter, OsSUT1, in the long-distance pathway for assimilate transport in rice. Journal of Experimental Botany. 2007;58:3155–3169. doi: 10.1093/jxb/erm153. [DOI] [PubMed] [Google Scholar]
- Setter TL, Ella ES, Valdez AP. Relationship between coleoptile elongation and alcoholic fermentation in rice exposed to anoxia. II. Cultivar differences. Annals of Botany. 1994;74:273–279. [Google Scholar]
- Setter TL, Ellis M, Laureles EV, Ella ES, Senadhira D, Mishra SB, Sarkarung SK, Datta S. Physiology and genetics of submergence tolerance in rice. Annals of Botany. 1997;79:67–77. [Google Scholar]
- Setter TL, Waters I, Greenway H, Atwell BJ, Kupkanchanakul T. Carbohydrate status of terrestrial plants during flooding. In: Crawford RMM, editor. Plant life in aquatic and amphibious habitats. 1987. Special Publication No. 5. British Ecological Society. Oxford, UK: Blackwell Scientific Publications, 411–433. [Google Scholar]
- Stoimenova M, Igamberdiev AU, Gupta KJ, Hill RD. Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta. 2007;226:465–474. doi: 10.1007/s00425-007-0496-0. [DOI] [PubMed] [Google Scholar]
- Trought M, Drew M. Alleviation of injury to young wheat plants in anaerobic solution cultures in relation to the supply of nitrate and other inorganic nutrients. Journal of Experimental Botany. 1981;32:509–522. [Google Scholar]
- Umeda M, Hara C, Matsubayashi Y, Li HH, Liu Q, Tadokoro F, Aotsuka S, Uchimiya H. Expressed sequence tags from cultured cells of rice (Oryza sativa L.) under stressed conditions: analysis of transcripts of genes engaged in ATP-generating pathways. Plant Molecular Biology. 1994;25:469–478. doi: 10.1007/BF00043875. [DOI] [PubMed] [Google Scholar]
- Wada S. Growth patterns of rice coleoptiles grown on water and under water. Scientific Reports of Tohoku University Series IV (Biology) 1961;27:199–207. [Google Scholar]
- Wang W, Scali M, Vignani R, Spadafora A, Sensi E, Mazzuca S, Cresti M. Protein extraction for two-dimensional electrophoresis from olive leaf, a plant tissue containing high levels of interfering compounds. Electrophoresis. 2003;24:2369–2375. doi: 10.1002/elps.200305500. [DOI] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heurer S, Ismail AM, Bailey-Serres J, Mackill D. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Greenway H. Membrane transport in anoxic rice coleoptiles and storage tissues of beetroot. Functional Plant Biology. 1995;22:965–975. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.