Abstract
Two enzymes of the gilvocarcin biosynthetic pathway, GilMT and GilM, with unclear functions were investigated by in vitro studies using purified, recombinant enzymes along with synthetically prepared intermediates. The studies revealed GilMT as a typical S-adenosylmethionine (SAM) dependent O-methyltransferase, but GilM was identified as a pivotal enzyme in the pathway that exhibits dual functionality in that it catalyzes a reduction of a quinone intermediate to a hydroquinone, which goes hand-in-hand with a stabilizing O-methylation and a hemiacetal formation. GilM mediates its reductive catalysis through the aid of GilR that provides FADH2 for the GilM reaction, through which FAD is regenerated for the next catalytic cycle. This unusual synergy eventually completes the biosynthesis of the polyketide derived defuco-gilvocarcin chromphore.
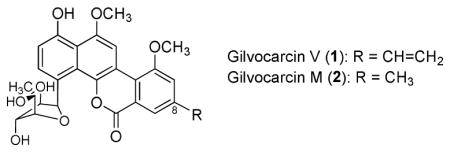
Gilvocarcin V (GV, 1), produced by Streptomyces griseoflavus and various other Streptomyces strains, is the prototype of the rare group of polycyclic aromatic polyketides with benzo[d]naphtha[1,2-b]pyran-6-one chromophore, referred to as gilvocarcin group. This group shows significant antitumor activities and remarkably low toxicity.1,2 Gilvocarcin V exhibits its light-induced anticancer activity by mediating crosslinking between DNA and histone H3.3,4 The vinyl group enhances the antitumor activity through formation of a photo-induced [2+2] cycloadduct with thymine residues of double stranded DNA,5 since the minor congeners gilvocarcin M (2) and gilvocarcin E, which lack the vinyl group, are considerably less active than GV.6 For the histone H3 interaction the D-fucofuranose moiety is considered essential.
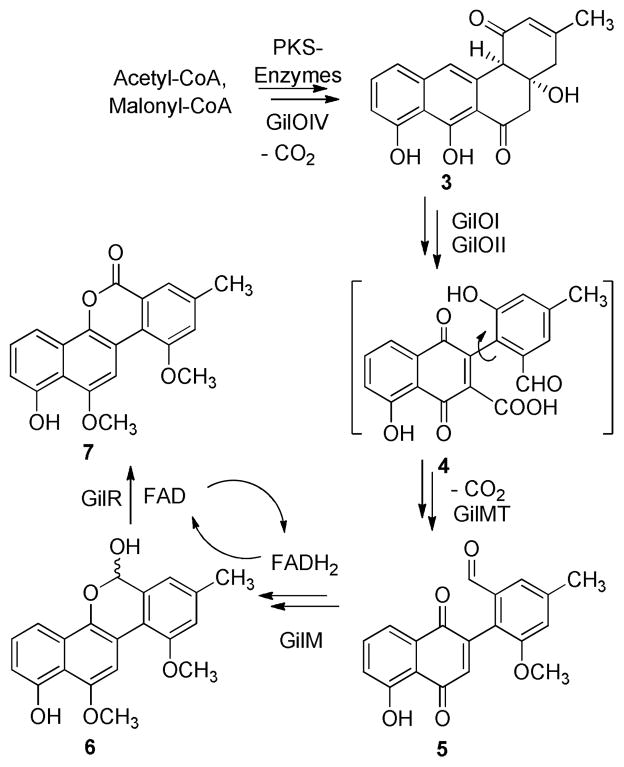
The aromatic skeleton of the gilvocarcins is produced by a type II polyketide synthase (PKS),1,7 which initially yields an angucycline biosynthetic intermediate (e.g., 3 in Scheme 1). Intriguing oxidative post-PKS modifications then convert this intermediate into the unique defuco-gilvocarcin chromophore typical for this class of anticancer drugs.8 The role of many of the post PKS gene products have been assigned based on gene inactivation, cross-feeding and labeling studies,1,7–12 and most of the genes of the three known biosynthetic clusters13 of this group have been assigned to certain enzymatic activities with gilM (encoding an enzyme of unknown function) and gilMT (encoding a methyltransferase) being notable exceptions. Thus, the functions of their genes products, GilM and GilMT, remained obscure. Both the enzymes appear to be crucial for the biosynthesis of gilvocarcin and were proposed to be involved in O-methylation reactions. However, mutant strains created by deletion of these individual genes did not accumulate any isolable metabolites, and thus provided no clue regarding their potential substrates and hence structure of key biosynthetic intermediates.
Scheme 1.
Key oxidative rearrangement and follow-up sequence of events involving GilMT and GilM reactions en route to defuco-gilvocarcin M (7)
However, a recent combinatorial biosynthetic enzymology approach led to the enzymatic total synthesis of defuco-gilvocarcin M (7) from acetyl-CoA and malonyl-CoA, a model compound with the completed gilvocarcin chromophore. Moreover, systematic variations of the enzyme mixtures revealed a hypothetical pathway suggesting that 2-aryl-5-hydroxy-1,4-naphthoquinone-3-carboxylic acid (4) is likely the product of the critical oxidative C-C bond cleavage and thereby a key intermediate of gilvocarcin V. The results also suggested that only three more enzymes, namely GilM, GilMT and GilR, were necessary to finish the biosynthesis of 7. To confirm this hypothesis and fully enlighten the “biosynthetic black box”, a potential model substrate, 2-aryl-5-hydroxy-1,4-naphthoquinone (15) was synthesized and subsequently used to interrogate the function of GilM and GilMT.
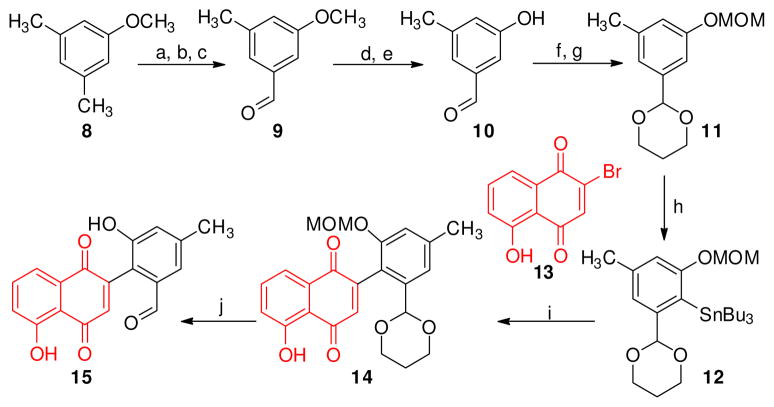
Synthesis of compound 15 began with commercially available dimethyl anisole (8).14 Bromination with NBS and benzoyl peroxide provided mono bromo benzyl derivative. A sequential hydroxylation followed by oxidation with PCC afforded 9 in 60% yield from 8.14 Removal of the methoxy group was achieved in 60% yield by treatment with BBr3 at r.t., followed by acidic hydrolysis. Protection of the aldehyde as a cyclic acetal and the phenolic OH as methoxylmethyl ether provided 11. An ortho-metalation with tributyltinchloride yielded the required stannane 12 in 88% yield.15 2-bromonaphthoquinone (13) was prepared from juglone using the reported protocol.16 A facile Stille coupling between 12 and 13 provided 14. 14 was then exposed to harsh acidic conditions (2.4 M HCl in CH3CN) for 4 min to provide fully unprotected 2-aryl-1,4-naphthaquinone 15 in 74% yield.15
With a potential substrate in hand, both gilM and gilMT genes were cloned into pET28a and expressed in E. coli to yield soluble proteins that were purified to near homogeneity. Synthetic 15 when incubated with purified GilM produced a complex mixture (Fig. 1, trace C). In contrast, HPLC-MS analysis of 15 with GilMT and S-adenosylmethionine (SAM) revealed a decrease in the amount of substrate and formation of a new product (Fig. 1, trace E), while in the absence of SAM, no product was formed (Fig. 1, trace D). The GilMT product was isolated and characterized using NMR and HRMS. The analysis revealed the product as mono-methylated derivative 5. Nuclear Overhauser enhancement (nOe) studies further confirmed that the hydroxyl group of the phenyl ring of substrate 15 was methylated.
Figure 1.
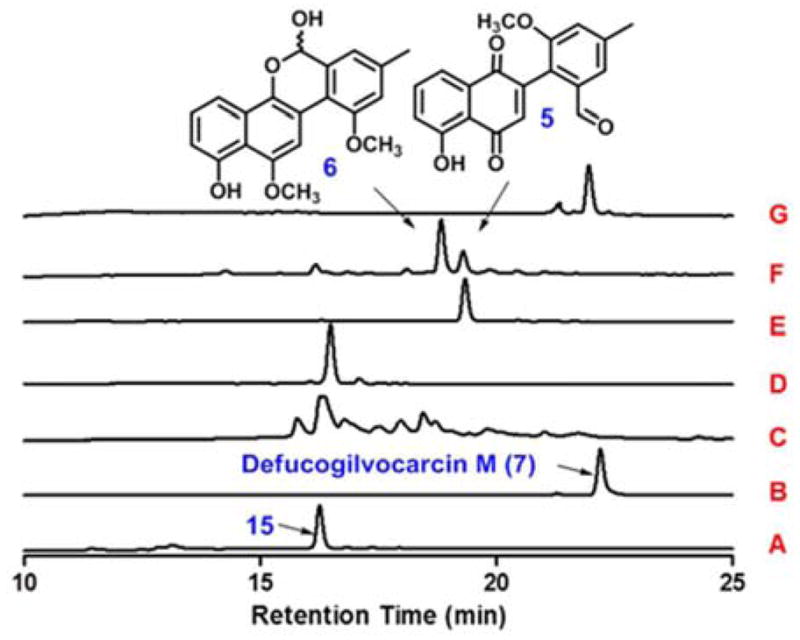
HPLC traces of the enzymatic reactions with 2-aryl-1,4-naphthaquinone (15): (A) standard 2-aryl-1,4-naphthaquinone (15); (B) standard defuco-gilvocarcin M (7); (C) 15 + GilM; (D) 15 + GilMT; (E) 15 + GilMT+ SAM; (F) 15 + GilMT + GilM + SAM; (G) 15 + GilMT + GilM + GilR + SAM.
When compound 15 was incubated with both GilM and GilMT along with SAM, a new product along with 5 accumulated (Fig. 1, trace F). The new compound was identified as defuco-pregilvocarcin M (6) by NMR experiments (1H NMR, HSQC). Adding GilR led to the accumulation of defuco-gilvocarcin M (7) (Fig. 1, trace G), further confirming the structure of the GilM/MT product as 6. In total the results suggested that GilMT is a typical SAM-dependent O-methyltransferase, while GilM was responsible for the reduction of the quinone moiety necessary for the observed second O-methylation. BLAST analysis17,18 revealed GilM to have low similarity (34–37% sequence identity) to nucleotidyl-S-transferases such as thiopurine-S-methyltransferases from Rhodococcus equi or Mycobacterium avium as well as to benzoquinone methyltransferases from Mycobacterium tuberculosis (33% sequence identity). The translated amino acid sequence of GilM contains VLDLGCGLG as residues 49–57, which appears to be a SAM binding motif (generally hh(D/E)hGXGXG, where h represents a hydrophobic residue). Thus, it remained unclear at this point whether GilMT or GilM catalyzes the second O-methylation. To solve this ambiguity, product 5 from GilMT reaction was used as substrate for GilM in the presence of SAM. The reaction accumulated 6, suggesting that GilM not only mediates reduction of the quinone but also catalyzes a second O-methylation. Surprisingly, the GilM reaction seemed to convert 5 to 6 even in absence of SAM, which prompted us to further investigate GilM for any bound SAM cofactor. The enzyme was boiled for 5 minutes and centrifuged (12000×g, 5 min). The supernatant when analyzed by HPLC showed UV-absorption at 260, typical of adenosine spectrum. A comparison with commercially available SAM verified that GilM co-purifies with SAM, thereby solving the mystery of the methyl source (Supporting information, Fig. 1).
To further analyze the reaction sequence catalyzed by GilM, compound 5 was incubated with GilM and the reaction was studied at different time points (Fig. 2). With stoichiometric enzyme quantities, the substrate was 80% converted into the product after 15 minutes (Fig. 2, trace D). When the reaction was analyzed by reversed phase HPLC-MS in 3–5 min intervals, a new peak (16) appeared with shorter retention time than the overall product 6 (Fig. 2). Although NMR analysis of 16 was impossible due to its instability, LC-MS analysis suggested it to be demethyl-defuco-pregilvocarcin M (m/z 323 [M-H]−). To prove that 16 was an intermediate en route to 6 and not a shunt product, 16 was incubated with GilM, and, as anticipated, was rapidly converted to 6 (Fig. 2, trace F). Overall, the results showed that GilM catalyzes a sequence of reactions: (i) quinone reduction, (ii) hemiacetal formation and (iii) O-methylation, to construct the tetracyclic core of the gilvocarcins.
Figure 2.
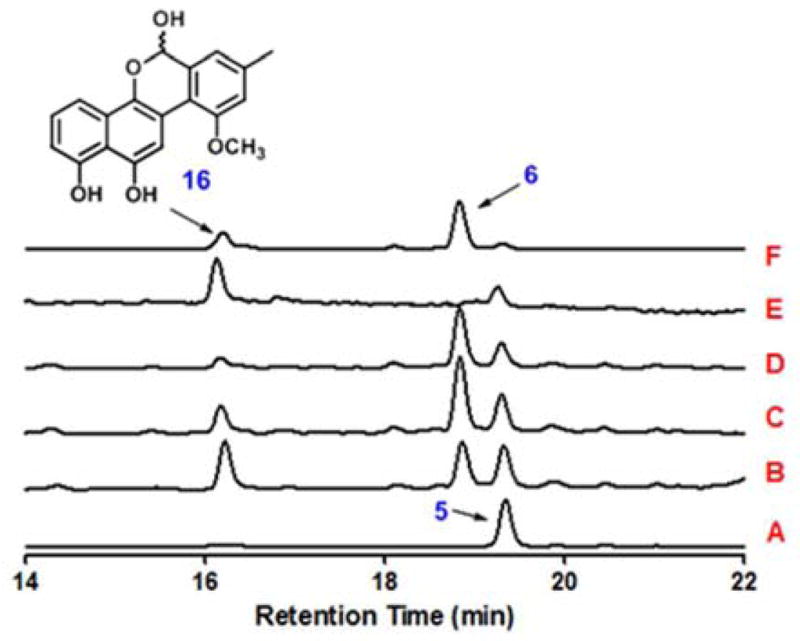
HPLC traces of the enzymatic reactions with 5: (A) standard 5; (B) 5 + GilM + SAM + 5 min; (C) 5 + GilM + SAM + 10 min; (D) 5 + GilM + SAM + 15 min; (E) intermediate (16) isolated from HPLC (mixture of closed and open form); (F) 16 + GilM.
The only remaining question was the regeneration of GilM after reduction of the quinone. Interestingly, the respective GilM-activity in the biosynthetic pathways of other structurally related compounds, chrysomycin A and ravidomycin V, is encoded on the same gene as the oxidoreductase GilR-activity,13 while in gilvocarcin pathway, gilM and gilR are two separate genes.1 This prompted us to propose that GilM could be working in conjunction with GilR, an FAD dependent oxidoreductase that catalyzes the very last step in gilvocarcin biosynthesis by converting pregilvocarcin to gilvocarcin (2), but also was shown to convert sugar free defuco-pregilvocarcins.9,19 We hypothesized that GilM utilizes the reduced flavin generated in the GilR reaction to reduce the quinone thereby regenerating oxidized flavin for the next catalytic cycle. To validate this hypothesis, compound 5 was incubated with GilM and GilR (Fig. 3). The assistance of GilR for the reducing capabilities of GilM was established by measuring the amounts of the products formed in the reactions. Catalytic amounts of GilM alone accumulated mostly starting material along with minor production of 6 (Fig. 3, trace C). In the absence of SAM, GilM and GilR accumulated a new peak (Fig. 3, trace D) that corresponds to demethyl-defuco-gilvocarcin M20 (17, m/z 321 [M-H]−). Adding SAM to the GilM-GilR reaction mixture led to the accumulation of 7 (Fig. 3, trace E). A 10-fold increase in the formation of 7 in the GilM-GilR reaction versus using GilM alone confirmed that GilM works synergistically with GilR.
Figure 3.
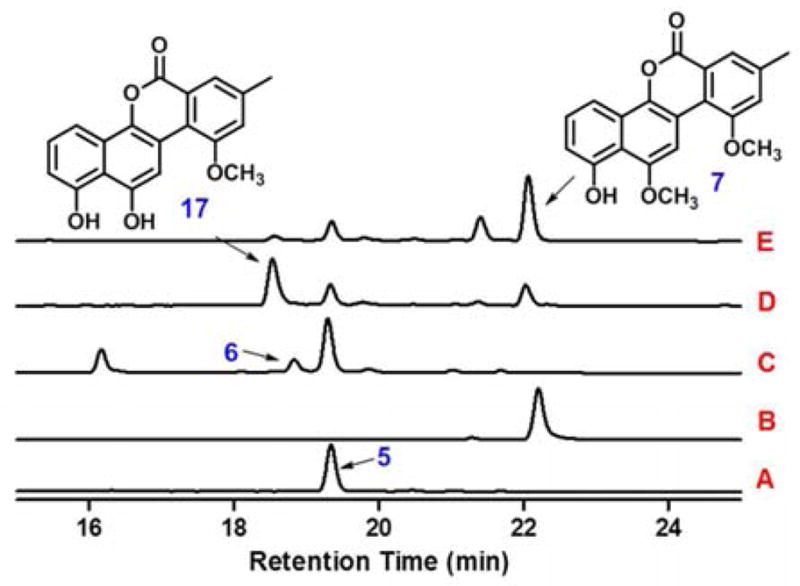
HPLC traces of the enzymatic reactions with 5: (A) standard 5; (B) standard defuco-gilvocarcin M (7); (C) 5 + GilM; (D) 5 + GilM + GilR; (E) 5 + GilM + GilR + SAM.
Overall, these results described here allowed us to fully prove the post-PKS reaction sequence of the gilvocarcin biosynthetic pathway as shown in Scheme 1. The fact that the easy to synthesize compound 15 is an intermediate of the gilvocarcin pathway opens up the future possibilities of generating gilvocarcin analogues through chemo-enzymatic synthesis or mutasynthesis,21,22 thus coupling the power of chemical synthesis with metabolic engineering.
Supplementary Material
Scheme 2. Reagents and Conditions.
(a) NBS, dibenzoyl peroxide, CCl4, reflux, 1h, 70%; (b) NaHCO3, H2O, Me2CO, reflux, 4h, 86%; (c) PCC, silica gel, CH2Cl2, 1h, quant.; (d) 1M BBr3 in CH2Cl2, 2h; (e) 1:1 HCl:AcOH, reflux, 10h, 60%; (f) 1,3-propanediol, TsOH (cat.), toluene, reflux, 12h, 75%; (g) chloro-methyl methyl ether, iPr2EtN, CH2Cl2, 40 °C, 24h, 95%; (h) n-BuLi, n-Bu3SnCl, hexane, 0°C, 88%; (i) Pd2(dba)3•CHCl3, PPh3, CuI, THF, 75 °C, 12h, 75%; (j) 2.4 M HCl in CH3CN, 74%.
Acknowledgments
This work was supported by NIH grants CA 102102 and CA 091901 to J.R. We thank Ms Manjula Sunkara as well as Drs. J. Goodman and Andrew Morris for the mass spectra.
ABBREVIATIONS
- BLAST
basic local alignment search tool
- NBS
N-bromosuccimide
- PKS
polyketide synthase
- SAM
S-adenosylmethionine
- PCC
pyridinium chlorochromate
Footnotes
Supporting Information. Synthetic procedures, kinetic studies of GilM and GilMT, enzymatic total synthesis of 6, and NMR spectra are supplied as Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Fischer C, Lipata F, Rohr J. J Am Chem Soc. 2003;125:7818. doi: 10.1021/ja034781q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakano H, Matsuda Y, Ito K, Ohkubo S, Morimoto M, Tomita F. J Antibiot. 1981;34:266. doi: 10.7164/antibiotics.34.266. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto A, Fujiwara Y, Elespuru RK, Hanawalt PC. Photochem Photobiol. 1994;60:225. doi: 10.1111/j.1751-1097.1994.tb05095.x. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto A, Hanawalt PC. Cancer Res. 2000;60:3921. [PubMed] [Google Scholar]
- 5.McGee LR, Misra R. J Am Chem Soc. 1990;112:2386. [Google Scholar]
- 6.Elespuru RK, Gonda SK. Science. 1984;223:69. doi: 10.1126/science.6229029. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd MD, Kharel MK, Zhu LL, Van Lanen SG, Rohr J. Org Biomol Chem. 2010;8:3851. doi: 10.1039/c0ob00036a. [DOI] [PubMed] [Google Scholar]
- 8.Pahari P, Kharel MK, Shepherd MD, Van Lanen SG, Rohr J. Angew Chem Int Ed Engl. 2012;51:1216. doi: 10.1002/anie.201105882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kharel MK, Pahari P, Lian H, Rohr J. ChemBioChem. 2009;10:1305. doi: 10.1002/cbic.200900130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kharel MK, Zhu L, Liu T, Rohr J. J Am Chem Soc. 2007;129:3780. doi: 10.1021/ja0680515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu T, Fischer C, Beninga C, Rohr J. J Am Chem Soc. 2004;126:12262. doi: 10.1021/ja0467521. [DOI] [PubMed] [Google Scholar]
- 12.Liu T, Kharel MK, Fischer C, McCormick A, Rohr J. ChemBioChem. 2006;7:1070. doi: 10.1002/cbic.200600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharel MK, Nybo SE, Shepherd MD, Rohr J. ChemBio-Chem. 2010;11:523. doi: 10.1002/cbic.200900673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srikrishna A, Ravikumar PC. Synthesis-Stuttgart. 2007. p. 65. [Google Scholar]
- 15.Shan M, Sharif EU, O’Doherty GA. Angew Chem Int Ed. 2010;49:9492. doi: 10.1002/anie.201005329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitani Y, Morita A, Kumamoto T, Ishikawa T. Helv Chim Acta. 2002;33:1186. [Google Scholar]
- 17.Altschul SF, Lipman DJ. Proc Natl Acad Sci USA. 1990;87:5509. doi: 10.1073/pnas.87.14.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ. Nucl Acid Res. 1997;25:3389. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noinaj N, Bosserman MA, Schickli MA, Piszczek G, Kharel MK, Pahari P, Buchanan SK, Rohr J. J Biol Chem. 2011;286:23533. doi: 10.1074/jbc.M111.247833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Frutos Ó, Atienza C, Echavarren AM. Eur J Org Chem. 2001:163–171. [Google Scholar]
- 21.Kirschning A, Hahn F. Angew Chem Int Ed. 2012;51:4012. doi: 10.1002/anie.201107386. [DOI] [PubMed] [Google Scholar]
- 22.Taft F, Harmrolfs K, Nickeleit I, Heutling A, Kiene M, Malek N, Sasse F, Kirschning A. Chem Eur J. 2012;18:880. doi: 10.1002/chem.201101640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.